Abstract
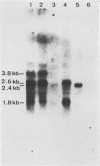
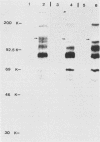
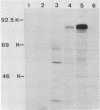
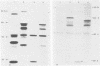
The unique short (Us) region of the pseudorabies virus (PRV) genome, which displays high transcriptional activity during the late phase of infection and has been found to code for glycoproteins, is partially deleted in the genomes of three vaccine strains (A57, Norden, and NIA-4). This deletion is located in the SalI subfragment 7A of BamHI fragment 7. To identify possible viral gene products involved in PRV virulence, we investigated the transcriptional and translational pattern of the deleted part of the Us region. Northern blots demonstrated that one major RNA species (3.8 kilobases) transcribed from fragment 7A was missing in the vaccine strains, whereas other transcripts were altered. Radioimmunoprecipitation of in vivo-labeled PRV glycoproteins and of in vitro-translated polypeptides with hyperimmune serum and monoclonal antibodies indicated a lack of glycoprotein gI. Hybrid-selection experiments with subcloned DNA fragments confirmed the absence of gI and of a 40,000-molecular-weight polypeptide. We suggest that both viral proteins are involved in the expression of PRV virulence.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ben-Porat T., Kaplan A. S. Synthesis of proteins in cells infected with herpesvirus. V. Viral glycoproteins. Virology. 1970 Jun;41(2):265–273. doi: 10.1016/0042-6822(70)90078-4. [DOI] [PubMed] [Google Scholar]
- Berns A., van den Ouweland A., Quint W., van Oirschot J., Gielkens A. Presence of markers for virulence in the unique short region or repeat region or both of pseudorabies hybrid viruses. J Virol. 1985 Jan;53(1):89–93. doi: 10.1128/jvi.53.1.89-93.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal N., Benyesh-Melnick M. Complementary nuclear RNA's of murine sarcoma-leukemia virus complex in transformed cells. Proc Natl Acad Sci U S A. 1969 Dec;64(4):1372–1379. doi: 10.1073/pnas.64.4.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmichael G. G., McMaster G. K. The analysis of nucleic acids in gels using glyoxal and acridine orange. Methods Enzymol. 1980;65(1):380–391. doi: 10.1016/s0076-6879(80)65049-6. [DOI] [PubMed] [Google Scholar]
- Field H. J., Wildy P. The pathogenicity of thymidine kinase-deficient mutants of herpes simplex virus in mice. J Hyg (Lond) 1978 Oct;81(2):267–277. doi: 10.1017/s0022172400025109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampl H., Ben-Porat T., Ehrlicher L., Habermehl K. O., Kaplan A. S. Characterization of the envelope proteins of pseudorabies virus. J Virol. 1984 Nov;52(2):583–590. doi: 10.1128/jvi.52.2.583-590.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland T. C., Sandri-Goldin R. M., Holland L. E., Marlin S. D., Levine M., Glorioso J. C. Physical mapping of the mutation in an antigenic variant of herpes simplex virus type 1 by use of an immunoreactive plaque assay. J Virol. 1983 May;46(2):649–652. doi: 10.1128/jvi.46.2.649-652.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isle H. B., Venkatesan S., Moss B. Cell-free translation of early and late mRNAs selected by hybridization to cloned DNA fragments derived from the left 14 million to 72 million daltons of the vaccinia virus genome. Virology. 1981 Jul 15;112(1):306–317. doi: 10.1016/0042-6822(81)90636-x. [DOI] [PubMed] [Google Scholar]
- Kessler S. W. Use of protein A-bearing staphylococci for the immunoprecipitation and isolation of antigens from cells. Methods Enzymol. 1981;73(Pt B):442–459. doi: 10.1016/0076-6879(81)73084-2. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lomniczi B., Blankenship M. L., Ben-Porat T. Deletions in the genomes of pseudorabies virus vaccine strains and existence of four isomers of the genomes. J Virol. 1984 Mar;49(3):970–979. doi: 10.1128/jvi.49.3.970-979.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomniczi B., Watanabe S., Ben-Porat T., Kaplan A. S. Genetic basis of the neurovirulence of pseudorabies virus. J Virol. 1984 Oct;52(1):198–205. doi: 10.1128/jvi.52.1.198-205.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukàcs N., Thiel H. J., Mettenleiter T. C., Rziha H. J. Demonstration of three major species of pseudorabies virus glycoproteins and identification of a disulfide-linked glycoprotein complex. J Virol. 1985 Jan;53(1):166–173. doi: 10.1128/jvi.53.1.166-173.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mettenleiter T. C., Lukacs N., Rziha H. J. Mapping of the structural gene of pseudorabies virus glycoprotein A and identification of two non-glycosylated precursor polypeptides. J Virol. 1985 Jan;53(1):52–57. doi: 10.1128/jvi.53.1.52-57.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rea T. J., Timmins J. G., Long G. W., Post L. E. Mapping and sequence of the gene for the pseudorabies virus glycoprotein which accumulates in the medium of infected cells. J Virol. 1985 Apr;54(1):21–29. doi: 10.1128/jvi.54.1.21-29.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Robbins A. K., Weis J. H., Enquist L. W., Watson R. J. Construction of E. coli expression plasmid libraries: localization of a pseudorabies virus glycoprotein gene. J Mol Appl Genet. 1984;2(5):485–496. [PubMed] [Google Scholar]
- Rösen A., Gelderblom H., Darai G. Transduction of virulence in herpes simplex virus type 1 from a pathogenic to an apathogenic strain by a cloned viral DNA fragment. Med Microbiol Immunol. 1985;173(5):257–278. doi: 10.1007/BF02124943. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Tenser R. B., Dunstan M. E. Herpes simplex virus thymidine kinase expression in infection of the trigeminal ganglion. Virology. 1979 Dec;99(2):417–422. doi: 10.1016/0042-6822(79)90021-7. [DOI] [PubMed] [Google Scholar]
- Tenser R. B., Ressel S. J., Fralish F. A., Jones J. C. The role of pseudorabies virus thymidine kinase expression in trigeminal ganglion infection. J Gen Virol. 1983 Jun;64(Pt 6):1369–1373. doi: 10.1099/0022-1317-64-6-1369. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson R. L., Wagner E. K., Stevens J. G. Physical location of a herpes simplex virus type-1 gene function(s) specifically associated with a 10 million-fold increase in HSV neurovirulence. Virology. 1983 Nov;131(1):180–192. doi: 10.1016/0042-6822(83)90544-5. [DOI] [PubMed] [Google Scholar]
- Wathen M. W., Wathen L. M. Isolation, characterization, and physical mapping of a pseudorabies virus mutant containing antigenically altered gp50. J Virol. 1984 Jul;51(1):57–62. doi: 10.1128/jvi.51.1.57-62.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittmann G., Ohlinger V., Rziha H. J. Occurrence and reactivation of latent Aujeszky's disease virus following challenge in previously vaccinated pigs. Arch Virol. 1983;75(1-2):29–41. doi: 10.1007/BF01314125. [DOI] [PubMed] [Google Scholar]