Abstract
The fission yeast Sty1 mitogen-activated protein (MAP) kinase (MAPK) and its activator the Wis1 MAP kinase kinase (MAPKK) are required for cell cycle control, initiation of sexual differentiation, and protection against cellular stress. Like the mammalian JNK/SAPK and p38/CSBP1 MAPKs, Sty1 is activated by a range of environmental insults including osmotic stress, hydrogen peroxide, UV light, menadione, heat shock, and the protein synthesis inhibitor anisomycin. We have recently identified two upstream regulators of the Wis1 MAPKK, namely the Wak1 MAPKKK and the Mcs4 response regulator. Cells lacking Mcs4 or Wak1, however, are able to proliferate under stressful conditions and undergo sexual differentiation, suggesting that additional pathway(s) control the Wis1 MAPKK. We now show that this additional signal information is provided, at least in part, by the Win1 mitotic regulator. We show that Wak1 and Win1 coordinately control activation of Sty1 in response to multiple environmental stresses, but that Wak1 and Win1 perform distinct roles in the control of Sty1 under poor nutritional conditions. Our results suggest that the stress-activated Sty1 MAPK integrates information from multiple signaling pathways.
INTRODUCTION
One of the most common mechanisms by which eucaryotic cells sense and respond to changes in the extracellular environment is via activation of a mitogen-activated protein (MAP) kinase cascade. Signal transduction through MAPK cascades involves sequential phosphorylation and activation of three distinct kinases; the MAP kinase kinase kinase (or MAPKKK), the MAP kinase kinase (or MAPKK), and the MAP kinase (MAPK) itself. Although the precise mechanisms by which plasma membrane-associated receptors induce activation of the MAPKKK are still unclear, MAPKKK activation leads to MAPKK activation by direct phosphorylation. The MAPKK, in turn, activates the MAPK by dual phosphorylation on two closely spaced residues, a threonine and a tyrosine. The most widely studied of the MAPKs in mammalian cells is the ERK family of kinases, which are activated by a wide range of peptide growth factors and hormones. More recently, a new family of MAPKs has been identified in metazoan cells that are activated by a variety of stress conditions including osmotic stress, heat shock, oxidative stress, UV radiation, and the protein synthesis inhibitor anisomycin, as well as by inflammatory cytokines and certain vasoactive neuropeptides (Dérijard et al., 1994; Galcheva-Gargova et al., 1994; Han et al., 1994; Kyriakis et al., 1994; Lee et al., 1994; Rouse et al., 1994). Pharmacological, biochemical, and genetic evidence indicates multiple roles for these stress-activated MAPKs (SAPKs) in a wide variety of physiological and pathological conditions including development, control of cell proliferation, cell death, inflammation, and response to ischemic injury. As such, these enzymes are drawing considerable attention as potential targets for novel therapeutics. The mechanism(s) by which this class of MAPK is activated is, however, unknown.
We have identified a stress-activated MAPK pathway in the unicellular fission yeast, Schizosaccharomyces pombe, the central elements of which are the Sty1 MAPK (also known as Spc1 and Phh1) and the Wis1 MAPKK (Warbrick and Fantes, 1991; Millar et al., 1995; Shiozaki and Russell, 1995; Kato et al., 1996). Importantly, the fission yeast Sty1 MAPK, like its mammalian counterparts, is activated by a range of environmental stimuli including osmotic stress, oxidative stress, UV light, certain DNA-damaging agents, heat shock, and the protein synthesis inhibitor anisomycin (Millar et al., 1995; Shiozaki and Russell, 1995; Degols et al., 1996, Degols and Russell, 1997; Shieh et al., 1997). This suggests that an evolutionarily conserved sensor may regulate both the mammalian and fission yeast SAPKs. This possibility is consistent with the recent finding that a direct phosphorylation target of fission yeast Sty1 is the Atf1 transcription factor, a structural homologue of human ATF2 that binds and is activated by the SAPKaII/JNK2 MAPK (Gupta et al., 1995; Shiozaki and Russell, 1996; Wilkinson et al., 1996). The Sty1 MAPK controls multiple cellular events in fission yeast including the initiation of sexual differentiation, prolonged viability in stationary phase, and the cellular response to environmental stress. Cells deleted for Atf1 also display many of these phenotypes, suggesting Atf1 is a physiologically important target for Sty1 (Shiozaki and Russell, 1996; Wilkinson et al., 1996). Importantly, cells lacking wis1 or sty1 are highly elongated at cell division. Since the timing of mitotic initiation in fission yeast requires attainment of a critical cell size, these observations suggest a crucial role for the stress-activated Sty1 MAPK pathway in control of the cell cycle (Nurse, 1975; Warbrick and Fantes, 1991; Millar et al., 1995; Shiozaki and Russell, 1995). Since mitotic initiation is triggered by activation of the catalytic subunit of the Cdc13 (Cyclin B)/Cdc2 kinase, genes that when mutated alter cell size at division are, by inference, required for the correct timing of Cdc2 activation. Two such genes are the wee1 and cdc25 mitotic regulators, which code for a tyrosine kinase and phosphatase, respectively, that directly control the activity of the Cdc13/Cdc2 complex (Russell and Nurse, 1987; Millar and Russell, 1992). At present, the mechanism by which the Sty1 MAPK influences mitotic initiation is unknown, but it is likely to be independent of both the Wee1 tyrosine kinase and Cdc25 protein phosphatase, since wis1 and sty1 mutations can reverse the suppression of cdc25–22 temperature-sensitive mutants by wee1 inactivation (Warbrick and Fantes, 1991; Shiozaki and Russell, 1995).
We have found that some of the upstream components of the fission yeast Sty1 pathway are structurally and functionally similar to those of the budding yeast HOG1 pathway, which is activated only by osmotic stress (Brewster et al., 1993; Schüller et al., 1994). These are the Mcs4 mitotic catastrophe suppressor and Wak1 MAPKKK (also know as Wik1). Mcs4 and Wak1 are structurally and functionally similar to the budding yeast SSK1 response regulator and SSK2/SSK22 MAPKKKs from budding yeast, respectively (Maeda et al., 1994, 1995; Shieh et al., 1997; Shiozaki et al., 1997). SSK1 acts as part of a “two-component phospho-relay system” that is initiated by inactivation of a transmembrane osmosensor, the SLN1 histidine kinase (Ota and Varshavsky, 1993; Posas et al., 1996). These results indicate that the fission yeast SAPK pathway is also controlled by a two-component system. It is not clear, however, whether the two-component system is responsible for activation of Sty1 by multiple stresses or whether additional pathways exist (Shieh et al., 1997). Importantly neither Wak1 nor Mcs4, however, are absolutely required for proliferation in stressful conditions, suggesting that additional elements do control the Wis1 MAPKK.
We have sought additional regulators of Sty1 with the goal of understanding how the pathway is activated by multiple environmental stresses. We focused initially on a recessive mutant, win1–1, that was found to be required for cell division in the simultaneous absence of both the Cdc25 phosphatase and Wee1 tyrosine kinase (Ogden and Fantes, 1986). Importantly, the cell cycle arrest phenotype of a wee1–50 cdc25–22 win1–1 strain is suppressed by overexpression of the Wis1 MAPKK (Warbrick and Fantes, 1991). In this paper we show that the Win1 mitotic regulator is a component of the Sty1 pathway and contributes to activation of Sty1 MAPK by multiple environmental stimuli.
MATERIALS AND METHODS
Media and General Techniques
Media and genetic methods for studying fission yeast have been reviewed recently (Moreno et al., 1991). General DNA methods were performed using standard techniques (Sambrook et al., 1989). Cell length measurements were made using log-phase cells with a Nikon (Garden City, NY) filar eyepiece drum micrometer at 1200× magnification. Transformations were regularly performed by the lithium acetate method (Moreno et al., 1991) or by electroporation (Prentice, 1991) using a Bio-Rad (Richmond, CA) Gene Pulser.
Assessment of Mating Efficiency
Homothallic (h90) cells were grown to log phase in liquid Edinburgh mimimal media (EMM) and then incubated in the same medium lacking NH4Cl as a nitrogen source. Mating efficiency was determined microscopically by the appearance of cells undergoing conjugation or spore-containing asci.
Overexpression of Tagged Wak1 Protein
The catalytic domain of the wak1 gene was cloned by polymerase chain reaction (PCR) amplification from S. pombe genomic DNA. The 5′ oligonucleotide TAACTAGATCTATGGCTTTCTGTTAACGCAT incorporated a BglII site (shown italicized) and hybridized to sequences 5′ to the catalytic domain, whereas the 3′ oligonucleotide TATTAGCGGCCGCGGTCAACACTATAGTTTATTGTG incorporated a NotI site (shown italicized) and hybridized to sequences surrounding the TGA termination codon. PCR amplification generated a fragment that was cleaved with BglII and NotI and cloned into the BglII and NotI sites of pREP41*(6HisHA) downstream of an attenuated version of the nmt1 promoter (Basi et al., 1993) to form pREP41-wak1(6HisHA). Expression of this protein in S. pombe was confirmed by Western blot analysis.
Integration and Detection of Tagged Sty1 Protein
A C-terminal fragment of a 6-six histidine and hemagglutinin (HA)- tagged sty1 gene was excised from pREP41-sty1(6HisHA) by digesting with NruI and BamHI (Millar et al., 1995) and cloned into the SmaI and BamHI sites of pBSSK-Ura4 to generate pBSSK-Ura4-sty1(6HisHA). pBSSK-Ura4-sty1(6HisHA) was linearized with PacI, and the resulting fragment was transformed into wild-type S. pombe cells bearing the ura4-D18 auxotrophic marker. Stable integration of the tagged sty1 gene at the genomic sty1 locus was confirmed by Southern blot analysis and PCR.
Detection of Tagged Sty1 Protein
The Sty1 protein was partially purified from cells bearing an integrated tagged version of sty1 (see above) using Ni++-nitrilo-tri-acetic acid (NTA) agarose exactly as previously described (Millar et al., 1995). Precipitated proteins were resolved by SDS-PAGE and transferred electrophoretically to nitrocellulose membranes. Membranes were probed with either a monoclonal antibody to the HA epitope (12CA5) or with a monoclonal antibody to phosphotyrosine (4G10, Upstate Biotechnology, New York, NY). Detection was performed using a peroxidase-conjugated anti-mouse IgG (Amersham, Buckinghamshire, U.K.) and enhanced chemiluminescence visualization (ECL, Amersham) according to the manufacturer’s instructions.
Assay of Endogenous Sty1 Kinase Activity
Endogenous Sty1 kinase was precipitated from cell extracts and activity was assayed using a glutathione-S-transferase (GST)-Atf1 fusion protein prebound to glutathione beads as previously described (Shieh et al., 1997). Protein concentration in cell extracts was measured by the Lowry assay and adjusted before precipitation. Precipitated proteins were washed three times with lysis buffer containing 0.5 M NaCl, washed once with kinase assay buffer without ATP, and then incubated in kinase assay buffer containing 50 mM HEPES, pH 7.4, 10 mM MgCl2, 10 mM MnCl2, 10 mM EGTA, 10 mM β-mercaptoethanol, 0.2 μCi/ml γ-32P-ATP for 20 min at 30°C. Reactions were terminated by the addition of SDS-sample buffer, and the products were separated by SDS-PAGE. Phosphorylation of Atf1 was determined by autoradiography.
Analysis of DNA Content by Flow Cytometry
Samples containing ∼107 cells were fixed with 70% ethanol, treated successively with RNase and pepsin, and stained with 50 mg/ml propidium iodide essentially as previously described (Corliss and White, 1981). DNA content was then analyzed with a Becton Dickinson (Oxford, U.K.) FACScan and CELL Quest software.
RNA Isolation and Hybridization
To isolate RNA, S. pombe cells were cultured in YEPD to exponential phase. Approximately 10 μg of total RNA were isolated and resolved by agarose gel electrophoresis before transfer to nitrocellulose for hybridization as previously described (Aves et al., 1985). Probes for pyp2 and ctt1 were as previously described (Millar et al., 1995; Takeda et al., 1995).
RESULTS
Evidence for an Additional Pathway Controlling the Wis1 MAPKK
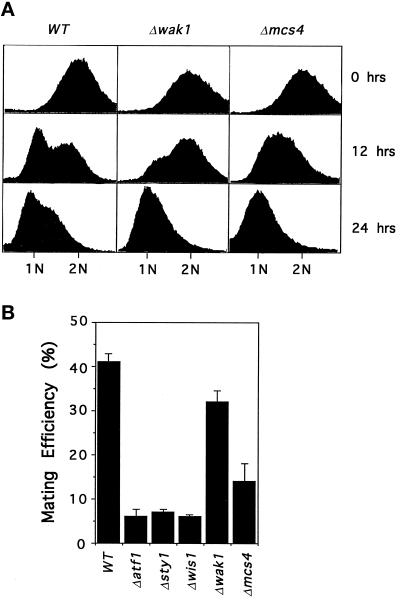
In poor nutritional conditions fission yeast cells enter a quiescent state either in the G1 or G2 phases of the cell cycle. In defined media, arrest in the G1 phase of the cell cycle can be promoted by depletion of an exogenous nitrogen source. Under these conditions cells lacking either the Wis1, Sty1, or Atf1 proteins fail to arrest in G1 and arrest preferentially in G2 (Takeda et al., 1995; Kato et al., 1996; Shiozaki and Russell, 1996). We have assessed the role of two upstream regulators of the Wis1-Sty1-Atf1 cascade, the Wak1 MAPKKK and Mcs4 response regulator, in this process. Analysis of DNA content by fluorescence-activated cell sorter indicates that cells deleted for either wak1 or mcs4 arrest normally with a 1 N content of DNA after either 12 or 24 h incubation in nitrogen-free medium, indicating a G1 arrest (Figure 1A). In contrast, approximately only 5% of Δwis1 or Δsty1 cells arrest in G1 under the same conditions as previously observed (our unpublished data; Shiozaki and Russell, 1996). In homothallic (h90) strains, entry into the G1 phase is accompanied by sexual conjugation and differentiation. Since the Wis1 MAPKK is required for proper arrest in G1, cells lacking either the Wis1, Sty1, or Atf1 proteins are severely defective in their ability to mate (Takeda et al., 1995; Kato et al., 1996; Figure 1B). In contrast, cells lacking the Wak1 MAPKKK are able to mate almost as effectively as wild-type cells (Figure 1B). Similarly, cells lacking Mcs4 are also able to mate more effectively than cells lacking Wis1, Sty1, or Atf1, pointing to the existence of an alternative pathway controlling the Wis1 MAPKK that is independent of either Wak1 or Mcs4 proteins. Mutants in the Sty1 pathway lose viability in stationary phase, which is likely to contribute to the mating deficiency (see below).
Figure 1.
Mcs4 and Wak1 are not required for sexual differentiation. (A) Cells lacking mcs4 or wak1 enter the G1 phase efficiently in nitrogen-limiting medium. Log phase cultures of wild-type (WT) (PR109), wak1::ura4 (Δwak1) (JM1436), or mcs4::his7 (Δmcs4) (JM 1468) cells growing in EMM medium at 30°C were analyzed for DNA content before (0 h) and after (12 or 24 h) incubation in the same medium lacking NH4Cl. (B) Mating efficiency of cells lacking Wak1 or Mcs4. Homothallic cultures of wild-type (WT) (JY878), atf1::ura4 (Δαtf1) (NT147), sty1::ura4 (Δsty1) (JM1263), wis1::ura4 (Δwis1) (JM1260), wak1::ura4 (Δwak1) (JM1505), or mcs4::ura4 (Δmcs4) (JM1355) cells were grown to log phase in liquid EMM and then transferred to the same medium lacking NH4Cl for 24 h at 30°C. Mating efficiency was assessed microscopically.
Win1 Mitotic Regulator Interacts Genetically with Components of the Sty1 Pathway
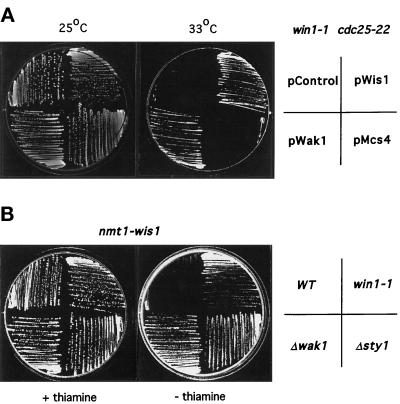
Cells deleted for either the Sty1 MAPK or Wis1 MAPKK are delayed in the timing of mitotic initiation. Since Δmcs4 and Δwak1 cells are shorter at division than Δwis1 or Δsty1 cells, we presumed that other genes controlling Wis1 may also control the timing of mitotic initiation. For this reason we focused initially on the win1 mitotic regulator in an attempt to identify other components of the Sty1 pathway (Ogden and Fantes, 1986). To examine genetic interactions of a win1–1 mutant with components of the Sty1 pathway, two approaches were taken: first, win1–1 cdc25–22 cells were transformed with plasmids expressing either the wis1, wak1, or the mcs4 genes. At 33°C win1–1 cdc25–22 cells undergo cell cycle arrest, whereas win1–1 and cdc25–22 single mutants are able to proliferate normally (our unpublished data). Overexpression of either wis1 or wak1 could suppress the cell cycle arrest of a win1–1 cdc25–22 strain at 33°C, indicating that ectopically increasing the activity of the Sty1 MAPK can bypass the mitotic delay of a win1–1 mutant. (Figure 2A). We have subsequently noticed that the restriction map of wak1 is identical to a previously identified multicopy suppressor of win1–1, namely wis4 (Warbrick and Fantes, 1992). In contrast, overexpression of mcs4 was unable to suppress the win1–1 cdc25–22 arrest, the reasons for which are discussed below.
Figure 2.
Win1 interacts with components of the StyI MAPK pathway. (A) Win1–1 is suppressed by wak1 but not mcs4. win1–1 cdc25–22 cells (JM1354) were transformed either with a control plasmid pREP41 (Control), pREP41-wis1 (pWis1), pREP41-wak1 (pWak1), or pREP41-mcs4 (pMcs4), and gene expression was regulated via the thiamine-repressible nmt1 promoter. Transformants were grown and streaked to minimal medium lacking thiamine and leucine and cultured for 3 d at either 25°C (left hand plate) or 33°C (right hand plate). (B) Loss of win1 suppresses overexpression of wis1. Wild-type (PR109), win1–1 (win1–1) (JM1413), wak1::ura4 (Δwak1) (JM 1436), or sty1::ura4 (Δsty1) (JM 1160) cells were transformed with pREP1-wis1. Transformants were selected on minimal medium lacking leucine containing 10 μM thiamine and then streaked onto the same medium (+ thiamine) or the same medium lacking thiamine (−thiamine), and growth of the cells was monitored after 3 d at 30°C.
In a second genetic test to assess the role of win1, wild-type, win1–1 mutants, or cells deleted for either wak1 or sty1 were transformed with a vector expressing wis1 from the strong thiamine-repressible nmt1 promoter (Maundrell, 1991). Hyperactivation of the Sty1 MAPK by massive overexpression of the Wis1 MAPKK is toxic to wild-type cells but not, for instance, to mcs4–13 cells, which are defective in Wis1 activation (Shieh et al., 1997). As the results in Figure 2B show, massive overexpression of wis1 is also not toxic in either win1–1 cells or in cells lacking either the Wak1 MAPKKK or Sty1 MAPK. These results confirm that win1 has an important role in controlling mitotic initiation in fission yeast and further suggest that win1 acts either as an activator or downstream target of the Sty1 pathway.
Win1 Controls Stress-induced Gene Expression and Activation of the Sty1 MAPK
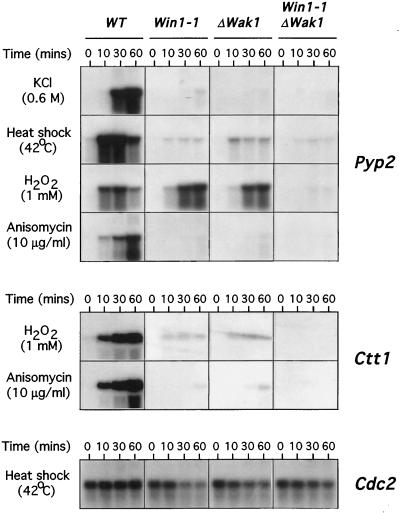
Activation of the Wis1-Sty1-Atf1 pathway by a variety of environmental insults including osmotic stress, heat shock, oxidative stress, and anisomycin causes induction of a number of genes including the pyp2 MAPK phosphatase, which acts in a feedback loop to attenuate Sty1 activity (Millar et al., 1992, 1995; Degols et al., 1996; Wilkinson et al., 1996). To examine whether win1 is required for activation of the Sty1 MAPK pathway, wild-type or win1–1 cells were incubated in the presence of either 0.6 M KCl, 1 mM H2O2, 10 μg/ml anisomycin or given a mild heat shock for various times and then the level of pyp2 expression was examined by Northern blot analysis. As the results in Figure 3, top, show, induction of pyp2 was dramatically reduced in win1–1 cells in response to osmotic or heat shock or to anisomycin, although significant delayed expression of the pyp2 mRNA was observed after stimulation with hydrogen peroxide (Figure 3). Notably, induction of pyp2 mRNA by these stresses is absolutely dependent on the Sty1 MAPK, as not even residual induction is observed in Δsty1 cells (Shieh et al., 1997). Northerns were reprobed with the cdc2, act1, or wis1 genes, the corresponding mRNA of which were not altered in win1–1 cells or in response to stress (Figure 3, bottom; our unpublished data). To confirm these results, we took advantage of previous observations that the catalase (ctt1) gene is also under control of the Wis1-Sty1-Atf1 pathway (Wilkinson et al., 1996; Shieh et al., 1997). Wild-type or win1–1 cells were stimulated with either 10 μg/ml anisomycin or 1 mM hydrogen peroxide and the induction of ctt1 was analyzed by Northern blot (Figure 3, middle). Stress-induced expression of ctt1 was also dramatically reduced in win1–1 cells relative to wild type, indicating that Win1 is required for induction of gene expression by multiple environmental stresses.
Figure 3.
Win1 is required for stress-induced gene expression. Expression of the Pyp2 MAPK phosphatase is attenuated in cells lacking win1. Top panel, log phase cultures growing in YEPD at 30°C of either wild-type (WT), (PR109), win1–1 (win1–1) (JM1413), wak1::ura4 (Δwak1) (JM 1436), or wak1::ura4 win1–1 (Δwak1 win1–1) (JM1504) cells were incubated in the same medium containing 0.6 M KCl, 1 mM H2O2, 10 μg/ml anisomycin or shifted to 42°C for the times indicated. Total RNA was extracted, and equal quantities were separated by electrophoresis and then probed using DNA specific to the pyp2 gene. Middle panel, induction of catalase (ctt1) is attenuated in cells lacking win1–1. Log phase cultures of wild type (WT) (PR109), win1–1 (win1–1) (JM1413), wak1::ura4 (Δwak1) (JM 1436), or wak1::ura4 win1–1 (Δwak1 win1–1) (JM1504) cells growing in YEPD were incubated in the same medium containing either 10 μg/ml anisomycin or 1 mM H2O2 for the times indicated. In this experiment total RNA was extracted as described previously and probed using DNA specific to the ctt1 gene. Bottom panel, blots were reprobed with a cdc2-specific probe to verify equal loading of RNA.
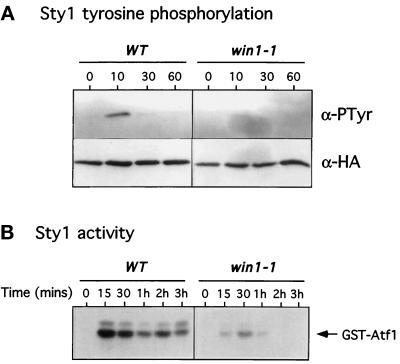
To assess whether the effect of win1–1 on stress-induced gene expression is at the level of transcription or via controlling the activity of the Sty1 MAPK, we measured both phosphotyrosine content and activity of Sty1. Strains bearing a single integrated C-terminally epitope-tagged sty1 gene in either wild-type or a win1–1 background were constructed. The Sty1 protein was affinity precipitated from log phase cultures of these strains after challenge with 0.6 M KCl. The phosphorylation state of Sty1 was assessed by Western blot using a monoclonal antibody to phosphotyrosine. Figure 4A demonstrates that the increase in phosphotyrosine on the Sty1 protein is dramatically reduced in win1–1 cells relative to wild type. Duplicate samples probed using a monoclonal antibody to the epitope (HA) tag showed that the level of protein did not change through the course of the experiment (Figure 4 A). Similar results were obtained when cells were stimulated with other stresses (our unpublished data). To confirm this result the activity of endogenous untagged Sty1 protein was determined by a coupled affinity precipitation-kinase assay using a GST-Atf1 fusion protein as a substrate, as previously described (Wilkinson et al., 1996). Wild-type and win1–1 cells were heat shocked at 42°C for various times, and the Sty1 protein was precipitated and its kinase activity determined. As the results in Figure 4B show, stimulation of Sty1 by heat shock was also dramatically reduced in win1–1 cells, although some residual induction was evident. Similar results were found when cells were challenged with either an osmotic stress or the protein synthesis inhibitor anisomycin (our unpublished data). Together these results indicate that Win1 is required for Sty1 MAPK activation in response to multiple independent environmental stresses.
Figure 4.
Win1 is required for stress-induced activation of the Sty1 MAPK. (A) Tyrosine phosphorylation of StyI in cells lacking win1. Log phase cultures of wild-type (WT) (JM1520) or win1–1 (win1–1) (MW 1539) cells bearing an integrated and epitope-tagged version of Sty1 were incubated in medium containing 0.6 M KCl for the times indicated. Approximately 2 × 108 cells were harvested and lysed at each time point, and the Sty1 protein was precipitated using Ni++-NTA agarose. Precipitates were probed by Western blot for the presence of phosphotyrosine (α-pTyr) or the HA epitope tag (α-HA). (B) Activation of Sty1 in cells lacking win1. Log phase cultures of either wild-type (WT) (PR109) or win1–1 (win1–1) (JM1413) cells growing in YEPD at 30°C were shifted to 42°C for the times indicated. Cell extracts were prepared as above, and Sty1 was precipitated with GST-Atf1 prebound to glutathione beads. Precipitates were washed extensively and incubated in the presence of [γ-32P]-ATP for 20 min at 30°C. Phosphorylation of Atf1 was visualized after SDS-PAGE and autoradiography.
Win1 and the Wak1 MAPKKK Cooperative to Control Activation of the Sty1 MAPK
Both Wak1 and Win1 are required for the correct timing of mitotic initiation (Ogden and Fantes, 1986; Shieh et al., 1997; Shiozaki et al., 1997). To assess the relationship of Win1 to the Wak1 MAPKKK, double Δwak1 win1–1 mutants were constructed and cell size at divison was analyzed. We observe that double mutant Δwak1 win1–1 cells divide at 21.4 + 1.8 μm, larger than either Δwak1 cells (16.5 + 0.5 μm) or win1–1 single mutants (17.1 ± 1.1 μm), indicating that the effect of Wak1 and Win1 on the timing of mitotic initiation is additive. To examine the relationship of wak1 and win1 in controlling Sty1 MAPK function, stress-induced expression of the pyp2 and ctt1 genes was determined in double Δwak1 win1–1 mutants and in Δwak1 and win1–1 single mutants. Expression of both pyp2 and ctt1 was virtually abolished in single wak1 and win1–1 mutants in response to osmotic stress, heat shock, or anisomycin (Figure 3). However, we found that considerable residual expression of pyp2 and to a lesser extent ctt1 was observed in both single mutants in response to hydrogen peroxide (Figure 3). Importantly this residual expression was also lost in double Δwak1 win1–1 mutants, suggesting that win1 contributes to acute activation of Sty1 in the presence or absence of wak1 (Figure 3).
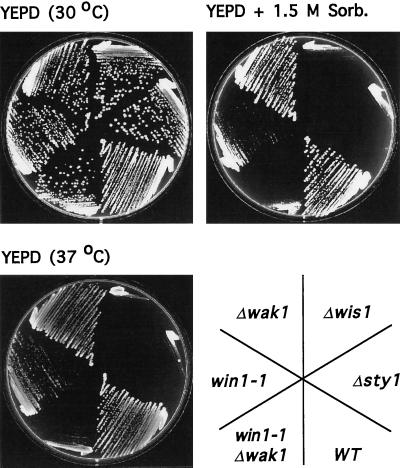
We have previously shown that Wak1 is not required for long-term survival either at high temperature or in high osmolarity medium (Shieh et al., 1997; Shiozaki et al., 1997). To assess the role of win1 in the long-term response of the cell to environmental stress, wild-type cells, Δwak1 and win1–1 single mutants, or Δwak1 win1–1 double mutants were grown either on rich medium at 30°C, on the same medium containing 1.5 M sorbitol, or on the same medium at 37°C. As previously observed neither Δwis1 nor Δsty1 cells were able to grow at high temperature or under hyperosmolar conditions whereas Δwak1 cells were unaffected (Millar et al., 1995; Figure 5). In contrast, win1–1 cells grew poorly at high temperature or on high osmolarity medium, and this effect was exacerbated in Δwak1 win1–1 double mutants (Figure 5). Together, these results indicate that Wak1 and Win1 act in concert to control stress-induced activity of the Sty1 MAPK in response to multiple environmental stimuli.
Figure 5.
Win1 and Wak1 act in concert to control stress resistance. Wild-type (WT) (PR109), sty1::ura4 (Δsty1) (JM 1160), wis1::ura4 (Δwis1) (JM 544), wak1::ura4 (Δwak1) (JM 1436), win1–1 (win1–1) (JM1413), or wak1::ura4 win1–1 (Δwak1 win1–1)(JM 1504) cells were grown on YEPD and then streaked to the same medium at 30°C (top left plate) or to the same medium containing 1.5 M sorbitol at 30°C (top right plate) or to YEPD at 37°C (bottom left plate) and incubated for 2 d.
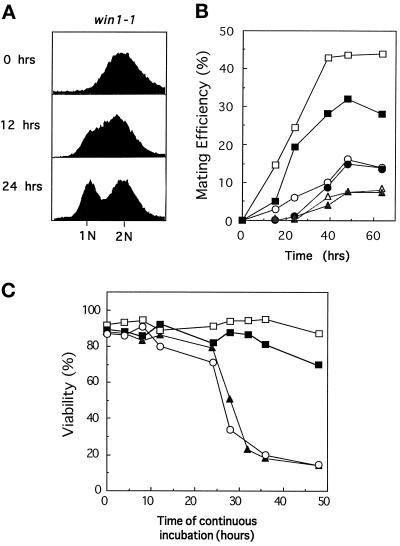
Win1 Is Crucial for Controlling Sty1 MAPK Activity in Stationary Phase
As previously demonstrated, cells lacking either the Wis1 MAPKK or the Sty1 MAPK fail to enter G1 when starved of a nitrogen source whereas cells lacking the Wak1 MAPKKK are able to do so (Warbrick and Fantes, 1991; Takeda et al., 1995; Kanoh et al., 1996). In parallel cultures to those shown in Figure 1A, win1–1 cells were grown to log phase in minimal medium, and the ability to arrest in G1 was determined by fluorescence-activated cell sorter analysis after either 12 or 24 h incubation in nitrogen-free medium. As the results in Figure 6A demonstrate, less than 50% of the cells had entered G1 after 24 h, suggesting that Wak1 and Win1 perform distinct functions in stationary phase (Figure 6C). To directly compare the relative roles of Wak1 and Win1 in stationary phase, the ability of mutant strains to initiate sexual conjugation and differentiation was assessed. Homothallic strains of wild-type cells, Δwak1 cells, win1–1 mutants, or Δwak1 win1–1 double mutants were grown to stationary phase in minimal medium lacking a nitrogen source, and the number of cells that had undergone sexual conjugation and meiosis were assessed after the times indicated. As the results in Figure 6B illustrate, win1–1 cells were profoundly defective in initiating sexual differentiation, but this was not exacerbated by simultaneous inactivation of wak1. In fission yeast, sexual conjugation is triggered in poor nutritional conditions that promote exit from the cell cycle and entry into a quiescent Go state. Importantly, cells lacking the Wis1 MAPKK, the Sty1 MAPK, or the Atf1 transcription factor fail to maintain viability in stationary phase, a phenotype that is also displayed by the win1–1 mutant (Ogden and Fantes, 1986; Warbrick and Fantes, 1991; Takeda et al., 1995; Kanoh et al., 1996). To directly compare the roles of Wak1 and Win1 in this process, wild-type cells, win1–1 mutants, or cells deleted for either the Wak1 MAPKKK or Wis1 MAPKK were grown in rich medium and cell viability assessed as the culture reached saturation and stationary phase. Counting of total cell number revealed that all cultures ceased dividing after continuous incubation for approximately 24 h (our unpublished data). As the results in Figure 6C demonstrate, after this time Δwis1 and win1–1 cells underwent a rapid loss of viability, whereas Δwak1 cells were mostly unaffected. It is likely that mating efficiency reflects both the ability to enter G1 phase of the cell cycle and to maintain viability in stationary phase. Regardless of this, these results indicate that Wak1 and Win1 perform distinct roles in controlling the Sty1 MAPK in poor nutritional conditions.
Figure 6.
Distinct roles for Wak1 and Win1 in the control of the StyI MAPK. (A) Win1–1 are partially defective in G1 arrest. Log phase cultures of win1–1 cells (JM1413) growing in EMM medium at 30°C were analyzed for DNA content before (0 h) and after (12 or 24 h) incubation in the same medium lacking NH4Cl. (B) Win1 is required for efficient sexual conjugation. Homothallic cultures of wild-type (open squares) (JY878), wak1::ura4 (closed squares) (JM1505), win1–1 (open circles) (ED623), wak1::ura4 win1–1 (closed circles) (JM1509), sty1::ura4 (open triangles) (JM1263), and wis1::ura4 (closed triangles) (JM1260) cells were grown to log phase in liquid EMM and transferred to the same medium lacking NH4Cl for the times indicated at 30°C, and mating efficiency was assessed microscopically. (C) Win1 but not Wak1 is required for long-term viability in stationary phase. Log phase (2 × 106 cells/ml) cultures of wild-type (open squares) (PR109), wis1::ura4 (closed triangles) (JM 544), wak1::ura4 (closed squares) (JM 1436), or win1–1 (open circles) (JM1413) were grown in YEPD at 30°C until the cultures reached stationary phase. At the times indicated, equal numbers of cells from these cultures were plated to fresh YEPD plates in triplicate, and viability was assessed by colony formation after an additional 3 d incubation at 30°C.
Wis1 and Sty1 Are Active at a Low Level in Δwak1 win1–1 Double Mutants
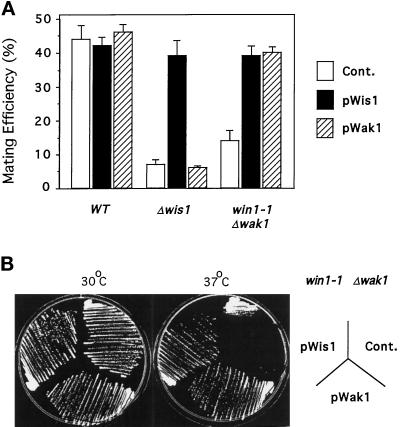
To assess whether Win1 is the only regulator of Sty1 in the absence of Wak1, the phenotypes of Δwak1 win1–1 cells were compared with those of Δsty1 and Δwis1 cells. First, we note that Δwak1 win1–1 cells divide at a smaller size than Δwis1 cells, suggesting that Sty1 is not inactive in Δwak1 win1–1 cells (Table 1). This is supported by the observation that Δwak1 win1–1 cdc25–22 cells can be propagated at 26°C in rich medium whereas Δwis1 cdc25–22 cannot (Table 1; Millar et al., 1995; Shiozaki and Russell, 1995). Second, Δwak1 win1–1 h90 cells were able to undergo sexual conjugation more effectively than Δwis1 h90or Δsty1 h90 cells (Figure 6B). These observations predict that increasing the expression of wis1 in double mutant Δwak1 win1–1 cells should reverse the phenotype resulting from simultaneous loss of wak1 and win1 function. Homothallic and heterothallic wild-type, Δwis1, or Δwak1 win1–1 cells were transformed with a vector expressing either wis1 or a truncated version of wak1 from the thiamine-repressible nmt1 promoter, and the ability of these cells to undergo sexual conjugation or grow at high temperature was assessed (Basi et al., 1993). Increasing the expression of wis1 completely suppresses the mating deficiency and thermosensitivity of Δwak1 win1–1 cells (Figure 7A and B). These effects are dependent on the catalytic activity of Wis1 since a catalytically inactive mutant in which the active site lysine has been mutated to an arginine is unable to complement these strains (our unpublished data). Overexpression of wak1 was also able to completely suppress the inability of win1–1 Δwak1 to mate or proliferate at high temperature, indicating that when overexpressed, wak1 can fully substitute for loss of win1 (Figure 7A and B). These data indicate that the Sty1 MAPK retains significant activity in the absence of both Wak1 and Win1, suggesting that either win1–1 is not a null allele or that additional elements control the Sty1 MAPK.
Table 1.
Win1 regulates cell size at division independently of Wak1
| T°C | Cell size at division (μm) | |
|---|---|---|
| wt | (30°C) | 14.2 ± 0.3 μm |
| win1-1 | (30°C) | 17.1 ± 1.1 μm |
| wak1::ura4 | (30°C) | 16.5 ± 0.5 μm |
| win1-1 wak1::ura4 | (30°C) | 21.4 ± 1.3 μm |
| wis1::ura4 | (30°C) | 23.1 ± 2.1 μm |
| cdc25-22 | (26°C) | 21.9 ± 0.8 μm |
| wak1::ura4 cdc25-22 | (26°C) | 28.5 ± 4.5 μm |
| win1-1 cdc25-22 | (26°C) | 33.2 ± 3.8 μm |
| win1-1 wak1::ura4 cdc25-22 | (26°C) | 37.2 ± 5.8 μm |
| wis1::ura4 cdc25-22 | (26°C) | cdc− |
Figure 7.
Evidence for a Wak1- and Win1- independent pathway controlling Wis1. (A) Wis1 suppresses the mating deficiency of a Δwak1 win1–1 strain. The homothallic strain Wak1::ura4 win1–1 leu1–32 h90 (Δwak1 win1–1) (JM 1509) was transformed either with a control plasmid pREP41 (Cont.) or with pREP41-wis1 (pWis1) or pREP41-wak1 (pWak1) (as above). Transformants were grown to log phase at 30°C in liquid EMM lacking leucine and transferred to the same medium lacking NH4Cl for 48 h, and mating efficiency was assessed microscopically. (B) Wis1 suppresses the temperature sensitivity of a Δwak1 win1–1 strain. Wak1::ura4 win1–1 leu1–32 (Δwak1 win1–1) (JM 1504) cells were transformed with a control plasmid pREP41 (Cont.) or either pREP41-wis1 (pWis1) or pREP41-wak1 (pWak1) in which the wis1 and wak1 genes were expressed from the thiamine-repressible nmt1 promoter. Transformants were streaked on minimal medium lacking thiamine and leucine, and colony formation was monitored after 3 d incubation at either 30°C (left hand plate) or 37°C (right hand plate).
DISCUSSION
The stress-activated Sty1 MAPK pathway of fission yeast displays some remarkably similar features to the mammalian SAPK pathways. Most significantly, the Sty1 MAPK is activated by a similar range of environmental insults including osmotic and oxidative stress, heat shock, UV light, certain DNA-damaging agents, and the protein synthesis inhibitor anisomycin. One of the goals of our research is to identify the upstream regulators of the Sty1 pathway and to determine how the stress signal is transduced to the Sty1 MAPK in the hope that this will provide important clues as to how the mammalian SAPK pathways are activated. The fission yeast Sty1 pathway participates in several seemingly independent cellular events. In particular, cells lacking Sty1 are delayed in the timing of mitotic initiation, are defective in both long- and short-term responses to environmental stress, and are unable to undergo sexual differentiation. In the search for gene products that regulate the Sty1 MAPK, we reasoned that mutants in these corresponding genes would also be defective for one or all of these functions.
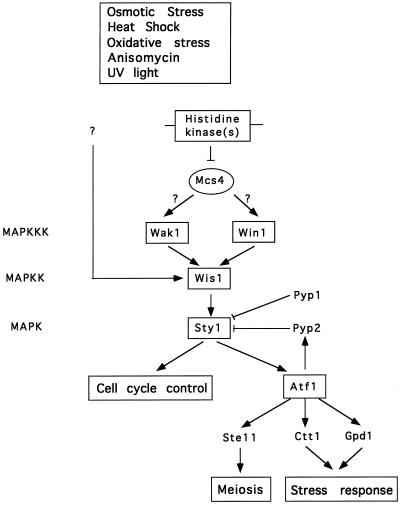
We have recently identified two upstream regulators of the Sty1 MAP kinase pathway as the Mcs4 response regulator and Wak1 MAPKKK. These results suggest that the architecture of the fission yeast Sty1 MAPK pathway is similar to the HOG1 MAPK pathway in the related budding yeast, and that both pathways are controlled by a conserved two-component system (Shieh et al., 1997). Notably, however, cells lacking either Mcs4 or Wak1 are able to proliferate under stressful conditions and have limited detectable defects in either entering G1 phase or initiating sexual conjugation. Together these data indicate the existence of alternative signaling pathways controlling the Wis1 MAPKK. Since cells lacking mcs4 or wak1 are not as severely delayed in the timing of mitotic initiation as are Δwis1 or Δsty1 cells, we presumed that this alternative pathway also controls the timing of mitotic initiation. For this reason we focused on a recessive mutant, win1–1, which is delayed in the timing of mitotic initiation and displays genetic interactions with the Wis1 MAPKK (Ogden and Fantes, 1986; Warbrick and Fantes, 1991). The following lines of evidence suggest that Win1 and the Wak1 MAPKKK cooperatively control the activity of the stress-activated Sty1 MAPK in response to multiple environmental stimuli. First, stress-mediated induction of several genes whose expression is regulated by Sty1, including pyp2, ctt1, and gpd1, is severely diminished in win1–1 mutant cells. Second, activation of the Sty1 MAPK by multiple environmental stresses is also dramatically attenuated in win1–1 cells, as assessed by phosphotyrosine content and ability to phosphorylate a GST-Atf1 fusion protein. To determine the relationship of Win1 to the Wak1 MAPKKK, win1–1 Δwak1 double mutants were constructed. These cells divided at a larger size than either wak1 or win1–1 single mutants alone, suggesting that Wak1 and Win1 act independently (Table 1). This conclusion is supported by the observation that induction of pyp2 mRNA in double Δwak1 win1–1 mutants is considerably lower than either win1–1 or Δwak1 single mutants alone, and that Δwak1 win1–1 double mutants proliferate very poorly either at high temperature or in high osmolarity, whereas win1–1 and Δwak1 single mutants are able to form colonies under these conditions. These data, together with genetic evidence that ectopically increasing the activity of Sty1 can bypass loss of win1 function, incontrovertibly establish Win1 as a component of the Sty1 pathway. One possible explanation for these data is that win1 may encode a structural component that tethers components of the Sty1 pathway together in a manner analogous to the role that the STE5 gene product plays in the budding yeast-mating pheromone pathway (Choi et al., 1994). We believe a more likely explanation is that since Wis1 is the only MAPKK needed for Sty1 activation, win1 encodes a second MAPKKK for Wis1 MAPKK. This is not unreasonable since wak1 when overexpressed can fully substitute for loss of win1. Indeed, three functionally overlapping MAPKKKs have been found to regulate the single PBS2 MAPKK in budding yeast in response to osmotic stress (Maeda et al., 1995; Posas and Saito, 1997). Moreover, our finding that win1–1 cells are epistatic to overexpression of mcs4 is consistent with the hypothesis that the Mcs4-response regulator acts upstream of both Wak1 and Win1 (Figure 8). This would be analogous to the relationship of the SSK1-response regulator with the SSK2 and SSK22 MAPKKKs in budding yeast. We have attempted to clone win1 by functional complementation of a win1–1 cdc25–22 mutant without success. We are currently attempting to genetically map the win1 locus. Although Wak1 and Win1 appear to have very similar functions in short-term activation of the Sty1 MAPK, wak1− and win1− mutants display some distinct phenotypes. Most notably, win1–1 mutant cells are profoundly defective in maintaining viability in stationary phase, whereas Δwak1 cells have little or no defect in this process. Second, win1–1 cells are partially sterile whereas Δwak1 are able to mate with almost wild-type efficiency. One possibility is that distinct regulators control Wak1 and Win1 in response to either environmental stress or nutrient deprivation. It is important to point out, however, that there is no formal proof that either Wak1 activity or Win1 function are stimulated by environmental stimuli, so that the mechanism by which the stress signal is transduced to the Sty1 MAPK is still unknown. The development of reagents to study the Wak1 and Win1 proteins in vivo will help resolve some of these issues.
Figure 8.
A model for the role of Win1 in controlling the fission yeast stress-activated Sty1 MAPK. We propose that Win1 controls the activity of Wis1 MAPKK in parallel with the Wak1 MAPKKK and that the Mcs4 response regulator acts upstream of both Wak1 and Win1. We also tentatively suggest that the Wis1 MAPKK may be controlled by an additional Wak1- and Win1-independent pathway.
A number of MAPKKKs that stimulate the JNK/SAPK and p38/CSBP1 MAPKs have been identified by transient transfection studies in mammalian cells, including MEKK1, TAK1, MUK, SPRK/MLK3, TPL2/COT1, and ASK1. It is curious that none of these enzymes have been shown to be catalytically stimulated by environmental stress (Yan et al., 1994; Yamaguchi et al., 1995; Hirai et al., 1996; Rana et al., 1996; Salmeron et al., 1996; Ichijo et al., 1997). It is possible that additional as-yet-undiscovered MAPKKKs control the SAPKs or that these pathways are triggered without necessarily activating a MAPKKK. In this regard it is intriguing that Sty1 activity is not abolished in win1–1 Δwak1 mutants. Specifically, win1–1 Δwak1 double mutants are not as severely delayed in mitotic initiation as Δwis1 or Δsty1 cells but are more effective in initiating sexual conjugation. In addition, overexpression of the wis1 MAPKK can rescue the phenotypes of win1–1 Δwak1 double mutant cells suggesting that either additional pathway(s) regulate the Sty1 MAPK or that the win1–1 mutant is not a complete loss-of-function allele. Further experimentation will be needed to establish which of these possibilities is true.
In conclusion, we have identified a new component of the stress-activated Sty1 MAPK pathway as the product of the mitotic regulator, Win1. Although we have yet to decipher precisely how Sty1 is activated, the similarity of the stimuli that activate both the fission yeast and mammalian SAPKs suggest that this information will dramatically improve our understanding of how the mammalian SAPK pathways are regulated. The amenability of fission yeast to genetic, biochemical, and immunocytochemical analysis indicates that this goal is attainable.
Table 2.
Strains used in this study
| Strain no. | Genotype | Reference/source |
|---|---|---|
| PR 109 | leu1-32 ura4-D18 h− | P. Russell |
| JM 1160 | leu1-32 ura4-D18 ade6-216 sty1::ura4 h− | Millar et al., 1995 |
| JM 544 | leu1-32 ura4-D18 wis1::ura4 h− | Millar et al., 1995 |
| JM 1436 | leu1-32 ura4-D18 ade6-M210 his7-366 wak1::ura4 h+ | Shieh et al., 1997 |
| JM 1439 | leu1-32 ura4-D18 ade6-M210 his7-366 wak1::LEU2 h+ | Shieh et al., 1997 |
| JM 1468 | leu1-32 ura4-D18 ade6-M210 his7-366 mcs4::his7 h+ | Shieh et al., 1997 |
| JM 1413 | leu1-32 ura4-D18 ade6-M210 his7-366 win1-1 h− | This study |
| JM 1504 | leu1-32 ura4-D18 ade6-M210 his7-366 wak1::ura4 win1-1 h− | This study |
| JM 1405 | leu1-32 ura4-D18 ade6-M216 his1-102 mcs4::ura4 win1-1 h− | This study |
| JY 878 | leu1-32 ura4-D18 ade6-M216 h90 | D. Hughes |
| NT 147 | leu1-32 ura4-D18 atf1::ura4 h90 | Takeda et al., 1995 |
| JM 1263 | leu1-32 ura4-D18 ade6-M216 sty1::ura4 h90 | This study |
| JM 1260 | leu1-32 ura4-D18 ade6-M216 wis1::ura4 h90 | This study |
| JM 1505 | leu1-32 ura4-D18 ade6-M216 wak1::ura4 h90 | This study |
| JM 1355 | leu1-32 ura4-D18 ade6-M216 mcs4::ura4 h90 | This study |
| ED 632 | h90 win1-1 | P. Fantes |
| JM 1509 | leu1-32 ura4-D18 ade6-M210 his7-366 wak1::ura4 win1-1 h90 | This study |
| JM 1520 | leu1-32 ura4-D18 ade6-M216 his7-366 sty1(6HisHA):ura4 h+ | This study |
| MW 1539 | leu1-32 ura4-D18 ade6-M216 his7-366 win1-1 sty1(6HisHA):ura4 h− | This study |
| JM 1351 | leu1-32 ura4-D18 ade6-M210 his7-366 cdc25-22 h+ | Shieh et al., 1997 |
| JM 1469 | leu1-32 ura4-D18 ade6-M210 his7-366 cdc25-22 wak1::ura4 h+ | Shieh et al., 1997 |
| JM 1354 | leu1-32 ura4-D18 ade6-M210 his7-366 cdc25-22 win1-1 h− | This study |
| JM 1646 | leu1-32 ura4-D18 ade6-M210 his7-366 cdc25-22 win1-1 wak1::ura4 | This study |
ACKNOWLEDGMENTS
The authors thank members of the Division of Yeast Genetics for helpful advice, discussions and, in particular, Vicky Buck for critical reading of the manuscript. The authors thank Dr. P. Fantes for the win1–1 strain. This research was supported by the Medical Research Council.
REFERENCES
- Aves SJ, Durkacz BW, Carr A, Nurse P. Cloning, sequencing and transcriptional control of the Schizosaccharomyces pombe cdc10 ’start’ gene. EMBO J. 1985;4:457–463. doi: 10.1002/j.1460-2075.1985.tb03651.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basi G, Schmid E, Maundrell K. TATA box mutations in the Schizosaccharomyces pombe nmt1 promoter affect transcriptional efficiency but not the transcription start point or thiamine repressibility. Gene. 1993;123:131–136. doi: 10.1016/0378-1119(93)90552-e. [DOI] [PubMed] [Google Scholar]
- Brewster JL, de Valoir T, Dwyer ND, Winter E, Gustin MC. An osmosensing signal transduction pathway in yeast. Science. 1993;259:1760–1763. doi: 10.1126/science.7681220. [DOI] [PubMed] [Google Scholar]
- Choi KY, Satterberg B, Lyons DM, Elion EA. Ste5 tethers multiple protein kinases in the MAP kinase cascade required for mating in S. cerevisiae. Cell. 1994;78:499–512. doi: 10.1016/0092-8674(94)90427-8. [DOI] [PubMed] [Google Scholar]
- Corliss DA, White WEJ. Fluorescence of yeast vitally stained with ethidium bromide and propidium iodide. J Histochem Cytochem. 1981;29:45–48. doi: 10.1177/29.1.6162881. [DOI] [PubMed] [Google Scholar]
- Davis RJ. MAPKs: new JNK expands the group. Trends Biochem Sci. 1994;19:470–473. doi: 10.1016/0968-0004(94)90132-5. [DOI] [PubMed] [Google Scholar]
- Dérijard B, Hibi M, Wu I-H, Barrett T, Su B, Deng T, Karin M, Davis RJ. JNK1: A protein kinase stimulated by UV light and Ha-Ras that binds and phosphorylates the c-jun activation domain. Cell. 1994;76:1025–1037. doi: 10.1016/0092-8674(94)90380-8. [DOI] [PubMed] [Google Scholar]
- Degols G, Russell P. Discrete roles of the Spc1 kinase and the Atf1 transcription factor in the UV response of Schizosaccharomyces pombe. Mol Cell Biol. 1997;17:3356–3363. doi: 10.1128/mcb.17.6.3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degols G, Shiozaki K, Russell P. Activation and feedback regulation of the Spc1 mitogen activated protein kinase in fission yeast. Mol Cell Biol. 1996;16:2870–2877. doi: 10.1128/mcb.16.6.2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galcheva-Gargova Z, Dérijard B, Wu I-H, Davis RJ. An osmosensing signal transduction pathway in mammalian cells. Science. 1994;265:806–808. doi: 10.1126/science.8047888. [DOI] [PubMed] [Google Scholar]
- Gupta S, Campbell D, Derijard B, Davies RJ. Transcription factor ATF2 regulation by the JNK signal transduction pathway. Science. 1995;267:389–393. doi: 10.1126/science.7824938. [DOI] [PubMed] [Google Scholar]
- Han J, Lee J-D, Bibbs L, Ulevitch RJ. A MAP kinase targeted by endotoxin and hyperosmolarity in mammalian cells. Science. 1994;265:808–811. doi: 10.1126/science.7914033. [DOI] [PubMed] [Google Scholar]
- Herskowitz I. MAP kinase pathways in yeast: for mating and more. Cell. 1995;80:187–197. doi: 10.1016/0092-8674(95)90402-6. [DOI] [PubMed] [Google Scholar]
- Hirai S-i, Izawa M, Osada S-i, Spyrou G, Ohno S. Activation of the JNK signalling pathway by distantly related protein kinases, MEKK and MUK. Oncogene. 1996;12:641–650. [PubMed] [Google Scholar]
- Ichijo H, Nishida E, Irie K, ten-Dijke P, Saitoh M, Moriguchi T, Takagi M, Matsumoto K, Miyazono K, Gotoh Y. Induction of apoptosis by ASK1, a mammalian MAPKKK that activates SAPK/JNK and p38 signaling pathways. Science. 1997;275:90–94. doi: 10.1126/science.275.5296.90. [DOI] [PubMed] [Google Scholar]
- Kanoh J, Watanabe Y, Ohsugi M, Lino Y, Yamamoto M. Schizosaccharomyces pombe gad7+ encodes a phosphoprotein with a bZIP domain, which is required for proper G1 arrest and gene expression under nitrogen starvation. Genes to Cells. 1996;1:391–408. doi: 10.1046/j.1365-2443.1996.d01-247.x. [DOI] [PubMed] [Google Scholar]
- Kato T, Okazaki K, Murakami H, Stettler S, Fantes P, Okayama H. Stress signal, mediated by a HOG1-like MAP kinase, controls sexual development in fission yeast. FEBS Lett. 1996;378:207–212. doi: 10.1016/0014-5793(95)01442-x. [DOI] [PubMed] [Google Scholar]
- Kyriakis JM, Banerjee P, Nikolakaki E, Dai-T., Rubie EA, Ahmad MF, Avruch J, Woodgett JR. The stress-activated protein kinase family of c-jun kinases. Nature. 1994;369:156–160. doi: 10.1038/369156a0. [DOI] [PubMed] [Google Scholar]
- Lee JC, Laydon JT, McDonnell PC, Gallagher TF, Kumar S, Green D, McNulty D, Blumenthal. MJ, Heys JR, Landvatter SW, Strickler JE, McLaughlin MM, Siemens IV, Fisher SM, Livi GP, White JR, Adams JL, Young PR. A protein kinase involved in the regulation of inflammatory cytokine biosynthesis. Nature. 1994;372:739–746. doi: 10.1038/372739a0. [DOI] [PubMed] [Google Scholar]
- Maeda T, Takekawa M, Saito H. Activation of yeast PBS2 MAPKK by MAPKKKs or by binding of an SH3-containing osmosensor. Science. 1995;269:554–558. doi: 10.1126/science.7624781. [DOI] [PubMed] [Google Scholar]
- Maeda T, Wurgler-Murphy SM, Saito H. A two-component system that regulates an osmosensing MAP kinase cascade in yeast. Nature. 1994;369:242–245. doi: 10.1038/369242a0. [DOI] [PubMed] [Google Scholar]
- Maundrell K. A highly transcribed gene completely repressed by thiamine. J Biol Chem. 1990;265:10857–10864. [PubMed] [Google Scholar]
- Millar JBA, Buck V, Wilkinson MG. Pyp1 and Pyp2 PTPases dephosphorylate an osmosensing MAP kinase controlling cell size at division in fission yeast. Genes Dev. 1995;9:2117–2130. doi: 10.1101/gad.9.17.2117. [DOI] [PubMed] [Google Scholar]
- Millar JBA, Russell P. The cdc25 mitotic inducer: an unconventional protein phosphatase. Cell. 1992;68:407–410. doi: 10.1016/0092-8674(92)90177-e. [DOI] [PubMed] [Google Scholar]
- Millar JBA, Russell P, Dixon JE, Guan K-L. Negative regulation of mitosis by two functionally overlapping PTPases in fission yeast. EMBO J. 1992;11:4943–4952. doi: 10.1002/j.1460-2075.1992.tb05601.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno S, Klar A, Nurse P. Molecular genetic analysis of fission yeast Schisosaccharomyces pombe. Methods Enzymol. 1991;194:795–823. doi: 10.1016/0076-6879(91)94059-l. [DOI] [PubMed] [Google Scholar]
- Nurse P. Genetic control of cell size at cell division. Nature. 1975;256:547–551. doi: 10.1038/256547a0. [DOI] [PubMed] [Google Scholar]
- Ogden JE, Fantes P. Isolation of a novel type of mutation in the mitotic control of Schizosaccharomyces pombe whose phenotypic expression is dependent on the genetic background and nutritional environment. Curr Genet. 1986;10:509–514. doi: 10.1007/BF00447384. [DOI] [PubMed] [Google Scholar]
- Ota IM, Varsharvsky A. A yeast protein similar to bacterial two-component regulators. Science. 1993;262:566–569. doi: 10.1126/science.8211183. [DOI] [PubMed] [Google Scholar]
- Posas F, Saito H. Osmotic activation of the HOG1 MAPK Pathway via Ste11p MAPKKK: scaffold role of Pbs2p MAPKK. Science. 1997;276:1702–1705. doi: 10.1126/science.276.5319.1702. [DOI] [PubMed] [Google Scholar]
- Posas F, Wurgler-Murphy SM, Maeda T, Witten EA, Thai TC, Saito H. Yeast HOG1 MAP kinase cascade is regulated by a multi-step phosphorelay mechanism in the SLN1-YPD1-SSK1 “two component” system. Cell. 1996;86:865–875. doi: 10.1016/s0092-8674(00)80162-2. [DOI] [PubMed] [Google Scholar]
- Prentice HL. High efficiency transformation of Schizosaccharomyces pombe by electroporation. Nucleic Acids Res. 1991;20:621. doi: 10.1093/nar/20.3.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rana A, Gallo K, Godowski P, Hirai S, Ohno S, Zon L, Kyriakis JM, Avruch J. The mixed lineage kinase SPRK phosphorylates and activates the stress-activated protein kinase activator, SEK-1. J Biol Chem. 1996;271:19025–19028. doi: 10.1074/jbc.271.32.19025. [DOI] [PubMed] [Google Scholar]
- Rouse J, Cohen P, Trigon S, Morange M, Alonso-Llamazares, Zamanillo D, Hunt T, Nebreda A. A novel kinase cascade triggered by stress and heat shock that stimulates MAPKAP kinase-2 and phosphorylation of the small heat shock proteins. Cell. 1994;78:1027–1037. doi: 10.1016/0092-8674(94)90277-1. [DOI] [PubMed] [Google Scholar]
- Russell P, Nurse P. Negative regulation of mitosis by wee1+, a gene encoding a protein kinase homolog. Cell. 1987;49:559–567. doi: 10.1016/0092-8674(87)90458-2. [DOI] [PubMed] [Google Scholar]
- Salmeron A, Ahmad TB, Carlile GW, Pappin D, Narsimham RP, Ley SC. Activation of MEK-1 and SEK-1 by Tp1-2 proto-oncoprotein, a novel MAP kinase kinase kinase. EMBO J. 1996;15:817–826. [PMC free article] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. In Molecular Cloning: A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Press; 1989. [Google Scholar]
- Schüller C, Brewster, Alexander JL, Gustin MR, MC, Ruis H. The HOG pathway controls osmotic regulation of transcription via the stress response element (STRE) of the Saccharomyces cerevisiae CTT1 gene. EMBO J. 1994;13:4382–4389. doi: 10.1002/j.1460-2075.1994.tb06758.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shieh J-C, Wilkinson MG, Buck V, Morgan B, Makino K, Millar JBA. The Mcs4 response regulator co-ordinately controls the stress activated Wak1-Wis1-Sty1 MAP kinase pathway and fission yeast cell cycle. Genes Dev. 1997;11:1008–1022. doi: 10.1101/gad.11.8.1008. [DOI] [PubMed] [Google Scholar]
- Shiozaki K, Russell P. Cell cycle control linked to extracellular environment by MAP kinase pathway in fission yeast. Nature. 1995;378:739–743. doi: 10.1038/378739a0. [DOI] [PubMed] [Google Scholar]
- Shiozaki K, Russell P. Conjugation, meiosis, and the osmotic stress response are regulated by Spc1 kinase through Atf1 transcription factor in fission yeast. Genes Dev. 1996;10:2276–2288. doi: 10.1101/gad.10.18.2276. [DOI] [PubMed] [Google Scholar]
- Shiozaki K, Shiozaki M, Russell P. Mcs4 mitotic catastrophe suppressor regulates the fission yeast cell cycle through the Wik1-Wis1-Spc1 MAP kinase cascade. Mol Biol Cell. 1997;8:409–419. doi: 10.1091/mbc.8.3.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda T, Toda T, Kominami K, Kohnosu A, Yanagida M, Jones N. Schizosaccharomuces pombe atf1+ encodes a transcription factor required for sexual differentiation and entry into stationary phase. EMBO J. 1995;14:6193–6208. doi: 10.1002/j.1460-2075.1995.tb00310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warbrick E, Fantes P. The Wis1 protein kinase is a dose-dependent regulator of mitosis in Schizosaccharomyces pombe. EMBO J. 1991;10:4291–4299. doi: 10.1002/j.1460-2075.1991.tb05007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warbrick E, Fantes P. Five novel elements involved in the regulation of mitosis in fission yeast. Mol Gen Genet. 1992;232:440–446. doi: 10.1007/BF00266249. [DOI] [PubMed] [Google Scholar]
- Wilkinson MG, Samuels M, Takeda T, Toda T, Toone MW, Shieh J-C, Millar JBA, Jones NC. The Atf1 transcription factor is a target for the Sty1 stress activated MAP kinase pathway in fission yeast. Genes Dev. 1996;10:2289–2301. doi: 10.1101/gad.10.18.2289. [DOI] [PubMed] [Google Scholar]
- Yamaguchi K, Shirakabe K, Shibuya H, Irie K, Oishi I, Ueno N, Taniguchi T, Nishida E, Matsumoto K. Identification of a member of the MAPKKK family as a mediator of TGF-β signal transduction. Science. 1995;270:2008–2011. doi: 10.1126/science.270.5244.2008. [DOI] [PubMed] [Google Scholar]
- Yan M, Dai T, Deak JC, Kyriakis JM, Zon LI, Woodgett JR, Templeton DJ. Activation of stress-activated protein kinase by MEKK1 phosphorylation of its activator SEK1. Nature. 1994;372:798–800. doi: 10.1038/372798a0. [DOI] [PubMed] [Google Scholar]