Abstract
Vascular adhesion protein 1 (VAP-1) is a human endothelial sialoglycoprotein whose cell surface expression is induced under inflammatory conditions. It has been shown previously to participate in lymphocyte recirculation by mediating the binding of lymphocytes to peripheral lymph node vascular endothelial cells in an L-selectin–independent fashion. We report here that the VAP-1 cDNA encodes a type II transmembrane protein of 84.6 kD with a single transmembrane domain located at the NH2-terminal end of the molecule and six potential N-glycosylation sites in the extracellular domain. In vivo, the protein exists predominantly as a homodimer of 170–180 kD. Ax endothelial cells transfected with a VAP-1 cDNA express VAP-1 on their cell surface and bind lymphocytes, and the binding can be partially inhibited with anti–VAP-1 mAbs. VAP-1 has no similarity to any currently known adhesion molecules, but has significant identity to the copper-containing amine oxidase family and has a monoamine oxidase activity. We propose that VAP-1 is a novel type of adhesion molecule with dual function. With the appropriate glycosylation and in the correct inflammatory setting, its expression on the lumenal endothelial cell surface allows it to mediate lymphocyte adhesion and to function as an adhesion receptor involved in lymphocyte recirculation. Its primary function in other locations where it is expressed, such as smooth muscle, may depend on its inherent monoamine oxidase activity.
Keywords: vascular adhesion protein 1, adhesion molecule, monoamine oxidase, sialoglycoprotein, endothelial
Continuous recirculation of lymphocytes between blood and tissue is critical for the functioning of the immune system. The adhesive interactions between multiple receptors on the circulating lymphocytes and their ligands expressed on the surface of endothelial cells in postcapillary venules provide both the means for the emigration process and a way to selectively control it. Although considerable progress has been made recently in describing the cascade of events required for a circulating leukocyte to pass into the tissue from freely flowing blood, much in this process remains to be discovered.
Present hypotheses suggest a multistep model of leukocyte adhesion to endothelium that relies on a cascade of sequential but overlapping molecular interactions between several receptor–ligand pairs (1–3). The initial transient and tethering interactions between a leukocyte in the blood stream and the vessel wall are performed principally by the selectins and their glycoprotein ligands (4–6). Integrins and other molecules may also have a role in this phase (7). This rolling and sampling step can, in the presence of the appropriate signals, be followed by firm adhesion mediated by the binding of activated integrins to their Ig superfamily ligands (4). Locally elevated levels of chemokines and other chemoattractants might be involved in initiating this activation, which is mediated by the appropriate receptors and subsequent transduction of signals within the cell (8–10). The final stage involves the transmigration of the bound cell through the endothelial lining into the tissue by mechanisms that are poorly understood. Tissue-specific recirculatory pathways, such as those of the gut and skin, rely on the regulated expression of particular adhesion molecule receptor–ligand pairs in the appropriate location (3).
We have described previously a murine mAb, 1B2, recognizing vascular adhesion protein 1 (VAP-1),1 a novel human cell adhesion molecule prominently expressed in high endothelial venules (HEV) of peripheral lymph nodes (PLN), through which much lymphocyte recirculation takes place. mAb 1B2 can block lymphocyte binding to tonsillar, synovial, and PLN HEV in a frozen section assay as well as to purified tonsillar VAP-1 (11–13). VAP-1 expression is upregulated on the endothelial cell surface under inflammatory conditions in nonlymphoid tissue such as the skin and synovium, suggesting that the molecule can also mediate the increased lymphocyte emigration found in inflamed tissue (12–14).
Two species of VAP-1 of differing molecular mass can be detected by mAb 1B2 immunoprecipitation of tonsil tissue, one of 90 and the other of 170–180 kD. However, after immunoblotting and detection with mAb 1B2 under nonreducing conditions, only the 170–180 kD species is detected (15). Immunoreactive VAP-1, with slightly different molecular masses, can also be found in other locations, particularly in the smooth muscle cells of the vasculature as well as in other smooth muscle–containing tissues (reference 12, and our unpublished observations). Studies of tonsillar VAP-1 using digestion with specific glycosidases have shown that VAP-1 is a sialoglycoprotein, probably containing both N- and O-linked sugars with abundant sialic acid residues of both the α2,3- and α2,6-linked type. It has also been shown that VAP-1 mediates lymphocyte binding to HEV in lymphatic tissues under nonstatic conditions and in a sialic acid–dependent manner, as the desialylated molecule can no longer support lymphocyte binding in the frozen section assay (15). In vivo, VAP-1 mediates the initial interactions between lymphocytes and inflamed vessels when analyzed by intravital microscopy (16). VAP-1 is distinct from the PLN addressin (PNAd) defined by the mAb MECA-79, though both VAP-1 and PNAd can mediate lymphocyte binding to PLN under shear conditions. However, in contrast to PNAd, VAP-1 can operate in an L-selectin– independent manner and support the binding of both L-selectin–negative and –positive lymphocytes (15, 16). Therefore, VAP-1 is a molecule with an adhesive function in an alternative pathway operating independently of L-selectin, and is likely to mediate early interactions in lymphocyte binding to PLN-type HEV and vessels in inflammatory foci. To analyze this protein at the molecular level, we have isolated a cDNA clone encoding it and show that VAP-1 defines a functional new adhesion molecule possessing a monoamine oxidase (MAO) activity.
Materials and Methods
Abs and Reagents.
Mouse mAb 1B2 against VAP-1 and negative control mAb 3G6 and 7C7 against chicken antigens have been described previously (11, 17). mAb TK8-14 against VAP-1 was produced by immunizing Balb/c mice with immunoaffinity-purified VAP-1 (17a). TK8-14 detects both the monomeric and dimeric forms of VAP-1, stains tissues in an identical manner to mAb 1B2, and blocks lymphocyte adhesion to PLN HEV in the Stamper-Woodruff frozen section assay. Its specificity was confirmed by a positive reaction with VAP-1 cDNA transfectants. The mAb M2 recognizing the FLAG peptide was obtained from KEBO Lab Oy (Espoo, Finland). Chemical reagents were from Sigma Chemical Co. (St. Louis, MO).
VAP-1 Purification and Sequencing.
Normal gut samples obtained from abdominal surgery were dissected free from the lamina propria, minced into small pieces, and lysed in a lysis buffer (150 mM NaCl, 10 mM Tris-base, pH 7.2, 1.5 mM MgCl2, 1% NP-40, 1% aprotinin, and 1 mM PMSF) overnight. After clarification, the lysate supernatant was applied sequentially to immunoaffinity columns containing 5 ml of CnBr-activated Sepharose beads armed with normal rat serum, nonbinding mAbs, and an anti–VAP-1 mAb (3 mg/ml beads). After washing with lysis buffer, the VAP-1 antigens were eluted with 50 mM triethylamine, frozen, and subsequently lyophilized. The sample was then dissolved in nonreducing Laemmli's sample buffer and separated on a 5–12.5% SDS-PAGE gel. After transfer to polyvinylidine difluoride membrane (Applied Biosystems, Inc., Foster City, CA) by electroblotting, the membrane was stained with Coomassie blue, and the 90- and 170–180-kD bands were excised. The whole of the 170–180-kD band and a portion of the 90-kD material were subjected to NH2-terminal and tryptic peptide sequencing (477A; Applied Biosystems, Inc.) as described previously (18). Matrix-assisted laser desorption mass spectrometry (Lasermat; Finnigan Corp., San Jose, CA) was used to confirm the predicted mass of some of the peptides.
Molecular Biology Techniques.
DNA cloning and manipulation were performed according to standard techniques (19) or by using commercially available kits following instructions supplied by the manufacturer. Plasmid DNA was sequenced using a Sequenase version 2.0 kit (US Biologicals, Cleveland, OH) or in the DNA sequencing facility of the University of Turku, Department of Medical Genetics. Sequence assembly and analysis were performed using the Wisconsin Package version 8.1 UNIX of the Genetics Computer Group (GCG, Madison, WI), and database comparisons were made using the BLAST server of the National Center for Biotechnology Information (http://www.ncbi.nlm. nih.gov/). Oligonucleotide primers for sequencing and PCR were obtained from KEBO Lab Oy. The primers used for amplifying VAP-1 cDNA fragment from smooth muscle mRNA by reverse transcription PCR were N2 (gctgtgatcacmatyttygc), designed from the VAP-1 NH2-terminal protein sequence AVITIFA (residues 13–19 of the complete protein), and T4 (ccggccctgrtagaasac), designed from the tryptic peptide sequence VFYQGR (residues 264–269). Amplification conditions with these primers were 94°C, 1 min; 55°C, 1 min; 72°C, 2 min for 30 cycles. Human multiple tissue Northern blots were obtained from Clontech (Palo Alto, CA) and hybridized with 32P-labeled probes as recommended by the manufacturer. The human cDNA library panel and lung (HL3004a) and heart (HL3026a) cDNA libraries were from Clontech.
Amine Oxidase Assays.
Confluent Chinese hamster ovary (CHO) or Ax cells stably expressing VAP-1 (10–15 × 106 per flask) were detached with trypsin-EDTA, washed in culture medium, and resuspended in 1.5 ml of lysis buffer. The lysate was clarified by centrifugation and used directly in enzyme assays (10– 50 μl per assay). Total protein concentrations were measured by the Bradford method using bovine gammaglobulin as a standard and a protein assay kit (Bio-Rad Laboratories, Hercules, CA). Amine oxidase activities were measured using a spectrophotometric method exactly as described by Holt et al. (20) in a volume of 200 μl in 96-well plates, and the absorbance change was monitored in a Victor multilabel plate counter at 490 nm (Wallac, Turku, Finland). Substrate concentrations were 1 mM, and samples were preincubated with amine oxidase inhibitors for 30 min before assay if required. All enzyme assays were performed in the presence of blanks containing boiled (5 min, 100°C) or mock sample in duplicate or triplicate. Tonsillar VAP-1 was purified using a Sepharose CNBr anti–VAP-1 mAb immunoaffinity column, as described above, but was assayed directly without elution from the beads. Controls of crude porcine kidney diamine oxidase (DAO) and bovine plasma MAO were obtained from Sigma Chemical Co. Detection of the quinone cofactor in purified VAP-1 was performed by the redox cycling method of Paz et al. (21).
Cell Culture and Expression of VAP-1 cDNAs in Mammalian Cells.
COS-7 (monkey fibroblasts) and CHO cells obtained from the American Type Culture Collection (Rockville, MD) and Ax (rat HEV–derived) cells originating in the laboratory of M. Miyasaka (Osaka University, Osaka, Japan; reference 22) and obtained with his permission from R. Renkonen, University of Helsinki (Helsinki, Finland), were used as hosts for transfection and expression of VAP-1. COS-7 and CHO cells were grown according to standard procedures. Ax cells were cultured as described (22). Expression plasmids consisting of the VAP-1 cDNA in pcDNA3 (Invitrogen Corp., San Diego, CA) were used for transient COS-7 cell transfections and generation of stably transfected CHO and Ax cell lines. Expression plasmids (20 μg) were used to transfect cells by electroporation (0.3 kV, 960 μF, 0.4-cm cuvette in RPMI plus 1 mM Na-pyruvate, 2 mM L-glutamine, without serum) or by the Lipofectamine method (GIBCO BRL, Gaithersburg, MD) using 2 μg of DNA. Transiently transfected cells were assayed 3 d after transfection. Stably transfected cells were selected by culturing in the presence of 0.5 mg/ml Geneticin (GIBCO BRL) for 4 wk.
Immunomagnetic Selections.
Stably transfected Ax and CHO cells were selected for uniformly brightly positive cells using magnetic activated cell sorting (MACS®; Miltenyi Biotec Inc., Auburn, CA). In brief, confluent monolayers of transfected cells were detached by a brief trypsin-EDTA treatment. Thereafter, the cells were incubated with a saturating concentration of anti– VAP-1 mAb. After washing, the cells were incubated with anti– mouse IgM–specific superparamagnetic microbeads (Miltenyi Biotec Inc.) and subjected to positive selection in an AS type MACS® column according to the manufacturer's instructions. Positively selected cells eluted from the column were stained for immunofluorescence using an FITC-labeled anti–VAP-1 mAb, TK8-14, which detects a different epitope on VAP-1 than mAb 1B2. Most positive cell lines were chosen for further culture, and the selection was repeated periodically to maintain high expression levels of the transfectants.
FACS® Analyses.
Stably or transiently transfected cells were detached from the culture flasks by a short trypsin-EDTA treatment (identical results were obtained using EDTA only, which indicates that brief trypsinization does not destroy VAP-1). After washings, normal surface staining was done as described (15). For detection of intracellular antigens, the cells were permeabilized by a 2-min incubation in −20°C acetone. The cells were then diluted immediately in an excess of the culturing medium and washed twice. After blocking nonspecific binding by a 20-min incubation on ice with an FCS-containing medium, the cells were stained normally using indirect immunofluorescence staining. The cells were finally fixed in 1% paraformaldehyde, and 104 cells were analyzed using FACScan® and Lysys II software (Becton Dickinson, Mountain View, CA). In all stainings, isotype-matched nonreactive negative control mAbs were used to set the level of nonspecific background staining.
Adhesion Assays.
Ax cells in which VAP-1 was stably expressed or mock control transfectants were plated within wax-pen circles drawn on gelatin-precoated microscope slides (2 × 104 cells per 2-cm-diameter circle). The cells were allowed to grow to confluence, and after two washings, 100 μl of RPMI 1640 medium containing 10% FCS and 10 mM Hepes (the assay medium) was added within each wax-pen circle to evenly cover the adherent cell monolayer. Meanwhile, PBL were isolated from freshly drawn blood using Ficoll centrifugation and adjusted to a concentration of 40 × 106 cells/ml in the assay medium. Thereafter, the slides were transferred to an orbital shaker operating at 60 rpm at 7°C, and 50 μl of the PBL suspension was applied onto each wax-pen circle. The assay was continued for 30 min with constant rotation. The slides were carefully decanted and dipped once in cold RPMI to remove nonadherent cells. Adherent cells were fixed to the sections by incubating the slides vertically in ice-cold PBS containing 1% glutaraldehyde overnight. The number of adherent cells was counted using an ocular grid (magnification ×200). The grid covers an area of 0.25 mm2. Nine predefined areas of 0.25 mm2 at the center of the circle where the transfectants formed a confluent monolayer were counted on each slide. Two slides per sample (total area, 4.5 mm2) were counted in each of five independent experiments. In certain experiments, the adhesion assay was performed in the presence of function-blocking mAbs against VAP-1 (a combination of 1B2 and TK8-14, both at 50 μg/ml diluted in the assay medium) or class-matched negative control mAbs (a combination of 3G6 and 7C7, both at 50 μg/ml). The mAbs were preincubated with the transfectant monolayer on the slides for 30 min at 7°C before the labeled lymphocytes were added. The number of adherent cells was counted in five independent experiments as described above.
Results
Isolation of a VAP-1 cDNA.
Human gut smooth muscle, in which VAP-1 is strongly expressed, was obtained from material removed in surgical procedures. Using an mAb immunoaffinity column, the 90- and 170–180-kD forms of VAP-1 were purified from detergent lysates of the tissue in sufficient quantities to obtain internal peptide sequence after digestion with trypsin and V8 protease. In addition, a portion of the 90-kD VAP-1 was subjected to NH2-terminal sequencing directly. The peptide elution profile from the HPLC column used to purify the peptides from both forms of VAP-1 was identical, as were the peptide sequences of the corresponding peaks, indicating that the protein is a homodimer composed of two 90-kD subunits. It should be noted that the NH2-terminal protein sequence is different from that reported previously for VAP-1 (11), which was later shown to be from an unrelated mouse protein of identical size to VAP-1 that coprecipitated with the mouse mAb used in the immunopurification (23).
VAP-1 peptide sequences were used to design partially degenerate oligonucleotide primers for reverse transcription PCR experiments on mRNA prepared from human gut smooth muscle. A single cDNA fragment of ∼700 bp was amplified. The partial cDNA sequence contained a continuous open reading frame that encoded a protein containing some of the sequences of the tryptic and V8 peptides of the immunopurified VAP-1 material, thereby confirming that the correct cDNA fragments had been amplified. A panel of 10 human cDNA libraries was analyzed by PCR in order to identify those containing VAP-1 cDNAs, and those giving the strongest signal were screened with the PCR-generated VAP-1 cDNA fragment. In this manner, a number of overlapping cDNA clones were isolated from human lung and heart cDNA libraries. Two overlapping lung cDNAs were sequenced completely on both strands. The resulting combined cDNA was 2,501 bp in length and contained a continuous open reading frame of 2,292 bp starting at an ATG methionine codon. This methionine was followed by the peptide sequence found at the NH2-terminal of the purified 90-kD VAP-1 protein, and the contiguous open reading frame encoded all of the VAP-1 tryptic and V8 peptides identified by protein sequencing (Fig. 1). A 5′ untranslated region of 80 bp and a 3′ untranslated region of 129 bp following the TAG stop codon were present in the clone. Neither a polyadenylation signal nor a poly A sequence was found at the 3′ end of the cDNA, suggesting that the native VAP-1 mRNA may be longer than indicated by the cDNA isolated here.
Figure 1.
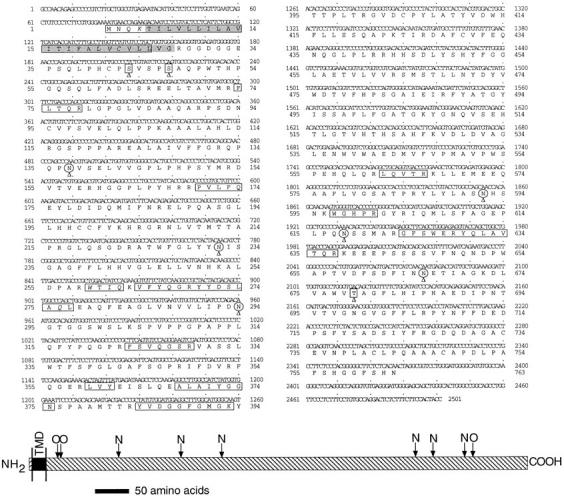
Sequence of the VAP-1 cDNA isolated from a human lung cDNA library and the predicted sequence of VAP-1 protein. Boxes, The NH2-terminal, tryptic and V8 peptides purified and sequenced from immunopurified VAP-1 protein. An italicized amino acid residue within a boxed region indicates that no amino acid could be assigned to that cycle in the peptide sequencing. Arrowed and circled asparagines, Potential N-glycosylation sites; arrowed squares, the putative O-glycosylation sites. Shading, The transmembrane domain between residues 5 and 27. Scale diagram (bottom) indicates the location of the transmembrane domain (shown by the filled transmembrane domain (TMD) region). N and O, The relative location of the putative glycosylation sites in the extracellular portion of the molecule, to show N- and O-linked sugar attachment sites, respectively. Scale bar, 50 amino acids. These sequence data have been submitted to DDBJ/EMBL/ GenBank as accession no. AF067406.
Adhesion Assays.
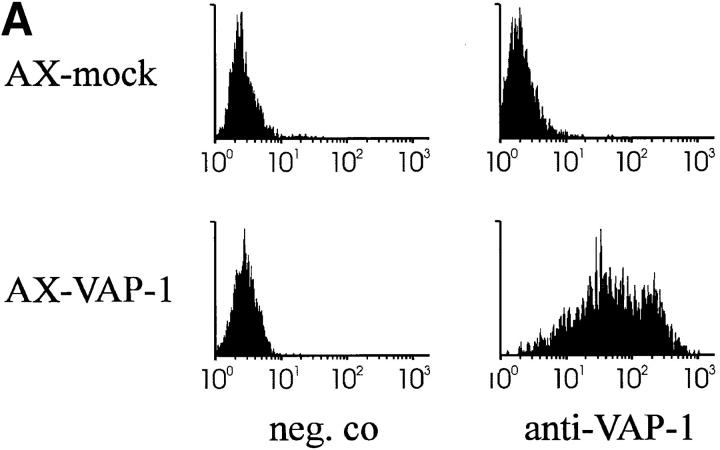
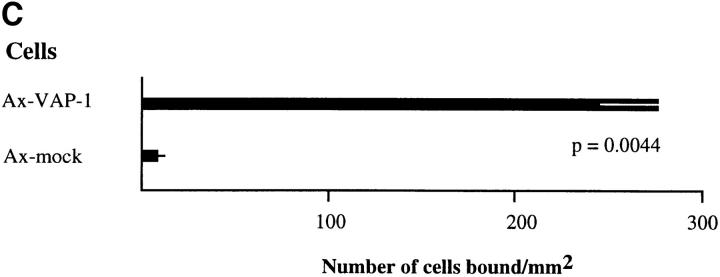
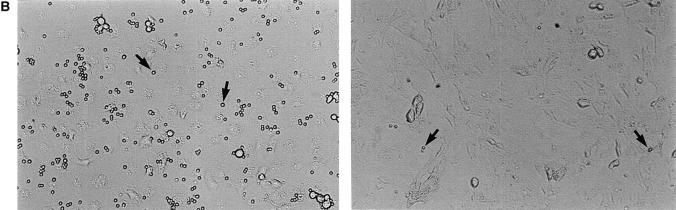
The VAP-1 cDNA in pcDNA3 was used to transfect Ax cells, a rat HEV–derived endothelial cell line (22) that probably provides a more natural functional environment for VAP-1 than other potential hosts such as CHO cells. Stable transfectants were obtained which expressed VAP-1 on their cell surface as determined by FACS® analysis (Fig. 2 A), and these were used in lymphocyte adhesion assays. When analyzed under rotatory conditions, PBL bound to VAP-1–transfected Ax cells 25.6 times better than to mock-transfected cells (Fig. 2, B and C). The enhanced binding to VAP-1 transfectants was statistically significantly inhibited, although not abolished completely, by anti–VAP-1 mAb treatment (inhibition 29.6 ± 10.7%, P = 0.05). These adhesion results are pooled from five independent experiments in which two to three parallel transfectant monolayers were analyzed each time using three independently transfected cell lines and PBLs from six different donors. Thus, these data show that the VAP-1 cDNA encodes a functional adhesion molecule which is located on the cell surface of transfected cells, and which when expressed in Ax cells, can directly mediate the binding of PBL.
Figure 2.
Ax cells transfected with the VAP-1 cDNA mediate lymphocyte adhesion. (A) Expression of VAP-1 on the cell surface of Ax cells stably transfected with VAP-1 cDNA or mock control (neg. co). x-axis, Intensity of staining on a log scale; y-axis, relative number of cells. (B) Increased VAP-1–dependent binding of lymphocytes to VAP-1 transfectants. Considerably more PBL (small round spheres on top of the monolayer, arrows) are bound to the VAP-1 transfectants (left) than to mock transfectants (right). Phase–contrast micrographs, original magnification ×100. (C) Quantitation of the binding. Results of five independent experiments are presented as mean ± SEM.
The VAP-1 Protein.
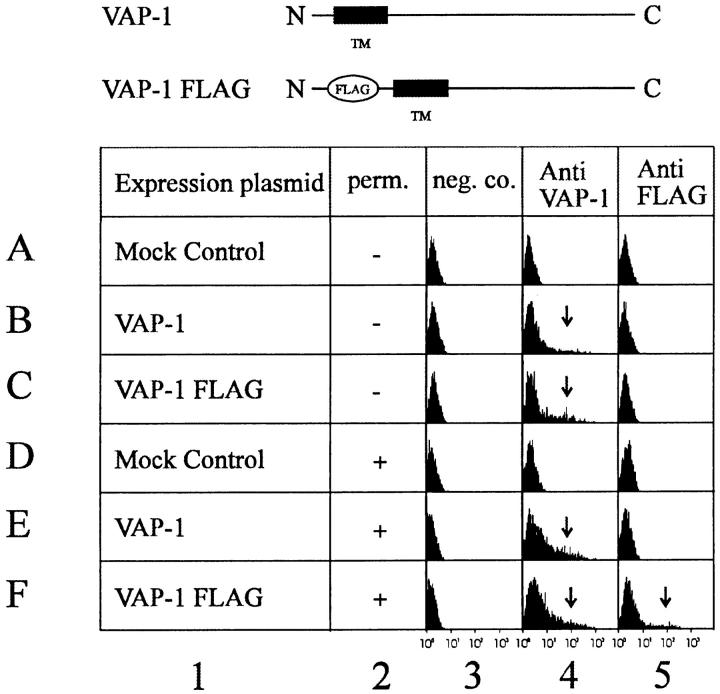
The open reading frame in the VAP-1 cDNA encoded a 763–amino acid protein of 84.6 kD (Fig. 1). Searching of the available protein sequence databases revealed that VAP-1 has significant identity to the copper-containing amine oxidase family (EC 1.4.3.6). This identity varied from 24% for Escherichia coli Cu–MAO to 41–81% for other mammalian members of this family. The highest identity, 81%, was found with bovine serum amine oxidase (BSAO), which exhibited significant conservation throughout the entire length of the protein except for a short region at the NH2-terminal end of the molecule. A recently published partial sequence of a rat adipocyte amine oxidase (24) had considerable sequence conservation (83.1% over 320 amino acids), including the NH2-terminal region, suggesting that this may be a rat homologue of VAP-1. A multiple alignment of VAP-1 with other mammalian members of the copper-containing amine oxidase family is shown in Fig. 3. VAP-1 has no significant identity to any currently known adhesion molecules, and contains none of the protein domains sometimes found within such proteins, although we note the occurrence of an RGD motif between residues 726 and 728. The functional significance with regard to integrin binding, if any, of this commonly occurring motif is unknown. The protein has six potential N-glycosylation sites and three putative O-glycosylation sites (determined using the O-glycosylation site prediction E-mail server at netoglyc@cbs.dtu.dk [25]) per monomer (Fig. 1). Examination of the protein sequence revealed no obvious areas with characteristics of membrane-spanning domains except for a region of 23 predominantly hydrophobic amino acids at the very NH2-terminal end (residues 5–27) of the molecule, indicated in Fig. 1. This region could be interpreted as either a cleavable secretion signal, because it contained a potential cleavage site at position 19 as determined by the method of von Heijne (26), or a transmembrane domain. The NH2-terminal protein sequence of the 90-kD VAP-1 protein showed that this hydrophobic region was not cleaved off in the material we had purified, and was thus unlikely to function as a normal cleavable signal sequence for secretion. In addition, the charge characteristics of the residues flanking the hydrophobic region suggested that it could be the membrane-spanning domain of a type II membrane protein (27) having a cytoplasmic NH2 terminus and a COOH-terminal extracellular domain. To determine if this hypothesis was correct, we made VAP-1 cDNA expression constructs in which a FLAG epitope (DYKDDDDK) recognized by mAb M2 was placed in frame after the initiating methionine codon on the side of the putative transmembrane domain predicted to be within the cytoplasm. This construct and a control construct in which the VAP-1 cDNA was placed in an inverse orientation in the vector were transfected into COS-7 cells, and the transient expression of the FLAG and VAP-1 epitopes, recognized by mAbs M2 and 1B2, respectively, was analyzed by FACS® analysis of permeabilized and nonpermeabilized cells (Fig. 4). A positive cell population with the anti-FLAG mAb M2 was seen with permeabilized cells only (Fig. 4, row F, column 5), whereas a VAP-1–positive cell population was seen in both permeabilized and nonpermeabilized cells (Fig. 4, rows C and F, column 4). Control transfected cells were negative with both the anti-FLAG and anti–VAP-1 mAbs (Fig. 4, rows A and D, columns 4 and 5). This shows that the NH2-terminal FLAG epitope is located on the cytoplasmic side of the cell membrane, the hydrophobic region spans the lipid bilayer, and there is a large COOH-terminal extracellular domain recognized by the adhesion blocking mAb 1B2. All the putative glycosylation sites are located in the extracellular portion of the molecule.
Figure 3.

Multiple alignment of the mammalian members of the copper amine oxidase family, including VAP-1. Labels (left) refer to the particular protein aligned in each row: BSAO; VAP-1; human placental diamine oxidase 1 (PDAO1); human placental diamine oxidase 2 (PDAO2); and rat DAO. Numbers, The first amino acid in each row of the aligned proteins. Sequences were extracted from the latest available database and aligned using GCG Pileup. Residues having identity with VAP-1 were highlighted using GCG Boxshade.
Figure 4.
FACS® analysis of VAP-1–transfected COS-7 cells to determine the membrane orientation and cellular location of VAP-1. Diagram (top) indicates the structure of the two VAP-1 expression plasmids used to transfect the COS-7 cells. VAP-1, The native VAP-1 expression construct; VAP-1 FLAG, the FLAG epitope–tagged VAP-1. The negative control for mock transfections was provided by a construct in which VAP-1 is in an inverse orientation in the expression vector. Column 1, The expression construct used in the transfection; column 2, no permeabilization (perm., −) or permeabilization (+) of the transfected cells; column 3, negative control mAb staining (neg. co.); column 4, anti–VAP-1 mAb 1B2 staining; column 5, anti-FLAG mAb M2 staining. Rows A–F, The expression construct or mock control used in transfection and the resulting staining pattern of the transfected cells. Arrows, The positively staining cell population.
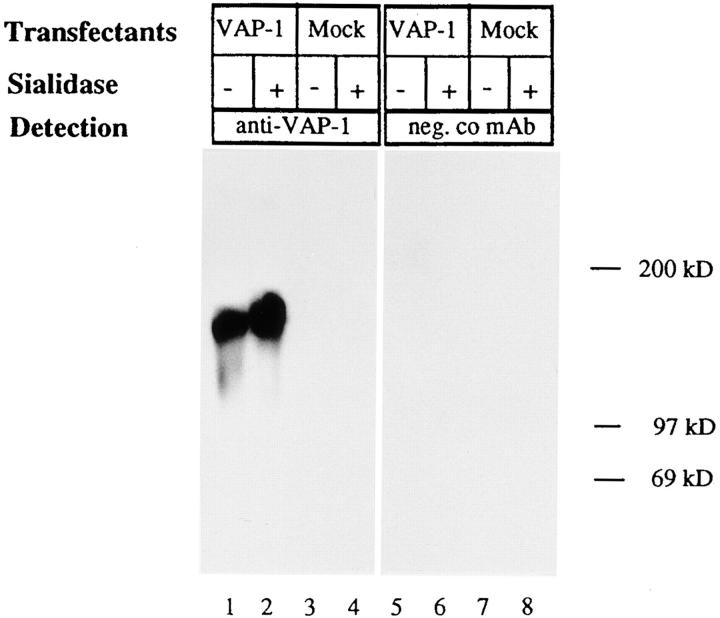
To determine the size and glycosylation status of recombinant VAP-1 transiently expressed in Ax cells, we immunoblotted SDS-PAGE–separated cell extracts of VAP-1 and mock-transfected cells with and without prior sialidase digestion (Fig. 5) using mAb 1B2. Consistent with the size of VAP-1 found in vivo, a band at 170–180 kD was detected which increased to 180–190 kD upon sialidase treatment. This indicates that recombinant VAP-1, like native VAP-1, is a sialoglycoprotein, and that the reduction in the net negative charge due to removal of the negatively charged sialic acids causes a decrease in the mobility of VAP-1 in SDS-PAGE. A similar effect upon sialidase treatment has been observed with tonsillar VAP-1 (15).
Figure 5.
Sialidase treatment of VAP-1 expressed in Ax cells. Cell lysates from VAP-1–transfected and mock-transfected Ax cells were treated with sialidase (+) or not (−) before SDS-PAGE, immunoblotting, and probing with the anti–VAP-1 mAb 1B2 (lanes 1–4) or negative control 3G6 mAb (neg. co, lanes 5–8).
VAP-1 Enzyme Activity.
The finding that VAP-1 has significant identity to the copper-containing amine oxidase family led us to examine if VAP-1 possessed amine oxidase activity. The copper-containing amine oxidases are distinguished by the presence of an unusual quinone cofactor, enzyme-bound copper, and activity only against primary polyamines or monoamines (28). Thus, they are distinct from the FAD-containing intracellular (mitochondrial) MAOs. A stable CHO cell line which expressed VAP-1 on the cell surface (determined by FACS® analysis, results not shown) was obtained by transfection of CHO cells with an expression vector containing the VAP-1 cDNA. Lysates of these cells were assayed for DAO activity using putrescine as a substrate, or MAO activity using benzylamine as a substrate. As positive controls, commercially available DAO and MAO were assayed, and negative controls were provided by mock plasmid–transfected CHO cell lysates. The results showed that the VAP-1–expressing cells had negligible activity towards putrescine, but significant activity was detected using the MAO substrate benzylamine (Table 1). Mock CHO cell lysates showed insignificant activity. In the presence of 100 μM semicarbazide and 10 μM hydroxylamine, specific inhibitors of copper-containing MAOs (28), VAP-1 in CHO cells had no activity against benzylamine (Table 1). In addition, we demonstrated that adhesion-competent VAP-1 expressed in Ax cells also possessed MAO activity against benzylamine which was inhibitable by semicarbazide and hydroxylamine (Table 1). The lower specific activities found in Ax cells probably reflect lower expression levels of VAP-1 in these cells. The semicarbazide-sensitive amine oxidase (SSAO) inhibitor hydroxylamine had no influence on the ability of VAP-1–expressing Ax cells to support lymphocyte binding in adhesion assays (results not shown), suggesting that the enzyme activity is not required for the adhesive properties of VAP-1 in these cells. Ax cells themselves appear to possess a native MAO activity against benzylamine which is not inhibitable by semicarbazide or hydroxylamine, as a low level of activity was detected in mock control cells (Table 1). To confirm that MAO activity is found in VAP-1 in vivo, we immunoaffinity-purified VAP-1 from tonsil and assayed the material in triplicate. Tonsillar VAP-1, like VAP-1 from transfected CHO cells, demonstrated activity against benzylamine with an A490 increase per hour of 0.09 at 37°C under the assay conditions used, which was 4.5 times greater than that of boiled sample. It was not possible to measure specific activities due to the very low yield of tonsillar VAP-1 protein obtained.
Table 1.
Assay of Amine Oxidase Activity in CHO and Ax Cells Expressing VAP-1
| Substrate | Cell type enzyme activity | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CHO VAP-1 | CHO mock | DAO | MAO | Ax VAP-1 | Ax mock | |||||||
| nmol product min−1 mg−1 protein | ||||||||||||
| Putrescine | 0.87 ± 0.17 | 1.45 ± 0.13 | 4.87 ± 0.10 | 0.29 ± 0.00 | ND | ND | ||||||
| Benzylamine | 26.94 ± 0.70 | 0.13 ± 0.13 | 0.57 ± 0.10 | 8.83 ± 0.00 | 2.15 ± 0.04 | 0.80 ± 0.80 | ||||||
| Benzylamine + SC* | 0.61 ± 0.17 | 1.32 ± 0.26 | ND | ND | 0.35 ± 0.09 | 1.00 ± 0.00 | ||||||
| Benzylamine + HA‡ | 0.35 ± 0.17 | 1.32 ± 0.26 | ND | ND | 0.31 ± 0.04 | 0.90 ± 0.10 | ||||||
VAP-1 cDNA–transfected CHO and Ax cells or mock-transfected cells were assayed for amine oxidase activity using putrescine as a polyamine substrate and benzylamine as a monoamine substrate, which was also assayed in the presence of MAO inhibitors. The experiment was performed at least twice on different samples with comparable results, and the results of one representative experiment are shown. Each assay was done in triplicate, and the mean specific activity ± SEM is shown.
Semicarbazide (SC) was at 100 μM.
Hydroxylamine (HA) was at 10 μM.
Benzylamine, although commonly used as a substrate for measuring amine oxidase activity, is not found in vivo. Thus, several biologically occurring endogenous amines were tested to see whether they could be used by VAP-1 (Table 2). Methylamine at 1 mM appeared to be readily used by VAP-1; however, no other amines tested demonstrated reactivity. The lower specific activities observed with benzylamine in these cell lysates compared with the lysates assayed in Table 1 reflects the variation in VAP-1 expression found in different batches of cells.
Table 2.
Substrate Specificity of VAP-1 Amine Oxidase Activity
| Substrate | Cell type enzyme activity | |||
|---|---|---|---|---|
| CHO VAP-1 | CHO mock | |||
| nmol product min−1 mg−1 protein | ||||
| Benzylamine | 4.57 ± 0.29 | 0.18 ± 0.62 | ||
| Methylamine | 4.28 ± 0.22 | 0.18 ± 0.44 | ||
| Tyramine | 0.04 ± 0.10 | 0.09 ± 0.35 | ||
| Tryptamine | 0.07 ± 0.04 | 0.35 ± 0.62 | ||
| β-Phenylethylamine | 0.10 ± 0.03 | 0.26 ± 0.00 | ||
| Histamine | 0.05 ± 0.03 | 0.20 ± 0.00 | ||
VAP-1 amine oxidase activity was measured in VAP-1 cDNA– and mock-transfected CHO cell lysates using different substrates. The experiment was performed twice on different samples with comparable results, and the results of one representative experiment are shown. Each assay was done in triplicate, and the mean specific activity ± SEM is given. Note that the activities cannot be directly compared with those in Table 1 because the cells are from different batches and therefore do not have the same level of VAP-1 expression.
The presence of a quinone cofactor in VAP-1 was shown by separating a portion of the immunopurified tonsillar VAP-1 material by SDS-PAGE under reducing conditions and transferring the material to nitrocellulose. The nitrocellulose filter was then stained with nitroblue tetrazolium/Na-glycinate under redox cycling conditions to specifically stain quinone moieties in the protein (21). The stain reacted with both monomeric 90-kD and dimeric 170–180-kD VAP-1, showing that tonsillar VAP-1 probably has a quinone cofactor in each subunit (Fig. 6).
Figure 6.
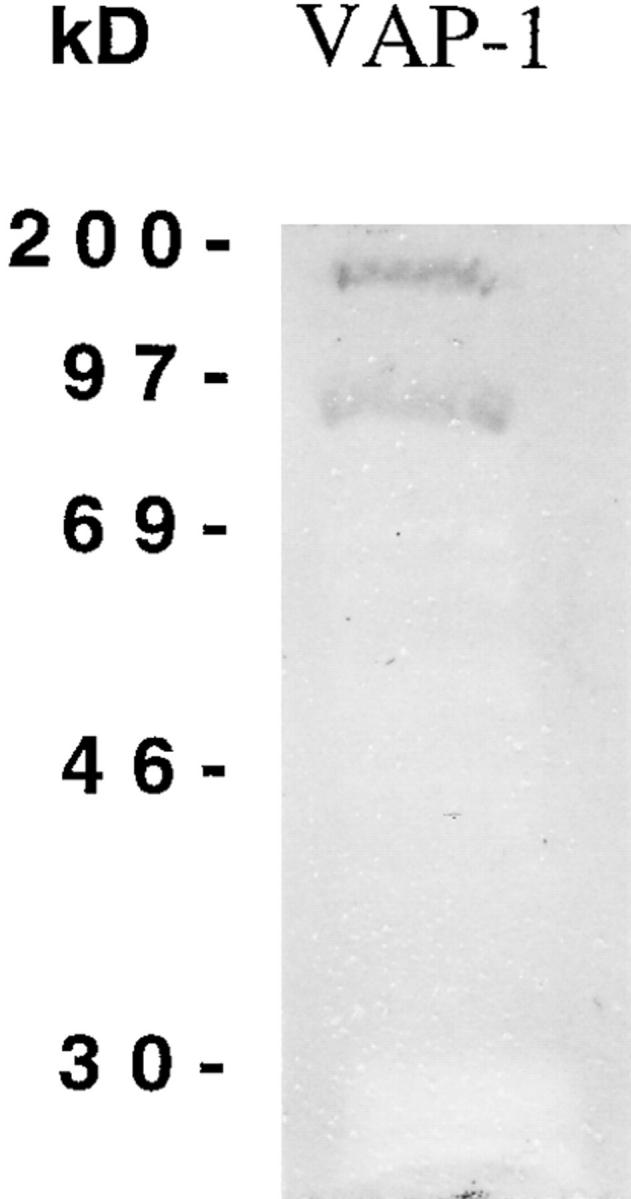
VAP-1 contains a quinone. Immunopurified tonsillar VAP-1 was separated by SDS-PAGE under reducing conditions and transferred to a nitrocellulose filter. The filter was stained with nitroblue tetrazolium, which results in a blue/purple staining of the filter at locations where quinones are present, as described in Materials and Methods. Two quinone-positive proteins are seen at 90 and 180 kD, corresponding to the size of tonsillar VAP-1 found in reducing conditions. The specificity of the reaction can be seen by the blockage of background staining of the nitrocellulose where other copurifying proteins are present but which do not give a quinone-positive reaction.
VAP-1 Expression.
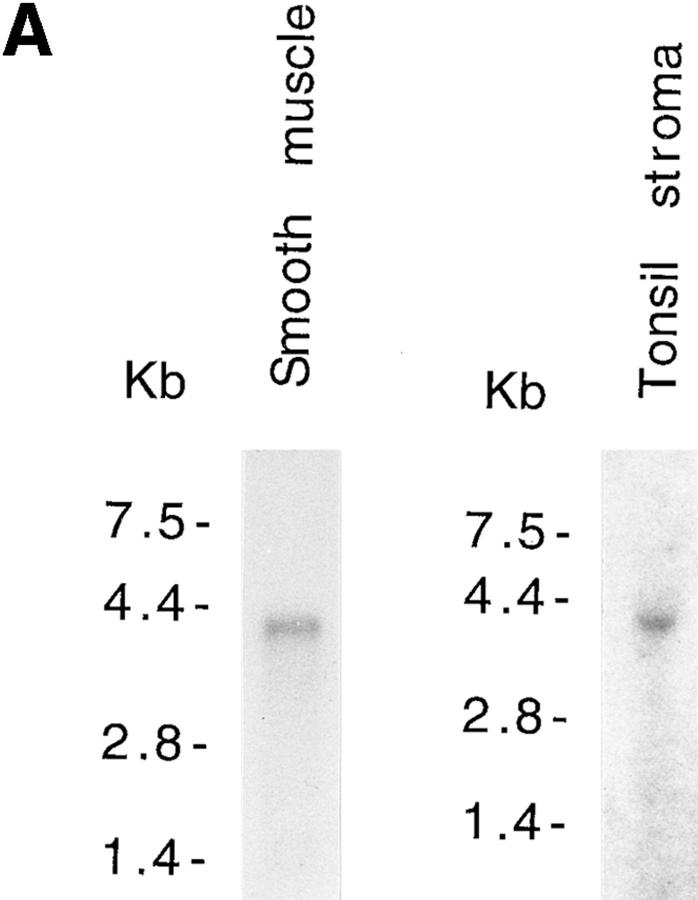
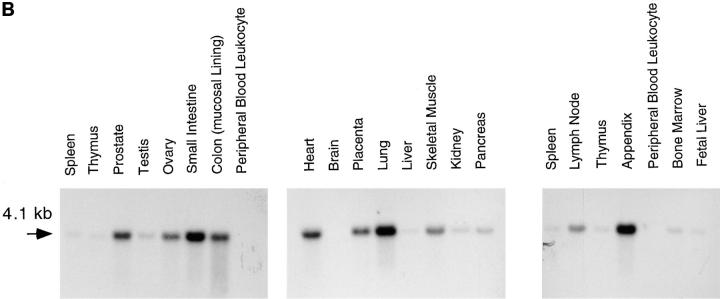
Northern blot analysis of mRNA isolated from human gut smooth muscle and lymphocyte-depleted tonsil stroma showed that the cloned VAP-1 cDNA hybridizes to a 4.1-kb mRNA in both tissues (Fig. 7 A). Further Northern blot analysis showed that a 4.1-kb VAP-1 mRNA is expressed in a wide range of human tissues. The message was not detectable in peripheral blood leukocytes or brain but was strongly expressed in lung, small intestine, and appendix compared with other tissues. Only low amounts of the VAP-1 mRNA were detected in spleen, thymus, testis, liver, pancreas, kidney, bone marrow, and fetal liver. An intermediate level of expression was seen in prostate, ovary, the mucosal lining of the colon, heart, placenta, skeletal muscle, and lymph node (Fig. 7 B). No other mRNA species of different size were detected even after prolonged autoradiography.
Figure 7.
VAP-1 has a widely expressed 4.1-kb mRNA. (A) Northern blot analysis of VAP-1 mRNA in poly A+ RNA extracted from human gut smooth muscle and tonsil stroma from which lymphocytes have been partially removed by washing and squeezing. A 4.1-kb hybridizing mRNA can be seen in both lanes. (B) Northern blot analysis of VAP-1 mRNA in different human tissues. Northern blots were obtained from Clontech, and equal amounts of mRNA are loaded in each lane. All the filters were probed with a 32P-labeled VAP-1 cDNA probe containing the entire coding sequence and washed at high stringency (posthybridization washing conditions were 0.1× SSC, 0.1% SDS at 65°C twice for 45 min).
Discussion
Prominent staining with the mAb 1B2 recognizing VAP-1 is found on the endothelial cells of vessels in several locations, particularly in PLN-type lymphoid tissues. However, as the total levels of VAP-1 found at these locations are relatively low, it proved difficult to isolate and purify sufficient quantities of endothelial VAP-1 from which to obtain protein sequence information. Of the other tissues in which VAP-1 is found, it is most abundant in the smooth muscle of the vasculature and gut-associated smooth muscle (12). VAP-1 from these sources has a marginally different molecular mass, probably due to glycosylation differences, but otherwise resembles the form analyzed previously in tonsil and other PLN-type tissues (our unpublished observations).
Using protein sequence information obtained from VAP-1 purified from gut smooth muscle, we isolated a cDNA encoding this adhesion molecule. Our evidence for this is based on the following. First, protein sequence obtained from immunopurified VAP-1 was found in the predicted protein sequence of the VAP-1 cDNA clone subsequently isolated. Second, transfected cells expressing the VAP-1 cDNA could be stained on their surface with the mAb 1B2 used originally to define VAP-1 (11), and VAP-1 immunoprecipitated from these cells had a similar molecular mass, 170–180 kD, to that found in vivo (15). Third, VAP-1 cDNA–transfected Ax cells could support the adherence of PBL, and this binding could be partially inhibited by anti–VAP-1 mAb, showing that the cDNA encodes a functional adhesion molecule. There is no conclusive evidence to suggest that there are forms of VAP-1 encoded by variant mRNAs. Thus, it seems likely that we have isolated a cDNA encoding the predominant form of VAP-1 protein studied previously by immunoblotting and immunoprecipitation.
After the completion of this work, a human MAO cDNA with an identical sequence to the VAP-1 cDNA was described recently (29). However, no functional data on the protein, its enzyme activity, or expression were presented by the authors, who concluded erroneously that the cDNA sequence encodes a secreted rather than a transmembrane protein. Thus, the VAP-1 cDNA isolated here is the first shown to encode a human membrane–bound protein of the copper-containing amine oxidase family.
VAP-1 is a large, dimeric, type II transmembrane protein having a membrane-spanning domain located at the NH2-terminal end of the molecule. The intracellular domain is particularly small, only four amino acids in length, leaving a large glycosylated extracellular domain of some 163 kD per dimer. All the potential glycosylation sites, 12 N-linked and 6 putative O-linked per dimer, are located in the extracellular domain. Although it is not currently known if all of these are used, previous data suggest that VAP-1 contains both N- and O-linked sugars and numerous sialic acid residues which are thought to play an important part in the adhesive function of VAP-1 protein (15). The correct posttranslational processing of VAP-1 may be critical for its adhesive function, as VAP-1 expressed in nonendothelial-derived cell lines such as CHO and COS cells was unable to support lymphocyte binding (results not shown). It may be that only endothelial cells, such as the Ax host cells used, contain the necessary precursors and express the essential glycosyltransferases for the correct oligosaccharide modifications required for VAP-1 adhesive function. The critical role of proper oligosaccharide modification for adhesion has been documented with other adhesion molecules such as E-selectin ligand 1 and P-selectin glycoprotein ligand 1 (30, 31).
The adhesion assays performed on VAP-1–expressing Ax cells indicated that the cDNA encodes a functional VAP-1 that can support interactions with its ligand on PBL and lead to stable binding of the PBL to the Ax cells. However, complete inhibition of this increased adhesion with anti– VAP-1 mAbs 1B2 and TK8-14 was not observed, suggesting that the VAP-1 molecule in rat-derived Ax cells is not functioning exactly as it does in its native environment. It may be that the carbohydrate modifications of the protein in Ax cells, the local membrane environment, or VAP-1 conformation are sufficiently different from that in human HEV such that the mAb can no longer block all VAP-1 interactions with its ligand. It is also possible that a subpopulation of VAP-1 molecules may exist on transfectants in a form lacking one or other of the epitopes recognized by mAbs 1B2 and TK8-14. Finally, we are left with the possibility that long-term overexpression of VAP-1 in stable transfectants alters the expression of other adhesion molecule(s) on the Ax cell surface. Of course, the function of this other putative adhesion molecule would not be inhibitable by anti–VAP-1 mAb. In HEV binding assays, VAP-1 has been shown to function independently of lymphocyte L-selectin, and it mediates the binding of CD8+ PBL much better than CD4+ PBL (16). Ax VAP-1 cDNA transfectants reproduce these observations, since analysis of immunomagnetically purified L-selectin–negative cells and CD8+ and CD4+ cells showed that L-selectin was not necessary for efficient binding to Ax transfectants, and that the CD8+ subset of PBL adhered severalfold better to VAP-1 transfectants than CD4+ cells (data not shown).
The intriguing finding that VAP-1 possesses an MAO activity suggests that the protein may have multiple physiological roles. In previous studies, we have clearly shown that VAP-1 is an inducible adhesion molecule mediating lymphocyte binding to PLN HEV as well as vessels in inflamed areas found in several inflammatory diseases (11, 12, 14–16). The expression of VAP-1 at these sites can help mediate and regulate lymphocyte extravasation, and this activity may be independent of the enzyme activity. In other tissues where VAP-1 is expressed, such as smooth muscle, its MAO activity may be its primary function. Whether the two activities are linked is unclear at present, but one can envisage that regulating amine levels could be of importance in inflamed tissue, where there may be accumulations of potentially toxic cellular amine metabolites liberated from cells damaged as a result of the inflammatory response. Another alternative is that the MAO activity may be involved in regulating the levels of a bioactive amine with a role in lymphocyte homing or inflammation.
We have shown that VAP-1 belongs to the widely distributed copper-containing amine oxidase family (28, 32) and that the extracellular domain of VAP-1 contains this activity. Thus VAP-1 is an ectoenzyme. A particular characteristic of these enzymes is the presence of an unusual carbonyl-containing topaquinone cofactor (33). In several mammalian species, including humans, a copper-containing MAO found both in the cell membrane and in a soluble form in serum has been identified (34–36). This activity is sensitive to inhibition by carbonyl-reactive agents such as semicarbazide and hydroxylamine, and the enzyme responsible is commonly referred to as an SSAO (28). We have shown that VAP-1 contains a covalently bound quinone, and these results, as well as the sensitivity of VAP-1 enzyme activity to the carbonyl-reactive agents semicarbazide and hydroxylamine, show that VAP-1 is a membrane-bound SSAO. It seems plausible that it is the same as the human membrane-bound SSAO described previously by biochemical studies and which has been reported to be expressed extensively in the vasculature, particularly in smooth muscle, although it has also been found in other cell types, including endothelial cells (28, 37). The physiological roles of SSAOs have been difficult to define, since little is known of their in vivo substrates, and since their substrate specificities vary considerably between species. We have shown that VAP-1 can use endogenously occurring methylamine as substrate but not several other endogenous amines such as tyramine, tryptamine, β-phenylethylamine, histamine, or putrescine. Identification of other substrates used by VAP-1 may provide a useful insight into the role of the amine oxidase activity.
VAP-1 would appear to belong to a growing family of adhesion molecules that have an intrinsic enzymic or other activity in addition to their adhesive ability. Such proteins include lymphocyte CD38 (38), CD73 (18), the E-selectin ligand ESL-1 (31), and heparanase (39).
The nature of the VAP-1 ligand is unknown at present. However, the previously reported finding that sialic acids are important for the adhesive ability of VAP-1 in sialidase-treated tonsil sections suggests that a ligand–carbohydrate interaction is important, at least for part of the adhesive mechanism. As this does not involve any of the known selectins, it is possible that another lectin-like molecule may well be a VAP-1 ligand. The isolation of a VAP-1 cDNA will facilitate studies to isolate the ligand and define further the role that this unusual and multifunctional new adhesion molecule plays in lymphocyte trafficking.
Acknowledgments
We wish to thank M. Pohjansalo, M. Mäkitalo, P. Käpylä, R. Lehvonen, K. Kaukonen, and M. Kolehmainen for skilled technical assistance, and Dr. Laura Airas for constructive criticism of the manuscript.
Abbreviations used in this paper
- BSAO
bovine serum amine oxidase
- CHO
Chinese hamster ovary
- DAO
diamine oxidase
- GCG
Genetics Computer Group
- HEV
high endothelial venule(s)
- MAO
monoamine oxidase
- PLN
peripheral lymph node(s)
- PNAd
PLN addressin
- SSAO
semicarbazide-sensitive amine oxidase
- VAP-1
vascular adhesion protein 1
Footnotes
D. Smith was a postdoctoral fellow of the European Molecular Biology Organisation and subsequently in receipt of a European Community Human Capital and Mobility Fellowship during the course of this work.
T. Leu's current address is Department of Medical Microbiology, University of Turku, FIN-20520 Turku, Finland.
References
- 1.Butcher EC. Leukocyte-endothelial cell recognition: three (or more) steps to specificity and diversity. Cell. 1991;67:1033–1036. doi: 10.1016/0092-8674(91)90279-8. [DOI] [PubMed] [Google Scholar]
- 2.Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 3.Butcher EG, Picker LJ. Lymphocyte homing and homeostasis. Science. 1996;272:60–66. doi: 10.1126/science.272.5258.60. [DOI] [PubMed] [Google Scholar]
- 4.Bargatze RF, Jutila MA, Butcher EC. Distinct roles of L-selectin and integrins alpha 4 beta 7 and LFA-1 in lymphocyte homing to Peyer's patch-HEV in situ: the multistep model confirmed and refined. Immunity. 1995;3:99–108. doi: 10.1016/1074-7613(95)90162-0. [DOI] [PubMed] [Google Scholar]
- 5.Lasky LA. Selectin-carbohydrate interactions and the initiation of the inflammatory response. Annu Rev Biochem. 1995;64:113–139. doi: 10.1146/annurev.bi.64.070195.000553. [DOI] [PubMed] [Google Scholar]
- 6.Ley K, Tedder TF. Leukocyte interactions with vascular endothelium. New insights into selectin-mediated attachment and rolling. J Immunol. 1995;155:525–528. [PubMed] [Google Scholar]
- 7.Berlin C, Bargatze RF, Campbell JJ, von Andrian UH, Szabo MC, Hasslen SR, Nelson RD, Berg EL, Erlandsen SL, Butcher EC. α4 integrins mediate lymphocyte attachment and rolling under physiologic flow. Cell. 1995;80:413–422. doi: 10.1016/0092-8674(95)90491-3. [DOI] [PubMed] [Google Scholar]
- 8.Schall TJ, Bacon KB. Chemokines, leukocyte trafficking, and inflammation. Curr Opin Immunol. 1994;6:865–873. doi: 10.1016/0952-7915(94)90006-x. [DOI] [PubMed] [Google Scholar]
- 9.Premack BA, Schall TJ. Chemokine receptors: gateways to inflammation and infection. Nat Med. 1996;2:1174–1178. doi: 10.1038/nm1196-1174. [DOI] [PubMed] [Google Scholar]
- 10.Förster R, Mattis AE, Kremmer E, Wolf E, Brem G, Lipp M. A putative chemokine receptor, BLR1, directs B cell migration to defined lymphoid organs and specific anatomic compartments of the spleen. Cell. 1996;87:1037–1047. doi: 10.1016/s0092-8674(00)81798-5. [DOI] [PubMed] [Google Scholar]
- 11.Salmi M, Jalkanen S. A 90-kilodalton endothelial cell molecule mediating lymphocyte binding in humans. Science. 1992;257:1407–1409. doi: 10.1126/science.1529341. [DOI] [PubMed] [Google Scholar]
- 12.Salmi M, Kalimo K, Jalkanen S. Induction and function of vascular adhesion protein-1 at sites of inflammation. J Exp Med. 1993;178:2255–2260. doi: 10.1084/jem.178.6.2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Salmi M, Rajala P, Jalkanen S. Homing of mucosal leukocytes to joints. Distinct endothelial ligands in synovium mediate leukocyte-subtype specific adhesion. J Clin Invest. 1997;99:2165–2172. doi: 10.1172/JCI119389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arvilommi A-M, Salmi M, Kalimo K, Jalkanen S. Lymphocyte binding to vascular endothelium in inflamed skin revisited: a central role for vascular adhesion protein-1 (VAP-1) Eur J Immunol. 1996;26:825–833. doi: 10.1002/eji.1830260415. [DOI] [PubMed] [Google Scholar]
- 15.Salmi M, Jalkanen S. Human vascular adhesion protein-1 (VAP-1) is a unique sialoglycoprotein that mediates carbohydrate-dependent binding of lymphocytes to endothelial cells. J Exp Med. 1996;183:569–579. doi: 10.1084/jem.183.2.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salmi M, Tohka S, Berg EL, Butcher EC, Jalkanen S. Vascular adhesion protein-1 (VAP-1) mediates lymphocyte subtype–specific, selectin-independent recognition of vascular endothelium in human lymph nodes. J Exp Med. 1997;186:589–600. doi: 10.1084/jem.186.4.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wuorela M, Jalkanen S, Pelliniemi LJ, Toivanen P. Nurse cells of the bursa of Fabricius: do they exist? . Eur J Immunol. 1990;20:913–917. doi: 10.1002/eji.1830200429. [DOI] [PubMed] [Google Scholar]
- 17a.Kurkijärvi, R., D.H. Adams, R. Leino, T. Möttönen, S. Jalkanen, and M. Salmi. 1998. Circulating form of human vascular adhesion protein-1 (VAP-1). Increased serum levels in inflammatory liver diseases. J. Immunol. In press. [PubMed]
- 18.Airas L, Hellman J, Salmi M, Bono P, Puurunen T, Smith DJ, Jalkanen S. CD73 is involved in lymphocyte binding to the endothelium: characterization of lymphocyte–vascular adhesion protein 2 identifies it as CD73. J Exp Med. 1995;182:1603–1608. doi: 10.1084/jem.182.5.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sambrook, J.F., T. Maniatis, and E.F. Fritsch. 1989. Molecular Cloning: Laboratory Manual. 2nd ed. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY.
- 20.Holt A, Sharman DF, Baker GB, Palcic MM. A continuous spectrophotometric assay for monoamine oxidase and related enzymes in tissue homogenates. Anal Biochem. 1997;244:384–392. doi: 10.1006/abio.1996.9911. [DOI] [PubMed] [Google Scholar]
- 21.Paz MA, Flückiger R, Boak A, Kagan HM, Gallop PM. Specific detection of quinoproteins by redox-cycling staining. J Biol Chem. 1991;266:689–692. [PubMed] [Google Scholar]
- 22.Ise Y, Yamaguchi K, Sato K, Yamamura Y, Kitamura F, Tamatani T, Miyasaka M. Molecular mechanisms underlying lymphocyte recirculation. I. Functional, phenotypical and morphological characterization of high endothelial cells cultured in vitro. . Eur J Immunol. 1988;18:1235–1244. doi: 10.1002/eji.1830180814. [DOI] [PubMed] [Google Scholar]
- 23.Salmi M, Smith DJ, Bono P, Leu T, Hellman J, Matikainen M-T, Jalkanen S. A mouse molecular mimic of human vascular adhesion protein-1. Mol Immunol. 1998;34:1227–1236. doi: 10.1016/s0161-5890(97)00060-6. [DOI] [PubMed] [Google Scholar]
- 24.Morris NJ, Ducret A, Aebersold R, Ross SA, Keller SR, Lienhard GE. Membrane amine oxidase cloning and identification as a major protein in the adipocyte plasma membrane. J Biol Chem. 1997;272:9388–9392. doi: 10.1074/jbc.272.14.9388. [DOI] [PubMed] [Google Scholar]
- 25.Hansen JE, Lund O, Engelbrecht J, Bohr H, Nielsen JO, Hansen J-ES, Brunak S. Prediction of O-glycosylation of mammalian proteins: specificity patterns of UDP-GaINAc: polypeptide N-acetylgalactosaminyltransferase. Biochem J. 1995;308:801–813. doi: 10.1042/bj3080801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.von Heijne G. A new method for predicting signal sequence cleavage sites. Nucleic Acids Res. 1986;14:4683–4690. doi: 10.1093/nar/14.11.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hartmann E, Rapoport TA, Lodish HF. Predicting the orientation of eukaryotic membrane-spanning proteins. Proc Natl Acad Sci USA. 1989;86:5786–5790. doi: 10.1073/pnas.86.15.5786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lyles GA. Mammalian plasma and tissue-bound semicarbazide-sensitive amine oxidases: biochemical, pharmacological and toxicological aspects. Int J Biochem Cell Biol. 1996;28:259–274. doi: 10.1016/1357-2725(95)00130-1. [DOI] [PubMed] [Google Scholar]
- 29.Zhang X, McIntire WS. Cloning and sequencing of a copper-containing, topa quinone-containing monoamine oxidase from human placenta. Gene (Amst) 1996;179:279–286. doi: 10.1016/s0378-1119(96)00387-3. [DOI] [PubMed] [Google Scholar]
- 30.Sako D, Chang X-J, Barone KM, Vachino G, White HM, Shaw G, Veldman GM, Bean KM, Ahern TJ, Furie B, et al. Expression cloning of a functional glycoprotein ligand for P-selectin. Cell. 1993;75:1179–1186. doi: 10.1016/0092-8674(93)90327-m. [DOI] [PubMed] [Google Scholar]
- 31.Steegmaier M, Levinovitz A, Isenmann S, Borges E, Lenter M, Kocher HP, Kleuser B, Vestweber D. The E-selectin-ligand ESL-1 is a variant of a receptor for fibroblast growth factor. Nature. 1995;373:615–620. doi: 10.1038/373615a0. [DOI] [PubMed] [Google Scholar]
- 32.Klinman JP, Mu D. Quinoenzymes in biology. Annu Rev Biochem. 1994;63:299–344. doi: 10.1146/annurev.bi.63.070194.001503. [DOI] [PubMed] [Google Scholar]
- 33.Janes SM, Palcic MM, Scaman CH, Smith AJ, Brown DE, Dooley DM, Mure M, Klinman JP. Identification of topaquinone and its consensus sequence in copper amine oxidases. Biochemistry. 1992;31:12147–12154. doi: 10.1021/bi00163a025. [DOI] [PubMed] [Google Scholar]
- 34.Suzuki O, Matsumoto T. Some properties of benzylamine oxidase in human aorta. Biog Amines. 1984;1:249–257. [Google Scholar]
- 35.Yu PH, Zuo D-M, Davis BA. Characterization of human serum and umbilical artery semicarbazide-sensitive amine oxidase (SSAO). Species heterogeneity and stereoisomeric specificity. Biochem Pharmacol. 1994;47:1055–1059. doi: 10.1016/0006-2952(94)90417-0. [DOI] [PubMed] [Google Scholar]
- 36.Lyles GA, Holt A, Marshall CMS. Further studies on the metabolism of methylamine by semicarbazide-sensitive amine oxidase activities in human plasma, umbilical artery and rat aorta. J Pharm Pharmacol. 1990;42:332–338. doi: 10.1111/j.2042-7158.1990.tb05421.x. [DOI] [PubMed] [Google Scholar]
- 37.Nakos G, Gossrau R. Light microscopic visualization of semicarbazide-sensitive amine oxidase (benzylamine oxidase) using a cerium method. Folia Histochem Cytobiol. 1994;32:3–10. [PubMed] [Google Scholar]
- 38.Dianzani U, Funaro A, DiFranco D, Garbarino G, Bragardo M, Redoglia V, Buonfiglio D, De Monte LB, Pileri A, Malavasi F. Interaction between endothelium and CD4+CD45RA+ lymphocytes. Role of the human CD38 molecule. J Immunol. 1994;153:952–959. [PubMed] [Google Scholar]
- 39.Gilat D, Hershkoviz R, Goldkorn I, Cahalon L, Korner G, Vlodavsky I, Lider O. Molecular behavior adapts to context: heparanase functions as an extracellular matrix-degrading enzyme or as a T cell adhesion molecule, depending on the local pH. J Exp Med. 1995;181:1929–1934. doi: 10.1084/jem.181.5.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]