Abstract
Autism spectrum disorders (ASD) are characterized by inflexible and repetitive behaviour. Response monitoring involves evaluating the consequences of behaviour and making adjustments to optimize outcomes. Deficiencies in this function, and abnormalities in the anterior cingulate cortex (ACC) on which it relies, have been reported as contributing factors to autistic disorders. We investigated whether ACC structure and function during response monitoring were associated with repetitive behaviour in ASD. We compared ACC activation to correct and erroneous antisaccades using rapid presentation event-related functional MRI in 14 control and ten ASD participants. Because response monitoring is the product of coordinated activity in ACC networks, we also examined the microstructural integrity of the white matter (WM) underlying this brain region using diffusion tensor imaging (DTI) measures of fractional anisotropy (FA) in 12 control and 12 adult ASD participants. ACC activation and FA were examined in relation to Autism Diagnostic Interview-Revised ratings of restricted and repetitive behaviour. Relative to controls, ASD participants: (i) made more antisaccade errors and responded more quickly on correct trials; (ii) showed reduced discrimination between error and correct responses in rostral ACC (rACC), which was primarily due to (iii) abnormally increased activation on correct trials and (iv) showed reduced FA in WM underlying ACC. Finally, in ASD (v) increased activation on correct trials and reduced FA in rACC WM were related to higher ratings of repetitive behaviour. These findings demonstrate functional and structural abnormalities of the ACC in ASD that may contribute to repetitive behaviour. rACC activity following errors is thought to reflect affective appraisal of the error. Thus, the hyperactive rACC response to correct trials can be interpreted as a misleading affective signal that something is awry, which may trigger repetitive attempts at correction. Another possible consequence of reduced affective discrimination between error and correct responses is that it might interfere with the reinforcement of responses that optimize outcomes. Furthermore, dysconnection of the ACC, as suggested by reduced FA, to regions involved in behavioural control might impair on-line modulations of response speed to optimize performance (i.e. speed-accuracy trade-off) and increase error likelihood. These findings suggest that in ASD, structural and functional abnormalities of the ACC compromise response monitoring and thereby contribute to behaviour that is rigid and repetitive rather than flexible and responsive to contingencies. Illuminating the mechanisms and clinical significance of abnormal response monitoring in ASD represents a fruitful avenue for further research.
Keywords: autism, anterior cingulate cortex, response monitoring, functional MRI, diffusion tensor imaging
Introduction
Autism spectrum disorders (ASD) are neurodevelopmental disorders characterized by the early onset of marked impairments in socialization and communication along with restricted and repetitive interests and activities. There is growing evidence that executive function deficits contribute to these core symptoms (Hill, 2004; Lopez et al., 2005; South et al., 2007). Executive function refers to diverse cognitive abilities involved in behavioural control including response monitoring—evaluating whether the consequences of behaviour are consistent with its intent, and instituting adjustments to optimize outcomes. Response monitoring is thought to rely on anterior cingulate cortex (ACC) on the basis of extensive evidence from functional neuroimaging, electrophysiological, lesion and intracranial recording studies (see Taylor et al., 2007 for a recent review). There is evidence of abnormal response monitoring in autism including increased latency (and amplitude in a subset) of the error-related negativity (ERN) (Henderson et al., 2006), an event-related potential that follows errors and is thought to be generated by the ACC (Gehring et al., 1993; Dehaene et al., 1994; van Veen and Carter, 2002); reduced error self-correction (Russell and Jarrold, 1998) and reduced post-error slowing, a compensatory mechanism to improve performance on the subsequent trial (Bogte et al., 2007). Since the evaluation of behaviour and its consequences is necessary to determine whether or not current strategies should be maintained, abnormal response monitoring may contribute to behavioural rigidity in ASD. The aims of the present study were: (i) to examine ACC function during response monitoring using functional MRI (fMRI); (ii) to assess the microstructural integrity of the white matter (WM) underlying the ACC using diffusion tensor imaging (DTI) and (iii) to investigate the relations of ACC function and WM microstructure to restricted, repetitive behaviour in ASD.
There is growing evidence of both functional and structural abnormalities of the ACC in ASD. Abnormal ACC activation has been observed during a range of cognitive tasks (Haznedar et al., 1997, 2000; Hall et al., 2003; Gomot et al., 2006; Kana et al., 2007; Kennedy et al., 2006; Silk et al., 2006; Ashwin et al., 2007; Dichter and Belger, 2007). There is also evidence of altered metabolism (Levitt et al., 2003), reduced 5-HT2A receptor binding (Murphy et al., 2006) and reduced volume of the ACC (Haznedar et al., 1997, 2000). A DTI study found reduced fractional anisotropy (FA—an index of WM microstructure: Beaulieu, 2002) of the WM adjacent to ACC (Barnea-Goraly et al., 2004). Aberrant connectivity is also suggested by findings of decreased functional connectivity (synchronization of activation) between the ACC and other structures during a task requiring response inhibition (Kana et al., 2007). These findings are consistent with the theory of abnormal inter-regional coordination in ASD (Courchesne and Pierce, 2005).
Abnormal ACC connectivity and function may contribute to the defining symptoms of autism. ACC dysfunction has been linked to indices of social impairment (Haznedar et al., 2000; Ohnishi et al., 2000; Kennedy et al., 2006), but we are aware of only one study linking it to restricted and repetitive behaviours in ASD (Shafritz et al., 2007). In this study restricted, repetitive behaviours were negatively correlated with fMRI activation in ACC during trials that required a switch in response. Such a link has face validity since the ACC contributes to evaluating and modifying behaviour based on its consequences and flexible behaviour depends on these functions. A link between abnormal ACC function and repetitive thoughts and behaviours has been more extensively documented in obsessive–compulsive disorder (OCD), which is often comorbid with ASD (Leyfer et al., 2006). Relative to controls, individuals with OCD show increased fMRI activation of the ACC and increased ERN amplitude not only to error trials (Gehring et al., 2000; Johannes et al., 2001; Fitzgerald et al., 2005; but see Nieuwenhuis et al., 2005 for a negative finding), but also to correct trials with high response-conflict (i.e. those that require suppression of a competing response) in some (Ursu et al., 2003; Maltby et al., 2005), but not all studies (Gehring et al., 2000; Fitzgerald et al., 2005). These exaggerated responses suggest hyperactive monitoring of responses. Moreover, both increased ERN to errors (Gehring et al., 2000) and increased ACC activation to error (Fitzgerald et al., 2005) and correct trials (Ursu et al., 2003) have been associated with the severity of obsessions and compulsions in OCD. These relations have been interpreted to reflect excessive or inappropriate error signalling that triggers a compulsion to repeat behaviours, even if they have already been successfully executed (Maltby et al., 2005). The findings that in OCD the ACC and associated regions show increased activation during symptom provocation (Breiter et al., 1996), and that cingulotomy relieves obsessions and compulsions (Dougherty et al., 2002) further support the link between ACC function and rigid, repetitive behaviours. Measurements of obsessive–compulsive behaviour have also been related to response monitoring in non-clinical samples. Obsessive characteristics are related to the amplitudes of ERN and error positivity (another event-related potential that follows errors and is thought to be generated by the ACC, Falkenstein et al., 1995; van Veen and Carter, 2002) in children (Santesso et al., 2006) and to the amplitude of ERN in college undergraduates (Hajcak and Simons, 2002). These findings suggest that abnormally increased activity of the ACC following response execution contributes to rigid, repetitive behaviour. Based on this literature, we expected to find increased ACC activation on both error and correct trials that is correlated with repetitive behaviour in ASD.
We investigated the role of the ACC in response monitoring using an antisaccade paradigm. Antisaccades require suppression of the prepotent response of looking towards a suddenly appearing visual stimulus and substitution of the novel behaviour of looking in the opposite direction (Hallett, 1978). Antisaccades are well-suited for examining response monitoring because they have a relatively well-delineated neuroanatomy and neurophysiology (for review see: Munoz and Everling, 2004), generate robust electrophyisological error markers, ERN, error positivity (Nieuwenhuis et al., 2001) and ACC hemodynamic responses (Polli et al., 2005) and involve a high degree of response conflict. Moreover, individuals with developmental disorders affecting social processing, including ASD, consistently show an increased rate of antisaccade errors (i.e. a failure to suppress the prepotent prosaccade) (Manoach et al., 1997, 2004; Minshew et al., 1999; Goldberg et al., 2002; Luna et al., 2007), which may reflect, in part, aberrant evaluation of performance and modification of prepotent stimulus-response mappings.
We examined group differences in ACC activation during correct and erroneous antisaccade trials using rapid presentation event-related fMRI. Comparisons were restricted to antisaccade trials since healthy and ASD participants produce too few prosaccade errors with this paradigm (Manoach et al., 2004). We examined activity in rostral and dorsal ACC (rACC, dACC) separately, given their differences in cytoarchitecture, function and connectivity (Devinsky et al., 1995; Bush et al., 1998; Whalen et al., 1998) and distinct patterns of haemodynamic activity during antisaccade error commission (Polli et al., 2005). Since response monitoring is the product of coordinated activity between the ACC and functionally related regions (Holroyd and Coles, 2002), and the ACC shows evidence of decreased structural and functional connectivity in autism (Barnea-Goraly et al., 2004; Kana et al., 2007), we also examined the microstructural integrity of the WM underlying the ACC using DTI. We expected ASD participants to show: (i) an increased antisaccade error rate; (ii) increased ACC activity during both correct and erroneous antisaccade trials and (iii) reduced FA in the WM underlying ACC. Finally, we hypothesized that (iv) structural and functional ACC abnormalities would be associated with rigid, repetitive behaviour in ASD.
Methods
Participants
Twelve adults with ASD and 14 healthy control (HC) participants were recruited by poster and website advertisements (see Table 1 for demographic data). Participants with ASD were diagnosed with autism (n = 8), Asperger's disorder (n = 2) or pervasive developmental disorder, not otherwise specified (n = 2) by an experienced clinician (R.M.J.) on the basis of current presentation and developmental history as determined by medical record review and clinical interview. Potential participants meeting DSM-IV criteria for co-morbid psychiatric conditions or substance abuse were excluded. ASD diagnoses were confirmed using the Autism Diagnostic Interview-Revised (ADI-R: Rutter et al., 2003) and the Autism Diagnostic Observation Schedule Module 4 (Lord et al., 1999) administered by research reliable personnel. Individuals with known autism-related medical conditions (e.g. Fragile-X syndrome, tuberous sclerosis) were not included. Four of the 12 ASD participants were taking the following medications: fluoxetine and lithium; buproprion and clonazepam; citalopram and sertraline and methylphenidate.
Table 1.
Means, standard deviations and group comparisons of demographic data
| Subject characteristics | HC (n = 14) | ASD (n = 12) | T | P |
|---|---|---|---|---|
| Age | 27 ± 8 | 30 ± 11 | −0.69 | 0.50 |
| Sex | 8M/6F | 10M/2F | φ = 0.28 | 0.22 |
| Laterality score (Handedness) | 75 ± 45 | 61 ± 38 | 0.86 | 0.40 |
| Parental SESa | 1.31 ± 0.48 | 1.17 ± 0.39 | z = −0.60 | 0.42 |
| Years of education | 16 ± 2 | 16 ± 3 | −0.51 | 0.62 |
| Estimated verbal IQb | 114 ± 9 | 116 ± 8 | −0.62 | 0.54 |
aA lower score denotes higher status. The Phi value is the result of a Fisher's exact test. The z-value is the result of a non-parametric Mann–Whitney U comparison. bBased on the American National Adult Reading Test.
HC participants were screened to exclude a history of autism or any other neurological or psychiatric condition. All participants were screened to exclude substance abuse or dependence within the preceding 6 months, and any independent condition that might affect brain function. ASD and control groups were matched for age, sex, handedness as measured by a laterality score on the modified Edinburgh Handedness Inventory (scores of −100 and +100 denote exclusive use of left or right hands, respectively) (Oldfield, 1971; White and Ashton, 1976), parental socioeconomic status on the Hollingshead Index (Hollingshead, 1965), years of education and estimated verbal intelligence quotient (IQ) based on a test of single word reading (American National Adult Reading Test: Blair and Spreen, 1989). All ASD participants had average or above estimates of verbal (124 ± 12, range: 106–141) and non-verbal (120 ± 10, range: 100–138) IQ as measured by the Wechsler Abbreviated Scale of Intelligence (Wechsler, 1999). The study was approved by the Partners Human Research Committee. All participants gave written informed consent after the experimental procedures had been fully explained.
fMRI data were unusable for one ASD participant due to eye tracking malfunction. For a second ASD participant, data were not acquired because scanner-safe glasses of the correct prescription were not available. fMRI analyses were conducted on the remaining 10 ASD and 14 control participants. Saccadic latency data for one control participant was omitted from analysis due to an acquisition problem. DTI data were acquired on all 12 ASD participants and 12 of the 14 controls.
Saccadic paradigm
Prior to scanning, the task was explained and participants practiced in a mock scanner until their performance indicated that they understood the directions and were comfortable with the task. Participants were instructed to respond as quickly and accurately as possible and told that they would receive a bonus of 5 cents for each correct response in addition to a base rate of pay. Each run of the task consisted of a pseudorandom sequence of prosaccade and antisaccade trials that were balanced for right and left movements. Figure 1 provides a graphic depiction of the task and a description of task parameters. Randomly interleaved with the saccadic trials were intervals of fixation lasting 2, 4 or 6 s. The fixation intervals provided a baseline and their variable length introduced ‘temporal jitter’, which optimizes the analysis of rapid presentation event-related fMRI designs (Buckner et al., 1998; Burock and Dale, 2000; Miezin et al., 2000). The schedule of events was determined using a technique to optimize the statistical efficiency of event-related designs (Dale, 1999). Participants performed six runs of the task, each lasting 5 min 22 s, with short rests between runs. The total experiment lasted about 40 min and generated a total of 211 prosaccade and 211 antisaccade trials, and 80 fixation intervals.
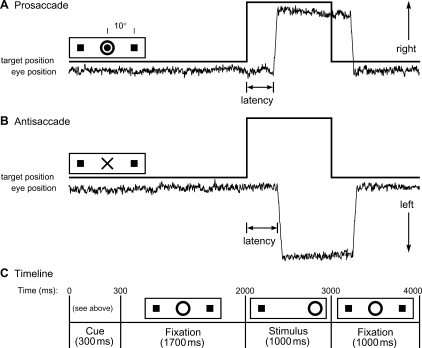
Fig. 1.
Saccadic paradigm with idealized eye position traces. Saccadic trials lasted 4000 ms and began with an instructional cue at the centre of the screen. For half of the participants, orange concentric rings were the cue for a prosaccade trial (A) and a blue cross was the cue for an antisaccade trial (B). These cues were reversed for the rest of the participants. The cue was flanked horizontally by two small green squares of 0.2° width that marked the potential locations of stimulus appearance, 10° left and right of centre. These squares remained on the screen for the duration of each run. (C) At 300 ms, the instructional cue was replaced by a green fixation ring at the centre of the screen, of 0.4° diameter and luminance of 20 cd/m2. After 1700 ms, the ring shifted to one of the two target locations, right or left, with equal probability. This was the stimulus to which the participant responded by either making a saccade to it (prosaccade) or to the square on the opposite side (antisaccade). The green ring remained in the peripheral location for 1000 ms and then returned to the centre, where participants were also to return their gaze for 1000 ms before the start of the next trial. Fixation intervals were simply a continuation of the fixation display that constituted the final second of the previous saccadic trial.
Stimulus display and eye tracking
Displays of the eye movement task were generated using the Vision Shell programming platform (www.visionshell.com, Watertown, MA, USA), and back-projected with a Sharp XG-2000 color LCD projector (Osaka, Japan) onto a screen at the rear of the bore that was viewed by the participant via a mirror on the head coil. The ISCAN fMRI Remote Eye Tracking Laboratory (ISCAN, Burlington, MA) recorded saccades during scanning. This system used a video camera mounted at the rear of the MRI bore. The camera imaged the eye of the participant via an optical combiner, a 45° cold transmissive mirror that reflects an infrared image of the eye, with the infrared illumination being provided by an LED mounted on the head coil. The system used passive optical components with no ferrous content within the bore to minimize artefacts in the MRI images. Eye position was sampled at a rate of 60 Hz. Eye images were processed by ISCAN's RK-726PCI high resolution Pupil/Corneal reflection tracker, located outside of the shielded MRI room. Stimuli presented by Vision Shell were digitally encoded and relayed to ISCAN as triggers that were inserted into the eye movement recordings.
Scoring and analysis of eye movement data
Eye movement data were scored in MATLAB (Mathworks, Natick, MA) using a partially automated programme that determined the directional accuracy of each saccade with respect to the required response and the latency from target onset. Saccades were identified as horizontal eye movements with velocities exceeding 47°/s. The onset of a saccade was defined as the point at which the velocity of the eye movement first exceeded 31°/s. Only trials with saccades in the desired direction and latencies over 130 ms were considered correct, and only correct saccades were included in the latency analyses. The cutoff of 130 ms excluded anticipatory saccades, which are executed too quickly to be a valid response to the appearance of the target (Fischer and Breitmeyer, 1987). We classified corrective saccades that followed antisaccade errors into short and long latency self-corrections (c.f. Polli et al., 2006): those with latencies (from error initiation) >130 ms suggesting that the correction was a conscious (aware) response to visual feedback regarding an error, and those with latencies <130 ms suggesting that the correction represented a concurrently programmed correct saccade and that the participant was unaware of having made an error (Mokler and Fischer, 1999; McPeek et al., 2000). Post-error slowing was calculated for each participant as the difference in latency between correct trials preceded by an error and correct trials preceded by a correct response. Error rate and latency of correct trials were analysed using randomized block ANOVAs with Group (ASD versus controls) as the between-subjects factor, Task (antisaccade versus prosaccade) as the within-subjects factor and subjects nested within group as the random factor. Post-error slowing and self-correction rates for antisaccades were compared by group using t-tests.
Image acquisition
Images were acquired with a 3.0T Siemens Trio whole body high-speed imaging device equipped for echo planar imaging (Siemens Medical Systems, Erlangen, Germany). Head stabilization was achieved with cushioning, and all participants wore earplugs (29 dB rating) to attenuate noise. Automated shimming procedures were performed and scout images were obtained. Two high-resolution structural images were acquired in the sagittal plane for slice prescription, spatial normalization (spherical and Talairach) and cortical surface reconstruction using a high resolution 3D magnetization prepared rapid gradient echo (MPRAGE) sequence (repetition time (TR), 2530 ms; echo spacing, 7.25 ms; echo time (TE), 3 ms; flip angle 7°) with an in-plane resolution of 1 and 1.3 mm slice thickness. T1- and T2-weighted structural images, with the same slice specifications as the Blood Oxygen Level Dependent (BOLD) scans, were obtained to assist in registering functional and structural images.
fMRI
Functional images were collected using a gradient echo T2* weighted sequence (TR/TE/Flip = 2000 ms/30 ms/90°). Twenty contiguous horizontal slices parallel to the intercommissural plane (voxel size: 3.13 × 3.13 × 5 mm) were acquired interleaved. The functional sequences included prospective acquisition correction for head motion (Thesen et al., 2000). Prospective acquisition correction adjusts slice position and orientation in real time during data acquisition. This reduces motion-induced effects on magnetization history.
DTI
Single-shot echo planar imaging DTI was acquired using a twice refocused spin echo sequence (Reese et al., 2003) with the following sequence parameters: TR/TE = 8400/82 ms; b = 700 s/mm2; NEX = 1; 10 T2 images acquired with b = 0; 72 diffusion directions; 128 × 128 matrix; 2 × 2 mm in-plane resolution; 64 axial oblique (AC-PC) slices; 2 mm (0 mm gap) slice thickness; scan duration 12′44". The n = 72 diffusion directions were obtained using the electrostatic shell algorithm (Jones, 2004).
Surface-based analyses for fMRI and DTI
Functional and FA volumes were aligned to the 3D structural image for each participant that was created by averaging the two MPRAGE scans after correcting for motion. The averaged MPRAGE scans were used to construct inflated (2D) models of individual cortical surfaces using previously described segmentation, surface reconstruction and inflation algorithms (Dale et al., 1999; Fischl et al., 1999a). To register data across participants, anatomical, functional and DTI scans were spatially normalized using a surface-based spherical coordinate system that employs a non-rigid alignment algorithm that explicitly aligns cortical folding patterns and is relatively robust to inter-individual differences in the gyral and sulcal anatomy of cingulate cortex (Dale et al., 1999; Fischl et al., 1999a, b). For both fMRI and DTI, the ACC was localized using an automated surface-based parcellation system that includes a label for the ACC (Fischl et al., 2004). The ACC label was divided into dorsal and rostral segments by drawing a line perpendicular to the intercommissural plane at the anterior boundary of the genu of the corpus callosum (Devinsky et al., 1995).
fMRI and DTI results were displayed on a template brain consisting of the averaged cortical surface of an independent sample of 40 adults from the Buckner laboratory at Washington University. To correct for multiple comparisons we ran 10 000 Monte Carlo simulations of synthesized white Gaussian noise using a P-value of ≤0.05 and the smoothing, resampling and averaging parameters of the functional analyses. This determines the likelihood that a cluster of a certain size would be found by chance for a given threshold. To test our a priori hypotheses concerning ACC function and structure, we restricted the simulations to our ACC ROIs. To determine whether other regions also showed significant group differences we also ran simulations to correct for multiple comparisons on the entire cortical surface. To facilitate comparison with other studies, approximate Talairach coordinates were derived by mapping the surface-based coordinates of activation and FA maxima back to the original structural volume for each participant, registering the volumes to the Montreal Neurological Institute (MNI305) atlas (Collins et al., 1994) and averaging the MNI305 coordinates that corresponded to the surface maxima across participants. The resulting coordinates were transformed to standard Talairach space using an algorithm developed by Matthew Brett (http://imaging.mrc-cbu.cam.ac.uk/imaging/MniTalairach).
fMRI data analyses
Analyses were conducted using FreeSurfer (Fischl et al., 1999a) and FreeSurfer Functional Analysis Stream (FS-FAST) software (Burock and Dale, 2000). In addition to on-line motion correction (prospective acquisition correction), functional scans were corrected retrospectively for motion using the AFNI algorithm (Cox and Jesmanowicz, 1999). To characterize average motion for each participant, total motion in millimetres for all six directions (x, y, z and three rotational directions) as determined by AFNI, was averaged across the six runs of the task and compared between groups. Following motion correction, scans were intensity normalized, and smoothed using a 3D 8 mm FWHM Gaussian kernel. Finite impulse response estimates (Burock and Dale, 2000; Miezin et al., 2000) of the event-related haemodynamic responses were calculated for each of the four trial types (correct prosaccades, error prosaccades, correct antisaccades and error antisaccades) for each participant. This involved using a linear model to provide unbiased estimates of the average signal intensity at each time point for each trial type without making a priori assumptions about the shape of the haemodynamic response. Haemodynamic response estimates were computed at 12 time points with an interval of 2 s (corresponding to the TR) ranging from 4 s prior to the start of a trial to 18 s after the start. Temporal correlations in the noise were accounted for by pre-whitening using a global estimate of the residual error autocorrelation function truncated at 30 s (Burock and Dale, 2000).
Registered group data were smoothed with a 2D 4.6 mm FWHM Gaussian kernel. We compared activation in error versus correct antisaccades, and in both correct and error antisaccades versus fixation, both within and between groups using a random effects model. We examined the 6 and 8 s time points that showed peak error-related activation in previous studies (Polli et al., 2005, 2008).
DTI analysis
The objective of the DTI analysis was to measure FA in the WM underlying specific regions using a vertex-wise analysis of the registered group data. FA was measured in the undeformed WM 2 mm below the WM/gray matter boundary. This strategy minimizes partial volume contributions from cortical gray matter and preserves regional specificity (the rationale for the surface-based approach is elaborated in Manoach et al., 2007a). DTI data were analysed using the following multi-step procedure:
Motion/eddy current distortion correction: Raw diffusion data were corrected for head motion and residual eddy current distortion by registering all images to the first T2 image, which was acquired with b = 0. The registration was performed using the FLIRT tool (Jenkinson and Smith, 2001) from the FSL software library (http://www.fmrib.ox.ac.uk/fsl). The registration used a global affine (12 df) transformation, a mutual information cost function and sinc resampling.
Tensor reconstruction: The diffusion tensor and FA volumes were reconstructed using the standard least-squares fit to the log diffusion signal (Basser et al., 1994).
Registration of FA and T1 volumes: FA volumes were registered to the high-resolution structural (T1) volumes for each participant using the T2 volume as an intermediary. This avoids the potential bias that might arise from using the FA volume in the registration, since FA is the variable of interest. In addition, the gray/white boundary in the T1 volume has better correspondence with the T2 volume than the FA volume, allowing for a more accurate registration. The T2 volume was taken from the b = 0 image in the DTI acquisition and was, therefore, in register with the FA volume. Each participant's averaged T2 volume was manually registered to the T1 volume with Tkregister2 (http://surfer.nmr.mgh.harvard.edu) using 9 df including translation, rotation and scaling. Manual registration was used to maximize anatomic agreement along the medial wall and because echo planar imaging susceptibility distortions in DTI can confound global registration procedures. The resulting spatial transformation was applied to the FA volume, thus bringing the FA and T1 volumes into register.
Projection of subcortical FA values onto the WM/gray matter interface: For each participant, a surface representation of the WM/gray matter boundary was derived from the T1 volume using the previously described segmentation, surface reconstruction and inflation algorithm (Dale et al., 1999; Fischl et al., 1999a). FA was sampled in the WM 2 mm below the WM/gray matter boundary for each vertex on the surface and then projected onto the WM/gray interface. FA values were smoothed using n = 50 iterations of replacement by nearest neighbours. This corresponds to smoothing by ∼10 mm FWHM on the surface.
Registration of surface FA maps across participants: This was achieved by applying the surface transformations from the spatial normalization of the T1 maps to the FA maps, which were in register with T1 maps (step 3).
To test for significant group differences in FA in the registered group data, a t-test was performed in the WM underlying each vertex.
Relations of ACC function and structure to restricted, repetitive behaviour in ASD
fMRI activation in each contrast at 6 and 8 s and FA values were regressed onto ADI-R diagnostic algorithm scores of restricted and repetitive behaviours for every vertex on the cortical surface. In the regression of FA values on ADI-R scores, age was used as a covariate given the documented relation of increasing age with decreasing FA (Pfefferbaum et al., 2000).
Results
Saccadic performance
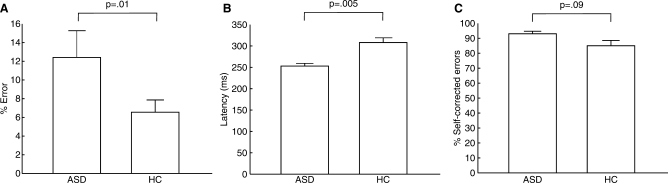
ASD participants made significantly more errors than HC [F(1,22) = 7.82, P = 0.008]. Although the group by task interaction was not significant [F(1,22) = 0.99, P = 0.32), ASD participants had a significantly higher antisaccade error rate than controls [Fig. 2A, t(22) = 2.68, P = 0.01, HC: 6.55 ± 4.94%, range 1.43–16.59%; ASD: 12.41 ± 9.02%, range 2.37–26.67%], but did not differ significantly in the error rate for prosaccades [t(22) = 1.62, P = 0.21, HC: 2.04 ± 1.50%, ASD: 4.82 ± 4.06%] (Fig. 2A). ASD participants responded more quickly on correct trials [F(1,21) = 9.74, P = 0.005] and there was a trend to a group by task interaction [F(1,21) = 3.33, P = 0.08] reflecting a greater group latency difference for antisaccade than prosaccade trials [antisaccade: t(21) = 3.47, P = 0.002, HC: 309 ± 40 ms, ASD: 253 ± 20 ms; prosaccade: t(21) = 2.56, P = 0.02, HC: 254 ± 50 ms, ASD: 212 ± 29 ms] (Fig. 2B). ASD participants were slightly more likely to correct antisaccade errors [trend: t(22) = 1.77, P = 0.09; HC: 85 ± 13%; ASD: 93 ± 5%] (Fig. 2C), and did not differ from controls in the proportion of long versus short self-corrections [t(22) = 1.50, P = 0.15, HC: 67 ± 29%; ASD: 51 ± 20%]. Controls did not show significant post-error slowing for either antisaccades [0 ± 26 ms, t(12) = 0.06, P = 0.95] or prosaccades [14 ± 29 ms, t(12) = 1.71, P = 0.11]. ASD participants showed post-error speeding for antisaccades [−11 ± 15 ms, t(9) = 2.48, P = 0.04] and no post-error change in latency for prosaccades [ASD: 3 ± 13 ms; t(9) = 0.73, P = 0.48].
Fig. 2.
Bar graphs of performance for each group as measured by mean and standard errors of (A) antisaccade error rate, (B) latency of correct antisaccades and (C) percentage of antisaccade errors that were self-corrected.
fMRI analyses of response monitoring in the ACC
Patients and controls did not differ in mean motion during functional scans [controls: 1.71 ± 0.81 mm, patients: 1.78 ± 0.47mm, t(22) = 0.62, P = 0.83]. As group differences in activation were more pronounced at the 8 s than the 6 s time point, we report findings from 8 s only.
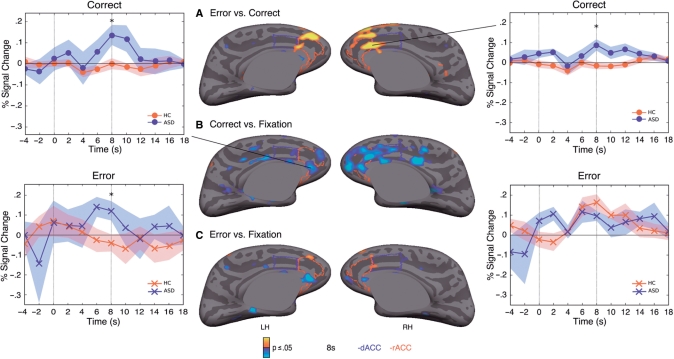
Controls showed a more differentiated response to error versus correct trials than ASD participants in the right rACC, extending into the dACC (Fig. 3A, Table 2). The basis of this group difference was that ASD participants showed significantly greater activation to correct responses in bilateral rACC (Fig. 3B, Table 2). In this region, the group difference in the response to errors was not significant (Fig. 3C), and within group analyses revealed that while both groups showed a significant response to errors bilaterally (Control: left maximum −9, 37, 12, cluster size = 1204 mm2, cluster-wise probability (CWP) = 0.0001; right maximum 7, 26, 18, cluster size = 1561 mm2, CWP = 0.0001; ASD: left maximum −5, 34, 5, cluster size = 1112 mm2, CWP = 0.0001; right maximum 9, 38, 12, cluster size = 850 mm2, CWP = 0.0001), only the ASD group showed a significant response to correct trials (left maximum −7, 40, 1, cluster size = 1107 mm2, CWP = 0.0001; right maximum 7, 39, 11, cluster size = 1498 mm2, CWP = 0.0001). Compared with controls, ASD participants also showed significantly greater activation for correct trials in another more anterior perigenual rACC region bilaterally (Fig. 3B), and in the left hemisphere this region also showed a significantly greater response to errors (Fig. 3C). Controls did not activate this perigenual rACC region for either correct or error responses. When multiple comparisons correction was applied to the entire cortical surface, the finding that the ASD group showed an exaggerated response to correct trials in right rACC extending into dACC remained significant (CWP = 0.0001). The only other significant group difference in cortical activation was a less differentiated response for ASD participants to error versus correct responses in the medial aspect of the right superior frontal gyrus.
Fig. 3.
Statistical maps of group differences in fMRI activation at 8 s for contrasts of (A) error versus correct antisaccades; (B) correct antisaccades versus fixation; (C) error antisaccades versus fixation. Statistical maps are displayed on the inflated medial cortical surfaces of the template brain at P ≤ 0.05. Regions of greater activation in controls are depicted in warm colours; greater activation in ASD patients is depicted in blue. The rACC is outlined in red and the dACC is outlined in blue. The gray masks cover non-surface regions in which activity is displaced. Haemodynamic response time course graphs with standard error bars are displayed for the indicated vertices with peak activation in the group comparisons.
Table 2.
Maxima and locations of clusters showing significant group differences and significant relations to ADI-R repetition scores in ASD
| Approximate Talairach coordinates |
||||||
|---|---|---|---|---|---|---|
| Cluster size (mm) | x | y | z | t-value (max) | CWP | |
| fMRI group comparisons | ||||||
| Error versus correct | ||||||
| HC > ASD | ||||||
| Right rostral anterior cingulate gyrus | 488 | 7 | 38 | 12 | 3.31 | 0.0001 |
| Unmedicated ASD participants | 424 | 7 | 38 | 12 | 2.18 | 0.008 |
| Right medial superior frontal gyrusa | 2071 | 9 | 50 | 38 | 3.53 | 0.001 |
| Correct versus fixation | ||||||
| ASD > HC | ||||||
| Left pericollosal sulcus (rACC) | 413 | −6 | 30 | −6 | 2.22 | 0.0001 |
| Unmedicated ASD participants | 102 | −6 | 31 | −6 | 1.59 | 0.41 |
| Right rostral anterior cingulate gyrusa | 1746 | 7 | 39 | 11 | 3.47 | 0.0001 |
| Unmedicated ASD participants | 912 | 9 | 39 | 10 | 3.61 | 0.0002 |
| Pericollosal anterior cingulate gyrus | 6 | 36 | −6 | |||
| Error versus fixation | ||||||
| ASD > HC | ||||||
| Left rostral anterior cingulate gyrus | 255 | −2 | 26 | −4 | 3.64 | 0.02 |
| Unmedicated ASD participants | 80 | −1 | 26 | −3 | 2.38 | 0.78 |
| DTI-FA group comparisonb | ||||||
| HC > ASD | ||||||
| Left rostral anterior cingulate gyrusa | 1598 | −7 | 39 | 6 | 6.05 | 0.0001 |
| Right rostral anterior cingulate gyrusa | 6620 | 7 | 38 | 3 | 5.64 | 0.0001 |
| Frontopolar transverse sulcus | 20 | 61 | 14 | |||
| Left transverse gyrus, frontopolara | 6152 | −20 | 59 | 6 | 7.2 | 0.0001 |
| Left precentral gyrusa | 1022 | −47 | −8 | 41 | 5.11 | 0.009 |
| Left intraparietal and parietal transverse sa | 4878 | −40 | −50 | 42 | 5.05 | 0.0001 |
| Right intraparietal and parietal transverse sa | 1465 | 21 | −56 | 52 | 4.06 | 0.0008 |
| ASD > HC | ||||||
| Right anterior circular sulcus, insula* | 838 | 35 | 31 | −2 | −2.91 | 0.05 |
| fMRI regressions with ADI-R | ||||||
| Correct versus fixation | ||||||
| Right rostral anterior cingulate gyrus | 684 | 6 | 37 | −7 | 3.23 | 0.0001 |
| Error versus correct | ||||||
| Right paracentral gryrusa | 1642 | 6 | −25 | 51 | −2.59 | 0.005 |
| DTI-FA regressions with ADI-R | ||||||
| Left subcollosal gyrus (rACC) | 148 | −5 | 18 | −8 | −2.04 | 0.05 |
aMeets correction for entire cortical surface. bCWP levels are based on correction for entire cortical surface. Important local maxima are indented. Results for fMRI group comparisons restricted to unmedicated ASD participants (n = 6) are indented and italicized.
Four of our ASD participants were taking medications that could affect ACC function. A re-analysis of the data with only unmedicated ASD participants confirmed the main findings that in the right rACC, ASD participants showed significantly less discrimination between correct and error trials and greater activation to correct trials (Table 2, Supplementary Figure).
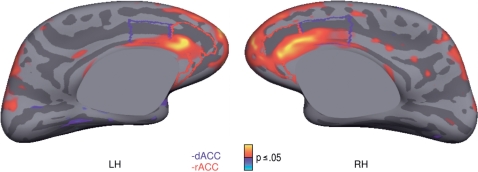
DTI analyses
ASD participants showed significantly reduced FA in the WM underlying rACC and dACC bilaterally (Fig. 4, Table 2). The maxima in both hemispheres were in rACC WM, near the border with dACC and FA reductions extended into the corpus callosum. These reductions met multiple comparisons correction for the entire cortical surface. Significantly reduced activation FA in ASD was also seen in the WM underlying bilateral frontopolar cortex, which, extended into dorsolateral and ventrolateral prefrontal cortex in the left hemisphere; bilateral intraparietal and parietal transverse sulci; and left precentral gyrus. The only region showing significantly greater FA in ASD participants versus controls was in the WM underlying right insula.
Fig. 4.
Statistical map of group differences in FA displayed on the inflated medial cortical surfaces of the template brain at a threshold of P ≤ 0.05. Regions of greater FA in controls are depicted in warm colours. The rACC is outlined in red and the dACC is outlined in blue.
Relations of ACC function and structure to ADI-R scores of restricted and repetitive behaviour in ASD
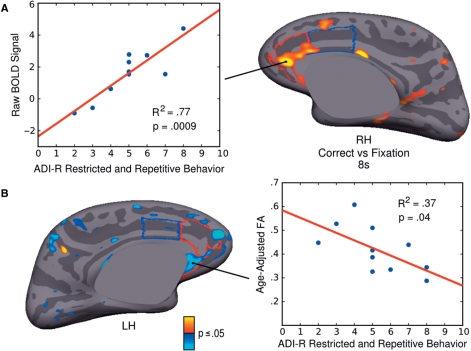
Regression analyses showed that in ASD, higher ADI-R restricted and repetitive behaviour scores were associated with greater activation during correct trials versus fixation at 8 s in the right rACC (Fig. 5A, Table 2). This right rACC region overlapped with the regions showing both significantly increased activation to correct trials and reduced FA. Repetitive behaviour scores were not significantly associated with ACC activation in other contrasts at either 6 or 8 s. The only other cortical region showing a significant relation at 8 s was the right paracentral gyrus extending into the postcentral gyrus in the error versus correct contrast.
Fig. 5.
Relations of ACC function and structure to restricted, repetitive behaviour in ASD. (A) Statistical map of regression of raw BOLD signal on ADI-R repetitive behaviour score. BOLD signal is from the correct antisaccade versus fixation contrast at 8 s. Results are displayed on the inflated right medial cortical surface. Scatter plot shows BOLD signal from the maximum vertex in right rACC for each ASD participant on the y-axis and ADI-R score on the x-axis. (B) Statistical map of regression of FA on ADI-R repetitive behaviour score. Results are displayed on the inflated left medial surface. Scatter plot shows FA from the maximum vertex in left rACC for each ASD participant on the y-axis and ADI-R score on the x-axis. The rACC is outlined in red and the dACC is outlined in blue.
Lower FA in the WM underlying ACC was significantly related to higher repetitive behaviour scores in left subgenual rACC, with a maximum vertex in subcallosal gyrus (Fig. 5B, Table 2). No other cortical regions showed significant relations in underlying WM.
The literature on repetitive behaviour in autism supports a division into ‘low-level’ repetitive motor behaviour and more complex behaviours that appear to be driven by ideation (for review see, Turner, 1999). To assess the relative importance of ‘sensorimotor’ versus ‘ideational’ forms of repetition in accounting for the observed relations with ACC activation and structure, we first divided each participants’ ADI-R repetitive behaviour score into ‘sensorimotor’ (i.e. D3/D4: sterotypies, mannerisms, repetitive object use, unusual sensory interests) and ‘ideational’ (i.e. D1/D2: narrow restricted interests and rituals) subscores (Hollander et al., 2003). On average, the ideational subscore accounted for 65 ± 14% of the total repetitive behaviour score. We then performed multiple regression analysis with BOLD or FA value at the ACC maxima of the regression with total score as the dependent variable, and the two subscores as covariates. Sensorimotor subscores were more strongly related to activation in the ACC (P = 0.04) than ideational subscores (P = 0.17). The opposite was true for FA, which showed a stronger correlations with ideational subscores (P = 0.05) than sensorimotor subscores (P = 0.18). The subscores did not differ significantly in their contribution to activation or FA, and neither improved on the relations seen with the total repetitive behaviour score (activation: P = 0.0009; FA: P = 0.04) suggesting that both subscores contributed to the relations observed.
Discussion
The present study demonstrates functional and structural abnormalities of the ACC in ASD that may contribute to restricted, repetitive and stereotyped patterns of behaviour, a defining feature of ASD. Compared with controls, ASD participants showed increased rACC activation to both correct and error responses and reduced microstructural integrity of the WM underlying ACC, including in regions showing abnormally increased response monitoring activation. Moreover, in rACC increased activation to correct trials and reduced FA were related to ratings of rigid, repetitive behaviour. These findings support the hypothesis of hyperactive response monitoring in ASD and link it to repetitive behaviour.
Relative to controls, ASD participants showed reduced discrimination between error and correct trials in a rACC region that responds to errors (Polli et al., 2005). This was primarily due to an exaggerated response to correct trials, rather than a blunted response to errors. Reduced discrimination between response outcomes may interfere with adjusting responses to obtain the most favourable outcome both with regard to subsequent trials and longer term modification of the strength of stimulus-response mappings. This could contribute to behaviour that is stimulus-bound and repetitive rather than flexible and responsive to contingencies. This provides a plausible explanation for the relation between increased rACC activity to correct trials and repetitive behaviour.
A complementary explanation has been offered for the relation of increased error signals to the severity of obsessions and compulsions in OCD (Gehring et al., 2000; Ursu et al., 2003). A longstanding theory of OCD posits that inappropriate and exaggerated error signals lead to a pervasive sense of incompleteness and self-doubt and behavioural repetition represents a vain attempt to reduce these error signals (Pitman, 1987). According to this theory, exaggerated ACC activation to correct trials reflects inappropriate error signalling and triggers a compulsion to repeat behaviours that were already successfully completed (Maltby et al., 2005). Similar to individuals with OCD, ASD participants showed an exaggerated ACC response to correct trials (Ursu et al., 2003; Maltby et al., 2005). While in OCD fMRI activation to correct trials was maximal in dACC (Maltby et al., 2005), in ASD it was maximal in rACC and was related to repetitive behaviour. While dACC activity following errors is thought to reflect either conflict or error detection (for review see: Ullsperger and von Cramon, 2004), rACC activity following errors has been interpreted as appraisal of the affective or motivational salience of errors (van Veen and Carter, 2002; Luu et al., 2003; Taylor et al., 2006). Thus, one interpretation of this relation is that it reflects an inappropriate affective response to correct performance that triggers behavioural repetition. This exaggerated affective response may occur even with awareness that one has performed correctly. Regardless of its basis, the relation between increased rACC responses to correct trials and repetitive behaviour suggests that aberrant response monitoring contributes to a defining feature of ASD.
Both ASD and OCD are characterized by behaviour that is rigid and not optimally responsive to contingency, and there is evidence of both phenomenological overlap and distinctions between the obsessions and compulsions of OCD and the restricted and repetitive behaviours of ASD, both in terms of subjective experience and type of behaviour (Baron-Cohen, 1989; McDougle et al., 1995; Russell et al., 2005; Zandt et al., 2007). Interestingly, a study of multiplex families of children with autism found that parents were significantly more likely to have obsessive–compulsive traits or disorder if the children had high ADI-R repetitive behaviour scores, and this was due to loading on ideational but not sensorimotor items (Hollander et al., 2003). This finding supports the distinction between repetition of movements and repetition of more complex, ideational behaviours (for review see, Turner, 1999) and suggests that the latter may share underlying mechanisms in OCD and ASD. In the present study, both sensorimotor and ideational subscores contributed to the relations seen with response monitoring activation and FA. At present, repetitive behaviours in autism are incompletely understood, and neurobiologically valid dimensions are yet to be delineated. The present findings suggest that ACC abnormalities may contribute to repetitive behaviour in ASD, possibly on the basis of their contribution to aberrant response monitoring, but linking ACC abnormalities to specific dimensions of repetitive behaviour and identifying the underlying mechanisms requires further study with large samples.
Abnormal response monitoring may also contribute to deficient cognitive control of behaviour in ASD, which, in the present study, was reflected in an increased antisaccade error rate, consistent with prior work (Manoach et al., 1997, 2004; Minshew et al., 1999; Goldberg et al., 2002; Luna et al., 2007) and possibly in reduced dynamic performance adjustment. In spite of making more errors, ASD participants responded more quickly than controls on correct trials suggesting a disruption in the speed-accuracy trade-off. This apparent difficulty in modulating response speed to improve accuracy is unlikely to reflect reduced error awareness since ASD participants were as, or more likely than controls to self-correct errors. Nor is it likely to reflect a blunted neural response to errors given both the increased response to errors in left perigenual rACC and previous work showing normal or increased amplitude of the ERN in ASD (Henderson et al., 2006). Deficient use of outcomes to adjust performance in a continuous, dynamic fashion may stem from response monitoring dysfunction in the ACC and/or from dysconnection of the ACC from ocular motor regions involved in saccade generation. Aberrant connectivity of ACC regions subserving response monitoring in ASD is suggested by our finding of reduced FA in the underlying WM. This finding is consistent with a previous report of reduced FA in ACC white matter in autism (Barnea-Goraly et al., 2004) and with evidence of decreased functional connectivity of the ACC to other regions during a task involving response inhibition (Kana et al., 2007).
How might information concerning the outcome of the prior response be communicated to ocular motor regions responsible for adjusting behaviour in the subsequent trial? Monkey electrophysiology studies suggest a basis for speed-accuracy trade-offs in the ocular motor system. Compared with prosaccades, antisaccades are associated with reduced pre-target activity of saccade-related neurons in the frontal eye field (Everling et al., 1999; Everling and Munoz, 2000) and superior colliculus (Everling et al., 1999), key regions in saccade generation. Moreover, lower pre-target frontal eye field activity is correlated with increased saccadic latency and fewer antisaccade errors. Reduced preparatory neural activity in response to the instruction to perform an antisaccade presumably makes it more difficult for activity related to the incorrect, but prepotent prosaccade to reach the threshold for triggering an eye movement. However, the price for this improved accuracy is that the activity generating the correct antisaccade also takes longer to reach threshold, resulting in prolonged saccadic latencies. Thus, we hypothesize that the slowing of responses that follow an antisaccade error (i.e. post-error slowing: Nieuwenhuis et al., 2001) stems from an ACC error response that signals the frontal eye field to reduce its preparatory activity during the subsequent trial. This hypothesis is consistent with previous work demonstrating that other aspects of trial history affect preparatory neural activity in the frontal eye field during antisaccades (Manoach et al., 2007b). How might error signals be conveyed to frontal eye field? The use of outcomes to dynamically adjust responses has been attributed to ACC acting with lateral prefrontal cortex (Gehring and Knight, 2000; Paus, 2001; Garavan et al., 2002) and lateral prefrontal cortex has been proposed to provide inhibitory input to frontal eye field during antisaccades (DeSouza et al., 2003). Thus, in ASD, aberrant communication between response evaluation in the ACC and regions initiating compensatory adjustments, possibly lateral prefrontal cortex, could result in a failure to optimally reduce preparatory firing rates in frontal eye field in response to a prior error. This reduced dynamic performance adjustment could lead to faster responding and more errors. We are presently testing this hypothesis using an antisaccade paradigm that gives rise to post-error slowing (Rabbitt, 1966) and will allow a more direct examination of compensatory behaviour and its supporting neural circuitry. Unfortunately, although post-error slowing is seen when antisaccades are the only task (Polli et al., 2006) the intermixed antisaccade and prosaccade paradigm employed here gives rise to other inter-trial factors that also affect latency (e.g. task-switching and prior antisaccades, Cherkasova et al., 2002; Fecteau et al., 2004; Barton et al., 2006; Manoach et al., 2007b) and may obscure the effects of a prior error. There is evidence of reduced post-error slowing in adults with high functioning autism, however, from a previous study that employed a memory search task (Bogte et al., 2007). Generalizing beyond the ocular motor system, failures of dynamic performance adjustment due to ACC dysfunction and dysconnectivity may contribute to the rigid and perseverative behaviours that characterize ASD.
Repetitive behaviour scores in ASD were also associated with reduced FA in the WM underlying left subgenual rACC. Subgenual rACC is part of a network that is thought to play an important role in generating autonomic responses to motivationally salient stimuli, evaluating their significance in predicting reward and in using this information to guide behaviour (Damasio et al., 1990; Drevets, 2000). An important consideration in interpreting this relation of FA to repetition is that subgenual rACC region was not functionally or structurally abnormal in this ASD sample. This suggests that even normal variation in FA of the subgenual rACC may contribute to a tendency to repeat unreinforced stimulus-response mappings. More generally, the relations we observed between behavioural repetition and ACC function and structure may not be specific to ASD. Accumulating evidence suggests that the three symptom clusters that define autism—social impairment, communication deficits and restricted, repetitive behaviour—arise from distinct genetic and cognitive mechanisms and may represent continuous traits in the population (for reviews see: Happe et al., 2006; London, 2007). Previous studies have reported relations between indices of response monitoring and repetition in OCD (Gehring et al., 2000; Ursu et al., 2003) and in non-clinically ascertained samples of children and adults with obsessive–compulsive characteristics (Hajcak and Simons, 2002; Santesso et al., 2006).
Our findings also raise the question of whether the response monitoring abnormalities that we observed are specific to ASD. Similar abnormalities have been reported in OCD (Maltby et al., 2005) and attention deficit hyperactivity disorder (van Meel et al., 2007). While methodological differences preclude a direct comparison of these findings, we previously studied patients with schizophrenia using the identical paradigm (Polli et al., 2008). Schizophrenia is also a neurodevelopmental disorder that is characterized by rigid and perseverative behaviour, response monitoring abnormalities (Carter et al., 2001), reduced FA in ACC WM (Manoach et al., 2007a) and a consistently elevated antisaccade error rate (Radant et al., 2007; Manoach et al. 2002). Although both schizophrenia and ASD samples made more antisaccade errors than controls, ASD participants performed correct trials faster and schizophrenia patients performed more slowly. While both groups showed reduced discrimination between error and correct trials in the ACC, this occurred for different reasons. In ASD this reflected a hyperactive response to correct trials, while in schizophrenia it reflected a blunted ACC response to errors, consistent with numerous previous reports (Laurens et al., 2003). These differences argue for distinct behavioural and neural signatures of response monitoring deficits in schizophrenia and ASD. These findings, along with those of recent neuroimaging studies suggesting genetic mediation of response monitoring (Fallgatter et al., 2004; Klein et al., 2007; Kramer et al., 2007), support the candidacy of neuroimaging-based indices of response monitoring as neurocognitive endophenotypes. Whether response monitoring abnormalities can be distinguished in ASD, OCD and other neuropsychiatric disorders, whether they are related to diagnosis or specific traits, and whether they are also present in unaffected family members remains to be seen.
Several additional limitations to and alternative interpretations of our findings merit consideration. First, might the hyperactive rACC response to correct antisaccades in ASD reflect a less efficient response to conflict rather than response monitoring? The ACC is thought to play a role in detecting conflict and engaging cognitive control to resolve it (for review see: Carter and van Veen, 2007). Antisaccades involve a high degree of conflict as one must choose between two competing responses and inhibit the prepotent one. Errors give rise to another type of conflict when the actual response is compared with the intended one. Thus, both correct and erroneous antisaccades engender conflict. While it is not possible to entirely rule out the conflict explanation, several observations argue against it. If this rACC region were detecting conflict it should respond to both correct and error trials. Instead, controls showed a significant response only to errors. In addition, the timing of the haemodynamic response to correct antisaccades in ASD, which started ∼4 s and peaked at ∼8 s (Fig. 3), argues for a role in response monitoring rather than successful conflict resolution. In this paradigm, differential fMRI activation for correct antisaccade versus prosaccade trials, including in dACC, presumably reflecting conflict detection, response inhibition and saccade generation, peaks at ∼4 s and is mostly gone by 8 s (Manoach et al., 2007b; see also Polli et al., 2005, for a discussion of ACC regions involved in saccade generation versus response monitoring). Finally, it is dorsal rather than rACC activation that is associated with engaging cognitive control on correct high-conflict and error trials (Carter and van Veen, 2007).
An important limitation is that the small sample size of the present study may have compromised our power to detect additional group differences and relations of interest, particularly in regions outside our a priori ACC regions of interest for which more stringent multiple comparisons corrections were applied. In spite of the relatively small sample size, our a priori hypotheses were confirmed. In addition, four of our ASD participants were taking medications that could affect ACC function. A re-analysis of the fMRI data with only unmedicated ASD participants, however, supported our main findings of significantly less discrimination between response outcomes in the rACC, and greater activation to correct trials. It is also important to note that our sample was limited to high functioning adults with ASD so it is not clear that our findings would generalize to lower functioning or younger samples. Although increased antisaccade error rates are seen in autism as early as ages 8–12 (Luna et al., 2007), we limited our study to adults since saccadic inhibition may not fully develop until late adolescence (Klein and Foerster, 2001) and larger samples would be necessary to discriminate between the effects of diagnosis and those due to normal development. Another limitation concerns our interpretation of FA as reflecting connectivity. FA reflects the degree of directional coherence of water diffusion in tissue and is, therefore, an indirect measure of WM microstructure. While FA correlates with axon myelination (Harsan et al., 2006), not all of its biophysical determinants are fully understood (Beaulieu, 2002). Thus, it is not possible to attribute FA differences or the relations we observed to a particular WM property.
In summary, the present study reports hyperactive response monitoring in the rACC in ASD that is related to restricted, repetitive behaviour and is accompanied by reduced FA in the underlying white matter. This suggests a structural correlate to abnormal function that may compromise connectivity in the circuitry subserving response monitoring. These findings suggest that structural and functional abnormalities of the ACC compromise response monitoring and contribute to behavioural repetition in ASD. Impairments in evaluating and learning from errors may lead to behaviour that is rigid and repetitive rather than optimally guided by outcomes, and may compromise performance across a wide range of tasks. Illuminating the neural basis and clinical significance of response monitoring deficits in ASD represents a fruitful avenue for further research.
Supplementary material
Supplementary material is available at Brain online.
Supplementary Material
Acknowledgements
The authors wish to thank Mark Vangel for statistical consultation, Robert Levy, Kim Ono, Christopher Sherman and Jeremy Young for help with manuscript preparation, and Matt Cain and Jay Edelman for technical assistance. Some of these data were presented at the annual meeting of the Society for Neuroscience in Washington, DC, in November, 2005. This study was supported by National Institute for Mental Health [R01 MH67720 (D.S.M.); MH72120 (F.E.P.)]; Mental Illness Neuroscience Discovery (MIND) Institute (DOE DE-FG02-99ER62764): The National Center for Research Resources (P41RR14075). Funding to pay the Open Access publication charges for this article was provided by the National Institute for Mental Health NRSA MH72120.
Glossary
Abbreviations:
- ACC
anterior cingulate cortex
- ADI-R
autism diagnostic interview-revised
- ASD
autism spectrum disorders
- BOLD
blood oxygen level dependent
- CWP
cluster-wise probability
- dACC
dorsal ACC
- DTI
diffusion tensor imaging
- ERN
error-related negativity
- HC
healthy control
- IQ
intelligence quotient
- MPRAGE
magnetization prepared rapid gradient echo
- OCD
obsessive–compulsive disorder
- rACC
rostral ACC
- TE
echo time
- TR
repetition time
- WM
white matter
References
- Ashwin C, Baron-Cohen S, Wheelwright S, O’Riordan M, Bullmore ET. Differential activation of the amygdala and the ‘social brain’ during fearful face-processing in Asperger syndrome. Neuropsychologia. 2007;45:2–14. doi: 10.1016/j.neuropsychologia.2006.04.014. [DOI] [PubMed] [Google Scholar]
- Barnea-Goraly N, Kwon H, Menon V, Eliez S, Lotspeich L, Reiss AL. White matter structure in autism: preliminary evidence from diffusion tensor imaging. Biol Psychiatry. 2004;55:323–6. doi: 10.1016/j.biopsych.2003.10.022. [DOI] [PubMed] [Google Scholar]
- Baron-Cohen S. Do autistic children have obsessions and compulsions? Br J Clin Psychol. 1989;28(Pt 3):193–200. doi: 10.1111/j.2044-8260.1989.tb01369.x. [DOI] [PubMed] [Google Scholar]
- Barton JJ, Greenzang C, Hefter R, Edelman J, Manoach DS. Switching, plasticity, and prediction in a saccadic task-switch paradigm. Exp Brain Res. 2006;168:76–87. doi: 10.1007/s00221-005-0091-1. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Mattiello J, LeBihan D. MR diffusion tensor spectroscopy and imaging. Biophys J. 1994;66:259–67. doi: 10.1016/S0006-3495(94)80775-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu C. The basis of anisotropic water diffusion in the nervous system - a technical review. NMR Biomed. 2002;15:435–55. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- Blair JR, Spreen O. Predicting premorbid IQ: a revision of the National Adult Reading Test. Clin Neuropsychol. 1989;3:129–136. [Google Scholar]
- Bogte H, Flamma B, van der Meere J, van Engeland H. Post-error adaptation in adults with high functioning autism. Neuropsychologia. 2007;45:1707–14. doi: 10.1016/j.neuropsychologia.2006.12.020. [DOI] [PubMed] [Google Scholar]
- Breiter HC, Rauch SL, Kwong KK, Baker JR, Weisskoff RM, Kennedy DN, et al. Functional magnetic resonance imaging of symptom provocation in obsessive-compulsive disorder. Arch Gen Psychiatry. 1996;53:595–606. doi: 10.1001/archpsyc.1996.01830070041008. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Goodman J, Burock M, Rotte M, Koutstaal W, Schacter D, et al. Functional-anatomic correlates of object priming in humans revealed by rapid presentation event-related fMRI. Neuron. 1998;20:285–96. doi: 10.1016/s0896-6273(00)80456-0. [DOI] [PubMed] [Google Scholar]
- Burock MA, Dale AM. Estimation and detection of event-related fMRI signals with temporally correlated noise: a statistically efficient and unbiased approach. Hum Brain Mapp. 2000;11:249–60. doi: 10.1002/1097-0193(200012)11:4<249::AID-HBM20>3.0.CO;2-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush G, Whalen PJ, Rosen BR, Jenike MA, McInerney SC, Rauch SL. The counting stroop: an interference task specialized for functional neuroimaging- -validation study with functional MRI. Hum Brain Mapp. 1998;6:270–82. doi: 10.1002/(SICI)1097-0193(1998)6:4<270::AID-HBM6>3.0.CO;2-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter CS, MacDonald AW, 3rd, Ross LL, Stenger VA. Anterior cingulate cortex activity and impaired self-monitoring of performance in patients with schizophrenia: an event-related fMRI study. Am J Psychiatry. 2001;158:1423–8. doi: 10.1176/appi.ajp.158.9.1423. [DOI] [PubMed] [Google Scholar]
- Carter CS, van Veen V. Anterior cingulate cortex and conflict detection: an update of theory and data. Cogn Affect Behav Neurosci. 2007;7:367–79. doi: 10.3758/cabn.7.4.367. [DOI] [PubMed] [Google Scholar]
- Cherkasova MV, Manoach DS, Intriligator JM, Barton JJS. Antisaccades and task-switching: interactions in controlled processing. Exp Brain Res. 2002;144:528–37. doi: 10.1007/s00221-002-1075-z. [DOI] [PubMed] [Google Scholar]
- Collins DL, Neelin P, Peters TM, Evans AC. Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space. J Comput Assist Tomogr. 1994;18:192–205. [PubMed] [Google Scholar]
- Courchesne E, Pierce K. Why the frontal cortex in autism might be talking only to itself: local over-connectivity but long-distance disconnection. Curr Opin Neurobiol. 2005;15:225–30. doi: 10.1016/j.conb.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Cox RW, Jesmanowicz A. Real-time 3D image registration for functional MRI. Magn Reson Med. 1999;42:1014–8. doi: 10.1002/(sici)1522-2594(199912)42:6<1014::aid-mrm4>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Dale AM. Optimal experimental design for event-related fMRI. Hum Brain Mapp. 1999;8:109–40. doi: 10.1002/(SICI)1097-0193(1999)8:2/3<109::AID-HBM7>3.0.CO;2-W. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–94. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Damasio AR, Tranel D, Damasio H. Individuals with sociopathic behavior caused by frontal damage fail to respond autonomically to social stimuli. Behav Brain Res. 1990;41:81–94. doi: 10.1016/0166-4328(90)90144-4. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Posner MI, Tucker DM. Localization of a neural system for error detection and compensation. Psychol Sci. 1994;5:303–5. [Google Scholar]
- DeSouza JF, Menon RS, Everling S. Preparatory set associated with pro-saccades and anti-saccades in humans investigated with event-related FMRI. J Neurophysiol. 2003;89:1016–23. doi: 10.1152/jn.00562.2002. [DOI] [PubMed] [Google Scholar]
- Devinsky O, Morrell MJ, Vogt BA. Contributions of anterior cingulate cortex to behaviour. Brain. 1995;118(Pt 1):279–306. doi: 10.1093/brain/118.1.279. [DOI] [PubMed] [Google Scholar]
- Dichter GS, Belger A. Social stimuli interfere with cognitive control in autism. Neuroimage. 2007;35:1219–30. doi: 10.1016/j.neuroimage.2006.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty DD, Baer L, Cosgrove GR, Cassem EH, Price BH, Nierenberg AA, et al. Prospective long-term follow-up of 44 patients who received cingulotomy for treatment-refractory obsessive-compulsive disorder. Am J Psychiatry. 2002;159:269–75. doi: 10.1176/appi.ajp.159.2.269. [DOI] [PubMed] [Google Scholar]
- Drevets WC. Neuroimaging studies of mood disorders. Biol Psychiatry. 2000;48:813–29. doi: 10.1016/s0006-3223(00)01020-9. [DOI] [PubMed] [Google Scholar]
- Everling S, Dorris MC, Klein RM, Munoz DP. Role of primate superior colliculus in preparation and execution of anti-saccades and pro-saccades. J Neurosci. 1999;19:2740–54. doi: 10.1523/JNEUROSCI.19-07-02740.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everling S, Munoz DP. Neuronal correlates for preparatory set associated with pro-saccades and anti-saccades in the primate frontal eye field. J Neurosci. 2000;20:387–400. doi: 10.1523/JNEUROSCI.20-01-00387.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkenstein M, Hohnsbein J, Hoormann J. Event-related correlates of errors in reaction tasks. In: Karmos G, Molnar M, Csepe I, Desmedt JE, editors. Perspectives of event-related potentials research. Amsterdam, The Netherlands: Elsevier Science; 1995. pp. 287–96. [Google Scholar]
- Fallgatter AJ, Herrmann MJ, Roemmler J, Ehlis AC, Wagener A, Heidrich A, et al. Allelic variation of serotonin transporter function modulates the brain electrical response for error processing. Neuropsychopharmacology. 2004;29:1506–11. doi: 10.1038/sj.npp.1300409. [DOI] [PubMed] [Google Scholar]
- Fecteau JH, Au C, Armstrong IT, Munoz DP. Sensory biases produce alternation advantage found in sequential saccadic eye movement tasks. Exp Brain Res. 2004;159:84–91. doi: 10.1007/s00221-004-1935-9. [DOI] [PubMed] [Google Scholar]
- Fischer B, Breitmeyer B. Mechanisms of visual attention revealed by saccadic eye movements. Neuropsychologia. 1987;25:73–83. doi: 10.1016/0028-3932(87)90044-3. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999a;9:195–207. doi: 10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Tootell RBH, Dale AM. High-resolution intersubject averaging and a coordinate system for the cortical surface. Hum Brain Mapp. 1999b;8:272–84. doi: 10.1002/(SICI)1097-0193(1999)8:4<272::AID-HBM10>3.0.CO;2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, van der Kouwe A, Destrieux C, Halgren E, Segonne F, Salat DH, et al. Automatically parcellating the human cerebral cortex. Cereb Cortex. 2004;14:11–22. doi: 10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- Fitzgerald KD, Welsh RC, Gehring WJ, Abelson JL, Himle JA, Liberzon I, et al. Error-related hyperactivity of the anterior cingulate cortex in obsessive-compulsive disorder. Biol Psychiatry. 2005;57:287–94. doi: 10.1016/j.biopsych.2004.10.038. [DOI] [PubMed] [Google Scholar]
- Garavan H, Ross TJ, Murphy K, Roche RA, Stein EA. Dissociable executive functions in the dynamic control of behavior: inhibition, error detection, and correction. NeuroImage. 2002;17:1820–9. doi: 10.1006/nimg.2002.1326. [DOI] [PubMed] [Google Scholar]
- Gehring WJ, Goss B, Coles MG, Meyer DE, Donchin E. A neural system for error detection and compensation. Psychol Sci. 1993;4:385–90. [Google Scholar]
- Gehring WJ, Himle J, Nisenson LG. Action-monitoring dysfunction in obsessive-compulsive disorder. Psychol Sci. 2000;11:1–6. doi: 10.1111/1467-9280.00206. [DOI] [PubMed] [Google Scholar]
- Gehring WJ, Knight RT. Prefrontal-cingulate interactions in action monitoring. Nat Neurosci. 2000;3:516–20. doi: 10.1038/74899. [DOI] [PubMed] [Google Scholar]
- Goldberg MC, Lasker AG, Zee DS, Garth E, Tien A, Landa RJ. Deficits in the initiation of eye movements in the absence of a visual target in adolescents with high functioning autism. Neuropsychologia. 2002;40:2039–49. doi: 10.1016/s0028-3932(02)00059-3. [DOI] [PubMed] [Google Scholar]
- Gomot M, Bernard FA, Davis MH, Belmonte MK, Ashwin C, Bullmore ET, et al. Change detection in children with autism: an auditory event-related fMRI study. Neuroimage. 2006;29:475–84. doi: 10.1016/j.neuroimage.2005.07.027. [DOI] [PubMed] [Google Scholar]
- Hajcak G, Simons RF. Error-related brain activity in obsessive-compulsive undergraduates. Psychiatry Res. 2002;110:63–72. doi: 10.1016/s0165-1781(02)00034-3. [DOI] [PubMed] [Google Scholar]
- Hall GB, Szechtman H, Nahmias C. Enhanced salience and emotion recognition in autism: a PET study. Am J Psychiatry. 2003;160:1439–41. doi: 10.1176/appi.ajp.160.8.1439. [DOI] [PubMed] [Google Scholar]
- Hallett PE. Primary and secondary saccades to goals defined by instructions. Vision Res. 1978;18:1279–96. doi: 10.1016/0042-6989(78)90218-3. [DOI] [PubMed] [Google Scholar]
- Happe F, Ronald A, Plomin R. Time to give up on a single explanation for autism. Nat Neurosci. 2006;9:1218–20. doi: 10.1038/nn1770. [DOI] [PubMed] [Google Scholar]
- Harsan LA, Poulet P, Guignard B, Steibel J, Parizel N, de Sousa PL, et al. Brain dysmyelination and recovery assessment by noninvasive in vivo diffusion tensor magnetic resonance imaging. J Neurosci Res. 2006;83:392–402. doi: 10.1002/jnr.20742. [DOI] [PubMed] [Google Scholar]
- Haznedar MM, Buchsbaum MS, Metzger M, Solimando A, Spiegel-Cohen J, Hollander E. Anterior cingulate gyrus volume and glucose metabolism in autistic disorder. Am J Psychiatry. 1997;154:1047–50. doi: 10.1176/ajp.154.8.1047. [DOI] [PubMed] [Google Scholar]
- Haznedar MM, Buchsbaum MS, Wei TC, Hof PR, Cartwright C, Bienstock CA, et al. Limbic circuitry in patients with autism spectrum disorders studied with positron emission tomography and magnetic resonance imaging. Am J Psychiatry. 2000;157:1994–2001. doi: 10.1176/appi.ajp.157.12.1994. [DOI] [PubMed] [Google Scholar]
- Henderson H, Schwartz C, Mundy P, Burnette C, Sutton S, Zahka N, et al. Response monitoring, the error-related negativity, and differences in social behavior in autism. Brain Cogn. 2006;61:96–109. doi: 10.1016/j.bandc.2005.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill EL. Executive dysfunction in autism. Trends Cogn Sci. 2004;8:26–32. doi: 10.1016/j.tics.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Hollander E, King A, Delaney K, Smith CJ, Silverman JM. Obsessive-compulsive behaviors in parents of multiplex autism families. Psychiatry Res. 2003;117:11–6. doi: 10.1016/s0165-1781(02)00304-9. [DOI] [PubMed] [Google Scholar]
- Hollingshead AB. Two factor index of social position. New Haven, CT: Yale University Press; 1965. [Google Scholar]
- Holroyd CB, Coles MG. The neural basis of human error processing: reinforcement learning, dopamine, and the error-related negativity. Psychol Rev. 2002;109:679–709. doi: 10.1037/0033-295X.109.4.679. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal. 2001;5:143–56. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Johannes S, Wieringa BM, Nager W, Rada D, Dengler R, Emrich HM, et al. Discrepant target detection and action monitoring in obsessive-compulsive disorder. Psychiatry Res. 2001;108:101–10. doi: 10.1016/s0925-4927(01)00117-2. [DOI] [PubMed] [Google Scholar]
- Jones DK. The effect of gradient sampling schemes on measures derived from diffusion tensor MRI: A Monte Carlo study. Magn Reson Med. 2004;51:807–15. doi: 10.1002/mrm.20033. [DOI] [PubMed] [Google Scholar]
- Kana RK, Keller TA, Minshew NJ, Just MA. Inhibitory control in high-functioning autism: decreased activation and underconnectivity in inhibition networks. Biol Psychiatry. 2007;62:198–206. doi: 10.1016/j.biopsych.2006.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy DP, Redcay E, Courchesne E. Failing to deactivate: resting functional abnormalities in autism. Proc Natl Acad Sci USA. 2006;103:8275–80. doi: 10.1073/pnas.0600674103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein C, Foerster F. Development of prosaccade and antisaccade task performance in participants aged 6 to 26 years. Psychophysiology. 2001;38:179–89. [PubMed] [Google Scholar]
- Klein TA, Neumann J, Reuter M, Hennig J, von Cramon DY, Ullsperger M. Genetically determined differences in learning from errors. Science. 2007;318:1642–5. doi: 10.1126/science.1145044. [DOI] [PubMed] [Google Scholar]
- Kramer UM, Cunillera T, Camara E, Marco-Pallares J, Cucurell D, Nager W, et al. The impact of catechol-O-methyltransferase and dopamine D4 receptor genotypes on neurophysiological markers of performance monitoring. J Neurosci. 2007;27:14190–8. doi: 10.1523/JNEUROSCI.4229-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurens KR, Ngan ET, Bates AT, Kiehl KA, Liddle PF. Rostral anterior cingulate cortex dysfunction during error processing in schizophrenia. Brain. 2003;126:610–22. doi: 10.1093/brain/awg056. [DOI] [PubMed] [Google Scholar]
- Levitt JG, O’Neill J, Blanton RE, Smalley S, Fadale D, McCracken JT, et al. Proton magnetic resonance spectroscopic imaging of the brain in childhood autism. Biol Psychiatry. 2003;54:1355–66. doi: 10.1016/s0006-3223(03)00688-7. [DOI] [PubMed] [Google Scholar]
- Leyfer OT, Folstein SE, Bacalman S, Davis NO, Dinh E, Morgan J, et al. Comorbid psychiatric disorders in children with autism: interview development and rates of disorders. J Autism Dev Disord. 2006;36:849–61. doi: 10.1007/s10803-006-0123-0. [DOI] [PubMed] [Google Scholar]
- London E. The role of the neurobiologist in redefining the diagnosis of autism. Brain Pathol. 2007;17:408–11. doi: 10.1111/j.1750-3639.2007.00103.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez BR, Lincoln AJ, Ozonoff S, Lai Z. Examining the relationship between executive functions and restricted, repetitive symptoms of Autistic Disorder. J Autism Dev Disord. 2005;35:445–60. doi: 10.1007/s10803-005-5035-x. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore PC, Risi S. Autism diagnostic observation schedule - WPS (ADOS-WPS). Los Angeles, CA: Western Psychological Services; 1999. [Google Scholar]
- Luna B, Doll SK, Hegedus SJ, Minshew NJ, Sweeney JA. Maturation of executive function in autism. Biol Psychiatry. 2007;61:474–81. doi: 10.1016/j.biopsych.2006.02.030. [DOI] [PubMed] [Google Scholar]
- Luu P, Tucker DM, Derryberry D, Reed M, Poulsen C. Electrophysiological responses to errors and feedback in the process of action regulation. Psychol Sci. 2003;14:47–53. doi: 10.1111/1467-9280.01417. [DOI] [PubMed] [Google Scholar]
- Maltby N, Tolin DF, Worhunsky P, O’Keefe TM, Kiehl KA. Dysfunctional action monitoring hyperactivates frontal-striatal circuits in obsessive-compulsive disorder: an event-related fMRI study. Neuroimage. 2005;24:495–503. doi: 10.1016/j.neuroimage.2004.08.041. [DOI] [PubMed] [Google Scholar]
- Manoach DS, Ketwaroo GA, Polli FE, Thakkar KN, Barton JJ, Goff DC, et al. Reduced microstructural integrity of the white matter underlying anterior cingulate cortex is associated with increased saccadic latency in schizophrenia. Neuroimage. 2007a;37:599–610. doi: 10.1016/j.neuroimage.2007.04.062. [DOI] [PubMed] [Google Scholar]
- Manoach DS, Lindgren KA, Barton JJ. Deficient saccadic inhibition in Asperger's disorder and the social-emotional processing disorder. J Neurol Neurosurg Psychiatry. 2004;75:1719–26. doi: 10.1136/jnnp.2003.025981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoach DS, Lindgren KA, Cherkasova MV, Goff DC, Halpern EF, Intriligator J, et al. Schizophrenic subjects show deficient inhibition but intact task-switching on saccadic tasks. Biol Psychiatry. 2002;51:816–26. doi: 10.1016/s0006-3223(01)01356-7. [DOI] [PubMed] [Google Scholar]
- Manoach DS, Thakkar KN, Cain MS, Polli FE, Edelman JA, Fischl B, et al. Neural activity is modulated by trial history: a functional magnetic resonance imaging study of the effects of a previous antisaccade. J Neurosci. 2007b;27:1791–8. doi: 10.1523/JNEUROSCI.3662-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manoach DS, Weintraub S, Daffner KR, Scinto LF. Deficient antisaccades in the social-emotional processing disorder. Neuroreport. 1997;8:901–5. doi: 10.1097/00001756-199703030-00017. [DOI] [PubMed] [Google Scholar]
- McDougle CJ, Kresch LE, Goodman WK, Naylor ST, Volkmar FR, Cohen DJ, et al. A case-controlled study of repetitive thoughts and behavior in adults with autistic disorder and obsessive-compulsive disorder. Am J Psychiatry. 1995;152:772–7. doi: 10.1176/ajp.152.5.772. [DOI] [PubMed] [Google Scholar]
- McPeek RM, Skavenski AA, Nakayama K. Concurrent processing of saccades in visual search. Vision Res. 2000;40:2499–516. doi: 10.1016/s0042-6989(00)00102-4. [DOI] [PubMed] [Google Scholar]
- Miezin FM, Maccotta L, Ollinger JM, Petersen SE, Buckner RL. Characterizing the hemodynamic response: effects of presentation rate, sampling procedure, and the possibility of ordering brain activity based on relative timing. Neuroimage. 2000;11:735–59. doi: 10.1006/nimg.2000.0568. [DOI] [PubMed] [Google Scholar]
- Minshew NJ, Luna B, Sweeney JA. Oculomotor evidence for neocortical systems but not cerebellar dysfunction in autism. Neurology. 1999;52:917–22. doi: 10.1212/wnl.52.5.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mokler A, Fischer B. The recognition and correction of involuntary prosaccades in an antisaccade task. Exp Brain Res. 1999;125:511–6. doi: 10.1007/s002210050709. [DOI] [PubMed] [Google Scholar]
- Munoz DP, Everling S. Look away: the anti-saccade task and the voluntary control of eye movement. Nat Rev Neurosci. 2004;5:218–28. doi: 10.1038/nrn1345. [DOI] [PubMed] [Google Scholar]
- Murphy DG, Daly E, Schmitz N, Toal F, Murphy K, Curran S, et al. Cortical serotonin 5-HT2A receptor binding and social communication in adults with Asperger's syndrome: an in vivo SPECT study. Am J Psychiatry. 2006;163:934–6. doi: 10.1176/ajp.2006.163.5.934. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S, Nielen MM, Mol N, Hajcak G, Veltman DJ. Performance monitoring in obsessive-compulsive disorder. Psychiatry Res. 2005;134:111–22. doi: 10.1016/j.psychres.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S, Ridderinkhof KR, Blom J, Band GP, Kok A. Error-related brain potentials are differentially related to awareness of response errors: evidence from an antisaccade task. Psychophysiology. 2001;38:752–60. [PubMed] [Google Scholar]
- Ohnishi T, Matsuda H, Hashimoto T, Kunihiro T, Nishikawa M, Uema T, et al. Abnormal regional cerebral blood flow in childhood autism. Brain. 2000;123(Pt 9):1838–44. doi: 10.1093/brain/123.9.1838. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: The Edinburgh Inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Paus T. Primate anterior cingulate cortex: where motor control, drive and cognition interface. Nat Rev Neurosci. 2001;2:417–24. doi: 10.1038/35077500. [DOI] [PubMed] [Google Scholar]
- Pfefferbaum A, Sullivan EV, Hedehus M, Lim KO, Adalsteinsson E, Moseley M. Age-related decline in brain white matter anisotropy measured with spatially corrected echo-planar diffusion tensor imaging. Magn Reson Med. 2000;44:259–68. doi: 10.1002/1522-2594(200008)44:2<259::aid-mrm13>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Pitman RK. A cybernetic model of obsessive-compulsive psychopathology. Compr Psychiatry. 1987;28:334–43. doi: 10.1016/0010-440x(87)90070-8. [DOI] [PubMed] [Google Scholar]
- Polli FE, Barton JJ, Cain MS, Thakkar KN, Rauch SL, Manoach DS. Rostral and dorsal anterior cingulate cortex make dissociable contributions during antisaccade error commission. Proc Natl Acad Sci USA. 2005;102:15700–5. doi: 10.1073/pnas.0503657102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polli FE, Barton JJ, Thakkar KN, Greve DN, Goff DC, Rauch SL, et al. Reduced error-related activation in two anterior cingulate circuits is related to impaired performance in schizophrenia. Brain. 2008;131:971–86. doi: 10.1093/brain/awm307. [DOI] [PubMed] [Google Scholar]
- Polli FE, Barton JJ, Vangel M, Goff DC, Iguchi L, Manoach DS. Schizophrenia patients show intact immediate error-related performance adjustments on an antisaccade task. Schizophr Res. 2006;82:191–201. doi: 10.1016/j.schres.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Rabbitt PM. Errors and error correction in choice-response tasks. J Exp Psychol. 1966;71:264–72. doi: 10.1037/h0022853. [DOI] [PubMed] [Google Scholar]
- Radant AD, Dobie DJ, Calkins ME, Olincy A, Braff DL, Cadenhead KS, et al. Successful multi-site measurement of antisaccade performance deficits in schizophrenia. Schizophr Res. 2007;89:320–9. doi: 10.1016/j.schres.2006.08.010. [DOI] [PubMed] [Google Scholar]
- Reese TG, Heid O, Weisskoff RM, Wedeen VJ. Reduction of eddy-current-induced distortion in diffusion MRI using a twice-refocused spin echo. Magn Reson Med. 2003;49:177–82. doi: 10.1002/mrm.10308. [DOI] [PubMed] [Google Scholar]
- Russell J, Jarrold C. Error-correction problems in autism: evidence for a monitoring impairment? J Autism Dev Disord. 1998;28:177–88. doi: 10.1023/a:1026009203333. [DOI] [PubMed] [Google Scholar]
- Russell AJ, Mataix-Cols D, Anson M, Murphy DG. Obsessions and compulsions in Asperger syndrome and high-functioning autism. Br J Psychiatry. 2005;186:525–8. doi: 10.1192/bjp.186.6.525. [DOI] [PubMed] [Google Scholar]
- Rutter M, Le Couteur A, Lord C. Autism diagnostic interview-revised. Los Angeles, CA: Western Psychological Services; 2003. [Google Scholar]
- Santesso DL, Segalowitz SJ, Schmidt LA. Error-related electrocortical responses are enhanced in children with obsessive-compulsive behaviors. Dev Neuropsychol. 2006;29:431–45. doi: 10.1207/s15326942dn2903_3. [DOI] [PubMed] [Google Scholar]
- Shafritz KM, Dichter GS, Baranek GT, Belger A. The neural circuitry mediating shifts in behavioral response and cognitive set in autism. Biol Psychiatry. 2007;63:974–80. doi: 10.1016/j.biopsych.2007.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silk TJ, Rinehart N, Bradshaw JL, Tonge B, Egan G, O’Boyle MW, et al. Visuospatial processing and the function of prefrontal-parietal networks in autism spectrum disorders: a functional MRI study. Am J Psychiatry. 2006;163:1440–3. doi: 10.1176/ajp.2006.163.8.1440. [DOI] [PubMed] [Google Scholar]
- South M, Ozonoff S, McMahon WM. The relationship between executive functioning, central coherence, and repetitive behaviors in the high-functioning autism spectrum. Autism. 2007;11:437–51. doi: 10.1177/1362361307079606. [DOI] [PubMed] [Google Scholar]
- Taylor SF, Martis B, Fitzgerald KD, Welsh RC, Abelson JL, Liberzon I, et al. Medial frontal cortex activity and loss-related responses to errors. J Neurosci. 2006;26:4063–70. doi: 10.1523/JNEUROSCI.4709-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SF, Stern ER, Gehring WJ. Neural systems for error monitoring: recent findings and theoretical perspectives. Neuroscientist. 2007;13:160–72. doi: 10.1177/1073858406298184. [DOI] [PubMed] [Google Scholar]
- Thesen S, Heid O, Mueller E, Schad LR. Prospective acquisition correction for head motion with image-based tracking for real-time fMRI. Magn Reson Med. 2000;44:457–65. doi: 10.1002/1522-2594(200009)44:3<457::aid-mrm17>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Turner M. Annotation: Repetitive behaviour in autism: a review of psychological research. J Child Psychol Psychiatry. 1999;40:839–49. [PubMed] [Google Scholar]
- Ullsperger M, von Cramon DY. Neuroimaging of performance monitoring: error detection and beyond. Cortex. 2004;40:593–604. doi: 10.1016/s0010-9452(08)70155-2. [DOI] [PubMed] [Google Scholar]
- Ursu S, Stenger VA, Shear MK, Jones MR, Carter CS. Overactive action monitoring in obsessive-compulsive disorder: evidence from functional magnetic resonance imaging. Psychol Sci. 2003;14:347–53. doi: 10.1111/1467-9280.24411. [DOI] [PubMed] [Google Scholar]
- Meel CS, Heslenfeld DJ, Oosterlaan J, Sergeant JA. Adaptive control deficits in attention-deficit/hyperactivity disorder (ADHD): the role of error processing. Psychiatry Res. 2007;151:211–20. doi: 10.1016/j.psychres.2006.05.011. [DOI] [PubMed] [Google Scholar]
- van Veen V, Carter CS. The timing of action-monitoring processes in the anterior cingulate cortex. J Cogn Neurosci. 2002;14:593–602. doi: 10.1162/08989290260045837. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of intelligence. San Antonio, TX: The Psychological Corporation; 1999. [Google Scholar]
- Whalen PJ, Bush G, McNally RJ, Wilhelm S, McInerney SC, Jenike MA, et al. The emotional counting Stroop paradigm: a functional magnetic resonance imaging probe of the anterior cingulate affective division. Biol Psychiatry. 1998;44:1219–28. doi: 10.1016/s0006-3223(98)00251-0. [DOI] [PubMed] [Google Scholar]
- White K, Ashton R. Handedness assessment inventory. Neuropsychologia. 1976;14:261–4. doi: 10.1016/0028-3932(76)90058-0. [DOI] [PubMed] [Google Scholar]
- Zandt F, Prior M, Kyrios M. Repetitive behaviour in children with high functioning autism and obsessive compulsive disorder. J Autism Dev Disord. 2007;37:251–9. doi: 10.1007/s10803-006-0158-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.