Abstract
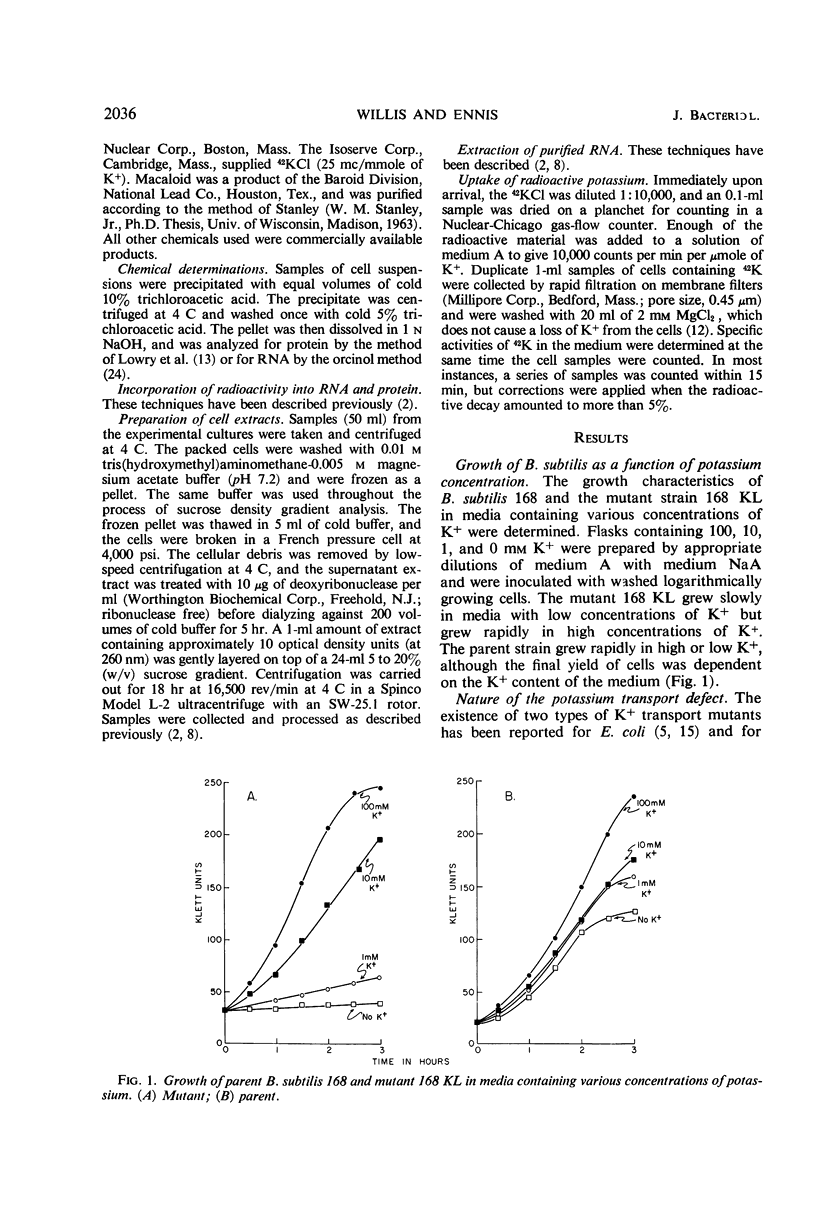
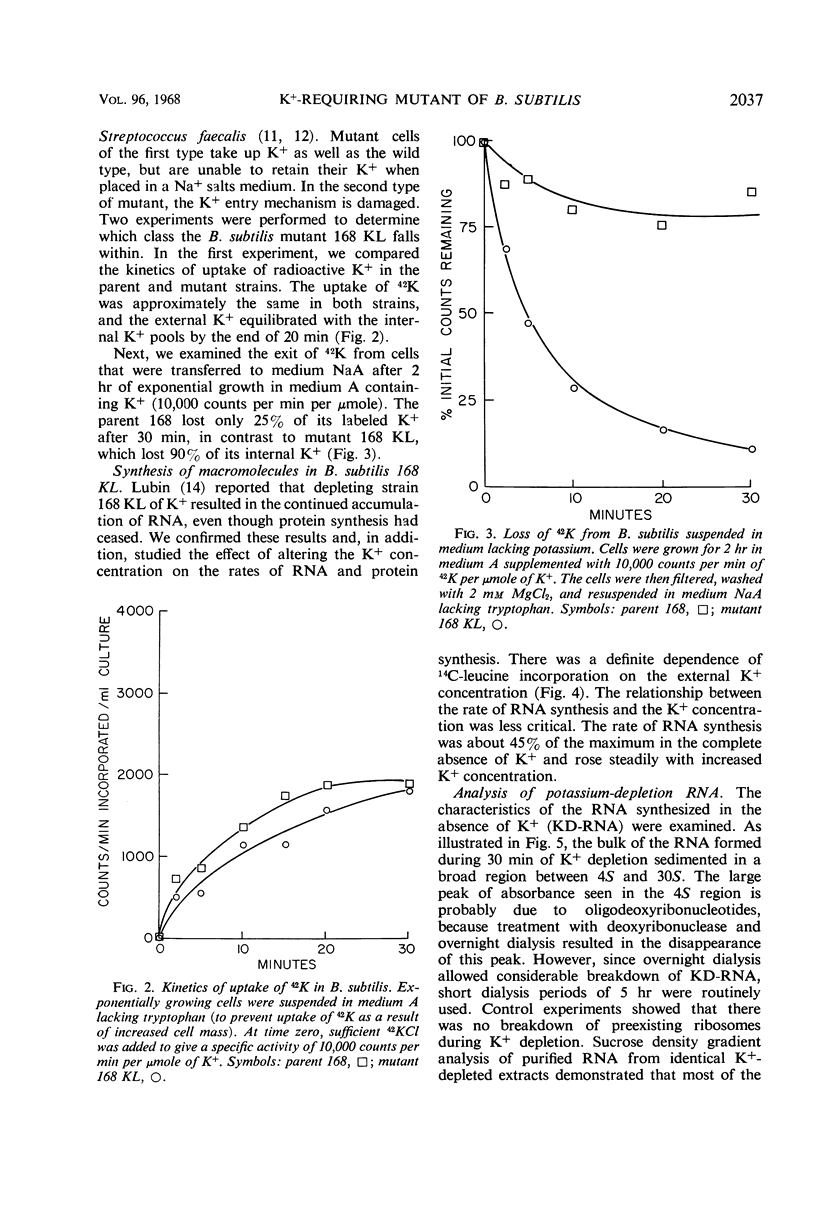
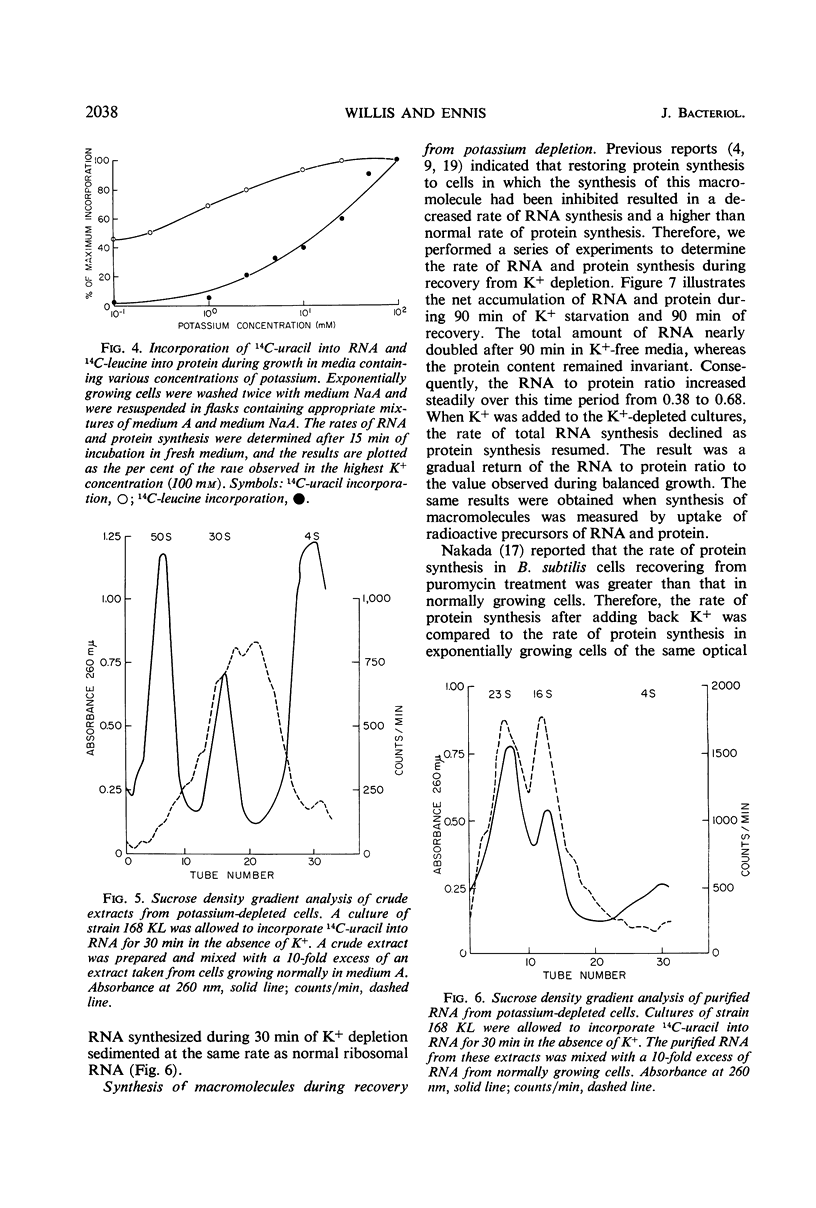
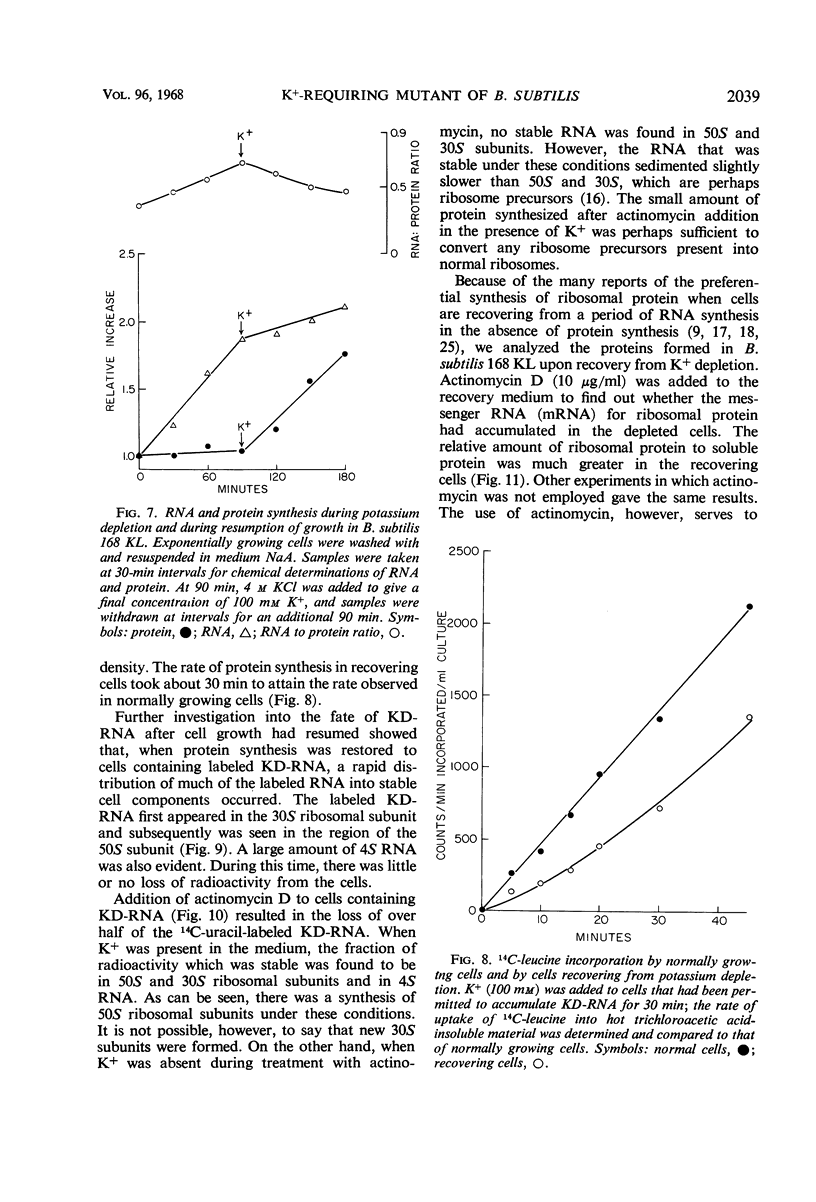
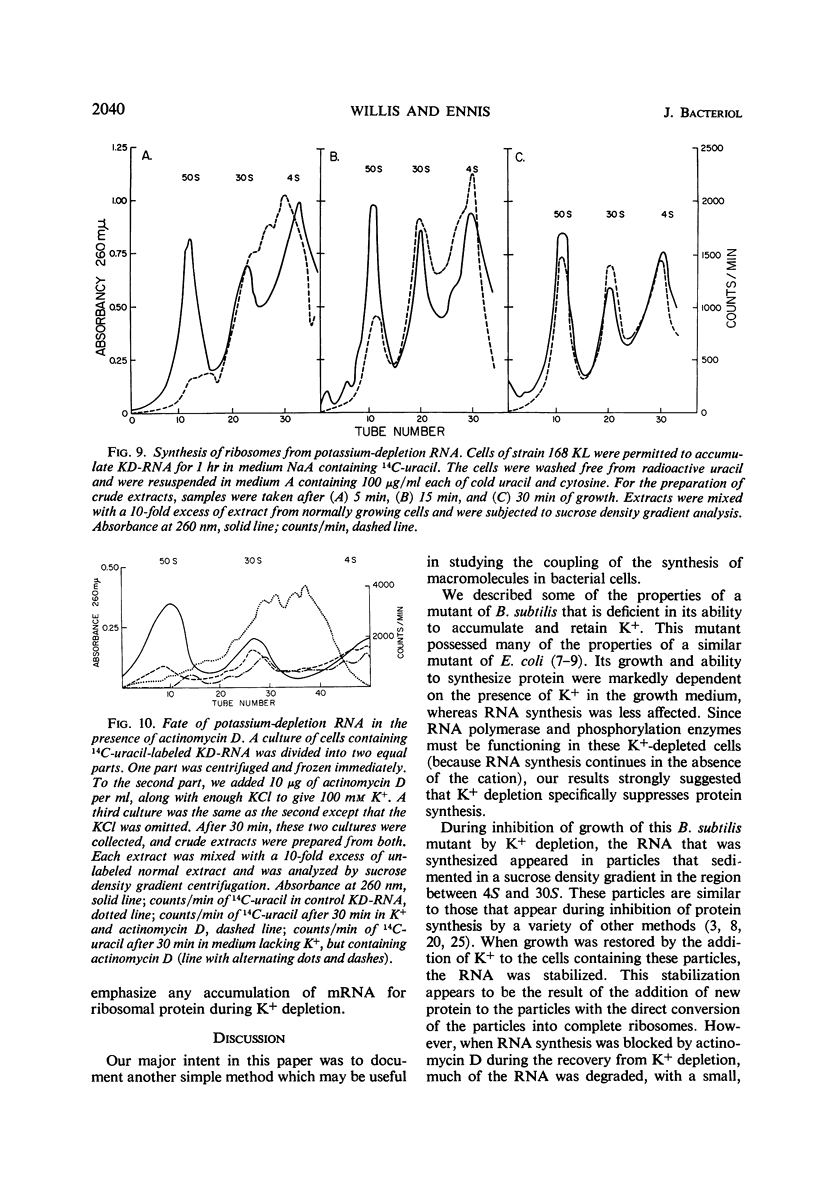
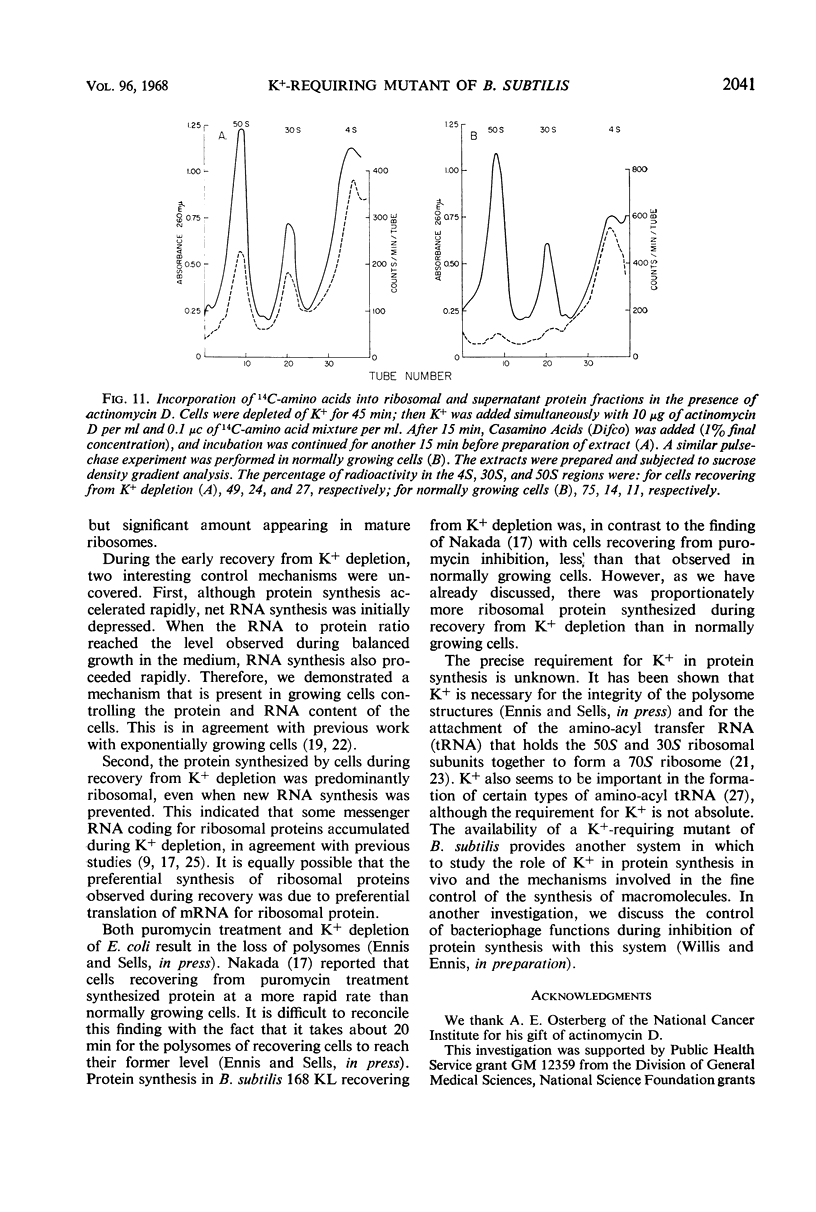
A mutant of Bacillus subtilis 168 (strain 168 KL), which had lost its normal capacity to accumulate K+, was used to explore the interrelationship between protein and ribonucleic acid (RNA) synthesis. In contrast to the wild type, the growth rate of strain 168 KL was markedly dependent on the K+ concentration in the medium. K+ uptake in the mutant strain was identical to that in the parent, but the mutant was unable to retain and accumulate K+. Protein synthesis was markedly dependent on the K+ concentration in the medium, whereas RNA synthesis was relatively unaffected by changes in the level of K+. Most of the RNA synthesized during K+ depletion was ribosomal RNA; it appeared in crude extracts in the form of ribonucleoproteins particles with sedimentation values between 4S and 30S. These particles were converted into mature ribosomes when growth was allowed to resume by the addition of K+. Simultaneous synthesis of RNA and protein was necessary for the quantitative conversion of the ribonucleoprotein particles into ribosomes. During recovery from K+ depletion, ribosomal protein was synthesized in preference to the other proteins of the cell.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BOREK E., RYAN A., ROCKENBACH J. Nucleic acid metabolism in relation to the lysogenic phenomenon. J Bacteriol. 1955 Apr;69(4):460–467. doi: 10.1128/jb.69.4.460-467.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen P. S., Ennis H. L. The requirement for potassium for bacteriophage T4 protein and deoxyribonucleic acid synthesis. Virology. 1965 Nov;27(3):282–289. doi: 10.1016/0042-6822(65)90107-8. [DOI] [PubMed] [Google Scholar]
- DAGLEY S., TURNOCK G., WILD D. G. THE ACCUMULATION OF RIBONUCLEIC ACID BY A MUTANT OF ESCHERICHIA COLI. Biochem J. 1963 Sep;88:555–566. doi: 10.1042/bj0880555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAGLEY S., WHITE A. E., WILD D. G., SYKES J. Synthesis of protein and ribosomes by bacteria. Nature. 1962 Apr 7;194:25–27. doi: 10.1038/194025a0. [DOI] [PubMed] [Google Scholar]
- DAVIS B. D., MINGIOLI E. S. Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol. 1950 Jul;60(1):17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damadian R. Ion metabolism in a potassium accumulation mutant of Escherichia coli B. I. Potassium metabolism. J Bacteriol. 1968 Jan;95(1):113–122. doi: 10.1128/jb.95.1.113-122.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ENNIS H. L., LUBIN M. Dissociation of ribonucleic acid and protein synthesis in bacteria deprived of potassium. Biochim Biophys Acta. 1961 Jun 24;50:399–402. doi: 10.1016/0006-3002(61)90355-9. [DOI] [PubMed] [Google Scholar]
- ENNIS H. L., LUBIN M. PRE-RIBOSOMAL PARTICLES FORMED IN POTASSIUM-DEPLETED CELLS. STUDIES ON DEGRADATION AND STABILIZATION. Biochim Biophys Acta. 1965 Apr 19;95:605–623. doi: 10.1016/0005-2787(65)90515-0. [DOI] [PubMed] [Google Scholar]
- ENNIS H. L., LUBIN M. SELECTIVE SYNTHESIS OF RIBOSOMAL PROTEIN DURING RECOVERY FROM UNBALANCED GROWTH. Biochim Biophys Acta. 1965 Apr 19;95:624–633. doi: 10.1016/0005-2787(65)90516-2. [DOI] [PubMed] [Google Scholar]
- GALE E. F., FOLKES J. P. The assimilation of amino-acids by bacteria. XV. Actions of antibiotics on nucleic acid and protein synthesis in Staphylococcus aureus. Biochem J. 1953 Feb;53(3):493–498. doi: 10.1042/bj0530493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harold F. M., Baarda J. R. Inhibition of potassium transport by sodium in a mutant of Streptococcus faecalis. Biochemistry. 1967 Oct;6(10):3107–3110. doi: 10.1021/bi00862a018. [DOI] [PubMed] [Google Scholar]
- Harold F. M., Harold R. L., Baarda J. R., Abrams A. A genetic defect in retention of potassium by Streptococcus faecalis. Biochemistry. 1967 Jun;6(6):1777–1784. doi: 10.1021/bi00858a028. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- LUBIN M. INTRACELLULAR POTASSIUM AND CONTROL OF PROTEIN SYNTHESIS. Fed Proc. 1964 Sep-Oct;23:994–1001. [PubMed] [Google Scholar]
- LUBOCHINSKY B., MEURY J., STOLKOWSKI J. CIN'ETIQUE DES 'ECHANGES DE POTASSIUM CHEZ L'ESCHERICHIA COLI, SOUCHE B 207, QUI NE PEUT CRO ITRE NORMALEMENT QU'EN PR'ESENCE DE CONCENTRATIONS 'ELEV'EES EN POTASSIUM. C R Hebd Seances Acad Sci. 1964 May 20;258:5106–5109. [PubMed] [Google Scholar]
- NAKADA D., ANDERSON I. A., MAGASANIK B. FATE OF THE RIBOSOMAL RNA PRODUCED BY A "RELAXED" MUTANT OF ESCHERICHIA COLI. J Mol Biol. 1964 Aug;9:472–488. doi: 10.1016/s0022-2836(64)80220-5. [DOI] [PubMed] [Google Scholar]
- NEIDHARDT F. C., MAGASANIK B. Studies on the role of ribonucleic acid in the growth of bacteria. Biochim Biophys Acta. 1960 Jul 29;42:99–116. doi: 10.1016/0006-3002(60)90757-5. [DOI] [PubMed] [Google Scholar]
- Nakada D. Ribosome formation by puromycin-treated Bacillus subtilis. Biochim Biophys Acta. 1965 Jul 15;103(3):455–465. doi: 10.1016/0005-2787(65)90138-3. [DOI] [PubMed] [Google Scholar]
- Pestka S., Nirenberg M. Regulatory mechanisms and protein synthesis. X. Codon recognition on 30 S ribosomes. J Mol Biol. 1966 Oct 28;21(1):145–171. doi: 10.1016/0022-2836(66)90085-4. [DOI] [PubMed] [Google Scholar]
- SCHAECHTER M., MAALOE O., KJELDGAARD N. O. Dependency on medium and temperature of cell size and chemical composition during balanced grown of Salmonella typhimurium. J Gen Microbiol. 1958 Dec;19(3):592–606. doi: 10.1099/00221287-19-3-592. [DOI] [PubMed] [Google Scholar]
- SELLS B. H. RNA SYNTHESIS AND RIBOSOME PRODUCTION IN PUROMYCIN-TREATED CELLS. Biochim Biophys Acta. 1964 Feb 17;80:230–241. doi: 10.1016/0926-6550(64)90095-7. [DOI] [PubMed] [Google Scholar]
- Schlessinger D., Mangiarotti G., Apirion D. The formation and stabilization of 30S and 50S ribosome couples in Escherichia coli. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1782–1789. doi: 10.1073/pnas.58.4.1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu C. T., Hirsh D. The stimulatory effect of ammonium or potassium ions on the activity of leucyl-tRNA synthetase from Escherichia coli. Biochim Biophys Acta. 1967 Jun 20;142(1):149–154. doi: 10.1016/0005-2787(67)90523-0. [DOI] [PubMed] [Google Scholar]



