Abstract
Background
In Japan, an increase in age-adjusted incidence rates of lung adenocarcinoma (ADC) and a decrease in lung squamous cell carcinoma (SQCC) have been reported.
Methods
The number of lung cancer incidence, age-adjusted rates, and age-specific rates by birth-cohort according to histological type were examined using the data from Osaka Cancer Registry.
Results
The numbers of lung cancer incidence among men and women have increased, particularly in ADC. The age-adjusted incidence rates of ADC among men and women have continuously increased, while those of SQCC and small cell carcinoma (SMCC) turned to decrease since 1990s. A trough of lung cancer incidence rates was observed among men in 1935–39 birth-cohorts. The declining trend appeared in 1955–59 birth-cohorts. Lung cancer incidence rates among women have increased since 1895–99 birth-cohorts, but those rates leveled off or decreased in 1950s birth-cohorts. Trends of ADC by birth-cohort were almost the same as those of all histological types. The SQCC among men peaked in 1915–19 birth-cohorts, and decreased in the subsequent birth-cohorts. The SMCC among men peaked in 1920s birth-cohorts, and decreased or leveled off in the subsequent birth-cohorts.
Conclusions
Lung cancer incidence rates by birth-cohorts were almost parallel to the smoking prevalence. However, those for ADC among young women in 1950s birth-cohorts were not parallel to the smoking prevalence, which requires careful monitoring to confirm such findings.
Key words: lung cancer, incidence, histological type, birth-cohort
BACKGROUND
Lung cancer is the leading cause of cancer deaths in Japan, with 45 927 men and 17 307 women dying from lung cancer in 2006. To date, increase in the incidence rates of lung adenocarcinoma (ADC) and decrease in the incidence rates of squamous cell carcinoma (SQCC) and small cell carcinoma (SMCC) have been reported in Japan (1,2). The same trend has been reported in Western countries also (3–5). Some previous studies reported that there was a trough of lung cancer incidence or mortality in Japanese male 1935–39 birth-cohorts because of the limited cigarette supply just after World War II (6–10). Soda et al. (7) reported the birth-cohort analysis by histological type using Nagasaki Cancer Registry in 2000. However, this study was based on the small number of registered lung cancer cases and excluded the cases without histological diagnoses.
In the present study, we updated the recent trends in lung cancer incidence by histological type and tried to clarify their characteristics by birth-cohort, using the data from Osaka Cancer Registry (OCR) with the large number of lung cancer incidence.
MATERIALS AND METHODS
OCR, which started in 1962, is the population-based cancer registry covering Osaka prefecture (population: 8.8 million, 2005 census). Using OCR data on lung cancer incidence (International Classification of Diseases 10th revision C33–C34) diagnosed between 1975 and 2003, we calculated the number of lung cancer incidence per year, age-adjusted rates and age-specific rates by birth-cohort according to histological type.
Histological types were categorized into three major types: ADC (ICD-O: 8140, 8141, 8200, 8211, 8250, 8251, 8260, 8310, 8323, 8440, 8470, 8480, 8481 and 8490), SQCC (ICD-O: 8050, 8052 and 8070–8076), SMCC (ICD-O: 8041–8045) and the others.
Incident years are divided into 5-year periods: 1975–78, 1979–83, 1989–93, 1994–98 and 1999–2003. Birth years were also divided into 5-year periods. The population data by age group in Osaka prefecture were obtained from the data of Population Census. For age-standardization, the Japanese model population in 1985 was used.
The data from OCR included the cases without specific histological diagnosis: a maximum of 60.4% in 1975–78 and a minimum of 31.4% in 1994–98. Based on the assumption that distributions of histological types in the same sex and age group were the same between those with and without a specific histological type, we compensated for the proportion of cases without a specific histological type. The detailed procedure was followed to the previous study (1); first, the sex-, age (5-year)- and incident year (or birth-cohort)-specific numbers of incidence were calculated for all histological types including the cases without histological diagnosis. Second, the sex-, age (10-year)- and incident year (or birth-cohort)-specific proportion of each histological type among the cases with histological diagnosis were calculated for three major histological types. Finally, the sex-, age (5-year)-, incident year (or birth-cohort)-specific number of incidence were multiplied by the corresponding sex-, age- and incident year (or birth-cohort)-specific proportion to approximate the number of incidence by histological type.
RESULTS
Table 1 shows the trends in the number of lung cancer incidence per year according to histological type. Lung cancer incidence per year for all histological types among men and women increased consistently; from 1086 in 1975–78 to 3487 in 1999–2003 among men and from 395 in 1975–78 to 1482 in 1999–2003 among women. As for histological type, the number of ADC incidence has increased remarkably among men and women. The shift in main histological type among men occurred in the 1990s.
Table 1.
Trends in the number of lung cancer incidence per year according to histological type
| Histological type | Incident year |
|||||
|---|---|---|---|---|---|---|
| 1975–78 | 1979–83 | 1984–88 | 1989–93 | 1994–98 | 1999–2003 | |
| Men | ||||||
| Adenocarcinoma (%) | 372 (34.2) | 510 (35.7) | 696 (35.5) | 853 (35.8) | 1191 (40.2) | 1497 (42.9) |
| Squamous cell carcinoma (%) | 474 (43.6) | 582 (40.8) | 760 (38.8) | 921 (38.6) | 1086 (36.6) | 1208 (34.7) |
| Small cell carcinoma (%) | 142 (13.0) | 203 (14.2) | 315 (16.1) | 410 (17.2) | 483 (16.3) | 543 (15.6) |
| Others (%) | 99 (9.1) | 132 (9.3) | 189 (9.7) | 200 (8.4) | 204 (6.9) | 239 (6.8) |
| All histological types (%) | 1086 (100) | 1428 (100) | 1961 (100) | 2383 (100) | 2964 (100) | 3487 (100) |
| Women | ||||||
| Adenocarcinoma (%) | 242 (61.2) | 328 (60.2) | 453 (58.7) | 579 (60.3) | 792 (64.8) | 996 (67.2) |
| Squamous cell carcinoma (%) | 86 (21.9) | 106 (19.5) | 163 (21.2) | 184 (19.2) | 218 (17.9) | 241 (16.3) |
| Small cell carcinoma (%) | 32 (8.0) | 73 (13.3) | 97 (12.5) | 132 (13.7) | 152 (12.5) | 178 (12.0) |
| Others (%) | 35 (8.9) | 38 (6.9) | 59 (7.6) | 65 (6.8) | 60 (4.9) | 66 (4.4) |
| All histological types (%) | 395 (100) | 545 (100) | 772 (100) | 961 (100) | 1223 (100) | 1482 (100) |
Table 2 shows the trends in the age-adjusted rates according to the histological type. The age-adjusted rates for all histological types peaked in 1994–98 and recently leveled off among men, while those consistently increased among women. The rates for ADC consistently increased among men and women. In contrast, the rates for SQCC and SMCC peaked in 1989–93 among men, and decreased subsequently. Those rates for SQCC and SMCC peaked in 1984–88 and 1989–93, respectively, among women, and decreased subsequently.
Table 2.
Trends in age-adjusted lung cancer incidence rates per 100 000 person-years according to histological type
| Histological type | Incident year |
|||||
|---|---|---|---|---|---|---|
| 1975–78 | 1979–83 | 1984–88 | 1989–93 | 1994–98 | 1999–2003 | |
| Men | ||||||
| Adenocarcinoma | 15.5 | 18.9 | 21.7 | 22.3 | 26.0 | 27.2 |
| Squamous cell carcinoma | 20.4 | 22.2 | 25.0 | 25.6 | 24.8 | 22.3 |
| Small cell carcinoma | 6.0 | 7.6 | 10.1 | 11.1 | 10.8 | 10.0 |
| All histological types | 46.2 | 53.7 | 62.9 | 64.7 | 66.5 | 64.3 |
| Women | ||||||
| Adenocarcinoma | 7.9 | 9.2 | 10.5 | 11.3 | 13.1 | 13.8 |
| Squamous cell carcinoma | 2.8 | 3.0 | 3.8 | 3.5 | 3.4 | 3.1 |
| Small cell carcinoma | 1.0 | 2.0 | 2.3 | 2.5 | 2.4 | 2.3 |
| All histological types | 12.9 | 15.2 | 17.9 | 18.6 | 19.9 | 20.2 |
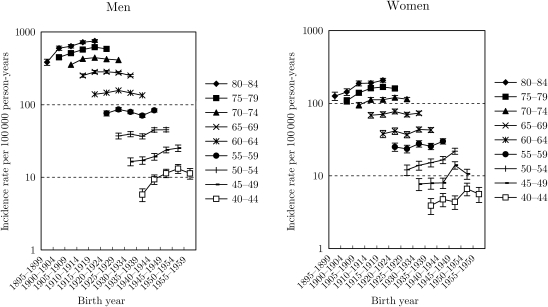
Fig. 1 shows the trends in the age-specific lung cancer incidence rates with 95% confidence interval by birth-cohort for all histological types. Among men, there was a trough in rates for all age groups in 1935–39 birth-cohorts, which was consistent with the previous findings (7,10). In the subsequent birth-cohorts, the rates increased for all age groups, but the declining tendency appeared in 1955–59 birth-cohorts. Among women, the trough in rates in 1935–39 birth-cohorts was not confirmed. The rates for aged ≥50 years increased gradually, while it seemed that the rates for aged <50 years turned to decrease or level off after 1950–54 birth-cohorts: however, these trends were unstable because of the wide confidence intervals due to the small number of incidence.
Figure 1.
Trends in age-group-specific lung cancer incidence rates with 95% confidence interval by birth-cohort for all histological types.
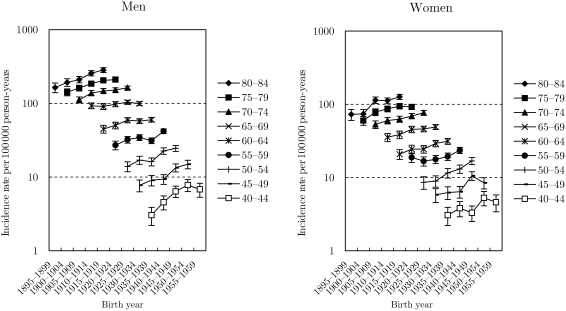
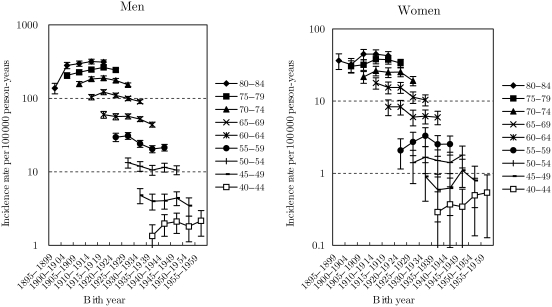
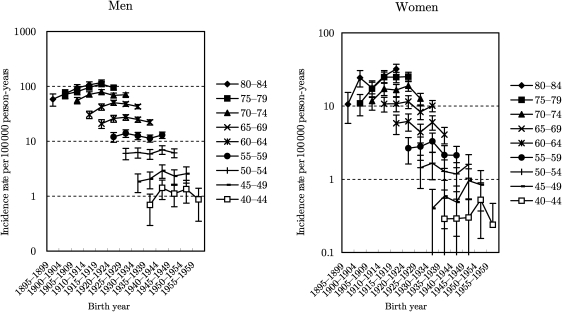
Figs 2–4 show the trends in the age-specific incidence rates with 95% confidence interval by birth-cohort for ADC, SQCC and SMCC, respectively. The rates for ADC among men increased gradually for all age groups, but the declining tendency appeared in 1955–59 birth-cohorts. Furthermore, it seemed that there was a slight trough in rates in 1935–39 birth-cohorts, as well as findings in all histological types. The trends in ADC among women were almost similar with those in all histological types. The rates for SQCC among men peaked in 1910–14 birth-cohorts and decreased in the subsequent birth-cohorts. The trough in rates during 1935–39 birth-cohorts was not clear for SQCC among men. Trends in the rates for SQCC among women aged ≥65 years were similar with those among men. The trends in aged <65 years were, however, unclear because of the wide confidence interval. The rates for SMCC among men peaked around 1920s birth-cohorts and turned to slightly decrease or level off in the subsequent birth-cohorts. The rates for SMCC among women were unclear because of the wide confidence interval.
Figure 2.
Trends in age-group-specific incidence rates with 95% confidence interval by birth-cohort for adenocarcinoma.
Figure 3.
Trends in age-group-specific incidence rates with 95% confidence interval by birth-cohort for squamous cell carcinoma.
Figure 4.
Trends in age-group-specific incidence rates with 95% confidence interval by birth-cohort for small cell carcinoma.
DISCUSSION
In the present study, we reported the population-based trends in lung cancer incidence including birth-cohort analyses according to histological type using OCR. The number of lung cancer incidence per year increased continuously because of the population aging. The main histological type of lung cancer switched from SQCC to ADC among men in 1990s. The declining trends for SQCC and SMCC continued in the updated present study.
Smoking prevalence by birth-cohort among Japanese men was reported to have two peaks: around the 1925 birth-cohort and around the 1950 birth-cohort (11). In addition, there was a trough of smoking prevalence in 1930–40 birth-cohorts because of the limited cigarette supply just after World War II (11). In general, the trends in lung cancer incidence or mortality by birth-cohort were parallel to the trends in the smoking prevalence. Our results were consistent with the findings from previous studies, showing that lung cancer mortality and incidence rates among men in 1935–39 birth-cohorts were lower than the subsequent birth-cohorts (6–10). Since the smoking prevalence among Japanese men was declining after 1950s birth-cohorts, the appearance of declining trends of lung cancer incidence among men in 1955–59 birth-cohorts was an expected result.
Classically, smoking behavior was considered to be more strongly associated with SQCC than with ADC. However, SQCC incidence rates by birth-cohort among men were not parallel to the smoking prevalence by birth-cohort. SQCC incidence rates among men after 1940–44 birth-cohorts leveled off, whereas the smoking prevalence among men after 1940–44 birth-cohorts increased. One reason would be the switching from non-filtered cigarettes to filtered cigarettes. Filtered cigarettes were considered to be associated with peripheral ADC because of the deep inhalation (3,12,13). According to the information from Japan Tobacco Inc., the switching from non-filtered cigarettes to filtered cigarettes occurred in the 1960s in Japan (14). The shift from SQCC to ADC among men in 1990s observed in the present study might have been the result of this shift in cigarette types.
The smoking prevalence by birth-cohort among women is continuously increasing after 1930s birth-cohorts (11). However, lung cancer incidence among women in 1950s birth-cohorts, particularly for ADC, seemed to be leveling off or decreasing. Marugame et al. (15) also reported the trends in lung cancer mortality by birth-cohort using the National Vital Statistics. In that study, lung cancer mortality trends appeared to be decreasing for female birth-cohorts born after 1960. Although our results were unstable because of the wide confidence intervals, those were not contradictory to this previous study. There is no clear explanation for these findings among younger Japanese women. There would be some factors other than active smoking for lung cancer incidence among them.
The present study has some limitations. First, there may be some missing cases in the OCR. The proportion of death certificate only for lung cancer in OCR was 19.3% in 1998–2002 (16). Therefore, lung cancer incidence may be under-estimated as a whole. Secondly, the trends by histological type among young women were unstable because of the small number of incidence; the number of lung cancer incidence among women aged <50 years per year was ∼80 cases in 2003. Finally, the data from OCR included many lung cancer cases without specific histological diagnoses. We had to use assumption in order to calculate the number of incidence according to histological type. The proportions of lung cancer cases without histological diagnoses for aged <80 years decreased between 1975–78 and 1999–2003; from 49.7 to 16.4% among aged 40–49 years, from 47.4 to 17.6% among aged 50–59 years, from 55.9 to 22.7% among aged 60–69 years and from 70.8 to 31.6% among aged 70–79 years, respectively. However, those for aged ≥80 years were still high: from 78.9 to 64.1%. Therefore, we require carefulness to interpret the findings, particularly for elderly.
In conclusion, we reported recent trends in lung cancer incidence according to histological type. The increase in ADC incidence and the decrease in SQCC and SMCC incidence were confirmed. The trends in lung cancer incidence among young women in 1950s birth-cohorts, particularly for ADC, were not parallel to the smoking prevalence. We need careful monitoring of the trends in lung cancer incidence, particularly for ADC among young women.
Funding
Funding to pay the Open Access publication charges for this article was provided by ‘The Third-Term Comprehensive 10-Year Strategy for Cancer Control’, Ministry of Health, Labour and Welfare, Japan.
Conflict of interest statement
None declared.
Acknowledgements
We would like to thank Dr Akira Oshima, Cancer Patients Information Service, Osaka Medical Center for Cancer and Cardiovascular Disease, and Professor Hiroyasu Iso, Public Health, Department of Social and Environmental Medicine, Osaka University Graduate School of Medicine for their valuable comments. We also thank the staff at the Osaka Cancer Registry for data collection.
References
- 1.Sobue T, Ajiki W, Tsukuma H, Oshima A, Hanai A, Fujimoto I. Trend of lung cancer incidence rate by histological type: a population-based study in Osaka, Japan. Jpn J Cancer Res. 1999;90:6–15. doi: 10.1111/j.1349-7006.1999.tb00659.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yoshimi I, Ohshima A, Ajiki W, Tsukuma H, Sobue T. A comparison of trends in the incidence rate of lung cancer by histological type in the Osaka Cancer Registry, Japan and in the Surveillance, Epidemiology and Ends Results Program, USA. Jpn J Clin Oncol. 2003;33:98–104. doi: 10.1093/jjco/hyg019. [DOI] [PubMed] [Google Scholar]
- 3.Thun MJ, Lally CA, Flannery JT, Calle EE, Flanders WD, Heath CW., Jr Cigarette smoking and changes in the histopathology of lung cancer. J Natl Cancer Inst. 1997;89:1580–6. doi: 10.1093/jnci/89.21.1580. [DOI] [PubMed] [Google Scholar]
- 4.Devesa SS, Bray F, Vizcaino AP, Parkin DM. International lung cancer trends by histological type: male:female differences diminishing and adenocarcinoma rising. Int J Cancer. 2005;117:294–9. doi: 10.1002/ijc.21183. [DOI] [PubMed] [Google Scholar]
- 5.Janssen-Heijnen MLG, Coebergh JWW. The changing epidemiology of lung cancer in Europe. Lung Cancer. 2003;41:245–58. doi: 10.1016/s0169-5002(03)00230-7. [DOI] [PubMed] [Google Scholar]
- 6.Marugame T, Sobue T. Mortality trend of mouth and pharynx, esophagus, stomach, larynx and lung cancer in Japan by birth-cohort. Jpn J Clin Oncol. 2004;34:432–8. doi: 10.1093/oxfordjournals.jjco.a003407. [DOI] [PubMed] [Google Scholar]
- 7.Soda H, Oka M, Soda M, Nakatomi K, Kawabata S, Suenaga M, et al. Birth-cohort effects on incidence of lung cancers: a population-based study in Nagasaki, Japan. Jpn J Cancer Res. 2000;91:960–5. doi: 10.1111/j.1349-7006.2000.tb00871.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaneko S, Ishikawa KB, Yoshimi I, Marugame T, Hamashima C, Kamo K, et al. Projection of lung cancer mortality in Japan. Cancer Sci. 2003;94:919–23. doi: 10.1111/j.1349-7006.2003.tb01376.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.IARC hand books of cancer prevention, Tobacco control volume 11. International Agency for Research on Cancer, World Health Organization; 2007. Trends in lung cancer mortality in selected countries; pp. 307–22. [Google Scholar]
- 10.Tsukuma H, Ajiki W, Oshima A. Cancer incidence in Japan. Gan To Kagaku Ryoho. 2004;31:840–6. (in Japanese) [PubMed] [Google Scholar]
- 11.Marugame T, Kamo K, Sobue T, Akiba S, Mizuno S, Satoh H, et al. Trends in smoking by birth-cohorts born between 1900 and 1977 in Japan. Preventive Med. 2006;42:120–7. doi: 10.1016/j.ypmed.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 12.Stellman SD, Muscat JE, Thompson S, Hoffmann D, Wynder EL. Risk of squamous cell carcinoma and adenocarcinoma of the lung in relation to lifetime filter cigarette smoking. Cancer. 1997;80:382–8. doi: 10.1002/(sici)1097-0142(19970801)80:3<382::aid-cncr5>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 13.Marugame T, Sobue T, Nakayama T, Suzuki T, Kuniyoshi H, Sunagawa K, et al. Filter cigarette smoking and lung cancer risk; a hospital-based case–control study in Japan. Br J Cancer. 2004;90:646–51. doi: 10.1038/sj.bjc.6601565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hanai A, Benn T, Fujimoto I, Muir CS. Comparison of lung cancer incidence rates by histological type in high and low incidence countries, with reference to the limited role of smoking. Jpn J Cancer Res. 1988;79:445–52. doi: 10.1111/j.1349-7006.1988.tb01612.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marugame T, Yoshimi I, Kamo K, Imamura Y, Kaneko S, Mizuno S, et al. Trends in lung cancer mortality among young adults in Japan. Jpn J Clin Oncol. 2005;35:177–80. doi: 10.1093/jjco/hyi054. [DOI] [PubMed] [Google Scholar]
- 16.Curado MP, Edwards B, Shin HR, Storm H, Ferlay J, Heanue M, et al. IX. Lyon: IARC; 2007. Cancer Incidence in Five Continents. IARC Scientific Publications No. 160 Available at http://www-dep.iarc.fr/CI5-IX/PDF/INDICES/I_07.pdf . [Google Scholar]