Abstract
Nucleoside diphosphate kinase from Halomonas sp. 593 (HaNDK) exhibits halophilic characteristics. Residues 134 and 135 in the carboxy-terminal region of HaNDK are Glu–Glu, while those of its homologous counterpart of non-halophilic Pseudomonas NDK (PaNDK) are Ala–Ala. The double mutation, E134A-E135A, in HaNDK results in the loss of the halophilic characteristics, and, conversely, the double mutation of A134E-A135E in PaNDK confers halophilic characters to this enzyme, indicating that the charged state of these two residues that are located in the C-terminal region plays a critical role in determining halophilic characteristics. The importance of these two residues versus the net negative charges will be discussed in relation to the halophilicity of NDK.
Keywords: halophilic, Halomonas, nucleoside diphosphate kinase, negative charge, solubility, reversibility, stability
Halophilic enzymes, especially those from the extremely halophilic archaea and the extracellular fractions of moderately halophilic bacteria, are highly stable and functional in the presence of salt at high concentrations, under which most non-halophilic enzymes often aggregate and become nonfunctional (Kushner 1978, 1985; Eisenberg et al. 1992; Mevarech et al. 2000). The amino acid composition of halophilic enzymes is in general characterized by an abundant content of acidic amino acid (Rao and Argos 1981), leading to high aqueous solubility without aggregation in both native and denatured states and hence allowing efficient renaturation of halophilic proteins after heat or denaturant treatment (Tokunaga et al. 2004). The electrostatic repulsion caused by the abundant negative charges on the surface of halophilic proteins is believed to be screened by the counter ions present in salt solution, resulting in a more stable, folded structure (Dym et al. 1995; Frolow et al. 1996; Ishibashi et al. 2002). Thus, the typical characteristics of halophilic enzymes are summarized as follows: (1) higher optimum salt concentrations for enzymatic activity, (2) higher reversibility to denaturation stresses, (3) higher stability in the presence of salt ions, and (4) slow mobility on SDS electrophoresis due to lower SDS binding (Tokunaga et al. 1999, 2006; Madern et al. 2000).
Nucleoside diphosphate kinase (NDK) is a well-known housekeeping enzyme that catalyzes the transfer of the γ-phosphate group of nucleoside triphosphate to nucleoside diphosphate (Parks Jr. and Agarwal 1973). It is now clear that NDK is a member of a multifunctional protein family with much broader functions (Lascu 2000; Kimura 2006; Wieland 2007). We have been studying the halophilic characteristics of NDKs from the extremely halophilic archaea, Halobacterium salinarum (Ishibashi et al. 2001), and moderately halophilic bacteria, such as Halomonas sp. 593 (HaNDK) (Yonezawa et al. 2001). The halophilic HaNDK (15,268 Da) has a high sequence homology (Yonezawa et al. 2003), i.e., 78% identity and 89% similarity, with PaNDK (15,592 Da), its non-halophilic counterpart from Pseudomonas aeruginosa (Stover et al. 2000). Despite their high sequence homology, they differ in their subunit structures; i.e., HaNDK forms a homo-dimer (Yonezawa et al. 2007) in contrast to the tetrameric structure of PaNDK and other NDKs from Gram-negative bacteria, whose subunit structures have been determined (Williams et al. 1993; Moynié et al. 2007). Recently, we found that residue 134 of HaNDK determines the dimer–tetramer assembly. Thus, HaNDK with E134 forms a dimeric assembly, while a mutation of this Glu to Ala converts HaNDK to tetrameric assembly. Conversely, tetrameric PaNDK which contains an A134 is converted to a dimer with the A134E mutation (Tokunaga et al. 2008).
In the course of this study, we found that HaNDK containing mutations at both residues 134 and 135, i.e., an E134A/E135A double mutation, migrates faster than the parent HaNDK, similar manner to the migration of non-halophilic PaNDK on SDS–polyacrylamide gel electrophoresis (SDS-PAGE). In contrast, PaNDK containing an A134E/A135E double mutation migrates as slowly as wild-type HaNDK on SDS-PAGE. These observations suggest that the negative charge at this C-terminal region affects the halophilic properties of NDK proteins. Here, we describe the critical role of these two amino acid residues, E134 and E135, at the carboxy-terminal region of NDKs in determining the halophilic characteristics of these proteins.
Results
Mobility of NDK proteins on SDS-PAGE and optimum salt concentrations for enzymatic activity
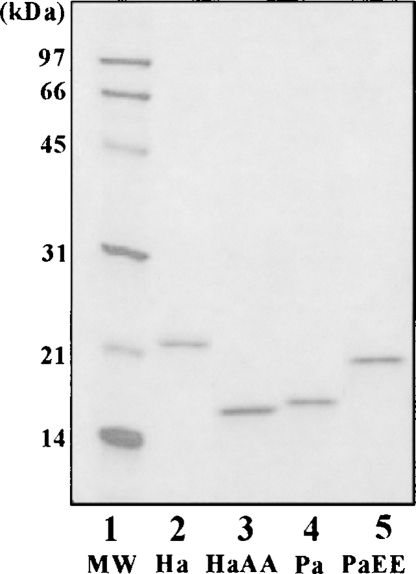
The sequence of amino acids 130 to 137 of Myxococcus NDK is reported to be involved in the tetrameric assembly of the NDK (Williams et al. 1993). The corresponding sequence of HaNDK is A-Y-F-F-E-E-S-E (Yonezawa et al. 2003), while the PaNDK sequence is A-Y-F-F-A-A-T-E (Stover et al. 2000). Thus, there are striking differences between these two NDKs, as underlined above. To analyze the roles of these two residues (134 and 135) in the halophilic properties of NDK proteins, HaNDK/AA and PaNDK/EE mutant NDK proteins were constructed, expressed in Escherichia coli, and purified. Figure 1 shows, in addition to the purity of proteins, the differences in the electrophoretic mobility of purified NDKs, albeit their similar molecular weight. Halophilic proteins usually show low mobility on SDS-PAGE relative to their molecular weight, since these proteins bind lower amounts of SDS probably due to their abundant negative charge (Tokunaga et al. 2006). Figure 1 shows that the HaNDK/AA double mutant has a faster mobility than the wild-type HaNDK/EE (cf. lanes 2,3), suggesting the loss of halophilic character by this double mutation. Conversely, the PaNDK/EE mutant has a slower mobility than the wild-type PaNDK/AA (lanes 4,5), suggesting the acquisition of halophilicity by this double mutation.
Figure 1.
SDS-PAGE analysis of wild-type and mutant NDKs. MW, molecular weight marker; Ha, HaNDK/EE wild type; Pa, PaNDK/AA wild type; HaAA, HaNDK/AA mutant; PaEE, PaNDK/EE mutant.
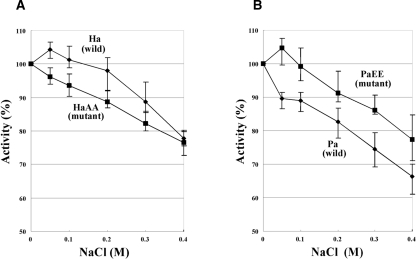
Since the electrophoretic mobility shown in Figure 1 suggested a change of halophilicity in the mutant NDKs, the salt dependence of enzyme activity, another halophilic characteristic, was examined; i.e., the enzymatic activities of wild-type and mutant NDK proteins were measured in the presence of NaCl at different concentrations. The specific enzymatic activities at 0 M NaCl of HaNDK and its mutants were determined to be ∼1000 μmol·min−1·mg protein−1, and those of PaNDK were determined to be ∼1100 μmol·min−1·mg protein−1; i.e., the activity is essentially independent of their wild or mutant sequences. As shown in Figure 2, both the wild-type HaNDK/EE and the PaNDK/EE mutant showed an optimum salt concentration at 50 mM, as expected for halophilic proteins requiring the presence of salt for optimal activity (Eisenberg et al. 1992; Mevarech et al. 2000). Conversely, the wild-type PaNDK/AA and the HaNDK/AA mutant gradually lost activity, in a monotone fashion, as the NaCl concentration was increased, consistent with the non-halophilic characters. These results demonstrate that localization of negative charges in the C-terminal region provides a slight but clear halophilic characteristic to the PaNDK/EE mutant as well as to the wild-type HaNDK/EE.
Figure 2.
NaCl concentration dependence of enzymatic activity. Concentration of KCl in the reaction mixture was 50 mM in this experiment. (A) HaNDK/EE (Ha) and its mutant HaNDK/AA (HaAA) are shown. (B) PaNDK/AA (Pa) and its mutant PaNDK/EE (PaEE) are shown. Vertical bars show the standard deviation of six measurements.
Reversibility of heat denaturation
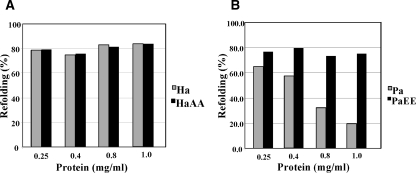
We have previously reported that the wild-type PaNDK/AA (1 mg protein/mL) formed aggregates and was irreversibly denatured when heat-treated at 85°C for 5 min and then cooled on ice (Tokunaga et al. 2006; Yonezawa et al. 2007). In contrast, the wild-type HaNDK/EE regained ∼80% enzymatic activity immediately upon cooling; such reversibility of thermal denaturation is one of the hallmarks of the halophilic proteins (Tokunaga et al. 2004). Here, we examined the recovery of enzymatic activity of HaNDK/AA and PaNDK/EE mutant proteins under the same conditions as described above. As shown in Figure 3B, the wild-type PaNDK/AA (gray bar) exhibited only ∼20% activity recovery when heat-treated at 1 mg/mL. However, the activity recovery gradually increased as the protein concentration was decreased. The recovery reached ∼65% at 0.25 mg/mL. Remarkably, the PaNDK/EE mutant (black bar) exhibited high reversibility (∼80%), similar to that of HaNDK/EE (see Fig. 3A, gray bar), independent of the protein concentration. It is thus evident that the substitution of the Glu residue for Ala134/135 clearly prevents aggregation of heat-denatured PaNDK, leading to higher reversibility. Although the HaNDK/AA mutant lost two negative charges at the C-terminal region, it still showed high reversibility (Fig. 3A, black bar), similar to that of the wild-type HaNDK/EE, indicating that the high reversibility of HaNDK depends on other factors, e.g., abundant net negative charges at the experimental pH of 7.5 even with these two mutations of EE to AA (9 negative charges vs. 7), as summarized in Table 1.
Figure 3.
Refolding efficiency of NDK proteins from heat denaturation. NDK proteins at protein concentrations of 0.25, 0.4, 0.8, and 1.0 mg/mL were heat-treated at 85°C for 5 min, cooled, kept on ice for 30 min, and then measured for enzymatic activity at 30°C. (A) Gray and black bars represent the HaNDK/EE wild type and HaNDK/AA mutant, respectively. (B) Gray and black bars represent the PaNDK/AA wild type and PaNDK/EE mutant, respectively. All of the experiments were carried out more than three times, and average values are shown.
Table 1.
Net charges and pI of NDK proteinsa
HaNDK/EE wild-type and PaNDK/EE mutant proteins were stabilized by NaCl
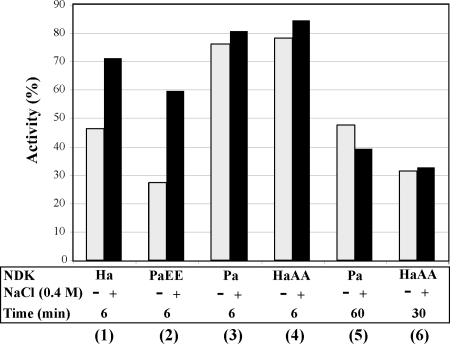
The NDK proteins were stable at high protein concentration but were gradually inactivated at low protein concentration. The NDK stock solution (0.5 mg/mL) was diluted 1000-fold with 50 mM Tris-HCl buffer, pH 8.0, to 0.5 μg/mL and incubated at 30°C for 6–60 min. After a 6-min incubation, the HaNDK/EE wild type (Fig. 4, lane 1, gray bar) and the PaNDK/EE mutant (lane 2, gray bar) were inactivated, respectively, to 47% and 28% of the activity level observed before dilution. However, these enzymes remained stable against dilution-induced inactivation in the presence of salt; the activity remained at 72% (HaDNK/EE) and 60% (PaNDK/EE) of the activity levels of the undiluted samples in the presence of 0.4 M NaCl (lanes 1,2, black bar). The PaNDK/AA wild type and HaNDK/AA mutant were much more stable against dilution than HaNDK/EE and PaNDK/EE (lanes 3,4) both with and without 0.4 M NaCl. The activity of PaNDK/AA was reduced to 48% of the control after a 60-min incubation (lane 5), and the activity of the HaNDK/AA mutant was reduced to 32% of the control after a 30-min incubation (lane 6), and neither activity loss was recovered with the addition of 0.4 M NaCl. These results clearly indicate that NDK/EE, but not NDK/AA, remains stable against dilution-induced inactivation by salt, which is one of the characteristics of the halophilic proteins.
Figure 4.
Effects of 0.4 M NaCl on the dilution-induced inactivation of NDKs. NDKs were incubated at 30°C for the time period (6–60 min) shown in the figure, in the presence (black bars) or absence (gray bars) of 0.4 M NaCl. Enzymatic activity immediately after dilution was taken as 100%. Lane 1, HaNDK/EE (Ha); lane 2, PaNDK/EE (PaEE); lanes 3 and 5, PaNDK/AA (Pa); lanes 4 and 6, HaNDK/AA (HaAA). Average values of more than three experiments are shown.
Secondary structure and thermal melting of the wild-type and mutant NDK proteins
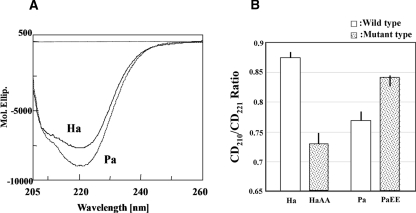
The far-UV CD spectra of the wild NDK proteins at 0.5 mg/mL, determined at 25°C, are shown in Figure 5A. There is a clear difference in the far-UV CD spectra between the two proteins. The CD spectrum of the wild-type PaNDK/AA has a much stronger negative signal at 221 nm than HaNDK/EE. The secondary-structure analysis indicated that PaNDK/AA contains more α-helix and less β-sheet structures. Such spectral differences may be characterized by the ratio of the CD intensity at 210 nm to that at 221 nm; CD210/CD221 was 0.77–0.78 for PaNDK/AA and 0.87–0.88 for HaNDK/EE. The far-UV CD spectra of the HaNDK/AA and PaNDK/EE mutants were determined (Fig. 5B), and the CD210/CD221 ratios obtained are plotted in Figure 5B. It is evident that the HaNDK/AA mutant has a significantly lower ratio than the wild-type protein, more similar to that of PaNDK/AA, whereas PaNDK/EE has a higher ratio, which is similar to that of HaNDK/EE. Thus, it may be concluded that the PaNDK/EE mutant is more similar to the HaNDK wild type carrying the EE sequence, and conversely, the HaNDK mutant with the AA sequence is more similar to the PaNDK/AA wild type.
Figure 5.
Circular dichroism profile of NDK proteins. (A) Far-UV CD spectra of wild-type NDKs are shown. Ha and Pa represent HaNDK/EE and PaNDK/AA, respectively. (B) CD210/CD221 ratios are shown. White bars represent wild-type HaNDK/EE (Ha) and PaNDK/AA (Pa). Dotted bars represent the mutant-type NDKs, HaNDK/AA (HaAA) and PaNDK/EE (PaEE). As the protein concentration was determined by the Lowry method, the mean residue ellipticity (Mol. Ellip.) is subject to uncertainty due to possible errors in the protein concentration.
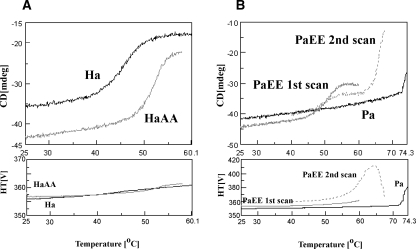
Thermal melting was followed by CD at 220 nm. The melting curves for the HaNDK wild type and HaNDK/AA mutant are shown in Figure 6A. As previously reported, wild-type HaNDK/EE melts reversibly at a low melting temperature of ∼40°C–50°C (Tokunaga et al. 2006). The thermally unfolded structure is reversed to the native structure upon cooling within 1–2 min, consistent with no aggregation evident from no change in absorbance (see lower panel). There was no second transition above 60°C (data not shown). The HaNDK/AA mutant, which showed a low CD210/CD221 ratio, unfolds at a melting temperature of ∼47°C–56°C and hence is significantly more stable than the wild type. The melting of this mutant was also highly reversible (data not shown). It thus appears that the melting temperature of HaNDK is raised by the double mutation. Reversibility is, however, not affected by this mutation, consistent with the high activity recovery from heating for both proteins (Fig. 3A).
Figure 6.
Thermal melting of NDK proteins. Thermal melting at a protein concentration of 0.5 mg/mL was followed at 220 nm. (A) HaNDK/EE (Ha) and its mutant HaNDK/AA (HaAA). (B) PaNDK/AA (Pa) and its mutant PaNDK/EE (PaEE). The first scan (“PaEE first scan”) was stopped at 60°C, cooled to 20°C, and rescanned from 40°C to 67°C (PaEE second scan).
Thermal melting of PaNDK is shown in Figure 6B. The wild-type PaNDK/AA shows a high onset temperature of melting at around 68°C, accompanied by extensive aggregation (as observed by a sharp increase in the absorbance and hence light scattering). The PaNDK/EE mutant, which showed a high CD210/CD221 ratio, displayed a low melting temperature of 44°C–56°C (T 1), which was similar to the HaNDK/EE wild type. There was a slight increase in the absorbance and light scattering (see gray line in lower panel). After stopping the scan at 60°C, the sample was cooled to 20°C and incubated for ∼15 min. The same sample was then rescanned from 40°C to 67°C (Fig. 6B, see PaEE second scan). There was a low-temperature transition (T 1 = 44°C–55°C), but with a smaller magnitude, followed by a sharp increase in CD at ∼62°C (T 2) and a gradual increase of light scattering at ∼56°C. Thus, it appears that the melting of the PaNDK/EE mutant has properties of both wild-type HaNDK/EE (i.e., low melting temperature) and PaNDK/AA (high melting temperature and aggregation).
Discussion
We have previously shown that residue 134 determines dimer–tetramer assembly of HaNDK and PaNDK (Tokunaga et al. 2008). In addition to the impact of residue 134 on the dimer–tetramer conversion, residue E134 together with E135 appears to confer halophilic characteristics to NDK proteins. Both the HaNDK/EE wild-type and PaNDK/EE mutant proteins showed optimum salt concentrations at 50 mM, meaning that the double mutation at these positions conferred PaNDK halophilic characteristics (Fig. 2B). The wild-type PaNDK/AA and the HaNDK/AA mutant lost activity in a monotone fashion as NaCl concentration was increased, meaning loss of halophilic characteristics in HaNDK/AA (Fig. 2A). These results indicate that the localization of negative charges in the C-terminal region provides halophilic characteristics to both NDKs. The wild-type HaNDK, which has an EE sequence, shows a high reversibility of thermal denaturation due to the inability of the denatured protein to aggregate. This has lead to full activity recovery of HaNDK/EE upon heat treatment (Fig. 3A). Thermal denaturation of the HaNDK/AA mutant (Fig. 6A) was also reversible, consistent with the heat-treatment experiment (Fig. 3A). On the other hand, PaNDK/AA readily aggregates under identical denaturation conditions (Fig. 6B), rendering the reaction irreversible, consistent with the activity loss after heat treatment (Fig. 3B). The PaNDK/EE mutant showed partial reversibility of thermal denaturation (Fig. 6B), indicating that these two EE residues make PaNDK more halophilic. There is a discrepancy between the thermal denaturation (Fig. 6B) and heat-treatment (Fig. 3B) experiments. Although the thermal denaturation experiment for PaNDK/EE at 0.5 mg/mL showed only a partial reversibility after heating to 60°C, heat-treated PaNDK/EE at 0.4–0.8 mg/mL regained higher activity. This is most likely due to differences between the two experiments: prolonged heating during the CD thermal scan and brief heating for the activity measurements.
In addition to the effects on activity, the stability of NDKs is also modulated by salt. Thus, the NDK/EE proteins, i.e., HaNDK/EE and the PaNDK/EE mutant, remained stable against dilution-induced inactivation in the presence of salt, while the NDK/AA proteins were destabilized by dilution regardless of the presence of NaCl. The stabilizing effect of salt is one of the typical properties of the halophilic proteins (Fig. 4). Altogether, localization of negative charges at the C-terminal region confers all four characteristics of halophilic proteins on NDK proteins, i.e., lower migration on SDS-PAGE, higher optimum salt concentration for enzymatic activity, higher reversibility from thermal denaturation, and higher stability in the presence of NaCl.
The calculated pIs of HaNDK/EE, HaNDK/AA, PaNDK/AA, and PaNDK/EE were 4.56, 4.70, 5.36, and 5.01, respectively, showing that PaNDK/EE (halophilic) possesses a higher calculated pI than HaNDK/AA (non-halophilic). However, a more relevant parameter for the acidic properties of NDK is the net negative charge at the experimental pH of 7.5–8.0 (Table 1). Although it appears clear that an increased net negative charge does contribute to the halophilic characters of NDK, the fact that PaEE, which is less acidic than HaAA (non-halophilic), is halophilic demonstrates that the acidic amino acid residues at a particular region, rather than the overall net negative charges, are responsible for the halophilicity in this particular case. The pI and net negative charge of the HaNDK/AA mutant do not change much, making this mutant overall still acidic, which is responsible for its observed reversibility (Fig. 3A).
Mutations at residues 134 and 135 changed the secondary structure of HaNDK and PaNDK. The structural consequence was evident in the CD data (Fig. 5). NDKs with the EE sequence have higher CD210/CD221 ratios, meaning that they contain less α-helix but more β-sheet structures. NDKs with the AA sequence have a lower ratio. As far as HaNDK is concerned, the mutation results in a higher melting temperature (Fig. 6A). It did not generate a second melting transition. Although the CD210/CD221 ratio of HaNDK/AA decreased to a level closer to that of PaNDK/AA (Fig. 5B), a high melting transition was not observed for HaNDK/AA. On the other hand, PaNDK/EE, with a high CD210/CD221 ratio, exhibited a low melting temperature, as has been observed with HaNDK, meaning that this PaNDK/EE mutant behaves like wild-type HaNDK (Fig. 6B). It is thus evident that the replacement of neutral amino acid residues at this C-terminal region by negatively charged residues destabilizes the NDK structure against heat treatment, while the reverse mutation (replacing the acidic amino acid with a neutral residue) stabilizes it.
Our present study demonstrates the possibility of molecular breeding of new halophilic enzymes by the addition of acidic amino acid residues at appropriate positions of non-halophilic enzymes to obtain better reversibility from denaturation or to allow functioning at higher salt concentrations.
Materials and Methods
Bacterial strains, medium, and plasmids
Escherichia coli JM109 and DH5α were used for DNA manipulation. E. coli BL21Star(DE3) was used for gene expression encoded on pET vectors (Novagen). LB-ampicillin (100 μg/mL) was used. For preculture of the transformant harboring pET-derived vectors, LB-ampicillin containing 0.4% glucose was used. The site-directed mutagenesis of ndk genes cloned on pET3a vector was performed using a QuikChange Site-Directed Mutagenesis kit (Stratagene) according to the manufacturer's instructions. All mutations were verified by determination of their nucleotide sequences.
The 134th and 135th residues of wild-type HaNDK are both glutamic acid; this wild-type HaNDK is expressed as HaNDK/EE. Residue 134 and 135 of wild-type PaNDK are both alanine; it is expressed as PaNDK/AA. In the expression of NDK/XX, the first X after the slash represents the amino acid residue 134, and the second X represents residue 135. The HaNDK double mutant, in which both Glu residues 134 and 135 are changed to Ala, is expressed as HaNDK/AA. PaNDK/EE contains A134E and A135E double mutations.
Expression and purification of NDK proteins
Wild-type and mutant NDK proteins were expressed in E. coli BL21Star(DE3) and purified using ATP-agarose affinity column chromatography in standard buffer solution (50 mM Tris-HCl buffer, pH 8.0, containing 0.2 M NaCl and 2 mM MgCl2) to homogeneity (Yonezawa et al. 2007). An overnight preculture of BL21Star(DE3) harboring plasmid grown in LB-ampicillin containing 0.4% glucose was inoculated to fresh LB-ampicillin medium and cultured at 37°C until A 600 ≈ 0.5. Isopropyl-1-thio-β-d-galactopyranoside (final concentration of 1 mM) was added to induce protein expression and growth continued for an additional 3 h. Then, the cells were disrupted in ice-cold standard buffer solution by sonication. The soluble fraction after centrifugation of the homogenate was applied to ATP affinity column chromatography. The column was washed with the same buffer and then eluted in a stepwise manner with 0.2–3.0 mM of ATP in the same buffer.
Enzymatic activity assay and refolding assay
Enzymatic activity was measured by the enzyme-coupling method at 30°C as described previously (Agarwal and Parks Jr. 1971; Ishibashi et al. 2001). A reaction mixture (0.5 mL) containing 0.4 mM TDP, 2 mM ATP, 25 mM MgCl2, 3 mM phosphoenolpyruvate, 0.15 mM NADH, 0.1 M KCl, 0.8 U pyruvate kinase, 2.3 U lactate dehydrogenase, 100 mM Tris-HCl buffer, pH 7.5, and the enzyme sample was incubated at 30°C in a cuvette placed in a photometer chamber. The reaction was followed by A 340. The enzymatic activity was also measured in a microtiter plate (96-well plate) with the same reaction mixture (0.2 mL) except with a higher NADH concentration of 0.24 mM and A 340 was measured by a plate reader (Bio-Rad Benchmark Plus) at 30°C.
For the refolding assay from heat denaturation, NDK proteins in standard buffer solution were heat-treated at 85°C for 5 min, kept on ice for 30 min, and then measured for enzymatic activity at 30°C by the enzyme-coupling method.
Circular dichroism and thermal melting
Circular dichroism (CD) measurements were carried out on a Jasco J-715 spectropolarimeter equipped with a Peltier cell holder and a PTC-348WI temperature controller. The samples were prepared at 0.5 mg/mL in 50 mM Tris-HCl, 2 mM MgCl2, 0.2 M NaCl, pH 8.0. Thermal melting followed at a fixed wavelength of 220 nm.
Other
SDS-PAGE was carried out according to the method of Laemmli (1970). The protein concentration was determined by Lowry et al. (1951), due to the limited quantity of the sample. As this method is less accurate than direct absorbance measurements, the CD calculation will be affected by this uncertainty.
Acknowledgments
This study was supported by the Salt Science Research Foundation (Program for Research on Halophilic Organisms) and by a Grant-in-Aid for Science Research (16580280) from MEXT Japan.
Footnotes
Reprint requests to: Masao Tokunaga, Applied and Molecular Microbiology, Faculty of Agriculture, Kagoshima University, 1-21-24 Korimoto, Kagoshima 890-0065, Japan; e-mail: tokunaga@chem.agri.kagoshima-u.ac.jp; fax: 81-99-285-8634.
Article and publication are at http://www.proteinscience.org/cgi/doi/10.1110/ps.035725.108.
References
- Agarwal, R.P., Parks R.E., Jr Erythrocytic nucleoside diphosphokinase. V. Some properties and behavior of the pI 7.3 isozyme. J. Biol. Chem. 1971;246:2258–2264. [PubMed] [Google Scholar]
- Dym, O., Mevarech, M., Sussman, J.L. Structure features that stabilize halophilic malate dehydrogenase from an archaebacterium. Science. 1995;267:1344–1346. doi: 10.1126/science.267.5202.1344. [DOI] [PubMed] [Google Scholar]
- Eisenberg, H., Mevarech, M., Zaccai, G. Biochemical, structural, and molecular genetic aspects of halophilism. Adv. Protein Chem. 1992;43:1–62. doi: 10.1016/s0065-3233(08)60553-7. [DOI] [PubMed] [Google Scholar]
- Frolow, F., Harel, M., Sussman, J.L., Mevarech, M., Shoham, M. Insights into protein adaptation to a saturated salt environment from the crystal structure of a halophilic 2Fe–2S ferredoxin. Nat. Struct. Biol. 1996;3:452–458. doi: 10.1038/nsb0596-452. [DOI] [PubMed] [Google Scholar]
- Ishibashi, M., Tokunaga, H., Hiratsuka, K., Yonezawa, Y., Turumaru, H., Arakawa, T., Tokunaga, M. NaCl-activated nucleoside diphosphate kinase from extremely halophilic archaeon, Halobacterium salinarum, maintain native conformation without salt. FEBS Lett. 2001;493:134–138. doi: 10.1016/s0014-5793(01)02292-x. [DOI] [PubMed] [Google Scholar]
- Ishibashi, M., Arakawa, T., Philo, J.S., Sakashita, K., Yonezawa, Y., Tokunaga, H., Tokunaga, M. Secondary and quaternary structural transition of the halophilic archaeon nucleoside diphosphate kinase under high- and low-salt conditions. FEMS Microbiol. Lett. 2002;216:235–241. doi: 10.1111/j.1574-6968.2002.tb11441.x. [DOI] [PubMed] [Google Scholar]
- Kimura, N. Introduction. J. Bioenerg. Biomembr. 2006;38:149–150. [Google Scholar]
- Kushner, D.J. Life in high salt and solute concentrations: Halophilic bacteria. In: Kushner D.J., editor. Microbial life in extreme environments. Academic Press; San Diego, CA: 1978. pp. 317–368. [Google Scholar]
- Kushner, D.J. The Halobacteriaceae. In: Woese C.R., Wolfe R.S., editors. The bacteria. Vol. 8. Academic Press; Orlando, FL: 1985. pp. 171–214. [Google Scholar]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lascu, I. The nucleoside diphosphate kinases 1973–2000. J. Bioenerg. Biomembr. 2000;32:213–214. [PubMed] [Google Scholar]
- Lowry, O.H., Rosebrough, N.J., Farr, A.L., Randall, R.J. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Madern, D., Ebel, C., Zaccai, G. Halophilic adaptation of enzymes. Extremophiles. 2000;4:91–98. doi: 10.1007/s007920050142. [DOI] [PubMed] [Google Scholar]
- Mevarech, M., Frolow, F., Gloss, L.M. Halophilic enzymes: Proteins with a grain of salt. Biophys. Chem. 2000;86:155–164. doi: 10.1016/s0301-4622(00)00126-5. [DOI] [PubMed] [Google Scholar]
- Moynié, L., Giraud, M.-F., Georgescauld, F., Lascu, I., Dautant, A. The structure of the Escherichia coli nucleoside diphosphate kinase reveals a new quaternary architecture for this enzyme family. Proteins. 2007;67:755–765. doi: 10.1002/prot.21316. [DOI] [PubMed] [Google Scholar]
- Parks R.E., Jr, Agarwal, R.P. Nucleoside diphosphokinases. In: Boyer P.D., editor. The enzymes. Vol. 8. Academic Press; New York: 1973. pp. 307–334. [Google Scholar]
- Rao, J.K.M., Argos, P. Structure stability of halophilic proteins. Biochemistry. 1981;20:6536–6543. doi: 10.1021/bi00526a004. [DOI] [PubMed] [Google Scholar]
- Stover, C.K., Pham, X.Q., Erwin, A.L., Mizoguchi, S.D., Warrener, P., Hickey, M.J., Brinkman, F.S., Hufnagle, W.O., Kowalik, D.J., Lagrou, M., et al. Complete genome sequence of Pseudomonas aeruginosa PAO1, an opportunistic pathogen. Nature. 2000;406:959–964. doi: 10.1038/35023079. [DOI] [PubMed] [Google Scholar]
- Tokunaga, H., Hara, S., Arakawa, T., Ishibashi, M., Gupta, R.S., Tokunaga, M. Identification and partial purification of DnaK homologue from extremely halophilic archaebacteria, Halobacterium cutirubrum . J. Protein Chem. 1999;18:837–844. doi: 10.1023/a:1020675128201. [DOI] [PubMed] [Google Scholar]
- Tokunaga, H., Ishibashi, M., Arakawa, T., Tokunaga, M. Highly efficient renaturation of β-lactamase isolated from moderately halophilic bacteria. FEBS Lett. 2004;558:7–12. doi: 10.1016/S0014-5793(03)01508-4. [DOI] [PubMed] [Google Scholar]
- Tokunaga, H., Oda, Y., Yonezawa, Y., Arakawa, T., Tokunaga, M. Contribution of halophilic nucleoside diphosphate kinase sequence to the heat stability of chimeric molecule. Protein Pept. Lett. 2006;13:525–530. doi: 10.2174/092986606776819628. [DOI] [PubMed] [Google Scholar]
- Tokunaga, H., Ishibashi, M., Arisaka, F., Arai, S., Kuroki, R., Arakawa, T., Tokunaga, M. Residue 134 determines the dimer–tetramer assembly of nucleoside diphosphate kinase from moderately halophilic bacteria. FEBS Lett. 2008;582:1049–1054. doi: 10.1016/j.febslet.2008.02.054. [DOI] [PubMed] [Google Scholar]
- Wieland, T. Interaction of nucleoside diphosphate kinase B with heterotrimeric G protein βγ dimers: Consequences on G protein activation and stability. Naunyn Schmiedebergs Arch. Pharmacol. 2007;374:373–383. doi: 10.1007/s00210-006-0126-6. [DOI] [PubMed] [Google Scholar]
- Williams, R.L., Oren, D.A., Munoz-Dorado, J., Inouye, S., Inouye, M., Arnold, E. Crystal structure of Myxococcus xanthus nucleoside diphosphate kinase and its interaction with a nucleotide substrate at 2.0 Å resolution. J. Mol. Biol. 1993;234:1230–1247. doi: 10.1006/jmbi.1993.1673. [DOI] [PubMed] [Google Scholar]
- Yonezawa, Y., Tokunaga, H., Ishibashi, M., Tokunaga, M. Characterization of nucleoside diphosphate kinase from moderately halophilic eubacteria. Biosci. Biotechnol. Biochem. 2001;65:2343–2346. doi: 10.1271/bbb.65.2343. [DOI] [PubMed] [Google Scholar]
- Yonezawa, Y., Tokunaga, H., Ishibashi, M., Taura, S., Tokunaga, M. Cloning, expression, and efficient purification in Escherichia coli of a halophilic nucleoside diphosphate kinase from the moderate halophile Halomonas sp. #593. Protein Expr. Purif. 2003;27:128–133. doi: 10.1016/s1046-5928(02)00594-6. [DOI] [PubMed] [Google Scholar]
- Yonezawa, Y., Izutsu, K., Tokunaga, H., Maeda, H., Arakawa, T., Tokunaga, M. Dimeric structure of nucleoside diphosphate kinase from moderately halophilic bacterium: Contrast to the tetrameric Pseudomonas counterpart. FEMS Microbiol. Lett. 2007;268:52–58. doi: 10.1111/j.1574-6968.2007.00626.x. [DOI] [PubMed] [Google Scholar]