Abstract
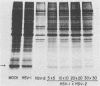
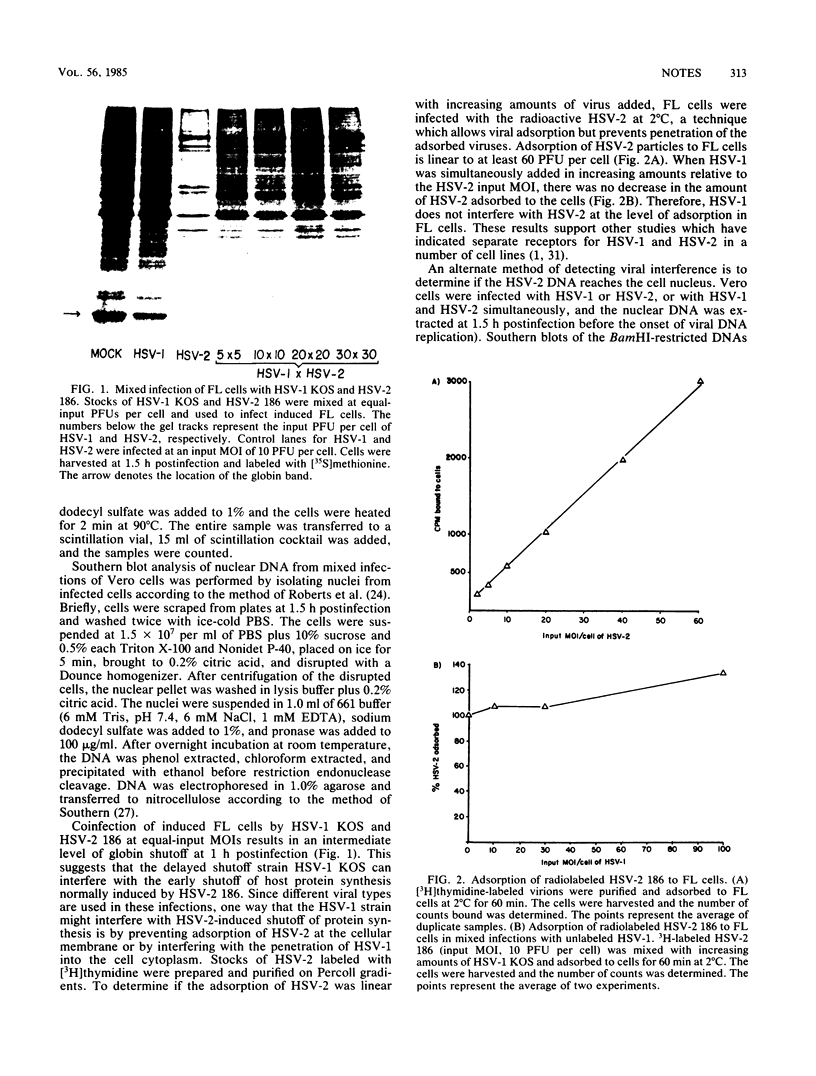
Herpes simplex virus (HSV) strains HSV type 1 (HSV-1) KOS and HSV-2 186 are representative of delayed and early shutoff strains, respectively, with regard to their ability to inhibit protein synthesis in Friend erythroleukemia cells. When these cells were simultaneously infected with HSV-1 KOS and HSV-2 186, HSV-1 KOS interfered with the rapid suppression of globin synthesis induced by HSV-2 186. The observed interference was competitive and not due to exclusion of HSV-2 by HSV-1 at the level of adsorption. Furthermore, UV-irradiated HSV-1 KOS was also effective at interfering with the early shutoff function of HSV-2 186, indicating that a virion component is responsible for the observed interference.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AURELIAN L., ROIZMAN B. ABORTIVE INFECTION OF CANINE CELLS BY HERPES SIMPLEX VIRUS. II. ALTERNATIVE SUPPRESSION OF SYNTHESIS OF INTERFERON AND VIRAL CONSTITUENTS. J Mol Biol. 1965 Mar;11:539–548. doi: 10.1016/s0022-2836(65)80009-2. [DOI] [PubMed] [Google Scholar]
- Addison C., Rixon F. J., Palfreyman J. W., O'Hara M., Preston V. G. Characterisation of a herpes simplex virus type 1 mutant which has a temperature-sensitive defect in penetration of cells and assembly of capsids. Virology. 1984 Oct 30;138(2):246–259. doi: 10.1016/0042-6822(84)90349-0. [DOI] [PubMed] [Google Scholar]
- Bablanian R., Coppola G., Scribani S., Esteban M. Inhibition of protein synthesis by vaccinia virus. IV. The role of low-molecular-weight viral RNA in the inhibition of protein synthesis. Virology. 1981 Jul 15;112(1):13–24. doi: 10.1016/0042-6822(81)90607-3. [DOI] [PubMed] [Google Scholar]
- Fenwick M. L., Clark J. Early and delayed shut-off of host protein synthesis in cells infected with herpes simplex virus. J Gen Virol. 1982 Jul;61(Pt 50):121–125. doi: 10.1099/0022-1317-61-1-121. [DOI] [PubMed] [Google Scholar]
- Fenwick M. L., Walker M. J. Suppression of the synthesis of cellular macromolecules by herpes simplex virus. J Gen Virol. 1978 Oct;41(1):37–51. doi: 10.1099/0022-1317-41-1-37. [DOI] [PubMed] [Google Scholar]
- Fenwick M., Morse L. S., Roizman B. Anatomy of herpes simplex virus DNA. XI. Apparent clustering of functions effecting rapid inhibition of host DNA and protein synthesis. J Virol. 1979 Feb;29(2):825–827. doi: 10.1128/jvi.29.2.825-827.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenkel N., Jacob R. J., Honess R. W., Hayward G. S., Locker H., Roizman B. Anatomy of herpes simplex virus DNA. III. Characterization of defective DNA molecules and biological properties of virus populations containing them. J Virol. 1975 Jul;16(1):153–167. doi: 10.1128/jvi.16.1.153-167.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galloway D. A., Swain M. Cloning of Herpes simplex virus 2 DNA fragments in a plasmid vector. Gene. 1980 Nov;11(3-4):253–257. doi: 10.1016/0378-1119(80)90065-7. [DOI] [PubMed] [Google Scholar]
- Hill T. M., Sinden R. R., Sadler J. R. Herpes simplex virus types 1 and 2 induce shutoff of host protein synthesis by different mechanisms in Friend erythroleukemia cells. J Virol. 1983 Jan;45(1):241–250. doi: 10.1128/jvi.45.1.241-250.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inglis S. C. Inhibition of host protein synthesis and degradation of cellular mRNAs during infection by influenza and herpes simplex virus. Mol Cell Biol. 1982 Dec;2(12):1644–1648. doi: 10.1128/mcb.2.12.1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isom H. C., Liao W. S., Taylor J. M., Willwerth G. E., Eadline T. S. Rapid and selective shutoff of plasma protein production in herpes simplex virus type 2-infected hepatoma cells. Virology. 1983 Apr 30;126(2):548–562. doi: 10.1016/s0042-6822(83)80012-9. [DOI] [PubMed] [Google Scholar]
- Jofre J. T., Courtney R. J., Schaffer P. A. A dominant lethal temperature-sensitive mutant of herpes simplex virus type 1. Virology. 1981 May;111(1):173–190. doi: 10.1016/0042-6822(81)90663-2. [DOI] [PubMed] [Google Scholar]
- Mocarski E. S., Post L. E., Roizman B. Molecular engineering of the herpes simplex virus genome: insertion of a second L-S junction into the genome causes additional genome inversions. Cell. 1980 Nov;22(1 Pt 1):243–255. doi: 10.1016/0092-8674(80)90172-5. [DOI] [PubMed] [Google Scholar]
- Morse L. S., Pereira L., Roizman B., Schaffer P. A. Anatomy of herpes simplex virus (HSV) DNA. X. Mapping of viral genes by analysis of polypeptides and functions specified by HSV-1 X HSV-2 recombinants. J Virol. 1978 May;26(2):389–410. doi: 10.1128/jvi.26.2.389-410.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray B. K., Biswal N., Bookout J. B., Lanford R. E., Courtney R. J., Melnick J. L. Cyclic appearance of defective interfering particles of herpes simplex virus and the concomitant accumulation of early polypeptide VP175. Intervirology. 1975;5(3-4):173–184. doi: 10.1159/000149894. [DOI] [PubMed] [Google Scholar]
- Nishioka Y., Jones G., Silverstein S. Inhibition by vesicular stomatitis virus of herpes simplex virus-directed protein synthesis. Virology. 1983 Jan 30;124(2):238–250. doi: 10.1016/0042-6822(83)90341-0. [DOI] [PubMed] [Google Scholar]
- Nishioka Y., Silverstein S. Alterations in the protein synthetic apparatus of Friend erythroleukemia cells infected with vesicular stomatitis virus or herpes simplex virus. J Virol. 1978 Jan;25(1):422–426. doi: 10.1128/jvi.25.1.422-426.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishioka Y., Silverstein S. Degradation of cellular mRNA during infection by herpes simplex virus. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2370–2374. doi: 10.1073/pnas.74.6.2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishioka Y., Silverstein S. Requirement of protein synthesis for the degradation of host mRNA in Friend erythroleukemia cells infected wtih herpes simplex virus type 1. J Virol. 1978 Sep;27(3):619–627. doi: 10.1128/jvi.27.3.619-627.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizer L. I., Beard P. The effect of herpes virus infection on mRNA in polyoma virus transformed cells. Virology. 1976 Dec;75(2):477–480. doi: 10.1016/0042-6822(76)90045-3. [DOI] [PubMed] [Google Scholar]
- Purifoy D. J., Powell K. L. Interference between strains of type 1 and type 2 herpes simplex virus. Virology. 1977 Mar;77(1):84–94. doi: 10.1016/0042-6822(77)90408-1. [DOI] [PubMed] [Google Scholar]
- ROIZMAN B., ROANE P. R., Jr THE MULTIPLICATION OF HERPES SIMPLEX VIRUS. II. THE RELATION BETWEEN PROTEIN SYNTHESIS AND THE DUPLICATION OF VIRAL DNA IN INFECTED HEP-2 CELLS. Virology. 1964 Feb;22:262–269. doi: 10.1016/0042-6822(64)90011-x. [DOI] [PubMed] [Google Scholar]
- Read G. S., Frenkel N. Herpes simplex virus mutants defective in the virion-associated shutoff of host polypeptide synthesis and exhibiting abnormal synthesis of alpha (immediate early) viral polypeptides. J Virol. 1983 May;46(2):498–512. doi: 10.1128/jvi.46.2.498-512.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts W. K., Newman J. F., Rueckert R. R. Synthesis of Maus-Elberfeld viral RNA in ascites tumor cells. J Mol Biol. 1966 Jan;15(1):92–101. doi: 10.1016/s0022-2836(66)80211-5. [DOI] [PubMed] [Google Scholar]
- Roizman B., Borman G. S., Rousta M. K. Macromolecular synthesis in cells infected with herpes simplex virus. Nature. 1965 Jun 26;206(991):1374–1375. doi: 10.1038/2061374a0. [DOI] [PubMed] [Google Scholar]
- Southern E. Gel electrophoresis of restriction fragments. Methods Enzymol. 1979;68:152–176. doi: 10.1016/0076-6879(79)68011-4. [DOI] [PubMed] [Google Scholar]
- Stenberg R. M., Pizer L. I. Herpes simplex virus-induced changes in cellular and adenovirus RNA metabolism in an adenovirus type 5-transformed human cell line. J Virol. 1982 May;42(2):474–487. doi: 10.1128/jvi.42.2.474-487.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sydiskis R. J., Roizman B. Polysomes and protein synthesis in cells infected with a DNA virus. Science. 1966 Jul 1;153(3731):76–78. doi: 10.1126/science.153.3731.76. [DOI] [PubMed] [Google Scholar]
- Vahlne A., Svennerholm B., Lycke E. Evidence for herpes simplex virus type-selective receptors on cellular plasma membranes. J Gen Virol. 1979 Jul;44(1):217–225. doi: 10.1099/0022-1317-44-1-217. [DOI] [PubMed] [Google Scholar]
- Wagner E. K., Roizman B. Ribonucleic acid synthesis in cells infected with herpes simplex virus. I. Patterns of ribonucleic acid synthesis in productively infected cells. J Virol. 1969 Jul;4(1):36–46. doi: 10.1128/jvi.4.1.36-46.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelená D., Roubal J., Vonka V. Inhibitory effect of herpes simplex virus type 1 on type 2 virus replication. J Gen Virol. 1976 Nov;33(2):249–257. doi: 10.1099/0022-1317-33-2-249. [DOI] [PubMed] [Google Scholar]