Abstract
Context
Impairment of incretin activity is now recognized as integral to the metabolic derangement underlying type 2 diabetes. Glucoregulatory agents that target the incretin system have recently been developed, and the place of these drugs in the treatment of type 2 diabetes can be assessed based on a growing body of clinical data.
Evidence Acquisition
A PubMed search was conducted to identify clinical studies of incretin therapies in type 2 diabetes. Article reference lists were also searched for relevant information, and supplemental material such as conference abstracts, drug prescribing information, and treatment guidelines were included as appropriate.
Evidence Synthesis
Two classes of therapies target the incretin system. The first, glucagon-like peptide-1 (GLP-1) agonists (exemplified by exenatide and liraglutide), have demonstrated considerable efficacy in clinical trials, reducing hemoglobin A1c (HbA1c) by up to 1.3%, decreasing fasting and postprandial glucose concentrations, reducing weight by approximately 3.0 kg, and improving cardiovascular risk factors. The second class, the dipeptidyl peptidase-4 inhibitors (such as sitagliptin and vildagliptin) rely on production of endogenous GLP-1 and act by reducing its turnover. The dipeptidyl peptidase-4 (DPP-4) inhibitors produce modest reductions in HbA1c (< 1%) compared with GLP-1 agonists and are generally weight-neutral. Neither GLP-1 agonists nor DPP-4 inhibitors cause hypoglycemia unless used with other agents known to increase risk.
Conclusions
GLP-1 agonists and DPP-4 inhibitors provide a valuable new treatment option for patients with type 2 diabetes and may be associated with a wider range of therapeutic benefits than older drugs.
Introduction
The current pandemic of diabetes mellitus and projections for future growth in the prevalence of the disease threaten to create a global public health crisis. In the United States alone, 20.8 million (9.6%) persons aged 20 years and older and 8.6 million (10.3%) of those aged 60 years and older have diabetes. In addition, approximately 54 million people are estimated to have prediabetes.[1] From 1995 to 2025 the number of individuals with diabetes is likely to rise by 42% (from 51 million to 72 million) in developed countries and by 170% (from 84 million to 228 million) in developing countries.[2] Type 2 diabetes accounts for between 90% and 95% of these cases.[3]
The chronic complications of diabetes are frequent, severe, progressive, and expensive. These complications – which prominently include macrovascular disease (coronary heart disease, stroke, and peripheral arterial disease), microvascular disease (nephropathy, neuropathy, retinopathy), as well as heart failure and periodontal disease – are responsible for a reduction in life expectancy of 12 years for men and 19 years for women in addition to considerable morbidity.[3] Of patients with diabetes, 65% die of heart disease or stroke.[1] Patients with diabetes are 2 to 4 times more likely to have coronary heart disease or peripheral arterial disease and, among patients < 55 years of age, are at a 10-fold increased risk for stroke.[4–6] Microvascular complications from diabetes are an additional important cause of morbidity and disability. Diabetes is the leading cause of end-stage renal disease, accounting for approximately 44% of new cases.[1] It is also the leading cause of blindness in working-age adults; approximately 55% of patients with type 2 diabetes have evidence of retinopathy within 15 years of diagnosis.[1]
Evidence from clinical trials provides clear, compelling evidence that intensive treatment of type 2 diabetes – which represents > 90% of cases in the United States – can profoundly reduce the risk for the development and progression of chronic complications. Given the impact of diabetes on both the individual and the healthcare system together with the projected exponential growth of the population with diabetes, it is clear that the availability of a broad spectrum of effective, safe antidiabetic agents is becoming increasingly important.
This review will examine the latest additions to the therapeutic armamentarium for type 2 diabetes: the incretin-modifying therapies, including glucagon-like peptide-1 (GLP-1) agonists and dipeptidyl peptidase-4 (DPP-4) inhibitors, with a view to how these agents can fit into the increasingly complex treatment regimens for type 2 diabetes. In the context of current therapy, in which many patients and prescribers are unwilling to initiate insulin therapy because of the complexity of treatment, requirement for additional teaching, and risk for hypoglycemia and weight gain, GLP-1 agonists offer an effective alternative to oral antidiabetic drugs without the risk for weight gain associated with many of the commonly used oral drugs. Indeed, GLP-1 agonists have consistently elicited weight reductions in clinical trials. Furthermore, GLP-1 agonists are associated with a low risk for hypoglycemia unless combined with a secretagogue or insulin.[7–9] The characteristics of GLP-1 agonists make them a viable alternative (or addition) to oral antidiabetic agents and/or insulin.
Conventional View of the Progression of Type 2 Diabetes
Type 2 diabetes is a progressive metabolic disorder that is typified by functional defects in multiple organs. Prominent abnormalities include progressive pancreatic islet dysfunction, characterized by both qualitative and quantitative abnormities in insulin secretion from beta-cells and unrestrained glucagon secretion from alpha-cells, insulin resistance in muscle and adipose tissue, and dysregulated hepatic glucose production. Clinically, type 2 diabetes progresses from asymptomatic insulin resistance and islet defects to mild postprandial hyperglycemia and to frank diabetes that requires pharmacologic intervention. The conventional view of the progression of type 2 diabetes has focused primarily on insulin resistance and progressive beta-cell failure resulting in insulin deficiency.
Insulin acts to reduce blood glucose by signaling peripheral tissues to increase glucose uptake; it also promotes glycogen formation from glucose (glycogenesis) in the liver and inhibits secretion of glucagon from pancreatic alpha-cells.[10] Glucagon acts as a counterbalancing force by enhancing hepatic glucose production. Under normal physiologic conditions, glucagon sustains plasma glucose under fasting conditions.[10]
Beyond Insulin and Glucagon The Role of Amylin and Incretin Hormones
Apart from insulin resistance and deficiency, glucose homeostasis is also influenced by amylin and incretin hormones. Amylin, a beta-cell hormone like insulin, was first identified in 1987.[10] Amylin is secreted in response to nutrient stimuli and works together with insulin to suppress glucagon secretion. It is also involved in the regulation of gastric emptying, thereby influencing the rate of glucose appearance in the circulation.[10] As with insulin, there are both qualitative and quantitative abnormalities in the secretion of amylin in type 2 diabetes.
The incretin hormones, glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP) are now widely recognized as important contributors to the maintenance of glucose homeostasis. These gut-produced hormones were first hypothesized to exist when it was noted that ingested glucose elicits a larger and longer-lasting insulin response compared with intravenous glucose, suggesting that a mechanism existed within the gut that enhanced insulin release in response to meals.[11,12]
Two incretin hormones were later identified: GIP and GLP-1. Levels of these hormones were shown to rise rapidly shortly after nutrient intake and then fall precipitously shortly thereafter as a result of inactivation by the enzyme DPP-4. Of the two incretin hormones, GLP-1 is secreted in greater concentrations and is generally considered more physiologically relevant in humans (Figure).[13] GLP-1 has been shown to enhance glucose-dependent insulin secretion, suppress postprandial glucagon secretion from pancreatic alpha-cells, slow gastric emptying, and reduce food intake and body weight. GLP-1 may also preserve and/or enhance beta-cell mass, by promoting beta-cell proliferation, and by decreasing beta-cell apoptosis.[14] In addition to its effects on the core defects in type 2 diabetes, GLP-1 also appears to have direct effects on other tissues. For example, GLP-1 has been shown to reduce blood pressure and triglyceride levels, and also may have a protective effect on the vasculature and kidneys.[15–20]
Figure.
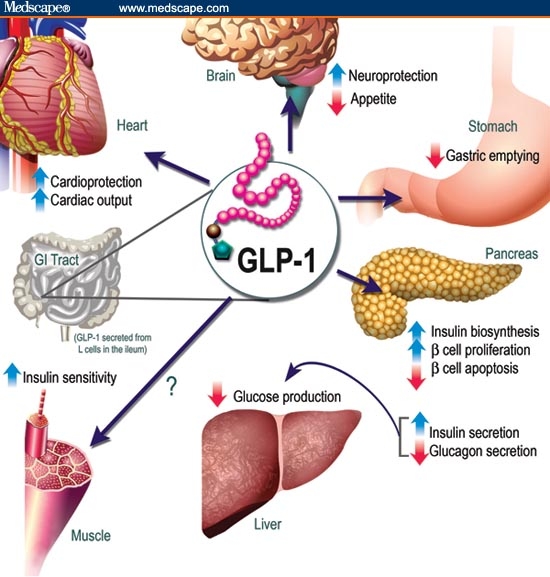
Figure: Physiology of glucagon-like peptide-1 (GLP-1) secretion and action on GLP-1 receptors in different organs and tissues. GI = gastrointestinal. Modified with permission from Drucker DJ. The biology of incretin hormones. Cell Metab. 2007;3:154.
As with insulin, glucagon, and amylin, derangements in GLP-1 function appear to contribute to the development of hyperglycemia. Type 2 diabetes is characterized by a marked blunting of the incretin effect, which is caused, at least in part, by decreased secretion of GLP-1.[21] This blunting of the incretin effect results in defective glucose-stimulated insulin secretion, reduced glucose clearance, increased levels of glucagon, and quicker gastric emptying.[22]
Initial evidence for the efficacy of supplementing incretin activity in patients with diabetes came from studies in which exogenous, native GLP-1 was administered by continuous intravenous or subcutaneous infusion. In these studies, exogenous GLP-1 normalized beta-cell responsiveness to glucose,[23] restored insulin response in patients with type 2 diabetes,[24] and reduced daytime plasma glucose levels to near normal.[25]
Although highly effective, the administration of native GLP-1 is limited because of the rapid degradation of native GLP-1 by DPP-4. Because continuous infusion of native GLP-1 is both expensive and impractical for the large majority of patients with type 2 diabetes, research has focused on developing compounds that could either mimic the activities of GLP-1 while being less susceptible to degradation by DPP-4 or that could limit turnover of endogenous GLP-1 by inhibiting DPP-4.
Limitations of Current Antidiabetic Agents
Collectively, the literature summarized above indicates that glucose homeostasis is governed by a complex interplay among insulin, glucagon, amylin, and incretin hormones. In addition to insulin replacement, a broad range of oral and injectable therapies is available, including insulin secretagogues and sensitizers, amylin analog, biguanides, and alpha-glucosidase inhibitors. Although all of these agents control hyperglycemia to varying degrees (with reductions in A1c ranging from about 0.5% to 1.5%), some are limited by adverse effects including weight increases (sulfonylureas, glinides, thiazolidinediones, and insulin) that may counteract the therapeutic gains elicited by these treatments as well as increased risk of hypoglycemia (sulfonylureas, glinides, amylin analog, and insulin). All of these antidiabetic agents are subject to declining efficacy with disease progression. Moreover, none of these therapies address the changes in incretin production, which is now known to constitute an integral part of the core defects underlying type 2 diabetes.
Perhaps the most important recent advances in the treatment of type 2 diabetes have been the development of GLP-1 agonists, which act by supplementing and/or replacing the activity of endogenous incretins, and DPP-4 inhibitors, which work by indirectly increasing levels of intact, physiologically active endogenous GLP-1 and GIP-1.
The GLP-1 Agonists Role in Type 2 Diabetes
Glucagon-like peptide-1 agonists represented the first advance in addressing defects in incretin secretion. The first of these agents, exenatide, is a naturally occurring peptide GLP-1 agonist originally identified in the venom of the Gila monster (Heloderma suspectum).[26] Exenatide has only 53% homology to the human GLP-1 amino acid sequence; as such, it is relatively more resistant to DPP-4 (with a half-life of 2.4 hours rather than 2–3 minutes for native GLP-1).[27] Owing to an enhanced pharmacokinetic profile, exenatide is more pharmacologically potent than native GLP-1.[28] A once-daily human GLP-1 analog, liraglutide, is in late clinical development; additional GLP-1 agonists, including a long-acting formulation of exenatide, are in phase 2 and 3 trials.
Exenatide: Synthetic Exendin-4
Exenatide has been studied extensively in humans and is currently approved as adjunctive therapy to improve glycemic control in patients with type 2 diabetes who are taking metformin, a sulfonylurea, a thiazolidinedione, or a combination of metformin and either a sulfonylurea or a thiazolidinedione, and who have not achieved adequate glycemic control.[27] It is administered initially as a 5-microgram (mcg) twice-daily injection within 60 minutes of the morning and evening meals and is usually titrated to 10 mcg twice daily as tolerated.[27] The Table summarizes outcomes in clinical trials of exenatide.[8,9,24,29–43]
Table.
Comparison of Incretin-Targeted Therapies
| Therapy | Baseline HbA1c | ΔHbA1cvs Baseline | Δ Body Weight vs Baseline | Most Common Adverse Events |
|---|---|---|---|---|
| Exenatide* | ||||
| Added to metformin[29,30] | 8.2% (30 weeks) | −0.8% (30 weeks) | −3.0 kg (30 weeks) to −5.3 kg (82 weeks) | Nausea (45%) |
| 8.1% (82 weeks) | −1.3% (82 weeks) | Nausea (14%) | ||
| Added to sulfonylurea[9] | 8.6% | −0.9% (30 weeks) | −1.6 kg (30 weeks) | Nausea (51%) |
| Mild-to-moderate hypoglycemia (36%) | ||||
| Added to metformin And sulfonylurea[8] | 8.5% | −0.8% (30 weeks) | −1.6 kg (30 weeks) | Nausea (49%) |
| Mild-to-moderate hypoglycemia (28%) | ||||
| Added to thiazolidinedione[31] | 7.9% | −0.9% (16 weeks) | −1.75 kg (16 weeks) | Nausea (40%) |
| Vomiting (13%) | ||||
| Monotherapy (LAR), 0.8 mg or 2.0 mg[32] | 8.3% | −1.7% (15 weeks) | −3.8 kg (15 weeks) | Mild-to-moderate nausea (19% and 27%, respectively) |
| Gastroenteritis (19% and 13%, respectively) | ||||
| Hypoglycemia (25% and 0%, respectively) | ||||
| Liraglutide* | ||||
| Monotherapy[52] | 8.5% | −1.4% (14 weeks) | −2.99 kg (14 weeks) | Diarrhea (21%) |
| Nausea (7.3%) | ||||
| Added to metformin[33] | 9.5% | −0.8%† (5 weeks) | −2.2 kg (5 weeks) | Nausea (35.3%) |
| Sitagliptin* | ||||
| Monotherapy[34] | 8.0% | −0.6% | Neutral | Few adverse events |
| Added to metformin[35] | 8.0% | −0.7% | Neutral | Few adverse events |
| Added to pioglitazone[36] | 8.1% | −0.8% | +1.8 kg | Few adverse events |
| Added to glimepiride + metformin[37] | 8.3% | −0.6% | +0.8 kg (24 weeks) | Hypoglycemia (16.4%) |
| Vildagliptin* | ||||
| Monotherapy[38] | 8.7% | −1.0% | Neutral | Few adverse events |
| Added to insulin[39] | 8.4% | −0.5% | +1.3 kg (24 weeks) | Hypoglycemia (22.9%) |
| Added to metformin[40] | 8.4% | −0.9% | +0.2 kg (24 weeks) | Few adverse events |
| Added to glimepiride[41] | 8.6% | −0.6% | +1.3 kg (24 weeks) | Asthenia (5.9%) |
| Nasopharyngitis (5.9%) | ||||
| Upper respiratory tract infection (5.3) | ||||
| Initiated with pioglitazone[42] | 8.8% | −1.9% | Neutral | Few adverse events |
| Added to pioglitazone[43] | 8.7% | −1.0% | +2.7 kg | Edema (7%) |
| UTI (5.1%) | ||||
Data given for 10-mcg dosage (exenatide) or for highest dosage used in a particular trial that does not exceed the currently indicated dosage, or for the highest dosage employed in a clinical trial (investigational agents). All values are approximate.
Reduction is given relative to metformin alone, not baseline.
LAR = long-acting release; HbA1c = hemoglobin A1c; UTI = urinary tract infection
Three 30-week, double-blind, placebo-controlled trials enrolling a total of 1446 patients have been conducted to evaluate the safety and efficacy of exenatide in patients with type 2 diabetes whose glycemic control was inadequate with metformin alone, a sulfonylurea alone, or metformin in combination with a sulfonylurea.[8,9,29] All of these trials were similarly designed: after a 4-week placebo lead-in period, patients were randomized to treatment with exenatide 5 mcg twice daily, exenatide 10 mcg twice daily (titrated from 5 mcg twice daily), or placebo before the morning and evening meals, in addition to their existing antidiabetic agent(s).[8,9,29] The primary outcome measure for all trials was glycemic control as assessed by change in hemoglobin A1c (HbA1c).
Regardless of the underlying oral antidiabetic therapy, patients who were randomized to treatment with exenatide experienced significant but modest incremental reductions in HbA1c. In the 5-mcg twice-daily groups, HbA1c reductions ranged from −0.40% when used with a sulfonylurea to −0.60% when used with the combination of metformin and a sulfonylurea, compared with no change or small gains in the placebo groups.[8,9] In patients who received the 10-mcg twice-daily dosage of exenatide, reductions in HbA1c ranged from −0.78% when combined with metformin alone to −0.86% when combined with a sulfonylurea. Small but consistent reductions in fasting glucose levels of approximately 7.0 to 10.0 mg/dL were also seen in the exenatide 5 mcg and 10 mcg groups; in the 2 studies that reported postprandial glucose levels, reductions of up to 34% were observed.[29,44] Notably, progressive weight losses of up to −2.8 kg were noted in all studies at the 10-mcg twice-daily dose.[8,9,29]
Across all studies, gastrointestinal side effects were relatively frequent, particularly during the early weeks of the trial with the 10-mcg dosage of exenatide. In the sulfonylurea add-on trial, nausea was reported in 51% of patients who received exenatide 10 mcg twice daily, compared with only 7% of those who received the sulfonylurea alone.[9] Similarly, nausea was more frequent when exenatide 10 mcg was used with metformin (45% vs 23%) and when used in combination with metformin plus a sulfonylurea (48.5% vs 20.6%).[29,44] Nausea was, however, mostly mild to moderate and generally decreased with increasing duration of treatment. The incidence of hypoglycemia (primarily mild to moderate) was higher among patients who received exenatide 10 mcg g twice daily in combination with a sulfonylurea (36% vs 3%) or the combination of metformin and a sulfonylurea (27.8% vs 12.6%), but the incidence was similar in patients who received exenatide plus metformin or metformin alone.[9,29,44]
Exenatide, like many nonhuman peptide therapeutics, appears to elicit an immune response. In the trials summarized, detectable antibodies to exenatide developed in a substantial percentage of patients (approximately 40% to 50%).[9,29,44] Although anti-exenatide antibodies did not influence glycemic response in these trials, it is noted in the exenatide prescribing information that high-titer anti-exenatide antibodies develop in between 6% and 9% of patients; in 3% to 9% of these patients, the glycemic response to exenatide is attenuated.[27]
Exenatide is not recommended in patients with severe renal insufficiency (creatinine clearance <30mL/minute) or end stage renal disease. In patients with end-stage renal disease on dialysis, exenatide 5 mcg has been poorly tolerated because of gastrointestinal side effects.[27]
Long-term extension studies of the exenatide/metformin study[29] and a report of patients enrolled in all 3 trials who were overweight[45], indicate that long-term exenatide treatment provides incremental reductions in HbA1c of up to 1.3% (when combined with metformin) and up to −1.1% in overweight patients after 82 weeks.[30,45] Notably – and unlike most weight-loss agents – continued progressive reductions in weight were observed in both studies. As is well known, long-term extensions are likely to enroll patients who experience good results during earlier study phases, and these data should be interpreted with caution.
Exenatide has also been compared with biphasic insulin aspart and insulin glargine.[46,47] In these studies, exenatide was non-inferior to insulins in HbA1c reductions and appeared to provide better postprandial glucose control, as well as weight reductions. However, the design of these trials has elicited some concern, as insulin therapy was not optimized in a manner consistent with other trials.[48]
Long-acting Exenatide
A once-weekly, long-acting formulation of exenatide (exenatide LAR) is in phase 3 clinical development, with a projected availability of 2009 to 2010. Although data are limited, phase 2 clinical trials indicated that exenatide LAR (at dosages of 0.8 and 2.0 mg) produced mean HbA1c reductions from baseline of 1.4% and 1.7%, respectively, and mean fasting plasma glucose reductions from baseline of 43.2 and 39.6 mg/dL, respectively. Relatively high rates of nausea (up to 27%) with low rates of hypoglycemia were reported with exenatide LAR as was frequent injection-site bruising (Table). Also, by the end of the study, 67% of subjects had anti-exenatide antibodies.[32]
Apart from mentioning that subcutaneous injections were administered by study personnel each week, few details were provided on the injection device. In practical terms, exenatide LAR is likely to be most useful in patients with demonstrated tolerance to the shorter-acting formulation because the long half-life of this drug may extend the adverse effects of treatment in exenatide-naive patients.
Liraglutide: A Human GLP-1 Analog
Once-daily liraglutide represents an approach to GLP-1 agonist therapy that is distinct from exenatide. Unlike exenatide, liraglutide is nearly identical to native human GLP-1, with only a single amino acid substitution and the addition of a glutamate-spaced acyl side chain to distinguish it from the native peptide. These minor substitutions have a dramatic effect on turnover, extending the time to maximum concentration to 9 to 12 hours and the half-life to around 13 hours after subcutaneous administration.[28,49] These pharmacokinetic improvements permit 24-hour glycemic control with a once-daily injection of the drug. Early dose-ranging studies provided initial evidence for the efficacy of liraglutide.[50,51] More recent phase 2 trials have evaluated the effects of liraglutide at clinically relevant dosages.[52] The table summarizes outcomes in phase II trials of liraglutide.
A phase 2 trial evaluated the effects of liraglutide monotherapy in patients who have not achieved glycemic control despite treatment with an oral antidiabetic agent.[52] Enrolled patients were either on diet with HbA1c 7.5% to 10% or on oral antidiabetic monotherapy with HbA1c 7.0% to 9.5%. At 1.90 mg once daily, liraglutide was associated with HbA1c reductions of 1.74% compared with placebo after 14 weeks of treatment; nearly half of patients who received liraglutide at the 2 highest dosages (1.25 or 1.90 mg once daily) achieved American Diabetes Association targets for postprandial control.[52] Fasting plasma glucose levels were significantly reduced vs placebo by 61.3 and 79.3 mg/dL with liraglutide 1.25 and 1.90 mg once daily, respectively (P < .0001). Liraglutide resulted in significant dose-dependent reductions in body weight of up to 3 kg at the end of treatment. Notably, the difference in the incidence of any gastrointestinal adverse event between patients in the liraglutide groups (29% to 37%) and the placebo group (23%) appears lower than that observed with exenatide.[52] Consistent with the fact that liraglutide has high homology with native GLP-1, anti-liraglutide antibodies have not been detected.[52]
Beyond Glycemic Control and Weight: Pleiotropic Effects of GLP-1 Agonists
Beyond their effects on glycemic control and weight, both exenatide and liraglutide have demonstrated pleiotropic effects that may enhance their therapeutic effect in patients with type 2 diabetes. The extension studies summarized above found marked improvements in lipid profiles for exenatide, with substantial reductions in apolipoprotein B (ApoB) (−5.2 mg/dL), triglycerides (−73 mg/dL), and increased high-density lipoprotein cholesterol (+4.5 mg/dL), together with small reductions in total cholesterol and low-density lipoprotein cholesterol. In addition, exenatide appears to be associated with substantial improvements in systolic and diastolic blood pressure, averaging −6.3/−4.1 mm Hg after 82 weeks of long-term treatment.[30] These improvements may, in part, be the result of the weight loss experienced by patients who remained on treatment in the extension studies. Much shorter-term phase 2 studies of liraglutide have also found substantial reductions in triglycerides of 22%, and reductions in blood pressure of about −7.9/2–3 mm Hg.[52] The observation that improvements in triglycerides and blood pressure occur after relatively short periods of treatment with liraglutide suggests that GLP-1 has direct effects that are not simply the result of weight loss. In addition, these studies have demonstrated reductions in emerging markers of cardiovascular risk and inflammation, including plasminogen activator inhibitor type 1, brain natiuretic peptide, and C-reactive protein.[53] The changes in cardiovascular risk factors seen with the GLP-1 agonists are particularly important given the substantially elevated risk for morbidity and mortality from cardiovascular disease seen in patients with type 2 diabetes.
In addition to cardiovascular effects, GLP-1 agonists appear to enhance beta-cell function. In preclinical studies, exenatide has been shown to inhibit beta-cell apoptosis and preserve function[54–56] and to improve beta-cell function in humans by up to 50%.[33], After 30 weeks of therapy with exenatide 10 mcg twice daily, insulin secretion rates were shown to increase by up to 72%.[56] Similarly, preclinical studies suggest that liraglutide has a positive effect on beta-cell function.[57–59] Liraglutide 1.9 mg/day increased beta-cell secretory capacity by up to 114% and increased first-phase insulin secretion by up to 118%.[60] In another study using a validated beta-cell model to evaluate liraglutide effects during conditions of normal living, insulin secretion was significantly increased from 189 to 322 pmol/minute/m2 (P < .005).[61] Whether the improvements observed in clinical studies are the direct effects of GLP-1 agonists on beta-cell function or are a result of improved glycemia remains to be elucidated.
The DPP-4 Inhibitors Role in Type 2 Diabetes
The DPP-4 inhibitors represent the first oral therapies targeted at increasing endogenous incretin levels. As the name suggests, these agents function by inhibiting the essential enzyme DPP-4, extending the physiologic half-life of endogenous GLP-1 and GIP by preventing their degradation. These agents are entirely dependent on secretion of endogenous incretins; thus, they may be best employed early in type 2 diabetes before substantial impairments in incretin secretion become apparent.
Sitagliptin
One DPP-4 inhibitor, sitagliptin, was approved in 2006 and is currently marketed in most countries worldwide. A fixed-dose combination of sitagliptin with metformin is also currently available. A second DPP-4 inhibitor, vildagliptin, has been approved in Europe but not in the United States. A broad range of DPP-4 inhibitors are currently in clinical development.
In the United States, sitagliptin is indicated as an adjunct to diet and exercise to improve glycemic control in patients with type 2 diabetes as monotherapy and in combination with metformin, glimepiride, pioglitazone, or metformin + glimepiride. It is also indicated as initial therapy in combination with metformin.[62] The recommended dose of sitagliptin is 100 mg once daily; in patients with moderate or severe renal insufficiency, the dose must be reduced to 50 mg and 25 mg once daily, respectively.[62]
Sitagliptin monotherapy (100 and 200 mg) provides significant (P < .001) reductions in HbA1c of 0.79% and 0.94%, respectively, and reductions in plasma fasting glucose of −17.1 to −21.3 mg/dL among drug-naive patients.[43] These benefits are accompanied by few hypoglycemic adverse events, even at the unapproved 200-mg dose. In general, sitagliptin is very well tolerated; slightly higher rates of certain adverse events, including constipation, nasopharyngitis, urinary tract infection, hypertension, and dizziness compared with placebo were seen in trials.[43] However, in postmarketing studies, more severe skin reactions, including some cases of Stevens-Johnson syndrome, have been reported. The relationship of these events to sitagliptin treatment is uncertain, however. In contrast to exenatide and liraglutide, sitagliptin does not appear to elicit meaningful changes in body weight.
Addition of sitagliptin in patients inadequately controlled on metformin or pioglitazone monotherapy also yields reductions in HbA1c of up to 0.65% to 0.70%, without an increased risk for gastrointestinal adverse events.[44,47] In head-to-head comparisons, sitagliptin plus metformin was non-inferior to the combination of glipizide 5 to 20 mg/day plus metformin in terms of HbA1c, and also provided small weight losses (−1.5 kg) compared with weight gain with glipizide (1.1 kg), with a clinically significant between-treatment difference of −2.5 kg (P < .001).[63] It is notable that in previous studies, relative to placebo, sitagliptin has been generally shown to have a weight-neutral effect as both monotherapy and as add-on therapy to metformin.[44,64] Sitagliptin and metformin combination therapy is associated with significant incremental reductions in HbA1c over initiation of either agent alone, without a substantial increase in adverse events.[65]
Vildagliptin
Vildagliptin is currently under investigation in the United States but was recently approved in Europe for the treatment of type 2 diabetes. Vildagliptin yields HbA1c reductions of approximately 1.0% compared with 1.4% with metformin after 1 year of treatment, with neutral effects on body weight and much lower rates of adverse events than metformin (primarily related to major reductions in the incidence of gastrointestinal adverse events compared with metformin).[46] Vildagliptin has also been shown to provide HbA1c reductions similar to those seen with the thiazolidinedione rosiglitazone (−1.1% vs −1.3%, respectively) while remaining weight-neutral compared with increases of 1.9 kg among patients who received rosiglitazone.[66]
Vildagliptin has also been studied in combination with pioglitazone. In a 24-week trial, vildagliptin 100 mg administered once daily with pioglitazone 30 mg yielded HbA1c reductions of −1.9%, with significant changes in fasting plasma glucose (−50 mg/dL) and overall weight gain (2.1 kg).[42] Another trial that tested vildagliptin (50 or 100 mg/day) as add-on therapy for patients poorly controlled with pioglitazone (45 mg/day) yielded incremental improvements in HbA1c, with small gains in weight (Table).[43]
DPP-4 inhibitors, like GLP-1 agonists, have been shown to improve beta-cell function and insulin sensitivity. In a 12-week study that examined the effects of vildagliptin on meal-related beta-cell function and insulin sensitivity in metformin-treated patients with type 2 diabetes, insulin secretion correlated with changes in HbA1c and increased with the addition of vildagliptin.[67] Sitagliptin 100 or 200 mg has also demonstrated improvements in homeostasis model assessment of beta-cell function vs placebo after 24 weeks of monotherapy in patients with type 2 diabetes.[43] As with GLP-1 agonists, the mechanism by which DPP-4 inhibitors improve beta-cell function requires further study.
Safety of DPP-4 Inhibitors
DPP-4 inhibitors have proven to be generally safe and well tolerated in clinical trials. Because sitagliptin is primarily excreted via renal elimination, the dosage must be adjusted in patients with moderate-to-severe renal insufficiency or end-stage renal disease.[62] A systematic meta-analysis suggests increased risks for nasopharyngitis and urinary tract infections, as well as an increased frequency of headache with short-term use of sitagliptin.[68] DPP-4 is also involved in multiple physiologic processes, with substrates beyond incretins that include, but are not limited to, certain neuropeptides, growth factors, and chemokines.[69] Whether DPP-4 plays an important physiologic role in the processing of these peptides remains to be determined. Although there have not been adverse events attributable to alterations in these off-target hormones, the potential long-term effects of chronic DPP-4 inhibition must be monitored carefully.
How Should Incretin-Targeted Therapies Be Used in the Clinic
The number of therapies available for type 2 diabetes has increased significantly over the last decade, which translates into more therapeutic options and complexity in making treatment decisions.
Although the 2007 American Diabetes Association guidelines do not contain specific guidance on the use of exenatide or sitagliptin, the American Association of Clinical Endocrinologists guidelines for the management of diabetes provide recommendations on the use of incretin-targeted therapies in patients with type 2 diabetes.[3] According to these guidelines, exenatide (in combination with a sulfonylurea, metformin, a sulfonylurea plus metformin, or a thiazolidinedione) is a therapeutic option for patients with type 2 diabetes who have not achieved glycemic goals with oral therapy alone.[3] Exenatide is not recommended for use in patients with end-stage renal disease or severe renal impairment. The DPP-4 inhibitor, sitagliptin, is recommended as initial monotherapy and as part of combination therapy with metformin, glimepiride, pioglitazone, or metformin + glimepiride. It can also be given as initial therapy in combination with metformin; dose reductions are required in patients with moderate or severe renal insufficiency.
Conclusions Integrating Incretin-Modifying Therapies Into Clinical Practice
Given the multiple therapeutic options available to treat type 2 diabetes, where do DPP-4 inhibitors and GLP-1 agonists fit into the overall treatment paradigm? Because the mechanism of action of DPP-4 inhibitors is dependent on endogenous incretin secretion, which may be impaired in patients with type 2 diabetes, DPP-4 inhibition achieves active GLP-1 levels that are, at best, in the high physiologic range. Moreover, DPP-4 inhibitors produce only minor increases in fasting active GLP-1 levels; their predominant effects are seen post-prandially. Thus, DPP-4 inhibitors lower A1c levels by only a modest degree. Nevertheless, because these drugs are orally available, given once daily, extremely well tolerated and, to date, have had a good safety profile, they will likely become important agents in the treatment paradigm for type 2 diabetes. Until long-term data are available, it is plausible to assume that DPP-4 inhibitors will work best early in the course of diabetes, given their dependence on endogenously produced GLP-1. They can work well as monotherapy for patients who are metformin intolerant or in those with renal insufficiency in whom metformin is contraindicated. Their weight-neutral action and low risk for hypoglycemia also make them appropriate monotherapy in patients for whom the weight gain and risk for hypoglycemia associated with sulfonylureas is undesirable. Likewise, their complementary mechanism of action and low risk for hypoglycemia is ideal in combination with either metformin or thiazolidinediones. Finally, DPP-4 inhibitors are well suited for treating elderly patients with type 2 diabetes because of their low risk for hypoglycemia as well as their tolerability profile and lack of significant drug-drug interactions.[70]
In contrast to DPP-4 inhibitors, GLP-1 agonists do not rely on endogenous incretin secretion, and pharmacologic levels of GLP-1 activity are achieved only after injection. Whereas the efficacy of exenatide is limited by its relatively short half-life and consequent minor effects on fasting glucose levels, longer-acting GLP-1 agonists, such as liraglutide, exenatide-LAR, and other molecules in development have robust effects on fasting glucose levels and may potentially provide superior efficacy to exenatide and most oral agents. In addition, an important attribute of members of this class is their ability to promote weight loss. Counterbalancing these benefits, all GLP-1 agonists must be given as injections, and the incidence of adverse events, particularly nausea, is higher. GLP-1 agonists are appropriate treatment for patients with type 2 diabetes when given in combination with oral agents such as metformin, sulfonylureas, and thiazolidinediones. They may be particularly beneficial in obese patients with type 2 diabetes because of the weight loss promoted by these agents, which can be substantial, and other ancillary benefits such as reducing blood pressure and improving lipid profiles. If further clinical studies of long-acting GLP-1 agonists confirm this initial impression of superior efficacy, these drugs could achieve widespread use as second-line, or even first-line treatments to bring more patients with type 2 diabetes to goal.
Acknowledgements
The author would like to thank John Ferguson for providing medical writing services and Adelphi Inc for providing editorial services paid for by Novo Nordisk.
Footnotes
Reader Comments on: Overview of Glucagon-like Peptide-1 Analogs and Dipeptidyl Peptidase-4 Inhibitors for Type 2 Diabetes See reader comments on this article and provide your own.
Readers are encouraged to respond to the author at richard.pratley@uvm.edu or to George Lundberg, MD, Editor in Chief of The Medscape Journal of Medicine, for the editor's eyes only or for possible publication as an actual Letter in the Medscape Journal via email: glundberg@medscape.net
References
- 1.American Diabetes Association. National Diabetes Fact Sheet. 2005 Available at: http://www.diabetes.org/uedocuments/NationalDiabetesFactSheetRev.pdf Accessed July 11, 2008.
- 2.King H, Aubert RE, Herman WH. Global burden of diabetes, 1995–2025: prevalence, numerical estimates, and projections. Diabetes Care. 1998;21:1414–1431. doi: 10.2337/diacare.21.9.1414. [DOI] [PubMed] [Google Scholar]
- 3.Rodbard HW, the AACE Diabetes Mellitus Clinical Practice Guidelines Task Force American Association of Clinical Endocrinologists medical guidelines for clinical practice for the management of diabetes mellitus. Endocrine Pract. 2007;13(suppl 1):3–68. doi: 10.4158/EP.13.S1.1. [DOI] [PubMed] [Google Scholar]
- 4.Feskens EJM, Kromhout D. Glucose tolerance and the risk of cardiovascular disease: the Zutphen Study. J Clin Epidemiol. 1992;45:1327–1334. doi: 10.1016/0895-4356(92)90173-k. [DOI] [PubMed] [Google Scholar]
- 5.Newman AB, Siscovick DS, Manolio TA, et al. for the Cardiovascular Health Study (CHS) Collaborative Research Group Ankle-arm index as a marker of atherosclerosis in the Cardiovascular Health Study. Circulation. 1993;88:837–845. doi: 10.1161/01.cir.88.3.837. [DOI] [PubMed] [Google Scholar]
- 6.You RX, McNeil JJ, O'Malley HM, Davis SM, Thrift AG, Donnan GA. Risk factors for stroke due to cerebral infarction in young adults. Stroke. 1997;28:1913–1918. doi: 10.1161/01.str.28.10.1913. [DOI] [PubMed] [Google Scholar]
- 7.Pratley R. Islet dysfunction: an underlying defect in the pathophysiology of type 2 diabetes. Endocrinol Metab Clin North Am. 2006;35(suppl 1):6–11. doi: 10.1016/s0889-8529(07)70004-x. [DOI] [PubMed] [Google Scholar]
- 8.Kendall DM, Riddle MC, Rosenstock J, et al. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in patients with type 2 diabetes treated with metformin and a sulfonylurea. Diabetes Care. 2005;28:1083–1091. doi: 10.2337/diacare.28.5.1083. [DOI] [PubMed] [Google Scholar]
- 9.Buse JB, Henry RR, Han J, Kim DD, Fineman MS, Baron AD, for the Exenatide-113 Clinical Study Group Effects of exenatide (exendin-4) on glycemic control over 30 weeks in sulfonylurea-treated patients with type 2 diabetes. Diabetes Care. 2004;27:2628–2635. doi: 10.2337/diacare.27.11.2628. [DOI] [PubMed] [Google Scholar]
- 10.Aronoff SL, Berkowitz K, Shreiner B, Want L. Glucose metabolism and regulation: beyond insulin and glucagon. Diabetes Spectrum. 2004;17:183–190. [Google Scholar]
- 11.Elrick H, Stimmler L, Hlad CJ, Jr, Arai Y. Plasma insulin response to oral and intravenous glucose administration. J Clin Endocrinol Metab. 1964;24:1076–1082. doi: 10.1210/jcem-24-10-1076. [DOI] [PubMed] [Google Scholar]
- 12.Nauck MA, Homberger E, Siegel EG, et al. Incretin effects of increasing glucose loads in man calculated from venous insulin and C-peptide responses. J Clin Endocrinol Metab. 1986;63:492–498. doi: 10.1210/jcem-63-2-492. [DOI] [PubMed] [Google Scholar]
- 13.Nauck MA, Heimesaat MM, Orskov C, Holst JJ, Ebert R, Creutzfeldt W. Preserved incretin activity of glucagon-like peptide 1 [7–36 amide] but not of synthetic human gastric inhibitory polypeptide in patients with type-2 diabetes mellitus. J Clin Invest. 1993;91:301–307. doi: 10.1172/JCI116186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Drucker DJ. Glucagon-like peptides: regulators of cell proliferation, differentiation, and apoptosis. Mol Endocrinol. 2003;17:161–171. doi: 10.1210/me.2002-0306. [DOI] [PubMed] [Google Scholar]
- 15.Bose AK, Mocanu MM, Carr RD, Brand CL, Yellon DM. Glucagon-like peptide 1 can directly protect the heart against ischemia/reperfusion injury. Diabetes. 2005;54:146–151. doi: 10.2337/diabetes.54.1.146. [DOI] [PubMed] [Google Scholar]
- 16.Thrainsdottir I, Malmberg K, Olsson A, Gutniak M, Ryden L. Initial experience with GLP-1 treatment on metabolic control and myocardial function in patients with type 2 diabetes mellitus and heart failure. Diab Vasc Dis Res. 2004;1:40–43. doi: 10.3132/dvdr.2004.005. [DOI] [PubMed] [Google Scholar]
- 17.Nystrom T, Gonon AT, Sjoholm A, Pernow J. Glucagon-like peptide-1 relaxes rat conduit arteries via an endothelium-independent mechanism. Regul Pept. 2005;125:173–177. doi: 10.1016/j.regpep.2004.08.024. [DOI] [PubMed] [Google Scholar]
- 18.Nystrom T, Gutniak MK, Zhang Q, et al. Effects of glucagon-like peptide-1 on endothelial function in type 2 diabetes patients with stable coronary artery disease. Am J Physiol Endocrinol Metab. 2004;287:E1209–1215. doi: 10.1152/ajpendo.00237.2004. [DOI] [PubMed] [Google Scholar]
- 19.Yu M, Moreno C, Hoagland KM, et al. Antihypertensive effect of glucagon-like peptide 1 in Dahl salt-sensitive rats. J Hypertens. 2003;21:1125–1135. doi: 10.1097/00004872-200306000-00012. [DOI] [PubMed] [Google Scholar]
- 20.Gutzwiller J-P, Tschopp S, Bock A, et al. Glucagon-like peptide 1 induces natriuresis in healthy subjects and in insulin-resistant obese men. J Clin Endocrinol Metab. 2004;89:3055–3061. doi: 10.1210/jc.2003-031403. [DOI] [PubMed] [Google Scholar]
- 21.Toft-Nielsen M-B, Damholt MB, Madsbad S, et al. Determinants of the impaired secretion of glucagon-like peptide-1 in type 2 diabetic patients. J Clin Endocrinol Metab. 2001;86:3717–3723. doi: 10.1210/jcem.86.8.7750. [DOI] [PubMed] [Google Scholar]
- 22.Schirra J, Sturm K, Leicht P, Arnold R, Goke B, Katschinski M. Exendin(9–39)amide is an antagonist of glucagon-like peptide-1(7–36)amide in humans. J Clin Invest. 1998;101:1421–1430. doi: 10.1172/JCI1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kjems LL, Holst JJ, Volund A, Madsbad S. The influence of GLP-1 on glucose-stimulated insulin secretion: effects on beta-cell sensitivity in type 2 and nondiabetic subjects. Diabetes. 2003;52:380–386. doi: 10.2337/diabetes.52.2.380. [DOI] [PubMed] [Google Scholar]
- 24.Vilsboll T, Knop FK, Krarup T, et al. The pathophysiology of diabetes involves a defective amplification of the late-phase insulin response to glucose by glucose-dependent insulinotropic polypeptide – regardless of etiology and phenotype. J Clin Endocrinol Metab. 2003;88:4897–4903. doi: 10.1210/jc.2003-030738. [DOI] [PubMed] [Google Scholar]
- 25.Rachman J, Barrow BA, Levy JC, Turner RC. Near-normalisation of diurnal glucose concentrations by continuous administration of glucagon-like peptide-1 (GLP-1) in subjects with NIDDM. Diabetologia. 1997;40:205–211. doi: 10.1007/s001250050664. [DOI] [PubMed] [Google Scholar]
- 26.Eng J, Kleinman WA, Singh L, Singh G, Raufman J-P. Isolation and characterization of exendin-4, an exendin-3 analogue, from Heloderma suspecturn venom. J Biol Chem. 1992;267:7402–7405. [PubMed] [Google Scholar]
- 27.Byetta (exenatide injection) Prescribing Information. San Diego, Cal: Amylin Pharmaceuticals; 2007. [Google Scholar]
- 28.Young AA, Gedulin BR, Bhavsar S, et al. Glucose-lowering and insulin-sensitizing actions of exendin-4: studies in obese diabetic (ob/ob, db/db) mice, diabetic fatty Zucker rats, and diabetic rhesus monkeys (Macaca mulatta) Diabetes. 1999;48:1026–1034. doi: 10.2337/diabetes.48.5.1026. [DOI] [PubMed] [Google Scholar]
- 29.DeFronzo RA, Ratner RE, Han J, Kim DD, Fineman MS, Baron AD. Effects of exenatide (exendin-4) on glycemic control and weight over 30 weeks in metformin-treated patients with type 2 diabetes. Diabetes Care. 2005;28:1092–1100. doi: 10.2337/diacare.28.5.1092. [DOI] [PubMed] [Google Scholar]
- 30.Ratner RE, Maggs D, Nielsen LL, et al. Long-term effects of exenatide therapy over 82 weeks on glycaemic control and weight in over-weight metformin-treated patients with type 2 diabetes mellitus. Diabetes Obes Metab. 2006;8:419–428. doi: 10.1111/j.1463-1326.2006.00589.x. [DOI] [PubMed] [Google Scholar]
- 31.Zinman B, Hoogwerf BJ, Duran Garcia S, et al. The effect of adding exenatide to a thiazolidinedione in suboptimally controlled type 2 diabetes: a randomized trial. Ann Intern Med. 2007;146:477–485. doi: 10.7326/0003-4819-146-7-200704030-00003. [DOI] [PubMed] [Google Scholar]
- 32.Kim D, MacConell L, Zhuang D, et al. Effects of once-weekly dosing of a long-acting release formulation of exenatide on glucose control and body weight in subjects with type 2 diabetes. Diabetes Care. 2007;30:1487–1493. doi: 10.2337/dc06-2375. [DOI] [PubMed] [Google Scholar]
- 33.Nauck MA, Hompesch M, Filipczak R, Le TDT, Zdravkovic M, Gumprecht J. Five weeks of treatment with the GLP-1 analogue liraglutide improves glycaemic control and lowers body weight in subjects with type 2 diabetes. Exp Clin Endocrinol Diabetes. 2006;114:417–423. doi: 10.1055/s-2006-924230. [DOI] [PubMed] [Google Scholar]
- 34.Aschner P, Kipnes MS, Lunceford JK, et al. Effect of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy on glycemic control in patients with type 2 diabetes. Diabetes Care. 2006;29:2632–2637. doi: 10.2337/dc06-0703. [DOI] [PubMed] [Google Scholar]
- 35.Charbonnel B, Karasik A, Liu J, Wu M, Meininger G, for the Sitagliptin Study 020 Group Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin added to ongoing metformin therapy in patients with type 2 diabetes inadequately controlled with metformin alone. Diabetes Care. 2006;29:2638–2643. doi: 10.2337/dc06-0706. [DOI] [PubMed] [Google Scholar]
- 36.Rosenstock J, Brazg R, Andryuk PJ, Lu K, Stein P, for the Sitagliptin Study 019 Group Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin added to ongoing pioglitazone therapy in patients with type 2 diabetes: a 24-week, multicenter, randomized, double-blind, placebo-controlled, parallel-group study. Clin Ther. 2006;28:1556–1568. doi: 10.1016/j.clinthera.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 37.Hermansen K, Kipnes M, Luo E, Fanurik D, Khatami H, Stein P, for the Sitagliptin Study 035 Group Efficacy and safety of the dipeptidyl peptidase-4 inhibitor, sitagliptin, in patients with type 2 diabetes mellitus inadequately controlled on glimepiride alone or on glimepiride and metformin. Diabetes Obes Metab. 2007;9:733–745. doi: 10.1111/j.1463-1326.2007.00744.x. [DOI] [PubMed] [Google Scholar]
- 38.Schweizer A, Couturier A, Foley JE, Dejager S. Comparison between vildagliptin and metformin to sustain reductions in HbA1c over 1 year in drug-naive patients with type 2 diabetes. Diabet Med. 2007;24:955–961. doi: 10.1111/j.1464-5491.2007.02191.x. [DOI] [PubMed] [Google Scholar]
- 39.Fonseca V, Schweizer A, Albrecht D, Baron MA, Chang I, Dejager S. Addition of vildagliptin to insulin improves glycaemic control in type 2 diabetes. Diabetologia. 2007;50:1148–1155. doi: 10.1007/s00125-007-0633-0. [DOI] [PubMed] [Google Scholar]
- 40.Bosi E, Camisasca RP, Collober C, Rochotte E, Garber AJ. Effects of vildagliptin on glucose control over 24 weeks in patients with type 2 diabetes inadequately controlled with metformin. Diabetes Care. 2007;30:890–895. doi: 10.2337/dc06-1732. [DOI] [PubMed] [Google Scholar]
- 41.Garber AJ, Foley J, Banerji MA, et al. Effects of vildagliptin on glucose control in patients with type 2 diabetes inadequately controlled with a sulfonylurea [published online ahead of print February 18, 2008] Diabetes Obes Metab. 2008 doi: 10.1111/j.1463-1326.2008.00859.x. [DOI] [PubMed] [Google Scholar]
- 42.Rosenstock J, Kim SW, Baron MA, et al. Efficacy and tolerability of initial combination therapy with vildagliptin and pioglitazone compared with component monotherapy in patients with type 2 diabetes. Diabetes Obes Metab. 2007;9:175–185. doi: 10.1111/j.1463-1326.2006.00698.x. [DOI] [PubMed] [Google Scholar]
- 43.Garber AJ, Schweizer A, Baron MA, Rochotte E, Dejager S. Vildagliptin in combination with pioglitazone improves glycaemic control in patients with type 2 diabetes failing thiazolidinedione monotherapy: a randomized, placebo-controlled study. Diabetes Obes Metab. 2007;9:166–174. doi: 10.1111/j.1463-1326.2006.00684.x. [DOI] [PubMed] [Google Scholar]
- 44.Kendall DM, Blonde L, Mac SM, et al. Improvements in cardiovascular risk factors accompanied improved glycemic control and weight reduction in patients with type 2 diabetes treated with exenatide for 3.5 y. Program and abstracts of the American Diabetes Association 67th Annual Scientific Sessions; June 22–26, 2007; Chicago, Illinois. Abstract 0557-P. [Google Scholar]
- 45.Blonde L, Klein EJ, Han J, et al. Interim analysis of the effects of exenatide treatment on A1C, weight and cardiovascular risk factors over 82 weeks in 314 overweight patients with type 2 diabetes. Diabetes Obes Metab. 2006;8:436–447. doi: 10.1111/j.1463-1326.2006.00602.x. [DOI] [PubMed] [Google Scholar]
- 46.Nauck MA, Duran S, Kim D, et al. A comparison of twice-daily exenatide and biphasic insulin aspart in patients with type 2 diabetes who were suboptimally controlled with sulfonylurea and metformin: a non-inferiority study. Diabetologia. 2007;50:259–267. doi: 10.1007/s00125-006-0510-2. [DOI] [PubMed] [Google Scholar]
- 47.Heine RJ, Van Gaal LF, Johns D, Mihm MJ, Widel MH, Brodows RG, for the GWAA Study Group Exenatide versus insulin glargine in patients with suboptimally controlled type 2 diabetes: a randomized trial. Ann Intern Med. 2005;143:559–569. doi: 10.7326/0003-4819-143-8-200510180-00006. [DOI] [PubMed] [Google Scholar]
- 48.Home PD. Comment on: Nauck MA, Duran S, Kim D et al (2007) A comparison of twice-daily exenatide and biphasic insulin aspart in patients with type 2 diabetes who were suboptimally controlled with sulfonylurea and metformin: a non-inferiority study. Diabetologia. 2007;50:1561–1562. doi: 10.1007/s00125-006-0510-2. [DOI] [PubMed] [Google Scholar]
- 49.Elbrond B, Jakobsen G, Larsen S, et al. Pharmacokinetics, pharmacodynamics, safety, and tolerability of a single-dose of NN2211, a long-acting glucagon-like peptide 1 derivative, in healthy male subjects. Diabetes Care. 2002;25:1398–1404. doi: 10.2337/diacare.25.8.1398. [DOI] [PubMed] [Google Scholar]
- 50.Madsbad S, Schmitz O, Ranstam J, Jakobsen G, Matthews DR, on behalf of the NN2211-13 International Study Group Improved glycemic control with no weight increase in patients with type 2 diabetes after once-daily treatment with the long-acting glucagon-like peptide 1 analog liraglutide (NN2211): a 12-week, double-blind, randomized, controlled trial. Diabetes Care. 2004;27:1335–1342. doi: 10.2337/diacare.27.6.1335. [DOI] [PubMed] [Google Scholar]
- 51.Feinglos MN, Saad MF, Pi-Sunyer FX, An B, Santiago O, on behalf of the Liraglutide Dose-Response Study Group Effects of liraglutide (NN2211), a long-acting GLP-1 analogue, on glycaemic control and bodyweight in subjects with type 2 diabetes. Diabet Med. 2005;22:1016–1023. doi: 10.1111/j.1464-5491.2005.01567.x. [DOI] [PubMed] [Google Scholar]
- 52.Vilsboll T, Zdravkovic M, Le-Thi T, et al. Liraglutide, a long-acting human glucagon-like peptide-1 analog, given as monotherapy significantly improves glycemic control and lowers body weight without risk of hypoglycemia in patients with type 2 diabetes. Diabetes Care. 2007;30:1608–1610. doi: 10.2337/dc06-2593. [DOI] [PubMed] [Google Scholar]
- 53.Vilsboll T, Zdravkovic M, Le-Thi T, et al. Beneficial effect of the GLP-1 analogue liraglutide on blood pressure and cardiovascular risk markers in subjects with type 2 diabetes. Diabetic Med. 2006;23(Suppl 4):696. [Google Scholar]
- 54.Chen J, Couto FM, Minn AH, Shalev A. Exenatide inhibits beta-cell apoptosis by decreasing thioredoxin-interacting protein. Biochem Biophys Res Comm. 2006;346:1067–1074. doi: 10.1016/j.bbrc.2006.06.027. [DOI] [PubMed] [Google Scholar]
- 55.Xu G, Stoffers DA, Habener JF, Bonner-Weir S. Exendin-4 stimulates both beta-cell replication and neogenesis, resulting in increased beta-cell mass and improved glucose tolerance in diabetic rats. Diabetes. 1999;48:2270–2276. doi: 10.2337/diabetes.48.12.2270. [DOI] [PubMed] [Google Scholar]
- 56.Mari A, Nielsen LL, Nanayakkara N, DeFronzo RA, Ferrannini E, Halseth A. Mathematical modeling shows exenatide improved beta-cell function in patients with type 2 diabetes treated with metformin or metformin and a sulfonylurea. Horm Metab Res. 2006;38:838–844. doi: 10.1055/s-2006-956505. [DOI] [PubMed] [Google Scholar]
- 57.Sturis J, Gotfredsen CF, Romer J, et al. GLP-1 derivative liraglutide in rats with beta-cell deficiencies: influence of metabolic state on beta-cell mass dynamics. Br J Pharmacol. 2003;140:123–132. doi: 10.1038/sj.bjp.0705397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rolin B, Larsen MO, Gotfredsen CF, et al. The long-acting GLP-1 derivative NN2211 ameliorates glycemia and increases beta-cell mass in diabetic mice. Am J Physiol Endocrinol Metab. 2002;283:E745–E752. doi: 10.1152/ajpendo.00030.2002. [DOI] [PubMed] [Google Scholar]
- 59.Bock T, Pakkenberg B, Buschard K. The endocrine pancreas in non-diabetic rats after short-term and long-term treatment with the long-acting GLP-1 derivative NN2211. APMIS. 2003;111:1117–1124. doi: 10.1111/j.1600-0463.2003.apm1111207.x. [DOI] [PubMed] [Google Scholar]
- 60.Vilsboll T, Birgitte B, Perrild H, et al. Liraglutide, a once-daily human GLP-1 analogue improves beta-cell function and arginine-stimulated insulin secretion at hyperglycaemia in patients with type 2 diabetes mellitus. Diabet Med. 2008;25:152–156. doi: 10.1111/j.1464-5491.2007.02333.x. [DOI] [PubMed] [Google Scholar]
- 61.Mari A, Degn K, Brock B, Rungby J, Ferrannini E, Schmitz O. Effects of the long-acting human glucagon-like peptide-1 analog liraglutide on beta-cell function in normal living conditions. Diabetes Care. 2007;30:2032–2033. doi: 10.2337/dc07-0310. [DOI] [PubMed] [Google Scholar]
- 62.Januvia (sitagliptin) product information. Whitehouse Station, NJ: Merck & Co.; 2007. [Google Scholar]
- 63.Nauck MA, Meininger G, Sheng D, Terranella L, Stein PP, for the Sitagliptin Study 024 Group Efficacy and safety of the dipeptidyl peptidase-4 inhibitor, sitagliptin, compared with the sulfonylurea, glipizide, in patients with type 2 diabetes inadequately controlled on metformin alone: a randomized, double-blind, non-inferiority trial. Diabetes Obes Metab. 2007;9:194–205. doi: 10.1111/j.1463-1326.2006.00704.x. [DOI] [PubMed] [Google Scholar]
- 64.Raz I, Hanefeld M, Xu L, Caria C, Williams-Herman D, Khatami H, the Sitagliptin Study 023 Group Efficacy and safety of the dipeptidyl peptidase-4 inhibitor sitagliptin as monotherapy in patients with type 2 diabetes mellitus. Diabetologia. 2006;49:2564–2571. doi: 10.1007/s00125-006-0416-z. [DOI] [PubMed] [Google Scholar]
- 65.Goldstein BJ, Feinglos MN, Lunceford JK, Johnson J, Williams-Herman DE, for the Sitagliptin 036 Study Group Effect of initial combination therapy with sitagliptin, a dipeptidyl peptidase-4 inhibitor, and metformin on glycemic control in patients with type 2 diabetes. Diabetes Care. 2007;30:1979–1987. doi: 10.2337/dc07-0627. [DOI] [PubMed] [Google Scholar]
- 66.Rosenstock J, Baron MA, Dejager S, Mills D, Schweizer A. Comparison of vildagliptin and rosiglitazone monotherapy in patients with type 2 diabetes: a 24-week, double-blind, randomized trial. Diabetes Care. 2007;30:217–223. doi: 10.2337/dc06-1815. [DOI] [PubMed] [Google Scholar]
- 67.Ahren B, Pacini G, Foley JE, Schweitzer A. Improved meal-related beta-cell function and insulin sensitivity by the dipeptidyl peptidase-IV inhibitor vildagliptin in metformin-treated patients with type 2 diabetes over 1 year. Diabetes Care. 2005;28:1936–1940. doi: 10.2337/diacare.28.8.1936. [DOI] [PubMed] [Google Scholar]
- 68.Amori RE, Lau J, Pittas AG. Efficacy and safety of incretin therapy in type 2 diabetes: systematic review and meta-analysis. JAMA. 2007;298:194–206. doi: 10.1001/jama.298.2.194. [DOI] [PubMed] [Google Scholar]
- 69.Lautar SL, Rojas C, Slusher BS, et al. DPP IV inhibitor blocks mescaline-induced scratching and amphetamine-induced hyperactivity in mice. Brain Res. 2005;1048:177–184. doi: 10.1016/j.brainres.2005.04.069. [DOI] [PubMed] [Google Scholar]
- 70.Pratley RE, Rosenstock J, Pi-Sunyer FX, et al. Management of type 2 diabetes in treatment-naive elderly patients: benefits and risks of vildagliptin monotherapy. Diabetes Care. 2007;30:3017–3022. doi: 10.2337/dc07-1188. [DOI] [PubMed] [Google Scholar]


