Abstract
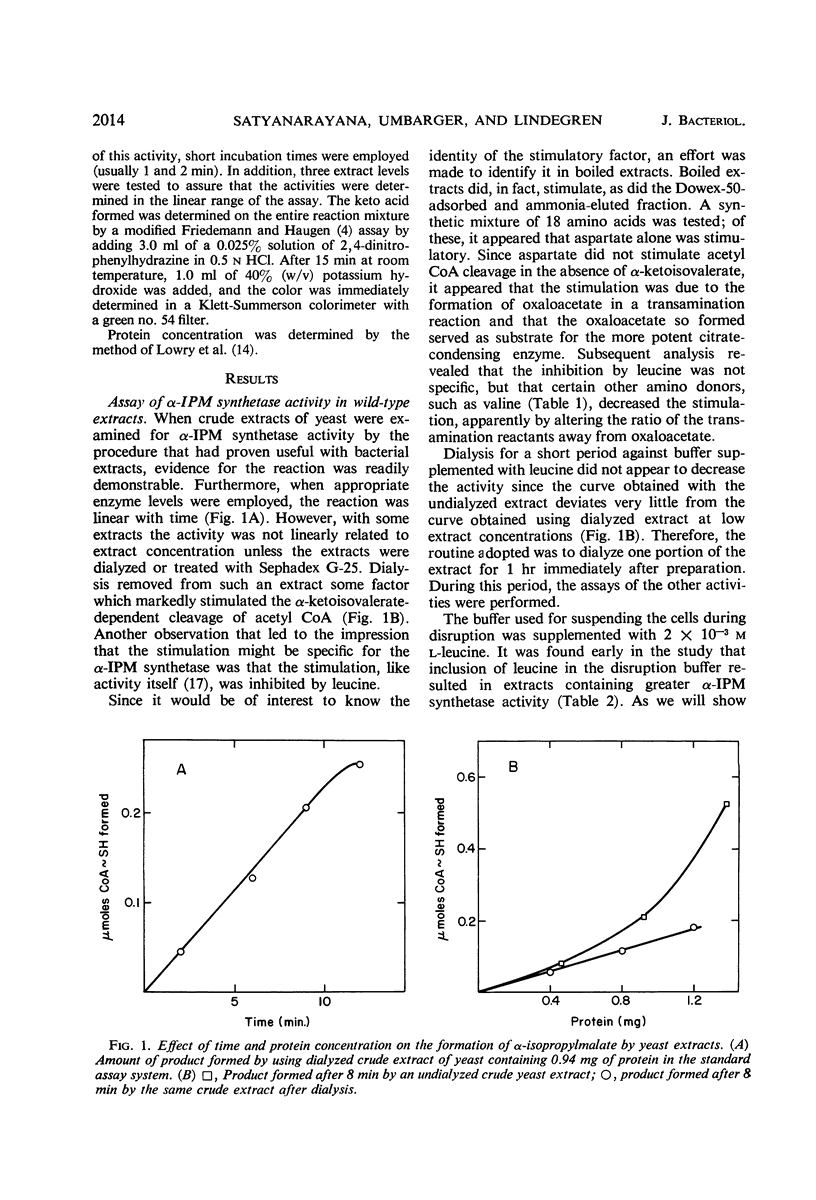
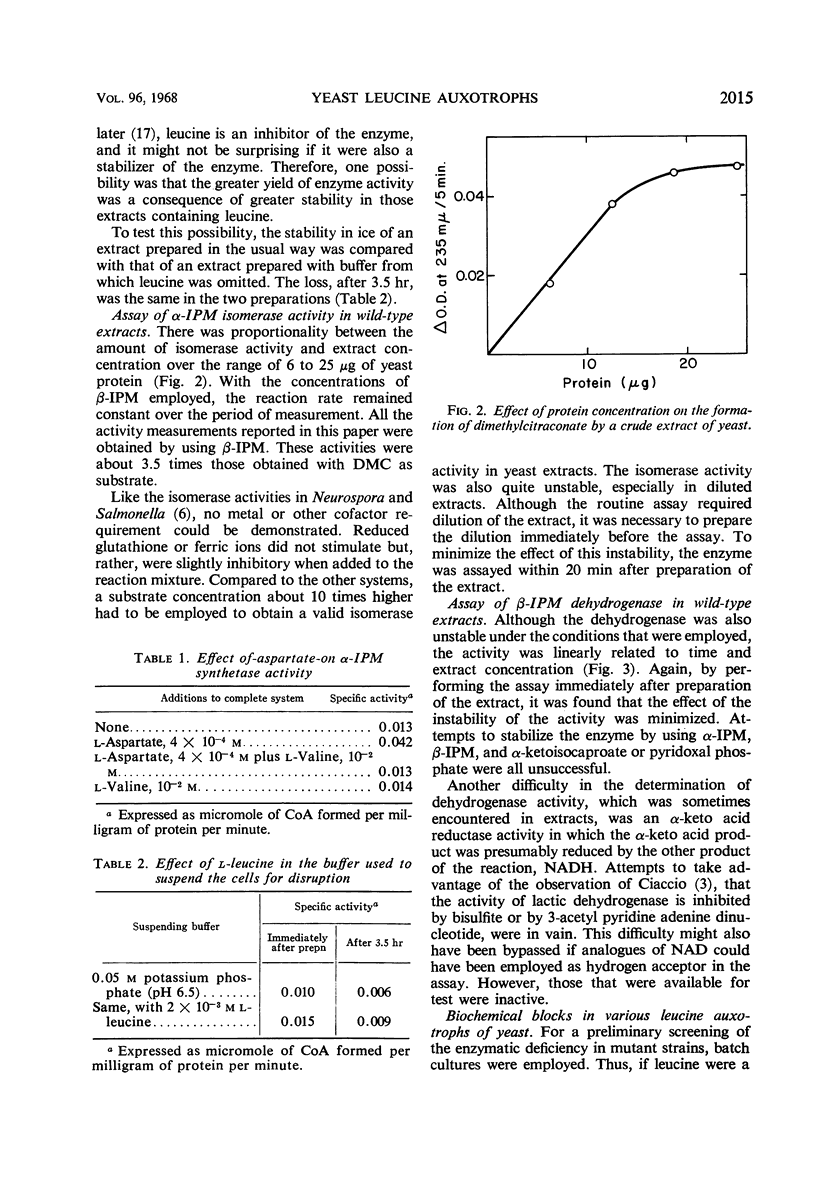
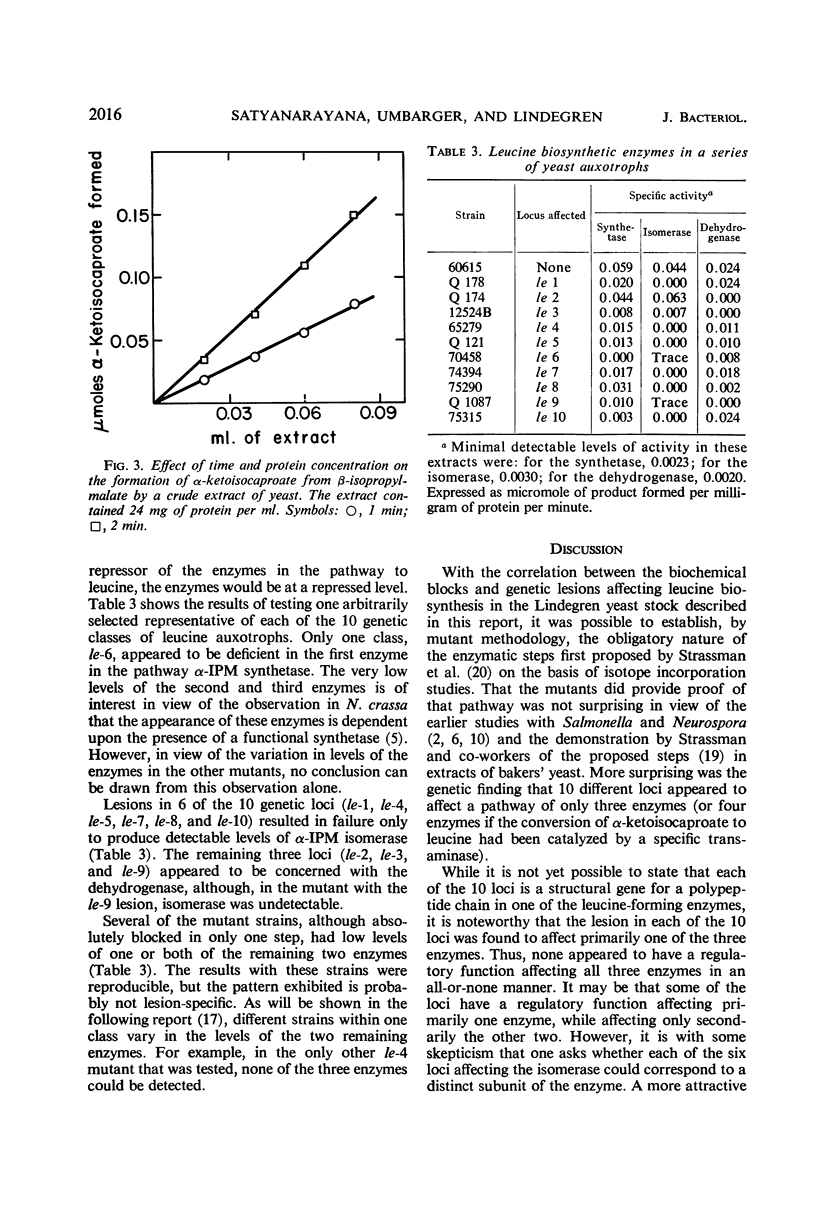
The three enzymatic steps in the conversion of α-ketoisovalerate to α-ketoisocaproate were examined in wild-type and in leucine auxotrophic stocks of yeast. Procedures for the reliable assay of each of the enzymatic steps in crude extracts were devised. Crude extracts of the prototrophic haploid stock catalyzed all three enzymatic steps. Examination of a series of leucine auxotrophs permitted a correlation between the three enzymatic steps and the genetic lesions affecting 10 different loci. This examination revealed that a single locus (le-6) affected primarily α-isopropylmalate synthetase, the first step in the pathway. Lesions in six loci (le-1, le-4, le-5, le-7, le-8, and le-10) lead primarily to a deficiency in the activity of the second enzyme in the pathway, α-isopropylmalate isomerase. Stocks with lesions in three loci (le-2, le-3, and le-9) were primarily blocked in the third step of the pathway, catalyzed by β-isopropylmalate dehydrogenase. The results with the mutants provide strong evidence that the pathway for leucine biosynthesis proposed by Strassman and his colleagues is the sole significant pathway in yeast.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURNS R. O., UMBARGER H. E., GROSS S. R. THE BIOSYNTHESIS OF LEUCINE. III. THE CONVERSION OF ALPHA-HYDROXY-BETA-CARBOXYISOCAPROATE TO ALPHA-KETOISOCAPROATE. Biochemistry. 1963 Sep-Oct;2:1053–1058. doi: 10.1021/bi00905a024. [DOI] [PubMed] [Google Scholar]
- Burns R. O., Calvo J., Margolin P., Umbarger H. E. Expression of the leucine operon. J Bacteriol. 1966 Apr;91(4):1570–1576. doi: 10.1128/jb.91.4.1570-1576.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciaccio E. I. The inhibition of lactate dehydrogenase by 3-acetylpyridine adenine dinucleotide and bisulfite. J Biol Chem. 1966 Apr 10;241(7):1581–1586. [PubMed] [Google Scholar]
- GROSS S. R., BURNS R. O., UMBARGER H. E. THE BIOSYNTHESIS OF LEUCINE. II. THE ENZYMIC ISOMERIZATION OF BETA-CARBOXY-BETA-HYDROXYISOCAPROATE AND ALPHA-HYDROXY-BETA-CARBOXYISOCAPROATE. Biochemistry. 1963 Sep-Oct;2:1046–1052. doi: 10.1021/bi00905a023. [DOI] [PubMed] [Google Scholar]
- GRUNERT R. R., PHILLIPS P. H. A modification of the nitroprusside method of analysis for glutathione. Arch Biochem. 1951 Feb;30(2):217–225. [PubMed] [Google Scholar]
- Gross S. R. The regulation of synthesis of leucine biosynthetic enzymes in Neurospora. Proc Natl Acad Sci U S A. 1965 Dec;54(6):1538–1546. doi: 10.1073/pnas.54.6.1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HALVORSON H. Studies on protein and nucleic acid turnover in growing cultures of yeast. Biochim Biophys Acta. 1958 Feb;27(2):267–276. doi: 10.1016/0006-3002(58)90333-0. [DOI] [PubMed] [Google Scholar]
- KIRITANI K., NARISE S., BERGQUIST A., WAGNER R. P. THE OVERALL IN VITRO SYNTHESIS OF VALINE FROM PYRUVATE BY NEUROSPORA HOMOGENATES. Biochim Biophys Acta. 1965 May 4;100:432–443. doi: 10.1016/0304-4165(65)90013-9. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- LUCAS J. M., SCHUURS A. H., SIMPSON M. V. A CELL-FREE AMINO ACID-INCORPORATING SYSTEM FROM SACCHAROMYCES CEREVISIAE. VARIATION IN RIBOSOMAL ACTIVITY AND IN RNA SYNTHESIS DURING LOGARITHMIC GROWTH. Biochemistry. 1964 Jul;3:959–967. doi: 10.1021/bi00895a020. [DOI] [PubMed] [Google Scholar]
- Lindegren G., Hwang Y. L., Oshima Y., Lindegren C. C. Genetical mutants induced by ethyl methanesulfonate in Saccharomyces. Can J Genet Cytol. 1965 Sep;7(3):491–499. doi: 10.1139/g65-064. [DOI] [PubMed] [Google Scholar]
- MARGOLIN P. Genetic fine structure of the leucine operon in Salmonella. Genetics. 1963 Mar;48:441–457. doi: 10.1093/genetics/48.3.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STADTMAN E. R. The coenzyme A transphorase system in Clostridium kluyveri. J Biol Chem. 1953 Jul;203(1):501–512. [PubMed] [Google Scholar]
- STRASSMAN M., CECI L. N. Enzymatic formation of alpha-isopropylmalic acid, an intermediate in leucine biosynthesis. J Biol Chem. 1963 Jul;238:2445–2452. [PubMed] [Google Scholar]
- Satyanarayana T., Umbarger H. E., Lindegren G. Biosynthesis of branched-chain amino acids in yeast: regulation of leucine biosynthesis in prototrophic and leucine auxotrophic strains. J Bacteriol. 1968 Dec;96(6):2018–2024. doi: 10.1128/jb.96.6.2018-2024.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]



