Abstract
Background
The use of aspirin alone and statins alone has been shown to reduce markers of inflammation, including C-reactive protein (CRP); however, their combination has been poorly studied.
Methods and Results
In a cross-sectional analysis of black and white adults ≥45 years from the REasons for Geographic And Racial Differences in Stroke (REGARDS) cohort, the associations of aspirin and statin use with CRP were examined. Individuals requiring nonsteroidal anti-inflammatory drug therapy or those taking aspirin for reasons other than cardioprotection were excluded from analysis. Participants were classified into one of four groups: aspirin only (n=3673), statin only (n=1898), both agents (n=3008), or neither agent (n=7718). Estimated mean CRP was 2.78 mg/L for subjects taking neither drug, 2.73 mg/L with aspirin only, 2.29 mg/L with statins only, and 2.03 mg/L for subjects taking both agents. The combined use of both agents was associated with an apparent synergistically lower CRP; the mean CRP level among these combined users was 0.21 mg/L lower than that anticipated from additive association related to aspirin and statins alone (P for interaction=0.01). Associations were larger among participants reporting a history of cardiovascular disease. Also, among statin users, the use of aspirin for >5 years compared to ≤5 years was associated with apparent significantly lower CRP concentrations (P=0.01).
Conclusions
The combined use of aspirin and statins was associated with a synergistically lower CRP concentration, especially among participants taking aspirin for >5 years. Given the limitations of this study and the modest associations, randomized controlled trial evidence is needed to confirm the findings.
Keywords: inflammation, aspirin, statins, C-reactive protein, cardiovascular diseases
Introduction
C-reactive protein (CRP) is a biomarker of inflammation, and elevated levels are recognized as an important mediator of cardiovascular disease risk.1,2 Many observational studies have demonstrated the predictive value of elevated CRP for cerebral, coronary and peripheral vascular diseases.3–7
The use of aspirin as an antiplatelet agent in the primary and secondary prevention of cardiovascular events is well-established.8,9 In contrast, the anti-inflammatory effects of low-dose aspirin (75–325 mg), including its effect on CRP levels, are less clear. Among several small clinical trials that evaluated the effect of aspirin on CRP levels, results have varied.10–14 In the large Physicians’ Health Study, however, low-dose aspirin was shown to reduce the risk of a first myocardial infarction (MI), particularly among men with elevated baseline CRP levels.15
HMG CoA reductase inhibitors or statins are a mainstay of therapy in the prevention of cardiovascular events in patients with and without elevated lipid levels.16,17 Several trials have shown that statins reduce CRP and other inflammatory markers, and this has been suggested as a mechanism of action in cardiovascular risk reduction.18–20 However, combination therapy with aspirin and statins has been poorly studied. The results of a meta-analysis of several pravastatin trials in secondary prevention settings concluded that the combination of aspirin with pravastatin synergistically reduced recurrent cardiovascular events.21
This study investigated the joint associations of aspirin and statins on CRP concentration in a cross-sectional analysis of individuals enrolled in the REasons for Geographic And Racial Differences in Stroke (REGARDS) study.
Methods
The characteristics of the subjects enrolled in the REGARDS study as well as the study design and objectives have been previously published.22 Briefly, REGARDS is a population-based, longitudinal cohort study of United States, black and white adults designed to evaluate the geographical and racial/ethnic differences in the incidence of stroke. Recruitment began in February 2003. The goal of REGARDS is to enroll 30,000 individuals ≥45 years, with equal representation of whites and blacks, and men and women. A total of 20% of the sample is selected from the “buckle” of the Stroke Belt [coastal plain region of North Carolina (NC), South Carolina (SC) and Georgia (GA)], 30% from the Stroke Belt states (remainder of NC, SC, and GA, plus Alabama, Mississippi, Tennessee, Arkansas, and Louisiana), and the remaining 50% from the other 40 contiguous states. The study methods were reviewed and approved by the institutional review boards of each collaborating institution. Informed consent was obtained verbally and later in writing from each study participant.
Aspirin use was established on the basis of the computer-assisted telephone interview (CATI) information. Participants who responded affirmatively to “Are you currently taking aspirin or aspirin containing products regularly, that is, at least two times each week?” were defined as regular aspirin users, and dosing information was collected. The parameter, 2 times/week was used as this was the question provided in the survey. The evaluation of more frequent use was not possible. Amongst these regular aspirin users, the subgroup taking aspirin only for cardiovascular protection was then identified; these subjects provided a negative response to a question about taking aspirin for pain, but a positive response to a question about taking aspirin to reduce the chance of heart attack or stroke. Participants were excluded from the present cross-sectional analysis if they were regular users (at least 2 times/week) of aspirin for any reason other than cardioprotection or regular users (at least 2 times/week) of NSAID therapy. Use of NSAIDs was ascertained by self reported intake during the telephone interview.
Statin use was determined on the basis of a drug inventory conducted at the in-home examination. Statin doses were not consistently reported and were not captured for this analysis. Participants were asked to retrieve the bottles of all prescription and over-the-counter medications taken in the 2 weeks prior to the in-home visit, and the names of these medications were recorded on study forms.
Venous blood samples were collected at the time of the in-home examination, with local centrifugation and separation of serum or plasma and shipment overnight to a central laboratory at the University of Vermont. Samples were then centrifuged at 30,000 × g at 4 degrees Celsius, with serum or plasma then stored at −80°C. High-sensitivity testing of CRP (hs-CRP) was performed in plasma at the central REGARDS laboratory via particle enhanced immunonephelometry on the BNII nephelometer (N High Sensitivity CRP; Dade Behring Inc., Deerfield, IL).
Statistical Methods
All statistical analyses were performed by a university-affiliated coauthor (GH) using SAS Version 9.1. The association of aspirin and statin use (alone and in combination) with CRP was made univariately, and after multivariate adjustment for potential confounding variables age, race, gender, cigarette smoking, body mass index (BMI), hypertension, diabetes, evidence of cardiovascular disease (CVD), weekly exercise, and socio-economic status and income. Because CRP values had a substantial right skew, values were natural log (Ln) transformed before statistical analysis; however, for graphical presentation, the estimated least-square mean values were transformed back (i.e., exponentiation) to ordinary CRP units (mg/L). Ln (CRP) values were evaluated using general linear models with a focus on assessing if there was evidence of synergy (effect modification or interaction) with the combined use of aspirin and statins. The potential of a synergistic effect of statin use and regular aspirin use on CRP levels was assessed by an interaction term (statin use by regular aspirin use), and the potential that this effect differs by either race or gender was assessed by the addition of three way interactions (and appropriate hierarchical two-way interactions) to the model (specifically race by statin use by regular aspirin use, and gender by statin use by regular aspirin use). These tests for interaction assess the likelihood that the combined effect of aspirin and statin on CRP differs from the response anticipated by their individual effects by an amount that is larger than would be anticipated by chance alone. That is, if use of aspirin alone was associated with a reduction of X units in CRP, and use of statins alone are associated with a reduction of Y units in CRP, then their combined effect would be expected to be X + Y. Using standard statistical tests,23 the test of interaction assesses if the observed level (Z) for those people on both aspirin and statins differs from this expected level (X + Y) by an amount that that is unlikely to have occurred by chance alone. We will define the combined effect of statins and aspirin to be “synergistic” if the reduction in CRP associated with their combined effect is larger than anticipated by their individual effects (i.e., if Z < X + Y).
Secondary analyses were performed assessing the potential differences in these relationships as a function of aspirin dose, duration of aspirin use, and self-reported cardiovascular disease. All secondary analyses were performed after multivariate adjustment for the potential confounding variables. The authors had full access to the data and take responsibility for its integrity. All authors have read and agree to the manuscript as written.
Results
Subjects
Between February 2003 and December 1, 2005, a total of 20,667 subjects were enrolled. Of these, 4,370 were excluded from this cross-sectional analysis: participants without a drug inventory (n=532), those reporting the regular use of NSAIDs (n = 2,844), and because the sources or chronic pain requiring regular aspirin use could be associated with inflammatory condition, use of aspirin for reasons other than cardioprotection (n = 1,307). Some participants were excluded for more than one reason. The final sample size for the present analysis was 16,297 subjects; their characteristics are provided in Table 1, stratified by aspirin and statin use. There were 6,681 participants using aspirin regularly for cardiovascular protection, and 9,616 non-users. There were 4,906 participants using statins and 11,391 non-users. There were 3,673 using aspirin only, 1,898 using statins only, 3,008 using both agents, and 7,718 using neither agent (Table 1). In general, individuals using one or both of the medications were more likely to be older, white, male, and have a more adverse cardiovascular risk profile, with smaller differences in socio-economic status and exercise level.
Table 1.
Baseline Characteristics According to Aspirin and Statin Use
| Characteristic | Neither | Statin Only | Aspirin Only | Both |
|---|---|---|---|---|
| N | 7718 | 1898 | 3673 | 3008 |
| Age, y | 64.9 ± 9.4 | 67.2 ± 8.7 | 67.6 ±8.7 | 68.2 ± 8.2 |
| African American, % | 47.7 | 45.6 | 38.3 | 35.7 |
| Female, % | 55.8 | 53.1 | 43.8 | 36.5 |
| Smoking status, % | ||||
| Never | 47.4 | 43.0 | 41.8 | 36.9 |
| Past | 36.4 | 44.9 | 44.8 | 51.2 |
| Current | 16.2 | 12.1 | 13.4 | 12.0 |
| Body Mass Index, kg/m2 | 28.6± 6.1 | 29.7± 5.8 | 28.7± 5.8 | 29.5± 5.7 |
| Hypertension, % | 48.5 | 69.1 | 62.7 | 72.3 |
| Diabetes, % | 15.5 | 31.8 | 22.1 | 36.0 |
| Cardiovascular Disease, % | 10.5 | 26.7 | 28.4 | 53.7 |
| Educational Category, % | ||||
| < High school | 13.5 | 15.5 | 13.2 | 13.9 |
| High school graduate | 26.1 | 27.1 | 25.2 | 26.8 |
| Some college | 27.6 | 25.1 | 24.4 | 25.5 |
| College or higher | 32.8 | 32.3 | 37.2 | 33.7 |
| Income Category, % | ||||
| <$20K | 22.3 | 23.0 | 20.9 | 20.0 |
| $20K–$35K | 29.2 | 29.6 | 27.4 | 28.9 |
| $35K–75K | 32.9 | 34.1 | 33.1 | 34.1 |
| >$75 | 15.6 | 13.3 | 18.6 | 17.0 |
| Weekly Exercise, % | ||||
| None | 34.2 | 39.0 | 30.7 | 33.4 |
| 1 to 3 times/week | 36.2 | 35.5 | 35.6 | 35.5 |
| ≥4 times/week | 29.6 | 25.5 | 33.7 | 31.1 |
| Ln (CRP) (unadjusted) mg/L | 0.85 ± 1.18 | 0.78 ± 1.15 | 0.84 ± 1.15 | 0.66 ± 1.16 |
Values are means ± SD, unless otherwise indicated.
Primary Analyses
The unadjusted mean Ln (CRP) values are provided in Table 1 and the exponentiated values are presented in Figure 1. Compared with non-users of both statins and aspirin, there was a significant difference in mean Ln (CRP) concentration among statin users (difference = 0.071 mg/L, P<0.0001), but not among the aspirin-only group (i.e., those not taking statins) (difference = 0.019 mg/L, P=0.45). The mean Ln (CRP) of subjects taking both aspirin and statins was 0.662 mg/L, a significantly (P<0.0007) lower concentration compared with the group not taking either agent. Furthermore, this value is 0.104 mg/L lower (P=0.017) than the expected mean Ln (CRP) of 0.766 mg/L under an additive hypothesis [i.e., 0.855 mg/L − (0.071 + 0.019) = 0.766], with no evidence of a difference in this synergistic effect by race or gender (p > 0.18).
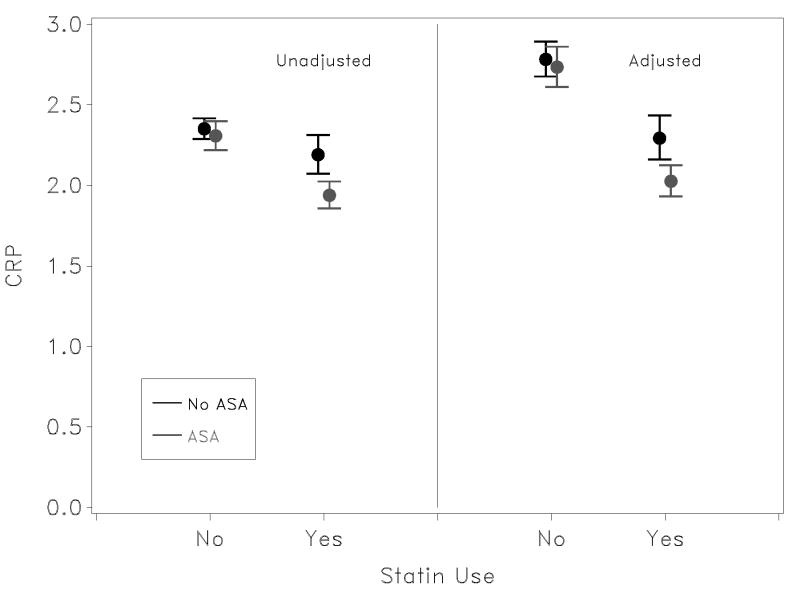
Figure 1.
Mean and 95% confidence limits on unadjusted and adjusted CRP levels among users of aspirin and statins. Estimates were made in log-space, and results exponentiated for graphic presentation. CRP levels are reported in mg/L. For unadjusted CRP levels, P-value for effect modification (interaction) is 0.017. For adjusted CRP values, P-value for effect modification (interaction) is 0.014.
Similar results were observed after multivariate adjustment for potential confounding factors. Compared with non-users of both statins and aspirin (adjusted Ln (CRP) = 1.024 mg/L), mean Ln (CRP) concentration was significantly lower among statin users (difference = 0.194 mg/L, P<0.0001), but not among those using aspirin only (difference = 0.018 mg/L, P=0.48). The mean Ln (CRP) among subjects taking both aspirin and statins was 0.706 mg/L, which was significantly lower compared with the group not taking either agent (P<0.0007). This observed mean Ln (CRP) of the combined aspirin-statin group was also significantly lower than the expected mean Ln (CRP) by an additive association of the joint monotherapy groups (0.812 Ln (CRP) [1.024 − (0.194 + 0.018)], P = 0.01)]), with no evidence of a difference in this synergistic effect by race or gender (p > 0.18).
Figure 1 shows the exponentiated results of the Ln of mean CRP concentrations for the 4 groups of subjects, without and with adjustment for covariates. Concentrations were generally higher in adjusted analyses, but differences by drug use were not altered with adjustment. The mean CRP concentrations for subjects not taking either aspirin or statins was 2.35 mg/L (2.78 mg/L adjusted), for subjects using aspirin only, 2.31 mg/L (2.73 mg/L adjusted), for subjects taking only statins, 2.19 mg/L (2.29 mg/L adjusted), and for subjects taking both drugs, 1.94 mg/L (2.03 mg/L adjusted).
Secondary Analyses
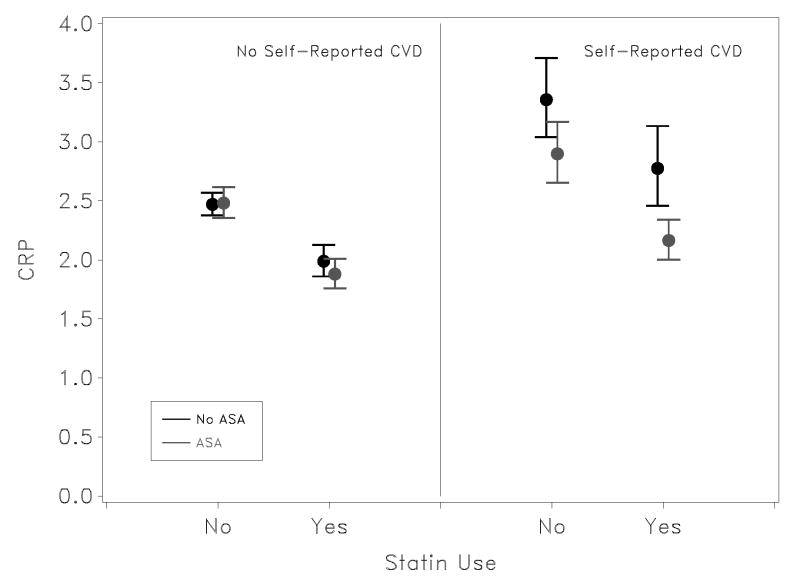
The association of aspirin use with CRP in the multivariate model differed significantly by self-reported CVD status (Pinteraction = 0.002); however, there was no evidence of a difference in the synergistic impact of aspirin and statins between those with and without CVD (p = 0.67). That is, while aspirin was associated with a larger difference in mean Ln CRP levels among those with CVD, there was not evidence of a difference in the observed synergistic effect of aspirin and statins among those with and without CVD. Specifically, as shown in Figure 2, there is a substantial difference in mean Ln CRP concentration associated with aspirin use compared with no drug use in those with self-reported CVD, but virtually no difference in those without self-reported CVD. In analysis presented in the previous section, adjustment for symptomatic status (i.e., prevalent CHD) had little impact on the magnitude of the aspirin-statin interaction, suggesting that confounding by indication through a primary pathway is not a major concern. However, after adjustment for the interaction between CVD and aspirin use the interaction between aspirin and statin use remains of marginal significance (p = 0.083), suggesting that confounding by indication through a more complex pathway may contribute to the observed synergistic effect of statins and aspirin.
Figure 2.
Mean and 95% confidence limits on CRP are shown stratified by previous cardiovascular symptoms and after multivariate adjustment. Estimates were made in log-space, and results exponentiated for graphic presentation. CRP level are reported in mg/L. P-value for effect modification of aspirin and statins for those without evidence of CVD is 0.25, and for those with a history of CVD is 0.16; however, the three-way interaction was not significant, suggesting the pooled interaction between ASA and statin use of 0.014 is appropriate for effect modification between these factors.
After adjustment for the factors in the multivariate model, CRP did not differ by aspirin dose. Compared with those taking neither statin nor aspirin, the average Ln (CRP) was 0.020 mg/L lower among those taking 81 mg of aspirin, and 0.014 mg/L lower among those taking >81 mg (i.e., predominately 325 mg), P 81vs 325 mg = 0.88. Similarly, among subjects taking statins, use of aspirin 81 mg was associated with a 0.123 mg/L lower Ln (CRP), while those using higher doses had a 0.132 mg/L lower Ln (CRP) (P 81 vs 325 mg= 0.85).
As shown in Table 2, among subjects taking statins and aspirin compared with those taking statins only, mean Ln (CRP) was 0.072 mg/L lower for those who reported taking aspirin for ≤5 years, and 0.189 mg/L for those taking aspirin for >5 years, a difference that was significantly larger (P=0.01). While there was a similar pattern for aspirin-users not taking statins, the differences did not reach statistical significance (P=0.31).
Table 2.
Adjusted Mean Differences in CRP by Duration of Aspirin Exposure and Statin Use*
| Drug | No Statin | Statin Use | ||
|---|---|---|---|---|
| Difference in mean log CRP | P-value | Difference in mean log CRP | P-value | |
|
| ||||
| No regular ASA use
|
Pnone-short = 0.99 | Pnone-short = 0.08 | ||
| Reference Group | Pnone-long = 0.25 | Reference Group | Pnone-long = <0.0001 | |
| Short-term ASA use (≤ 5 years)
|
Pshort-long = 0.31 | Pshort-long = 0.01 | ||
| 0.000 | −0.072 | |||
| Long-term ASA use (> 5 years) | −0.042 | −0.189 | ||
Adjusted for race, gender, smoking status, body mass index, hypertension, diabetes, previous cardiovascular symptoms or procedures, education, income, and physical activity, shown by regular aspirin use for cardiovascular protection and statin use.
Discussion
In this cross-sectional study, combination therapy with aspirin and statins was associated with an apparent synergistically lower CRP concentration. The association was larger among those who had been taking aspirin for >5 years compared with ≤5 years. Furthermore, among participants with self-reported CVD, the synergistic association of aspirin and statin use was larger than among those without self-reported CVD but failed to reach statistical significance because of smaller sample size after stratification.
The combination of aspirin and statin therapy on cardiovascular outcomes has been previously studied, although not prospectively.21 A meta-analysis of pravastatin trials evaluated the effects of combination therapy in patients at high risk for coronary heart disease events and showed a 31% reduction for pravastatin plus aspirin vs. aspirin alone and 26% for pravastatin plus aspirin vs. pravastatin alone - a synergy of 0.92 for the endpoint of fatal and nonfatal MI.21
The effects of aspirin and statins alone and in combination on mediators of inflammation have been explored.24–26 One important mediator is oxidized-low density lipoprotein (ox-LDL), which is believed to play a role in atherosclerosis and associated inflammatory reactions. Ox-LDL up-regulates the expression of its receptor, lectin-like oxidized-low density lipoprotein receptor-1 (LOX-1), and through this receptor, ox-LDL mediates its inflammatory effects, including up-regulation of the proinflammatory CD40/CD40 ligand pathway.27 In vitro studies have found that treatment with aspirin alone inhibited ox-LDL expression of LOX-1 and p38 mitogen-activated protein (p38 MAP) kinase,24 while treatment with statins alone inhibited LOX-126 and NFκB.28
We also observed that among users of both statins and aspirin, the long-term use of aspirin for >5 years was associated with a significantly lower CRP level compared with no aspirin use (i.e., subjects in the statin only group) or use of aspirin for ≤ 5 years (P=0.01). These observations suggest that at least some favorable effects related to aspirin use may only manifest over long periods of time, which would be in agreement with the findings of others.10,11 This is in contrast to the common belief that the beneficial effects of aspirin are related only to irreversible inhibition of platelet aggregation, which lasts for the lifetime of the platelet (≈10 days).29 In vivo and in vitro studies have suggested that aspirin may have anti-oxidant as well as nitric oxide (NO)-promoting properties.24,30,31 Thus, the anti-inflammatory effect of aspirin may work in concert with these additional properties to improve cardiovascular health.
Furthermore, individuals with CVD may be more sensitive to the combined pleiotropic effect of statins and aspirin due to their general proinflammatory condition. In contrast, the clinical benefits related to statin and especially aspirin therapy in healthy subjects may be more closely linked to the primary mechanisms of the drugs (i.e., lipid lowering and antiplatelet effects, respectively). In one study of subjects with stable angina, aspirin 300 mg daily for 6 weeks reduced levels of CRP concentration by 29% compared with placebo (P<0.05).10 Likewise, Solheim and colleagues observed significantly (P≤0.03) decreased CRP levels in post-MI patients taking aspirin 160 mg/day compared with those taking warfarin alone after 3 months and after 4 years following randomization.11 Consistent with our findings in participants without self-reported CVD, studies of healthy subjects suggest no significant effect of aspirin on CRP concentration.12–14 It is not clear whether this finding relates to the potential for a threshold effect. Additional study is needed.
Limitations of our study include the cross-sectional design and the reliance on self-reported medical information, including the self-reported frequency and duration of medication use and application of the multiple statistical tests. The aspirin survey data provided a definition of regular use as at least 2 times per week. This could result in a variety use patterns but it is likely that most low dose aspirin users were taking aspirin daily. We were concerned that confounding by indication would play an important role. The significance of the interaction between statin and aspirin use remained significant after adjustment for prevalent cardiovascular disease, suggesting that the direct confounding by indication is not present. However, further adjustment for the interaction between aspirin use and prevalent cardiovascular disease reduced the interaction of aspirin and statins to a level that is not typically considered significant (p = 0.083). This suggests that confounding by indication may play a complex role in mediating the synergistic effects of aspirin and statins. Also, CRP was measured only once and more precise estimates of an individual’s true value can be obtained with repeat measures. Although we adjusted for established cardiovascular risk factors and potential confounders, residual confounding could, as in other observational studies, impact the results in unpredictable ways. Strengths of our study include the reliable measurement of CRP levels in a central laboratory and the large, carefully characterized, population-based bi-racial REGARDS cohort.
In summary, in this large, population-based cohort, the combined use of aspirin and statin therapy was associated with a synergistically lower CRP level. The association was strongest in those using aspirin for >5 years. Given the limitations of this analysis, we consider these findings as weakly supporting the hypothesis that aspirin and statin use exert a synergistic effect on inflammation as reflected by hsCRP. Randomized controlled trials are required to confirm our findings and to assess the specific value of combination therapy with aspirin and statins in cardiovascular event prevention.
Acknowledgments
Funding Source: This research project is supported by a cooperative agreement U01 NS041588 from the National Institute of Neurological Disorders and Stroke, National Institutes of Health, Department of Health and Human Services. The authors acknowledge the participating investigators and institutions: University of Alabama at Birmingham, Birmingham, Alabama (Study PI, Data Coordinating Center, Survey Research Unit ): George Howard, Leslie McClure, Virginia Howard, Libby Wagner, Virginia Wadley, Rodney Go); University of Vermont (Central Laboratory): Mary Cushman; Wake Forest University (ECG Reading Center): Ron Prineas; Alabama Neurological Institute (Stroke Validation Center, Medical Monitoring): Camilo Gomez, David Rhodes, Susanna Bowling, Sean Orr; University of Arkansas for Medical Sciences (Survey Research): LeaVonne Pulley; Examination Management Services Incorporated (In Home Visits): Andra Graham; National Institute of Neurological Disorders and Stroke, National Institutes of Health (funding agency): Claudia Moy.
Abbreviations
- BMI
body mass index
- CRP
C-reactive protein
- CVD
cardiovascular disease
- LDL
low density lipoprotein
- LOX-1
lectin-like oxidized-low density lipoprotein receptor-1
- MI
myocardial infarction
- NO
nitric oxide
- NSAID
nonsteroidal anti-inflammatory drug
- REGARDS
Reasons for Geographic and Racial Differences in Stroke
Footnotes
Financial Disclosures:
Matt Fisher is an employee of Bayer HealthCare and Volker Knappertz is a former employee of Bayer HealthCare.
Fisher, Joint Associations of ASA/statins on CRP
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Tsimikas S, Willerson JT, Ridker PM. C-reactive protein and other emerging blood biomarkers to optimize risk stratification of vulnerable patients. J Am Coll Cardiol. 2006;47(8 Suppl):C19–31. doi: 10.1016/j.jacc.2005.10.066. [DOI] [PubMed] [Google Scholar]
- 2.Yeh ETH. CRP as a mediator of disease. Circulation. 2004;109:11–14. doi: 10.1161/01.CIR.0000129507.12719.80. [DOI] [PubMed] [Google Scholar]
- 3.Cao JJ, Thach C, Manolio TA, Psaty BM, Kuller LH, Chaves PH, Polak JF, Sutton-Tyrrell K, Herrington DM, Price TR, Cushman M. C-reactive protein, carotid intima-media thickness, and incidence of ischemic stroke in the elderly: the Cardiovascular Health Study. Circulation. 2003;108:166–170. doi: 10.1161/01.CIR.0000079160.07364.6A. [DOI] [PubMed] [Google Scholar]
- 4.Koenig W, Sund M, Fröhlich M, Fischer H-G, Löwel H, Döring A, Hutchinson WL, Pepys MB. C-reactive protein, a sensitive marker of inflammation, predicts future risk of coronary heart disease in initially healthy middle-aged men: results from the MONICA (Monitoring Trends and Determinants of Cardiovascular Disease) Augsberg Cohort Study, 1984 to1992. Circulation. 1999;99:237–242. doi: 10.1161/01.cir.99.2.237. [DOI] [PubMed] [Google Scholar]
- 5.Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342:836–843. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- 6.Ridker PM, Rifai N, Rose L, Buring JE, Cook NR. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N Engl J Med. 2002;347:1557–1565. doi: 10.1056/NEJMoa021993. [DOI] [PubMed] [Google Scholar]
- 7.Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH. Plasma concentration of C-reactive protein and risk of developing peripheral vascular disease. Circulation. 1998;97:425–428. doi: 10.1161/01.cir.97.5.425. [DOI] [PubMed] [Google Scholar]
- 8.Eidelman RS, Herbert PR, Weisman SM, Hennekens CH. An update on aspirin in the primary prevention of cardiovascular disease. Arch Intern Med. 2003;163:2006–2010. doi: 10.1001/archinte.163.17.2006. [DOI] [PubMed] [Google Scholar]
- 9.Antithrombotic Trialists’ Collaboration. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ. 2002;524:71–86. doi: 10.1136/bmj.324.7329.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ikonomidis I, Andreotti F, Economou E, Stephanadis C, Toutouzas P, Nihoyannopoulos P. Increased proinflammatory cytokines in patients with chronic stable angina and their reduction by aspirin. Circulation. 1999;100:793–798. doi: 10.1161/01.cir.100.8.793. [DOI] [PubMed] [Google Scholar]
- 11.Solheim S, Arnesen H, Eikvar L, Hurlen M, Seljeflot I. Influence of aspirin on inflammatory markers in patients after acute myocardial infarction. Am J Cardiol. 2003;92:843–845. doi: 10.1016/s0002-9149(03)00897-x. [DOI] [PubMed] [Google Scholar]
- 12.Azar RR, Klayme S, Germanos M, Kassab R, Tawm S, Aboujaoude S, Naman R. Effects of aspirin (325 mg/day) on serum high-sensitivity C-reactive protein, cytokines, and adhesion molecules in healthy volunteers. Am J Cardiol. 2003;92:236–239. doi: 10.1016/s0002-9149(03)00549-6. [DOI] [PubMed] [Google Scholar]
- 13.Feldman M, Jialal I, Devaraj S, Cryer B. Effects of low-dose aspirin on serum C-reactive protein and thromboxane B2 concentrations: a placebo-controlled study using a highly sensitive C-reactive protein assay. J Am Coll Cardiol. 2001;37:2036–2041. doi: 10.1016/s0735-1097(01)01289-x. [DOI] [PubMed] [Google Scholar]
- 14.Feng D, Tracy RP, Lipinska I, Murillo J, McKenna C, Tofler GH. Effect of short-term aspirin use on C-reactive protein. J Thromb Thrombolysis. 2000;9:37–41. doi: 10.1023/a:1018644212794. [DOI] [PubMed] [Google Scholar]
- 15.Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med. 1997;336:973–979. doi: 10.1056/NEJM199704033361401. Erratum in: N Engl J Med 1997;337–356. [DOI] [PubMed] [Google Scholar]
- 16.National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143–3421. [PubMed] [Google Scholar]
- 17.Grundy SM, Cleeman JI, Merz CN, Brewer HB, Jr, Clark LT, Hunninghake DB, Pasternak RC, Smith SC, Jr, Stone NJ National Heart, Lung, and Blood Institute; American College of Cardiology Foundation; American Heart Association. Implications of Recent Clinical Trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation. 2004;110:227–239. doi: 10.1161/01.CIR.0000133317.49796.0E. Erratum in: Circulation. 2004;110:6–763. [DOI] [PubMed] [Google Scholar]
- 18.Plenge JK, Hernandez TL, Weil KM, Poirier P, Grunwald GK, Marcovina SM, Eckel RH. Simvastatin lowers C-reactive protein within 14 days: an effect independent of low-density lipoprotein cholesterol reduction. Circulation. 2002;106:1447–1452. doi: 10.1161/01.cir.0000029743.68247.31. [DOI] [PubMed] [Google Scholar]
- 19.Kent SM, Flaherty PJ, Coyle LC, Markwood TT, Taylor AJ. Effect of atorvastatin and pravastatin on serum C-reactive protein. Am Heart J. 2003;145:e8. doi: 10.1067/mhj.2003.34. [DOI] [PubMed] [Google Scholar]
- 20.Ballantyne CM, Houri J, Notarbartolo A, Melani L, Lipka LJ, Suresh R, Sun S, LeBeaut AP, Sager PT, Veltri EP Ezetimibe Study Group. Effect of ezetimibe coadministered with atorvastatin in 628 patients with primary hypercholesterolemia. Circulation. 2003;107:2409–2415. doi: 10.1161/01.CIR.0000068312.21969.C8. [DOI] [PubMed] [Google Scholar]
- 21.Hennekens CH, Sacks FM, Tonkin A, Jukema JW, Byington RP, Pitt B, Berry DA, Berry SM, Ford NF, Walker AJ, Natarajan K, Sheng-Lin C, Fiedorek FT, Belder R. Additive benefits of pravastatin and aspirin to decrease risks of cardiovascular disease. Arch Intern Med. 2004;164:40–44. doi: 10.1001/archinte.164.1.40. [DOI] [PubMed] [Google Scholar]
- 22.Howard VJ, Cushman M, Pulley LV, Gomez CR, Go RC, Prineas RJ, Graham A, Moy CS, Howard G. The REasons for Geographic and Racial Differences in Stroke (REGARDS) Study: Objectives and Design. Neuroepidemiology. 2005;25:135–143. doi: 10.1159/000086678. [DOI] [PubMed] [Google Scholar]
- 23.Neter J, Kutner MH, Wasserman W. Applied linear statistical models. 4. McGraw-Hill; 2004. [Google Scholar]
- 24.Mehta JL, Chen J, Yu F, Li DY. Aspirin inhibits ox-LDL-mediated LOX-1 expression and metalloproteinase-1 in human coronary endothelial cells. Cardiovasc Res. 2004;64:243–249. doi: 10.1016/j.cardiores.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 25.Chen J, Liu H, Liu Y, Hermonat P, Mehta JL. Combination of aspirin and pravastatin dramatically reduces oxidative stress and the expression of LOX-1 and adhesion molecules: novel insight into the mechanism of action of aspirin and statins [abstract] J Am Coll Cardiol. 2006;47:340A. [Google Scholar]
- 26.Hofnagel O, Luechtenborg B, Eschert H, Weissen-Plenz G, Severs NJ, Robenek H. Pravastatin inhibits expression of lectin-like oxidized low-density lipoprotein receptor-1 (LOX-1) in Watanabe heritable hyperlipidemic rabbits: a new pleiotropic effect of statins. Arterioscler Thromb Vasc Biol. 2006;26:604–610. doi: 10.1161/01.ATV.0000201073.45862.8b. [DOI] [PubMed] [Google Scholar]
- 27.Li D, Liu L, Chen H, Sawamura T, Mehta JL. LOX-1, an oxidized LDL endothelial receptor, induces CD40/CD40L signaling in human coronary artery endothelial cells. Arterioscler Thromb Vasc Biol. 2003;23:816–821. doi: 10.1161/01.ATV.0000066685.13434.FA. [DOI] [PubMed] [Google Scholar]
- 28.Lin R, Liu J, Peng N, Yang G, Gan W, Wang W. Lovastatin reduces nuclear factor kappaB activation induced by C-reactive protein in human vascular endothelial cells. Biol Pharm Bull. 2005;28:1630–1634. doi: 10.1248/bpb.28.1630. [DOI] [PubMed] [Google Scholar]
- 29.Awtry E, Loscalzo J. Aspirin. Circulation. 2000;101:1206–1218. doi: 10.1161/01.cir.101.10.1206. [DOI] [PubMed] [Google Scholar]
- 30.Taubert D, Berkels R, Grosser N, Schroder H, Grundemann D, Schomig E. Aspirin induces nitric oxide release from vascular endothelium: a novel mechanism of action. Br J Pharmacol. 2004;143:159–165. doi: 10.1038/sj.bjp.0705907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chiang N, Burmudez EA, Ridker PM, Hurwitz S, Serhan CN. Aspirin triggers antiinflammatory 15-epi-lipoxin A4 and inhibits thromboxane in a randomized human trial. Proc Natl Acad Sci USA. 2004;101:15178–15183. doi: 10.1073/pnas.0405445101. [DOI] [PMC free article] [PubMed] [Google Scholar]