Abstract
Physiologically, the lymphatic system regulates fluid volume in the interstitium and provides a conduit for immune cells to travel to lymph nodes, but pathologically, the lymphatic system serves as a primary escape route for cancer cells. Lymphatic capillaries have a thin discontinuous basement membrane, lack pericyte coverage, and often contain endothelial cell gaps that can be invaded by immune cells (or tumor cells). In addition, tumor cells and stromal cells in the tumor microenvironment secrete factors that stimulate lymphangiogenesis, the growth of lymphatic endothelial cells and the sprouting of lymphatic capillaries. As a result, many tumors are surrounded by large, hyperplastic, peri-tumoral lymphatic vessels and less frequently are invaded by intra-tumoral lymphatic vessels. Carcinoma cells commonly metastasize through these lymphatic vessels to regional lymph nodes. The presence of metastatic cells in the sentinel lymph node is a prognostic indicator for many types of cancer, and the degree of dissemination determines the therapeutic course of action. Lymphangiogenesis is currently at the frontier of metastasis research. Recent strides in this field have uncovered numerous signaling pathways specific for lymphatic endothelial cells and vascular endothelial cells. This review will provide an overview of tumor lymphangiogenesis and current strategies aimed at inhibiting lymphatic metastasis. Novel therapeutic approaches that target the tumor cells as well as the vascular and lymphatic endothelial compartments are discussed.
INTRODUCTION
The Cutaneous Lymphatic System
Although the vascular system and the lymphatic system are both lined with endothelial cells, the two systems differ quite dramatically. The vascular system is a closed, circulatory system in which the heart pumps blood around the body through arteries, capillaries, and veins. In contrast, the lymphatic system is an open-ended, unidirectional system in which fluid flows from tissues back to the blood stream (Rusznyak, 1967). The cutaneous lymphatic system is depicted in Figure 1A. Initial lymphatics are blind-ended, finger-shaped vessels that protrude into the upper dermis near the epidermis. These lymphatic capillaries are lined with a thin, single layer of endothelial cells that form interdigitating, overlapping, and end-to-end-type junctions (Sauter et al., 1998). Terminal lymphatics drain the interstitial fluid and proteinous exudate that leaks from blood capillaries. Lymphatic endothelial cells (LEC) in the capillaries attach to collagen fibers in the dermal extracellular matrix via anchoring filaments composed of elastic fibers. These fibers are responsible for increasing luminal diameters of lymphatic vessels when interstitial fluid volumes are increased (Swartz and Skobe, 2001). In addition, lymphatic capillaries have an incomplete basement membrane, lack pericyte coverage, and contain frequent gaps between neighboring endothelial cells (Daróczy, 1988; Sauter et al., 1998; Schacht et al., 2004). Immune cells such as Langerhans cells in the skin can invade these interendothelial openings (Stoitzner et al., 2002).
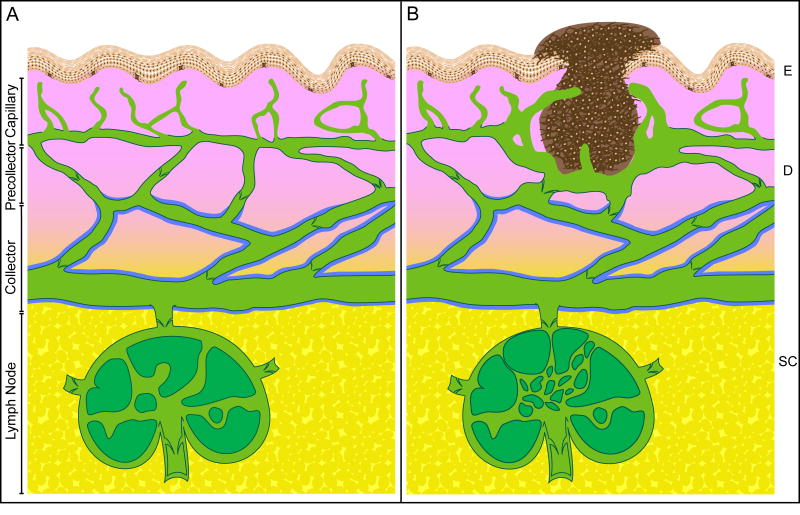
Figure 1.
Structural diagram of the cutaneous lymphatic system under physiological and pathological (tumor-bearing) conditions. A. Interstitial fluid is drained through wide luminal capillaries (green color) that extend up near the epidermis (E, peach color). Capillaries are composed of thin layers of endothelial cells connecting to the extracellular matrix through anchoring filaments. Capillaries possess inter-endothelial cell gaps, discontinuous basement membrane, no valves, and no pericyte coverage. In the dermis (D, pink color), capillaries drain into lymphatic vessels called precollectors that have a continuous basement membrane (denoted by dark green line) and valves that prevent the reflux of lymph. At the border to the subcutis (SC, yellow color), precollectors drain into collecting lymphatic vessels that are surrounded with smooth muscle cells or pericytes (denoted by blue line) that constrict to propel the lymph along to regional lymph nodes. B. An invasive melanoma (dark brown color) is shown. Tumor cells metastasize through peri-tumoral and intratumor lymphatic capillaries. Lymphatic capillary density around the tumor is increased and tumor-associated lymphatic capillaries are dilated and hyperplastic. A few lymphatic capillaries have sprouted into the tumor. The sentinel lymph node is shown with lymphangiogenic vessels as well. Note: this diagram is not drawn exactly to scale.
The superficial lymphatic plexus is located in the upper dermis (near the arterial plexus) and includes a network of valve-less, lymphatic capillaries that interconnect to ensure adequate drainage even in the event that one becomes occluded (Haagensen et al., 1972). In general, veins outnumber lymphatics in the skin, but in certain regions such as the fingers, palms, soles, and pubic areas the density of lymphatic capillaries is abundant (Haagensen et al., 1972; Rusznyak, 1967). Lymphatic vessels are often found in close proximity to blood vessels, yet the two systems never intermix within the skin (Rafii and Skobe, 2003). In the dermis, lymphatic capillaries drain into larger lymphatic vessels called precollectors. The precollectors have a continuous basal lamina and contain endothelial cell protrusions into the vessel lumen that function as valves to maintain the unidirectional flow of lymph and to protect against reflux. In the skin, valves are present every 2-3 mm (Daróczy, 1988).
A deeper lymphatic plexus is found at the cutis-subcutis boundary, where precollectors drain into thicker lymphatic vessels of varying caliber called collectors (Figure 1A). Collectors have a continuous membrane, valves, and are surrounded by smooth muscle cells that contract to propel lymph toward afferent vessels of regional lymph nodes (Daróczy, 1988). The subcutaneous space contains no lymphatic capillaries, but the large collecting lymphatics in this region are found sparsely distributed and follow venous routes (Rusznyak, 1967). Lymphatic fluid enters the lymph node through several afferent vessels and usually exits via a single efferent vessel (Figure 1A). After the lymph is filtered through several lymph nodes, it is drained into larger lymphatic trunks that lead to the left lymphatic duct (thoracic duct) or right lymphatic duct and then into the subclavian veins. In this way, the lymphatic system helps to return approximately 10% of the fluid volume that escapes from the tissue capillary beds to the vascular system.
The Pathogenesis of a Lymphatic Metastasis
Metastasis, the spread of tumor cells from the primary site to distant organ environments, is the leading cause of death from cancer. Tumors are heterogeneous in that some tumor foci within the parent neoplasm have more invasive and metastatic properties than others (Fidler, 1990; Fidler, 2002). The metastatic cascade involving the vascular system has been well established (Fidler et al., 2000). But in many solid tumors, metastasis via the lymphatic system precedes metastasis via the vascular system.
Here, we outline our current understanding and the likely events in the pathogenesis of a lymphatic metastasis. Following transformation, a tumor cell continues to proliferate and receive its nutrients and oxygen by diffusion until the nodule reaches approximately 2 mm in diameter. In order to grow further, the tumor must recruit a new blood capillary network from the surrounding tissue, a process called angiogenesis (Folkman, 1971). Tumors promote angiogenesis by secreting molecules from tumor cells and stromal cells that attract vascular capillaries and stimulate their sprouting. Many of the same molecules that induce vascular endothelial cell (VEC) growth also stimulate LEC growth (Table 1) (McColl et al., 2005; Van der Auwera et al., 2006). In some patients, tumor-derived factors stimulate intra-tumoral lymphatic vessels (Dadras et al., 2003; Maula et al., 2003; Kyzas et al., 2005b; Sipos et al., 2004), while most tumors induce hyperplastic peri-tumoral lymphatic vessels (depicted in Figure 1B). The lymphatic capillaries surrounding the tumor can reach sizes of 10-50 times that of a normal lymphatic capillary. This increased lumen size may be the result of dilation, LEC proliferation, high tumor-induced interstitial pressure, or the consequence of tumor-secreted extracellular matrix molecules that pull on the lymphatic focal adhesions keeping them in a constant state of openness.
Table 1.
Lymphangiogenic factors.
| Growth factor/cytokine | Selected references | |
|---|---|---|
| VEGF | ||
| • | VEGF-A | (Nagy et al., 2002; Bjorndahl et al., 2005b; Hirakawa et al., 2005) |
| • | VEGF-C | (Joukov et al., 1996; Jeltsch et al., 1997; Skobe et al., 2001b) |
| • | VEGF-D | (Achen et al., 1998; Stacker et al., 2001; Rissanen et al., 2003) |
| PDGF | (Cao et al., 2004) | |
| HGF | (Jiang et al., 2005; Kajiya et al., 2005; Cao et al., 2006) | |
| Ang-1 | (Morisada et al., 2005; Tammela et al., 2005b) | |
| Ang-2 | (Gale et al., 2002) | |
| IGF-1/2 | (Akagi et al., 1998; Bjorndahl et al., 2005a) | |
| FGF-2 (bFGF) | (Kubo et al., 2002; Chang et al., 2004) | |
Tumor cells capable of invading the host stroma may encounter these hyperplastic lymphatic capillaries as well as normal lymphatic capillaries. Terminal lymphatics do not have a continuous basement membrane and contain intercellular gaps that allow for fluid drainage and the infiltration of immune cells such as dendritic cells and Langerhans cells. These structural characteristics may make it easier for a tumor cell to enter the lymphatic system than the blood system (Sleeman, 2000). The basement membrane of tumor-associated lymphatic capillaries lacks laminin and collagen XVIII (Skobe et al., 2001b) and is completely lacking in some areas; therefore, tumor cells may not require as many invasive properties to invade the lymphatic system. In addition, LEC secrete chemotactic agents that attract malignant tumor cells toward areas of high lymphatic vessel density (Shields et al., 2007). Tumor cells must detach in order to be carried away in the lymph fluid, likely as small emboli, toward the afferent vessels of the sentinel lymph node. Fewer than 0.1% of tumor cells entering the blood circulation actually form metastases (Fidler, 1970), while dissemination through the lymphatic system appears much more efficient (Haagensen et al., 1972). The high flow rate and high serum concentrations in the blood stream are often toxic to tumor cells, whereas the relatively passive, low shear stress of the lymph fluid may allow for tumor cell survival (Sleeman, 2000).
Just as the lymph node acts as a filter for pathogens and immune cells, it also filters tumor cells. This filter function may initially protect the patient and prevent the early dissemination of tumor cells throughout the body. Hematogenous metastasis requires that the tumor cell extravasate through the vascular capillary out into the new organ environment, while lymphatic metastasis may not require such extravasation (Sleeman, 2000). The lymph node may simply concentrate all shed tumor cells into the same location in the reticular fibers of the marginal sinus where the emboli get trapped and subsequently proliferate in situ. The pooling of tumor cells in one site in the parenchyma of the node likely promotes their survival and further growth. Eventually, the entire node may become replaced by tumor. Tumor cells may escape from the efferent vessels of the draining lymph node to the next lymph node or to the thoracic duct and into the venous blood system for dissemination throughout the body. Alternatively, tumor cells may invade the blood vessels within the lymph node itself and metastasize to distant organs (Haagensen et al., 1972; Tobler and Detmar, 2006).
DISCUSSION
The Occurrence of Lymph Node Metastasis in Solid Tumors
Tumor cell metastasis to regional lymph nodes often marks the first step in tumor cell progression (Table 2). In general, carcinomas metastasize through the lymphatic system more often than sarcomas. In fact, carcinoma in the large intestine metastasizes almost exclusively via the lymphatics. Some carcinomas also metastasize via the vascular system. In breast cancer, the metastases usually result from early lymphatic dissemination followed by extensive spread into the vascular system in more advanced disease stages (Haagensen et al., 1972; Hess et al., 2006). Shortly after tumor formation, gastric carcinomas metastasize through both routes. Even within a certain type of cancer, location of the primary neoplasm can influence the route of metastasis. For instance, squamous cell carcinoma (SCC) in the lung metastasizes early through both hematogenous and lymphogenous paths (Haagensen et al., 1972), while SCC in the skin or cervix metastasizes late and mainly to sentinel lymph nodes (Weinberg et al., 2007).
Table 2.
Solid tumors that metastasize to lymph nodes.
| Tumor type | Selected references |
|---|---|
| Common: | |
| Bladder carcinoma | (Shariat et al., 2006; Jones et al., 2005) |
| Breast carcinoma | (Chen et al., 2006; Hess et al., 2006) |
| Cervical carcinoma | (Pandit-Taskar, 2005) |
| Colorectal carcinoma | (Chen et al., 2006; Hess et al., 2006) |
| Endometrial carcinoma | (Sohaib et al., 2007) |
| Esophageal carcinoma | (Hatakeyama et al., 2006) |
| Head and Neck carcinomas | (Chen et al., 2006) |
| Hepatocellular carcinoma | (Uka et al., 2007) |
| Melanoma | (Chen et al., 2006; Lin et al., 2005) |
| Non-small cell lung carcinoma | (Hess et al., 2006; Renyi-Vamos et al., 2005) |
| Ovarian carcinoma | (Onda et al., 1996) |
| Pancreatic ductual adenocarcinoma | (Sipos et al., 2005; Von Marschall et al., 2005) |
| Prostate carcinoma | (Morisawa et al., 2006) |
| Thyroid carcinoma* | (Rodriguez et al., 2000; Scollo et al., 2003) |
| Less common: | |
| Gastric Carcinoma | (Saito et al., 2007) |
| Renal cell carcinoma | (Denzinger et al., 2007) |
| Testicular carcinoma** | (Jones et al., 2000) |
Papillary thyroid carcinoma, medullary thyroid carcinoma and anaplastic thyroid carcinoma metastasize to sentinel lymph nodes.
Testicular carcinoma has a near 100% cure rate, but the primary site of relapse is the para-aortic lymph node.
Head and neck carcinomas frequently metastasize to lymph nodes (Table 2). Whether this is due to the fact that these tumors express high levels of lymphatic growth factors such as VEGF-C or VEGF-D (discussed below) or whether this is partly due to structural considerations since this region contains 300 lymph nodes, is unclear. Conversely, liposarcoma, fibrosarcoma and osteosarcoma seldom metastasize to lymph nodes (Fong et al., 1993). Two possible explanations may account for the lack of lymphatic metastasis in sarcomas: their low expression of lymphatic stimulators such as VEGF-C or their secretion of endogenous lymphangiogenic inhibitors. In fact, T241 fibrosarcoma cells growing subcutaneously in a syngeneic mouse could prevent lymphangiogenesis at a distant site (cornea) in that mouse, presumably by the production of a systemic inhibitor (Chang et al., 2002). However, when T241 fibrosarcoma cells were transfected with VEGF-C, this inhibition was overridden as tumors readily metastasized to regional lymph nodes (Padera et al., 2002).
Predicting the metastatic potential of a patient's tumor is a challenging task. Evidence of tumor cells in a lymph node is often the first indicator of cancer spread. Lymph node metastasis is correlated with an increased risk of distant metastasis and a poor clinical outcome (Chen et al., 2006). For instance, 75% of prostate cancer patients with lymph node metastasis at diagnosis will possess bone metastasis in 5 years (Smith et al., 1983). According to the sentinel lymph node theory, tumor cells invade lymph nodes in sequence with the closest or draining lymph node (called the sentinel node) first, followed by the next node in line with the drainage flow, etc. (Schauer et al., 2005). Sentinel lymph node biopsy or lymphadenectomy is performed for many cancers, and the absence of tumor cells in the sentinel node predicts that other downstream nodes will likely be free of metastasis as well (Chen et al., 2006). Tumor foci in the lymph node are categorized as micrometastases (0.2 mm to ≤2 mm) or macrometastases (>2 mm). While isolated tumor cells (for example, single keratin-positive tumor cells) in the lymph node are not currently considered as micrometastases, and these lymph nodes are usually designated as “tumor-free” (Chen et al., 2006). Metastasis to any lymph node other than the regional node is considered a distant metastasis.
(Tumor-Derived) Stimulators of Lymphangiogenesis
Not all tumors metastasize to lymph nodes. Understanding the molecular cues that cause some tumors to induce lymphangiogenesis while others do not may result in the discovery and application of new strategies for disease prevention. For this reason, it is important to study the factors that regulate lymphatic metastasis (Table 1). The first proteins identified as stimulators of lymphangiogenesis were VEGF-C and VEGF-D (Joukov et al., 1996; Achen et al., 1998). Both are members of the VEGF family and bind VEGFR-3, inducing proliferation and migration of LECs. Knock-out models for VEGF-C fail to form initial lymphatic vessels during embryogenesis, indicating that VEGF-C is a vital factor in lymphatic development (Karkkainen et al., 2004). VEGF-D, on the other hand, is not required for lymphatic vessel formation during embryogenesis (Baldwin et al., 2005), but is the strongest inducer of lymphangiogenesis in the adult when given via adenoviral delivery into mouse skeletal muscle (Rissanen et al., 2003).
VEGF-C or VEGF-D can stimulate tumor lymphangiogenesis (Skobe et al., 2001a; Skobe et al., 2001b; Stacker et al., 2001; Karpanen et al., 2001; Mattila et al., 2002; He et al., 2002) and increase the metastatic spread of tumor cells to sentinel lymph nodes (Skobe et al., 2001b; Stacker et al., 2001; Mandriota et al., 2001; Mattila et al., 2002; Krishnan et al., 2003). VEGF-D expression correlates with lymph node metastasis in many human cancers including invasive breast, ovarian, cervical, undifferentiated gastric, and lung adenocarinoma (Renyi-Vamos et al., 2005; Tammela et al., 2005a; Hess et al., 2006). VEGF-C expression correlates with lymphatic vessel invasion or lymph node metastasis in human breast, cervical, colon, prostate, endometrial, esophageal, gall bladder, ovarian, pancreatic, and non-small cell lung carcinomas (Ristimaki et al., 1998; Swartz and Skobe, 2001; Stacker et al., 2002; Tammela et al., 2005a). On the other hand, VEGF-C expression did not correlate with lymph node metastasis in neuroblastoma tumors, which typically lack lymphatic vessels (Tammela et al., 2005a). VEGF-C expression also correlates with poor prognosis or poor patient survival in many tumors, especially breast, cervical, esophageal and non-small cell lung cancer (Tammela et al., 2005a).
In some tumors there appears to be a lymphangiogenic switch. Van Trappen and colleagues report that pre-cancerous cells in the cervix (CIN-1 and CIN-2) seldom express VEGF-C and VEGF-D, but pre-invasive carcinoma in situ lesions (CIN-3) upregulate VEGF-C/D expression (Van Trappen et al., 2003). Presumably, following this switch, tumor cells recruit and invade lymphatic capillaries and travel to lymph nodes. The increase in lymph node metastasis caused by VEGF-C may be due in part to its ability to stimulate LEC proliferation thereby increasing the size of tumor-associated lymphatic vessels and increasing the chance that a tumor cell will come in contact with a LEC. Other mechanisms may include the upregulation of chemokines by VEGF-C that attract immune cells and tumor cells toward the lymphatic capillary (Shields et al., 2007). In addition, VEGF-C is a permeability factor that causes blood vessels to leak fluid thereby raising the interstitial pressure, dilating lymphatic capillaries, and forcing a greater flow of fluid to lymph nodes (Hoshida et al., 2006). Tumor-secreted VEGF-C has recently been shown to act systemically by inducing lymphangiogenesis in the sentinel lymph node even prior to tumor cell invasion (Hirakawa et al., 2007). This may prepare the lymph node for disseminated cells to come and may facilitate the further spread of cells to other nodes (Tobler and Detmar, 2006; Harrell et al., 2007).
When fully processed, VEGF-C and VEGF-D, can also bind to VEGFR-2. VEGFR-2 is expressed on both VECs and LECs (Saaristo et al., 2002). In fact, high expression of VEGF-C or VEGF-D results in increased angiogenesis in addition to increased lymphangiogenesis in some models (Skobe et al., 2001a; Roberts et al., 2006; Achen et al., 2002;). VEGF-C and VEGF-D also bind to a non-tyrosine kinase receptor called Neuropilin 2 (NRP2) (Karpanen et al., 2006a). NRP2 acts as a co-receptor with VEGFR-2 and VEGFR-3 (Soker et al., 1998; Favier et al., 2006). NRP2 knock-out mice have decreased numbers of lymphatic capillaries and veins, but surprisingly do not show edema (Yuan et al., 2002).
VEGF-A is a potent inducer of tumor angiogenesis. Recent studies demonstrate that tumor-derived VEGF-A can also increase intra- and peri-tumoral lymphangiogenesis and increase lymphatic metastasis (Bjorndahl et al., 2005b; Hirakawa et al., 2005). However, in 293EBNA tumors VEGF-A over-expression was insufficient to induce lymphangiogenesis whereas VEGF-D effectively stimulated lymphangiogenesis and lymph node metastasis (Stacker et al., 2001). Dramatic lymphangiogenesis is found when VEGF-A (165 isoform) is delivered to mice using adenoviral strategies. Surprisingly, the newly formed lymphatics do not regress for >1 year although the adenoviral VEGF-A is depleted in approximately 2 weeks (Nagy et al., 2002). VEGF-A also induces lymphangiogenesis in other non-tumor murine models including adenoviral delivery to skeletal muscle, K14-VEGF-A transgenic skin, UV-B irradiation, and corneal pellet models (Rissanen et al., 2003; Kunstfeld et al., 2004; Kajiya et al., 2006; Rogers et al., 2003). The effects of VEGF-A on lymphangiogenesis may be direct through VEGFR-2 and NRP2 expression on LECs or indirect as VEGF-A causes upregulation of VEGF-C/D in inflammatory cells (Cursiefen et al., 2004).
Most tumors express fibroblast growth factor-2 (FGF-2), also called basic FGF (for review Abuharbeid et al., 2006). FGF-2 is a powerful mitogen for VECs and also stimulates the growth of LECs (Chang et al., 2004). Although there are currently no reports linking tumor-derived FGF-2 with lymphangiogenesis and lymph node metastasis, injection of FGF-2 protein during the early phases of tumor progression stimulated neovascularization and metastasis (Tsunoda et al., 2007). In addition, mouse corneal lymphangiogenesis is strongly stimulated with FGF-2 (Kubo et al., 2002; Chang et al., 2004). Interestingly, low-dose FGF-2 pellets can induce lymphatic vessel sprouting in the absence of blood vessel sprouting (Chang et al., 2004; Chang et al., 2002). FGF-2 may directly affect the growth of lymphatic vessels since LEC express FGFR-3 (Shin et al., 2006). Alternately, FGF-2 upregulates VEGF-C and VEGF-D in the cornea (Kubo et al., 2002; Chang et al., 2004) and upregulates VEGF-A in tumor stroma (Tsunoda et al., 2007).
The angiopoietins (Ang) may also stimulate lymphatics partly through VEGFR-3. Two angiopoietins are known, Ang-1 and Ang-2, and both play a role in vascular development in combination with VEGF-A (Sato et al., 1995). Ang-1 (adenovirus) promotes LEC proliferation, lymphatic vessel enlargement, and lymphatic vessel sprouting in vivo in adult mouse ears (Tammela et al., 2005b). Inhibitors of the VEGF-C/VEGFR-3 pathway block Ang-1-stimulated lymphangiogenesis (Tammela et al., 2005b). Alternatively, Ang may affect lymphangiogenesis directly since embryonic and adult LECs express the tyrosine kinase angiopoietin receptor, Tie2 (Morisada et al., 2005; Tammela et al., 2005b). Ang-1 protein stimulates the growth of LEC in vitro and in vivo in a mouse corneal pocket model (Morisada et al., 2005), and genetically engineered mice expressing Ang-1 in basal keratinocytes have hyperplastic dermal lymphatic vessels (Tammela et al., 2005b). Transgenic mice deficient in Ang-2 expression fail to form large collecting lymphatic vessels and have defective patterning of small lymphatic capillaries, possibly indicating that Ang-2 functions in the maturation of lymphatic vessels (Gale et al., 2002). Although the majority of tumors show an increase in Ang-1 and Ang-2 expression (for review Tait and Jones, 2004), a direct correlation between either of these factors and tumor lymphangiogenesis is lacking.
Pathways other than the classical VEGF-C/VEGFR-3 signal transduction can stimulate lymphangiogenesis as well. These include the platelet-derived growth factor (PDGF)/PDGFR pathway, the hepatocyte growth factor (HGF)/c-met pathway, and the insulin-like growth factor (IGF)/IGFR pathway. PDGF-AA, PDGF-AB and PDGF-BB induce mouse corneal lymphangiogenesis, with PDGF-AA being the least effective stimulator (Cao et al., 2004). Mouse fibrosarcoma-derived PDGF-BB induced peri- and intra-tumoral lymphangiogenesis, possibly through activation of the PDGFR, and promoted lymphatic metastatasis (Cao et al., 2004). Recently, HGF was identified as a lymphangiogenic factor. In vitro, HGF stimulates LEC to proliferate, migrate and form tube-like structures (Kajiya et al., 2005). HGF did not upregulate VEGF-C or VEGF-D in LEC, and therefore the effect of HGF was assumed to be directly through its receptor, c-met. On the other hand, corneal mouse models demonstrate lymphangiogenesis toward HGF-releasing pellets, but the neo-lymphatic vessels did not express c-met. This corneal HGF-stimulated lymphangiogenesis could be partially blocked with soluble VEGFR-3, indicating an indirect effect of HGF (Cao et al., 2006). HGF was associated with tumor lymphangiogenesis in transgenic mammary tumors (Cao et al., 2006). Infusion of rhHGF into tumors or co-injection of a fibroblast cell line producing HGF with tumor cells resulted in increased tumor lymphangiogenesis (Jiang et al., 2005). IGF-1 and IGF-2 stimulate the proliferation and migration of primary LECs that express IGFR-1 and IGFR-2 (Bjorndahl et al., 2005a). In vivo, IGF-1 and IGF-2 could both stimulate corneal lymphangiogenesis independently (Bjorndahl et al., 2005a). Although the effect of IGF-1 on tumor lymphangiogenesis has yet to be shown, tumor secreted-IGF-1 may indirectly affect LEC by the upregulation of VEGF-A and activation of VEGFR-2 similarly to the way it affects VEC (Akagi et al., 1998).
Potential Therapies and New Possibilities
The tumor-associated lymphatic endothelium represents a novel target for cancer therapy. Metastasis to lymph nodes results from tumor cell invasion into lymphatic capillaries. Lymphangiogenesis is documented in numerous animal models and in many human cancers as well. For many neoplasms, the lymphatic system is the primary and only route of metastasis. The existence and functionality of intra-tumoral lymphatic vessels has been vehemently debated (Padera et al., 2002; Schneider et al., 2006), but the fact that many tumors are surrounded with grossly enlarged lymphatic vessels cannot be denied (Figure 2). Large peri-tumoral lymphatic vessels may result from: 1) pre-existing capillaries that become highly dilated due to increased interstitial pressure, 2) pre-existing capillaries that have increased their luminal diameter by proliferation of LECs (hyperplasia), and 3) neovascularization or sprouting of existing vessels toward a stimulus (tumor) that results in the increased size or density of vessels in the vicinity. Regardless of the origin, these vessels represent an increased opportunity for tumor cells to enter the lymphatic system and the increased chance of systemic dissemination. Therapies targeted toward the tumor associated-lymphatic compartment may inhibit metastasis if given early and may prevent further spread of tumor cells even if given at later time points.
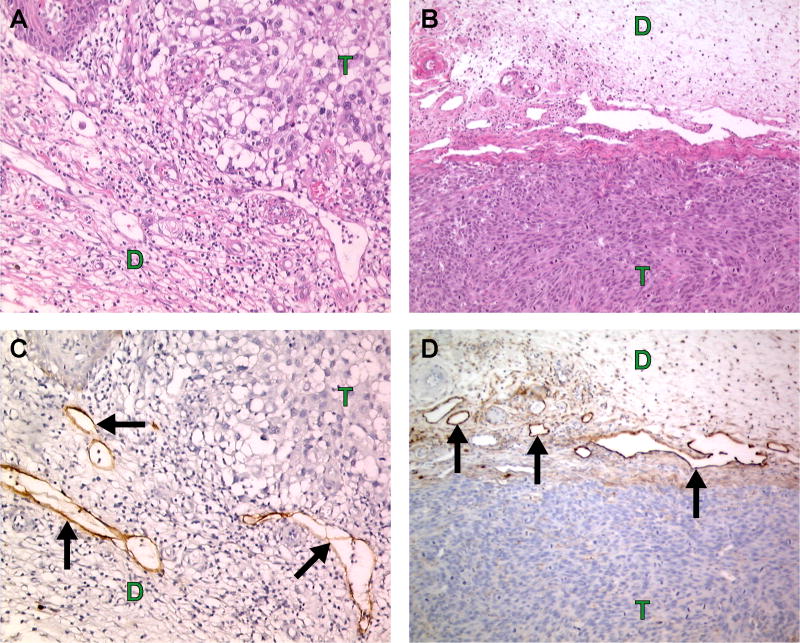
Figure 2.
Human melanoma (A, C) and human melanoma (A375SM) xenograft (B, D) are surrounded with large peri-tumoral lymphatics (arrows). A, B. Hematoxylin and eosin. C. Human podoplanin (D2-40 antibody, Signet Laboratories) staining (brown color). D. Murine podoplanin (Reliatech) staining (brown color). T = tumor, D = dermis.
Angiogenesis inhibitors have been intensely studied over the last decades. Currently, there are more than twenty-five anti-angiogenic drugs in clinical trials (Folkman, 2004; Morabito et al., 2006). The recent FDA-approval of the first exclusively anti-angiogenic agent, bevacizumab (Avastin™), a humanized monoclonal antibody to VEGF-A to be used in combination with chemotherapy for the treatment of advanced stage colon cancer, has proven beneficial to patients and pivotal for the field of vascular biology (Slevin and Payne, 2004). This clinical “proof of concept” has paved the way for other novel anti-vascular strategies and opened a new frontier in cancer therapy (Folkman, 2004). Lymphangiogenesis research has lagged behind angiogenesis, but the discovery of new lymphatic markers and an increased understanding of the growth factor signaling pathways in LEC have poised scientists to begin testing many potential anti-lymphangiogenic therapeutic strategies (Table 3). New trials will be needed to validate these pre-clinical studies and determine their medicinal significance.
Table 3.
Potential targets and therapeutic strategies for anti-lymphangiogenesis.
| Target | Strategy | Anti-
|
||
|---|---|---|---|---|
| Angiogenesis | Lymphangiogenesis | Tumor | ||
| Ligands | ||||
| HGF | Antibody to HGF * | + | + | + |
| NK4 | + | + | + | |
| VEGF-A | Antibody to VEGF-A | ++ | + | |
| Soluble VEGFR-2 | ++ | + | +/- | |
| VEGF trap (Soluble R1/R2) | ++ | + | ||
| Soluble NRP1 | ++ | + | ||
| Soluble NRP2 * | + | ++ | ||
| Semaphorin 3F | + | + | + | |
| VEGF-C | Antibody to VEGF-C * | + | ++ | |
| Soluble VEGFR-3 | + | ++ | ||
| Soluble NRP2 * | + | ++ | ||
| Semaphorin 3F | + | + | + | |
| VEGF-D | Antibody to VEGF-D | + | ++ | |
| Soluble VEGFR-3 | + | ++ | ||
| Soluble NRP2 * | + | ++ | ||
|
| ||||
| Receptors | ||||
| c-met | Antibody to c-met * | + | ++ | |
| c-met kinase inhibitors | + | ++ | ||
| Neuropilin 2 | Antibody to NRP2 * | + | + | |
| Semophorin 3F | + | + | + | |
| PDGFRα/β | Antibody to PDGFR * | + | + | ++ |
| PDGFR kinase inhibitors | + | + | ++ | |
| VEGFR-3 | Antibody to VEGFR-3 | + | ++ | |
| VEGFR-3 kinase inhibitors | + | ++ | ||
| VEGFR-2 | Antibody to VEGFR-2 | ++ | + | + |
| VEGFR-2 kinase inhibitors | ++ | + | + | |
| Integrins | Antibody to integrin-α1/α2 | + | ||
|
| ||||
| Others | ||||
| Angiostatin | ++ | + | ||
| COX-2 inhibitors | + | + | + | |
| Endostatin | ++ | + | ||
| Glucocorticoids | + | + | ||
| Interferon α/β | ++ | + | ++ | |
| Platelet-factor 4 | ++ | + | ||
| Semaphorin 3F | + | + | + | |
These strategies have not yet been reported for anti-lymphangiogenesis.
Targeting Lymphangiogenic Ligands
VEGF-A is a major tumor-derived angiogenic factor, while VEGF-C is a major tumor-derived lymphangiogenic factor. Indeed, many other growth factors (FGF-2, Ang-1, VEGF-A, IGF-1, HGF) stimulate lymphangiogenesis indirectly through VEGF-C. VEGF-C is secreted by other cells in the tumor microenvironment besides neoplastic cells including fibroblasts, macrophages, platelets, and keratinocytes (Skobe and Detmar, 2000; Sleeman, 2006). VEGF-C not only induces the hyperplasia of peri-tumoral vessels, but also increases the volumetric flow rate of fluid in lymphatics that results in the increased delivery of tumor cells to the lymph node (Hoshida et al., 2006). In fact, tumors over-expressing VEGF-C were found to deliver 200-fold more tumor cells to the regional lymph node and increase lymph node metastasis 4-fold (Hoshida et al., 2006). Since angiogenic inhibitors targeting VEGF-A (ie., antibodies, kinase inhibitors, and soluble receptors) have proven effective (for review Manley et al., 2002), strategies targeting the VEGF-C pathway may prove equally effective against tumor lymphangiogenesis.
Most anti-lymphangiogenic pre-clinical studies to date have targeted the VEGF-C/VEGF-D/VEGFR-3 signaling pathway (Table 3). These strategies have included antibodies to VEGF-D, knock-down of VEGF-C using RNAi, and soluble receptors, all aimed to neutralize the effects of these ligands. VD1, a monoclonal VEGF-D antibody, inhibited binding of VEGF-D to both VEGFR-2 and VEGFR-3 (Achen et al., 2000). This antibody blocked lymphatic spread of VEGF-D over-expressing human 293EBNA cells in immunocompromised mice (Stacker et al., 2001). The down-modulation of VEGF-C with stable RNAi transfection in murine breast cancer cells results in reduced tumor lymphangiogenesis and lymph node metastasis (Chen et al., 2005). In a prostate cancer model, VEGF-C silencing completely abolished intra-tumoral lymphatic vessels when cells were implanted subcutaneously, but reduced lymphatic vessel density by only 50% when implanted orthotopically (Wong et al., 2005). The reduction of lymphatic vessels in the orthotopic model was insufficient to inhibit the incidence of lymph node metastasis. These results may indicate that stromal cells of different organ environments may differ in their VEGF-C expression. Additionally, peri-tumoral vessels may be sufficient for tumor cell invasion and metastasis in some cases. It is important to note that RNAi strategies have not yet been approved for clinical use in human patients.
Inhibiting Tumor Lymphangiogenesis with Soluble Receptors
Targeting an individual ligand (eg., VEGF-C) can neutralize its effects on several receptors (eg., VEGFR-3, VEGFR-2, and NRP2), but will not affect the ability of another ligand (eg., VEGF-D) to stimulate these same receptors. Targeting a specific receptor (eg., VEGFR-2) with a kinase inhibitor or blocking antibody will inhibit its activation by multiple ligands (eg., VEGF-C, VEGF-D, or VEGF-A), but will not affect the ability of these ligands to stimulate another receptor such as NRP2 or VEGFR-3. Soluble receptors may prove more effective since they bind multiple ligands (for example, sVEGFR-2 binds VEGF-A, C, and D) and compete for binding with multiple receptors (for example, sVEGFR-2 may sequester VEGF-C away from VEGFR-2, VEGFR-3, and NRP2).
sVEGFR-3 proteins and adenoviral constructs have been the most widely used strategy for targeting tumor lymphangiogenesis. sVEGFR-3 globulin protein inhibited lymphangiogenesis and lymph node metastasis in rat and human breast cancer models (Karpanen et al., 2001; Krishnan et al., 2003). sVEGFR-3 protein was effective even when the tumor cells were engineered to express high levels of VEGF-C (Karpanen et al., 2001). Blocking lymph node metastasis in breast tumors with sVEGFR-3 protein inhibited subsequent distant lung metastases as well (Krishnan et al., 2003). The same strategy did not inhibit distant metastases in a lung carcinoma model (He et al., 2002). These results may indicate that sVEGFR-3 strategies may be more useful in cancers like breast and prostate carcinomas that initially metastasize to lymph nodes and later to distant sites.
The mode of delivery of sVEGFR-3 may influence its therapeutic potential. Tumor cells transfected with sVEGFR-3 suppressed lymph node metastasis by 66% (from 12/12 lymph node metastases in controls to 4/12 lymph node metastases in transfected mice), while sVEGFR-3 given systemically in an adenovirus completely inhibited lymph node metastasis (from 11/14 metastases in Adeno-LacZ mice to 0/28 lymph node metastases in Adeno-sVEGFR-3 mice) (He et al., 2002). At this time, transfection or viral therapies may not be desirable for human patients, but sVEGFR-3 given as a protein may be a viable strategy. sVEGFR-3 protein inhibits lymphangiogenesis in a dose-dependent manner with the most effective dose depending on the VEGF-C levels in the tumor (Karpanen et al., 2001; Krishnan et al., 2003; He et al., 2005; Karpanen et al., 2006b). sVEGFR-3 therapy can inhibit multiple stimulators of lymphangiogenesis including HGF, FGF-2, IGF-1, and Ang-1, which may prove useful considering that tumors do not always express VEGF-C directly (Bjorndahl et al., 2005a; Cao et al., 2006; Chang et al., 2004; Tammela et al., 2005b).
The timing of sVEGFR-3 delivery is also quite important. When sVEGFR-3-Fc adenovirus or adeno-associated virus was given prior to tumor cell inoculation or early in tumor progression, lymphangiogenesis and lymph node metastasis could be suppressed in several tumor models including melanoma, prostate, renal cell, and lung carcinoma (He et al., 2005; Lin et al., 2005). When systemic sVEGFR-3 therapy was started too late, tumor cells had already reached regional lymph nodes and metastasis was not affected (He et al., 2005).
Targeting Receptors on Lymphatic Endothelium
To date, nearly all attempts to target receptors on the lymphatic endothelium of tumors have been via VEGFR-3. VEGFR-3 was the first lymphatic-specific marker identified (Kaipainen et al., 1995). Blocking antibodies that target the ligand-binding domain of murine VEGFR-3 inhibit tumor lymphangiogenesis (Shimizu et al., 2004; Hoshida et al., 2006; Pytowski et al., 2005; Roberts et al., 2006; Laakkonen et al., 2007), FGF-2 induced corneal lymphangiogenesis (Kubo et al., 2002), spontaneous corneal lymphangiogenesis in corn1 mice (Cursiefen et al., 2005), and UV-B-induced (VEGF-A-mediated) lymphangiogenesis (Kajiya et al., 2006). Early intervention (7 days after tumor cell injection) with VEGFR-3 antibodies reduced lymphatic vessel density and inhibited lymph node metastasis in highly metastatic gastric carcinomas (Shimizu et al., 2004). Peri-tumoral lymphatic vessel hyperplasia was also reduced with VEGFR-3 antibodies. The smaller vessel area resulted in fewer GFP-labeled tumor cells delivered to regional lymph nodes (Hoshida et al., 2006; Roberts et al., 2006).
VEGFR-3 is expressed by tumor-associated blood vessels as well as lymphatic blood vessels (Clarijs et al., 2002; Partanen et al., 1999). Therefore, VEGFR-3 antibodies inhibited tumor angiogenesis and primary tumor growth in some models (Kubo et al., 2000; Roberts et al., 2006; Laakkonen et al., 2007). Treated tumors showed reduced microvessel density and in some cases micro-hemorrhaging throughout the tumor. VEGF-C can induce tumor angiogenesis via VEGFR-2 as well as VEGFR-3 on tumor endothelial cells and a blocking antibody to VEGFR-2, called DC101, was also effective at inhibiting VEGF-C-induced tumor angiogenesis (Kadambi et al., 2001; Roberts et al., 2006). LECs express VEGFR-2, although preferentially on peri-tumoral lymphatics and less so on intra-tumoral lymphatics; and accordingly, DC101 was able to decrease tumor lymphangiogenesis (Roberts et al., 2006). In addition, DC101 was found to inhibit lymphatic vessel regeneration in cutaneous wounds in K14-VEGF-A transgenic mice (Hong et al., 2004). Lymphangiogenesis in cutaneous delayed-type hypersensitivity reactions, however, could not be inhibited with VEGFR-2 antibodies alone and required the combination of both VEGFR-2 and VEGFR-1 antibodies (Kunstfeld et al., 2004).
When comparing VEGFR-3 versus VEGFR-2 antibodies, VEGFR-3 antibodies were more effective at inhibiting regional lymphatic metastases and distant metastases than VEGFR-2 antibodies (Roberts et al., 2006). VEGFR-2 antibodies were more effective at inhibiting tumor growth and angiogenesis. Interestingly, even though tumor volume was reduced by 84% and tumor blood vessel area was decreased by 71% in the anti-VEGFR-2 treated group, these small weakly vascularized tumors still managed to metastasize to regional lymph nodes in 8/10 mice compared with 10/10 mice in the controls (Roberts et al., 2006). These results underscore the importance of blocking lymphangiogenesis in addition to angiogenesis. The combination of both antibodies was even more effective at inhibiting metastasis than either antibody alone (Roberts et al., 2006). A diabody, a bi-specific antibody that recognizes both VEGFR-2 and VEGFR-3 and can block the interaction of VEGF-C/VEGFR-2, VEGF-C/VEGFR-3, and VEGF-A/VEGFR-2 was recently produced by Imclone (Jimenez et al., 2005), but the efficacy of this antibody at inhibiting metastasis or prolonging survival has not been reported yet. Strategies such as these that utilize both anti-angiogenic and anti-lymphangiogenic approaches may be the most potent methods for blocking the deadly spread of cancer cells (see Table 3).
Using RNA microarray technology, investigators have compared the expression profiles of VECs and LECs (Hirakawa et al., 2003; Podgrabinska et al., 2002). VECs express α5 and αv integrins, whereas cultured LECs express α1 and α2 integrins (which partner with β1 subunits). Lymphatic capillaries have an incomplete basement membrane and lie in close contact to interstitial collagen. VEGF-A enhances the expression of collagen type I binding-subunits, α1 and α2, on cultured LECs (Hong et al., 2004; Senger et al., 1997). Monoclonal antibodies targeting α1 and α2, used in combination, inhibited physiologic lymphangiogenesis in a wound healing model and inhibited pathologic lymphangiogenesis in a skin tumor model (Hong et al., 2004).
Small compounds have been used to block the kinase domains of cell surface receptors. These kinase inhibitors generally target one or more receptors specifically, but can have non-specific effects on other receptors. Many kinase inhibitors are currently in clinical trials. Several compounds inhibit the activities of VEGF receptors including: Semaxanib (SU5416), Sumitnib (SU11248), Vatalanib (PTK/ZK), Sorafenib (BAY 43-9006), Recentin (AZD2171), SU6668, AG-013736, MAZ51, CEP-7055 (for review Ahmed et al., 2004; Baka et al., 2006; Morabito et al., 2006). The relative effects of these inhibitors on tumor lymphangiogenesis versus tumor angiogenesis are unclear. Kinase inhibitors targeting the c-met receptor, the PDGFR, and the IGFR may target LECs in addition to tumor cells (Table 3).
Other Strategies to Suppress Lymphangiogenesis
Many different strategies are being explored to target tumor lymphangiogenesis and prevent lymphatic metastases. These potential therapies include using competive ligands of lymphangiogenic stimulators, inflammatory regulators, and endogenous inhibitors of angiogenesis/lymphangiogenesis. A novel approach may be to select those inhibitors that target both vascular and lymphatic endothelial cells as well as tumor cells (Table 3).
Competitive Ligands
Semaphorin 3F (SEMA3F) is a competitive ligand of VEGF-A and VEGF-C for Neuropilin 2 (NRP2). NRP2 is a co-receptor for VEGF-A with VEGFR-2 and a co-receptor for VEGF-C with VEGFR-3. NRP2 is also a co-receptor for SEMA3F with plexins, a family of transmembrane receptors involved in neuronal guidance. In addition to competing for VEGF binding, SEMA3F induces repulsion of NRP2-expressing VECs and LECs (Bielenberg et al., 2004). Human melanoma cells over-expressing SEMA3F have reduced microvessel density and a complete inhibition of lymph node metastasis and lung metastasis. SEMA3F-expressing tumors have no intra-tumoral lymphatic vessels and reduced peri-tumoral lymphatic vessel area as compared to control A375SM tumors that have large peri-tumoral lymphatic vessels (Figure 2) and intra-tumoral lymphatic vessels (manuscript submitted, Bielenberg and Klagsbrun). The SEMA3F chromosomal loci, 3p21.3, is often deleted in lung cancer (Roche et al., 1996; Sekido et al., 1996; Xiang et al., 1996), therefore SEMA3F has been suggested to act as a tumor suppressor. SEMA3F protein may be a novel therapy capable of inhibiting angiogenesis, lymphangiogenesis, tumorigenicity, and metastasis (Bielenberg et al., 2006).
NK4 is a competitive inhibitor of HGF binding to c-met and was effective at inhibiting HGF-induced tumor angiogenesis and tumor lymphangiogenesis (Jiang et al., 2005; Kuba et al., 2000). NK4 may also reduce neovascularization independently of HGF as it decreased VEGF- and FGF-stimulated proliferation and migration of EC in vitro as well (Kuba et al., 2000).
Endothelial Inhibitors
Surprisingly few studies have compared the endogenous or classical angiogenesis inhibitors in lymphangiogenesis models. Some in vitro studies do suggest that these compounds may affect both endothelial cell compartments. For example, Shao and colleges reported that angiostatin, endostatin, and platelet-factor 4 could inhibit LEC proliferation (MTT assay) and migration (scratch assay) in a dose-dependent manner (Shao and Chi, 2005; Shao and Xie, 2005). On the other hand, these compounds need to be examined in vivo to truly establish their potency. For instance, thalidomide inhibited LEC growth in vitro (Shao and Xie, 2005), but was inefficient at inhibiting FGF-2 induced corneal lymphangiogenesis (Chang et al., 2004).
Interferon (IFN) α and β have been shown to regress cutaneous hemangiomas in children and to block tumor angiogenesis and metastasis in murine models by down-regulating FGF-2 and matrix metalloproteinases (Ezekowitz et al., 1992; Ezekowitz et al., 1995; Dinney et al., 1998; Bielenberg et al., 1999; Dong et al., 1999). FGF-2 injection during the initial phase of tumorigenesis increases tumor growth and metastasis (Tsunoda et al., 2007). Tumor-secreted FGF-2 may also stimulate tumor lymphangiogenesis. IFNα inhibits LEC growth and induces apoptosis in vitro (Shao and Liu, 2006), but its effect on tumor lymphangiogenesis remains to be seen.
Thrombospondin-1 (TSP-1) is a competent angiogenesis inhibitor, and tumors resulting from chemical-induced carcinogenesis in K14-TSP-1 mice have reduced microvessel density (Hawighorst et al., 2002). Lymphangiogenesis and lymph node metastasis, on the other hand, was not diminished in tumors from TSP-1 transgenic mice possibly due to the fact that cutaneous lymphatic capillaries lack CD36, one of the TSP-1 receptors.
Inflammatory Regulators
Cyclooxygenase-2 (COX-2) expression correlates with VEGF-C expression and lymph node metastasis in numerous human cancers including lung adenocarcinoma, head and neck squamous cell carcinoma, esophageal carcinoma, gastric carcinoma and colon carcinoma (Su et al., 2004; Kyzas et al., 2005a; von Rahden et al., 2005; Zhang et al., 2005; Soumaoro et al., 2006). COX-2 over-expression induces VEGF-C but not VEGF-D in human lung adenocarcinomas and correlates with lymphatic vessel density (Su et al., 2004). COX-2 inhibitors decrease VEGF-C levels (Timoshenko et al., 2006). When three esophageal cell lines were treated in vitro with COX-2 inhibitors such as diclofenac, rofecoxib, and SC560, VEGF-A and VEGF-C levels decreased (von Rahden et al., 2005). The COX-2 inhibitor, celecoxib (Celebrex, Pfizer), powerfully blocked FGF-2 induced corneal lymphangiogenesis (Chang et al., 2004). Celecoxib inhibited tumor angiogenesis and microvessel density by causing apoptosis in tumor endothelial cells (Raut et al., 2004). Celecoxib also inhibits tumor lymphangiogenesis as assessed by Q-PCR and immunohistochemistry in MCF7 and MDAMB231 breast cancers (Barnes et al., 2007).
Glucocorticoids, including prednisone, hydrocortisone, and dexamethasone, inhibit tumor angiogenesis by down-regulating VEGF-A and IL-8 (Yano et al., 2006). Dexamethasone reduced VEGF-C expression in Du145 prostate tumors by 50% and inhibited tumor lymphangiogenesis (Yano et al., 2006).
Summary and Perspective
Two safety concerns that arise when considering any therapy against lymphangiogenesis is whether the therapy will specifically target abnormal tumor lymphatic vessels and spare normal lymphatic vessels or blood vessels and whether such therapy will result in lymphedema by reducing lymphatic flow and accumulating interstitial fluid (Gershenwald and Fidler, 2002). Several recent studies have indirectly addressed these concerns. sVEGFR-3 in an adenoviral construct (which results in high expression) did not appear to affect normal lymphatic vessels once mice reached adolescence, approximately 4 weeks of age (Karpanen et al., 2006b). In addition, tumor studies in adult animals using sVEGFR-3 therapy have not reported normal lymphatic vessel damage or non-specific effects on normal blood vessels (Karpanen et al., 2001; Krishnan et al., 2003; He et al., 2005; Karpanen et al., 2006b). Edema formation was not reported in any of the anti-tumor lymphangiogenic studies using sVEGFR-3, but was found in newborn transgenic mice. When mice were engineered to express sVEGFR-3-Ig under the keratin 14-promoter, which results in transgene expression in all basal keratinocytes of the skin, normal lymphatic vessels regressed and edema resulted (Makinen et al., 2001). Using locally administered protein strategies (such as soluble receptors, antibodies, or competitive inhibitors) instead of adenoviral or transgenic strategies and appropriate dosing schedules may alleviate these unwanted edema reactions.
Angiogenesis and lymphangiogenesis are both important processes contributing to tumor progression and metastasis. In order to prevent the deadly spread of cancer cells, therapeutic strategies combining inhibitors of both these endothelial compartments may be necessary. Alternatively, single compounds that target both tumor-associated blood vessels and tumor-associated lymphatic vessels may decrease tumor size and decrease the incidence of local and distant metastases (Table 3). Other strategies such as “low-dose chemotherapy” or “metronomic chemotherapy” may target the lymphatic endothelium as well as the vascular endothelium (Gately and Kerbel, 2001; Kerbel and Kamen, 2004; Kieran et al., 2005). Future studies will require the use of metastatic tumor models to elucidate the most effective strategies of inhibiting tumor progression.
Acknowledgments
This work was supported by a Howard Temin Award (CA118732-01A1) from the National Cancer Institute and by the Robert Leet and Clara Guthrie Patterson Trust Award. The authors would like to acknowledge Ethan Bickford and Kristin Johnson for illustrations and Ricardo Sanchez for histology.
Abbreviations
- Ang
angiopoietins
- COX-2
cyclooxygenase-2
- EGF
epidermal growth factor
- FGF-2
fibroblast growth factor-2, also called basic fibroblast growth factor
- FGFR
fibroblast growth factor receptor
- HGF
hepatocyte growth factor
- IGF
insulin-like growth factor
- IGFR
insulin-like growth factor receptor
- IL-8
interleukin-8
- LEC
lymphatic endothelial cell
- NRP
neuropilin
- PDGF
platelet-derived growth factor
- PDGFR
platelet-derived growth factor receptor
- SEMA3F
semaphorin 3F
- TSP-1
thrombospondin-1
- VEC
vascular endothelial cell
- VEGF
vascular endothelial growth factor
- VEGFR
vascular endothelial growth factor receptor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abuharbeid S, Czubayko F, Aigner A. The fibroblast growth factor-binding protein FGF-BP. Int J Biochem Cell Biol. 2006;38:1463–8. doi: 10.1016/j.biocel.2005.10.017. [DOI] [PubMed] [Google Scholar]
- Achen MG, Williams RA, Baldwin ME, Lai P, Roufail S, Alitalo K, Stacker SA. The angiogenic and lymphangiogenic factor vascular endothelial growth factor-D exhibits a paracrine mode of action in cancer. Growth Factors. 2002;20:99–107. doi: 10.1080/08977190290031969. [DOI] [PubMed] [Google Scholar]
- Achen MG, Jeltsch M, Kukk E, Makinen T, Vitali A, Wilks AF, Alitalo K, Stacker SA. Vascular endothelial growth factor D (VEGF-D) is a ligand for the tyrosine kinases VEGF receptor 2 (Flk1) and VEGF receptor 3 (Flt4) Proc Natl Acad Sci U S A. 1998;95:548–53. doi: 10.1073/pnas.95.2.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achen MG, Roufail S, Domagala T, Catimel B, Nice EC, Geleick DM, Murphy R, Scott AM, Caesar C, Makinen T, Alitalo K, Stacker SA. Monoclonal antibodies to vascular endothelial growth factor-D block its interactions with both VEGF receptor-2 and VEGF receptor-3. Eur J Biochem. 2000;267:2505–15. doi: 10.1046/j.1432-1327.2000.01257.x. [DOI] [PubMed] [Google Scholar]
- Ahmed SI, Thomas AL, Steward WP. Vascular endothelial growth factor (VEGF) inhibition by small molecules. J Chemother. 2004;16 4:59–63. doi: 10.1179/joc.2004.16.Supplement-1.59. [DOI] [PubMed] [Google Scholar]
- Akagi Y, Liu W, Zebrowski B, Xie K, Ellis LM. Regulation of vascular endothelial growth factor expression in human colon cancer by insulin-like growth factor-I. Cancer Res. 1998;58:4008–14. [PubMed] [Google Scholar]
- Baka S, Clamp AR, Jayson GC. A review of the latest clinical compounds to inhibit VEGF in pathological angiogenesis. Expert Opin Ther Targets. 2006;10:867–76. doi: 10.1517/14728222.10.6.867. [DOI] [PubMed] [Google Scholar]
- Baldwin ME, Halford MM, Roufail S, Williams RA, Hibbs ML, Grail D, Kubo H, Stacker SA, Achen MG. Vascular endothelial growth factor D is dispensable for development of the lymphatic system. Mol Cell Biol. 2005;25:2441–9. doi: 10.1128/MCB.25.6.2441-2449.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes NL, Warnberg F, Farnie G, White D, Jiang W, Anderson E, Bundred NJ. Cyclooxygenase-2 inhibition: effects on tumour growth, cell cycling and lymphangiogenesis in a xenograft model of breast cancer. Br J Cancer. 2007;96:575–82. doi: 10.1038/sj.bjc.6603593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bielenberg DR, Pettaway CA, Takashima S, Klagsbrun M. Neuropilins in neoplasms: expression, regulation, and function. Exp Cell Res. 2006;312:584–93. doi: 10.1016/j.yexcr.2005.11.024. [DOI] [PubMed] [Google Scholar]
- Bielenberg DR, Bucana CD, Sanchez R, Mulliken JB, Folkman J, Fidler IJ. Progressive growth of infantile cutaneous hemangiomas is directly correlated with hyperplasia and angiogenesis of adjacent epidermis and inversely correlated with expression of the endogenous angiogenesis inhibitor, IFN-beta. Int J Oncol. 1999;14:401–8. doi: 10.3892/ijo.14.3.401. [DOI] [PubMed] [Google Scholar]
- Bielenberg DR, Hida Y, Shimizu A, Kaipainen A, Kreuter M, Kim CC, Klagsbrun M. Semaphorin 3F, a chemorepulsant for endothelial cells, induces a poorly vascularized, encapsulated, nonmetastatic tumor phenotype. J Clin Invest. 2004;114:1260–71. doi: 10.1172/JCI21378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorndahl M, Cao R, Nissen LJ, Clasper S, Johnson LA, Xue Y, Zhou Z, Jackson D, Hansen AJ, Cao Y. Insulin-like growth factors 1 and 2 induce lymphangiogenesis in vivo. Proc Natl Acad Sci U S A. 2005a;102:15593–8. doi: 10.1073/pnas.0507865102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorndahl MA, Cao R, Burton JB, Brakenhielm E, Religa P, Galter D, Wu L, Cao Y. Vascular endothelial growth factor-A promotes peritumoral lymphangiogenesis and lymphatic metastasis. Cancer Res. 2005b;65:9261–8. doi: 10.1158/0008-5472.CAN-04-2345. [DOI] [PubMed] [Google Scholar]
- Cao R, Bjorndahl MA, Gallego MI, Chen S, Religa P, Hansen AJ, Cao Y. Hepatocyte growth factor is a lymphangiogenic factor with an indirect mechanism of action. Blood. 2006;107:3531–6. doi: 10.1182/blood-2005-06-2538. [DOI] [PubMed] [Google Scholar]
- Cao R, Bjorndahl MA, Religa P, Clasper S, Garvin S, Galter D, Meister B, Ikomi F, Tritsaris K, Dissing S, Ohhashi T, Jackson DG, Cao Y. PDGF-BB induces intratumoral lymphangiogenesis and promotes lymphatic metastasis. Cancer Cell. 2004;6:333–45. doi: 10.1016/j.ccr.2004.08.034. [DOI] [PubMed] [Google Scholar]
- Chang L, Kaipainen A, Folkman J. Lymphangiogenesis new mechanisms. Ann N Y Acad Sci. 2002;979:111–9. doi: 10.1111/j.1749-6632.2002.tb04872.x. [DOI] [PubMed] [Google Scholar]
- Chang LK, Garcia-Cardena G, Farnebo F, Fannon M, Chen EJ, Butterfield C, Moses MA, Mulligan RC, Folkman J, Kaipainen A. Dose-dependent response of FGF-2 for lymphangiogenesis. Proc Natl Acad Sci U S A. 2004;101:11658–63. doi: 10.1073/pnas.0404272101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SL, Iddings DM, Scheri RP, Bilchik AJ. Lymphatic mapping and sentinel node analysis: current concepts and applications. CA Cancer J Clin. 2006;56:292–309. doi: 10.3322/canjclin.56.5.292. [DOI] [PubMed] [Google Scholar]
- Chen Z, Varney ML, Backora MW, Cowan K, Solheim JC, Talmadge JE, Singh RK. Down-regulation of vascular endothelial cell growth factor-C expression using small interfering RNA vectors in mammary tumors inhibits tumor lymphangiogenesis and spontaneous metastasis and enhances survival. Cancer Res. 2005;65:9004–11. doi: 10.1158/0008-5472.CAN-05-0885. [DOI] [PubMed] [Google Scholar]
- Clarijs R, Schalkwijk L, Hofmann UB, Ruiter DJ, de Waal RM. Induction of vascular endothelial growth factor receptor-3 expression on tumor microvasculature as a new progression marker in human cutaneous melanoma. Cancer Res. 2002;62:7059–65. [PubMed] [Google Scholar]
- Cursiefen C, Chen L, Borges LP, Jackson D, Cao J, Radziejewski C, D'Amore PA, Dana MR, Wiegand SJ, Streilein JW. VEGF-A stimulates lymphangiogenesis and hemangiogenesis in inflammatory neovascularization via macrophage recruitment. J Clin Invest. 2004;113:1040–50. doi: 10.1172/JCI20465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cursiefen C, Ikeda S, Nishina PM, Smith RS, Ikeda A, Jackson D, Mo JS, Chen L, Dana MR, Pytowski B, Kruse FE, Streilein JW. Spontaneous corneal hem- and lymphangiogenesis in mice with destrin-mutation depend on VEGFR3 signaling. Am J Pathol. 2005;166:1367–77. doi: 10.1016/S0002-9440(10)62355-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dadras SS, Paul T, Bertoncini J, Brown LF, Muzikansky A, Jackson DG, Ellwanger U, Garbe C, Mihm MC, Detmar M. Tumor lymphangiogenesis: a novel prognostic indicator for cutaneous melanoma metastasis and survival. Am J Pathol. 2003;162:1951–60. doi: 10.1016/S0002-9440(10)64328-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daróczy J. The Dermal Lymphatic Capillaries. Springer-Verlag; Berlin Heidelberg: 1988. p. 157. [Google Scholar]
- Denzinger S, Otto W, Burger M, Hammerschmied C, Junker K, Hartmann A, Wieland WF, Walter B. Sporadic renal cell carcinoma in young and elderly patients: are there different clinicopathological features and disease specific survival rates? World J Surg Oncol. 2007;5:16. doi: 10.1186/1477-7819-5-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinney CP, Bielenberg DR, Perrotte P, Reich R, Eve BY, Bucana CD, Fidler IJ. Inhibition of basic fibroblast growth factor expression, angiogenesis, and growth of human bladder carcinoma in mice by systemic interferon-alpha administration. Cancer Res. 1998;58:808–14. [PubMed] [Google Scholar]
- Dong Z, Greene G, Pettaway C, Dinney CP, Eue I, Lu W, Bucana CD, Balbay MD, Bielenberg D, Fidler IJ. Suppression of angiogenesis, tumorigenicity, and metastasis by human prostate cancer cells engineered to produce interferon-beta. Cancer Res. 1999;59:872–9. [PubMed] [Google Scholar]
- Ezekowitz A, Mulliken J, Folkman J. Additional corrections: interferon for hemangiomas of infancy. N Engl J Med. 1995;333:595–6. doi: 10.1056/NEJM199508313330913. [DOI] [PubMed] [Google Scholar]
- Ezekowitz RA, Mulliken JB, Folkman J. Interferon alfa-2a therapy for life-threatening hemangiomas of infancy. N Engl J Med. 1992;326:1456–63. doi: 10.1056/NEJM199205283262203. [DOI] [PubMed] [Google Scholar]
- Favier B, Alam A, Barron P, Bonnin J, Laboudie P, Fons P, Mandron M, Herault JP, Neufeld G, Savi P, Herbert JM, Bono F. Neuropilin-2 interacts with VEGFR-2 and VEGFR-3 and promotes human endothelial cell survival and migration. Blood. 2006;108:1243–50. doi: 10.1182/blood-2005-11-4447. [DOI] [PubMed] [Google Scholar]
- Fidler IJ. Metastasis: quantitative analysis of distribution and fate of tumor emboli labeled with 125 I-5-iodo-2′-deoxyuridine. J Natl Cancer Inst. 1970;45:773–82. [PubMed] [Google Scholar]
- Fidler IJ. Critical factors in the biology of human cancer metastasis: twenty-eighth G.H.A. Clowes memorial award lecture. Cancer Res. 1990;50:6130–8. [PubMed] [Google Scholar]
- Fidler IJ. Critical determinants of metastasis. Semin Cancer Biol. 2002;12:89–96. doi: 10.1006/scbi.2001.0416. [DOI] [PubMed] [Google Scholar]
- Fidler IJ, Singh RK, Yoneda J, Kumar R, Xu L, Dong Z, Bielenberg DR, McCarty M, Ellis LM. Critical determinants of neoplastic angiogenesis. Cancer J. 2000;6 3:S225–36. [PubMed] [Google Scholar]
- Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285:1182–6. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- Folkman J. Endogenous angiogenesis inhibitors. APMIS. 2004;112:496–507. doi: 10.1111/j.1600-0463.2004.apm11207-0809.x. [DOI] [PubMed] [Google Scholar]
- Fong Y, Coit DG, Woodruff JM, Brennan MF. Lymph node metastasis from soft tissue sarcoma in adults. Analysis of data from a prospective database of 1772 sarcoma patients. Ann Surg. 1993;217:72–7. doi: 10.1097/00000658-199301000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale NW, Thurston G, Hackett SF, Renard R, Wang Q, McClain J, Martin C, Witte C, Witte MH, Jackson D, Suri C, Campochiaro PA, Wiegand SJ, Yancopoulos GD. Angiopoietin-2 is required for postnatal angiogenesis and lymphatic patterning, and only the latter role is rescued by Angiopoietin-1. Dev Cell. 2002;3:411–23. doi: 10.1016/s1534-5807(02)00217-4. [DOI] [PubMed] [Google Scholar]
- Gately S, Kerbel R. Antiangiogenic scheduling of lower dose cancer chemotherapy. Cancer J. 2001;7:427–36. [PubMed] [Google Scholar]
- Gershenwald JE, Fidler IJ. Cancer. Targeting lymphatic metastasis. Science. 2002;296:1811–2. doi: 10.1126/science.10731318. [DOI] [PubMed] [Google Scholar]
- Haagensen CD, Feind KR, Herter FP, Slanets CA, Weinberg JA. The lymphatics in Cancer. Saunders; Philadelphia: 1972. p. 583. [Google Scholar]
- Harrell MI, Iritani BM, Ruddell A. Tumor-induced sentinel lymph node lymphangiogenesis and increased lymph flow precede melanoma metastasis. Am J Pathol. 2007;170:774–86. doi: 10.2353/ajpath.2007.060761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatakeyama H, Kondo T, Fujii K, Nakanishi Y, Kato H, Fukuda S, Hirohashi S. Protein clusters associated with carcinogenesis, histological differentiation and nodal metastasis in esophageal cancer. Proteomics. 2006;6:6300–16. doi: 10.1002/pmic.200600488. [DOI] [PubMed] [Google Scholar]
- Hawighorst T, Oura H, Streit M, Janes L, Nguyen L, Brown LF, Oliver G, Jackson DG, Detmar M. Thrombospondin-1 selectively inhibits early-stage carcinogenesis and angiogenesis but not tumor lymphangiogenesis and lymphatic metastasis in transgenic mice. Oncogene. 2002;21:7945–56. doi: 10.1038/sj.onc.1205956. [DOI] [PubMed] [Google Scholar]
- He Y, Kozaki K, Karpanen T, Koshikawa K, Yla-Herttuala S, Takahashi T, Alitalo K. Suppression of tumor lymphangiogenesis and lymph node metastasis by blocking vascular endothelial growth factor receptor 3 signaling. J Natl Cancer Inst. 2002;94:819–25. doi: 10.1093/jnci/94.11.819. [DOI] [PubMed] [Google Scholar]
- He Y, Rajantie I, Pajusola K, Jeltsch M, Holopainen T, Yla-Herttuala S, Harding T, Jooss K, Takahashi T, Alitalo K. Vascular endothelial cell growth factor receptor 3-mediated activation of lymphatic endothelium is crucial for tumor cell entry and spread via lymphatic vessels. Cancer Res. 2005;65:4739–46. doi: 10.1158/0008-5472.CAN-04-4576. [DOI] [PubMed] [Google Scholar]
- Hess KR, Varadhachary GR, Taylor SH, Wei W, Raber MN, Lenzi R, Abbruzzese JL. Metastatic patterns in adenocarcinoma. Cancer. 2006;106:1624–33. doi: 10.1002/cncr.21778. [DOI] [PubMed] [Google Scholar]
- Hirakawa S, Kodama S, Kunstfeld R, Kajiya K, Brown LF, Detmar M. VEGF-A induces tumor and sentinel lymph node lymphangiogenesis and promotes lymphatic metastasis. J Exp Med. 2005;201:1089–99. doi: 10.1084/jem.20041896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirakawa S, Brown LF, Kodama S, Paavonen K, Alitalo K, Detmar M. VEGF-C-induced lymphangiogenesis in sentinel lymph nodes promotes tumor metastasis to distant sites. Blood. 2007;109:1010–7. doi: 10.1182/blood-2006-05-021758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirakawa S, Hong YK, Harvey N, Schacht V, Matsuda K, Libermann T, Detmar M. Identification of vascular lineage-specific genes by transcriptional profiling of isolated blood vascular and lymphatic endothelial cells. Am J Pathol. 2003;162:575–86. doi: 10.1016/S0002-9440(10)63851-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong YK, Lange-Asschenfeldt B, Velasco P, Hirakawa S, Kunstfeld R, Brown LF, Bohlen P, Senger DR, Detmar M. VEGF-A promotes tissue repair-associated lymphatic vessel formation via VEGFR-2 and the alpha1beta1 and alpha2beta1 integrins. Faseb J. 2004;18:1111–3. doi: 10.1096/fj.03-1179fje. [DOI] [PubMed] [Google Scholar]
- Hoshida T, Isaka N, Hagendoorn J, di Tomaso E, Chen YL, Pytowski B, Fukumura D, Padera TP, Jain RK. Imaging steps of lymphatic metastasis reveals that vascular endothelial growth factor-C increases metastasis by increasing delivery of cancer cells to lymph nodes: therapeutic implications. Cancer Res. 2006;66:8065–75. doi: 10.1158/0008-5472.CAN-06-1392. [DOI] [PubMed] [Google Scholar]
- Jeltsch M, Kaipainen A, Joukov V, Meng X, Lakso M, Rauvala H, Swartz M, Fukumura D, Jain RK, Alitalo K. Hyperplasia of lymphatic vessels in VEGF-C transgenic mice. Science. 1997;276:1423–5. doi: 10.1126/science.276.5317.1423. [DOI] [PubMed] [Google Scholar]
- Jiang WG, Davies G, Martin TA, Parr C, Watkins G, Mansel RE, Mason MD. The potential lymphangiogenic effects of hepatocyte growth factor/scatter factor in vitro and in vivo. Int J Mol Med. 2005;16:723–8. [PubMed] [Google Scholar]
- Jimenez X, Lu D, Brennan L, Persaud K, Liu M, Miao H, Witte L, Zhu Z. A recombinant, fully human, bispecific antibody neutralizes the biological activities mediated by both vascular endothelial growth factor receptors 2 and 3. Mol Cancer Ther. 2005;4:427–34. doi: 10.1158/1535-7163.MCT-04-0261. [DOI] [PubMed] [Google Scholar]
- Jones A, Fujiyama C, Turner K, Fuggle S, Cranston D, Turley H, Valtola R, Bicknell R, Harris AL. Angiogenesis and lymphangiogenesis in stage 1 germ cell tumours of the testis. BJU Int. 2000;86:80–6. doi: 10.1046/j.1464-410x.2000.00660.x. [DOI] [PubMed] [Google Scholar]
- Jones TD, Carr MD, Eble JN, Wang M, Lopez-Beltran A, Cheng L. Clonal origin of lymph node metastases in bladder carcinoma. Cancer. 2005;104:1901–10. doi: 10.1002/cncr.21466. [DOI] [PubMed] [Google Scholar]
- Joukov V, Pajusola K, Kaipainen A, Chilov D, Lahtinen I, Kukk E, Saksela O, Kalkkinen N, Alitalo K. A novel vascular endothelial growth factor, VEGF-C, is a ligand for the Flt4 (VEGFR-3) and KDR (VEGFR-2) receptor tyrosine kinases. Embo J. 1996;15:290–98. [PMC free article] [PubMed] [Google Scholar]
- Kadambi A, Mouta Carreira C, Yun CO, Padera TP, Dolmans DE, Carmeliet P, Fukumura D, Jain RK. Vascular endothelial growth factor (VEGF)-C differentially affects tumor vascular function and leukocyte recruitment: role of VEGF-receptor 2 and host VEGF-A. Cancer Res. 2001;61:2404–8. [PubMed] [Google Scholar]
- Kaipainen A, Korhonen J, Mustonen T, van Hinsbergh VW, Fang GH, Dumont D, Breitman M, Alitalo K. Expression of the fms-like tyrosine kinase 4 gene becomes restricted to lymphatic endothelium during development. Proc Natl Acad Sci U S A. 1995;92:3566–70. doi: 10.1073/pnas.92.8.3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajiya K, Hirakawa S, Detmar M. Vascular endothelial growth factor-A mediates ultraviolet B-induced impairment of lymphatic vessel function. Am J Pathol. 2006;169:1496–503. doi: 10.2353/ajpath.2006.060197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajiya K, Hirakawa S, Ma B, Drinnenberg I, Detmar M. Hepatocyte growth factor promotes lymphatic vessel formation and function. Embo J. 2005;24:2885–95. doi: 10.1038/sj.emboj.7600763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karkkainen MJ, Haiko P, Sainio K, Partanen J, Taipale J, Petrova TV, Jeltsch M, Jackson DG, Talikka M, Rauvala H, Betsholtz C, Alitalo K. Vascular endothelial growth factor C is required for sprouting of the first lymphatic vessels from embryonic veins. Nat Immunol. 2004;5:74–80. doi: 10.1038/ni1013. [DOI] [PubMed] [Google Scholar]
- Karpanen T, Egeblad M, Karkkainen MJ, Kubo H, Yla-Herttuala S, Jaattela M, Alitalo K. Vascular endothelial growth factor C promotes tumor lymphangiogenesis and intralymphatic tumor growth. Cancer Res. 2001;61:1786–90. [PubMed] [Google Scholar]
- Karpanen T, Heckman CA, Keskitalo S, Jeltsch M, Ollila H, Neufeld G, Tamagnone L, Alitalo K. Functional interaction of VEGF-C and VEGF-D with neuropilin receptors. Faseb J. 2006a;20:1462–72. doi: 10.1096/fj.05-5646com. [DOI] [PubMed] [Google Scholar]
- Karpanen T, Wirzenius M, Makinen T, Veikkola T, Haisma HJ, Achen MG, Stacker SA, Pytowski B, Yla-Herttuala S, Alitalo K. Lymphangiogenic growth factor responsiveness is modulated by postnatal lymphatic vessel maturation. Am J Pathol. 2006b;169:708–18. doi: 10.2353/ajpath.2006.051200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerbel RS, Kamen BA. The anti-angiogenic basis of metronomic chemotherapy. Nat Rev Cancer. 2004;4:423–36. doi: 10.1038/nrc1369. [DOI] [PubMed] [Google Scholar]
- Kieran MW, Turner CD, Rubin JB, Chi SN, Zimmerman MA, Chordas C, Klement G, Laforme A, Gordon A, Thomas A, Neuberg D, Browder T, Folkman J. A feasibility trial of antiangiogenic (metronomic) chemotherapy in pediatric patients with recurrent or progressive cancer. J Pediatr Hematol Oncol. 2005;27:573–81. doi: 10.1097/01.mph.0000183863.10792.d4. [DOI] [PubMed] [Google Scholar]
- Krishnan J, Kirkin V, Steffen A, Hegen M, Weih D, Tomarev S, Wilting J, Sleeman JP. Differential in vivo and in vitro expression of vascular endothelial growth factor (VEGF)-C and VEGF-D in tumors and its relationship to lymphatic metastasis in immunocompetent rats. Cancer Res. 2003;63:713–22. [PubMed] [Google Scholar]
- Kuba K, Matsumoto K, Date K, Shimura H, Tanaka M, Nakamura T. HGF/NK4, a four-kringle antagonist of hepatocyte growth factor, is an angiogenesis inhibitor that suppresses tumor growth and metastasis in mice. Cancer Res. 2000;60:6737–43. [PubMed] [Google Scholar]
- Kubo H, Cao R, Brakenhielm E, Makinen T, Cao Y, Alitalo K. Blockade of vascular endothelial growth factor receptor-3 signaling inhibits fibroblast growth factor-2-induced lymphangiogenesis in mouse cornea. Proc Natl Acad Sci U S A. 2002;99:8868–73. doi: 10.1073/pnas.062040199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo H, Fujiwara T, Jussila L, Hashi H, Ogawa M, Shimizu K, Awane M, Sakai Y, Takabayashi A, Alitalo K, Yamaoka Y, Nishikawa SI. Involvement of vascular endothelial growth factor receptor-3 in maintenance of integrity of endothelial cell lining during tumor angiogenesis. Blood. 2000;96:546–53. [PubMed] [Google Scholar]
- Kunstfeld R, Hirakawa S, Hong YK, Schacht V, Lange-Asschenfeldt B, Velasco P, Lin C, Fiebiger E, Wei X, Wu Y, Hicklin D, Bohlen P, Detmar M. Induction of cutaneous delayed-type hypersensitivity reactions in VEGF-A transgenic mice results in chronic skin inflammation associated with persistent lymphatic hyperplasia. Blood. 2004;104:1048–57. doi: 10.1182/blood-2003-08-2964. [DOI] [PubMed] [Google Scholar]
- Kyzas PA, Stefanou D, Agnantis NJ. COX-2 expression correlates with VEGF-C and lymph node metastases in patients with head and neck squamous cell carcinoma. Mod Pathol. 2005a;18:153–60. doi: 10.1038/modpathol.3800244. [DOI] [PubMed] [Google Scholar]
- Kyzas PA, Geleff S, Batistatou A, Agnantis NJ, Stefanou D. Evidence for lymphangiogenesis and its prognostic implications in head and neck squamous cell carcinoma. J Pathol. 2005b;206:170–7. doi: 10.1002/path.1776. [DOI] [PubMed] [Google Scholar]
- Laakkonen P, Waltari M, Holopainen T, Takahashi T, Pytowski B, Steiner P, Hicklin D, Persaud K, Tonra JR, Witte L, Alitalo K. Vascular endothelial growth factor receptor 3 is involved in tumor angiogenesis and growth. Cancer Res. 2007;67:593–9. doi: 10.1158/0008-5472.CAN-06-3567. [DOI] [PubMed] [Google Scholar]
- Lin J, Lalani AS, Harding TC, Gonzalez M, Wu WW, Luan B, Tu GH, Koprivnikar K, VanRoey MJ, He Y, Alitalo K, Jooss K. Inhibition of lymphogenous metastasis using adeno-associated virus-mediated gene transfer of a soluble VEGFR-3 decoy receptor. Cancer Res. 2005;65:6901–9. doi: 10.1158/0008-5472.CAN-05-0408. [DOI] [PubMed] [Google Scholar]
- Makinen T, Jussila L, Veikkola T, Karpanen T, Kettunen MI, Pulkkanen KJ, Kauppinen R, Jackson DG, Kubo H, Nishikawa S, Yla-Herttuala S, Alitalo K. Inhibition of lymphangiogenesis with resulting lymphedema in transgenic mice expressing soluble VEGF receptor-3. Nat Med. 2001;7:199–205. doi: 10.1038/84651. [DOI] [PubMed] [Google Scholar]
- Mandriota SJ, Jussila L, Jeltsch M, Compagni A, Baetens D, Prevo R, Banerji S, Huarte J, Montesano R, Jackson DG, Orci L, Alitalo K, Christofori G, Pepper MS. Vascular endothelial growth factor-C-mediated lymphangiogenesis promotes tumour metastasis. Embo J. 2001;20:672–82. doi: 10.1093/emboj/20.4.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manley PW, Martiny-Baron G, Schlaeppi JM, Wood JM. Therapies directed at vascular endothelial growth factor. Expert Opin Investig Drugs. 2002;11:1715–36. doi: 10.1517/13543784.11.12.1715. [DOI] [PubMed] [Google Scholar]
- Mattila MM, Ruohola JK, Karpanen T, Jackson DG, Alitalo K, Harkonen PL. VEGF-C induced lymphangiogenesis is associated with lymph node metastasis in orthotopic MCF-7 tumors. Int J Cancer. 2002;98:946–51. doi: 10.1002/ijc.10283. [DOI] [PubMed] [Google Scholar]
- Maula SM, Luukkaa M, Grenman R, Jackson D, Jalkanen S, Ristamaki R. Intratumoral lymphatics are essential for the metastatic spread and prognosis in squamous cell carcinomas of the head and neck region. Cancer Res. 2003;63:1920–6. [PubMed] [Google Scholar]
- McColl BK, Loughran SJ, Davydova N, Stacker SA, Achen MG. Mechanisms of lymphangiogenesis: targets for blocking the metastatic spread of cancer. Curr Cancer Drug Targets. 2005;5:561–71. doi: 10.2174/156800905774932833. [DOI] [PubMed] [Google Scholar]
- Morabito A, De Maio E, Di Maio M, Normanno N, Perrone F. Tyrosine kinase inhibitors of vascular endothelial growth factor receptors in clinical trials: current status and future directions. Oncologist. 2006;11:753–64. doi: 10.1634/theoncologist.11-7-753. [DOI] [PubMed] [Google Scholar]
- Morisada T, Oike Y, Yamada Y, Urano T, Akao M, Kubota Y, Maekawa H, Kimura Y, Ohmura M, Miyamoto T, Nozawa S, Koh GY, Alitalo K, Suda T. Angiopoietin-1 promotes LYVE-1-positive lymphatic vessel formation. Blood. 2005;105:4649–56. doi: 10.1182/blood-2004-08-3382. [DOI] [PubMed] [Google Scholar]
- Morisawa N, Koyama T, Togashi K. Metastatic lymph nodes in urogenital cancers: contribution of imaging findings. Abdom Imaging. 2006;31:620–9. doi: 10.1007/s00261-005-0244-5. [DOI] [PubMed] [Google Scholar]
- Nagy JA, Vasile E, Feng D, Sundberg C, Brown LF, Detmar MJ, Lawitts JA, Benjamin L, Tan X, Manseau EJ, Dvorak AM, Dvorak HF. Vascular permeability factor/vascular endothelial growth factor induces lymphangiogenesis as well as angiogenesis. J Exp Med. 2002;196:1497–506. doi: 10.1084/jem.20021244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onda T, Yoshikawa H, Yokota H, Yasugi T, Taketani Y. Assessment of metastases to aortic and pelvic lymph nodes in epithelial ovarian carcinoma. A proposal for essential sites for lymph node biopsy. Cancer. 1996;78:803–8. doi: 10.1002/(SICI)1097-0142(19960815)78:4<803::AID-CNCR17>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Padera TP, Kadambi A, di Tomaso E, Carreira CM, Brown EB, Boucher Y, Choi NC, Mathisen D, Wain J, Mark EJ, Munn LL, Jain RK. Lymphatic metastasis in the absence of functional intratumor lymphatics. Science. 2002;296:1883–6. doi: 10.1126/science.1071420. [DOI] [PubMed] [Google Scholar]
- Pandit-Taskar N. Oncologic imaging in gynecologic malignancies. J Nucl Med. 2005;46:1842–50. [PubMed] [Google Scholar]
- Partanen TA, Alitalo K, Miettinen M. Lack of lymphatic vascular specificity of vascular endothelial growth factor receptor 3 in 185 vascular tumors. Cancer. 1999;86:2406–12. [PubMed] [Google Scholar]
- Podgrabinska S, Braun P, Velasco P, Kloos B, Pepper MS, Skobe M. Molecular characterization of lymphatic endothelial cells. Proc Natl Acad Sci U S A. 2002;99:16069–74. doi: 10.1073/pnas.242401399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pytowski B, Goldman J, Persaud K, Wu Y, Witte L, Hicklin DJ, Skobe M, Boardman KC, Swartz MA. Complete and specific inhibition of adult lymphatic regeneration by a novel VEGFR-3 neutralizing antibody. J Natl Cancer Inst. 2005;97:14–21. doi: 10.1093/jnci/dji003. [DOI] [PubMed] [Google Scholar]
- Rafii S, Skobe M. Splitting vessels: keeping lymph apart from blood. Nat Med. 2003;9:166–8. doi: 10.1038/nm0203-166. [DOI] [PubMed] [Google Scholar]
- Raut CP, Nawrocki S, Lashinger LM, Davis DW, Khanbolooki S, Xiong H, Ellis LM, McConkey DJ. Celecoxib inhibits angiogenesis by inducing endothelial cell apoptosis in human pancreatic tumor xenografts. Cancer Biol Ther. 2004;3:1217–24. doi: 10.4161/cbt.3.12.1221. [DOI] [PubMed] [Google Scholar]
- Renyi-Vamos F, Tovari J, Fillinger J, Timar J, Paku S, Kenessey I, Ostoros G, Agocs L, Soltesz I, Dome B. Lymphangiogenesis correlates with lymph node metastasis, prognosis, and angiogenic phenotype in human non-small cell lung cancer. Clin Cancer Res. 2005;11:7344–53. doi: 10.1158/1078-0432.CCR-05-1077. [DOI] [PubMed] [Google Scholar]
- Rissanen TT, Markkanen JE, Gruchala M, Heikura T, Puranen A, Kettunen MI, Kholova I, Kauppinen RA, Achen MG, Stacker SA, Alitalo K, Yla-Herttuala S. VEGF-D is the strongest angiogenic and lymphangiogenic effector among VEGFs delivered into skeletal muscle via adenoviruses. Circ Res. 2003;92:1098–106. doi: 10.1161/01.RES.0000073584.46059.E3. [DOI] [PubMed] [Google Scholar]
- Ristimaki A, Narko K, Enholm B, Joukov V, Alitalo K. Proinflammatory cytokines regulate expression of the lymphatic endothelial mitogen vascular endothelial growth factor-C. J Biol Chem. 1998;273:8413–8. doi: 10.1074/jbc.273.14.8413. [DOI] [PubMed] [Google Scholar]
- Roberts N, Kloos B, Cassella M, Podgrabinska S, Persaud K, Wu Y, Pytowski B, Skobe M. Inhibition of VEGFR-3 activation with the antagonistic antibody more potently suppresses lymph node and distant metastases than inactivation of VEGFR-2. Cancer Res. 2006;66:2650–7. doi: 10.1158/0008-5472.CAN-05-1843. [DOI] [PubMed] [Google Scholar]
- Roche J, Boldog F, Robinson M, Robinson L, Varella-Garcia M, Swanton M, Waggoner B, Fishel R, Franklin W, Gemmill R, Drabkin H. Distinct 3p21.3 deletions in lung cancer and identification of a new human semaphorin. Oncogene. 1996;12:1289–97. [PubMed] [Google Scholar]
- Rodriguez JM, Pinero A, Ortiz S, Moreno A, Sola J, Soria T, Robles R, Parrilla P. Clinical and histological differences in anaplastic thyroid carcinoma. Eur J Surg. 2000;166:34–8. doi: 10.1080/110241500750009672. [DOI] [PubMed] [Google Scholar]
- Rogers MS, Rohan RM, Birsner AE, D'Amato RJ. Genetic loci that control vascular endothelial growth factor-induced angiogenesis. Faseb J. 2003;17:2112–4. doi: 10.1096/fj.03-0246fje. [DOI] [PubMed] [Google Scholar]
- Rusznyak I, Foldi M, Szabo G. In: Lymphatics and lymph circulation; physiology and pathology. Youlton L, editor. Pergamon Press; Oxford, New York: 1967. [Google Scholar]
- Saaristo A, Veikkola T, Tammela T, Enholm B, Karkkainen MJ, Pajusola K, Bueler H, Yla-Herttuala S, Alitalo K. Lymphangiogenic gene therapy with minimal blood vascular side effects. J Exp Med. 2002;196:719–30. doi: 10.1084/jem.20020587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito H, Osaki T, Murakami D, Sakamoto T, Kanaji S, Ohro S, Tatebe S, Tsujitani S, Ikeguchi M. Prediction of sites of recurrence in gastric carcinoma using immunohistochemical parameters. J Surg Oncol. 2007;95:123–8. doi: 10.1002/jso.20612. [DOI] [PubMed] [Google Scholar]
- Sato TN, Tozawa Y, Deutsch U, Wolburg-Buchholz K, Fujiwara Y, Gendron-Maguire M, Gridley T, Wolburg H, Risau W, Qin Y. Distinct roles of the receptor tyrosine kinases Tie-1 and Tie-2 in blood vessel formation. Nature. 1995;376:70–4. doi: 10.1038/376070a0. [DOI] [PubMed] [Google Scholar]
- Sauter B, Foedinger D, Sterniczky B, Wolff K, Rappersberger K. Immunoelectron microscopic characterization of human dermal lymphatic microvascular endothelial cells: differential expression of CD31, CD34, and type IV collagen with lymphatic endothelial cells vs blood capillary endothelial cells in normal human skin, lymphangioma, and hemangioma in situ. J Histochem Cytochem. 1998;46:165–76. doi: 10.1177/002215549804600205. [DOI] [PubMed] [Google Scholar]
- Schacht V, von Rautenfeld DB, Abels C. The lymphatic system in the dorsal skinfold chamber of the Syrian golden hamster in vivo. Arch Dermatol Res. 2004;295:542–8. doi: 10.1007/s00403-004-0453-8. [DOI] [PubMed] [Google Scholar]
- Schauer AJ, Becker W, Reiser M, Possinger K. In: The Sentinel Lymph Node Concept. Heilmann U, editor. Springer--Verlag; Berlin Heidelberg New York: 2005. p. 565. [Google Scholar]
- Schneider M, Buchler P, Giese N, Giese T, Wilting J, Buchler MW, Friess H. Role of lymphangiogenesis and lymphangiogenic factors during pancreatic cancer progression and lymphatic spread. Int J Oncol. 2006;28:883–90. [PubMed] [Google Scholar]
- Scollo C, Baudin E, Travagli JP, Caillou B, Bellon N, Leboulleux S, Schlumberger M. Rationale for central and bilateral lymph node dissection in sporadic and hereditary medullary thyroid cancer. J Clin Endocrinol Metab. 2003;88:2070–5. doi: 10.1210/jc.2002-021713. [DOI] [PubMed] [Google Scholar]
- Sekido Y, Bader S, Latif F, Chen JY, Duh FM, Wei MH, Albanesi JP, Lee CC, Lerman MI, Minna JD. Human semaphorins A(V) and IV reside in the 3p21.3 small cell lung cancer deletion region and demonstrate distinct expression patterns. Proc Natl Acad Sci U S A. 1996;93:4120–5. doi: 10.1073/pnas.93.9.4120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senger DR, Claffey KP, Benes JE, Perruzzi CA, Sergiou AP, Detmar M. Angiogenesis promoted by vascular endothelial growth factor: regulation through alpha1beta1 and alpha2beta1 integrins. Proc Natl Acad Sci U S A. 1997;94:13612–7. doi: 10.1073/pnas.94.25.13612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao X, Liu C. Influence of IFN- alpha and IFN- gamma on lymphangiogenesis. J Interferon Cytokine Res. 2006;26:568–74. doi: 10.1089/jir.2006.26.568. [DOI] [PubMed] [Google Scholar]
- Shao XJ, Xie FM. Influence of angiogenesis inhibitors, endostatin and PF-4, on lymphangiogenesis. Lymphology. 2005;38:1–8. [PubMed] [Google Scholar]
- Shao XJ, Chi XY. Influence of angiostatin and thalidomide on lymphangiogenesis. Lymphology. 2005;38:146–55. [PubMed] [Google Scholar]
- Shariat SF, Karakiewicz PI, Palapattu GS, Lotan Y, Rogers CG, Amiel GE, Vazina A, Gupta A, Bastian PJ, Sagalowsky AI, Schoenberg MP, Lerner SP. Outcomes of radical cystectomy for transitional cell carcinoma of the bladder: a contemporary series from the Bladder Cancer Research Consortium. J Urol. 2006;176:2414–22. doi: 10.1016/j.juro.2006.08.004. discussion 2422. [DOI] [PubMed] [Google Scholar]
- Shields JD, Emmett MS, Dunn DB, Joory KD, Sage LM, Rigby H, Mortimer PS, Orlando A, Levick JR, Bates DO. Chemokine-mediated migration of melanoma cells towards lymphatics - a mechanism contributing to metastasis. Oncogene. 2007;26:2997–3005. doi: 10.1038/sj.onc.1210114. [DOI] [PubMed] [Google Scholar]
- Shimizu K, Kubo H, Yamaguchi K, Kawashima K, Ueda Y, Matsuo K, Awane M, Shimahara Y, Takabayashi A, Yamaoka Y, Satoh S. Suppression of VEGFR-3 signaling inhibits lymph node metastasis in gastric cancer. Cancer Sci. 2004;95:328–33. doi: 10.1111/j.1349-7006.2004.tb03211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin JW, Min M, Larrieu-Lahargue F, Canron X, Kunstfeld R, Nguyen L, Henderson JE, Bikfalvi A, Detmar M, Hong YK. Prox1 promotes lineage-specific expression of fibroblast growth factor (FGF) receptor-3 in lymphatic endothelium: a role for FGF signaling in lymphangiogenesis. Mol Biol Cell. 2006;17:576–84. doi: 10.1091/mbc.E05-04-0368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipos B, Klapper W, Kruse ML, Kalthoff H, Kerjaschki D, Kloppel G. Expression of lymphangiogenic factors and evidence of intratumoral lymphangiogenesis in pancreatic endocrine tumors. Am J Pathol. 2004;165:1187–97. doi: 10.1016/S0002-9440(10)63379-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sipos B, Kojima M, Tiemann K, Klapper W, Kruse ML, Kalthoff H, Schniewind B, Tepel J, Weich H, Kerjaschki D, Kloppel G. Lymphatic spread of ductal pancreatic adenocarcinoma is independent of lymphangiogenesis. J Pathol. 2005;207:301–12. doi: 10.1002/path.1840. [DOI] [PubMed] [Google Scholar]
- Skobe M, Detmar M. Structure, function, and molecular control of the skin lymphatic system. J Investig Dermatol Symp Proc. 2000;5:14–9. doi: 10.1046/j.1087-0024.2000.00001.x. [DOI] [PubMed] [Google Scholar]
- Skobe M, Hamberg LM, Hawighorst T, Schirner M, Wolf GL, Alitalo K, Detmar M. Concurrent induction of lymphangiogenesis, angiogenesis, and macrophage recruitment by vascular endothelial growth factor-C in melanoma. Am J Pathol. 2001a;159:893–903. doi: 10.1016/S0002-9440(10)61765-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skobe M, Hawighorst T, Jackson DG, Prevo R, Janes L, Velasco P, Riccardi L, Alitalo K, Claffey K, Detmar M. Induction of tumor lymphangiogenesis by VEGF-C promotes breast cancer metastasis. Nat Med. 2001b;7:192–8. doi: 10.1038/84643. [DOI] [PubMed] [Google Scholar]
- Sleeman JP. The Lymph Node as a Bridgehead in the Metastatic Dissemination of Tumors. In: Schlag PM, Veronesi U, editors. Lymphatic Metastasis and Sentinel Lymphonodectomy. Springer-Verlag; Berlin Heidelberg: 2000. p. 309. [Google Scholar]
- Sleeman JP. The relationship between tumors and the lymphatics: what more is there to know? Lymphology. 2006;39:62–8. [PubMed] [Google Scholar]
- Slevin M, Payne S. New treatments for colon cancer. Bmj. 2004;329:124–6. doi: 10.1136/bmj.329.7458.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JA, Jr, Seaman JP, Gleidman JB, Middleton RG. Pelvic lymph node metastasis from prostatic cancer: influence of tumor grade and stage in 452 consecutive patients. J Urol. 1983;130:290–2. doi: 10.1016/s0022-5347(17)51112-x. [DOI] [PubMed] [Google Scholar]
- Sohaib SA, Houghton SL, Meroni R, Rockall AG, Blake P, Reznek RH. Recurrent endometrial cancer: patterns of recurrent disease and assessment of prognosis. Clin Radiol. 2007;62:28–34. doi: 10.1016/j.crad.2006.06.015. discussion 35-6. [DOI] [PubMed] [Google Scholar]
- Soker S, Takashima S, Miao HQ, Neufeld G, Klagsbrun M. Neuropilin-1 is expressed by endothelial and tumor cells as an isoform-specific receptor for vascular endothelial growth factor. Cell. 1998;92:735–45. doi: 10.1016/s0092-8674(00)81402-6. [DOI] [PubMed] [Google Scholar]
- Soumaoro LT, Uetake H, Takagi Y, Iida S, Higuchi T, Yasuno M, Enomoto M, Sugihara K. Coexpression of VEGF-C and Cox-2 in human colorectal cancer and its association with lymph node metastasis. Dis Colon Rectum. 2006;49:392–8. doi: 10.1007/s10350-005-0247-x. [DOI] [PubMed] [Google Scholar]
- Stacker SA, Achen MG, Jussila L, Baldwin ME, Alitalo K. Lymphangiogenesis and cancer metastasis. Nat Rev Cancer. 2002;2:573–83. doi: 10.1038/nrc863. [DOI] [PubMed] [Google Scholar]
- Stacker SA, Caesar C, Baldwin ME, Thornton GE, Williams RA, Prevo R, Jackson DG, Nishikawa S, Kubo H, Achen MG. VEGF-D promotes the metastatic spread of tumor cells via the lymphatics. Nat Med. 2001;7:186–91. doi: 10.1038/84635. [DOI] [PubMed] [Google Scholar]
- Stoitzner P, Pfaller K, Stossel H, Romani N. A close-up view of migrating Langerhans cells in the skin. J Invest Dermatol. 2002;118:117–25. doi: 10.1046/j.0022-202x.2001.01631.x. [DOI] [PubMed] [Google Scholar]
- Su JL, Shih JY, Yen ML, Jeng YM, Chang CC, Hsieh CY, Wei LH, Yang PC, Kuo ML. Cyclooxygenase-2 induces EP1- and HER-2/Neu-dependent vascular endothelial growth factor-C up-regulation: a novel mechanism of lymphangiogenesis in lung adenocarcinoma. Cancer Res. 2004;64:554–64. doi: 10.1158/0008-5472.can-03-1301. [DOI] [PubMed] [Google Scholar]
- Swartz MA, Skobe M. Lymphatic function, lymphangiogenesis, and cancer metastasis. Microsc Res Tech. 2001;55:92–9. doi: 10.1002/jemt.1160. [DOI] [PubMed] [Google Scholar]
- Tait CR, Jones PF. Angiopoietins in tumours: the angiogenic switch. J Pathol. 2004;204:1–10. doi: 10.1002/path.1618. [DOI] [PubMed] [Google Scholar]
- Tammela T, Heckman CA, Alitalo K. American Association for Cancer Research Educational Book. 2005a. Lymphangiogenesis and Metastasis; pp. 67–73. [Google Scholar]
- Tammela T, Saaristo A, Lohela M, Morisada T, Tornberg J, Norrmen C, Oike Y, Pajusola K, Thurston G, Suda T, Yla-Herttuala S, Alitalo K. Angiopoietin-1 promotes lymphatic sprouting and hyperplasia. Blood. 2005b;105:4642–8. doi: 10.1182/blood-2004-08-3327. [DOI] [PubMed] [Google Scholar]
- Timoshenko AV, Chakraborty C, Wagner GF, Lala PK. COX-2-mediated stimulation of the lymphangiogenic factor VEGF-C in human breast cancer. Br J Cancer. 2006;94:1154–63. doi: 10.1038/sj.bjc.6603067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobler NE, Detmar M. Tumor and lymph node lymphangiogenesis--impact on cancer metastasis. J Leukoc Biol. 2006;80:691–6. doi: 10.1189/jlb.1105653. [DOI] [PubMed] [Google Scholar]
- Tsunoda S, Nakamura T, Sakurai H, Saiki I. Fibroblast growth factor-2-induced host stroma reaction during initial tumor growth promotes progression of mouse melanoma via vascular endothelial growth factor A-dependent neovascularization. Cancer Sci. 2007;98:541–8. doi: 10.1111/j.1349-7006.2007.00432.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uka K, Aikata H, Takaki S, Shirakawa H, Jeong SC, Yamashina K, Hiramatsu A, Kodama H, Takahashi S, Chayama K. Clinical features and prognosis of patients with extrahepatic metastases from hepatocellular carcinoma. World J Gastroenterol. 2007;13:414–20. doi: 10.3748/wjg.v13.i3.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Auwera I, Cao Y, Tille JC, Pepper MS, Jackson DG, Fox SB, Harris AL, Dirix LY, Vermeulen PB. First international consensus on the methodology of lymphangiogenesis quantification in solid human tumours. Br J Cancer. 2006;95:1611–25. doi: 10.1038/sj.bjc.6603445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Trappen PO, Steele D, Lowe DG, Baithun S, Beasley N, Thiele W, Weich H, Krishnan J, Shepherd JH, Pepper MS, Jackson DG, Sleeman JP, Jacobs IJ. Expression of vascular endothelial growth factor (VEGF)-C and VEGF-D, and their receptor VEGFR-3, during different stages of cervical carcinogenesis. J Pathol. 2003;201:544–54. doi: 10.1002/path.1467. [DOI] [PubMed] [Google Scholar]
- Von Marschall Z, Scholz A, Stacker SA, Achen MG, Jackson DG, Alves F, Schirner M, Haberey M, Thierauch KH, Wiedenmann B, Rosewicz S. Vascular endothelial growth factor-D induces lymphangiogenesis and lymphatic metastasis in models of ductal pancreatic cancer. Int J Oncol. 2005;27:669–79. [PubMed] [Google Scholar]
- von Rahden BH, Stein HJ, Puhringer F, Koch I, Langer R, Piontek G, Siewert JR, Hofler H, Sarbia M. Coexpression of cyclooxygenases (COX-1, COX-2) and vascular endothelial growth factors (VEGF-A, VEGF-C) in esophageal adenocarcinoma. Cancer Res. 2005;65:5038–44. doi: 10.1158/0008-5472.CAN-04-1107. [DOI] [PubMed] [Google Scholar]
- Weinberg AS, Ogle CA, Shim EK. Metastatic cutaneous squamous cell carcinoma: an update. Dermatol Surg. 2007;33:885–99. doi: 10.1111/j.1524-4725.2007.33190.x. [DOI] [PubMed] [Google Scholar]
- Wong SY, Haack H, Crowley D, Barry M, Bronson RT, Hynes RO. Tumor-secreted vascular endothelial growth factor-C is necessary for prostate cancer lymphangiogenesis, but lymphangiogenesis is unnecessary for lymph node metastasis. Cancer Res. 2005;65:9789–98. doi: 10.1158/0008-5472.CAN-05-0901. [DOI] [PubMed] [Google Scholar]
- Xiang RH, Hensel CH, Garcia DK, Carlson HC, Kok K, Daly MC, Kerbacher K, van den Berg A, Veldhuis P, Buys CH, Naylor SL. Isolation of the human semaphorin III/F gene (SEMA3F) at chromosome 3p21, a region deleted in lung cancer. Genomics. 1996;32:39–48. doi: 10.1006/geno.1996.0074. [DOI] [PubMed] [Google Scholar]
- Yano A, Fujii Y, Iwai A, Kawakami S, Kageyama Y, Kihara K. Glucocorticoids suppress tumor lymphangiogenesis of prostate cancer cells. Clin Cancer Res. 2006;12:6012–7. doi: 10.1158/1078-0432.CCR-06-0749. [DOI] [PubMed] [Google Scholar]
- Yuan L, Moyon D, Pardanaud L, Breant C, Karkkainen MJ, Alitalo K, Eichmann A. Abnormal lymphatic vessel development in neuropilin 2 mutant mice. Development. 2002;129:4797–806. doi: 10.1242/dev.129.20.4797. [DOI] [PubMed] [Google Scholar]
- Zhang J, Ji J, Yuan F, Zhu L, Yan C, Yu YY, Liu BY, Zhu ZG, Lin YZ. Cyclooxygenase-2 expression is associated with VEGF-C and lymph node metastases in gastric cancer patients. Biomed Pharmacother. 2005;59 2:S285–8. doi: 10.1016/s0753-3322(05)80047-2. [DOI] [PubMed] [Google Scholar]