Abstract
Using a PCR approach we have isolated racF1, a novel member of the Rho family in Dictyostelium. The racF1 gene encodes a protein of 193 amino acids and is constitutively expressed throughout the Dictyostelium life cycle. Highest identity (94%) was found to a RacF2 isoform, to Dictyostelium Rac1A, Rac1B, and Rac1C (70%), and to Rac proteins of animal species (64–69%). To investigate the role of RacF1 in cytoskeleton-dependent processes, we have fused it at its amino-terminus with green fluorescent protein (GFP) and studied the dynamics of subcellular redistribution using a confocal laser scanning microscope and a double-view microscope system. GFP–RacF1 was homogeneously distributed in the cytosol and accumulated at the plasma membrane, especially at regions of transient intercellular contacts. GFP–RacF1 also localized transiently to macropinosomes and phagocytic cups and was gradually released within <1 min after formation of the endocytic vesicle or the phagosome, respectively. On stimulation with cAMP, no enrichment of GFP–RacF1 was observed in leading fronts, from which it was found to be initially excluded. Cell lines were obtained using homologous recombination that expressed a truncated racF1 gene lacking sequences encoding the carboxyl-terminal region responsible for membrane targeting. These cells displayed normal phagocytosis, endocytosis, and exocytosis rates. Our results suggest that RacF1 associates with dynamic structures that are formed during pinocytosis and phagocytosis. Although RacF1 appears not to be essential, it might act in concert and/or share functions with other members of the Rho family in the regulation of a subset of cytoskeletal rearrangements that are required for these processes.
INTRODUCTION
Dictyostelium cells are equipped with an actin cytoskeleton comparable to the one found in mammalian cells, and there is an extensive body of literature dealing with structural and functional aspects of various components of the microfilament system (Noegel and Luna, 1995); however, we lack a clear picture of the link between signal transduction pathways and the reorganization of the actin cytoskeleton that takes place in response to various internal and external signals. Evidence has accumulated during the last few years regarding the role of small GTP-binding proteins of the Ras superfamily in these reorganizations. Ras-related small GTPases are molecular switches that control signaling pathways involved in a diversity of cellular processes (Macara et al., 1996). On the basis of structural and functional relationships, five main groups of small GTPases can be distinguished. Ras regulates signal transduction pathways linking plasma membrane receptors to growth and differentiation responses. Rab and ARF proteins have roles in budding and fusion of vesicles that move between different membrane compartments, and Ran proteins regulate the transport through the nuclear pore. Finally, proteins of the Rho family, composed of Rho, Rac, and Cdc42, control the cytoskeletal organization.
Microinjection studies in Swiss 3T3 cells revealed an ordered GTPase cascade in which Cdc42 acts upstream of Rac, which is upstream of Rho. In these cells Cdc42 induces filopodia formation, Rac stimulates formation of lamellipodia, and Rho induces production of stress fibers and adhesion plaques (Machesky and Hall, 1996). A considerable degree of cross-talk between family members appears to exist, particularly with Ras, which explains that in addition to controlling cytoskeletal organization Rac and Rho proteins are involved in vesicle trafficking, morphogenesis, neutrophil activation, mitogenesis, transformation, protein kinase cascades, phosphatidylinositol phosphate metabolism, and transcriptional activation (Van Aelst and D’Souza-Schorey, 1997). These GTPases interact with a multitude of guanine nucleotide exchange factors (GEFs),1 GTPase-activating proteins (GAPs), and other effectors. GEFs catalyze the conversion to the GTP-bound “on” state, and GAPs accelerate the intrinsic rate of hydrolysis of bound GTP to GDP. Additionally, GDP-dissociation inhibitors (GDIs) have been described that capture GDP-bound Rho and maintain it in an inactive form. An increasing number of downstream effectors have been identified, most of them protein kinases that establish a link to protein kinase cascades, which in turn exert their actions on cytoskeletal components (Tapon and Hall, 1997; Hall, 1998; Mackay and Hall, 1998).
In Dictyostelium discoideum, several genes coding for Rac-related proteins have been identified (Rivero and Noegel, 1998). By means of a PCR-based approach using oligonucleotide primers derived from a region corresponding to human H-Ras, amino acid 57–62, which codes for one of the most conserved domains of GTP-binding proteins, Bush et al. (1993) isolated seven rho-related genes. The sequences in this particular report (rac1A, rac1B, rac1C, and racA to racD) are highly homologous, and their patterns of expression during growth and development seem to be unique for each member, suggesting specific roles during the different stages of the Dictyostelium life cycle. RacE, another member belonging to this family, was identified as the gene being affected in a cytokinesis mutant (Larochelle et al., 1996). Except for RacC and RacE, the data available to date are insufficient to assign a functional role for each of the Dictyostelium Rac proteins. RacE appears to be essential for cytokinesis, but not for other processes such as phagocytosis, chemotaxis, or development (Larochelle et al., 1996, 1997). A role in actin cytoskeleton organization, pinocytosis, and phagocytosis has been proposed for RacC, based on a study carried on with RacC overexpressor cell lines (Seastone et al., 1998).
Using a PCR approach we have identified yet another member of the Rac subfamily, RacF1. In addition, we have identified RacF2, an isoform of RacF1, during the analysis of racF1− mutant cells. Four additional novel rac genes, racG through racJ, were found during the screening of the sequence database of the Dictyostelium cDNA project (University of Tsukuba, Japan), increasing the number of Rac proteins known in Dictyostelium to 14. The racF1 gene is constitutively expressed throughout the complete Dictyostelium developmental cycle and codes for a protein of 193 amino acids that shows highest homology to Rac1 and Rac2 proteins described in animal species. To investigate the role of RacF1 in cytoskeleton-dependent processes, we have fused it at its amino-terminus with green fluorescent protein (GFP) and studied the dynamics of subcellular redistribution using a confocal laser scanning microscope and a double-view microscope system. GFP–RacF1 was evenly distributed in the cytosol and was particularly enriched at the cell cortex, in regions of transient intercellular contacts, and on macropinosomes and phagocytic cups. By contrast, GFP–RacF1 was initially excluded from pseudopods. To investigate the function of RacF1 we have used homologous recombination to generate a cell line that expresses a truncated inactive RacF1. These cells display normal rates of pinocytosis and phagocytosis. Our results suggest that, although not essential, RacF1 might be involved in the regulation of actin cytoskeleton rearrangements that take place in a particular subset of cellular processes, through interaction with a specific array of regulating and target proteins.
MATERIALS AND METHODS
Dictyostelium Strains and Growth Conditions
Cells of Dictyostelium discoideum strain AX2-214 (referred to as wild type), an axenically growing derivative of wild strain NC-4, and all transformants used in this study were grown either in liquid nutrient medium at 21°C with shaking at 160 rpm (Claviez et al., 1982), or on SM agar plates with Klebsiella aerogenes (Williams and Newell, 1976).
Gene Cloning
PCR was performed using a Dictyostelium cDNA library as template and degenerate primers rac5 (5′-GAYGGTGCWGTTGGTAAAACYTG-3′) and rac3 (5′-TTGACCWGCRGTATCCCARAG-3′) corresponding to highly conserved regions of the sequences of several small GTPases of the Rho family known to be involved in GTP-binding (see Figure 2). The 153-bp PCR product obtained was used to screen a genomic DNA library containing HindIII fragments. A 1.16-kb clone was obtained that spanned ∼600 bp of 5′ noncoding sequences and an open reading frame interrupted by an intron. Because this clone ended at amino acid residue 157, we screened a genomic DNA library containing KpnI fragments to clone the rest of the gene. As a probe, a PCR-amplified DNA fragment downstream of the KpnI site of the 1.16-kb clone was used. A 920-bp clone was obtained that overlapped ∼0.5 kb with the HindIII clone. To fuse both overlapping clones, the HindIII clone was cut with KpnI, liberating a 0.5-kb fragment that included sequences of the polylinker of the pUC19 cloning vector. The KpnI clone was subsequently ligated into this vector.
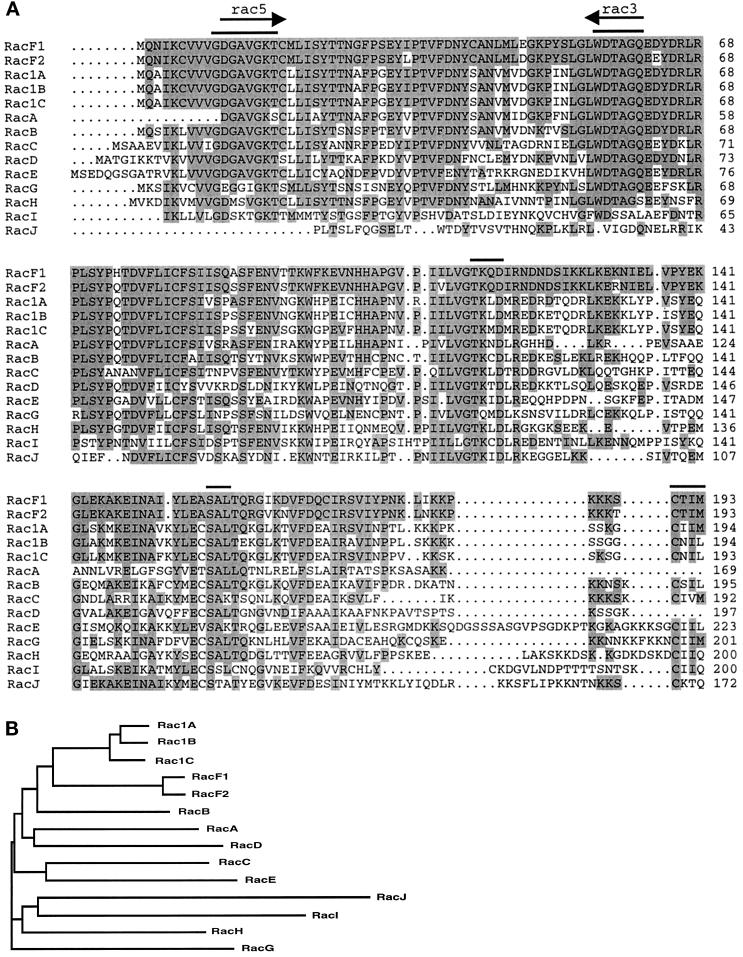
Figure 2.
(A) Alignment of RacF1 with members of the Rac subfamily in Dictyostelium. Residues that are identical between RacF1 and any of the other members of the Rac subfamily are shadowed. Arrows indicate the sequences used to design the degenerate primers for cloning the racF1 gene. Bars indicate conserved functional elements discussed in the text. GenBank accession numbers: RacF1, AF037042; RacF2, C94218; Rac1A, L11588; Rac1B, L11589; Rac1C, L11590; RacA, L11591; RacB, L11592; RacC, L11593; RacD, L11594; RacE, U41222; RacG, C92890; RacH, C92764; RacI, C84883; RacJ, C91054. Sequences corresponding to RacG, RacH, RacI, and RacJ, as well as the amino termini of Rac1C and RacD, were found after screening of the Dictyostelium sequence databank of the Japanese cDNA sequencing project (University of Tsukuba, Japan). (B) Phylogenetic analysis of the Dictyostelium Rac subfamily. The sequences most related to that of RacF1 and RacF2 are those from Rac1A, Rac1B, Rac1C, and RacB. Sequences were aligned using the Clustal W program (Wisconsin Package version 9.0), and the phylogenetic tree was displayed using TreeView (Institute of Biomedical and Life Sciences, University of Glasgow, Glasgow, United Kingdom).
The plasmid DNAs were sequenced with gene-specific primers using an automated sequencer (ABI 377 PRISM, Perkin Elmer-Cetus, Norwalk, CT). The Wisconsin Package Version 9.0 of the Genetics Computer Group (University of Wisconsin, Madison, WI) was used for sequence analysis.
Western, Southern, and Northern Blotting
DNA and RNA were isolated as described (Noegel et al., 1985), transferred onto nylon membranes (Biodyne B, Pall Filtron, Dreieich, Germany), and incubated with 32P probes generated using a random prime labeling kit (Stratagene, La Jolla, CA). Hybridization was performed at 37°C for 12–16 h in hybridization buffer containing 50% formamide and 2× SSC. The blots were washed twice for 5 min in 2× SSC containing 0.1% SDS at room temperature and for 60 min in a buffer containing 50% formamide and 2× SSC at 37°C.
Development of Dictyostelium discoideum
Cells were grown to a density of 2–3 × 106 cells/ml, washed in 17 mM Soerensen phosphate buffer, pH 6.0, and 0.8 × 108 cells were deposited on nitrocellulose filters (Millipore type HA, Millipore, Molsheim, France) and allowed to develop at 21°C as described (Newell et al., 1969). For development in shaking suspension, cells were washed as above, resuspended at 1 × 107 cells/ml in Soerensen phosphate buffer, and shaken at 160 rpm at 21°C. To test the effect of cAMP on racF1 expression, cells were starved in suspension and stimulated with 2 × 10−8 M cAMP pulses using a syringe attached to a perfusion pump.
Construction of a Vector Allowing Expression of a GFP–RacF1 Fusion
A vector was constructed that allowed expression of GFP–RacF1 in Dictyostelium cells under the control of the actin-15 promoter and the actin-8 terminator. PCR was used to amplify the coding sequence of racF1. A forward primer was designed to avoid sequences from the intron that interrupts the initiation methionine codon. The PCR product was cloned in frame at its 5′ end to the coding region of the red-shifted S65T mutant of Aequoria victoria GFP (Westphal et al., 1997). The continuous reading frame composed of GFP, the octapeptide linker KLGGRRIP derived from the cloning procedure, and RacF1 was ligated into the EcoRI site of pDEX RH (Faix et al., 1992). The resulting vector was introduced into AX2 cells by electroporation. After selection for growth in the presence of G418, transformants were confirmed by visual inspection under a fluorescence microscope.
Disruption of the racF1 Gene
For construction of a racF1 targeting vector, a 4.4-kb clone was obtained after screening a genomic DNA library containing EcoRI fragments in pUC19. A unique BglII site placed at Ser 174 was blunt-ended with Klenow before insertion of the blasticidin resistance cassette (Adachi et al., 1994). The resulting vector was introduced into AX2 cells by electroporation. Southern blot analysis was used for screening after selection for growth in the presence of blasticidin (ICN Biomedicals, Aurora, OH). In ∼40% of the transformants tested the racF1 gene was disrupted.
Fluorescence Microscopy
To record distribution of GFP–RacF1 in living cells, cells were grown to a density of 2–3 × 106 cells/ml, washed in Soerensen phosphate buffer, resuspended at a density of 1 × 107 cells/ml, and starved for 3 h with shaking. Starvation before observation was necessary to allow cells to digest endocytosed nutrient medium, which is autofluorescent. Cells were transferred onto 5 × 5-cm glass coverslips with a plastic ring for observation. For analysis of distribution of GFP–RacF1 during phagocytosis, Saccharomyces cerevisiae cells labeled with TRITC were added to the coverslips. To follow distribution of GFP–RacF1 on chemotactic stimulation, cells were handled as above, except that starvation was for 6 h. Cells were then transferred onto 5 × 5-cm glass coverslips and stimulated with a micropipette filled with 0.1 M cAMP (Gerisch et al., 1995). For studies on fixed cells, cells were fixed either in cold methanol (−20°C) or at room temperature with picric acid/paraformaldehyde (15% vol/vol of a saturated aqueous solution of picric acid/2% paraformaldehyde, pH 6.0) followed by 70% ethanol. Actin distribution was determined either by incubation with TRITC- or FITC-phalloidin (Sigma, St. Louis, MO) or by incubation with an actin-specific monoclonal antibody (Simpson et al., 1984) followed by incubation with Cy3-labeled anti-mouse IgG. Nuclei were stained with 4′,6-diamidino-2-phenylindole (Sigma).
Confocal images were taken with an inverted Zeiss LSM 410 laser scanning microscope with a 40× Neofluar 1.3 oil-immersion objective (Carl Zeiss Jena GmbH, Jena, Germany) or with an inverted Leica TCS-SP laser scanning microscope with a 63× PL Fluotar 1.32 oil-immersion objective (Leica Lasertechnik GmbH, Heidelberg, Germany). Conditions for image acquisition and processing were as described previously (Maniak et al., 1995).
For simultaneous recording of fluorescence and phase-contrast images, a double-view system was set up in an inverted fluorescence microscope (Zeiss Axiovert 100). For the phase-contrast image, red light was used. The light was filtered with a Cy5 excitation filter (HQ 620/60, AHF, Tübingen, Germany) that blocks out light also in the blue region, and a low-pass filter (LP590, Zeiss) to suppress the remaining green light. These two filters together suppress the green light from the phase-contrast image sufficiently so that the sensitive camera used for observing GFP is not excited. For exciting GFP, the normal mercury lamp was replaced with an adjustable tungsten lamp, and a narrow filter designed for red-shifted GFP (D 485/20, AHF, Tübingen, Germany) was used. The microscope was equipped with two dichroic mirrors. The first one was the one from the FITC filter set (Q 505/LP, AHF) of the microscope. The second one (FT 580, Zeiss) was placed into the beam splitter in the head of the microscope near the video output. The emission filter for GFP (HQ 535/50, AHF) was moved from its original location in the filter block directly to the front of the silicon intensifier target tube camera. Phase-contrast and fluorescence images were captured, respectively, with a charge-coupled device camera (XC-75E, Sony, Tokyo, Japan) and a silicon intensifier target tube camera (C2400–08, Hamamatsu Photonics, Hamamatsu, Japan). Observing the phase-contrast image directly through the eye piece during the experiment was also possible. A modified color frame grabber (MVC-Image Capture PCI, Imaging Technology, Bedford, MA) served as a digitizer for both cameras. The images were stored as raw data in a PC (Pentium, 120 MHz, 32 Mbyte RAM from Stemmer, Puchheim, Germany) and converted to the TIFF format after the experiment.
Phagocytosis, Fluid-Phase Endocytosis, and Exocytosis Assays
Phagocytosis was assayed as described using TRITC-labeled yeast cells (Maniak et al., 1995). Fluid-phase endocytosis and exocytosis assays were performed using FITC-dextran according to Aubry et al. (1993).
Miscellaneous Methods
Standard molecular biology methods were as described by Sambrook et al. (1989). Cell size was determined as described previously (Rivero et al., 1996).
RESULTS
Sequence and Structural Features of Dictyostelium RacF1
We used a PCR approach to isolate a 153-bp DNA fragment of racF1, a novel member of the Dictyostelium Rac subfamily of small GTP-binding proteins. This fragment was used to obtain two overlapping genomic clones that, assembled together, comprise 1.6 kb and contain the open reading frame of the racF1 gene, encoding a protein of 193 amino acids (Figure 1). Assignment of the translation start codon was assisted by comparison to other known Dictyostelium Rac amino acid sequences. The open reading frame is interrupted at the start codon by a 79-bp-long intron and is flanked by noncoding sequences containing extensive homopolymeric A+T rich stretches, as is characteristic of Dictyostelium intergenic regions (Kimmel and Firtel, 1983). We have identified a putative TATA box, 124 bp upstream of the start codon, followed by a homopolymeric T stretch, a feature that is present in many Dictyostelium promoter regions (Kimmel and Firtel, 1983). Four tandemly arranged polyadenylation signals were found immediately after the stop codon.
Figure 1.
Sequence of the Dictyostelium racF1 gene. The open reading frame (capital letters) of the racF1 gene is interrupted by a short intron. The 5′ and 3′ flanking sequences are shown in lower case letters. The predicted amino acid sequence is shown below the corresponding coding sequence. A putative TATA box followed by a poly-T stretch is shown in bold. A tandem of polyadenylation signals is underlined. The sequence is available from EMBL/GenBank/DDBJ under accession number AF037042.
Comparison to other members of the Rho family revealed that RacF1 is most closely related to animal Rac1 and Rac2 (64–69% identity). The identity to diverse Rac proteins of plant origin was lower (52–57%). Identity to members of the Cdc42 subfamily, with 58–61%, was also high, and dropped to 37–53% when compared with members of the Rho subfamily. Consequently, we initially named the gene described here racF, following the nomenclature of the different rac genes described in Dictyostelium. During the analysis of a racF1− mutant (see below), we isolated the gene corresponding to a RacF isoform. We therefore renamed both isoforms RacF1 and RacF2; they are 94% identical to each other. Comparison to the sequence database of the Dictyostelium cDNA project (University of Tsukuba, Japan) with the racF1 sequence allowed us to retrieve a sequence identical to racF2. This prompted us to extend the screening of this database for additional novel members of the Rho family. Four new clones were found that did not show strong similarity to each other or to any of the already known Rac proteins; they have consequently been named RacG through RacJ.
Figure 2A shows an alignment of the RacF1 protein sequence with all other members of the Rac subfamily in Dictyostelium whose sequence has been published (Bush et al., 1993; Larochelle et al., 1996) or released so far. After RacF2, the highest identity was found to Rac1A, Rac1B, and Rac1C (∼70%), followed by RacB (64%). RacA, RacC, and RacH scored 56–58% identity. The most divergent family members (42–50% identity) were RacD, RacE, RacG, RacI, and RacJ. These relationships are summarized in the phylogenetic analysis shown in Figure 2B.
The alignment also shows that the four conserved regions involved in GTP-binding (Bourne et al., 1991) are present in RacF1. These regions comprise the sequence G(X)4GKS/T (amino acids 10–17) that constitutes the phosphate binding loop L1, the sequence WDTAGQE (amino acids 56–62) that interacts with the gamma phosphate, the N/TKXD sequence (amino acids 115–118) or guanine specificity region, and the SAK/L sequence (amino acids 157–159). Moreover, RacF ends with a CAAX prenylation motif characteristic of all Rho superfamily members. This motif constitutes a signal for attachment of a lipid moiety. In the case of RacF1, both geranylgeranylation and farnesylation are possible according to the rules of prenylation of CAAX boxes (Moores et al., 1991). This motif is immediately preceded by a polybasic domain consisting of 6 lysine residues within 11 residues. Prenylation and the polybasic domain have been demonstrated to contribute to the association of Rho proteins with membranes (Hancock et al., 1991).
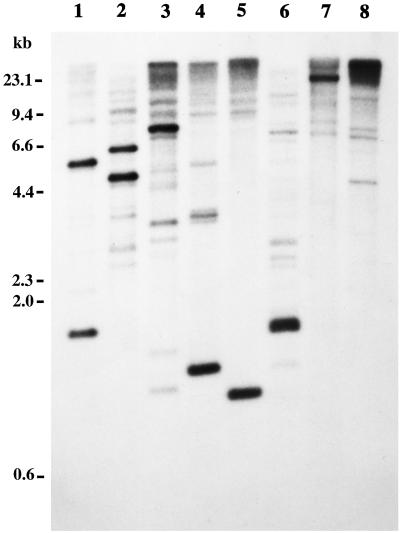
The genomic organization of the racF1 gene was studied by Southern blot analysis. Genomic DNA was cut with various restriction enzymes and hybridized under stringent conditions with a probe corresponding to the coding region of racF1 (Figure 3). The racF1 probe hybridized predominantly to one or two DNA fragments. The 1.16-kb HindIII fragment and the 920-bp KpnI fragment used to clone racF1, as well as the 4.4-kb EcoRI fragment used for generation of a knockout vector, can be clearly appreciated. Besides, additional bands are visible in every lane, indicating the presence of related genes in the Dictyostelium genome. In particular, two intense bands, a 1.6-kb BglII fragment and a 6.6-kb EcoRI fragment, cannot be explained from the sequence information available for racF1, and most probably arise by hybridization to the racF2 gene.
Figure 3.
Genomic organization of the racF1 gene. Genomic DNA was digested, and the fragments were resolved on 0.7% agarose gels and blotted onto nylon membrane. Hybridization under stringent conditions was performed with a 32P-radiolabeled probe corresponding to the coding region of racF1 generated by PCR. Genomic DNA was digested with (1) BglII, (2) EcoRI, (3) EcoRV, (4) HindIII, (5) KpnI, (6) NdeI, (7) PstI, and (8) XbaI. The 1.16-kb HindIII fragment and the 920-bp KpnI fragment used to clone racF1, as well as the 4.4-kb EcoRI fragment used for generation of a knockout vector, can be clearly appreciated. Additional bands visible in every lane indicate the presence of related genes in the Dictyostelium genome. In particular, a 1.6-kb BglII fragment and a 6.6-kb EcoRI fragment probably arise by hybridization to the racF2 gene.
Expression of racF1 during Development
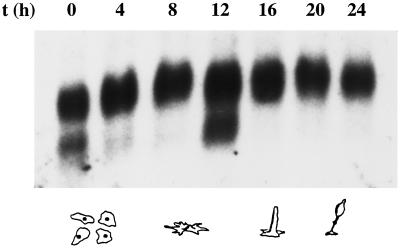
One prominent feature of the Dictyostelium life cycle is the transition from single-cell amebas to a multicellular fruiting body consisting of at least two differentiated cell types. This transition is induced by starvation of the cells and involves coordinated transcription of certain genes and differentiation and sorting out of cell populations, a process strongly dependent on the integrity of the actin cytoskeleton. We therefore used Northern blot analysis to study the expression of the racF1 gene during synchronized development on nitrocellulose filters. As shown in Figure 4, a transcript of 0.9 kb is constitutively expressed throughout the Dictyostelium developmental cycle. Additionally, a 0.7-kb transcript is present in vegetative cells and at the tipped mound stage (12 h development). When development was analyzed in cells starved in suspension, the 0.7-kb transcript was observed after 9-h starvation. We did not notice an effect of cAMP on either transcript after stimulation of aggregating cells with cAMP pulses (our unpublished results). The presence of transcripts of different sizes has been reported previously for Dictyostelium rac1C and racD (Bush et al., 1993) and can be attributed to alternative splicing or to the use of more than one promoter. Although we have identified a putative transcription initiation site (Figure 1), the existence of other possible sites in the 5′ flanking region cannot be ruled out. Finally, high quantities of RNA and prolonged exposure times after hybridization were necessary to detect racF1 mRNA, indicative of low levels of expression.
Figure 4.
Northern blot analysis of racF1 mRNA isolated from cells developed on nitrocellulose filters. Cells were washed off the filters at the times indicated for RNA extraction. Total RNA (30 μg) was resolved by gel electrophoresis, blotted onto a nylon membrane, and hybridized as described for Figure 3.
Subcellular Localization of GFP–RacF1 in Vegetative Cells
We have used a GFP tag to study the localization of RacF1 in vivo. Because the carboxyl-terminus of Rho-related proteins contains structural elements responsible for membrane association (Hancock et al., 1991), a fusion of GFP at the amino-terminal end of RacF1 was chosen. Fusion of GFP or an epitope tag at the amino terminus does not appear to disturb the function of Rac proteins, as has been shown by others for Dictyostelium RacE and RacC (Larochelle et al., 1997; Seastone et al., 1998). The average intensity of the GFP–RacF1 signal in our transformants was weak, despite several attempts to improve it by selection for higher copy numbers of the integrated expression vectors. This suggests a tight regulation of the RacF1 levels, a phenomenon also observed by Larochelle et al. (1997) for RacE. Additionally, we observed that fluorescence levels varied broadly from cell to cell (Figure 5), a common phenomenon probably related to the actin-15 promoter used to drive the expression of the GFP fusion (Westphal et al., 1997). Only occasionally could we record cells displaying very intense GFP–RacF1 fluorescence (Figure 6 shows an example). The low intensity of the GFP–RacF1 fluorescence also precluded further studies with fixed cells.
Figure 5.
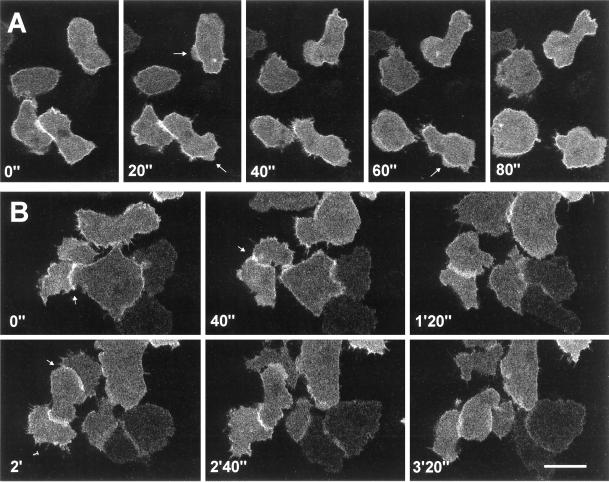
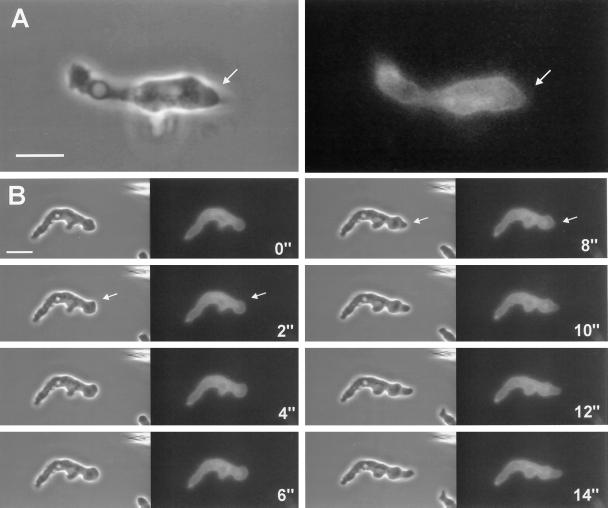
Localization of GFP–racF1 in vegetative cells. Dictyostelium cells were starved for 3 h and allowed to sit on glass coverslips. Images were taken with a confocal laser scanning microscope. (A) Distribution of GFP–RacF1 during pseudopod formation. Arrows indicate newly forming protrusions. (B) GFP–RacF1 enrichment at sites of cell-to-cell contacts, indicated by arrows. Bar, 10 μm.
Figure 6.
Dynamics of subcellular localization of GFP–RacF1 in aggregation-competent cells. Cells were starved for 6 h, allowed to sit, and stimulated with a micropipette filled with 0.1 mM cAMP. Images were taken using a double-view microscope system as described in MATERIALS AND METHODS. Each pair of images is composed of a phase-contrast (left) and a fluorescence (right) image. Image of a single cell (A) and time series (B) of a cell migrating toward the pipette, which is visible at the top right of the phase contrast image. Outbreaking protrusions (arrows) are not filled with GFP–RacF1. Bar, 10 μm.
In vegetative cells GFP–RacF1 was specifically enriched at the cell cortex, although no uniform label was observed (Figures 5 and 7), and was present in filopods (Figure 5). In addition GFP–RacF1 was homogeneously distributed throughout the cytoplasm. This pattern of distribution contrasts with the one of GFP that, being evenly distributed throughout the cytoplasm, shows no particular accumulation at specific sites (Maniak et al., 1995; Westphal et al., 1997). A cortical enrichment has been also reported for RacC and RacE (Larochelle et al., 1997), and Seastone et al. (1998) showed that 70% of RacC localizes to the membrane fraction, where it behaves like an integral protein. During pseudopod formation in vegetative cells, GFP–RacF1 enrichment along the plasma membrane exhibits a distinct pattern of relocalization. At initial stages of pseudopod formation, the local cortical enrichment of GFP-RacF1 appears unaltered; however, during outgrowth of the pseudopod, labeling of the membrane disappears. GFP–RacF1 reaccumulates in the cortex region at the front of the protrusion when the cell stops moving in the direction given by the pseudopod (Figure 5A). We noted another unique feature of GFP–RacF1 dynamics in living cells when they formed transitory cell-to-cell contacts. A strong cortical enrichment in the contact area is apparent as soon as two cells contact each other and lasts as long as the cells remain attached. On separation, GFP–RacF1 is released from the contact zones into the cytoplasm. When a cell established contacts with more than one cell simultaneously, it exhibited GFP–RacF1 enrichment in all contact areas (Figure 5B).
Figure 7.
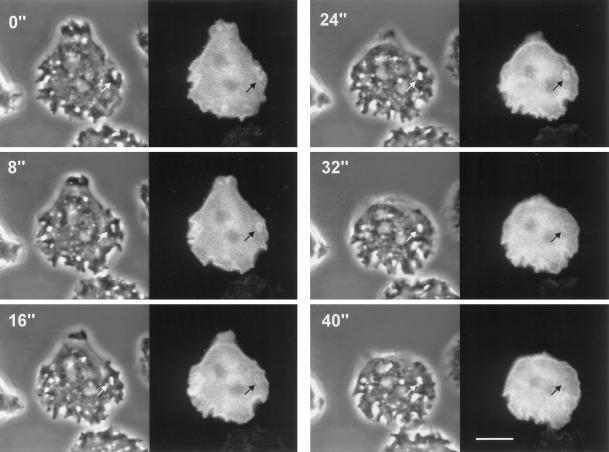
Dynamics of subcellular localization of GFP–RacF1 during endocytosis. Dictyostelium cells were starved for 3 h and allowed to sit on glass coverslips. Images were taken using a double-view microscope system as described in MATERIALS AND METHODS. Left, phase-contrast image; right, fluorescence image. Note the strong enrichment of GFP–RacF1 at the plasma membrane and the formation of endocytic vesicles. Redistribution of GFP–RacF1 is especially apparent in the vesicle indicated by arrows. Fluorescence around this vesicle is no longer visible after 32 s. Bar, 10 μm.
Redistribution of GFP–RacF1 during Chemotaxis
On starvation Dictyostelium enters a developmental program that confers on cells the capability to sense and respond to cAMP gradients. Aggregation-competent cells are elongated and locomote by extension of pseudopods in the direction of the chemoattractant, a process that depends on reorganization of the cortical actin cytoskeleton and constitutes therefore a potential target for regulation by RacF1. To study the distribution of GFP–RacF1 during chemotaxis toward a cAMP source, we have combined a micropipette assay with a double-view microscope for simultaneous visualization of fluorescence and transmitted light images.
In addition to cytoplasmic and cortical GFP–RacF1 distribution, GFP–RacF1-expressing aggregation-competent cells display one salient feature: migrating fronts are devoid of GFP–RacF1 during early stages of pseudopod formation (Figure 6A). This observation was made possible by the simultaneous recording of phase-contrast and fluorescence images and can be appreciated in more detail in the time series of Figure 6B. New protrusions, initially devoid of GFP–RacF1 (Figure 6, arrows), are progressively filled with fluorescent signal, and at the end the cortical staining around the pseudopod is reestablished.
Redistribution of GFP–RacF1 during Fluid-Phase Endocytosis
In Dictyostelium cells, macropinocytosis accounts for most of the fluid-phase uptake (Hacker et al., 1997). Because fluid-phase endocytosis in Dictyostelium is dependent on rearrangements of the actin cytoskeleton, we examined the dynamics of GFP–RacF1 distribution in vegetative cells during this process. To this end we again made use of the double-view microscope system. Figure 7 shows a time series of an actively endocytosing cell. The cortical enrichment of GFP–RacF1 is clearly apparent in this cell. The GFP–RacF1 redistribution during formation of a macropinosome can be followed for the vesicle indicated by arrows. At the beginning of the series the plasma membrane is invaginating (0 s), and at 8 s it has pinched off the membrane. The newly formed vesicle stays surrounded by GFP–RacF1 for at least 24 s. At 32 s the fluorescence is barely observable and completely absent at 40 s, although the vesicle is still present, as can be observed in the corresponding phase-contrast images. The same phenomenon can be observed with vesicles formed at the lower and left margins of the same cell. This process is highly suggestive of an involvement of RacF1 at early stages of endocytosis; on maturation of the endosome, RacF1 detaches and returns to the plasma membrane or remains in the cytoplasm.
Redistribution of GFP–RacF1 during Particle Uptake
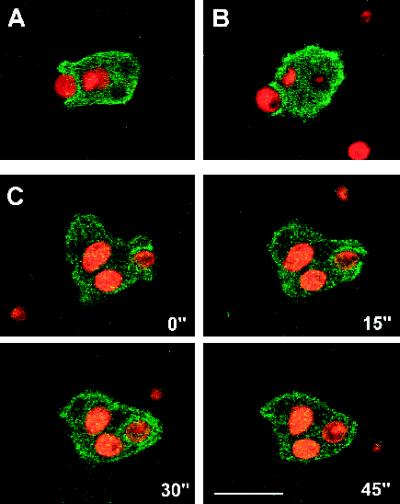
Like fluid-phase endocytosis, particle uptake in Dictyostelium, as in other cells, involves rearrangements of the actin cytoskeleton. We studied the distribution of GFP–RacF1 during phagocytosis of TRITC-labeled yeast cells (Figure 8). In a confocal laser scanning microscope the emitted light from TRITC and from GFP can be separated, allowing simultaneous and independent localization of GFP–RacF1 and yeast cells. Because of the low average levels of GFP–RacF1, we had to optimize the conditions for excitation and scanning time so that we did not damage cells. This explains the strongly pixelled aspect of the images obtained. Figure 8, A and B, shows two examples of Dictyostelium amebas engulfing a fluorescent yeast particle. In both images a predominantly cortical localization of GFP–RacF1, which is more intense at the site of yeast attachment, can be appreciated. In Figure 8A internalization of the particle occurred, whereas in the example shown in Figure 8B the yeast particle was finally rejected, indicating that relocalization of RacF1 does not irreversibly influence the molecular events in which it is involved, in agreement with a zipper mechanism of phagocytosis as proposed by Swanson and Baer (1995). In Figure 8C a series of confocal images documents the dynamics of GFP–RacF1 localization during the complete process of uptake and internalization of a yeast particle. At the beginning of the sequence (0 s), a cell already filled with two yeast particles is engulfing a new particle; attachment of this particle to the cell surface had begun at least 45 s earlier; 15 s later surface protrusions that formed around the yeast cell were about to fuse, producing an early phagosome (30 s). During the engulfment process, accumulation of GFP–RacF1 around the yeast particle was evident. Thereafter (45 s) the GFP–RacF1 had dissociated from the phagosome, suggestive of a relocalization of RacF on maturation of the phagosome, similar to what we observed during fluid-phase endocytosis (Figure 7). Indeed, GFP–RacF1 was in no case observed around yeast particles except for the very early stages of phagocytosis.
Figure 8.
Localization of GFP–RacF1 during phagocytosis of yeast cells. Dictyostelium cells were allowed to sit on glass coverslips and challenged with TRITC-labeled yeast cells. Images were taken with a confocal laser scanning microscope. Images from green and red channels were independently attributed with color codes (green for GFP and red for TRITC) and superimposed. (A and B) Accumulation of GFP–RacF1 at the phagocytic cup. (C) Time series showing the dynamics of GFP–RacF1 redistribution on uptake of a yeast cell. GFP–RacF1 fluorescence localizes around the ingested particle only at early stages of phagocytosis. Bar, 10 μm.
Generation of a racF1− Mutant
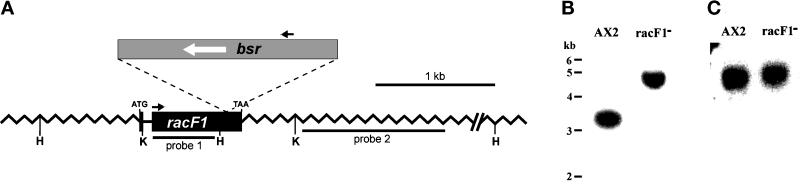
To investigate the function of RacF1 in vivo we have generated Dictyostelium knockout cell lines by homologous recombination. We made a construct in which the blasticidin resistance cassette (bsr) was inserted into a 4.4-kb genomic fragment containing the racF1 gene. Two independent mutants, 1C6 and 1D2, were isolated and further characterized. Because both behaved identically in the analyses performed, only the results obtained with mutant 1C6 are presented. Southern blot analysis was used to characterize the recombination event, and the deduced genomic organization of the replaced gene is depicted in Figure 9A. Insertion of the bsr cassette caused a shift of a 3.3-kb HindIII fragment to a 4.7-kb fragment in the mutant (Figure 9B). Because no signal was observed in the Southern blot after probing with pUC19 vector sequences, we conclude that a double cross-over event has taken place. In a further attempt to confirm the replacement of the racF1 gene we performed PCR on racF1− genomic DNA using a 5′ primer from the start codon and a 3′ primer from the stop codon of racF1. To our surprise, the PCR reaction yielded a product of the size expected for the racF1 cDNA. Sequencing of this PCR product indicated the presence of an isoform (96.4% identity at the DNA level) of RacF1, which we have termed RacF2 (Figure 2A).
Figure 9.
Disruption of the racF1 gene. (A) Diagram of the Dictyostelium racF1 gene and its disruption by the blasticidin resistance cassette (bsr) in the racF1− mutant. A construct was made in which the blasticidin resistance cassette was inserted into a unique BglII site of a 4.4-kb genomic clone. H, HindIII; K, KpnI restriction sites. Arrows indicate oligonucleotides used for PCR to confirm the insertion of the resistance cassette. (B) Southern blot analysis demonstrated that a gene replacement event had occurred. Genomic DNA was digested with HindIII, and the blot was probed with the 32P-labeled probe 2. Insertion of the bsr cassette causes a shift of a 3.3-kb band to a 4.7-kb band in the mutant. No signal was observed after probing with pUC19 vector. (C) Replacement of the racF1 gene results in an altered mRNA. Northern blot containing 30 μg RNA per lane was hybridized with probe 1. RacF1− mutant expresses a slightly larger mRNA that corresponds to a truncated racF1 plus sequences of the actin-8 terminator provided by the bsr cassette.
Northern blot analysis indicated that replacement of the racF1 gene resulted in a slightly larger mRNA as compared with the wild-type strain (Figure 9C). The explanation for this result is that because in the mutant the racF1 and the bsr genes are oriented in opposite directions, the actin-8 terminator provided by the bsr cassette is also functional for the truncated racF1 gene. This was confirmed using PCR on racF1− genomic DNA to amplify and sequence a fragment that encompasses bsr, the actin-8 terminator and the truncated racF1 gene (Figure 9A). Because antibodies specific for RacF1 are not available, we cannot determine how efficiently this altered mRNA is translated. In any case, this mRNA would give rise to a Rac protein lacking the carboxyl-terminal polybasic stretch and the prenylation signal responsible for membrane association. It is well established that members of the Ras, Rho, and Rab families require prenylation for membrane association, and disruption of prenylation prevents posttranslational modifications necessary for targeting, leading to cytosolic, inactive GTPases (Seabra, 1998). Furthermore, it has been demonstrated for mammalian proteins of the Rho family that correct carboxyl-terminal processing is essential for interaction with exchange or target proteins (Hori et al., 1991; Heyworth et al., 1993; Zigmond et al., 1997). Therefore, the putative product of the truncated racF1 gene of our mutant would be expected to be an inactive RacF1 protein.
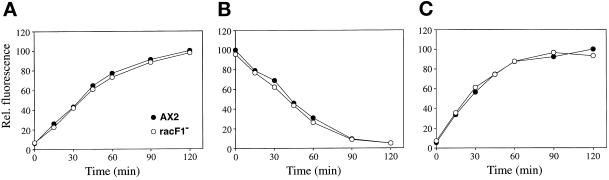
Because localization studies suggest that RacF1 participates in fluid- and solid-phase endocytosis, we quantitated both processes, as well as fluid-phase exocytosis, in the racF1− mutant. We found that racF1− cells were able to internalize and release the fluid-phase marker FITC-dextran at the same rate as AX2 cells (Figure 10, A and B). Similarly, phagocytosis of TRITC-labeled yeast cells occurred at the same rate in AX2 and racF1− cells (Figure 10C). In addition, growth of racF1− cells both in axenic medium and in suspension with Escherichia coli was comparable to wild-type cells, further supporting the finding that pinocytosis and phagocytosis are unimpaired in the mutant. No differences between wild-type and mutant cells were noted in actin distribution during the formation of macropinosomes and phagosomes (our unpublished results).
Figure 10.
RacF1 is not essential for fluid- and solid-phase uptake. Pinocytosis, exocytosis, and phagocytosis rates were assayed in AX2 and racF1− cells. (A) Pinocytosis of FITC-dextran. Cells were resuspended in fresh axenic medium at 5 × 106 cells/ml in the presence of 2 mg/ml FITC-dextran. Fluorescence from the internalized marker was measured at selected time points. (B) Fluid-phase exocytosis of FITC-dextran. Cells were pulsed with FITC-dextran (2 mg/ml) for 3 h, washed, and resuspended in fresh axenic medium. Fluorescence from the marker remaining in the cell was measured. (C) Phagocytosis of TRITC-labeled yeast cells. Dictyostelium cells were resuspended at 2 × 106 cells/ml in fresh axenic medium and challenged with fivefold excess fluorescent yeast cells. Fluorescence from internalized yeasts was measured at the designated time points. Data are presented as relative fluorescence, AX2 being considered 100%. All values are the average of three independent experiments.
Finally, we observed no differences in either the size or number of multinucleate cells between mutant and wild-type cells grown in suspension. RacF1− mutant cells developed normally, producing fruiting bodies of a morphology similar to those of the wild-type strain. Taken together, our results on racF1− cells indicate that RacF1 is not essential for endocytosis, cytokinesis, and development, its function probably been overtaken by RacF2 or other members of the Dictyostelium Rho family.
DISCUSSION
We have used a PCR approach to clone RacF1, a novel member of the Ras superfamily of small GTPases, and characterization of a racF1− mutant led to the isolation of a 94% identical RacF2 isoform. Eight different rac genes have been identified previously in Dictyostelium (Rivero and Noegel, 1998), and our work, combined with the search of public sequence databases, has allowed us to increase this number to 14. On the basis of sequence homology, RacF1 and RacF2 are more closely related to Dictyostelium Rac1A, Rac1B, and Rac1C (70%), which in turn are related to Rac1 and Rac2 proteins described in animal species. Most of the data available on the functional role of Rac proteins derive from studies performed in mammalian cells (Hall, 1994), and except for RacE, which is essential for cytokinesis (Larochelle et al., 1996), and RacC, which appears to be involved in pinocytosis and phagocytosis (Seastone et al., 1998), nothing is known about the function of the Dictyostelium Rac proteins. A GFP–RacF1 fusion allowed us to study the in vivo dynamics during several processes controlled by the actin cytoskeleton and to establish the involvement of RacF1 in pinocytosis, phagocytosis, and formation of cell-to-cell contacts.
In Dictyostelium many cytoskeletal proteins relocate in response to a chemoattractant and become enriched, together with actin, at the leading front of newly formed protrusions, where they contribute to the stabilization of actin meshworks (Noegel and Luna, 1995). In the case of RacF1, no accumulation of the GFP fusion protein could be detected at regions of cell protrusion, either cytosol or plasma membrane. In GFP–RacF1-expressing cells the local enrichment otherwise present at the cell cortex is dissipated as long as the protrusion is actively forming and is regained later, when the front stabilizes; this applies both to cells spontaneously protruding or in response to a chemoattractant. This is in line with results of Xiao et al. (1997), who reported a transient drop in fluorescence signal of a cAMP receptor type 1 GFP fusion, otherwise evenly distributed on the cell surface, in newly extended pseudopods. These observations can be attributed to a transient thinning or outstretching of the cell front during protrusion and would represent an epiphenomenon without functional significance.
Fluid-phase and particle uptake are very active processes in Dictyostelium amebas and, as in other cells, depend on the integrity of the actin system, as shown by treatment of cells with cytochalasin A (Maniak et al., 1995; Hacker et al., 1997). Besides actin, various actin-associated proteins are involved in macropinosome and phagosome formation, as revealed by immunolocalization studies and data obtained from knockout mutants (Noegel and Luna, 1995), but so far dynamic studies are available only for coronin. We have found a transient association of GFP–RacF1 to phagosomes and macropinosomes. Endocytic vesicles are initially coated with GFP–RacF1; within <1 min after internalization, this coat dissociates and the protein distributes within the cell. This pattern of redistribution strongly resembles observations made with GFP–coronin (Maniak et al., 1995; Hacker et al., 1997) and is in line with data on actin association with biochemically purified pinosomes (Nolta et al., 1994). Taken together these data support a model in which, on activation, RacF1 has the potential to promote actin polymerization at regions of endocytic activity. Once the endocytic vesicle is internalized, RacF1 becomes inactivated and dissociates from the membrane, eliciting actin disassembly and enabling fusion of the vesicle with the lysosomal compartment. The same mechanism could account for the accumulation of RacF1 in regions of transient intercellular contacts. RacF1 cycles between the cytosol and regions of the cell cortex underlying intercellular contacts where the protein could exert its actions on the actin cytoskeleton, and RacF1 becomes inactivated and dissociates when the cell contacts break. Such a model has been proposed previously for Rho GTPases (Adamson et al., 1992), and studies in cell-free systems suggest that translocation of Rho from the cytosol to the membrane fraction is controlled by its activation state (Bokoch et al., 1994). Furthermore, in neutrophils, Rac, which is localized primarily in the cytosol, translocates to the plasma membrane on stimulation (Quinn et al., 1993). At least in mammalian cells, the Rho GTPase–Rho GDI system appears to play an important role in this model of cycling of Rho-like proteins, regulating their activation state and their translocation between the cytosol and the plasma membrane (Sasaki and Takai, 1998). Rho GDIs are cytosolic proteins that preferentially bind GTPases in their inactive GDP-bound form. On stimulation the Rho–GDI complex dissociates, allowing translocation and nucleotide exchange by a GEF; once activated, the GTPase interacts with cellular targets. The GTP-bound form is then inactivated by the action of a GAP and can then be sequestered by a GDI and translocated to the cytosol, closing the cycle (Sasaki and Takai, 1998; Mackay and Hall, 1998).
A requirement of Rho-like GTPases for phagocytosis has been reported in mammalian cells. Rho was found to be essential for accumulation of F-actin around phagocytic cups and for Fcγ receptor-mediated calcium signaling in macrophages (Hackam et al., 1997), and in leukocytes expression of dominant-negative forms of Rac1 or Cdc42 partially inhibited accumulation of F-actin–rich phagocytic cups (Cox et al., 1997). It has been shown recently that myc-tagged Rho, Rac, and Cdc42 are recruited along with actin to nascent phagosomes (Caron and Hall, 1998). These authors have also demonstrated that Rho GTPases are essential for phagocytosis and have defined two different mechanisms controlled by distinct Rho GTPases: phagocytosis through the Ig receptor is mediated by Cdc42 and Rac, whereas phagocytosis through the complement receptor is mediated by Rho. Additionally, in phagocytic cells of the immune system, Rac1 and Rac2 are required for activation of NADPH oxidase, an enzyme essential for phagocytic function (Abo et al., 1991). Dictyostelium is a professional phagocyte, but a role for Rac proteins in NADPH oxidase activation in this species has not been investigated. Data from other systems also indicate an involvement of Rho-like proteins in pinocytosis. Very high levels of pinocytosis have been reported in mammalian cells overexpressing Rac (for review, see Hall, 1994); RhoB and RhoD localize to early endosomes, suggesting a potential role for these GTPases in endocytic trafficking (Adamson et al., 1992; Murphy et al., 1996). In Xenopus oocytes, RhoA acts by enhancing clathrin-independent endocytosis (Schmalzing et al., 1995), whereas in mammalian cells Rho and Rac inhibit receptor-mediated endocytosis of clathrin-coated vesicles (Lamaze et al., 1996). Studies on the possible coordinated regulation of actin-dependent events by Rac proteins in Dictyostelium have only very recently been undertaken. Overexpression of RacC resulted in cell lines with a decreased rate of endocytosis and exocytosis of a fluid-phase marker and an increase in the rate of phagocytosis. In addition, these cells displayed an abnormal morphology and changes in the actin cytoskeleton (Seastone et al., 1998). In line with the experimental data summarized above, we provide evidence that RacF1 associates with phagosomes and macropinosomes at distinct stages of the endocytic process, suggesting that this GTPase plays a role in the regulation of fluid-phase and particle uptake in Dictyostelium. The fact that racF1− mutant cells do not display alterations in pinocytosis, phagocytosis, and actin distribution is not surprising, considering the existence in Dictyostelium of a RacF2 isoform and a large number of other Rac proteins that could overtake the function of RacF1. Clearly, much work is still needed to establish the unique and redundant roles of all of these Rac proteins in the reorganization of the actin cytoskeleton in response to a diversity of stimuli.
Presently it is unknown through which mechanisms RacF1 could link signal transduction events and actin assembly around vesicles, but recent data exist that might provide some clues. Dictyostelium double-mutant cells deficient in DdPIK1 and DdPIK2, two phosphatidylinositide 3 (PI3) kinases, are impaired in pinocytosis and show additional defects related to alterations in actin cytoskeleton organization (Buczynski et al., 1997). It has been postulated that PI3 kinases participate in cytoskeleton reorganization via exchange factors that regulate Rac (Vanhaesebroeck et al., 1997), and they are potential candidates acting upstream of Rac proteins in Dictyostelium. With regard to components acting downstream of Rac, it has been recently shown that p21-activated kinase 1 (PAK1), a direct target of active Rac and Cdc42, is localized to pinocytic vesicles, among other sites, in fibroblasts (Dharmawardhane et al., 1997), and PRK1, a serine/threonine kinase, is targeted to endosomes by RhoB, where it becomes activated, suggesting a role for PRK1 in the control of endosomal trafficking (Mellor et al., 1998). Interestingly, one myosin I heavy chain kinase has been described in Dictyostelium that is closely related to PAK and activates myosin IB and ID, two myosin isoforms involved in endocytosis (Lee et al., 1996). Further studies are necessary to identify the counterparts of the mammalian targets of Rho-like proteins, determine their interaction with the Rac proteins present in Dictyostelium, and establish their contribution to endocytosis.
In summary, the in vivo study of the subcellular localization of GFP–RacF1 has allowed us to gain insights into the potential roles of this small GTP-binding protein in the rearrangement of the Dictyostelium actin cytoskeleton. Our results suggest a participation of RacF1, in concert with other members of the Rac subfamily, during fluid-phase and particle uptake, but not in pseudopod formation. It appears that specific interactions with downstream and upstream regulatory components control the unique or common pathways in which the different Dictyostelium Rac proteins participate, and further studies will be directed toward the identification and characterization of these components.
ACKNOWLEDGMENTS
We are grateful to Monika Westphal for supplying the red-shifted GFP mutant gene. This work was supported by the Deutsche Forschungsgemeinschaft No. 113/5-5, a grant from the European Community in the Human Capital and Mobility Program (CHRX-CT93-0250), and Project Grant No. 40 from the Zentrum für Molekularbiologische Medizin Köln.
Abbreviations used:
- GAP
GTPase-activating protein
- GDI
GDP-dissociation inhibitor GEF
- guanine nucleotide exchange factor
GFP, green fluorescent protein
REFERENCES
- Abo A, Pick E, Hall A, Totty N, Teahan CG, Segal AW. Activation of the NADPH oxidase involves the small GTP-binding protein p21rac1. Nature. 1991;353:668–669. doi: 10.1038/353668a0. [DOI] [PubMed] [Google Scholar]
- Adachi H, Hasebe T, Yoshinaga K, Ohta T, Sutoh K. Isolation of Dictyostelium discoideum cytokinesis mutants by restriction enzyme-mediated integration of the blasticidin S resistance marker. Biochem Biophys Res Commun. 1994;205:1808–1814. doi: 10.1006/bbrc.1994.2880. [DOI] [PubMed] [Google Scholar]
- Adamson P, Paterson HF, Hall A. Intracellular localization of the P21rho proteins. J Cell Biol. 1992;119:617–627. doi: 10.1083/jcb.119.3.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubry L, Klein G, Martiel J-L, Satre M. Kinetics of endosomal pH evolution in Dictyostelium discoideum amoebae. Study by fluorescence spectroscopy. J Cell Sci. 1993;105:861–866. doi: 10.1242/jcs.105.3.861. [DOI] [PubMed] [Google Scholar]
- Bokoch GM, Bohl BP, Chuang TH. Guanine nucleotide exchange regulates membrane translocation of Rac/Rho GTP-binding proteins. J Biol Chem. 1994;269:31674–31679. [PubMed] [Google Scholar]
- Bourne HR, Sanders DA, McCormick F. The GTPase superfamily: conserved structure and molecular mechanism. Nature. 1991;394:117–127. doi: 10.1038/349117a0. [DOI] [PubMed] [Google Scholar]
- Buczynski G, Grove B, Nomura A, Kleve M, Bush J, Firtel RA, Cardelli J. Inactivation of two Dictyostelium discoideum genes, DdPIK1 and DdPIK2, encoding proteins related to mammalian phosphatidylinositide 3-kinases, results in defects in endocytosis, lysosome to postlysosome transport, and actin cytoskeleton organization. J Cell Biol. 1997;136:1271–1286. doi: 10.1083/jcb.136.6.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush J, Franek K, Cardelli J. Cloning and characterization of seven novel Dictyostelium discoideum rac-related genes belonging to the rho family of GTPases. Gene. 1993;136:61–68. doi: 10.1016/0378-1119(93)90448-c. [DOI] [PubMed] [Google Scholar]
- Caron E, Hall A. Identification of two distinct mechanisms of phagocytosis controlled by different Rho GTPases. Science. 1998;282:1717–1721. doi: 10.1126/science.282.5394.1717. [DOI] [PubMed] [Google Scholar]
- Claviez M, Pagh K, Maruta H, Baltes W, Fisher P, Gerisch G. Electron microscopic mapping of monoclonal antibodies on the tail region of Dictyostelium myosin. EMBO J. 1982;1:1017–1022. doi: 10.1002/j.1460-2075.1982.tb01287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox D, Chang P, Zhang Q, Reddy PG, Bokoch GM, Greenberg S. Requirement for both rac1 and cdc42 in membrane ruffling and phagocytosis in leukocytes. J Exp Med. 1997;186:1487–1494. doi: 10.1084/jem.186.9.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dharmawardhane S, Sanders LC, Martin SS, Daniels RH, Bokoch GM. Localization of p21-activated kinase 1 (PAK1) to pinocytic vesicles and cortical actin structures in stimulated cells. J Cell Biol. 1997;138:1265–1278. doi: 10.1083/jcb.138.6.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faix J, Gerisch G, Noegel AA. Overexpression of the csA cell adhesion molecule under its own cAMP-regulated promoter impairs morphogenesis in Dictyostelium discoideum. J Cell Sci. 1992;102:203–214. doi: 10.1242/jcs.102.2.203. [DOI] [PubMed] [Google Scholar]
- Gerisch G, Albrecht R, Heizer C, Hodgkinson S, Maniak M. Chemoattractant-controlled accumulation of coronin at the leading edge of Dictyostelium cells monitored using a green fluorescent protein-coronin fusion protein. Curr Biol. 1995;5:1280–1285. doi: 10.1016/s0960-9822(95)00254-5. [DOI] [PubMed] [Google Scholar]
- Hackam DJ, Rotstein OD, Schreiber A, Zhang WJ, Grinstein S. Rho is required for the initiation of calcium signaling and phagocytosis by Fcγ receptors in macrophages. J Exp Med. 1997;186:955–966. doi: 10.1084/jem.186.6.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hacker U, Albrecht R, Maniak M. Fluid-phase uptake by macropinocytosis in Dictyostelium. J Cell Sci. 1997;110:105–112. doi: 10.1242/jcs.110.2.105. [DOI] [PubMed] [Google Scholar]
- Hall A. Small GTP-binding proteins and the regulation of the actin cytoskeleton. Annu Rev Cell Biol. 1994;10:31–54. doi: 10.1146/annurev.cb.10.110194.000335. [DOI] [PubMed] [Google Scholar]
- Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- Hancock JF, Cadwallader K, Paterson H, Marshall CJ. A CAAX or CAAL motif and a second signal are sufficient for plasma membrane targeting of ras proteins. EMBO J. 1991;10:4033–4039. doi: 10.1002/j.1460-2075.1991.tb04979.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyworth PG, Knaus UG, Xu X, Uhlinger DJ, Conroy L, Bokoch GM, Curnutte JT. Requirement for posttranslational processing of Rac GTP-binding proteins for activation of human neutrophil NADPH oxidase. Mol Biol Cell. 1993;4:261–269. doi: 10.1091/mbc.4.3.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hori Y, Kikuchi A, Isomura M, Katayama M, Miura Y, Fujioka H, Kaibuchi K, Takai Y. Posttranslational modifications of the C-terminal region of the rho protein are important for ist interaction with membranes and the stimulatory and inhibitory GDP/GTP exchange proteins. Oncogene. 1991;6:515–522. [PubMed] [Google Scholar]
- Kimmel AR, Firtel RA. Sequence organization in Dictyostelium: unique structure at the 5′-ends of protein coding genes. Nucleic Acids Res. 1983;11:541–552. doi: 10.1093/nar/11.2.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamaze C, Chuang TH, Terlecky LJ, Bokoch GM, Schmid SL. Regulation of receptor-mediated endocytosis by Rho and Rac. Nature. 1996;382:177–179. doi: 10.1038/382177a0. [DOI] [PubMed] [Google Scholar]
- Larochelle DA, Vithalani KK, De Lozanne A. A novel member of the rho family of small GTP-binding protein is specifically required for cytokinesis. J Cell Biol. 1996;133:1321–1329. doi: 10.1083/jcb.133.6.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larochelle DA, Vithalani KK, De Lozanne A. Role of the Dictyostelium racE in cytokinesis: mutational analysis and localization studies by use of green fluorescent protein. Mol Biol Cell. 1997;8:935–944. doi: 10.1091/mbc.8.5.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SF, Egelhoff TT, Mahasneh A, Coté GP. Cloning and characterization of a Dictyostelium myosin I heavy chain kinase activated by cdc42 and rac. J Biol Chem. 1996;271:27044–27048. doi: 10.1074/jbc.271.43.27044. [DOI] [PubMed] [Google Scholar]
- Macara IG, Lounsbury KM, Richards SA, McKiernan C, Bar-Sagi D. The Ras superfamily of GTPases. FASEB J. 1996;10:625–630. doi: 10.1096/fasebj.10.5.8621061. [DOI] [PubMed] [Google Scholar]
- Machesky LM, Hall A. Rho: a connection between membrane receptor signaling and the cytoskeleton. Trends Cell Biol. 1996;6:304–310. doi: 10.1016/0962-8924(96)10026-x. [DOI] [PubMed] [Google Scholar]
- Mackay DJG, Hall A. Rho GTPases. J Biol Chem. 1998;33:20685–20688. doi: 10.1074/jbc.273.33.20685. [DOI] [PubMed] [Google Scholar]
- Maniak M, Rauchenberger R, Albrecht R, Murphy J, Gerisch G. Coronin involved in phagocytosis: dynamics of particle-induced relocalization visualized by green fluorescent protein tag. Cell. 1995;83:915–924. doi: 10.1016/0092-8674(95)90207-4. [DOI] [PubMed] [Google Scholar]
- Mellor H, Flynn P, Nobes CD, Hall A, Parker PJ. PRK1 is targeted to endosomes by the small GTPase, RhoB. J Biol Chem. 1998;273:4811–4814. doi: 10.1074/jbc.273.9.4811. [DOI] [PubMed] [Google Scholar]
- Moores SL, Shaber MD, Mosser SD, Rands E, O’Hara MB, Garsky VM, Marshal MS, Pompliano DL, Gibbs JB. Sequence dependence of protein isoprenylation. J Biol Chem. 1991;266:14603–14610. [PubMed] [Google Scholar]
- Murphy C, et al. Endosome dynamics regulated by a Rho protein. Nature. 1996;384:427–432. doi: 10.1038/384427a0. [DOI] [PubMed] [Google Scholar]
- Newell PC, Telser A, Sussmann M. Alternative developmental pathways determined by environmental conditions in the cellular slime mold Dictyostelium discoideum. J Bacteriol. 1969;100:763–768. doi: 10.1128/jb.100.2.763-768.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noegel AA, Luna E. The Dictyostelium cytoskeleton. Experientia. 1995;51:1135–1143. doi: 10.1007/BF01944731. [DOI] [PubMed] [Google Scholar]
- Noegel AA, Metz BA, Williams KL. Developmentally regulated transcription of Dictyostelium discoideum plasmid Ddp1. EMBO J. 1985;4:3797–3803. doi: 10.1002/j.1460-2075.1985.tb04150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolta KV, Rodriguez-Paris JM, Steck TL. Analysis of successive endocytic compartments isolated from Dictyostelium discoideum by magnetic fractionation. Biochem Biophys Acta. 1994;1224:237–246. doi: 10.1016/0167-4889(94)90196-1. [DOI] [PubMed] [Google Scholar]
- Quinn MT, Evans T, Loetterle LR, Jesaitis AJ, Bokoch GM. Translocation of rac correlates with NADPH oxidase activation. J Biol Chem. 1993;268:20983–20987. [PubMed] [Google Scholar]
- Rivero F, Noegel AA. Small GTP-binding proteins of the Rho family in the Dictyostelium cytoskeleton. Protist. 1998;149:11–15. doi: 10.1016/S1434-4610(98)70004-5. [DOI] [PubMed] [Google Scholar]
- Rivero F, Furukawa R, Noegel AA, Fechheimer M. Dictyostelium discoideum cells lacking the 34,000-Dalton actin-binding protein can grow, locomote and develop, but exhibit defects in regulation of cell structure and movement: a case of partial redundancy. J Cell Biol. 1996;135:965–980. doi: 10.1083/jcb.135.4.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning: A Laboratory Manual. 2nd ed. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- Sasaki T, Takai Y. The Rho small G protein family-Rho GDI system as a temporal and spatial determinant for cytoskeletal control. Biochem Biophys Res Commun. 1998;245:641–645. doi: 10.1006/bbrc.1998.8253. [DOI] [PubMed] [Google Scholar]
- Schmalzing G, Richter HP, Hansen A, Schwarz W, Just I, Aktories K. Involvement of the GTP binding protein Rho in constitutive endocytosis in Xenopus laevis oocytes. J Cell Biol. 1995;130:1319–1332. doi: 10.1083/jcb.130.6.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seabra MC. Membrane association and targeting of prenylated Ras-like GTPases. Cell Signal. 1998;10:167–172. doi: 10.1016/s0898-6568(97)00120-4. [DOI] [PubMed] [Google Scholar]
- Seastone DJ, Lee E, Bush J, Knecht D, Cardelli J. Overexpression of a novel Rho family GTPase, RacC, induces unusual actin-based structures and positively affects phagocytosis in Dictyostelium discoideum. Mol Biol Cell. 1998;9:2891–2904. doi: 10.1091/mbc.9.10.2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson PA, Spudich JA, Parham P. Monoclonal antibodies prepared against Dictyostelium actin: characterization and interaction with actin. J Cell Biol. 1984;99:287–295. doi: 10.1083/jcb.99.1.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson JA, Baer SC. Phagocytosis by zippers and triggers. Trends Cell Biol. 1995;5:89–93. doi: 10.1016/s0962-8924(00)88956-4. [DOI] [PubMed] [Google Scholar]
- Tapon N, Hall A. Rho, Rac and Cdc42 GTPases regulate the organization of the actin cytoskeleton. Curr Opin Cell Biol. 1997;9:86–92. doi: 10.1016/s0955-0674(97)80156-1. [DOI] [PubMed] [Google Scholar]
- Van Aelst L, D’Souza-Schorey C. Rho GTPases and signaling networks. Genes Dev. 1997;11:2295–2322. doi: 10.1101/gad.11.18.2295. [DOI] [PubMed] [Google Scholar]
- Vanhaesebroeck B, Leevers SJ, Panayotu G, Waterfield MD. Phosphoinositide 3-kinases: a conserved family of signal transducers. Trends Biochem Sci. 1997;22:267–272. doi: 10.1016/s0968-0004(97)01061-x. [DOI] [PubMed] [Google Scholar]
- Westphal M, Jungbluth A, Heidecker M, Mühlbauer B, Heizer C, Schwarz J-M, Marriot G, Gerisch G. Microfilament dynamics during cell movement and chemotaxis monitored using a GFP-actin fusion. Curr Biol. 1997;7:176–183. doi: 10.1016/s0960-9822(97)70088-5. [DOI] [PubMed] [Google Scholar]
- Williams KL, Newell PC. A genetic study in the cellular slime mold Dictyostelium discoideum using complementation analysis. Genetics. 1976;82:287–307. doi: 10.1093/genetics/82.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Z, Zhang N, Murphy D, Devreotes PN. Dynamic distribution of chemoattractant receptors in living cells during chemotaxis and persistent stimulation. J Cell Biol. 1997;139:365–374. doi: 10.1083/jcb.139.2.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigmond SH, Joyce M, Borleis J, Bokoch GM, Devreotes PN. Regulation of actin polymerization in cell-free systems by GTPγS and Cdc42. J Cell Biol. 1997;138:363–374. doi: 10.1083/jcb.138.2.363. [DOI] [PMC free article] [PubMed] [Google Scholar]