Abstract
Movement of T and B lymphocytes through secondary lymphoid tissues is likely to involve multiple cues that help the cells navigate to appropriate compartments. Epstein-Barr virus– induced molecule 1 (EBI-1) ligand chemokine (ELC/MIP3β) is expressed constitutively within lymphoid tissues and may act as such a guidance cue. Here, we have isolated mouse ELC and characterized its expression pattern and chemotactic properties. ELC is expressed constitutively in dendritic cells within the T cell zone of secondary lymphoid tissues. Recombinant ELC was strongly chemotactic for naive (L-selectinhi) CD4 T cells and for CD8 T cells and weakly attractive for resting B cells and memory (L-selectinlo) CD4 T cells. After activation through the B cell receptor, the chemotactic response of B cells was enhanced. Like its human counterpart, murine ELC stimulated cells transfected with EBI-1/CC chemokine receptor 7 (CCR7). Our findings suggest a central role for ELC in promoting encounters between recirculating T cells and dendritic cells and in the migration of activated B cells into the T zone of secondary lymphoid tissues.
Keywords: chemokine, dendritic cells, lymphoid tissue, T zone, lymphocyte
Akey function of secondary lymphoid tissues is to bring together antigen, antigen-presenting cells, and rare antigen-specific lymphocytes so that an immune response can be mounted. Antigen-bearing dendritic cells (DCs)1 drain into lymphoid tissues from peripheral sites of infection, migrate into the T cell–rich zone, and present antigen on surface MHC molecules (1–3). Naive T cells enter lymphoid tissues from the blood and spend several hours migrating through the T zone, making contact with multiple DCs before returning to circulation. A successful recognition event between a T cell and an antigen-presenting DC leads to T cell retention and activation (3, 4). Resting B cells enter lymphoid tissues through the same pathways as T cells but rapidly home into B cell–rich microenvironments, lymphoid follicles, where they reside for ∼12 h before returning to circulation (5, 6). The tropism of B cells is altered markedly upon binding antigen, with the cells relocating to the outer T zone (7, 8). Localization of B cells in the outer T zone may play an important role in promoting encounter between antigen-specific B and T cells. The factors regulating this highly orchestrated movement of cells within the T zone and between T zone and follicles have been poorly defined, but several recent studies indicate an important role for chemokines and chemokine receptors (9).
Chemokines constitute a family of chemotactic cytokines that are subdivided based on the number and spacing of NH2-terminal cysteines into C, CC, CXC, and CXXXC chemokines (10–12). The majority of chemokines that have been shown to attract lymphocytes (e.g., RANTES [for regulated upon activation, normal T cell expressed and secreted], macrophage-inflammatory protein [MIP]1α, monocyte chemoattractant protein [MCP]-1) preferentially attract activated or memory cells and are unlikely to play a central role in guiding naive T or B lymphocytes through secondary lymphoid tissues (13–15). However, over the last year, several new chemokines have been identified that are constitutively expressed within secondary lymphoid tissues and that strongly attract naive, resting lymphocytes, including secondary lymphoid tissue chemokine (SLC)/6C-kine/exodus-2/thymus-derived chemotactic agent 4 (16–20), stromal cell–derived factor (SDF)1α/pre–B cell growth-stimulating factor (21–23), dendritic cell–derived CC chemokine (DCCK)1/pulmonary and activation–regulated chemokine (PARC) (24, 25), and B lymphocyte chemoattractant (BLC)/ B cell–attracting chemokine (BCA)-1 (26, 27). SLC has been found to strongly attract naive T cells and to more weakly attract B cells. SLC is expressed by high endothelial venules (HEV) in LNs and Peyer's patches and at lower levels by other cells in the T zone of spleen, LNs, and Peyer's patches (17). These properties suggest SLC plays a role in promoting lymphocyte passage across HEV and entry into the T cell region of lymphoid tissues. SDF1α plays a critical role in B cell and myeloid cell development (28). SDF1α is an efficacious attractant of mature lymphocytes (17, 23) and of pre-B cells (29), and in a recent study, has been found expressed in reticular cells located outside germinal centers in human tonsil, suggesting a role in lymphocyte trafficking to this site (30). DCCK1 or PARC is expressed by DCs within germinal centers of human tonsil and LN that may either be follicular DCs (25) or germinal center DCs (24). Expression in T zone DCs was reported in tonsil (24) but not LNs, and no expression was detected in spleen (25). Since DCCK1 attracts T cells, it may play a role in promoting DC–T cell interactions (24, 25). BLC/ BCA-1 is a recently defined chemokine expressed by stromal cells within the follicles of secondary lymphoid tissues that strongly attracts resting B cells but few other cell types (26, 27). BLC is a ligand for Burkitt's lymphoma receptor (BLR)1, a receptor required for B cell homing to follicles in spleen and Peyer's patches (31), and this chemokine is likely to play a key role in promoting the compartmentalization of B cells into follicles (26).
ELC (also called MIP3β) is another CC chemokine that is highly expressed in lymphoid tissues. Expression analysis of human ELC has identified LNs and appendix as especially rich sources (32, 33). Expression is also high in human thymus, whereas expression in spleen is either low (32) or negative (33). Among various cell populations tested, monocytes activated by LPS and IFN-γ in the presence of IL-10–blocking antibodies were the only cells that expressed significant amounts of ELC (33).
The only receptor characterized to date for ELC, EBV-induced molecule 1 (EBI-1) or CC chemokine receptor (CCR)7 (32), was first identified as a molecule upregulated in EBV-transformed Burkitt's lymphoma cells (34). The gene was later isolated from CD4 T cells that had been infected with herpesvirus (35) and by PCR as a molecule homologous to BLR1 (36). Northern blot analysis demonstrated that CCR7 is expressed in several B and T cell lines but not on monocytic or myeloid cell lines or cells of nonlymphoid origin. Freshly isolated PBLs show measurable CCR7 mRNA expression, and this can be upregulated by treatment with phorbol ester, PHA, or anti-CD3 (36).
The expression of ELC within secondary lymphoid tissues and the expression of its receptor CCR7 in lymphocytes suggests that ELC may function as a lymphocyte chemoattractant, perhaps promoting cell compartmentalization within these tissues. However, the only cell type that has been described to date to respond chemotactically to ELC is the T cell lymphoma line HUT78 (32). We report here the identification of mouse ELC, and demonstrate that this CCR7 ligand is a potent chemoattractant for resting and acutely activated lymphocytes. Furthermore, we find that mouse ELC is expressed in DCs in the T cell zone of LNs, spleen, and Peyer's patches.
Materials and Methods
Clone Identification and Sequence Analysis.
BLAST searches of the National Center for Biological Information expressed sequence tag (EST) database using human ELC as a query retrieved contiguous mouse ESTs for ELC. IMAGE Consortium (LLNL) cDNA clones 1088818 and 77575 were obtained from Genome Systems, Inc. (St. Louis, MO) as EcoR1-Not1 inserts in the pT7T3-Pac vector, and sequenced.
RNA Expression Studies.
For Northern blot analysis, 10–15 μg of total RNA from mouse tissues or purified cells was subjected to gel electrophoresis, transferred to Hybond N+ membranes (Amersham Corp., Arlington Heights, IL), and probed using randomly primed 32P-labeled mouse ELC EST 1088818, which spans bases 1–755 of the ELC cDNA. To control for loading and RNA integrity, membranes were stripped and reprobed with a mouse elongation factor (EF)-1α probe. For in situ hybridizations, frozen sections (5 μm) from C57BL/6 mice were treated as described (26). In brief, sections were fixed in 4% paraformaldehyde, washed in PBS, prehybridized for 1–3 h, and hybridized overnight at 60°C with sense or antisense digoxigenin-labeled riboprobes in hybridization solution. After washing at high stringency, sections were incubated with sheep antidigoxigenin antibody (Boehringer Mannheim Biochemicals, Indianapolis, IN) followed by alkaline phosphatase–coupled donkey anti– sheep antibody (Jackson Immunoresearch Labs, West Grove, PA) and developed with nitroblue tetrazolium/5-bromo-4-chloro-3-indoyl phosphate (NBT/BCIP). Immunohistochemistry with anti-CD3, anti-B220, and anti-CD11c (PharMingen, San Diego, CA), anti-DEC205 (kindly provided by R. Steinman, Rockefeller University, NY), and MOMA-1 (kindly provided by G. Kraal, Free University, Amsterdam, The Netherlands) antibodies was as described (37). Reverse transcription (RT)-PCR analysis of RNA from purified cell populations was with an AdvantageTM RT-for-PCR kit (Clontech, Palo Alto, CA) using a mixture of primers specific for ELC (5′ primer, ggtgctaatgatgcggaagac; and 3′ primer, agacacagggctccttctggt) and hypoxanthine-guanine phosphoribosyl transferase (5′ primer, cctgctggattacatcaaagcactg; and 3′ primer, tccaacacttcgtggggtcct).
Production of Recombinant ELC.
Sequence encoding amino acids 26–108, which are predicted to encompass mature mouse ELC based on comparison with the signal peptide cleavage site of human ELC (32), was isolated by PCR and subcloned into Nde1-Xho1 sites of the pET23b vector (Novagen, Inc., Madison, WI) in-frame with the COOH-terminal HIS6-tag. TAP302 cells (kindly provided by Tracy Handel, University of California, Berkeley, CA) were transformed with the vector, the HIS-tagged chemokine was purified using NiNTA agarose (QIAGEN, Inc., Chatsworth, CA) according to the manufacturer's instructions, and elution was with 250 mM imidazole (Fisher Scientific Co., Pittsburgh, PA). Further purification was achieved using a C-18 reverse-phase HPLC column (Vydac, Hesperia, CA) and elution with an acetonitrile gradient. SDS-PAGE of this preparation showed a single band of the expected molecular mass for ELC (∼9 kD) that represented >90% of the total protein. Protein concentration was measured using a protein assay (Bio-Rad Laboratories, Hercules, CA).
Cell Purification.
B cells were purified by staining non-B cells with anti–CD43-biotin (PharMingen) and depletion using streptavidin-MACS® beads and a MACS® (Miltenyi Biotec, Inc., Auburn, CA). T cells were purified by staining B cells, macrophages, and granulocytes with biotinylated antibodies to B220, Mac-1, and Gran-1 (PharMingen) and depletion by MACS®, except for the RT-PCR analysis where T cells were isolated by positive selection for CD3 (Caltag Laboratories, Inc., South San Francisco, CA) by MACS®. DCs used as a source of RNA for Northern blotting were purified as described (38). In brief, spleen or LN suspensions were prepared in RPMI containing 10% FCS, 10 mM Hepes, and antibiotics and adjusted with sodium chloride to mouse osmolarity (39) by gently pressing through a 70-μM mesh cell strainer (Fisher Scientific Co.). The suspension was layered on 2 ml of metrizamide (14.5% by weight in the same medium) solution (Accurate Chemical and Science Corp., Westbury, NY) and spun at 600 g for 10 min, and the cells at and above the interface were isolated. Flow cytometry confirmed that ∼70% of the cells were CD11c+ DCs. For RT-PCR analysis, in addition to isolation from cell suspensions as above, DCs were purified from the tissue fragments that did not go into suspension by digesting in collagenase D (Boehringer Mannheim Biochemicals) at 1.6 mg/ml and bovine pancreas DNAse (Sigma Chemical Co.) at 100 μg/ml for 30 min at room temperature. EDTA was then added to a concentration of 10 mM for 5 min (40), and the digested preparation was mashed through a cell strainer. Cells were then stained with antibodies to B220, TcR, and CD11c (PharMingen) and purified to >93% CD11c+B220−TcR− by sorting on a FACStar plus® (Becton Dickinson, San Jose, CA).
Chemotaxis.
Cell suspensions were prepared from spleen, LNs, thymus, or bone marrow of C57BL/6 mice as described (8). In the case of spleen and bone marrow, red blood cells were lysed using Tris-ammonium chloride. Chemotaxis assays were performed as described (23) using 106 total cells per 5-μM transwell (Corning Costar Corp., Acton, MA). Cells that migrated to the lower chamber were enumerated by collecting events for a fixed time (60 s) on a FACScan®. By counting a 1:5 dilution of input cells in the same way, the absolute number of cells that transmigrated could be determined. To determine which subsets of cells were migrating, a fraction of the cells that migrated to the lower chamber was stained and analyzed by flow cytometry. Cells from resting and activated spleen were stained with B220-FITC (clone 6B2), CD4-PE and CD8-PE (Caltag Laboratories, Inc.), and L-selectin–biotin/streptavidin cychrome (PharMingen). Migrating thymocytes were stained with CD8-FITC, CD4-PE (Caltag Laboratories, Inc.), and CD3-biotin (PharMingen), and bone marrow cells were stained with IgDb-FITC (PharMingen), B220-PE, and IgM-biotin (Caltag Laboratories, Inc.). Granulocytes were identified by their characteristic large side scatter. For macrophage chemotaxis assays, spleen cells were first depleted of B220+, CD4+, and CD8+ cells by MACS® (Miltenyi Biotec, Inc.), and migrating cells were identified with Mac-1–PE (Caltag Laboratories, Inc.). In some experiments, cells were preactivated by incubation with phorbol-12,13-dibutyrate (PDBu, 100 ng/ml; Calbiochem Corp., San Diego, CA), 17 μg/ml polyclonal anti–mouse IgM (Jackson ImmunoResearch Labs), or 20 μg/ml LPS. Synthetic human SDF1α (N33A) synthesized by chemical ligation (a gift from M. Siani, Gryphon Sciences, South San Francisco, CA) was used as a control. This SDF1α preparation maximally attracts lymphocytes, macrophages, and granulocytes at 100–500 ng/ml (26) and therefore was used at 300 ng/ml in these studies.
Mouse EBI-1 Transfection and Calcium Fluorimetry.
Mouse EBI-1 (41) was isolated by RT-PCR using a 3′ primer downstream of the stop codon and a 5′ primer starting at amino acid 26 of the full-length protein, truncating the hydrophobic NH2-terminal (possible leader) peptide. Both primers contained Sal-1 sites, and the PCR product was cloned into a modified form of pREP4 (Invitrogen Corp., San Diego, CA) that contains an NH2-terminal prolactin leader sequence and FLAG epitope followed by an in-frame Sal-1 site (42) (provided by S. Coughlin, University of California, San Francisco, San Francisco, CA). Human embryonic kidney 293 cells were transiently transfected with this construct using LipoTAXI according to the manufacturer's instructions (Stratagene Inc., La Jolla, CA). EBI-1 surface expression 2 d after transfection was confirmed by flow cytometry with anti-FLAG antibody M2 (VWR Scientific Products, West Chester, PA). Calcium mobilization studies were performed on Indo-1–loaded cells as described (43) using a spectrometer (model 4500; Hitachi Ltd., Tokyo, Japan). Intracellular calcium concentrations were calculated using the Hitachi 4500 intracellular cation measurement program.
Results
Identification of Murine ELC Homologue.
To permit the expression pattern and chemotactic activity of mouse ELC to be studied, we searched for ESTs that may encode mouse ELC using human ELC as the query sequence (32). Seven mouse ESTs were identified in the GenBank EST database (EST nos. 1088818, 906750, 885418, 1183472, 775758, 807269, and 822508) that could be aligned into a 755-bp contig encoding a predicted protein of 108 amino acids. The high nucleotide sequence identity (84%) and amino acid identity (78%) of this sequence with human ELC (Fig. 1) strongly suggested that we had identified mouse ELC. The proteins with greatest homology to both mouse and human ELC are mouse and human SLC (Fig. 1). A major difference between ELC and SLC is the extended cysteine-containing COOH-terminal region unique to SLC. When this region is excluded from the comparison, ELC and SLC are 33% identical. The next closest match to ELC is MIP3α (33), with 23% identity. The similarity between mouse and human ELC and SLC, together with the observation that the human genes colocalize on chromosome 9 (16, 32), is consistent with the possibility that these molecules arose from a gene-duplication event, and suggests they may have related functions.
Figure 1.

Mouse ELC sequence and alignment with human ELC and SLC. (A) Nucleotide and deduced amino acid sequence of mouse ELC. The predicted signal sequence is underlined, cysteines are shown in bold, and a single potential N-linked glycosylation site is underlined. (B) Alignment of mouse ELC (mELC) protein sequence with those of human ELC (hELC) and mouse and human SLC (mSLC and hSLC). Identical amino acids are shown as hyphens, and dots represent gaps inserted for optimum alignment. Numbering is with respect to the first amino acid shown in the full-length protein. These sequence data are available from EMBL/ GenBank/DDBJ under accession no. AF059208.
ELC Is Expressed in T Cell Zones of Secondary Lymphoid Tissues.
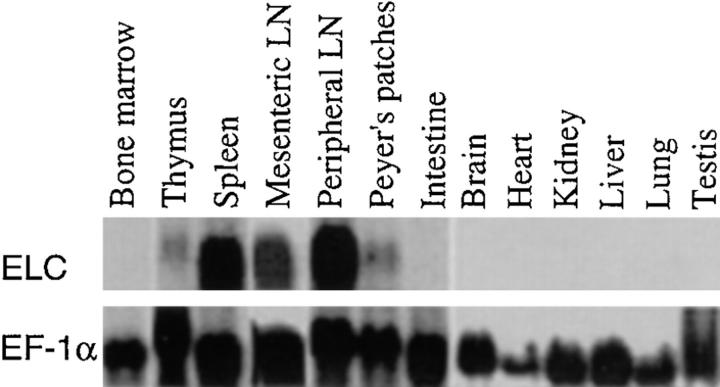
Northern blotting analysis revealed that mouse ELC was constitutively expressed at high levels in both mesenteric and peripheral LNs (Fig. 2). Significant expression was also identified in spleen and Peyer's patches, and low expression was seen in the thymus (Fig. 2). No expression was detected in bone marrow or in several nonlymphoid tissues (Fig. 2). To determine whether ELC was produced by a restricted set of cells within lymphoid tissues, which might suggest a role in controlling cell positioning, in situ hybridization analysis was performed (Fig. 3). Strikingly, the ELC probe hybridized strongly to a subset of cells within the T cell zone (or periarteriolar lymphatic sheath) of spleen but not to cells in the B cell follicles or the red pulp (Fig. 3 A). Within LNs, ELC-expressing cells were distributed evenly throughout the T zone (or paracortex) and were absent from follicles (Fig. 3 B). Similarly, in Peyer's patches, a hybridization signal was detected in the T cell region (the interfollicular zone) but not within follicles or in the subepithelial dome region (Fig. 3 C). No hybridization signal to T zones of secondary lymphoid tissues was seen with a control (sense) probe (Fig. 3 I).
Figure 2.
Northern blotting analysis of ELC expression in mouse tissues. Top, Hybridization with ELC probe. Bottom, Hybridization with EF-1α to control for amounts of RNA loaded.
Figure 3.
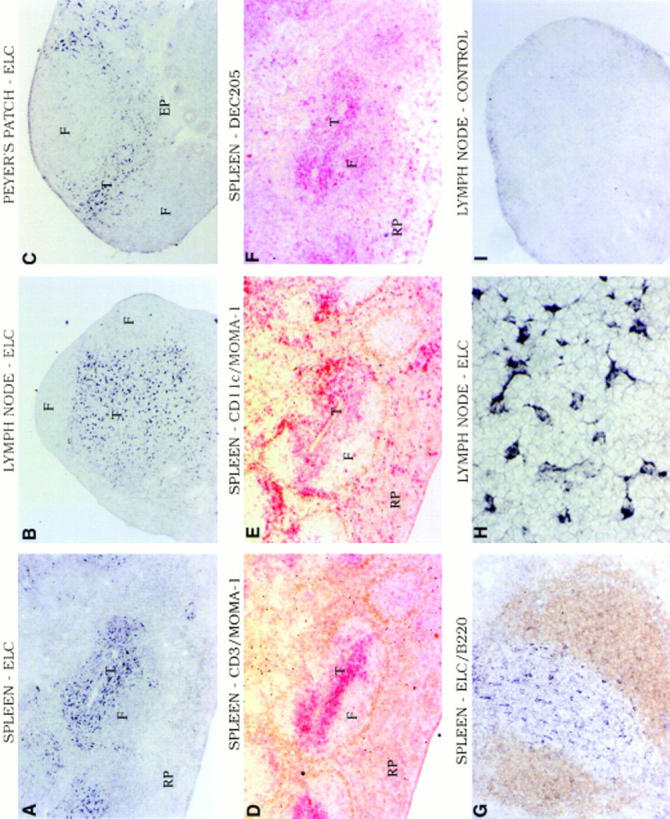
In situ hybridization analysis of ELC expression pattern in mouse lymphoid tissues. (A–C and G–I) Bright-field micrographs showing hybridization with digoxigenin-labeled antisense (A–C, G, and H) or sense (I) ELC probe to sections of spleen (A and G), mesenteric LN (B, H, and I), and Peyer's patch (C). Signal is seen as black staining. (D–F) Immunohistochemistry of spleen sections serial to A to detect in brown MOMA-1–positive marginal metallophilic macrophages (D and E) and in red CD3+ T cells (D), CD11c-expressing DCs (E) and DEC205-expressing DCs (F). The spleen section in G was double stained to detect B220 (brown) and ELC (black). T, T zone; F, follicle; RP, red pulp; EP, epithelium. Original magnifications: A–F and I, ×5 objective; G, ×10 objective; and H, ×40 objective.
ELC Is Expressed by Dendritic Cells.
The distribution of ELC-expressing cells in spleen, LNs, and Peyer's patches appeared similar to the distribution of T zone DCs in these tissues (3). To further investigate this similarity, spleen sections serial to those used for hybridization were stained to detect T cells (Fig 3. D, red) or with antibodies to CD11c (44) to detect DCs (Fig. 3 E, red). These sections were also stained with antibody to the marginal metallophilic macrophage marker, MOMA-1 (45), to outline the white pulp cords (Fig. 3, D and E, brown). The distribution of ELC-positive cells (Fig. 3 A) was very similar to the distribution of CD11c+ DCs located in the T zone (Fig. 3, A, D, and E). Interestingly, although clusters of CD11c+ DCs were also located in the bridging channels between T zone and red pulp (Fig. 3 E) as observed previously (3, 44), little ELC hybridization signal was detected in these regions (Fig. 3, A and E). Staining of a further serial section with the DC marker DEC205 that is expressed most highly on DCs in the T zone (3, 46) revealed a close concordance between the ELC hybridization pattern and the distribution of DEC205-expressing cells (Fig. 3, A and F). Combined staining for the fixation-resistant marker B220 and for ELC confirmed that the ELC-expressing cells were distributed across the T cell zone but did not extend into the B cell area of the splenic white pulp (Fig. 3 G). Close examination of the ELC hybridization signal in the T zone showed that many of the cells had a striking dendritic morphology (Fig. 3 H).
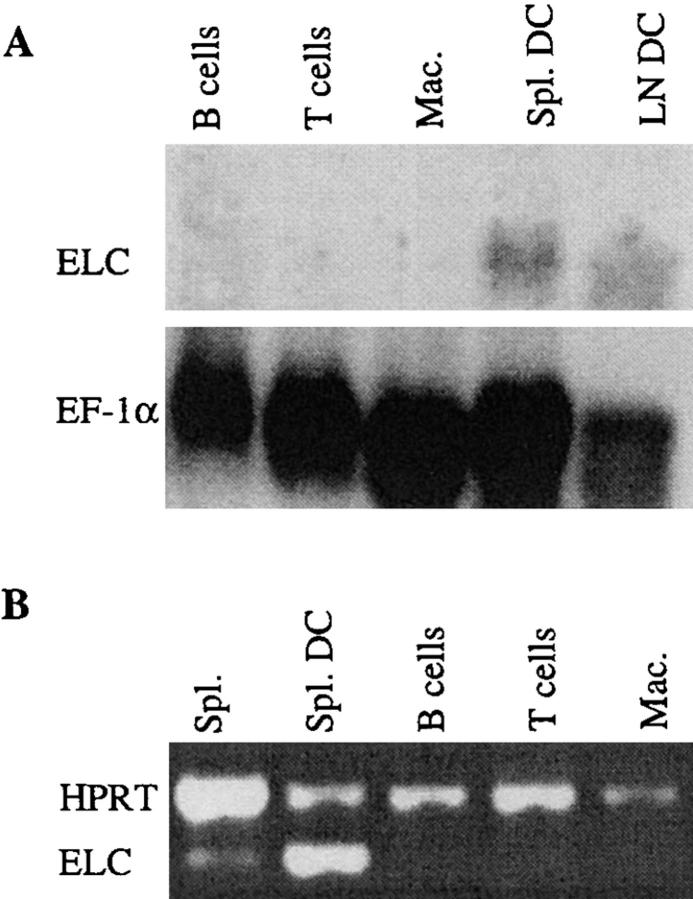
To directly test whether DCs were a source of ELC, Northern blot analysis was performed with RNA from partially purified spleen and LN DCs (Fig. 4 A). ELC expression was detected in the DC preparations, whereas purified B cells, T cells, and macrophages did not express detectable amounts of ELC (Fig. 4 A). To rule out the possibility that the Northern blot signal derived from a small population of contaminating cells, CD11c+ splenic DCs were FACS® sorted, and ELC expression was measured by RT-PCR. In agreement with the Northern blot result, ELC expression was detected in the purified DCs but was undetectable in purified T cells, B cells, or macrophages (Fig. 4 B). It was anticipated that if DCs were the major ELC-expressing cells in the spleen, the PCR signal in the sorted DCs would be increased compared with the unsorted spleen preparation, and this is what was observed (Fig. 4 B). Taking the findings from the in situ hybridization, Northern blot, and RT-PCR analysis together, we conclude that DCs present within the T zone are the major source of ELC in secondary lymphoid tissues.
Figure 4.
ELC expression in purified cells. (A) Northern blot analysis of ELC expression in MACS®-purified spleen B cells and T cells, mouse peritoneal macrophages (Mac.), and spleen (Spl.) and LN DCs purified to ∼70%. EF-1α hybridization is shown to control for amounts of RNA loaded. (B) RT-PCR analysis of ELC expression in total spleen before sorting (Spl.) and in splenic CD11c+ DCs isolated by FACS® sorting (Spl. DC), and lack of expression in purified spleen B cells and T cells and peritoneal macrophages (Mac.). Primers specific for HPRT were included in each sample as a reaction control.
ELC Strongly Attracts T Cells and Weakly Attracts B Cells.
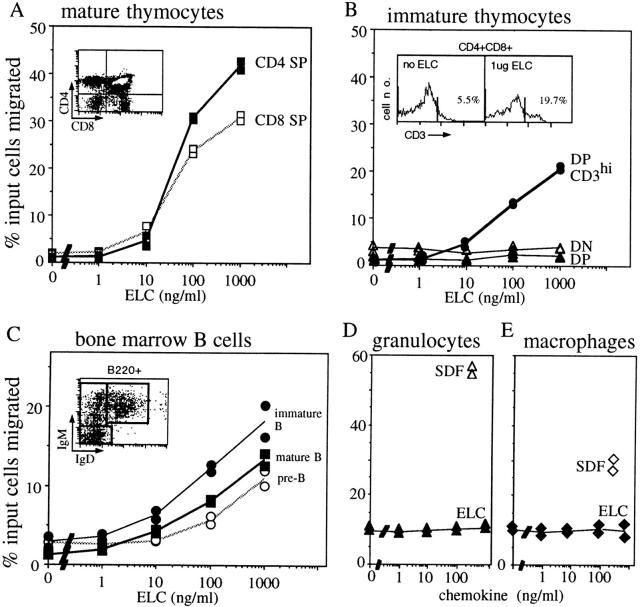
To characterize the chemotactic properties of mouse ELC, a COOH-terminal HIS-tagged version was expressed in Escherichia coli and purified to >90% by affinity- and high-pressure liquid chromatography. In migration assays with mouse spleen cells, a strong response was identified in CD4 and CD8 T cells (Fig. 5 A). Interestingly, CD4 cells were reproducibly found to be more sensitive to low ELC concentrations than CD8 cells (Fig. 5 A). B cells showed a weaker chemotactic response to ELC than both CD4 and CD8 T cells (Fig. 5 A). Strikingly, CD4 T cells of a naive (L-selectinhi) phenotype showed a stronger migratory response than CD4 T cells of a postactivation or memory (L-selectinlo) phenotype (Fig. 5 B). Lymphocytes from blood and LNs showed a similar response as cells from the spleen (data not shown).
Figure 5.
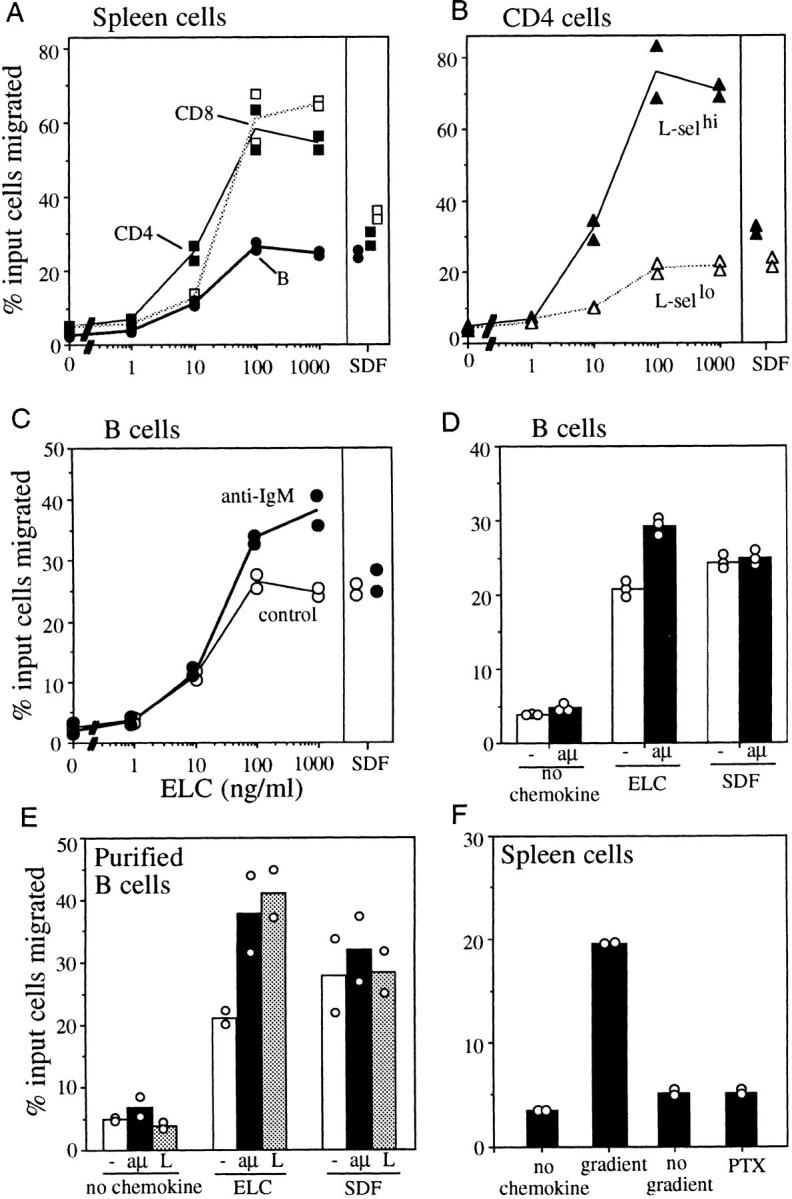
Chemotactic activity of ELC on resting and acutely activated mouse lymphocytes. Results are expressed as the percentage of input cells of each subtype migrating to the lower chamber of a transwell filter. Panels show migration of spleen lymphocyte subsets: (A) CD4 T cells, CD8 T cells, and B cells; (B) L-selectinhi and L-selectinlo CD4 T cells; (C) B cells preincubated with anti-IgM (17 μg/ml) or media alone (control) for 4 h; (D) duplicate experiment to C with cells preincubated with media alone (−) or anti-IgM (aμ) for 4 h; (E) purified B cells preincubated with media alone (−), anti-IgM at 17 μg/ml (aμ), or LPS at 20 μg/ml (L) for 6 h; (F) total spleen cells in the absence of a gradient (no gradient, equal concentration of ELC in upper and lower chamber) or preincubated with pertussis toxin at 200 ng/ml (PTX) for 2 h. In A–E, SDF1α was included at 300 ng/ml as a positive control. In D, ELC was at 200 ng/ml, and in E and F at 300 ng/ml. The results in A–C are representative of three independent experiments, and results in D–F of two experiments.
Activated Lymphocytes Have an Enhanced Chemotactic Response to ELC.
The ELC receptor was identified originally as a molecule upregulated in EBV-transformed B cells (34). Other studies indicated that CCR7 is also upregulated by treatment of PBLs with phorbol ester, PHA, or anti-CD3 (35, 36). In preliminary experiments with cells cultured overnight in phorbol ester, we observed an increase in the magnitude of the B cell response to ELC (data not shown). This experiment encouraged us to test whether more physiological stimuli also increased B cell responsiveness. This was of particular interest because after engagement by antigen in vivo, B cells migrate rapidly into the outer T zone (7, 8). Cells activated through the B cell receptor by pretreatment with anti-IgM for 4–6 h showed a 1.5-fold increase in the magnitude of their response to ELC (Fig. 5, C and D). Similar findings were made using purified B cells (Fig. 5 E), confirming that this effect was B cell intrinsic. Activation of B cells in vivo by LPS treatment also promotes migration of some cells into the T zone (47), and LPS activation was also found to enhance the B cell chemotactic response to ELC (Fig. 5 E). The increased ELC responsiveness of activated B cells did not simply reflect an overall increase in B cell motility, as there was little difference in the frequency of activated and resting cells that migrated in the absence of chemokine (Fig. 5, C–E). Furthermore, the activated cells did not demonstrate increased responsiveness to SDF1α (Fig. 5, C–E), indicating that the enhanced response was ELC specific.
ELC Responsiveness of Thymocytes and Immature B Cells.
Since ELC expression was also detected in the thymus (Fig. 2), the chemotactic response of thymocytes was measured (Fig. 6). Mature single positive thymocytes responded to ELC similarly to mature T cells in the periphery (Fig. 6 A), whereas immature (CD4−CD8− double negative) thymocytes and most CD4/CD8 double positive thymocytes did not show a measurable response (Fig. 6 B). Interestingly, however, when the responding cells were stained with anti-CD3 antibodies, an enrichment of CD3-bright CD4/CD8 double positive cells was detected in the population that migrated in response to ELC (Fig. 6 B), indicating that the most mature fraction of double positive cells had acquired responsiveness to ELC. We also measured the ELC responsiveness of immature B lineage cells in the bone marrow. B220+IgM−IgD− pre-B cells and B220+IgM+IgD− immature B cells showed a similar dose-dependent migratory response towards ELC as mature B cells (Fig. 6 C), indicating that the cells acquire responsiveness to this chemokine early in development. Consistent with the failure to detect CCR7 expression in myeloid cells (34, 41), bone marrow granulocytes (Fig. 6 D) and spleen monocytes/macrophages (Fig. 6 E) did not respond to ELC.
Figure 6.
Chemotactic activity of ELC on immature T and B cells and lack of activity on granulocytes and macrophages. Results are expressed as the percentage of input cells of each subtype migrating to the lower chamber of a transwell filter. Panels show response of (A) mature CD4 and CD8 single positive (SP) thymocytes; (B) immature CD4/CD8 double negative (DN) thymocytes, total CD4/CD8 double positive (DP) thymocytes, and CD3hi double positive thymocytes; (C) bone marrow B220+IgM−IgD− cells (pre-B), B220+IgM+IgD− cells (immature B), and B220+IgM+IgD+ cells (mature B); (D) bone marrow granulocytes; and (E) spleen macrophages. Insets in A and C show flow cytometric profiles of input cells and gates used to measure the frequency of each cell type. The profile in C has already been gated for B220+ cells. The inset in B shows the gate used to identify high CD3 expression on double positive thymocytes and, as an example, the fraction of CD3hi double positive cells that migrated in the absence of ELC or in response to 1 μg/ml ELC. In D and E, SDF1α (300 ng/ml) is included as a positive control. Each experiment was performed a minimum of two times.
Murine ELC Stimulates Murine CCR7–transfected Cells.
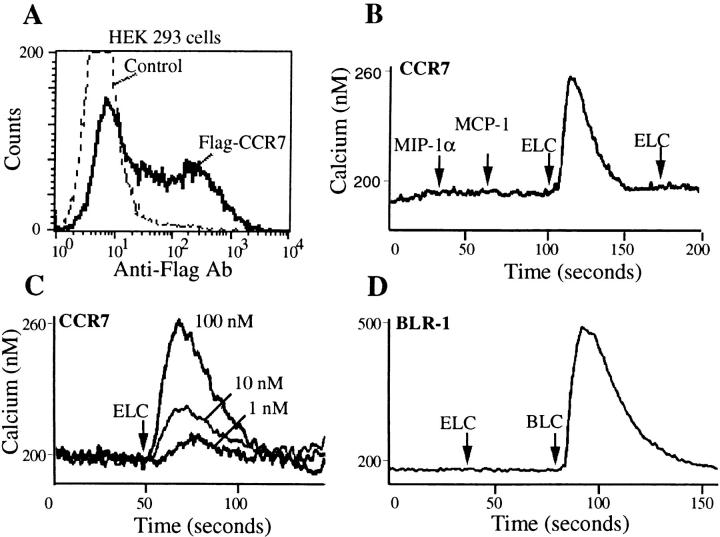
The migration response of lymphocytes to mouse ELC was chemotactic since the cells failed to migrate when incubated in ELC in the absence of a gradient (Fig. 5 F), and the pertussis toxin sensitivity of the response (Fig. 5 F) indicated the expected involvement of a Gi-coupled receptor. To test whether the mouse homologue of human ELC we had identified was a ligand for mouse CCR7/EBI-1, 293 cells were transiently transfected with a FLAG epitope– tagged form of the receptor and assayed for mouse ELC responsiveness. CCR7-expressing cells (Fig. 7 A) showed a rapid rise in intracellular calcium in response to ELC but not to two other CC chemokines, MIP1α and MCP1 (Fig 7 B). The response of the transfected cells was dose sensitive (Fig. 7 C), and exposure to high dose ELC fully desensitized the cells to further ELC exposure (Fig. 7 B). ELC did not stimulate a calcium flux in 293 cells transiently transfected with BLR1, showing that the response of CCR7-transfected cells to ELC was specific (Fig. 7 D). These results establish that mouse ELC can stimulate cells through mouse CCR7/EBI-1.
Figure 7.
Mouse CCR7/EBI-1 mediated calcium mobilization in response to ELC. (A) Flow cytometric analysis of FLAG-tagged CCR7 expression on HEK293 cells transiently transfected with CCR7 expression vector (Flag-CCR7) compared with cells stained without primary antibody (Control). (B) Calcium flux of CCR7-expressing cells in response to mouse ELC (1 μg/ml) but not MIP1α (0.2 μg/ml) or MCP1 (0.2 μg/ml). (C) Calcium flux of CCR7-expressing cells as a function of ELC concentration. (D) Lack of response of BLR1-transfected HEK293 cells to ELC (1 μg/ml). BLC (2 μg/ml) was used as positive control.
Discussion
In this report, we have identified mouse ELC and demonstrated that this CCR7 ligand is highly expressed by interdigitating DCs in the T zone of secondary lymphoid tissues. ELC strongly attracts resting T cells and more weakly attracts B cells. However, acute activation increases B cell responsiveness to ELC. These findings suggest two major roles for ELC: (a) to attract naive T cells into the T zone of lymphoid tissues and promote T cell–DC encounters, and (b) to attract antigen-binding B cells into the T zone of lymphoid tissues.
T and B lymphocytes enter LNs and Peyer's patches by crossing HEV located predominantly in the T zone (6, 48). Lymphocyte attachment to HEV involves chemokine- induced activation of integrin-mediated adhesion (49). The physiologically relevant chemokine on HEV that mediates integrin activation has not been defined, but recent studies demonstrated that SLC, SDF, and ELC could each promote integrin-mediated arrest of human lymphocytes in in vitro rolling chambers (17, 50). The high expression of SLC by HEV cells (17) together with our failure to detect ELC expression by HEV (Fig. 3) suggests that SLC may play the more prominent role in triggering lymphocyte adhesion to HEV. Whether SDF is expressed by HEV has not yet been well characterized, and its importance at this site remains to be established. Once lymphocytes have attached to HEV, they then migrate rapidly into the T zone. Our finding that ELC is highly expressed by T zone DCs and that ELC attracts naive lymphocytes suggests that this chemokine plays an important role in attracting cells away from HEV into the T zone and into contact with DCs. Interestingly, ELC and SLC have a strikingly similar chemotaxis profile, since both molecules attract naive T cells more efficiently than memory cells, and attract B cells more weakly than T cells. In this regard, it is interesting to note that SLC is the closest homologue of ELC (Fig. 1) and that both chemokines colocalize on chromosome 9 (16, 32), whereas most other CC chemokines cluster on chromosome 17 (11). We are currently investigating the possibility that SLC is also a ligand for CCR7/EBI-1. Since SLC is expressed at highest levels by HEV and at lower levels by stromal cells in the T zone (17), this chemokine may be inefficient at attracting cells into the T zone once they have crossed HEV. The existence of DC-derived ELC gradients may facilitate their movement away from the HEV and in amongst the T zone DCs. A further chemokine, PARC/ DCCK1, that is expressed by DCs in germinal centers and possibly also T zone DCs, and that can attract naive T cells (24, 25), may work with ELC to promote naive T cell–DC encounters. However, in contrast to the concentrated expression of ELC in secondary lymphoid tissues in humans (32) and mice (Figs. 2 and 3), PARC/DCCK1 is expressed at highest levels in the lung (25), suggesting distinct roles for the two molecules.
Lymphocytes enter the spleen by a different route to LNs or Peyer's patches, being released in the marginal sinus that surrounds the white pulp, or at terminal arterioles in the red pulp, and then migrating into the T zone (5, 52). However, as with entry into LNs, entry into the lymphoid region of the spleen is pertussis toxin sensitive (53, 54), implicating the involvement of chemokines. The expression of ELC in DCs within the splenic T zone indicates that ELC is likely to play a similar role in spleen and LNs, attracting lymphocytes into the T zone and promoting T cell encounter with DCs. Interestingly, despite the absence of HEV in the spleen, SLC expression is also detected in stromal cells in the splenic T zone (17), suggesting that these two chemokines may again act in concert to attract cells into the T zone.
The responsiveness of single positive thymocytes to ELC is likely to be important in ensuring that when these cells leave the thymus, they can enter into the T zone of secondary lymphoid tissues and interact with DCs. Whether ELC plays a role in thymocyte movement within the thymus is less clear. The expression level of ELC in mouse thymus was low, and we were unable to obtain a reproducible in situ hybridization signal (data not shown). However, the acquisition of ELC responsiveness by mouse thymocytes at the CD3hi, CD4/CD8 double positive stage suggests ELC may have a role in cell positioning within the mouse thymus. In this regard, ELC may function with several other chemokines identified recently in this tissue (20, 55–59), to help compartmentalize the cells appropriately for each developmental/selection event. Interestingly, ELC appears to be expressed at much higher levels in human thymus (32, 33), suggesting a more prominent role for ELC in human T cell development.
The weak but significant responsiveness of resting B cells to ELC is consistent with the original finding of EBI-1/ CCR7 expression in B lymphocytes (34). Previous studies have shown SLC also weakly attracts resting B cells (17), and it seems likely that ELC and SLC work together to promote migration of B cells into the T zone after they have crossed HEV or entered the marginal sinus of the spleen. Once in the T zone, the B cells may then enter the gradient of BLC that attracts the cells into follicles (26). Interestingly, immature B cells which do not express BLR1 (60, 61) are able to migrate into the T zone of the spleen but fail to enter follicles (62, 63). Positioning within the T zone may play an important role in the maturation or selection of these cells (63). Since immature B cells in the bone marrow are responsive to ELC (Fig. 6 C), it is reasonable to propose that once these cells leave the marrow and enter peripheral lymphoid tissues (especially the spleen), they are able to migrate into the T zone along a gradient of ELC.
Upon binding antigen, the migration of mature recirculating B cells is altered dramatically. Rather than moving into follicles, the cells accumulate within 6–8 h in the outer T zone (7, 37). This repositioning is likely to be critical for antigen-specific B and T cells to find each other. One mechanism by which antigen-engaged B cells might relocate to the outer T zone is through increased responsiveness to T zone–expressed chemokines. Our finding that stimulating cells through the antigen receptor increased the B cell response to ELC raises the possibility that ELC may participate in recruiting antigen-engaged B cells to this site.
In summary, our findings suggest a central role for ELC in secondary lymphoid tissue function. The high expression by T zone DCs and the strong chemotactic activity for naive T cells suggest that ELC attracts T cells into the T zone and promotes T cell–DC encounters. The weaker responsiveness of both immature and mature B cells supports a role for ELC in attracting these cells into the T zone as the necessary first step before they migrate into follicles, whereas the enhanced response of activated B cells implicates ELC as an important cue that promotes localization of antigen-engaged cells in the T cell zone. Understanding the factors that regulate ELC expression by DCs and the signals that control ELC responsiveness of lymphocytes is likely to teach us much about how secondary lymphoid tissues function in immunity.
Acknowledgments
We thank Elijah Wasson and Dana Avrahami-Tzfati for help in preparation of recombinant ELC, Mike Siani for the generous gift of SDF1α, Chris Turck for HPLC, and Art Weiss for use of the Hitachi 4500 spectrometer. We also thank M. Dee Gunn, Rich Locksley, and members of the laboratory for comments on the manuscript. J.G. Cyster is a Pew Scholar in the Biomedical Sciences.
This work was supported in part by a grant from the National Institutes of Health (AI40098).
Abbreviations used in this paper
- BLC
B lymphocyte chemoattractant
- BLR
Burkitt's lymphoma receptor
- CCR
CC chemokine receptor
- DC
dendritic cell
- DCCK
dendritic cell–derived CC chemokine
- EBI-1
EBV-induced molecule 1
- ELC
EBI-1 ligand chemokine
- EF
elongation factor
- EST
expressed sequence tag
- HEV
high endothelial venules
- MCP
monocyte chemoattractant protein
- MIP
macrophage-inflammatory protein
- PARC
pulmonary and activation–regulated chemokine
- RT
reverse transcription
- SDF
stromal cell–derived factor
- SLC
secondary lymphoid tissue chemokine
Footnotes
Note added in proof. After submission of this manuscript, Kim et al. reported that human ELC is chemotactic for human T and B lymphocytes ( J. Immunol. 160:2418–2424), and Yoshida et al. demonstrated that SLC is a second ligand for CCR7 ( J. Biol. Chem. 273:7118–7122).
References
- 1.Austyn JM. New insights into the mobilization and phagocytic activity of dendritic cells. J Exp Med. 1996;183:1287–1292. doi: 10.1084/jem.183.4.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hart DN. Dendritic cells: unique leukocyte populations which control the primary immune response. Blood. 1997;90:3245–3287. [PubMed] [Google Scholar]
- 3.Steinman RM, Pack M, Inaba K. Dendritic cells in the T-cell areas of lymphoid organs. Immunol Rev. 1997;156:25–37. doi: 10.1111/j.1600-065x.1997.tb00956.x. [DOI] [PubMed] [Google Scholar]
- 4.Ingulli E, Mondino A, Khoruts A, Jenkins MK. In vivo detection of dendritic cell antigen presentation to CD4(+) T cells. J Exp Med. 1997;185:2133–2141. doi: 10.1084/jem.185.12.2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Niewenhuis P, Ford WL. Comparative migration of B- and T-lymphocytes in the rat spleen and lymph nodes. Cell Immunol. 1976;23:254–267. doi: 10.1016/0008-8749(76)90191-x. [DOI] [PubMed] [Google Scholar]
- 6.Picker LJ, Butcher EC. Physiological and molecular mechanisms of lymphocyte homing. Annu Rev Immunol. 1992;10:561–591. doi: 10.1146/annurev.iy.10.040192.003021. [DOI] [PubMed] [Google Scholar]
- 7.Liu Y-J, Zhang J, Lane PJL, Chan EY-T, MacLennan ICM. Sites of specific B cell activation in primary and secondary responses to T cell-dependent and T cell-independent antigens. Eur J Immunol. 1991;21:2951–2962. doi: 10.1002/eji.1830211209. [DOI] [PubMed] [Google Scholar]
- 8.Cyster JG, Hartley SB, Goodnow CC. Competition for follicular niches excludes self-reactive cells from the recirculating B-cell repertoire. Nature. 1994;371:389–395. doi: 10.1038/371389a0. [DOI] [PubMed] [Google Scholar]
- 9.Goodnow CC, Cyster JG. Lymphocyte homing: the scent of a follicle. Curr Biol. 1997;7:R219–R222. doi: 10.1016/s0960-9822(06)00105-9. [DOI] [PubMed] [Google Scholar]
- 10.Bacon KB, Schall TJ. Chemokines as mediators of allergic inflammation. Int Arch Allergy Immunol. 1996;109:97–109. doi: 10.1159/000237207. [DOI] [PubMed] [Google Scholar]
- 11.Baggiolini M, Dewald B, Moser B. Human chemokines: an update. Annu Rev Immunol. 1997;15:675–705. doi: 10.1146/annurev.immunol.15.1.675. [DOI] [PubMed] [Google Scholar]
- 12.Mackay CR. Chemokines: what chemokine is that? . Curr Biol. 1997;7:R384–R386. doi: 10.1016/s0960-9822(06)00181-3. [DOI] [PubMed] [Google Scholar]
- 13.Schall TJ, Bacon K, Toy KJ, Goeddel DV. Selective attraction of monocytes and T lymphocytes of the memory phenotype by cytokine RANTES. Nature. 1990;347:669–671. doi: 10.1038/347669a0. [DOI] [PubMed] [Google Scholar]
- 14.Taub DD, Conlon K, Lloyd AR, Oppenheim JJ, Kelvin DJ. Preferential migration of activated CD4+ and CD8+ T cells in response to MIP-1α and MIP-1β. Science. 1993;260:355–358. doi: 10.1126/science.7682337. [DOI] [PubMed] [Google Scholar]
- 15.Roth SJ, Carr MC, Springer TA. C-C chemokines, but not C-X-C chemokines interleukin-8 and interferon-γ inducible protein-10, stimulate transendothelial chemotaxis of T lymphocytes. Eur J Immunol. 1995;25:3482–3488. doi: 10.1002/eji.1830251241. [DOI] [PubMed] [Google Scholar]
- 16.Nagira M, Imai T, Hieshima K, Kusuda J, Ridanpaa M, Takagi S, Nishimura M, Kakizaki M, Nomiyama H, Yoshie O. Molecular cloning of a novel human CC chemokine secondary lymphoid-tissue chemokine that is a potent chemoattractant for lymphocytes and mapped to chromosome 9p13. J Biol Chem. 1997;272:19518–19524. doi: 10.1074/jbc.272.31.19518. [DOI] [PubMed] [Google Scholar]
- 17.Gunn MD, Tangemann K, Tam C, Cyster JG, Rosen S, Williams LT. A chemokine expressed in lymphoid high endothelial venules promotes the adhesion and chemotaxis of naive T lymphocytes. Proc Natl Acad Sci USA. 1998;95:258–263. doi: 10.1073/pnas.95.1.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hedrick JA, Zlotnik A. Identification and characterization of a novel beta chemokine containing six conserved cysteines. J Immunol. 1997;159:1589–1593. [PubMed] [Google Scholar]
- 19.Hromas R, Kim CH, Klemsz M, Krathwohl M, Fife K, Cooper S, Schnizlein-Bick C, Broxmeyer HE. Isolation and characterization of Exodus-2, a novel C-C chemokine with a unique 37-amino acid carboxyl-terminal extension. J Immunol. 1997;159:2554–2558. [PubMed] [Google Scholar]
- 20.Tanabe S, Lu Z, Luo Y, Quackenbush EJ, Berman MA, Collins-Racie LA, Mi S, Reilly C, Lo D, Jacobs KA, Dorf ME. Identification of a new mouse β-chemokine, thymus-derived chemotactic agent 4, with activity on T lymphocytes and mesangial cells. J Immunol. 1997;159:5671–5679. [PubMed] [Google Scholar]
- 21.Tashiro K, Tada H, Heilker R, Shirozu M, Nakano T, Honjo T. Signal sequence trap: a cloning strategy for secreted proteins and type I membrane proteins. Science. 1993;261:600–603. doi: 10.1126/science.8342023. [DOI] [PubMed] [Google Scholar]
- 22.Nagasawa T, Kikutani H, Kishimoto T. Molecular cloning and structure of a pre-B-cell growth-stimulating factor. Proc Natl Acad Sci USA. 1994;91:2305–2309. doi: 10.1073/pnas.91.6.2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bleul CC, Fuhlbrigge RC, Casasnovas JM, Aiuti A, Springer TA. A highly efficacious lymphocyte chemoattractant, stromal cell–derived factor 1 (SDF-1) J Exp Med. 1996;184:1101–1109. doi: 10.1084/jem.184.3.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Adema GJ, Hartgers F, Verstraten R, de Vries E, Marland G, Menon S, Foster J, Xu Y, Nooyen P, McClanahan T, et al. A dendritic cell-derived C-C chemokine that preferentially attracts naive T cells. Nature. 1997;387:713–717. doi: 10.1038/42716. [DOI] [PubMed] [Google Scholar]
- 25.Hieshima K, Imai T, Baba M, Shoudai K, Ishizuka K, Nakagawa T, Tsuruta J, Takeya M, Sakaki Y, Takatsuki K, et al. A novel human CC chemokine PARC that is most homologous to macrophage-inflammatory protein-1 alpha/LD78 alpha and chemotactic for T lymphocytes, but not for monocytes. J Immunol. 1997;159:1140–1149. [PubMed] [Google Scholar]
- 26.Gunn MD, Ngo VN, Ansel KM, Ekland EH, Cyster JG, Williams LT. A B-cell homing chemokine made in lymphoid follicles activates Burkitt's lymphoma receptor-1. Nature. 1998;391:799–803. doi: 10.1038/35876. [DOI] [PubMed] [Google Scholar]
- 27.Legler DF, Loetscher M, Roos RS, Clark-Lewis I, Baggiolini M, Moser B. B cell–attracting chemokine 1, a human CXC chemokine expressed in lymphoid tissues, selectively attracts B lymphocytes via BLR1/CXCR5. J Exp Med. 1998;187:655–660. doi: 10.1084/jem.187.4.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nagasawa T, Hirota S, Tachibana K, Takakura N, Nishikawa S, Kitamura Y, Yoshida N, Kikutani H, Kishimoto T. Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature. 1996;382:635–638. doi: 10.1038/382635a0. [DOI] [PubMed] [Google Scholar]
- 29.D'Apuzzo M, Rolink A, Loetscher M, Hoxie JA, Clark-Lewis I, Melchers F, Baggiolini M, Moser B. The chemokine SDF-1, stromal cell-derived factor 1, attracts early stage B cell precursors via the chemokine receptor CXCR4. Eur J Immunol. 1997;27:1788–1793. doi: 10.1002/eji.1830270729. [DOI] [PubMed] [Google Scholar]
- 30.Bleul CC, Schultze JL, Springer TA. B lymphocyte chemotaxis regulated in association with microanatomic localization, differentiation state, and B cell receptor engagement. J Exp Med. 1998;187:753–762. doi: 10.1084/jem.187.5.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Forster R, Mattis AE, Kremmer E, Wolf E, Brem G, Lipp M. A putative chemokine receptor, BLR1, directs B cell migration to defined lymphoid organs and specific anatomic compartments of the spleen. Cell. 1996;87:1–20. doi: 10.1016/s0092-8674(00)81798-5. [DOI] [PubMed] [Google Scholar]
- 32.Yoshida R, Imai T, Hieshima K, Kusuda J, Baba M, Kitaura M, Nishimura M, Kakizaki M, Nomiyama H, Yoshie O. Molecular cloning of a novel human CC chemokine EBI1-ligand chemokine that is a specific functional ligand for EBI1, CCR7. J Biol Chem. 1997;272:13803–13809. doi: 10.1074/jbc.272.21.13803. [DOI] [PubMed] [Google Scholar]
- 33.Rossi DL, Vicari AP, Franz-Bacon K, McClanahan TK, Zlotnik A. Identification through bioinformatics of two new macrophage proinflammatory human chemokines: MIP-3α and MIP-3β. J Immunol. 1997;158:1033–1036. [PubMed] [Google Scholar]
- 34.Birkenbach M, Josefsen K, Yalamanchili R, Lenoir G, Kieff E. Epstein-Barr virus-induced genes: first lymphocyte-specific G protein-coupled peptide receptors. J Virol. 1993;67:2209–2220. doi: 10.1128/jvi.67.4.2209-2220.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hasegawa H, Utsunomiya Y, Yasukawa M, Yanagisawa K, Fujita S. Induction of G protein-coupled peptide receptor EBI 1 by human herpesvirus 6 and 7 infection in CD4+ T cells. J Virol. 1994;68:5326–5329. doi: 10.1128/jvi.68.8.5326-5329.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Burgstahler R, Kempkes B, Steube K, Lipp M. Expression of the chemokine receptor BLR2/EBI1 is specifically transactivated by Epstein-Barr virus nuclear antigen 2. Biochem Biophys Res Commun. 1995;215:737–743. doi: 10.1006/bbrc.1995.2525. [DOI] [PubMed] [Google Scholar]
- 37.Cyster JG, Goodnow CC. Antigen-induced exclusion from follicles and anergy are separate and complementary processes that influence peripheral B cell fate. Immunity. 1995;3:691–701. doi: 10.1016/1074-7613(95)90059-4. [DOI] [PubMed] [Google Scholar]
- 38.Macatonia SE, Knight SC, Edwards AJ, Griffiths S, Fryer P. Localization of antigen on lymph node dendritic cells after exposure to the contact sensitizer fluorescein isothiocyanate. J Exp Med. 1987;166:1654–1667. doi: 10.1084/jem.166.6.1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shortman K. Analytical and preparative equilibrium density separation of lymphoid cells on albumin and metrizamide. Methods Enzymol. 1984;108:102–117. doi: 10.1016/s0076-6879(84)08077-0. [DOI] [PubMed] [Google Scholar]
- 40.Vremec D, Shortman K. Dendritic cell subtypes in mouse lymphoid organs: cross-correlation of surface markers, changes with incubation, and differences among thymus, spleen, and lymph nodes. J Immunol. 1997;159:565–573. [PubMed] [Google Scholar]
- 41.Schweickart VL, Raport CJ, Godiska R, Byers MG, Eddy RL, Jr, Shows TB, Gray PW. Cloning of human and mouse EBI1, a lymphoid-specific G-protein-coupled receptor encoded on human chromosome 17q12-q21.2. Genomics. 1994;23:643–650. doi: 10.1006/geno.1994.1553. [DOI] [PubMed] [Google Scholar]
- 42.Ishii K, Hein L, Kobilka B, Coughlin SR. Kinetics of thrombin receptor cleavage on intact cells. Relation to signaling. J Biol Chem. 1993;268:9780–9786. [PubMed] [Google Scholar]
- 43.Myers SJ, Wong LM, Charo IF. Signal transduction and ligand specificity of the human monocyte chemoattractant protein-1 receptor in transfected embryonic kidney cells. J Biol Chem. 1995;270:5786–5792. doi: 10.1074/jbc.270.11.5786. [DOI] [PubMed] [Google Scholar]
- 44.Metlay JP, Witmer-Pack MD, Agger R, Crowley MT, Lawless D, Steinman RM. The distinct leukocyte integrins of mouse spleen dendritic cells as identified with new hamster monoclonal antibodies. J Exp Med. 1990;171:1753–1771. doi: 10.1084/jem.171.5.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kraal G. Cells in the marginal zone of the spleen. Int Rev Cytol. 1992;132:31–74. doi: 10.1016/s0074-7696(08)62453-5. [DOI] [PubMed] [Google Scholar]
- 46.Kraal G, Breel M, Janse M, Bruin G. Langerhans' cells, veiled cells, and interdigitating cells in the mouse recognized by a monoclonal antibody. J Exp Med. 1986;163:981–997. doi: 10.1084/jem.163.4.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gray D, Kumararatne DS, Lortan J, Khan M, MacLennan ICM. Relation of intra-splenic migration of marginal zone B cells to antigen localization on follicular dendritic cells. Immunology. 1984;52:659–669. [PMC free article] [PubMed] [Google Scholar]
- 48.Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell. 1994;76:301–314. doi: 10.1016/0092-8674(94)90337-9. [DOI] [PubMed] [Google Scholar]
- 49.Bargatze RF, Butcher EC. Rapid G protein– regulated activation event involved in lymphocyte binding to high endothelial venules. J Exp Med. 1993;178:367–372. doi: 10.1084/jem.178.1.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Campbell JJ, Hedrick J, Zlotnik A, Siani MA, Thompson DA, Butcher EC. Chemokines and the arrest of lymphocytes rolling under flow conditions. Science. 1998;279:381–384. doi: 10.1126/science.279.5349.381. [DOI] [PubMed] [Google Scholar]
- 51.Deleted in proof.
- 52.Pellas TC, Weiss L. Migration pathways of recirculating murine B cells and CD4+ and CD8+ T lymphocytes. Am J Anat. 1990;187:355–373. doi: 10.1002/aja.1001870405. [DOI] [PubMed] [Google Scholar]
- 53.Cyster JG, Goodnow CC. Pertussis toxin inhibits migration of B and T lymphocytes into splenic white pulp cords. J Exp Med. 1995;182:581–586. doi: 10.1084/jem.182.2.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lyons AB, Parish CR. Are murine marginal-zone macrophages the splenic white pulp analog of high endothelial venules? . Eur J Immunol. 1995;25:3165–3172. doi: 10.1002/eji.1830251127. [DOI] [PubMed] [Google Scholar]
- 55.Gattass CR, King LB, Luster AD, Ashwell JD. Constitutive expression of interferon γ–inducible protein 10 in lymphoid organs and inducible expression in T cells and thymocytes. J Exp Med. 1994;179:1373–1378. doi: 10.1084/jem.179.4.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Imai T, Yoshida T, Baba M, Nishimura M, Kakizaki M, Yoshie O. Molecular cloning of a novel T cell- directed CC chemokine expressed in thymus by signal sequence trap using Epstein-Barr virus vector. J Biol Chem. 1996;271:21514–21521. doi: 10.1074/jbc.271.35.21514. [DOI] [PubMed] [Google Scholar]
- 57.Imai T, Baba M, Nishimura M, Kakizaki M, Takagi S, Yoshie O. The T cell-directed CC chemokine TARC is a highly specific biological ligand for CC chemokine receptor 4. J Biol Chem. 1997;272:15036–15042. doi: 10.1074/jbc.272.23.15036. [DOI] [PubMed] [Google Scholar]
- 58.Vicari AP, Figueroa DJ, Hedrick JA, Foster JS, Singh KP, Menon S, Copeland NG, Gilbert DJ, Jenkins NA, Bacon KB, Zlotnik A. TECK: a novel CC chemokine specifically expressed by thymic dendritic cells and potentially involved in T cell development. Immunity. 1997;7:291–301. doi: 10.1016/s1074-7613(00)80531-2. [DOI] [PubMed] [Google Scholar]
- 59.Chang M, McNinch J, Elias C, III, Manthey CL, Grosshans D, Meng T, Boone T, Andrew DP. Molecular cloning and functional characterization of a novel CC chemokine, stimulated T cell chemotactic protein (STCP-1) that specifically acts on activated T lymphocytes. J Biol Chem. 1997;272:25229–25237. doi: 10.1074/jbc.272.40.25229. [DOI] [PubMed] [Google Scholar]
- 60.Forster R, Emrich T, Kremmer E, Lipp M. Expression of the G-protein-coupled receptor BLR1 defines mature, recirculating B cells and a subset of T-helper memory cells. Blood. 1994;84:830–840. [PubMed] [Google Scholar]
- 61.Schmidt KN, Hsu CW, Griffin CT, Goodnow CC, Cyster JG. Spontaneous follicular exclusion of SHP1-deficient B cells is conditional on the presence of competitor wild-type B cells. J Exp Med. 1998;187:929–937. doi: 10.1084/jem.187.6.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lortran JE, Roobottom CA, Oldfield S, MacLennan ICM. Newly produced virgin B cells migrate to secondary lymphoid organs but their capacity to enter follicles is restricted. Eur J Immunol. 1987;17:1311–1316. doi: 10.1002/eji.1830170914. [DOI] [PubMed] [Google Scholar]
- 63.Cyster JG. Signaling thresholds and interclonal competition in preimmune B cell selection. Immunol Rev. 1997;156:87–101. doi: 10.1111/j.1600-065x.1997.tb00961.x. [DOI] [PubMed] [Google Scholar]