Abstract
Activity of motor proteins must be tightly regulated in the cells, in order to prevent unnecessary energy consumption and to maintain proper distribution of cellular components. Loading of the cargo molecule is one likely mechanism to activate an inactive motor. Here, we report that the activity of the kinesin-3 motor protein, GAKIN, is regulated by the direct binding of its protein cargo, human Discs large (hDlg) tumor suppressor. Recombinant GAKIN exhibits potent microtubule gliding activity but has little microtubule-stimulated ATPase activity in solution, suggesting that it exists in an auto-inhibitory form. In vitro binding measurements revealed that defined segments of GAKIN, particularly the MAGUK binding stalk (MBS) domain and the motor domain, mediate intramolecular interactions to confer globular protein conformation. Direct binding of the SH3-I3-GUK module of hDlg to the MBS domain of GAKIN activates the microtubule-stimulated ATPase activity of GAKIN by ~10 fold. We propose that the cargo-mediated regulation of motor activity constitutes a general paradigm for the activation of kinesins.
Kinesin motors mediate the intracellular transport of cargo molecules along microtubule tracks (1). The mechanism that regulates the activation of kinesin motors remains an issue of fundamental importance (2, 3). It has been reported that the full length conventional kinesin, kinesin-1, has very little microtubule-stimulated ATPase activity in solution as compared to its shorter motor domain constructs (4, 5). The intramolecular interactions mediated by the motor and tail domains keep the kinesin-1 in a compactly-folded inhibitory state (6–8). It is well established that the mechanical attachment of the inactive full length motor to a glass surface or latex beads is sufficient to transform the inactive motor to an active state, presumably by disrupting its inhibitory conformation (8, 9). Therefore, it is reasonable to speculate that this mode of self-inhibition of kinesin-1 is the general feature conserved in all kinesin-like proteins to keep their motor activity regulated in vivo.
GAKIN/KIF13B (10) belongs to the kinesin-3 family. The family also includes KIF1A/Unc104, which is responsible for the transport of synaptic vesicles (11). Our previous studies have identified two cargo molecules for GAKIN; the membrane-associated guanylate kinase homologue (MAGUK) scaffolding protein hDlg/SAP97 (12) and a PIP3 binding protein termed PIP3BP (13). Both MAGUKs and PIP3 have been implicated in the regulation of cell polarity pathways, and their subcellular localization is considered to be important for their functions in vivo. Recently, we have shown that the transport of PIP3 containing vesicles to the distal ends of neurites by the GAKIN-PIP3BP complex regulates the axon-dendrite polarity determination in the hippocampal neurons (13).
Human Dlg is an important component of cell-cell contact sites such as epithelial adherence junctions and neuronal synaptic membranes, where it forms a multi-protein complex containing transmembrane receptors and cytoskeletal proteins (14). The loss of Drosophila Dlg disrupts epithelial apical-basal polarity resulting in the tumor-like overgrowth in imaginal discs (15). MAGUK proteins are also implicated in the transport of multi-protein complexes to specialized sites. For example, SAP97, the rat orthologue of hDlg, regulates the transport of AMPA receptor complex to the synapse (16–18). A scaffolding complex containing Lin-2, another MAGUK, mediates the transport of the NMDA receptor (19). These findings prompted us to investigate the regulatory mechanism of the hDlg-GAKIN cargo-motor complex and its role in the transport phenomenon. Here, we report that a specific segment of hDlg encodes the activation signal for converting an inactive GAKIN to an active motor in vitro. To our knowledge, this is the first demonstration of the regulation of a kinesin motor by direct interaction of its natural cargo using in vitro measurements of purified components.
EXPERIMENTAL PROCEDURES
Tissue culture and transfections
HEK293T cells were maintained in Dulbecco’s modified Eagle’s medium (DMEM) (Invitrogen) supplemented with 10% Fetal Calf Serum (FCS) (Hyclone). DNA transfection was performed by the calcium phosphate method.
Plasmids
GFP-motor2 (1–486) and GFP-motor-FHA (1–557) of GAKIN are in pEGFP-C1 (Clonetech). His-stalk1 (487–989) is in pRSET-A (Invitrogen). S-motor1 (1–368), S-MBS (607–831), S-stalk2 (607–989), S-stalk3 (746–989), S-FHA (423–557) and S-CT (1414–1826 aa) of GAKIN are in pET32a (Novagen). GST-motor2 (1–486), GST-CT (1414–1826) of GAKIN; and GST-SH3-I2-GUK, GST-SH3-I3-GUK, GST-I3-GUK, GST-GUK of hDlg; and GST-PIP3BP are in pGEX2T (Amersham Biosciences/GE Healthcare). GST-SH3-GUK of PSD-95 is a gift from Drs. O. Olsen and D. Bredt (20).
Recombinant protein expression in bacteria
GST fusion proteins were expressed in DH5α and purified using glutathione-Sepharose 4B (Pharmacia). His tag proteins, S-motor, S-FHA, and S-CT were expressed in BL21 (DE3) (Stratagene) and purified using Ni-NTA agarose (Invitrogen). S-stalk2, S-stalk3, and S-MBS were purified under denaturing conditions and renatured by dialysis as described previously (12) except for modification of lysis buffer (20 mM Tris-HCl pH 7.9, 100 mM NaCl, and 6 M urea) and dialysis buffer (100 mM Tris-HCl pH 7.9, 10 mM EDTA, and 0.2 M L-arginine) containing decreasing concentrations of urea. Purification of fusion proteins containing the motor domain was performed in the presence of 0.1 mM Mg-ATP.
Recombinant protein expression in insect cells
His-full length GAKIN (1–1826) and His-motor-FHA (1–557) were expressed in Sf9 cells. Corresponding cDNAs were inserted in pFastbac HTb and the recombinant viruses were prepared using the Bac-to-Bac baculovirus expression system (Invitrogen). Sf9 cells were maintained in SF-900 II SFM (Invitrogen) at 27°C. 48 hours after the infection of the recombinant virus, Sf9 cells were lysed by sonication at 4°C in the purification buffer (10 mM PIPES-KOH pH 6.9, 1 mM MgCl2, 1 mM EGTA-KOH, 10% glycerol and 0.1 mM Mg-ATP). Protein purification from the cleared lysate was performed by single step Ni++ affinity purification using Ni-NTA agarose (Invitrogen).
In vitro binding assays
For S-resin pull-down experiments from the cell lysate, 293T cells expressing GFP-motor or GFP-motor-FHA were lysed in the lysis buffer (PBS supplemented with 0.1% Triton-X100). S-GAKIN fusion proteins immobilized on S-protein agarose resin (Novagen) were incubated with cleared lysate for 4 h at 4°C and the beads were recovered and washed by centrifugation. GFP-fused proteins recovered with the beads were detected by Western blot using anti GFP pAb (Invitrogen). For the direct binding assay of GST-fusion proteins with S-tag proteins, purified GST fusion proteins were incubated with S-GAKIN fusion proteins immobilized on S-protein agarose resin in the lysis buffer and the beads were recovered by centrifugation. Recovered GST-fusion proteins were detected by anti-GST pAb (Santa Cruz). A pull-down assay from the rabbit reticulocyte lysate expressing the 35S labeled protein was performed as described before (12).
Microtubule-stimulated ATPase assay
Tubulin was prepared from porcine brain and taxol stabilized microtubules were prepared (21). MT-stimulated ATPase activity was measured as described previously (22). Briefly, full length GAKIN (50 nM) was mixed with MTs (1 μM, otherwise indicated) in A25 buffer (25 mM ACES-KOH, pH 6.9, 2.0 mM magnesium acetate, 2.0 mM EGTA, 0.1 mM EDTA) supplemented with 8.0 μM Taxol, 1.0 mM ATP, 10 μg/ml pyruvate kinase, and 2.0 mM phosphoenolpyruvate followed by incubation at 25°C. Aliquots of 20 μl were taken out at defined time intervals, and the amount of Pi was determined using the malachite-green method. To test the effect of GAKIN binding proteins, purified GST fusion proteins were added in the ATPase assay reaction. The total protein concentration in the reaction was adjusted to 250 μg/ml by adding the appropriate amount of BSA. The concentration of the proteins used in the assay was measured by the Bradford method (BioRad) using BSA as a standard.
Microtubule gliding assay
Microtubule gliding assay using fluorescent labeled Taxol-stabilized microtubules was done as described (13). To test the effect of SH3-GUK, full length GAKIN was incubated with or without 4 times of SH3-I2-GUK or SH3-I3-GUK and coated on the glass surface.
Dimerization assay
Dimerization assay using cross-linker O-phenanthroline was performed as described (23, 24). To test the effect of SH3-GUK, full length GAKIN was incubated with or without 4 times of SH3-I2-GUK or SH3-I3-GUK and incubated with 10 μM CuSO4 and 50 μM O-phenanthroline at room temperature for 1 hour. Cross-linking reaction was terminated by the addition of 2 mM EDTA. Samples were loaded in 4% SDS-PAGE gel without DTT or β-mercaptoethanol.
RESULTS AND DISCUSSION
Recombinant GAKIN is an autoinhibited motor in solution
We expressed a kinesin-3 protein, human GAKIN/KIF13B, in order to characterize its biochemical and inhibitory properties. Full length GAKIN, and its truncated form, motor-FHA domain, were expressed in insect cells and purified through nickel affinity chromatography (Figure 1A). Purified motor-FHA protein exhibited the microtubule-stimulated ATPase activity in solution; however, the full length GAKIN showed little activity, which is about 1/10 compared to the motor-FHA domain (Figure 1B). The activity of motor-FHA module was calculated as kcat = 4.6 S−1, K0.5(MT) = 0.8 μM, whereas it was kcat = 0.3 S−1 for full length GAKIN (Figure 1C).
FIGURE 1.
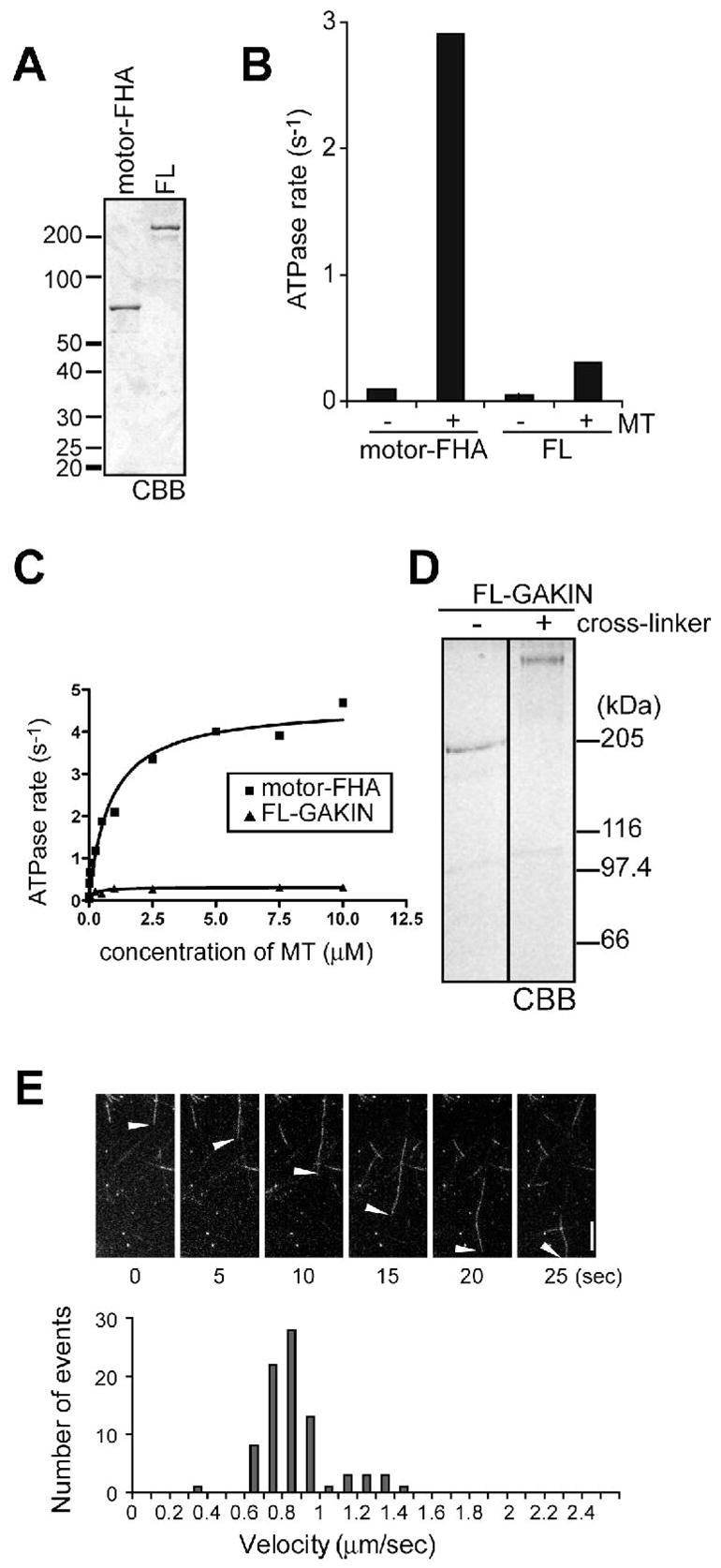
Autoinhibition of full length GAKIN protein. (A) Expression and purification of GAKIN. Motor-FHA and Full length (FL)-GAKIN recombinant proteins were expressed in insect cells and purified with Ni++ affinity purification. CBB stained 8% SDS-PAGE gel is shown. (B) Microtubule stimulated ATPase activity of recombinant GAKIN proteins. Motor-FHA and/or FL-GAKIN were incubated with or without 2.5 μM of microtubules and 1.0 mM MgATP. ATPase activity was determined by measuring phosphate release by the malachite green method. (C) Microtubule stimulated ATPase activity of motor-FHA and FL-GAKIN as a function of microtubule concentration. (D) Chemical cross-linking of full length GAKIN. Full length GAKIN was incubated with or without cross-linker and separated in 4% SDS-PAGE gel. (E) Microtubule gliding activity of FL-GAKIN. Movement of the fluorescent labeled Taxol-stabilized microtubules was recorded and their velocity distribution is shown in the histogram. Scale bar represents 5 μm.
Generally, most processive kinesins are known to function as dimer. To examine if GAKIN is a dimer or monomer in solution, recombinant full length GAKIN was tested by chemical cross-linking using copper/phenanthroline (Figure 1D). The copper/phenanthroline induces reversible oxidation of the sulfydryl groups forming disulfide bonds between near neighbors in solution (23, 24). When separated by 4% gel SDS-PAGE, cross-linked full length GAKIN migrated at approximately twice the size (Figure 1D, right) of the corresponding non-cross-linked form (Figure 1D, left), suggesting that the recombinant GAKIN molecule is a dimer. The full length recombinant GAKIN is active in the microtubule gliding assay where GAKIN is attached to the glass surface (Figure 1E). The average velocity of GAKIN was calculated as 0.79 μm/sec, which is slower than the average of the motor-FHA domain (1.66 μm/sec) that we reported previously (13). These results suggest that the full length GAKIN expressed in Sf9 insect cells contains an active motor domain, but its motor activity is inhibited presumably in the context of the full length molecule in solution.
Intra-molecular interactions of GAKIN
Next we examined whether defined segments of GAKIN could participate in intramolecular interactions. A series of constructs derived from human GAKIN were tested for in vitro protein-protein interactions (Figure 2A and Table 1). We found that the recombinant S-tagged motor domain (motor1) and C-terminus tail domain (CT), expressed in bacteria, interacted with a segment of the stalk region (stalk1), which was expressed using the rabbit reticulocyte lysate (Figure 2B). The stalk1 region contains two important cargo binding motifs. These motifs include a portion of the FHA domain, which interacts with PIP3BP/Centaurin-α (13), and the MAGUK Binding Stalk (MBS) domain, which interacts with hDlg tumor suppressor (12). To test whether either of these motifs mediates the intramolecular interaction with the motor domain of GAKIN, we expressed individual S-tagged fusions of FHA and stalk2 region, which includes the MBS domain, and performed an S-tag pull-down experiment. The GFP-motor2 domain was expressed in 293T cells. Binding of the GFP-motor2 domain was detected with the MBS containing stalk2 construct, but not with the motor1, CT or control S (Figure 2C) constructs. A relatively weak interaction with the GFP-motor2 and Trx-FHA domains was also observed. A longer construct of GFP-motor-FHA domain also interacted with the stalk2, but did not interact with the FHA, presumably because the motor-FHA domain might be self-folded. We further narrowed the binding site for the motor domain within stalk2. The stalk2 construct was divided in two segments named MBS and stalk3 (Figure 2A), where the MBS corresponds to the minimum-binding domain for hDlg (12). The GST-motor2 domain interacts with the S-tagged stalk2 domain and MBS domain, but not with stalk3 domain (Figure 2D), demonstrating that the MBS domain of GAKIN can interact with its own motor domain as well as with the GUK domain of hDlg. Direct interaction between CT and stalk2 domains was also confirmed (Figure 2E). The CT domain showed an interaction with itself (Figure 2E), which might contribute to the dimerization of full length GAKIN (Figure 1D) since one of the coiled-coil regions exists in the CT domain (Figure 2A). Taken together, these results suggest that defined segments of GAKIN interact with one another to form an intramolecular folded state, particularly involving the motor and MBS domains.
FIGURE 2.
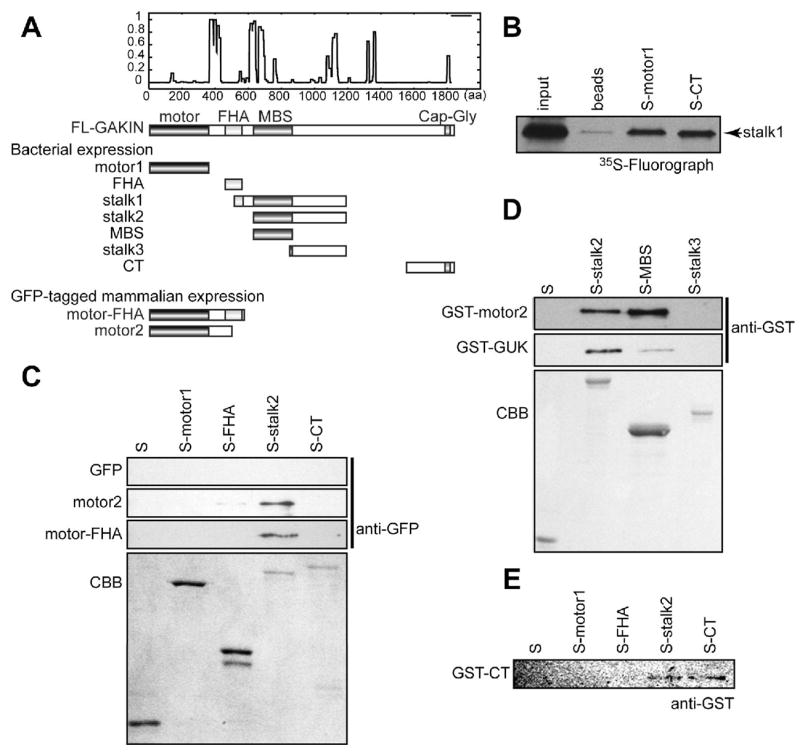
Intramolecular interactions mediated by MBS domain of GAKIN. (A) Schematic representation of GAKIN truncated constructs used for the binding assay. (B) Binding of stalk1 region of GAKIN with motor and CT. 35S labeled stalk1 was expressed in the rabbit reticulocyte lysate in vitro translation system (STP3, Novagen), and S-tag pull down was performed with S-motor1 or S-CT immobilized on S-protein agarose beads or plain beads as a control. Bound 35S-labeled stalk1 protein was detected by fluorography. (C) Pull down assay from 293T cell lysate. GFP-fused motor2 and motor-FHA were transiently expressed in 293T cells, and S-tag pull down was performed with control S, S-motor1, S-FHA, S-stalk2 and S-CT. Bound proteins were detected by Western blot using anti-GFP antibody. The bottom panel shows the CBB stained gel of the S-fusion protein used for binding. (D) Direct binding of the GST-motor2 and GST-GUK (hDlg) with Trx-fusion proteins of GAKIN. Purified recombinant proteins, GST-motor2 and GST-GUK (hDlg) were used for direct binding with S, S-stalk2, S-MBS and S-stalk3, immobilized on S-protein agarose. Bound proteins were detected by anti-GST Western blot. (E) Direct binding of GST-CT with S-stalk2.
TABLE 1.
list of the constructs used in this study
| name | boundary | domains included | vector | references |
|---|---|---|---|---|
| GAKIN | ||||
| FL-GAKIN | 1–1826 | Full length | pFastBacHTb | this study |
| motor-FHA | 1–557 | motor, FHA | pFastBacHTb | (13) |
| GFP-motor2 | 1–486 | motor | pEGFP-C1 | (13) |
| GFP-motor-FHA | 1–557 | motor, FHA | pEGFP-C1 | (13) |
| S-motor1 | 1–368 | motor | pET32a | (12) |
| S-stalk2 | 607–989 | MBS, stalk | pET32a | (12) |
| S-MBS | 607–831 | MBS | pET32a | (12) |
| S-stalk3 | 746–989 | stalk | pET32a | (12) |
| S-FHA | 423–557 | FHA | pET32a | (13) |
| S-CT | 1414–1826 | Cap-Gly | (13) | |
| His-stalk1 | 487–989 | half of FHA, MBS, stalk | pRSET-A | (12) |
| GST-motor2 | 1–486 | motor | pGEX2T | this study |
| GST-CT | 1414–1826 | Cap-Gly | pGEX2T | (10) |
| hDlg | ||||
| GST-SH3-I2-GUK | 584–904 | SH3, I2, GUK | GEX2T | (33) |
| GST-SH3-I3-GUK | 566–989 | SH3, I3, GUK | pGEX2T | (12) |
| GST-I3-GUK, | 669–915 | I3, GUK | pGEX2T | (10) |
| GST-GUK | 733–915 | GUK | pGEX2T | (10) |
| FL-I2 | 1–904 | full length with I2 insert | pGEX2T | (25) |
| FL-I3 | 1–926 | full length with I3 insert | pGEX2T | (25) |
| FL I2 L613P | 1–904 | point mutation in SH3 | pGEX2T | this study |
| FL I3 L613P | 1–926 | point mutation in SH3 | pGEX2T | this study |
Binding of a specific segment of hDlg activates GAKIN
The formation of intramolecular interactions of the motor domain with the MBS domain, and its relatively weak binding to the FHA domain, implies that the binding partners for these domains might regulate intramolecular interactions of GAKIN. First, we tested the effects of the FHA domain binder, PIP3BP, (13) and the MBS domain binder, hDlg (12) on the microtubule-stimulated ATPase activity of the recombinant full length GAKIN. Direct interactions of GST-fused PIP3BP and various hDlg constructs (Figure 3A) with GAKIN were confirmed by the in vitro pull-down assays (Figure 3B). The results of the ATPase assay revealed that the PIP3BP had little effect on the microtubule-stimulated ATPase activity of full length GAKIN, whereas, the SH3-I3-GUK module of hDlg enhanced the GAKIN activity about 10 fold (Figure 3C). Although the GUK domain of hDlg alone is sufficient for its interaction with GAKIN (10) and all constructs containing the GUK domain interacted with GAKIN (Figure 3A), the activation of GAKIN specifically requires the entire SH3-I3-GUK region, as the shorter constructs consisting of GUK, I3-GUK, and the alternatively spliced isoform, SH3-I2-GUK (25), did not activate GAKIN (Figure 3C). PSD-95, a close homologue of hDlg, also had no effect on GAKIN activation. These results suggest that the SH3-I3-GUK module of hDlg has a specific function to convert an inactive form of GAKIN into an active form. The concentration of SH3-I3-GUK module required for half maximal activation of GAKIN is 140 nM, which is approximately 3 times in excess of the amount of GAKIN used in the ATPase reaction (50 nM) (Figure 3D). In the presence of a saturating concentration of SH3-I3-GUK module (1,000 nM), the microtubule-stimulated ATPase activity of full length GAKIN as a function of microtubule concentration showed a pattern with a kcat = 3.2 S−1 and K0.5(MT) = 0.4 μM that is similar to the motor-FHA domain (Figures 3E and 1C). This result suggests that the motor domain of the full length GAKIN bound to the SH3-I3-GUK module becomes accessible to microtubules at the same level as the motor-FHA domain, indicating the full release of the motor domain from the MBS-mediated intramolecular inhibition. In contrast, we observed no effect of SH3-I3-GUK module on the velocity of full length GAKIN in the microtubule-gliding assay, where GAKIN is attached on the glass surface (data not shown).
FIGURE 3.
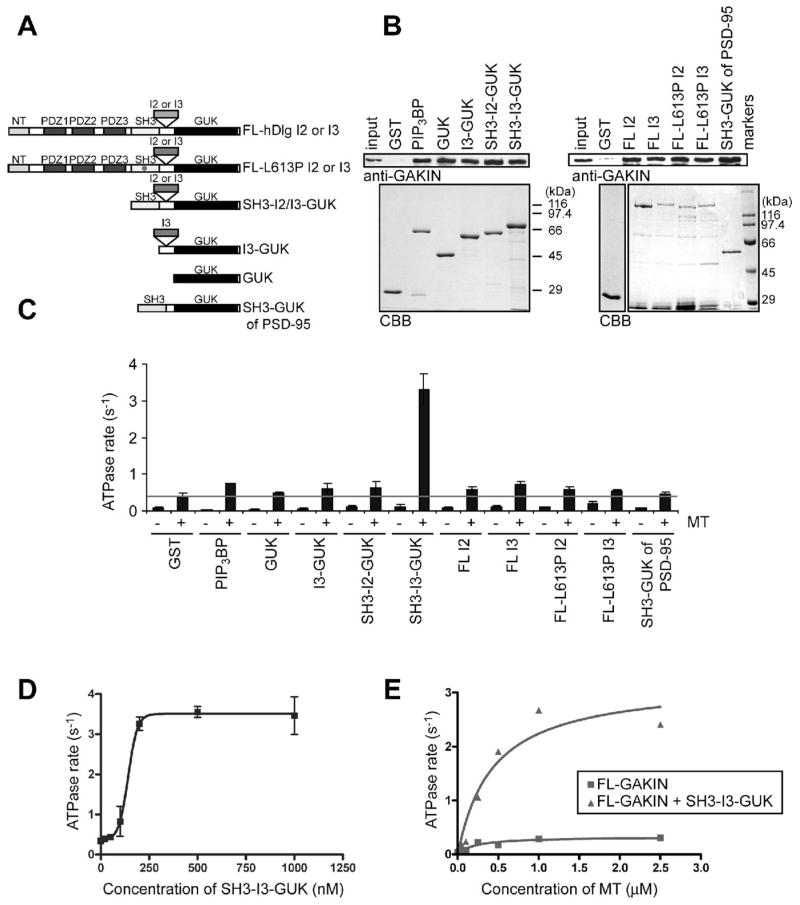
Activation of GAKIN by direct interaction of hDlg. (A) Schematic representation of GST fused hDlg constructs used in the study. (B) Binding of GST-PIP3BP and GST-hDlg proteins with GAKIN. Purified FL-GAKIN was used for direct binding with GST-fusion proteins immobilized on the beads. GAKIN was detected with Western blot using anti-GAKIN pAb (10). In the bottom panel, GST-fusion proteins were shown in the CBB stained gel. (C) Microtubule stimulated ATPase activity of FL-GAKIN in the presence of GST-fusion proteins. GST-fusion proteins of PIP3BP and hDlg (1 μM, except for FL-I3 and FL-I3-L613P, which were 0.5 μM) mixed with FL-GAKIN (50 nM) were incubated with or without microtubules (1 μM). (D) Microtubule dependent ATPase activity as a function of SH3-I3-GUK concentration. FL-GAKIN (50 nM) and microtubules (1 μM) were used in the reaction. (E) Microtubule concentration dependent ATPase activity of FL-GAKIN with and without SH3-I3-GUK. FL-GAKIN (50 nM) and GST-SH3-I3-GUK of hDlg (1 μM) were used in the reaction.
A functional requirement of the SH3-I3-GUK region of hDlg suggests that simple binding mediated by the GUK domain is not sufficient for the activation of GAKIN. The SH3-GUK domains are known to self-associate and form a single unit via an intra-molecularly folded closed conformation (20, 26, 27). One possible mechanism could be envisaged whereby the I3 insertion physically interferes or interacts with a segment of GAKIN that can induce sufficient conformational change in GAKIN to release it from the inhibited state. Alternatively, the SH3-I3-GUK module might display a folded structure that is different from the SH3-I2-GUK or PSD-95 SH3-GUK modules, which are predicted to be closed form monomers. As proposed previously, a closed conformation of SH3-GUK module could in theory change into an open conformation that forms an anti-parallel dimer or even a large polymer (20, 28). There is also a possibility that the SH3-I3-GUK unit represents a constitutively open conformation. Interestingly, full length hDlg, both the I2 and I3 isoforms, did not activate GAKIN (Figure 3C), suggesting that the full length hDlg-I3 protein has its SH3-I3-GUK module locked in an inhibited closed state in vitro. This result is consistent with the previous observations stating that various domains of hDlg participate in a series of intramolecular interactions that functionally regulate the full length hDlg, which exists in a compactly folded structure (18, 27). In the Drosophila Dlg tumor suppressor, a point mutation in the SH3 domain named m30 allele was identified as sufficient to abrogate its tumor suppressor function (15). It was suggested that this SH3 domain mutation may affect the intramolecular interactions of Dlg (27). We introduced a corresponding mutation in the hDlg (L613P); however, the mutant hDlg did not activate GAKIN irrespective of whether the mutation was introduced in the I2 or I3 containing hDlg isoforms (Figure 3C). Further characterization of the hDlg structure will be required to fully define the nature of the closed conformation. Nonetheless, the available data suggest that the nature of intramolecular interactions of hDlg is critical for the regulation of GAKIN motor activity. At this stage, we do not know the identity of the intracellular signal that activates the auto-inhibited state of the hDlg-I3 isoform. We can speculate that specific binding partners of hDlg, such as the PDZ binding transmembrane receptors, might change the conformation of hDlg, which in turn would activate GAKIN to initiate the translocation of the motor-cargo complex in vivo (model shown in Figure 4).
FIGURE 4.
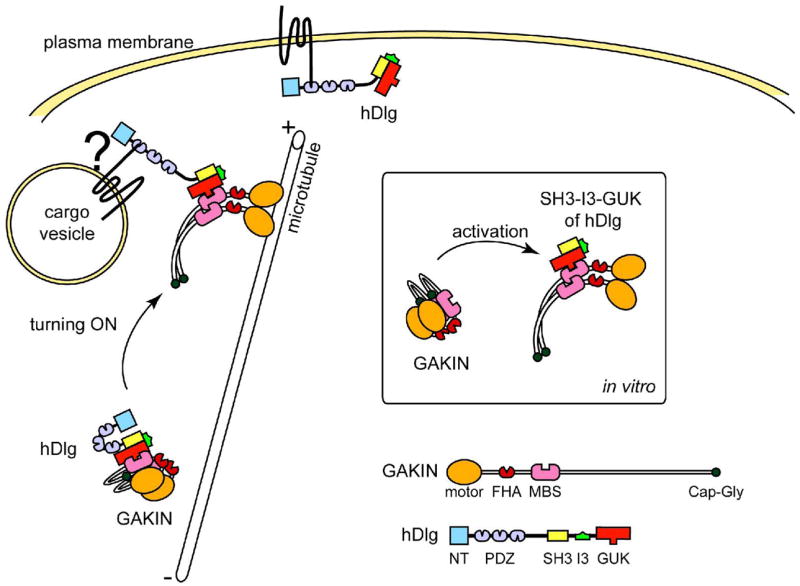
Proposed model of the regulation of GAKIN by hDlg binding. GAKIN forms intramolecular interactions and is inactive in solution in vitro. Binding of SH3-I3-GUK of hDlg relieves the intramolecular inhibition of GAKIN and frees its motor domain accessible to microtubules. Because the binding of full length hDlg is not sufficient to activate GAKIN, some as yet unknown mechanism must alter the conformation of full length hDlg so that it can activate GAKIN in vivo. Stable interactions of hDlg with proteins residing on the membrane vesicles might trigger the conformational change of hDlg, which in turn activate GAKIN, thereby initiating the trafficking of the vesicles along microtubules.
Functional differences between the two isoforms of hDlg (hDlg-I2 and hDlg-I3) have been reported previously (17, 25, 27, 29, 30). The I3 insert binds to the FERM domain of protein 4.1, and this binding sequence is conserved in several other MAGUKs that may mediate their physical linkage to the actin cytoskeleton (29). Emerging evidence also suggests that the I3 insert performs regulatory functions that are independent of its protein 4.1-binding activity. For example, the binding of GKAP to the GUK domain of SAP97 is strongly inhibited by an intramolecular interaction in the I3 isoform, whereas significantly less inhibition was observed in the I2 isoform (27). Adenovirus type 9 E4-ORF1 mediated transformation pathway, which leads to PI3-kinase activation, requires the participation of the I3 isoform of hDlg but not the I2 isoform (30). The I3 isoform of hDlg targets to the initial cell-cell contact sites in non-confluent MDCK epithelial cells much more efficiently than the I2 isoform (25). Furthermore, over-expression of the I3 isoform of SAP97 in neurons enhances the size of dendritic spines, the number of surface AMPA receptors, and synaptic function, whereas the I2 isoform does not participate in any of these functions (17). We speculate that some of these phenomena may be attributed to the function of the hDlg-I3 isoform to activate GAKIN-dependent intracellular transport in vivo.
Our results, as presented here, demonstrate that the motor activity of GAKIN is autoinhibited by intramolecular interactions, particularly via interactions between the motor and MBS domains. Direct binding of the SH3-I3-GUK module of hDlg relieves inhibition and activates GAKIN. This is the first demonstration showing the regulation of a kinesin motor by direct interaction of its protein cargo using reconstituted purified components. Accumulating evidence now suggests that this form of regulation is a common feature for various kinesins. Kinesin-1, the most well studied kinesin for its regulatory mechanism, forms an inhibitory conformation via its tail region of the heavy chain and also by its light chain (6, 31). Simultaneous interactions with two binding partners, the light chain binder, JIP1, and the heavy chain binder, FEZ1, activates kinesin-1 in the intact cells as well as lysates derived from the transfected cells (32). OSM-3, a kinesin-2 protein of C. elegans, is also an inactive motor in its native form in vitro, but can be turned on to an active motor by attaching an artificial cargo at the tail domain or introducing a point mutation in the stalk region that disrupts its intramolecular fold (9). KIF1A, a kinesin-3 protein, also participates in intramolecular interactions and mutations designed to disrupt these interactions convert it to an active motor in neuronal cells (11). Together, our results provide a proof of principle evidence that may govern generally conserved regulatory mechanisms for the activation of kinesins across species.
Acknowledgments
We thank Drs. Hae kyung Lee, Noriyuki Asaba, and Atsuko Takeuchi for their contributions in the early phase of this work. We are also grateful to Dr. David Bredt for sharing the PSD-95 plasmid. We thank Dena Inempolidis for help with the preparation of this manuscript.
Abbreviations
- FHA
forkhead associated
- FL
full length
- GAKIN
guanylate kinase associated kinesin
- GUK
guanylate kinase
- KHC
kinesin heavy chain
- KLC
kinesin light chain
- KIF
kinesin family
- hDlg
human discs large
- MAGUK
membrane-associated guanylate kinase homologue
- MBS
MAGUK binding stalk domain
- MT
microtubule
- PSD-95
post synaptic density 95
- SAP97
synapse associated protein 97
- SH
src homology
Footnotes
This work was supported by the National Institutes of Health grants CA 94414 and HL60755. T.H. is a recipient of Campus Research Board Award from the University of Illinois at Chicago (2004–2005).
References
- 1.Hirokawa N, Takemura R. Molecular motors and mechanisms of directional transport in neurons. Nat Rev Neurosci. 2005;6:201–214. doi: 10.1038/nrn1624. [DOI] [PubMed] [Google Scholar]
- 2.Hackney DD. Jump-starting kinesin. J Cell Biol. 2007;176:7–9. doi: 10.1083/jcb.200611082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cross R, Scholey J. Kinesin: the tail unfolds. Nat Cell Biol. 1999;1:E119–121. doi: 10.1038/12947. [DOI] [PubMed] [Google Scholar]
- 4.Stock MF, Guerrero J, Cobb B, Eggers CT, Huang TG, Li X, Hackney DD. Formation of the compact confomer of kinesin requires a COOH-terminal heavy chain domain and inhibits microtubule-stimulated ATPase activity. J Biol Chem. 1999;274:14617–14623. doi: 10.1074/jbc.274.21.14617. [DOI] [PubMed] [Google Scholar]
- 5.Hackney DD, Stock MF. Kinesin’s IAK tail domain inhibits initial microtubule-stimulated ADP release. Nat Cell Biol. 2000;2:257–260. doi: 10.1038/35010525. [DOI] [PubMed] [Google Scholar]
- 6.Hackney DD, Levitt JD, Suhan J. Kinesin undergoes a 9 S to 6 S conformational transition. J Biol Chem. 1992;267:8696–8701. [PubMed] [Google Scholar]
- 7.Friedman DS, Vale RD. Single-molecule analysis of kinesin motility reveals regulation by the cargo-binding tail domain. Nat Cell Biol. 1999;1:293–297. doi: 10.1038/13008. [DOI] [PubMed] [Google Scholar]
- 8.Coy DL, Hancock WO, Wagenbach M, Howard J. Kinesin’s tail domain is an inhibitory regulator of the motor domain. Nat Cell Biol. 1999;1:288–292. doi: 10.1038/13001. [DOI] [PubMed] [Google Scholar]
- 9.Imanishi M, Endres NF, Gennerich A, Vale RD. Autoinhibition regulates the motility of the C. elegans intraflagellar transport motor OSM-3. J Cell Biol. 2006;174:931–937. doi: 10.1083/jcb.200605179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hanada T, Lin L, Tibaldi EV, Reinherz EL, Chishti AH. GAKIN, a novel kinesin-like protein associates with the human homologue of the Drosophila discs large tumor suppressor in T lymphocytes. J Biol Chem. 2000;275:28774–28784. doi: 10.1074/jbc.M000715200. [DOI] [PubMed] [Google Scholar]
- 11.Lee JR, Shin H, Choi J, Ko J, Kim S, Lee HW, Kim K, Rho SH, Lee JH, Song HE, Eom SH, Kim E. An intramolecular interaction between the FHA domain and a coiled coil negatively regulates the kinesin motor KIF1A. Embo J. 2004;23:1506–1515. doi: 10.1038/sj.emboj.7600164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Asaba N, Hanada T, Takeuchi A, Chishti AH. Direct interaction with a kinesin-related motor mediates transport of mammalian discs large tumor suppressor homologue in epithelial cells. J Biol Chem. 2003;278:8395–8400. doi: 10.1074/jbc.M210362200. [DOI] [PubMed] [Google Scholar]
- 13.Horiguchi K, Hanada T, Fukui Y, Chishti AH. Transport of PIP3 by GAKIN, a kinesin-3 family protein, regulates neuronal cell polarity. J Cell Biol. 2006;174:425–436. doi: 10.1083/jcb.200604031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Montgomery JM, Zamorano PL, Garner CC. MAGUKs in synapse assembly and function: an emerging view. Cell Mol Life Sci. 2004;61:911–929. doi: 10.1007/s00018-003-3364-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Woods DF, Hough C, Peel D, Callaini G, Bryant PJ. Dlg protein is required for junction structure, cell polarity, and proliferation control in Drosophila epithelia. J Cell Biol. 1996;134:1469–1482. doi: 10.1083/jcb.134.6.1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sans N, Racca C, Petralia RS, Wang YX, McCallum J, Wenthold RJ. Synapse-associated protein 97 selectively associates with a subset of AMPA receptors early in their biosynthetic pathway. J Neurosci. 2001;21:7506–7516. doi: 10.1523/JNEUROSCI.21-19-07506.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rumbaugh G, Sia GM, Garner CC, Huganir RL. Synapse-associated protein-97 isoform-specific regulation of surface AMPA receptors and synaptic function in cultured neurons. J Neurosci. 2003;23:4567–4576. doi: 10.1523/JNEUROSCI.23-11-04567.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakagawa T, Futai K, Lashuel HA, Lo I, Okamoto K, Walz T, Hayashi Y, Sheng M. Quaternary structure, protein dynamics, and synaptic function of SAP97 controlled by L27 domain interactions. Neuron. 2004;44:453–467. doi: 10.1016/j.neuron.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 19.Setou M, Nakagawa T, Seog DH, Hirokawa N. Kinesin superfamily motor protein KIF17 and mLin-10 in NMDA receptor-containing vesicle transport. Science. 2000;288:1796–1802. doi: 10.1126/science.288.5472.1796. [DOI] [PubMed] [Google Scholar]
- 20.McGee AW, Dakoji SR, Olsen O, Bredt DS, Lim WA, Prehoda KE. Structure of the SH3-guanylate kinase module from PSD-95 suggests a mechanism for regulated assembly of MAGUK scaffolding proteins. Mol Cell. 2001;8:1291–1301. doi: 10.1016/s1097-2765(01)00411-7. [DOI] [PubMed] [Google Scholar]
- 21.Williams RC, Jr, Lee JC. Preparation of tubulin from brain. Methods Enzymol. 1982;85(Pt B):376–385. doi: 10.1016/0076-6879(82)85038-6. [DOI] [PubMed] [Google Scholar]
- 22.Hackney DD, Jiang W. Assays for kinesin microtubule-stimulated ATPase activity. Methods Mol Biol. 2001;164:65–71. [PubMed] [Google Scholar]
- 23.Marfatia SM, Byron O, Campbell G, Liu SC, Chishti AH. Human homologue of the Drosophila discs large tumor suppressor protein forms an oligomer in solution. Identification of the self-association site. J Biol Chem. 2000;275:13759–13770. doi: 10.1074/jbc.275.18.13759. [DOI] [PubMed] [Google Scholar]
- 24.Khanna R, Chang SH, Andrabi S, Azam M, Kim A, Rivera A, Brugnara C, Low PS, Liu SC, Chishti AH. Headpiece domain of dematin is required for the stability of the erythrocyte membrane. Proc Natl Acad Sci U S A. 2002;99:6637–6642. doi: 10.1073/pnas.052155999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hanada T, Takeuchi A, Sondarva G, Chishti AH. Protein 4.1-mediated membrane targeting of human discs large in epithelial cells. J Biol Chem. 2003;278:34445–34450. doi: 10.1074/jbc.M305209200. [DOI] [PubMed] [Google Scholar]
- 26.McGee AW, Bredt DS. Identification of an intramolecular interaction between the SH3 and guanylate kinase domains of PSD-95. J Biol Chem. 1999;274:17431–17436. doi: 10.1074/jbc.274.25.17431. [DOI] [PubMed] [Google Scholar]
- 27.Wu H, Reissner C, Kuhlendahl S, Coblentz B, Reuver S, Kindler S, Gundelfinger ED, Garner CC. Intramolecular interactions regulate SAP97 binding to GKAP. Embo J. 2000;19:5740–5751. doi: 10.1093/emboj/19.21.5740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nix SL, Chishti AH, Anderson JM, Walther Z. hCASK and hDlg associate in epithelia, and their src homology 3 and guanylate kinase domains participate in both intramolecular and intermolecular interactions. J Biol Chem. 2000;275:41192–41200. doi: 10.1074/jbc.M002078200. [DOI] [PubMed] [Google Scholar]
- 29.Lue RA, Marfatia SM, Branton D, Chishti AH. Cloning and characterization of hdlg: the human homologue of the Drosophila discs large tumor suppressor binds to protein 4.1. Proc Natl Acad Sci U S A. 1994;91:9818–9822. doi: 10.1073/pnas.91.21.9818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Frese KK, Latorre IJ, Chung SH, Caruana G, Bernstein A, Jones SN, Donehower LA, Justice MJ, Garner CC, Javier RT. Oncogenic function for the Dlg1 mammalian homolog of the Drosophila discs-large tumor suppressor. Embo J. 2006;25:1406–1417. doi: 10.1038/sj.emboj.7601030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Verhey KJ, Lizotte DL, Abramson T, Barenboim L, Schnapp BJ, Rapoport TA. Light chain-dependent regulation of Kinesin’s interaction with microtubules. J Cell Biol. 1998;143:1053–1066. doi: 10.1083/jcb.143.4.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blasius TL, Cai D, Jih GT, Toret CP, Verhey KJ. Two binding partners cooperate to activate the molecular motor Kinesin-1. J Cell Biol. 2007;176:11–17. doi: 10.1083/jcb.200605099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marfatia SM, Morais Cabral JH, Lin L, Hough C, Bryant PJ, Stolz L, Chishti AH. Modular organization of the PDZ domains in the human discs-large protein suggests a mechanism for coupling PDZ domain-binding proteins to ATP and the membrane cytoskeleton. J Cell Biol. 1996;135:753–766. doi: 10.1083/jcb.135.3.753. [DOI] [PMC free article] [PubMed] [Google Scholar]


