The menstrual cycle has long-been viewed to influence the experience of nausea and motion sickness, but empirical support for this view is mixed. Retrospective self-report surveys indicate that women are more susceptible to dizziness, fatigue, and vomiting (i.e., motion sickness) than men during situations that create a mismatch between visually-perceived movement and vestibular encoding of movement (Collins & Lentz, 1977). This sex difference has been reported for real-life situations, including air travel (Lindseth & Lindseth, 1995), travel across water (Grunfeld & Gresty, 1998; Lawther & Griffin, 1988), and terrestrial coach travel (Turner & Griffin, 1999). Post-operative nausea and vomiting (PONV), which is more severe in patients with a history of motion sickness (Rita, Goodarzi, & Seleny, 1981), is also more common in women than men (Janhumen & Tammisto, 1972; Palazzo & Strunin, 1984). Although results from studies that experimentally induce acute motion sickness are somewhat more ambiguous as they relate to sex differences, women generally report more motion sickness symptoms than men (Clemes & Howarth, 2005; Flanagan, May, & Dobie, 2005; Jokerst et al., 1999). Notably, a comparable sex difference has even been observed in non-human animals: female musk shrews (Suncus suncus) express more emesis (vomiting) and exhibit shorter latencies to emesis after exposure to a horizontal motion stimulus (Javid & Naylor, 1999; but see Ordy & Brizzee, 1980 for contrary results with squirrel monkeys).
Explanations for sex differences in nausea and motion sickness susceptibility, which are arguably untenable in light of recent empirical evidence, have included: i.) different exposure histories to various forms of transit, ii.) developmental differences in the engagement of physical, “rough and tumble” activities, and iii.) a reporting bias wherein women are more likely to express or report their symptoms of malaise (Dobie et al., 2001). More plausibly, such sex differences may be more closely linked to cyclic variations in reproductive hormones during the menstrual cycle, which have been shown to moderate autonomic and cardiovascular reactivity, gastrointestinal motility, and visceral perception (Girdler & Light, 1994; Hastrup & Light, 1984; Heitkemper & Jarett, 1992; Litschauer et al., 1998; Mayer et al., 1999; Mills & Berry, 1996). Interestingly, emetic episodes tend to increase as females approach menarche (Rita et al., 1981) and decrease during the menopausal transition (Forrest et al., 1990). Additionally, higher levels of estrogen and progesterone during pregnancy (as compared to post-partum or voluntary interruption of pregnancy status) have been linked to gastrointestinal phenomena related to subjective distress, including prolonged gastrointestinal transit times (Wald et al., 1982), reduced lower esophageal sphincter pressures in response to pharmacological and physical stimulation (Fisher et al., 1978), and more unstable electrogastrographic (EGG) activity (Riezzo et al., 1992). In parallel to human evidence, ovarian steroid administration in rodent models not only increases gastrointestinal motility (Bruce & Beshudi, 1981), but also inhibits gastric emptying, probably by increasing plasma levels of cholecystokinin (CCK) and CCK receptor expression (Yang, Liu, & Dong, 2006).
Given the lack of a definitive understanding of these issues, we reasoned that a logical line of inquiry would be to examine susceptibility to nausea associated with motion sickness as a function of the phase of the menstrual cycle. To date, however, the findings regarding the relationship between phase of the menstrual cycle and susceptibility to nausea and other related symptoms have been mixed. For example, more severe PONV, which is more common in people with a history of motion sickness (Rita et al., 1981), has been found for the peri-ovulatory period (approximate menstrual dates 11– 24) after middle ear surgery (Honkavaara, Pyykko, & Rutanen, 1996), extraction of wisdom teeth (Ramsay, McDonald, & Faragher, 1994), and gynaecological laparoscopy (Honkavaara, Lehtinen, & Hovorka, 1991). In contrast, more PONV has also been found in the peri-menstrual period for gynaecological laparoscopy (Beattie et al., 1991; Beattie et al., 1993). Other studies (e.g., Janhumen & Tammisto, 1972) indicate that phase of the menstrual cycle has no effect on PONV (although there was a non-significant trend towards more severe symptoms near menses in this study).
Differences in surgery type, anesthesia, definition of menstrual cycle stages, and/or employment of within versus independent-groups designs may account for divergent findings of PONV risk across the menstrual cycle. Although the mechanisms and neural pathways of nausea experienced during vection-induced motion sickness and those experienced during post-surgery are unlikely to be identical, there may be significant similarities for these two conditions. Of etiological relevance is the finding that antimotion sickness drugs can reduce the symptoms of PONV (Rubin & Metz-Rubin, 1951), although they are probably not as effective as traditional dopamine antagonists such as phenothiazines (Bellville, 1961). Decompression sickness incidents also increase in female divers near menses, but only for women not on oral contraceptive pills (Lee et al., 2003).
Concerning the menstrual cycle and motion sickness, Schwab (1954) described an anecdotal report of an army medical corps nurse who crossed the Atlantic in rough weather with no symptoms of motion sickness, but who later experienced severe vomiting and nausea in the quiet waters of the Mediterranean during her menstrual period. Unfortunately, few systematic studies have attempted to explore the influence of menstrual cycle stage on susceptibility to motion sickness under these conditions of actual motion or vection, a visually-induced false sense of movement. Grunfeld and Gresty (1998) examined female sailors in a “round-the-world” yacht race, finding more self-reported motion sickness and migraine headaches near menstruation. In two independent laboratory investigations using Coriolis-induced sickness, Cheung et al. (2001) found no effect for menstrual cycle phase in women, whereas Golding, Kadzere, and Gresty (2005) found increased susceptibility near menses. Clemes and Howarth (2001) found more susceptibility to virtual stimulation sickness on menstrual day 12 than on days 5, 19, or 26. In an elaboration of this study, Clemes and Howarth (2005) again used a nauseogenic virtual stimulation (video game) to induce motion sickness. However, menstrual phase was objectively confirmed by assaying salivary estradiol and progresterone levels. Women were more susceptible to motion sickness than men, and women also showed more susceptibility when ovulating (day 12 of the menstrual cycle). However, this increase in malaise was only noted for day 12 women in which estrogen was elevated (n = 9), as indicated by the hormonal assays. Day 12 women (n = 7) with long menstrual cycles (and thus no elevated estrogen on day 12) did not show increased susceptibility across the menstrual cycle, nor did a group of women on oral contraceptives. These results suggest that fluctuating reproductive hormones like estrogen may modulate motion sickness susceptibility.
The present study evaluated the effects of stage of the menstrual cycle on subjective reports of motion sickness during exposure to a rotating optokinetic drum. Confirmation of a menstrual cycle phase effect would be valuable for theoretical and practical reasons. The significance of establishing any putative influence of the menstrual cycle on susceptibility to motion sickness is reflected by the fact that women are increasingly being employed in military and civilian settings where personnel are exposed to stimulation capable of provoking nausea and motion sickness. Based upon the majority of prior research (e.g., Beattie et al., 1993; Golding et al., 2005), it was hypothesized that women in the peri-menses phase of the menstrual cycle would report greater symptoms of motion sickness compared to women in the peri-ovulatory phase.
Method
Participants
Ninety female students (median age = 19 years; range = 18 – 22) at a large public research university in the mid-Atlantic States provided informed consent and participated for course credit. The study protocol was approved in advance by the local Institutional Review Board and was executed in accordance with the ethical standards of the 1964 Declaration of Helsinki. Participants were screened to exclude those with a history of menstrual cycle irregularities, and neurological, cardiovascular, and gastrointestinal disorders. Participants were asked to refrain from consuming caffeine and alcoholic beverages, and to abstain from smoking cigarettes for a minimum of 24 hours prior to testing. They were also asked to refrain from eating for a minimum of three hours prior to testing. None of the participants were experiencing symptoms of nausea or motion sickness when they arrived at the laboratory. After the testing session, each participant was contacted periodically by a female experimenter until the date of the beginning of the next menses was reported. This date was used to estimate the phase of the participant's menstrual cycle during her participation in the experiment, assuming an approximate 28-day cycle. Using this method, participants were categorized as being in the peri-menses (days 25-10; n = 52) or peri-ovulatory (days 11-24; n =38) phase of the menstrual cycle. Twenty-six women in the peri-menses group and 16 women in the peri-ovulatory group were using oral contraceptives.
Apparatus
Symptoms of motion sickness (nausea, dizziness, drowsiness, headache, warmth, and sweating) were assessed once before, and every three minutes during, exposure to the rotating drum by the Subjective Symptoms of Motion Sickness (SSMS) questionnaire (Graybiel, Wood, Miller, & Cramer, 1968). Participants were asked, for each symptom, whether they were currently experiencing none, mild, moderate, or severe levels of the symptoms. The final ratings of the symptoms were used to compute a composite index of the severity of the nausea and motion sickness experienced. Symptoms were also assessed retrospectively (five minutes following exposure to the rotating drum) by the Nausea Profile (NP) (Muth, Stern, Thayer, & Koch, 1996). Responses on the NP was used to derive total nausea scores and subscale scores reflecting three dimensions of nausea: gastrointestinal distress (sick, queasy, ill, stomach discomfort, vomiting), somatic distress (shaky, lightheaded, sweaty, tired/fatigued, weak, warmth), and emotional distress (upset, worried, hopeless, panicked, nervous, scared/afraid).
Nausea and motion sickness symptoms were induced by a rotating optokinetic drum. The drum, which is 91.5 cm in height and 76 cm in diameter, is lined with alternating 3.8 cm black and 6.2 cm white vertical stripes. With the participants seated inside on a stationary stool, the drum rotated at a speed of 10 rotations per minute. Viewing of the motion of the drum induces the illusion of self-motion, or vection, in the direction opposite the drum’s rotation, and is sufficient for susceptible individuals to experience symptoms.
Procedure
After providing informed consent, participants were seated inside the motionless drum for a six-minute baseline period, followed by sixteen minutes of drum rotation, or shorter if early termination was requested by the participant. After ten minutes of rotation, participants began a series of head movements until the rotation period ended. Head movements involved the tilting of the head from shoulder to shoulder to the beat of a metronome at a rate of approximately 24 head movements per minute.
Data Quantification and Analysis
SSMS scores were determined by summing the individual symptom scores from the final assessment of symptom severity. The maximum score is 35. NP scores were quantified as percentages of the maximum score for the Total NP, and for each of the Gastrointestinal, Emotional, and Somatic NP subscales. One-way ANOVAs on NP and SSMS scores were used to compare women in the peri-menses and peri-ovulatory phases of the menstrual cycle for women not taking oral contraceptives and also for women taking oral contraceptives. P-values less than .05 were considered statistically significant, and those between .05 and .10 were considered marginally significant.
Results
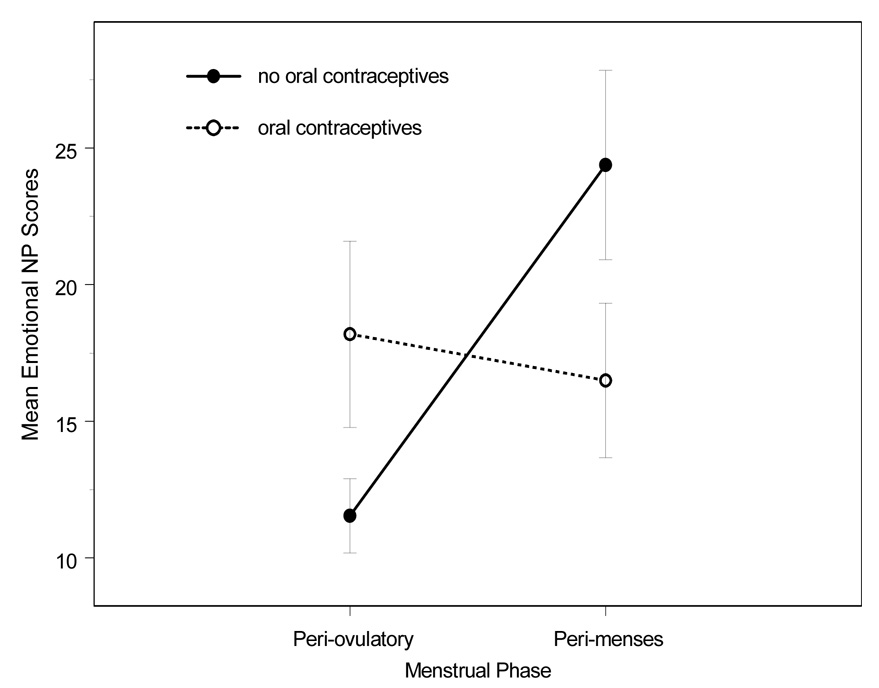
Table 1 shows the mean NP and SSMS scores reported by women in the peri-menses and peri-ovulatory phases of the menstrual cycle. A marginally significant difference was observed between Total NP scores of women in the peri-menses phase and women in the peri-ovulatory phase, such that the peri-menses phase was associated with more severe symptoms, F(1, 46) = 3.20, p =.08. Emotional NP scores were significantly higher among women in the peri-menses phase than women in the peri-ovulatory phase, F(1, 46) = 5.81, p = 02. A marginally significant difference was also observed between SSMS scores of women in the peri-menses phase and women in the peri-ovulatory phase, such that the peri-menses phase was associated with more severe symptoms, F(1, 46) = 2.70 , p =.10. There were no differences in nausea and motion sickness symptoms as a function of menstrual cycle phase for women using oral contraceptives (all p > .05). Figure 1 shows Emotional NP scores for women using and not using oral contraceptives.
Table 1.
Means and SEMs of the NP Total and Subscale Scores and the SSMS questionnaire as a Function of Stage of the Menstrual Cycle and Oral Contraceptive Use
| No Oral Contraceptives |
Oral Contraceptives |
|||
|---|---|---|---|---|
| Peri-Menses | Peri-ovulatory | Peri-Menses | Peri-Ovulatory | |
| Subjective Measure | (n = 26) | (n = 22) | (n = 26) | (n = 16) |
| Nausea Profile | ||||
| Total | 35.71 * (4.23) | 25.89 (3.25) | 31.63 (4.16) | 36.65 (5.75) |
| Somatic | 39.98 (3.94) | 34.13 (4.03) | 39.40 (4.59) | 38.73 (5.06) |
| Gastrointestinal | 43.90 (6.08) | 33.25 (5.60) | 40.16 (6.22) | 49.76 (8.07) |
| Emotional | 24.39 ** (4.47) | 11.54 (2.36) | 16.50 (3.83) | 18.19 (4.41) |
| SSMS | 12.27 * (2.23) | 7.91 (1.21) | 12.54 (1.81) | 13.81 (2.97) |
Note: SSMS = Subjective Symptoms of Motion Sickness questionnaire scores at end of drum rotation; SEM = standard error of the mean
= marginal significance (p < .05 – .10) and
= p < .05 between peri-menses and peri-ovulatory groups within oral contraceptive conditions. Standard errors of the means are in parentheses.
Figure 1.
Mean Emotional NP scores for peri-menses and peri-ovulatiory menstrual phases for oral contraceptive and non-oral contraceptive women. Error bars indicate standard error of the mean.
Forty-four percent of the participants requested drum cessation before the full 16 minutes (range = 5 to 15 minutes into drum rotation). Bivariate correlations between latency to request termination scores and SMSS and NP scores were all negative (i.e., shorter latencies to request termination were associated with more severe symptoms). However, only the correlation with the final SMSS scores was statistically significant (r = − .48, p < .01). A chi-square analysis indicated that a participant’s decision whether or not to terminate drum rotation did not vary according to phase of the menstrual cycle or oral contraceptive use (all p > .05).
Discussion
The main finding of the present study was that women not using oral contraceptives reported more symptoms of nausea and motion sickness during exposure to a rotating optokinetic drum in the peri-menses phase of the menstrual cycle than women in the peri-ovulatory phase of their menstrual cycle. Specifically, peri-menses phase women endorsed higher total nausea symptoms, higher emotion-related nausea symptoms, and higher motion sickness symptoms than women in the peri-ovulatory phase. By convention, the average SSMS score of 12 among peri-menstrual women not on oral contraceptives is typically representative of moderate-to-severe nausea and moderate-to-severe levels of at least two other motion sickness symptoms. A score of 8, which was the representative score for women not on oral contraceptives in the peri-ovulatory phase, often indicates little or no nausea and moderate levels of two other motion sickness symptoms.
At present, it is unclear why somatic and gastrointestinal symptoms did not vary as a function of phase of the menstrual cycle. One study found that individuals who are susceptible to motion sickness had higher State-Trait anxiety scores (Collins & Lentz, 1977), and an older study (e.g., Schwab, 1954) reported more “neurotic” traits in people susceptible to motion sickness. However, an inspection of Table 1 shows a trend toward higher scores during menses for all of the subjective measures of nausea. This same trend is not observed for women on oral contraceptives, which is not surprising given their attenuation of cyclic menstrual hormones.
Although we did not have data on type of oral contraceptive, traditional birth control pills (“combination pills” that contain both estrogen and progesterone) allow for vaginal bleeding similar to a regular menstrual cycle, unlike oral contraceptives such as Seasonale (Anderson, Gibbons, & Portman, 2006). It did not escape our attention that for some NP subscales there was little difference between any participant groups except peri-ovulatory women not on oral contraceptives. Women on oral contraceptives, under most circumstances, do not ovulate, and therefore, may not benefit from any protective effect of ovulation against the development of nausea and motion sickness. During menses, oral contraceptive and non-oral contraceptive women may be somewhat similar, hormonally and in regard to reported motion sickness symptomatology. Moreover, type of hormone dose received by the oral contraceptive group was unknown in the current study. Combination pills can be monophasic (with one constant hormone dose), biphasic (two hormone doses across the cycle), or triphasic (three different horomone doses). Presumably, the ideal oral contraceptive control group would be to employ only monophasic oral contraceptive women (see Clemes & Howarth, 2005). Type of oral contraceptive could prove to be an important research variable for those interested in nausea susceptibility and reproductive hormones.
The present results are consistent with Grunfeld and Gresty’s (1998) study that employed female sailors in a yacht race as participants, perhaps lending some external validity to the current findings. Our results are also consistent with laboratory studies using Coriolis-induced sickness (e.g., Golding et al., 2005) and various studies finding more PONV near menses (e.g., Beattie et al., 1991; Beattie et al., 1993), as well as more decompression sickness near menses (Lee et al., 2003). Our results also indicate that reported symptoms of nausea and motion sickness did not vary across the menstrual cycle for women who were taking oral contraceptives, congruent with prior research (e.g., Clemes & Howarth, 2005; Lee et al., 2003). In addition to our current report, there are three recent studies on the menstrual cycle and motion sickness, all three with conflicting results (e.g., Golding et al., 2005 – more nausea during menses; Cheung et al., 2005 – no change in nausea across the menstrual cycle; Clemes & Howarth, 2005 – more nausea near ovulation). Golding et al. (2005) postulated that these differences may stem from different types of nauseogenic stimuli. For example, in the Cheung et al. (2005) study, a very potent stimulus (120° s−1 during cross-coupled motion) was used that may have masked any subtle effect of the menstrual cycle. A weaker manipulation (self-directed video game) was used in the Clemes and Howarth (2005) study that was more under control of the research participants than traditionally-used nauseogenic stimuli. Further systematic research is clearly needed to understand the variables that contribute to these inter-laboratory differences.
Because of resource limitations, stage of the menstrual cycle was not objectively confirmed by examination of circulating hormone levels or variations in temperature in the present study; thus, individual differences in cycle length may have resulted in a misclassification of women into a particular phase. The classification procedure employed here, however, approximates those employed by other investigations reporting an effect of the menstrual cycle on motion sickness (Grunfeld & Gresty, 1998; Grunfeld, Price, Goadsby, & Gresty, 1998) and PONV symptoms (Honkavaara et al., 1991; Honkavaara et al., 1996; Ramsay et al., 1994). Thus, it seems unlikely that differences in symptom reports were obscured by the present method of categorization. It would, though, be more informative for future research to utilize objective hormonal measures of the menstrual cycle rather than, or in addition to self-reports. For example, younger women are more likely to have a higher prevalence of anovulatory cycles as measured by progesterone profiles (Lipson & Ellison, 1992; Vuorento, Lahti, Hovatta, & Huhtaniemi, 1989), and the majority of studies on nausea and the menstrual cycle employ young women and rely on self-reported menstrual cycle data, including the current study.
The present study used an independent-groups design, whereas other laboratory studies of nausea and the menstrual cycle employed within-groups designs (e.g., Cheung et al., 2001; Golding et al., 2005; Clemes & Howarth, 2005). This design was necessary, in part, because of the practical difficulties associated with repeated exposures of the same participants to the rotating drum. Moreover, it is known that with repeated exposure to a nauseogenic stimulus, habituation to a motion-sickness inducing stimulus can occur (Caillet et al., 2006; Hu, Grant, Stern, & Koch, 1991), as well as sensitization in high susceptibility individuals (Zhao & Stern, 1999). Each of these potential confounds has been avoided by the employment of the present design. In light of these methodological considerations, we extend prior work in this laboratory-based, independent-groups study of motion sickness by providing convergent validation to the emerging literature finding a link between the menstrual cycle and motion sickness.
Taken together, the present study and others (e.g., Clemes & Howarth, 2005; Golding et al., 2005) suggest that cyclic fluctuation of hormones may mediate susceptibility to various forms of nausea-inducing stimuli. The specific mechanisms underlying this mediation remain uncertain. Intriguingly, though, estrogen can increase the number of dopamine receptors (Hruska & Silbergeld, 1980), while antiemetic drugs such as Droperidol, which are commonly used to treat PONV in adults (Tornetta, 1977), inhibit dopamine (D2) receptors. Rita et al. (1981) found that a low dose (i.e., .005 mg.kg −1) of droperidol effectively treated PONV in children 11–15 years old undergoing puberty, but not in the 1 – 5 and 6 – 10 year old groups. Moreover, near ovulation, women tend to experience an increased sensitivity in auditory (Swanson & Dengerink, 1988), olfactory (Navarrete-Palacios et al., 2003), and visual (Eisner, Burke, & Tommey, 2004) including color vision (Giuffre, Rosa, & Fiorino, 2007) modalities, possibly exacerbating any mismatches between visual and vestibular perception. Given any putative effects of estrogen, it is still unclear as to why some studies find increased motion sickness and PONV near menses (e.g., Beattie et al., 1991; Golding et al., 2005). Beattie et al. (1991) postulate a nauseogenic effect of estrogen but argue that the initial rise or change in estrogen concentrations near the end of menses, rather than an absolute high concentration near ovulation, may sensitize vomiting centers and the chemoreceptor trigger zone in the medulla (Bellville, 1961). In addition, Gianni, Colleoni, Golding, and Goldhirsch (2005) reported that tamoxifen (an estrogen antagonist) relieved the symptoms of motion sickness in two breast cancer patients, further implicating estrogen in the etiology of motion sickness.
The present results encourage future work on the relationship between nausea and motion sickness susceptibility and phase of the menstrual cycle, including a careful evaluation of the potential effects of experimental design differences and various forms of nauseogenic stimuli. On a theoretical level, future research will offer important insights into the link between ovarian functioning and the functional physiology of the vestibular and gastrointestinal systems, as well as medullary systems important for vomiting and other clinically relevant syndromes. On a more applied level, future chronobiological research may inform women in military and civilian work settings, as well as in personal environments, with valuable information about potential risk for nausea and motion sickness.
Acknowledgments
This research was supported partially by a National Research Service Award from the National Institute of Health to the third author. We thank Kelly Burke and Allison Reh for their assistance in study execution.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson FD, Gibbons W, Portman D. Long-term safety of an extended-cycle oral contraceptive (Seasonale): A 2-year multicenter open-label extension trial. American Journal of Obstetrics and Gynecology. 2006;195:92–96. doi: 10.1016/j.ajog.2005.12.045. [DOI] [PubMed] [Google Scholar]
- Beattie WS, Lindblad T, Buckley DN, Forrest JB. The incidence of post-operative nausea and vomiting in women undergoing laparoscopy is influenced by the day of the menstrual cycle. Canadian Journal of Anaesthesiology. 1991;28:298–302. doi: 10.1007/BF03007618. [DOI] [PubMed] [Google Scholar]
- Beattie WS, Lindblad T, Buckley DN, Forrest JB. Menstruation increases the risk of nausea and vomiting after laparoscopy: A prospective randomized study. Anesthesiology. 1993;78:272–276. doi: 10.1097/00000542-199302000-00010. [DOI] [PubMed] [Google Scholar]
- Bellville JW. Postanesthetic nausea and vomiting. Anesthesiology, ?? 1961:773–780. doi: 10.1097/00000542-196109000-00011. [DOI] [PubMed] [Google Scholar]
- Bruce LA, Beshudi FM. Increased gastrointestinal motility in vitro following chronic estrogen treatment in male rats. Proceedings of the Society for Experimental Biology and Medicine. 1981;166:355–359. doi: 10.3181/00379727-166-41073. [DOI] [PubMed] [Google Scholar]
- Caillet G, Bosser G, Gauchard GC, Chau N, Benamghar L, Perrin PP. Effect of sporting activity practice on susceptibility to motion sickness. Brain Research Bulletin. 2006;69:288–293. doi: 10.1016/j.brainresbull.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Clemes SA, Howarth PA. Changes in virtual simulator sickness susceptibility throughout the menstrual cycle; Paper presented at the 36th United Kingdom Group Meeting on Human Responses to Vibration, Centre for Human Sciences; September 12–14; QinetiQ, Farnborough, UK. 2001. [Google Scholar]
- Clemes SA, Howarth PA. The menstrual cycle and susceptibility to virtual simulation sickness. Journal of Biological Rhythms. 2005;20:71–82. doi: 10.1177/0748730404272567. [DOI] [PubMed] [Google Scholar]
- Collins WE, Lentz JM. Some psychological correlates of motion sickness susceptibility. Aviation, Space, and Environmental Medicine. 1977;48:587–594. [PubMed] [Google Scholar]
- Cheung B, Heskin R, Hofer K, Gagnon M. The menstrual cycle and susceptibility to coriolis-induced sickness. Journal of Vestibular Research. 2005;11:129–136. [PubMed] [Google Scholar]
- Dobie T, McBride D, Dobie T, Jr, May J. The effects of age and sex on susceptibility to motion sickness. Aviation, Space, and Environmental Medicine. 2001;72:13–20. [PubMed] [Google Scholar]
- Eisner A, Burke SN, Toomey MD. Visual sensitivity across the menstrual cycle. Vison Neuroscience. 2004;21:513–531. doi: 10.1017/S0952523804214031. [DOI] [PubMed] [Google Scholar]
- Fisher RS, Roberts GS, Grabowski CJ, Cohen S. Altered lower esophageal sphincter function during early pregnancy. Gastroenterology. 1978;74:1233–1237. [PubMed] [Google Scholar]
- Flanagan MB, May JG, Dobie TG. Sex differences in tolerance to visually-induced motion sickness. Aviation, Space, and Environmental Medicine. 2005;76:642–646. [PubMed] [Google Scholar]
- Gianni L, Colleoni M, Golding JF, Goldhirsch A. Can tomoxifen relieve motion sickness ? Annals of Oncology. 2005;16:1713–1714. doi: 10.1093/annonc/mdi304. [DOI] [PubMed] [Google Scholar]
- Girdler SS, Light KC. Hemodynamic stress responses in men and women examined as a function of the female menstrual cycle. International Journal of Psychophysiology. 1994;17:233–248. doi: 10.1016/0167-8760(94)90066-3. [DOI] [PubMed] [Google Scholar]
- Giuffre G, Di Rosa L, Fiorino F. Changes in colour discrimination during the menstrual cycle. Ophthalmologica. 2007;221:47–50. doi: 10.1159/000096522. [DOI] [PubMed] [Google Scholar]
- Golding JF, Kadzere P, Gresty MA. Motion sickness susceptibility fluctuates through the menstrual cycle. Aviation, Space, and Environmental Medicine. 2005;76:970–973. [PubMed] [Google Scholar]
- Graybiel A, Wood CD, Miller EF, Cramer DB. Diagnostic criteria for grading the severity of acute motion sickness. Aerospace Medicine. 1968;39:453–455. [PubMed] [Google Scholar]
- Grunfeld E, Gresty MA. Relationship between motion sickness, migraine, and menstruation in crew members of a “round-the-world” yacht race. Brain Research Bulletin. 1998;47:433–436. doi: 10.1016/s0361-9230(98)00099-9. [DOI] [PubMed] [Google Scholar]
- Hastrup JL, Light KC. Sex differences in cardiovascular stress responses: Modulation as a function of menstrual cycle phases. Journal of Psychosomatic Research. 1984;28:475–483. doi: 10.1016/0022-3999(84)90081-3. [DOI] [PubMed] [Google Scholar]
- Heitkemper MM, Jarett M, Caudell KA, Bond E. Women with gastrointestinal symptoms: Implications for nursing research and practice. Gastroenterology Nursing, ?? 1993:226–232. [PubMed] [Google Scholar]
- Honkavaara P, Lehtinen AM, Hovorka J. Nausea and vomiting after gynaecological laparoscopy depends upon the phase of the menstrual cycle. Canadian Journal of Anaesthesiology. 1991;38:876–879. doi: 10.1007/BF03036963. [DOI] [PubMed] [Google Scholar]
- Honkavaara P, Pyykko I, Rutanen EM. Increased incidence of retching and vomiting during peri-ovulatory phase after middle ear surgery. Canadian Journal of Anaesthesiology. 1996;43:1108–1114. doi: 10.1007/BF03011836. [DOI] [PubMed] [Google Scholar]
- Howarth PA, Clemes SA. Susceptibility to induced visual discomfort during the menstrual cycle while viewing a visual display unit. Optometry and Vision Science. 2006;83:190–194. doi: 10.1097/01.opx.0000208626.35109.21. [DOI] [PubMed] [Google Scholar]
- Hruska RE, Silbergeld EK. Increased dopamine receptor sensitivity after estrogen treatment using the rat rotation model. Science. 1980;208:1466–1468. doi: 10.1126/science.7189902. [DOI] [PubMed] [Google Scholar]
- Hu S, Grant WF, Stern RM, Koch KL. Motion sickness severity and physiological correlates during repeated exposures to a rotating optokinetic drum. Aviation, Space, and Environmental Medicine. 1991;62:308–314. [PubMed] [Google Scholar]
- Janhunen L, Tammisto T. Postoperative vomiting after different modes of general anaesthesia. Annales Chirurgiae et Gynaecologiae Fenniae. 1972;61:152–159. [PubMed] [Google Scholar]
- Javid FA, Naylor RJ. Variables of movement amplitude and frequency in the development of motion sickness in Suncus murinus. Pharmacology, Biochemistry, and Behavior. 1999;64:115–122. doi: 10.1016/s0091-3057(99)00066-0. [DOI] [PubMed] [Google Scholar]
- Jokerst MD, Gatto M, Fazio R, Gianaros PJ, Stern RM, Koch KL. Effects of gender on susceptibility to motion sickness. Aviation, Space, and Environmental Medicine. 1999;70:962–965. [PubMed] [Google Scholar]
- Lawther A, Griffin MJ. The motion of a ship at sea and the consequent motion sickness amongst passengers. Ergonomics. 1986;29:535–552. doi: 10.1080/00140138608968289. [DOI] [PubMed] [Google Scholar]
- Lee V, Dowse MSL, Edge C, Gunby A, Bryson P. Decompression sickness in women : A possible relationship with the menstrual cycle. Aviation, Space, and Environmental Medicine. 2003;74:1177–1182. [PubMed] [Google Scholar]
- Lindseth G, Lindseth PD. The relationship of diet to airsickness. Aviation, Space, and Environmental Medicine. 1995;66:537–541. [PubMed] [Google Scholar]
- Lipson SF, Ellison PT. Normative study of age variation in salivary progesterone profiles. Journal of Biosocial Science. 1992;24:233–244. doi: 10.1017/s0021932000019751. [DOI] [PubMed] [Google Scholar]
- Litschauer B, Zauchner S, Huemer KH, Kafka-Lutzow A. Cardiovascular, endocrine, and receptor measures as related to sex and the menstrual cycle phase. Psychosomatic Medicine. 1998;60:219–226. doi: 10.1097/00006842-199803000-00019. [DOI] [PubMed] [Google Scholar]
- Mayer EA, Naliboff B, Lee O, Munakata J, Chang L. Review article: Gender-related differences in functional gastrointestinal disorders. Aliment Pharmacol Ther. 1999;13:65–69. doi: 10.1046/j.1365-2036.1999.00008.x. [DOI] [PubMed] [Google Scholar]
- Mills PJ, Berry CC. Menstrual cycle, race, and task recovery effects on blood pressure recovery from acute stress. Journal of Psychosomatic Research. 1999;46:445–454. doi: 10.1016/s0022-3999(99)00015-x. [DOI] [PubMed] [Google Scholar]
- Muth ER, Stern RM, Thayer JF, Koch KL. Assessment of the multiple dimensions of nausea: The nausea profile (NP) Journal of Psychosomatic Research. 1996;40:511–520. doi: 10.1016/0022-3999(95)00638-9. [DOI] [PubMed] [Google Scholar]
- Navarrete-Palacios E, Hudson R, Reyes-Guerrero G, Guevara-Guzman R. Lower olfactory thresholds during the ovulatory phase of the menstrual cycle. Biological Psychology. 2003;63:269–279. doi: 10.1016/s0301-0511(03)00076-0. [DOI] [PubMed] [Google Scholar]
- Ordy JM, Brizzee KR. Motion sickness in the squirrel monkey. Aviation, Space, and Environmental Medicine. 1980;51:215–223. [PubMed] [Google Scholar]
- Palazzo MGA, Strunin L. Anaesthesia and emesis II: Prevention and management. Canadian anaesthetic Society Journal. 1984;31:407–415. doi: 10.1007/BF03015417. [DOI] [PubMed] [Google Scholar]
- Ramsay TM, McDonald PF, Faragher EB. The menstrual cycle and nausea or vomiting after wisdom teeth extraction. Canadian Journal of Anaesthesiology. 1994;41:798–801. doi: 10.1007/BF03011586. [DOI] [PubMed] [Google Scholar]
- Riezzo G, Pezzolla F, Darconza G, Giorgio I. Gastric myoelectrical activity in the first trimester of pregnancy: A cutaneous electrogastrographic study. American Journal of Gastroenterology. 1992;87:702–707. [PubMed] [Google Scholar]
- Rita L, Goodarzi M, Seleny F. Effect of low dose droperidol on postoperative vomiting in children. Canadian Anaesthetics Society Journal. 1981;28:259–262. doi: 10.1007/BF03005511. [DOI] [PubMed] [Google Scholar]
- Rubin A, Metz-Rubin H. The effect of Dramamine upon postoperative nausea and vomiting: A controlled study of 250 consecutive surgical patients. Surgical Gynecological Obstetrics. 1951;92:415–418. [PubMed] [Google Scholar]
- Schwab RS. The nonlabyrinthine causes of motion sickness. International Record of Medicine. 1954;167:631–637. [PubMed] [Google Scholar]
- Swanson SJ, Dengerink HA. Changes in pure-tone thresholds and temporary threshold shifts as a function of menstrual cycle and oral contraceptives. Journal of Speech and Hearing Research. 1988;31:569–574. [PubMed] [Google Scholar]
- Tornetta FJ. A comparison of droperidol, diazepam, and hydroxyzine hydrochloride as premedication. Anesthesia and Analgesia. 1977;56:496–450. doi: 10.1213/00000539-197707000-00007. [DOI] [PubMed] [Google Scholar]
- Turner M, Griffin MJ. Motion sickness in public road transport: Passenger behaviour and susceptibility. Ergonomics. 1999;42:444–461. doi: 10.1080/001401399185586. [DOI] [PubMed] [Google Scholar]
- Vuorento T, Lahti A, Hovatta O, Huhtaniemi I. Daily measurements of salivary progesterone reveal a high rate of anovulation in healthy students. Scandinavian Journal of Clinical and Laboratory Investigation. 1989;49:395–401. doi: 10.3109/00365518909089113. [DOI] [PubMed] [Google Scholar]
- Wald A, Van Thiel DH, Hoechstetter L, Gavaler JS, Egler KM, Verm R, Scott L, Lester R. Effect of pregnancy on gastrointestinal transit. Digestive Diseases and Sciences. 1982;27:1015–1018. doi: 10.1007/BF01391748. [DOI] [PubMed] [Google Scholar]
- Yang X, Liu R, Dong Y. Regulative effects of ovarian steroids on rat gastric motility and sensitivity. Acta Physiolgica Sinica. 2006;58:275–280. [PubMed] [Google Scholar]
- Zhao L, Stern RM. Absence of habituation to repeated exposures to a rotating optokinetic drum with brief intersession intervals. Perceptual and Motor Skills. 1999;89:778–782. doi: 10.2466/pms.1999.89.3.778. [DOI] [PubMed] [Google Scholar]