Abstract
Cytotoxic T lymphocyte antigen 4 (CTLA4) appears to negatively regulate T cell activation. One mechanism by which CTLA4 might antagonize T cell function is through inhibition of CD28 signaling by competing for their shared ligands B7-1 and B7-2. In addition, CTLA4 ligation could initiate a signaling cascade that inhibits T cell activation. To address whether CTLA4 could inhibit immune responses in the absence of CD28, rejection of heart allografts was studied in CD28-deficient mice. H-2q hearts were transplanted into allogeneic wild-type or CD28-deficient mice (H-2b). Graft rejection was delayed in CD28-deficient compared with wild-type mice. Treatment of wild-type recipients with CTLA4-immunoglobulin (Ig), or with anti–B7-1 plus anti–B7-2 mAbs significantly prolonged allograft survival. In contrast, treatment of CD28-deficient mice with CTLA4-Ig, anti–B7-1 plus anti–B7-2 mAbs, or a blocking anti-CTLA4 mAb induced acceleration of allograft rejection. This increased rate of graft rejection was associated with more severe mononuclear cell infiltration and enhanced levels of IFN-γ and IL-6 transcripts in donor hearts of untreated wild-type and CTLA4-Ig– or anti-CTLA4 mAb–treated CD28-deficient mice. Thus, the negative regulatory role of CTLA4 extends beyond its potential ability to prevent CD28 activation through ligand competition. Even in the absence of CD28, CTLA4 plays an inhibitory role in the regulation of allograft rejection.
Keywords: cytotoxic T lymphocyte antigen 4, CD28-deficient, cytotoxic T lymphocyte antigen 4–immunoglobulin, transplantation, T lymphocyte
Cytotoxic T lymphocyte antigen 4 (CTLA4) and CD28 are T cell molecules that share sequence homology and bind to the same ligands, B7-1 (CD80) and B7-2 (CD86). Unlike the constitutively expressed CD28, CTLA4 expression is induced on T lymphocytes by TCR stimulation, and its upregulation in vitro depends on the presence of IL-2 and CD28 ligation (1). Furthermore, in contrast to the costimulatory activity of CD28, CTLA4 appears to function as a negative regulator of T cell activation (2). CTLA4 blocking antibodies administered in vivo to wild-type mice have been reported to increase antitumor (3) and antiparasite responses (4), to accelerate the onset of diabetes in TCR transgenic nonobese diabetic mice (NOD mice; reference 5), and to exacerbate disease in an experimental allergic encephalomyelitis model (EAE; reference 6). In addition, CTLA4-deficient mice develop a lymphoproliferative disease (7, 8), whereas CD28-deficient T cells exhibit decreased proliferative responses to mitogens (9).
The mechanisms underlying the inhibitory activity of CTLA4 are not clearly understood. It has been proposed that CTLA4 decreases T cell responses by inhibiting CD28 signaling (10). This may occur either through preferential binding of CTLA4 to B7-1 and B7-2, as the affinity of CTLA4 for B7 family members is ∼10 times greater than that of CD28 (11); through competition for common intracellular enzymes (for example, both CD28 [12] and CTLA4 [13] can bind phosphatidylinositol 3-kinase [PI3-kinase]); or through the activation of specific molecules that might directly inhibit CD28 intracellular signaling. An alternate hypothesis is that CTLA4 may counteract TCR signals independently of CD28. One way to distinguish these possibilities is to analyze the role of CTLA4 in T cells from CD28-deficient mice.
CD28-deficient mice can mount effective immune responses, including the clearance of viruses (9) and the rejection of skin grafts (14), albeit less vigorously than wild-type mice. This suggests that additional, alternative, or compensatory costimulatory mechanisms to CD28 signaling do exist in vivo. It has been reported previously that CTLA4 does not have any function on T cells from CD28-deficient mice during primary stimulations in vitro (15). However, we reasoned that chronic stimulation in vivo might reveal a function for CTLA4 independent of the presence of CD28 on T cells. To address whether CTLA4 could inhibit TCR-driven responses in a CD28-independent manner in vivo, cardiac allografts were transplanted into CD28+/+ and CD28−/− mice under conditions in which CTLA4 binding to B7 family members was prevented. CTLA4 blockade was found to accelerate the acute rejection of cardiac allografts in CD28-deficient mice. This strongly indicates an inhibitory role for CTLA4 that is independent of its potential effects on CD28.
Materials and Methods
Mice.
CD28-deficient mice were generated as described previously (9) and back-crossed to C57BL/6 (H-2b) mice for six generations. CD1 (H-2q) mice were purchased from Charles River Laboratories (Wilmington, MA), and C57BL/6 mice were obtained from The Jackson Laboratory (Bar Harbor, ME). Animals were all housed in a specific pathogen–free facility and used at 10–14 wk of age. Animals received humane care in compliance with the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (Bethesda, MD).
Reagents.
Human CTLA4-Ig, a fusion protein between the extracellular domain of human CTLA4 and the Fc portion of human IgG1, as well as anti–mouse B7-1 and anti–mouse B7-2, were generated by Genetics Institute. L6, a control human IgG1 had been obtained previously from Repligen Corp. (Needham, MA). The blocking anti-CTLA4 mAb 4F10 (2) was purchased from PharMingen (San Diego, CA).
Heart Transplantation.
C57BL/6 CD28+/+ or CD28−/− mice were anesthetized and mechanically ventilated. CD1 donor hearts were heterotopically transplanted into a cervical location using a microvascular technique, as described previously (16). Allograft survival was assessed by daily palpation. Rejection was defined as cessation of heart beat. Animals were treated with CTLA4-Ig (200 μg i.p. on day 2 after transplant), anti–B7-1 plus anti–B7-2 (100 μg i.p. each on days 0, 2, and 4), anti-CTLA4 mAb (100 μg i.p./d for 7 d), or control human IgG1 (200 μg i.p. on day 2).
Flow Cytometry.
Splenocytes from C57BL/6 CD28+/+ and CD28−/− mice (25 × 106 cells) were incubated in upright flasks (Costar Corp., Cambridge, MA) for 6 d in the presence of irradiated (2,000 rads) CD1 splenocytes (25 × 106 cells) in complete medium supplemented with recombinant human IL-2 (20 U/ml) to maintain cell viability. Live cells were restimulated with fresh irradiated CD1 splenocytes (106 each) and human IL-2 (20 U/ml) in 24-well plates (Costar Corp.). After 72 h, cells were stained for surface CD4 and total CTLA4, as described previously (1). Two-color flow cytometry was performed using a FACScan® flow cytometer (Becton Dickinson, Mountain View, CA). Data analysis was performed using CellQuest software (Becton Dickinson).
Histology.
Hearts were harvested and sectioned transversally at the maximal circumference of the ventricle, fixed in 4% formalin, embedded in paraffin, stained with hematoxylin and eosin, and analyzed using an optical microscope.
RNase Protection Assay.
Cardiac grafts harvested at different days after transplantation were quick frozen in dry ice and kept at −80°C until assayed. Frozen hearts were homogenized in 3–5 ml of Trizol (Life Technologies, Inc., Gaithersburg, MD), and RNA was extracted according to the manufacturer's protocol. Samples were normalized by optical density and by visualizing 18S and 28S RNA on a 1% agarose gel before use. The RNase protection assay was performed on 5 μg of RNA/sample using a multiprobe RNase protection assay system (Riboquant; PharMingen). L32 and GAPDH probes were used to assess the total amount of RNA loaded per lane.
Statistical Analysis.
Mean cardiac graft survival and SE were calculated for each group using the Kaplan-Meier estimate. Graft survival in the different groups was compared using the log–rank test. Differences were considered to be statistically significant at a confidence interval of 95% (P <0.05).
Results
Delayed Cardiac Allograft Rejection in CD28-deficient Mice Compared with that in Wild-type Mice.
Hearts from CD1 mice (H-2q) were transplanted into fully allogeneic C57BL/6 wild-type mice (H-2b). The allografts were promptly rejected, as evidenced by cessation of cardiac contraction at 1 wk after transplantation (mean survival ± SE of 6 ± 1 d; Table 1). In contrast, significant prolongation of cardiac allograft survival was observed in C57BL/6 CD28-deficient animals (mean survival of 15 ± 3 d, P <0.001).
Table 1.
Delayed Cardiac Allograft Rejection in CD28-deficient Mice
| Cardiac allograft recipient | Graft survival | |
|---|---|---|
| d | ||
| C57BL/6 wild-type | 5,6,5,7,5,6,5,6,6,6,6 | |
| C57BL/6 CD28-deficient | 12,28,11,10,10,38,10,9,9,17,12,11 |
Wild-type or CD28-deficient mice (H-2b) received a heart allograft (H-2q). Animals were examined daily; rejection was defined by cessation of beating of the allograft, and was confirmed by histology.
CD28-deficient Cells Can Upregulate CTLA4 in Response to Allostimulation.
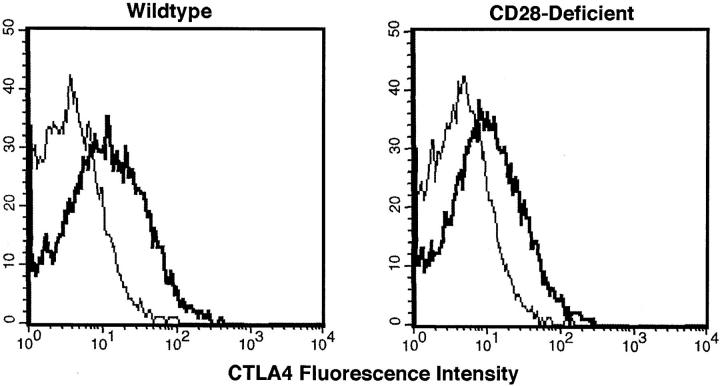
To determine if CTLA4 can be upregulated in CD28-deficient T cells in response to allogeneic challenge, splenocytes from C57BL/6 CD28-expressing and CD28-deficient mice (H-2b) were stimulated in vitro with irradiated allogeneic CD1 (H-2q) splenocytes for 6 d. A secondary stimulation was performed for 3 d on the expanded allogeneic-specific surviving cells, and CTLA4 expression on T cells was assessed by flow cytometry at 72 h. Both wild-type and CD28-deficient T cells displayed similar upregulation of CTLA4 in response to an allogeneic stimulation (Fig. 1). This result was consistent with the possibility that CTLA4 might affect allogeneic immune responses in CD28-deficient mice.
Figure 1.
Upregulation of CTLA4 in response to alloantigen by wild-type and CD28-deficient T cells. Splenocytes from wild-type and CD28−/− mice underwent two rounds of stimulation in vitro with irradiated allogeneic splenocytes, as described in Materials and Methods. 72 h after the second stimulation, samples were analyzed by flow cytometry. The histograms represent the fluorescence emitted by wild-type or CD28-deficient CD4+ cells after staining with hamster IgG (thin line) or anti-CTLA4 mAb (heavy line).
CTLA4-Ig and Anti–B7-1 plus Anti–B7-2 Exert Opposite Effects in CD28+ /+ and CD28− /− Mice.
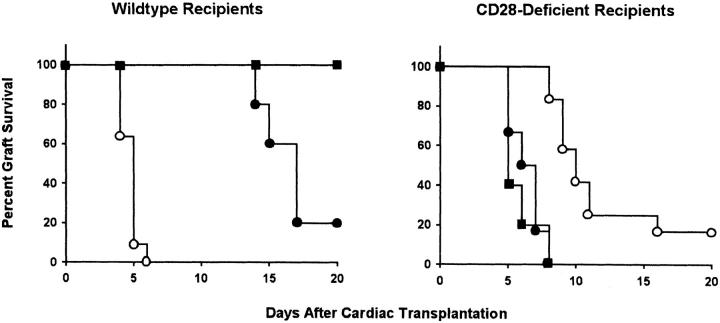
To investigate whether CTLA4 plays a regulatory role in allograft rejection independent of CD28, C57BL/6 CD28+/+ and CD28−/− mice were transplanted with CD1 cardiac allografts and subsequently treated with either CTLA4-Ig, or anti–B7-1 plus anti–B7-2 mAbs. Control animals were left untreated or received control human IgG1. Mean graft survival of IgG1-treated animals was similar to that of untreated mice in both CD28+/+ (mean graft survival = 6 ± 1 d in both cases) and CD28−/− groups (15 ± 3 and 12 ± 1 d, respectively; data not shown). CTLA4-Ig binds to B7 family members, and has been shown to prolong allograft survival in other model systems (17). As shown in Fig. 2, significant prolongation of cardiac allograft survival was achieved by CTLA4-Ig treatment of wild-type mice (mean graft survival = 21 ± 4 d, P <0.001) or by the combination of anti-B7 mAbs (graft survival >24 d for all mice, P <0.05). The difference in allograft survival between wild-type mice treated with CTLA4-Ig and untreated CD28-deficient animals was not statistically significant (P = 0.226). In contrast to its effect in wild-type animals, treatment with CTLA4-Ig markedly accelerated graft rejection in CD28-deficient mice (mean graft survival = 7 ± 1 d, P <0.001). Similar results were obtained in mice treated with a combination of anti–B7-1 plus anti–B7-2 (8 ± 1 d, P <0.001).
Figure 2.
CTLA4-Ig and anti–B7-1 plus anti–B7-2 prolong allograft survival in wild-type mice and accelerate graft rejection in CD28-deficient mice. Wild-type and CD28-deficient mice transplanted with allogeneic hearts were either left untreated (open circles; n = 11 and n = 12, respectively), or treated with CTLA4-Ig (filled circles; n = 5 and n = 6, respectively) or with a combination of anti–B7-1 and anti–B7-2 mAbs (filled squares; n = 3 and n = 5, respectively). As additional controls, transplanted wild-type and CD28-deficient animals were treated with control human IgG1. Graft survival curves in IgG1-treated animals were indistinguishable from those of untreated mice (data not shown).
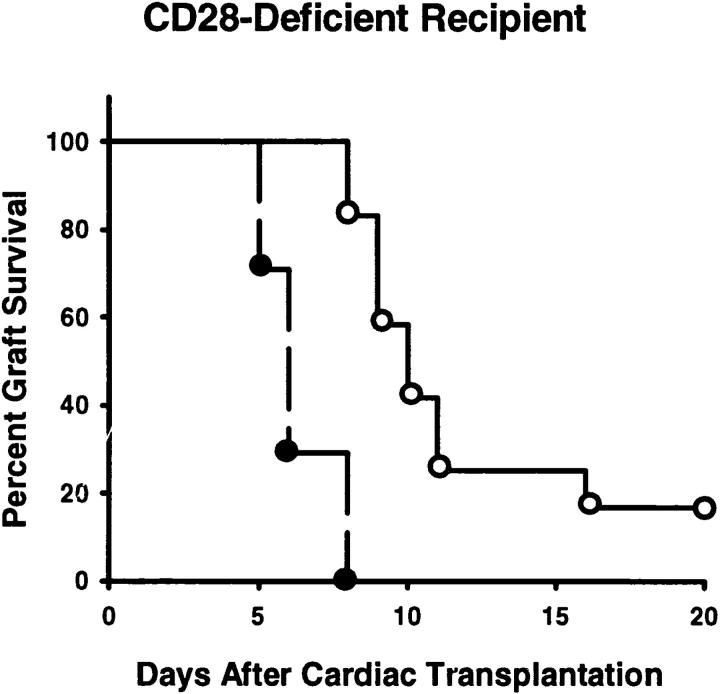
To confirm that CTLA4 was the B7 receptor responsible for the inhibition of allograft responses in CD28-deficient mice, animals were treated with a blocking anti-CTLA4 mAb. Previous results have indicated that whole mAb mediates the same effects as Fab fragments (2), consistent with the notion that this mAb is blocking rather than agonistic. In keeping with the hypothesis that CTLA4 was suppressing T cell responses independent of CD28, treatment with blocking anti-CTLA4 mAb also accelerated graft rejection in CD28-deficient mice (8 ± 1 d, P <0.001; Fig. 3).
Figure 3.
A blocking anti-CTLA4 mAb accelerates cardiac allograft rejection in CD28-deficient mice. CD28-deficient mice transplanted with allogeneic cardiac allografts were either not treated (open circles; n = 12) or treated with blocking anti-CTLA4 mAb (filled circles; n = 7).
CTLA4-Ig and Anti-CTLA4 mAb Increase the Cellular Infiltrate of Allografts in CD28-deficient Mice.
Histological examination of cardiac allografts from untreated mice or from animals treated with CTLA4-Ig or anti-CTLA4 mAb was performed on 7-d grafts removed before loss of palpable heart beat (Fig. 4). Analysis of CD1 allografts in C57BL/6 wild-type and CD28-deficient mice was compatible with different degrees of acute cellular rejection in all cases. The heart parenchyma was infiltrated by mononuclear cells, including lymphocytes and macrophages. These cellular infiltrates were associated with a significant destruction of cardiomyocytes. Allografts removed from untreated wild-type mice (Fig. 4 C) showed more severe histological signs of rejection than those from untreated CD28-deficient mice (Fig. 4 D). In addition, more extensive mononuclear cell infiltration and cardiomyocyte necrosis were observed in cardiac allografts harvested from CTLA4-Ig (Fig. 4 E) or anti-CTLA4–treated (Fig. 4 F) CD28-deficient animals compared with grafts from untreated CD28-deficient mice, correlating with the accelerated graft rejection we observed. Signs of acute cellular rejection were absent from syngeneic grafts, although some fibrosis was observed at later time points (100 d, Fig. 4 B).
Figure 4.
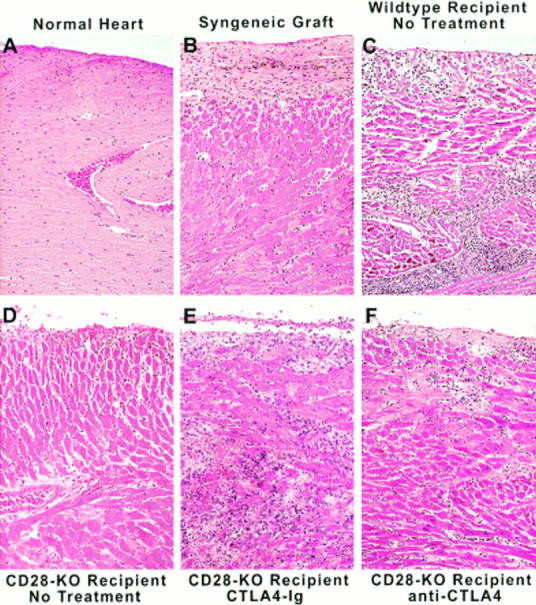
Increased cellular infiltrate on allografts of CTLA4-Ig– and anti-CTLA4–treated CD28-deficient mice. Cardiac allografts were removed from untreated wild-type animals and from untreated or CTLA4-Ig– or anti-CTLA4–treated CD28-deficient recipients on day 7 after transplantation. As controls, a syngeneic graft was removed at 100 d, and an orthotopic heart was taken from a normal mouse. The hearts were processed as described in Materials and Methods, and examined using an optical microscope at a magnification of 200. KO, Knockout.
CTLA4-Ig and Anti-CTLA4 mAb Treatments Upregulate Intraallograft IFN-γ Expression in Cardiac Allografts Transplanted into CD28-deficient Recipients.
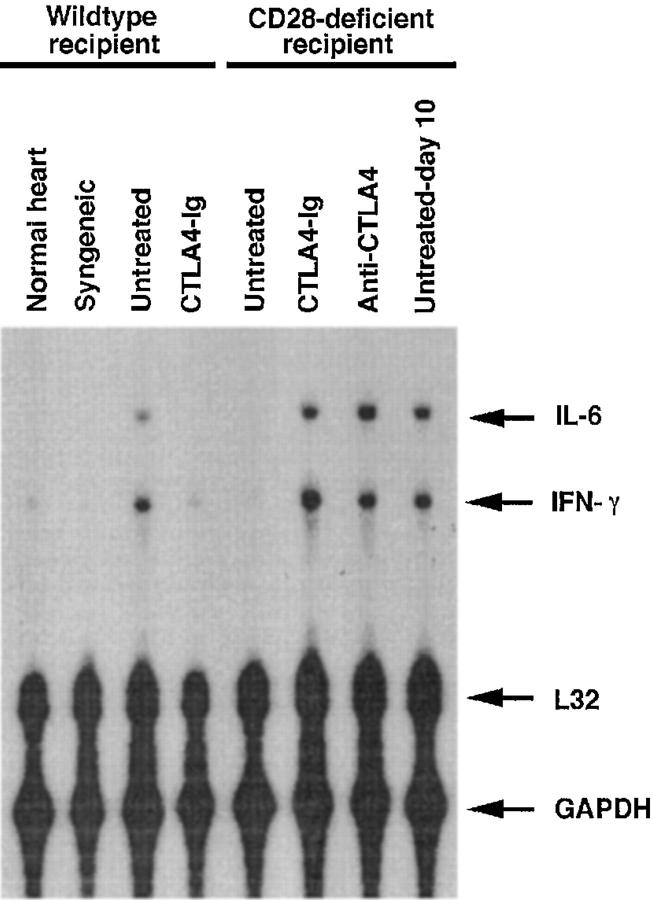
Th1-type cytokines such as IFN-γ are associated with acute allograft rejection (18). To quantitate the pattern of cytokines, RNA was extracted from cardiac allografts 7 d after transplantation (day 10 is shown in one case for comparison) and from a syngeneic graft removed at 100 d. Cytokine transcripts present in the graft at the time of harvest and reflective of infiltrating mononuclear cells were visualized by RNase protection using probes for IL-4, IL-6, and IFN-γ. Consistent with the histology results, levels of IFN-γ and the monocyte-derived inflammatory cytokine IL-6 mRNA were upregulated in animals from groups in which accelerated rejection was observed (Fig. 5). Hearts from CD28-deficient animals had decreased levels of IFN-γ and IL-6 mRNA compared with wild-type mice. Treatment of CD28-deficient mice with CTLA4-Ig or anti-CTLA4 mAb induced an increase in the levels of both IFN-γ and IL-6 mRNA. When hearts from untreated CD28-deficient mice were harvested at later time points (day 10), i.e., closer to the time of rejection, upregulation of the same cytokine transcripts was observed. IFN-γ and IL-6 transcripts could be detected in some control CD28-deficient mice at day 7 (data not shown), correlating with the fact that some CD28−/− animals reject allografts at earlier time points (see Table 1). No IL-4 mRNA was detected in any of the samples studied (data not shown). These results suggest that blockade of B7 family members induces a decrease in intraallograft Th1-type responses and global inflammation in wild-type but not in CD28-deficient mice.
Figure 5.
CTLA4-Ig and anti-CTLA4 mAb induce upregulation of IFN-γ transcripts in allografts from CD28-deficient recipients. Cardiac allografts removed 7 d (10 d in one case) after transplantation. As controls, a syngeneic graft was removed at 100 d, and an orthotopic heart was taken from a normal mouse. Samples were homogenized, RNA was extracted, and levels of cytokine transcripts were determined by RNase protection.
Discussion
Inhibition of the interaction of CTLA4 with B7 family members induces acceleration of graft rejection in CD28-deficient mice. Thus, in the absence of CD28, CTLA4 appears to retain its ability to inhibit T cell responses, indicating that the function of CTLA4 is not solely to counteract CD28 signaling. This inhibitory effect of CTLA4 could, in principle, be achieved through the direct inhibition of TCR signaling or, alternatively, by antagonizing a compensatory costimulatory receptor on CD28-deficient T cells.
Although the role of CD28 as a positive costimulatory receptor is well established, CTLA4 appears to be a negative regulator of T cell responses. Recently, the negative role of CTLA4 on T cell activation has become controversial. Indeed, contradictory reports have been published, indicating either that CTLA4 downregulates T cell responses (10, 19) or that CTLA4 can act as a weak costimulatory molecule in some systems (20). However, if a positive role of CTLA4 was masked under normal circumstances by the more potent costimulatory molecule CD28, one would have expected blockade of CTLA4 to induce prolongation of allograft survival in our model of cardiac transplantation in CD28-deficient mice. In contrast, whether CTLA4 ligation was prevented by blocking B7 family members on the APC side with anti–B7-1 plus anti–B7-2 mAbs or CTLA4-Ig, or by blocking CTLA4 directly on the T cell side with anti-CTLA4 mAb, the outcome was acceleration of graft rejection, further supporting an inhibitory role for CTLA4 on T cell responses. It is of interest that blockade of ligation of both CD28 and CTLA4 by CTLA4-Ig or by anti–B7-1 plus anti–B7-2 mAbs led to different outcomes in wild-type mice compared with CD28-deficient mice. Treatment in wild-type mice resulted in prolonged allograft survival comparable to that of untreated CD28-deficient recipients, whereas treatment in CD28-deficient animals led to rapid graft rejection similar to that of untreated wild-type mice. These results suggest a dominant role of CD28 in wild-type T cells, whereas a distinctive inhibitory role of CTLA4 is revealed in CD28-deficient T cells.
Little is known about how CTLA4 inhibits T cell responses. Because CTLA4 has a higher affinity for B7 family members than CD28, it has been hypothesized that downregulation of T cell activation could be mediated through competition for ligand and decreased CD28 costimulation. Recent reports have suggested an additional signaling role for CTLA4. First, the cytoplasmic tail of CTLA4 has been found to associate with the intracellular enzymes PI3-kinase (13) and protein tyrosine phosphatase 2 (SHP-2) (21). Second, CTLA4 cross-linking has been shown to decrease the activity of extracellular signal–regulated kinase and jun NH2-terminal kinase induced by TCR stimulation of preactivated T cells in vitro (22).
It has been reported recently that in an alternative mouse cardiac allograft model, CTLA4-Ig treatment did not have any detectable effect in CD28-deficient mice compared with animals receiving control IgG (23). The reasons for the different findings in the two models remain unclear. However, Pearson and co-workers positioned the cardiac allografts intraperitoneally rather than in a cervical location. It is conceivable that peritoneal macrophages may contribute to rejection regardless of CTLA4 expression on T cells in this setting. In addition, it is possible that the H-2k into H-2b combination used by Pearson et al. is less potent at inducing CTLA4 expression than is H-2q into H-2b.
The levels of intragraft cytokine transcripts correlated with the numbers of mononuclear cells infiltrating the allografts. Increased levels of the proinflammatory cytokines IFN-γ and IL-6 were observed in grafts from CD28-deficient animals treated with blocking anti-CTLA4 mAb. However, detectable levels of IL-4 were not observed in any hearts (data not shown), indicating absence of infiltrating Th2-type cells. This argues for the preferential generation of Th1-type cytokines in this model, as it appears that anti-CTLA4 mAb can augment cytokine production by both Th1- and Th2-type cells (references 4 and 6, and Alegre, M.L., H. Shiels, C.B. Thompson, and T.F. Gajewski, manuscript submitted for publication). Therefore, one would expect anti-CTLA4 mAb also to increase Th2-type cytokine production, if Th2-type cells were generated after allograft challenge.
Our findings provide evidence for a role of CTLA4 independent of that of CD28 in vivo. The data strongly support the hypothesis that the function of CTLA4 as a negative regulator extends beyond its potential ability to inhibit ligand binding to CD28, and can be exerted in the absence of CD28.
Acknowledgments
The authors wish to thank Jeffrey Ying and Mandel Davis for expert technical assistance with animal care, Xiantang Li for histological analysis of the grafts, and Robert Arch, Ellen Chuang, Ameeta Kelekar, David Nuñez, and Steven Reiner for critical reading of the manuscript and helpful discussions.
This work was supported in part by a grant from the American Heart Association of Metropolitan Chicago to H. Lin, and by a grant from the National Institute of Allergy and Infectious Diseases (R37AI29637) to J.M. Leiden.
References
- 1.Alegre ML, Noel PJ, Eisfelder BJ, Chuang E, Clark MR, Reiner SL, Thompson CB. Regulation of surface and intracellular expression of CTLA4 on mouse T cells. J Immunol. 1996;157:4762–4770. [PubMed] [Google Scholar]
- 2.Walunas TL, Lenschow DJ, Bakker CY, Linsley PS, Freeman GJ, Green JM, Thompson CB, Bluestone JA. CTLA-4 can function as a negative regulator of T cell activation. Immunity. 1994;1:405–413. doi: 10.1016/1074-7613(94)90071-x. [DOI] [PubMed] [Google Scholar]
- 3.Leach DR, Krummel MF, Allison JP. Enhancement of antitumor immunity by CTLA-4 blockade. Science. 1996;271:1734–1736. doi: 10.1126/science.271.5256.1734. [DOI] [PubMed] [Google Scholar]
- 4.McCoy K, Camberis M, Gros GL. Protective immunity to nematode infection is induced by CTLA-4 blockade. J Exp Med. 1997;186:183–187. doi: 10.1084/jem.186.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Luhder F, Hoglund P, Allison JP, Benoist C, Mathis D. Cytotoxic T lymphocyte–associated antigen 4 (CTLA-4) regulates the unfolding of autoimmune diabetes. J Exp Med. 1998;187:427–432. doi: 10.1084/jem.187.3.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Perrin PJ, Maldonado JH, Davis TA, June CH, Racke MK. CTLA-4 blockade enhances clinical disease and cytokine production during experimental allergic encephalomyelitis. J Immunol. 1996;157:1333–1336. [PubMed] [Google Scholar]
- 7.Tivol EA, Borriello F, Schweitzer AN, Lynch WP, Bluestone JA, Sharpe AH. Loss of CTLA4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA4. Immunity. 1995;3:541–547. doi: 10.1016/1074-7613(95)90125-6. [DOI] [PubMed] [Google Scholar]
- 8.Waterhouse P, Penninger JM, Timms E, Wakeham A, Shahinian A, Lee KP, Thompson CB, Griesser H, Mak TW. Lymphoproliferative disorders with early lethality in mice deficient in CTLA-4. Science. 1995;270:985–988. doi: 10.1126/science.270.5238.985. [DOI] [PubMed] [Google Scholar]
- 9.Shahinian A, Pfeffer K, Lee KP, Kundig TM, Kishihara K, Wakeham A, Kawai K, Ohashi PS, Thompson CB, Mak TW. Differential T cell costimulatory requirements in CD28-deficient mice. Science. 1993;261:609–612. doi: 10.1126/science.7688139. [DOI] [PubMed] [Google Scholar]
- 10.Walunas TL, Bakker CY, Bluestone JA. CTLA-4 ligation blocks CD28-dependent T cell activation. J Exp Med. 1996;183:2541–2550. doi: 10.1084/jem.183.6.2541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lenschow DJ, Walunas TL, Bluestone JA. CD28/B7 system of T cell costimulation. Annu Rev Immunol. 1996;14:233–258. doi: 10.1146/annurev.immunol.14.1.233. [DOI] [PubMed] [Google Scholar]
- 12.Cai Y, Cefai D, Schneider H, Raab M, Nabavi N, Rudd CE. Selective CD28pYMNM mutations implicate phosphatidylinositol 3-kinase in CD86-CD28-mediated costimulation. Immunity. 1995;3:417–426. doi: 10.1016/1074-7613(95)90171-x. [DOI] [PubMed] [Google Scholar]
- 13.Schneider H, Prasad KVS, Shoelson SE, Rudd CE. CTLA-4 binding to the lipid kinase phosphatidylinositol 3-kinase in T cells. J Exp Med. 1995;181:351–355. doi: 10.1084/jem.181.1.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kawai K, Shahinian A, Mak TW, Ohashi PS. Skin allograft rejection in CD28-deficient mice. Transplantation (Baltimore) 1996;61:352–355. doi: 10.1097/00007890-199602150-00003. [DOI] [PubMed] [Google Scholar]
- 15.Green JM, Noel PJ, Sperling AI, Walunas TL, Gray GS, Bluestone JA, Thompson CB. Absence of B7-dependent responses in CD28-deficient mice. Immunity. 1994;1:501–508. doi: 10.1016/1074-7613(94)90092-2. [DOI] [PubMed] [Google Scholar]
- 16.Lin H, Iammattoni MD, Modblum JR, Bolling SF. Experimental heterotopic heart transplantation without ischemia or reperfusion. J Heart Transplant. 1990;9:720–723. [PubMed] [Google Scholar]
- 17.Judge TA, Tang A, Turka LA. Immunosuppression through blockade of CD28:B7-mediated costimulatory signals. Immunol Res. 1996;15:38–49. doi: 10.1007/BF02918283. [DOI] [PubMed] [Google Scholar]
- 18.Dallman MJ. Cytokines and transplantation: Th1/ Th2 regulation of the immune response to solid organ transplants in the adult. Curr Opin Immunol. 1995;7:632–638. doi: 10.1016/0952-7915(95)80069-7. [DOI] [PubMed] [Google Scholar]
- 19.Krummel MF, Allison JP. CTLA-4 engagement inhibits IL-2 accumulation and cell cycle progression upon activation of resting T cells. J Exp Med. 1996;183:2533–2540. doi: 10.1084/jem.183.6.2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu Y, Guo Y, Huang A, Zheng P, Liu Y. CTLA-4–B7 interaction is sufficient to costimulate T cell clonal expansion. J Exp Med. 1997;185:1327–1335. doi: 10.1084/jem.185.7.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marengere LE, Waterhouse P, Duncan GS, Mittrucker H-W, Feng G, Mak TW. Regulation of T cell receptor signaling by tyrosine phosphatase SYP association with CTLA-4. Science. 1996;272:1170–1173. doi: 10.1126/science.272.5265.1170. [DOI] [PubMed] [Google Scholar]
- 22.Revilla Calvo, C., D. Amsen, and A.M. Kruisbeek. Cytotoxic T lymphocyte antigen 4 (CTLA-4) interferes with extracellular signal–regulated kinase (ERK) and jun NH2-terminal kinase (JNK) activation, but does not affect phosphorylation of T cell receptor zeta and ZAP70. J Exp Med. 1997;186:1645–1653. doi: 10.1084/jem.186.10.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pearson TC, Alexander DZ, Corbascio M, Hendrix R, Ritchie SC, Linsley PS, Faherty D, Larsen CP. Analysis of the B7 costimulatory pathway in allograft rejection. Transplantation (Baltimore) 1997;63:1463–1469. doi: 10.1097/00007890-199705270-00016. [DOI] [PubMed] [Google Scholar]