Abstract
Ligation of cytotoxic T lymphocyte antigen 4 (CTLA4) appears to inhibit T cell responses. Four mechanisms have been proposed to explain the inhibitory activity of CTLA4: competition for B7-1 and B7-2 binding by CD28; sequestration of signaling molecules away from CD28 via endocytosis; delivery of a signal that antagonizes a CD28 signal; and delivery of a signal that antagonizes a T cell receptor (TCR) signal. As three of these potential mechanisms involve functional antagonism of CD28, an experimental model was designed to determine whether CTLA4 could inhibit T cell function in the absence of CD28. TCR transgenic/recombinase activating gene 2–deficient/CD28–wild-type or CD28-deficient mice were generated and immunized with an antigen-expressing tumor. Primed T cells from both types of mice produced cytokines and proliferated in response to stimulator cells lacking B7 expression. However, whereas the response of CD28+/+ T cells was augmented by costimulation with B7-1, the response of the CD28−/− T cells was strongly inhibited. This inhibition was reversed by monoclonal antibody against B7-1 or CTLA4. Thus, CTLA4 can potently inhibit T cell activation in the absence of CD28, indicating that antagonism of a TCR-mediated signal is sufficient to explain the inhibitory effect of CTLA4.
Keywords: T lymphocytes, costimulation, cytotoxic T lymphocyte antigen 4, B7, transgenic/knockout
CD28 is a costimulatory receptor expressed constitutively on naive and on differentiated T cells. Ligation of CD28 on naive T cells using specific mAb or via its natural ligands B7-1 or B7-2 augments cytokine production and proliferation, and promotes differentiation into effector cells (1). In vitro, CD28 ligation appears to promote T cell survival via upregulation of Bcl-xL (2). CD28 costimulation also prevents induction of anergy in differentiated Th1 clones (3–5), whereas proliferation of differentiated Th2 cells can be relatively CD28 independent (5).
CD28-deficient mice have been generated by homologous recombination (6). Although B7-dependent activation of naive CD28−/− T cells in vitro does not occur (7), CD28−/− mice do appear capable of mounting effective cellular immune responses in vivo, albeit with slower kinetics (8). Thus, either some immune responses can occur in the absence of any costimulatory receptor engagement, or alternative costimulator molecules exist that can compensate for the absence of CD28 in vivo.
Cytotoxic T lymphocyte antigen 4 (CTLA4) is an alternative counterreceptor for B7-1 and B7-2 that is expressed on T cells only after activation and that binds these ligands at higher affinity than does CD28 (9, 10). CTLA4 expression is induced transcriptionally upon TCR–CD3 ligation, but accumulates predominantly as an intracellular pool in activated cells. A fraction of CTLA4 molecules is constantly transported to the surface and is subsequently endocytosed by a clathrin-mediated mechanism (11, 12). In contrast to CD28, ligation of CTLA4 appears to inhibit T cell activation events (13). Perhaps the best evidence that CTLA4 is a negative regulator of T cell activation has come from the generation of CTLA4-deficient mice, which exhibit a profound lymphoproliferative syndrome resulting in death within several weeks of age (14, 15). Other experimental models analyzing CTLA4 function have used stimulation of normal LN T cells with specific mAb against CD3 and CD28, with or without the addition of anti-CTLA4 mAb (16, 17). Under these conditions, CTLA4 ligation inhibits IL-2 production and proliferation, prevents sustained IL-2R induction, and induces G1 cell cycle arrest. We have observed recently that differentiated Th1 and Th2 clones are both susceptible to inhibition by CTLA4 (Alegre, M.L., H. Shiels, C.B. Thompson, and T.F. Gajewski, manuscript submitted for publication). Cross-linking of CTLA4 inhibited production of all measurable cytokines by both T cell subsets, suggesting that the biochemical mechanism by which CTLA4 antagonizes T cell activation involves a central signaling pathway vital for multiple TCR-mediated functions.
At least four mechanisms have been proposed to explain the inhibitory activity of CTLA4: competition for ligand access to CD28, thus preventing CD28 from binding to B7-1 and B7-2; “stealing” of signaling molecules away from CD28 via the rapid endocytosis of CTLA4; delivery of a signal that antagonizes or aborts a CD28 signal; and delivery of a signal that antagonizes or aborts a TCR-delivered signal. As the first three of these putative mechanisms depend on the presence of CD28, an experimental model was designed to determine whether CTLA4 could inhibit T cell function in the absence of CD28.
The 2C TCR transgenic (Tg) mouse expresses a TCR that recognizes the alloantigen Ld bound to a ubiquitous self peptide from α-ketoglutarate dehydrogenase (18). These mice were bred to a recombinase activating gene 2 (Rag2)- deficient background, to extinguish expression of endogenous TCR genes and to ensure a naive T cell phenotype. 2C × Rag2-deficient (2C/Rag) mice successfully reject Ld-expressing P815 tumors in a B7-independent fashion (19). 2C/Rag mice were also crossed with CD28-deficient mice (generating 2C/Rag/CD28−/− mice), which were found to reject P815 tumors in vivo as well. The majority of T cells from both 2C/Rag/CD28+/+ and 2C/Rag/ CD28−/− mice appeared to be primed after tumor rejection, and produced cytokines and proliferated in vitro in response to P815 stimulator cells lacking B7 expression. However, whereas the response of primed CD28+/+ T cells in vitro was augmented by costimulation with B7-1, the response of primed CD28−/− T cells was strongly inhibited. This inhibition was reversed by mAb against B7-1 or CTLA4. Thus, CTLA4 can potently inhibit the activation of primed T cells in the absence of CD28, indicating that antagonism of a TCR-mediated signal is sufficient to explain its inhibitory effect.
Materials and Methods
Mice.
2C TCR Tg mice were developed as described previously (18) and obtained from Dr. D. Loh (Washington University School of Medicine, St. Louis, MO). These were intercrossed with Rag2-deficient mice (kindly provided by Dr. C. Simon, University of Chicago) to obtain 2C × Rag2−/− mice (2C/Rag mice). CD28-deficient mice (obtained from Drs. J. Bluestone and C. Thompson, University of Chicago) were bred to 2C/Rag mice to obtain 2C × Rag2−/− × CD28−/− mice (2C/Rag/ CD28−/− mice). All Tg mice were bred in a specific pathogen– free barrier facility at the University of Chicago. Mice were used at 6–8 wk of age for experiments.
Cell Lines and Transfectants.
P815 mastocytoma cells were cultured in DMEM supplemented with 10% FCS and incubated at 37°C in an 8% CO2 atmosphere. P815.B7-1 was derived and maintained as described (Fields, P.E., S. Wolf, T.F. Gajewski, and F.W. Fitch, manuscript submitted for publication). P1.HTR is a highly transfectable variant of P815 that grows semiadherent in tissue culture and as a solid tumor mass when implanted subcutaneously in vivo (20), and was used for in vivo tumor rejection.
In Vivo Priming of 2C/Rag/CD28–wild-type or CD28-deficient Mice.
2C/Rag or 2C/Rag/CD28−/− mice were immunized subcutaneously in the left flank with 10 × 106 living P1.HTR tumor cells in 100 μl Dulbecco's PBS. The animals were killed 10 d after immunization, and splenic CD8+ T cells were purified. After confirmation of purity by flow cytometry, cells were stimulated to assess proliferation and cytokine production.
Purification of CD8+ T Cells.
CD8+ cells were isolated by negative enrichment using a magnetic separation system (StemCell Technologies, Vancouver, B.C., Canada) according to the manufacturer's protocol. The purity of the eluted fraction, determined by flow cytometry using the clonotypic Ab 1B2 or anti-CD8 mAb, ranged between 92 and 96%.
Antibodies and Flow Cytometry.
For flow cytometry, PE-coupled anti-CTLA4 mAb 4F10 (PharMingen, San Diego, CA), FITC-coupled 1B2 (prepared in our laboratory), and biotinylated anti-CD28 mAb (PharMingen) were used. Expression of the TCR and of CD28 was determined by normal surface staining, whereas CTLA4 expression was assessed after saponin permeabilization, as described (12). In both cases, nonspecific staining was reduced by blocking with the anti-FcRγ mAb 2.4G2. Samples were analyzed on a FACScan® (Becton Dickinson, San Jose, CA), and data were analyzed using Lysis II software (Becton Dickinson). Live cells were selected for analysis using forward versus side scatter gating. Nonconjugated mAb against B7-1 (16-10A1) or CTLA4 (4F10) were used for in vitro blocking experiments.
T Cell Proliferation Assay.
CD8+ T cells (3 × 104) were mixed with 3 × 104 mitomycin C–treated P815 or P815.B7-1 tumor cells along with the indicated concentrations of various reagents in microtiter plates in a final volume of 200 μl. Cultures were pulsed at various times with 1 μCi of [3H]thymidine and harvested 8 h later onto glass filters using a 96-well plate harvester (Packard, Meriden, CT). Incorporated radioactivity was assessed using a Top Count microplate scintillation counter (Packard).
Stimulation of T Cells for Lymphokine Production and CTLA4 Expression.
For lymphokine production, purified CD8+ T cells (2.5 × 105) were stimulated with mitomycin C–treated P815 or P815-B7.1 tumor cells (2.5 × 105) in a total volume of 1 ml. In some experiments, stimulation was performed in the presence of anti-CTLA4 mAb (50 μg/ml final), anti–B7-1 mAb (1 μg/ml final), or the combination. Supernatants were collected at 24 h to determine IL-2 production and at 48 h to assess IFN-γ content.
To stimulate for measurement of CTLA4 expression, purified CD8+ T cells (5 × 105) were restimulated in vitro in the presence of mitomycin C–treated P815-B7.1 (2.5 × 105) tumor cells for 48–72 h in a total volume of 1.5 ml. Flow cytometric analysis was then performed on permeabilized cells using anti-CTLA4 mAb.
Lymphokine Assays.
IFN-γ concentrations were determined using an ELISA developed by Dr. R. Schreiber (Washington University), who also provided the reagents. IL-2 was measured using an ELISA with antibody pairs obtained from PharMingen. Concentrations were expressed in U/ml as determined using the respective recombinant cytokines as standards.
Results
Characterization of 2C/Rag/CD28–wild-type and CD28-deficient Mice.
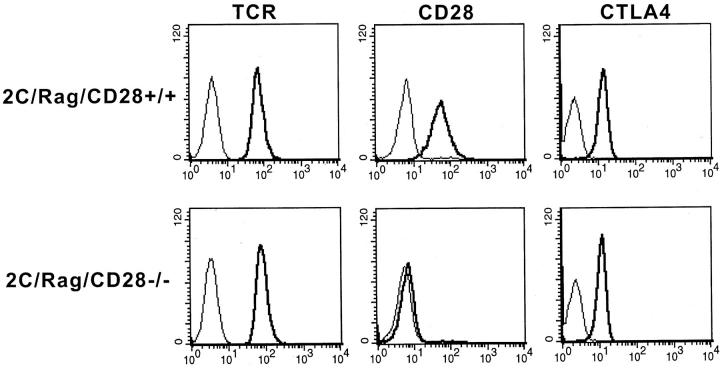
2C TCR Tg mice were bred to a Rag2-deficient background to extinguish expression of endogenous TCR genes and ensure a naive phenotype (reference 19, and data not shown). We have observed previously that 2C/Rag mice vigorously reject Ld-expressing P815 tumors, resulting in priming of the majority of the T cells in the mouse based on surface expression of CD44 and CD62L as well as induction of cytolytic activity (19). Thus, tumor rejection provides a convenient means by which to immunize these mice. Priming occurred even in mice treated with CTLA4-Ig, arguing that it can be achieved in the absence of B7–CD28 interactions. Indeed, 2C/Rag/CD28−/− mice also were found to reject P815 tumors in vivo, resulting in comparable induction of activation markers and cytolytic activity as is seen with the 2C/Rag/CD28+/+ mice (data not shown). Use of primed T cells from these immunized mice provided a means by which to examine a potential inhibitory role of CTLA4 in the absence of CD28. To determine whether expression of CTLA4 could indeed be induced in 2C/Rag/CD28−/− T cells, flow cytometric analysis was performed after restimulation in vitro with P815 cells. As shown in Fig. 1, surface expression of the TCR, as detected by the clonotypic mAb 1B2, was comparable on the CD28+/+ and CD28−/− T cells, whereas CD28 was expressed only on the CD28+/+ T cells, as expected. Interestingly, total cellular CTLA4 expression analyzed on permeabilized cells was comparable in T cells from both mice (Fig. 1), indicating that CTLA4 indeed has an opportunity to antagonize T cell function even in CD28-deficient T cells.
Figure 1.
Flow cytometric analysis of T cells from mutant mice. 2C/Rag/CD28+/+ and CD28−/− mice were immunized by in vivo rejection of P1.HTR tumor cells. Splenic T cells were purified and restimulated for 2 d with mitomycin C–treated P815 cells to assess expression of TCR, CD28, and CTLA4. Flow cytometric analysis was performed after surface staining using the clonotypic TCR antibody 1B2 or the anti-CD28 mAb 37.51, or after intracellular staining with the anti-CTLA4 mAb 4F10. Similar results were obtained in three separate experiments.
Response of 2C/Rag/CD28+/+ or 2C/Rag/CD28−/− T Cells to B7-1 Costimulation In Vitro.
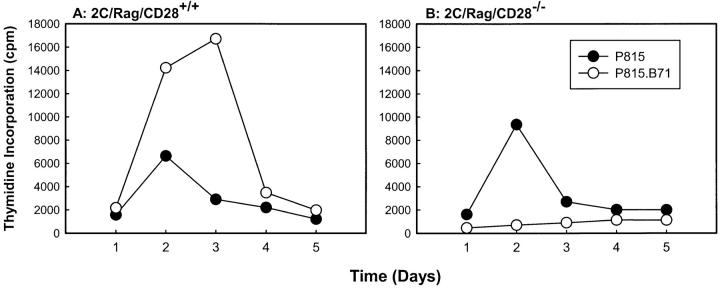
We have observed recently that in contrast to naive T cells which are dependent on CD28 costimulation for primary activation in vitro, secondary (primed) T cells proliferate and produce IL-2 after TCR stimulation alone (Fields, P.E., S. Wolf, T.F. Gajewski, and F.W. Fitch, manuscript submitted for publication). To determine the effect of B7-1 on the response of primed T cells that express or do not express CD28, in vivo–primed 2C/Rag/CD28–wild-type or CD28-deficient T cells were stimulated with P815 or P815.B7-1 cells, and proliferation was measured over time. As shown in Fig. 2 A, primed CD28+/+ T cells proliferated in response to P815, with a peak response observed on day 2. This proliferation was augmented and extended if B7-1 was expressed on the P815 stimulator cells. The CD28−/− cells also proliferated in response to nontransfected P815 cells (Fig. 2 B). However, in this case, expression of B7-1 on the stimulator cells resulted in nearly complete inhibition of the proliferative response.
Figure 2.
Time course of proliferation of CD28+/+ or CD28−/− TCR Tg T cells in response to B7-1+ or B7-1− stimulator cells. CD8+ T cells purified by negative selection from primed CD28+/+ or CD28−/− 2C/Rag2−/− mice were stimulated with mitomycin C–treated P815 or P815.B71 cells. Replicate cultures were pulsed daily with [3H]thymidine and harvested, and incorporated radioactivity was assessed. Similar results were obtained in at least two experiments.
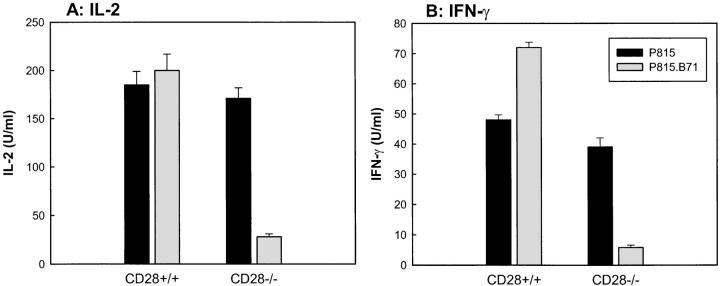
When cytokine production was examined, the effect of B7-1 on the response of CD28+/+ and CD28−/− T cells was similar to the effect seen on the proliferation of these cells. As shown in Fig. 3, P815.B7-1 cells generally stimulated equivalent or increased levels of IL-2 (A) and IFN-γ (B) production compared with nontransfected P815 cells when primed 2C/Rag/CD28+/+ T cells were examined. The magnitude of augmentation of detectable IL-2 production by B7-1 was variable between experiments, presumably due to variable rates of IL-2 consumption by the proliferating T cells. In contrast, when 2C/Rag/CD28−/− T cells were examined, P815.B7-1 cells induced profoundly reduced levels of IL-2 and IFN-γ release compared with those induced by nontransfected P815 stimulators (Fig. 3). These reduced levels were usually near the background levels of detection for the respective assays. Thus, the effect of B7-1 on primed CD28-deficient T cells is inhibitory rather than stimulatory for cytokine production and proliferation.
Figure 3.
Cytokine production by CD28+/+ or CD28−/− TCR Tg T cells in response to B7-1+ or B7-1− stimulator cells. CD8+ T cells purified as in Fig. 2 were stimulated with mitomycin C–treated P815 or P815.B71 cells. Supernatants were collected, and IL-2 and IFN-γ content were assessed. Similar results were obtained in at least three experiments.
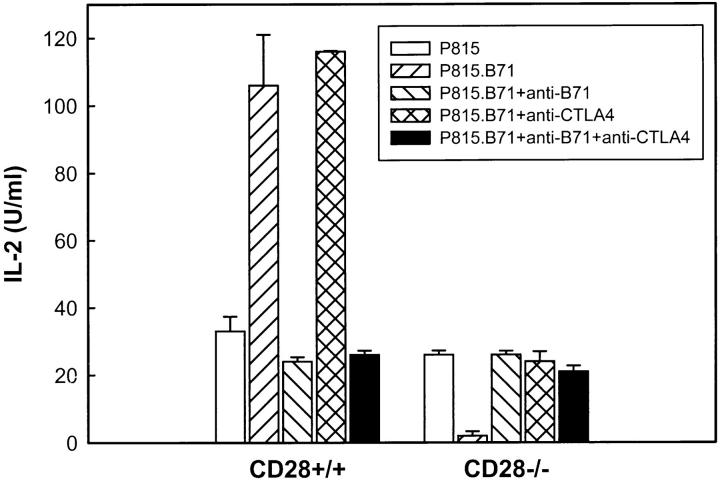
Inhibition of Activation of 2C/Rag/CD28−/− T Cells by P815.B7-1 Cells Is Reversed by Anti–B7-1 or Anti-CTLA4 mAb.
To confirm that the decreased ability of P815.B7-1 to stimulate 2C/Rag/CD28−/− T cells was directly mediated by B7-1 and CTLA4, neutralizing mAbs against these respective molecules were included during T cell stimulation. As shown in Fig. 4, the augmented IL-2 production by CD28+/+ cells promoted by B7-1 costimulation was blocked by anti–B7-1 mAb. In contrast, the decreased IL-2 production by CD28−/− T cells in the presence of B7-1 costimulation was reversed with the addition of anti–B7-1 mAb. Likewise, whereas anti-CTLA4 mAb had a slight augmentative effect on IL-2 production by the CD28+/+ T cells, the decreased IL-2 production of CD28−/− T cells in the presence of P815.B7-1 was reversed completely in the presence of anti-CTLA4 mAb (Fig. 4). The combination of both mAbs gave similar results to those seen with anti– B7-1 mAb alone. Thus, B7-1 expression on stimulator cells apparently can inhibit the activation of CD28-deficient T cells via ligation of CTLA4.
Figure 4.
Inhibition of cytokine production of CD28−/− T cells by B7-1 transfectants is prevented by anti–B7-1 or by anti-CTLA4 mAb. T cell stimulations were performed as in Fig. 3 without additions, or in the presence of anti–B7-1 mAb, anti-CTLA4 mAb, or the combination. IL-2 production was determined at 24 h. Similar results were obtained for IFN-γ production (data not shown), and in at least two separate experiments.
Discussion
Most experimental evidence obtained to date is consistent with a negative regulatory function of CTLA4 on T cells. Blockade of CTLA4 interactions using neutralizing mAb augments T cell proliferation in vitro (21), promotes tumor rejection in vivo (22), and appears to prevent antigen-specific tolerance in vivo (23, 24). Conversely, cross-linking of CTLA4 inhibits IL-2 production and proliferation of normal LN T cells in vitro (16, 25). In each of these settings, T cell responses were first optimized by ensuring CD28 ligation, either with anti-CD28 mAb or via presumed interaction with natural ligands, and CTLA4 was manipulated within that context. Therefore, these experimental systems have led to the proposal that CTLA4 may antagonize a CD28-mediated signal. Such a model appears intuitive as well; B7 ligands could first engage the positive receptor CD28, and then subsequent induction of CTLA4 could force the same B7 ligands to mediate a negative response through CTLA4, reversing the CD28 effect.
In this study, we took advantage of the observation that primed T cells exhibit a relaxed requirement for CD28 costimulation, such that substantial cytokine production and proliferation can be observed in response to TCR ligation alone. In this way, the potential for CTLA4 to inhibit T cell function in the absence of CD28 could be examined. In addition, by using primed CD28-deficient T cells, CTLA4 could be stimulated with the natural ligand B7-1 rather than using cross-linked anti-CTLA4 mAb. Indeed, B7-1 clearly inhibited rather than augmented T cell activation in the absence of CD28, in a CTLA4-dependent fashion. These data support previous observations that CTLA4 is a negative regulator of T cell activation. However, these results also indicate that CTLA4 can exert its inhibitory effect in the absence of CD28.
It is likely that CTLA4 antagonizes a signal delivered through the TCR–CD3 complex. However, our studies do not exclude the possibility that CTLA4 signaling can inhibit the function of an undefined alternative costimulatory receptor, or that CTLA4 can additionally inhibit a CD28 signal. Given the conclusions of this study, we have examined recently whether cross-linking of CTLA4 can abort ongoing signaling events initiated through CD3 cross-linking alone. Preliminary results have revealed that the normal prolonged activation of extracellular signal–regulated kinase (ERK)1 and ERK2 in response to CD3 ligation becomes inactivated once CTLA4 expression emerges and is itself cross-linked (data not shown). These results are consistent with a recent published report indicating that, using 3-d–stimulated T cells that have maximal surface expression of CTLA4, restimulation in the presence of CTLA4 cross-linking results in reduced activation of ERK and jun NH2-terminal kinase (26). Thus, blockade of a TCR–CD3-mediated signal is sufficient to explain the inhibitory effect of CTLA4.
The biochemical mechanism by which CTLA4 antagonizes T cell responses is not yet understood. Like CD28, the cytoplasmic tail of CTLA4 contains a binding motif for phosphatidylinositol (PI)3-kinase, and interaction between phosphorylated CTLA4 and PI3-kinase has been demonstrated (27, 28). CTLA4 can be phosphorylated in the presence of activated Lck in vivo (29). However, since CD28 also binds PI3-kinase, it is unclear how this interaction would mediate a negative signal for CTLA4. The protein tyrosine phosphatase PTP1D has been reported to interact with CTLA4 (30), although this interaction may be nonspecific (data not shown). Nonetheless, it is attractive to speculate that CTLA4 may bring a phosphatase into the TCR–CD3 complex through cocapping, thus dephosphorylating a key substrate that is necessary to sustain T cell signaling. We have observed recently, using differentiated Th1 and Th2 clones, that production of all cytokines was modulated by CTLA4 manipulation. Since activation of ERK1/ERK2 is easily demonstrated in both T cell subsets, and since this pathway may be required for cytokine gene expression, a substrate downstream from the TCR but proximal to ERK1/ERK2 may be one target of CTLA4 inhibition. This target is likely to be downstream from CD3ζ phosphorylation and ZAP70 recruitment, since these events have been reported to be unaffected by CTLA4 ligation (26).
In summary, our results support a model in which CTLA4 ligation antagonizes a TCR-driven signal rather than a CD28-dependent signaling event. Thus, rather than CD28 costimulation augmenting a TCR signal and CTLA4 inhibiting this CD28 costimulus, CD28 and CTLA4 may instead antagonistically regulate the same TCR-mediated events. A better understanding of how CTLA4 functions should aid in the development of agents that either inhibit or augment ongoing immune responses.
Acknowledgments
The authors thank J. Bluestone and C. Thompson for providing the CD28-deficient mice, C. Simon for providing the Rag2-deficient mice, and J. O'Keefe and A. Ashikari for their assistance in mouse screening.
Footnotes
T. Gajewski is a recipient of a McDonnell Foundation Scholar Award in molecular oncology. This work was also supported by grant P01 AI35294-05 from the National Institutes of Health, and by a grant from Pfizer Corporation.
References
- 1.Lenschow DJ, Walunas TL, Bluestone JA. CD28/B7 system of T cell costimulation. Annu Rev Immunol. 1996;14:233–258. doi: 10.1146/annurev.immunol.14.1.233. [DOI] [PubMed] [Google Scholar]
- 2.Boise LH, Minn AJ, Noel PJ, June CH, Accavitti MA, Lindsten T, Thompson CB. CD28 stimulation can enhance T cell survival by inducing expression of Bcl-xL . Immunity. 1995;3:87–98. doi: 10.1016/1074-7613(95)90161-2. [DOI] [PubMed] [Google Scholar]
- 3.Harding FA, McArthur JG, Gross JA, Raulet DH, Allison JP. CD28-mediated signalling co-stimulates murine T cells and prevents induction of anergy in T-cell clones. Nature. 1992;356:607–609. doi: 10.1038/356607a0. [DOI] [PubMed] [Google Scholar]
- 4.Tan P, Anasetti C, Hansen JA, Melrose J, Brunvand M, Bradshaw J, Ledbetter JA, Linsley PS. Induction of alloantigen-specific hyporesponsiveness in human T lymphocytes by blocking interaction of CD28 with its natural ligand B7/BB1. J Exp Med. 1993;177:165–173. doi: 10.1084/jem.177.1.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gajewski TF, Lancki DW, Stack R, Fitch FW. “Anergy” of TH0 helper T lymphocytes induces downregulation of TH1 characteristics and a transition to a TH2-like phenotype. J Exp Med. 1994;179:481–491. doi: 10.1084/jem.179.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shahinian A, Pfeffer K, Lee KP, Kundig TM, Kishihara K, Wakeham A, Kawai K, Ohashi PS, Thompson CB, Mak TW. Differential T cell costimulatory requirements in CD28-deficient mice. Science. 1993;261:609–612. doi: 10.1126/science.7688139. [DOI] [PubMed] [Google Scholar]
- 7.Green JM, Noel PJ, Sperling AI, Walunas TL, Gray GS, Bluestone JA, Thompson CB. Absence of B7-dependent responses in CD28-deficient mice. Immunity. 1994;1:501–508. doi: 10.1016/1074-7613(94)90092-2. [DOI] [PubMed] [Google Scholar]
- 8.Kawai K, Shahinian A, Mak TW, Ohashi PS. Skin allograft rejection in CD28-deficient mice. Transplantation (Baltimore) 1996;61:352–355. doi: 10.1097/00007890-199602150-00003. [DOI] [PubMed] [Google Scholar]
- 9.Linsley PS, Brady W, Urnes M, Grosmaire L, Damle NK, Ledbetter JA. CTLA-4 is a second receptor for the B cell activation antigen B7. J Exp Med. 1991;174:561–569. doi: 10.1084/jem.174.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Linsley PS, Greene JL, Brady W, Bajorath J, Ledbetter JA, Peach R. Human B7-1 (CD80) and B7-2 (CD86) bind with similar avidities but distinct kinetics to CD28 and CTLA-4 receptors. Immunity. 1994;1:793–801. doi: 10.1016/s1074-7613(94)80021-9. [DOI] [PubMed] [Google Scholar]
- 11.Chuang E, Alegre ML, Duckett CS, Noel PJ, Vander MG, Heiden, Thompson CB. Interaction of CTLA-4 with the clathrin-associated protein AP50 results in ligand-independent endocytosis that limits surface expression. J Immunol. 1997;159:144–151. [PubMed] [Google Scholar]
- 12.Alegre ML, Noel PJ, Eisfelder BJ, Chuang E, Clark MR, Reiner SL, Thompson CB. Regulation of surface expression and intracellular expression of CTLA4 on mouse T cells. J Immunol. 1996;157:4762–4770. [PubMed] [Google Scholar]
- 13.Thompson CB, Allison JP. The emerging role of CTLA-4 as an immune attenuator. Immunity. 1997;7:445–450. doi: 10.1016/s1074-7613(00)80366-0. [DOI] [PubMed] [Google Scholar]
- 14.Tivol EA, Borriello F, Schweitzer AN, Lynch WP, Bluestone JA, Sharpe AH. Loss of CTLA4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA4. Immunity. 1995;3:541–547. doi: 10.1016/1074-7613(95)90125-6. [DOI] [PubMed] [Google Scholar]
- 15.Waterhouse P, Penninger JM, Timms E, Wakeham A, Shahinian A, Lee KP, Thompson CB, Griesser H, Mak TW. Lymphoproliferative disorders with early lethality in mice deficient in CTLA-4. Science. 1995;270:985–988. doi: 10.1126/science.270.5238.985. [DOI] [PubMed] [Google Scholar]
- 16.Walunas TL, Bakker CY, Bluestone JA. CTLA-4 ligation blocks CD28-dependent T cell activation. J Exp Med. 1996;183:2541–2550. doi: 10.1084/jem.183.6.2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krummel MF, Allison JP. CTLA-4 engagement inhibits IL-2 accumulation and cell cycle progression upon activation of resting T cells. J Exp Med. 1996;183:2533–2540. doi: 10.1084/jem.183.6.2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sha WC, Nelson CA, Newberry RD, Kranz DM, Russell JH, Loh DY. Selective expression of an antigen receptor on CD8-bearing T lymphocytes in transgenic mice. Nature. 1988;335:271–275. doi: 10.1038/335271a0. [DOI] [PubMed] [Google Scholar]
- 19.Manning TC, Rund LA, Gruber MM, Fallarino F, Gajewski TF, Kranz DM. Antigen recognition and allogeneic tumor rejection in CD8+ TCR transgenic/Rag−/−mice. J Immunol. 1997;159:4665–4675. [PubMed] [Google Scholar]
- 20.Gajewski TF, Uyttenhove C, Fallarino F, Boon T. Tumor rejection requires a CTLA4 ligand provided by the host or expressed on the tumor: superiority of B7-1 over B7-2 for active tumor immunization. J Immunol. 1996;156:2909–2917. [PubMed] [Google Scholar]
- 21.Walunas TL, Lenschow DJ, Bakker CY, Linsley PS, Freeman GJ, Green JM, Thompson CB, Bluestone JA. CTLA-4 can function as a negative regulator of T cell activation. Immunity. 1994;1:405–413. doi: 10.1016/1074-7613(94)90071-x. [DOI] [PubMed] [Google Scholar]
- 22.Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271:1734–1736. doi: 10.1126/science.271.5256.1734. [DOI] [PubMed] [Google Scholar]
- 23.Perez VL, Van Parijs L, Biuckians A, Zheng XX, Strom TB, Abbas AK. Induction of peripheral T cell tolerance in vivo requires CTLA-4 engagement. Immunity. 1997;6:411–417. doi: 10.1016/s1074-7613(00)80284-8. [DOI] [PubMed] [Google Scholar]
- 24.Bluestone JA. Is CTLA-4 a master switch for peripheral T cell tolerance? . J Immunol. 1997;158:1989–1993. [PubMed] [Google Scholar]
- 25.Krummel MF, Allison JP. CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J Exp Med. 1995;182:459–465. doi: 10.1084/jem.182.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Calvo CR, Amsen D, Kruisbeek AM. Cytotoxic T lymphocyte antigen 4 (CTLA-4) interferes with extracellular signal-regulated kinase (ERK) and jun NH2-terminal kinase (JNK) activation, but does not affect phosphorylation of T cell receptor ζ and ZAP70. J Exp Med. 1997;186:1645–1653. doi: 10.1084/jem.186.10.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schneider H, Prasad KVS, Shoelson SE, Rudd CE. CTLA-4 binding to the lipid kinase phosphatidylinositol 3-kinase in T cells. J Exp Med. 1995;181:351–355. doi: 10.1084/jem.181.1.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hutchcroft JE, Bierer BE. Signaling through CD28/CTLA-4 family receptors. Puzzling participation of phosphatidylinositol-3 kinase. J Immunol. 1996;157:4071–4074. [PubMed] [Google Scholar]
- 29.Rudd CE, Hu H, Raab M, Cefai D, Schneider H. CD28 and CTLA-4 regulation of interleukin 2 production in T cells. J Allergy Clin Immunol. 1997;99:S206. . (Abstr.) [Google Scholar]
- 30.Marengere LEM, Waterhouse P, Duncan GS, Mittrucker HW, Feng GS, Mak TW. Regulation of T cell receptor signaling by tyrosine phosphatase SYP association with CTLA-4. Science. 1996;272:1170–1173. doi: 10.1126/science.272.5265.1170. [DOI] [PubMed] [Google Scholar]