Abstract
CD8 memory T cells are tightly regulated in young, healthy individuals but are often perturbed in aged animals by the appearance of large CD8 T cell clones. These clones are associated with impaired immunity in the aged. The molecular basis of this phenomenon remains unclear. Here, it is shown that the issue is confused by the fact that the clones are heterogeneous. Some clones bear high, and others, low levels of integrin α4 (itgα4). These subtypes differ by multiple criteria. They appear in mice of different ages, concentrate in different tissues, and have different stabilities in vivo and responses to stimulation in vitro. itgα4high, but not itgα4low, CD8 clonal expansions have several characteristics consistent with a chronically stimulated phenotype. These properties include lowered levels of CD8, decreased expression of some cytokine receptors, and elevated expression of various inhibitory receptors, including the programmed death-1 (PD1) receptor and the killer cell lectin-like receptor G1 (KLRG1). The characteristics of itgα4high clonal expansions suggest that they may arise from age-dependent alterations in antigen expression and tolerance. These data redefine CD8 clonal expansions into at least two distinct entities and indicate that there are multiple mechanisms that drive age-related alterations of CD8 T cell homeostasis.
Keywords: aging, CD8 T cell, homeostasis, T cell memory
CD8 T cells are important in controlling both primary and secondary infections by a variety of pathogens. After initial exposure, naïve CD8 T cells can differentiate into CD8 memory T cells, which can provide enhanced immunological protection on reexposure to that same pathogen (1). CD8 memory T cells can proliferate and survive for months to years after antigen exposure, a phenomenon facilitated by the cytokines IL-15 and IL-7 (2).
Although the abundance of any single CD8 memory T cell is typically maintained at a low frequency, many aged individuals develop large, monoclonal expansions of CD8 memory T cells (subsequently referred to as TCEs) (3, 4). TCEs vary in size but can occupy a large portion of the total CD8 T cell pool (up to 50% in humans, up to 90% in mice) (unpublished data) (5). Individuals with TCEs have no change in the total number of CD8 T cells (6).
Although TCEs are not associated with overt disease, they may influence immune competence in the aged. For example, aged humans with TCEs are less likely to respond successfully to influenza vaccination (7). Moreover, studies in mice indicate that the presence of TCEs may result in narrow holes in the T cell repertoire that, in some cases, may result in poor responses to pathogens that elicit a highly focused T cell response (6). TCEs are not tumors and do not appear to progress to malignancy over at least nine years (8).
The molecular alterations within TCEs are poorly defined. One challenge to understanding this phenomenon is the heterogeneity between different TCEs. For example, in mice, TCEs can have wide variation in their stability in vivo and their proliferative capacity in vitro (3, 9), suggesting that there may be multiple types of TCEs. Here, we present data that TCEs exist in at least two different subtypes with distinct biological properties. These TCEs differ in their cell surface profiles, relative stability in vivo, and responsiveness to polyclonal stimulation in vitro. These studies provide an important refinement of our current understanding of TCEs and provide evidence that multiple, independent mechanisms can result in age-associated alterations to CD8 memory T cell homeostasis.
Results
Identification, Purification and Microarray Analysis of TCEs.
To define the molecular alterations within TCEs, we isolated four large TCEs from independent, aged mice, as well as the remaining polyclonal CD8 memory-phenotype (MP) T cells from the same aged mice [supporting information (SI) Fig. S1A and Table S1]. TCEs were defined as an increased percentage of CD8 T cells bearing a particular T cell receptor Vβ that was at least 3 standard deviations above the mean Vβ use in young mice (Fig. S1B). To identify genes whose expression was consistently altered in TCEs, we compared gene expression in each TCE relative to paired, age-matched CD8 MP T cells by using high-density microarrays. Of the four TCEs we thus analyzed, three had normal levels of CD8α on their surfaces (TCEs from mice 2, 4, and 5) (Table S1). In these TCEs, the expression of 123 probesets was changed at least 2-fold relative to aged-matched polyclonal CD8 MP T cells (Table S2 and Table S3).
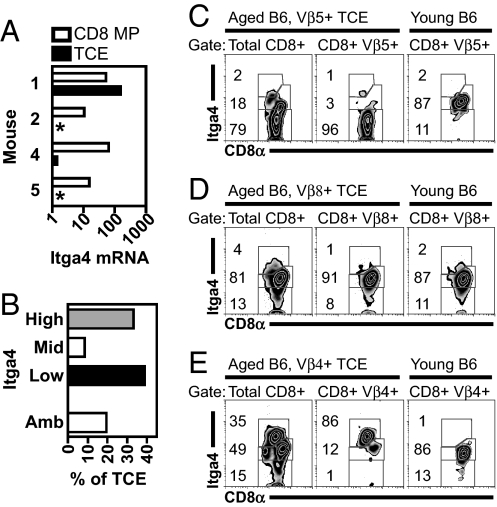
We were interested in cell-surface proteins that might allow better identification of TCEs. Integrin α4 mRNA [itgα4, also referred to as very late antigen 4 (VLA-4) or CD49d] was decreased in all three of the TCEs bearing normal levels of CD8 (TCEs 2, 4, and 5) (Fig. 1A) relative to polyclonal CD8 MP T cells isolated from the same mice. In contrast, itgα4 mRNA was slightly increased in the CD8low TCE identified during this analysis (TCE 1) (Fig. 1A). These data suggested that different TCEs may express different levels of itgα4 and that itgα4 expression may identify different types of TCEs.
Fig. 1.
TCEs express variable levels of itgα4. (A) TCEs express variable levels of itgα4 mRNA. Data represent itgα4 mRNA abundance for TCEs (solid bars) and aged-matched polyclonal CD8 MP T cells (open bars), plotting normalized signal values from microarray analysis. Sample numbers indicate paired samples from individual mice (Table S1). The TCE from mouse #1 had low CD8α expression, whereas all other TCEs had normal CD8α expression (data not shown). An asterisk indicates TCEs with no detectable signal. Shown is data from Affymetrix probe set 1421194_at; similar data obtained with a second probe set. (B) Total distribution of itgα4-defined TCEs. Shown is itgα4 phenotype of large TCEs, occupying at least 5% of the CD8 T cell repertoire as determined by flow cytometry (n = 36 TCEs, in mice aged 11.5–32.5 months). Ambiguous (Amb) TCEs were those that could not be given a single itgα4 phenotype. Examples are shown of itgα4low (C), itgα4mid (D), and itgα4high (E) TCEs. (C–E) In each example, data include the itgα4 profile on bulk CD8α+ events (Left) within the aged mouse with the TCE, the itgα4 profile of the TCE in the same aged mouse defined by the indicated Vβ (Center), and the itgα4 profile of CD8α+ events within the same Vβ in a young mouse (Right). Numbers within each plot indicate the percent of CD8α+ cells in each gate, rounded to the nearest integer.
When we analyzed itgα4 protein expression by many TCEs, we identified TCEs with three distinct itgα4 profiles: low, middle, and high (Fig. 1 B–E). itgα4high and itgα4low TCEs were the most common, whereas itgα4mid TCEs were rare (Fig. 1B). Although itgα4high and itgα4low TCEs were CD44high (Fig. S2), a marker of activated and memory CD8 T cells, itgα4high TCEs frequently had a 2–3-fold decrease in CD8α cell-surface expression (Fig. 1E), reminiscent of TCE 1 from our initial microarray analysis (data not shown). A subset of TCEs could not be given a definitive itgα4 phenotype (ambiguous, see Materials and Methods). Such TCEs were often small in size and therefore difficult to distinguish from their nonclonal counterparts bearing the same Vβ. Previous work has demonstrated that TCEs are clonal by various analyses (unpublished data) (9, 10). In a limited analysis, we have evidence of clonality for both itgα4high and itgα4low TCEs (unpublished data). TCEs with distinct itgα4 phenotypes will be referred to as TCE subtypes.
The Prevalence of itgα4high and itgα4low TCEs Varies with Age.
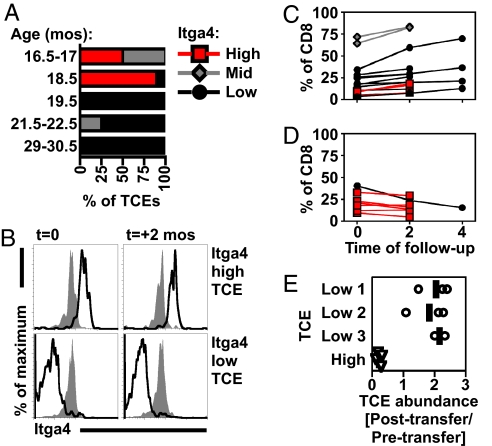
TCEs are found in mice that are 16 months and older (3). Although itgα4high and itgα4low TCEs were equally abundant (Fig. 1B), the prevalence of these TCEs was highly influenced by the age of the mouse (Fig. 2A). In a cross-sectional analysis, itgα4high TCEs were found in mice 16.5–18.5 months old, whereas itgα4low TCEs were identified in mice 19.5 months of age or older (Fig. 2A). To date, the oldest mouse with an itgα4high TCE was 20.5 months old. In contrast, we have identified itgα4low TCEs in mice as old as 36 months (Table S4).
Fig. 2.
TCE subtypes occur at distinct ages and have unequal stability. (A) Cross-sectional analysis of itgα4high (red), itgα4mid (gray), and itgα4low (black) TCEs as a function of age (n = 27 TCEs with a definitive itgα4 phenotype from Fig. 1B, mice aged 16.5–30.5 months). TCEs per timepoint were: 16.5–17 (n = 4), 18.5 (n = 9), 19.5 (n = 7), 21.5–22.5 (n = 4), and 29–30.5 (n = 3). (B) TCEs have a stable itgα4 phenotype over time. Shown is the itgα4 phenotype of a Vβ9+ itgα4high TCE (Top, black) or a Vβ5+ itgα4low TCE (Bottom, black) over 2 months, compared with CD8 T cells expressing the same Vβ in a young mouse (gray). Data shown were obtained during longitudinal analysis of TCEs (C–D). Stable itgα4 phenotypes were observed in 5 itgα4high and 7 itgα4low TCEs over 2 months. (C and D) Longitudinal analysis of TCE size showed that itgα4-defined TCEs increase in size (C) (n = 15) or fail to grow/decrease in size (D) (n = 7) over 2–4 months. TCEs were identified and analyzed sequentially in 19 C57BL/6J mice (analysis began with mice 16.5–30.5 months old). (E) Stability of TCEs after transfer into young recipients. TCEs with indicated itgα4 phenotypes were harvested from aged B6 mice and transferred into B6.PL recipients (2–4 recipients per TCE). At 3 months after transfer, spleens were harvested, and the size of the TCE within the transferred population was determined (percent of CD8+ Thy1.2+ cells that expressed the Vβ of the original TCE). The relative abundance of TCE in transferred population (posttransfer/pretransfer) is shown, with a vertical line for each TCE indicating mean fold change. TCEs that did not change in predominance would have a fold change of 1.
The itgα4 Phenotype of TCEs is Correlated with Growth Dynamics in Vivo.
The age-associated alterations in the itgα4 phenotype of TCEs suggested either that TCEs differentiate from an itgα4high to an itgα4low phenotype, or that itgα4high TCEs are inherently unstable, disappearing after their initial outgrowth, and that itgα4low TCEs may arise independently and later. To discriminate between these possibilities, we analyzed the itgα4 phenotype and size of TCEs over 2–4 months. This analysis revealed: (i) that TCEs uniformly retained their original itgα4 phenotype over time (Fig. 2B), and (ii) that TCEs that failed to grow with time (32% of TCEs) were frequently (6 of 7) itgα4high TCEs (Fig. 2D). During this analysis, only 33% of itgα4high TCEs (3 of 9) increased in size, whereas 91% of itgα4low TCEs (10 of 11) increased in size over this same period.
To analyze the dynamics of TCE subtypes in vivo more extensively, we adoptively transferred itgα4high and itgα4low TCEs into young recipients. Whereas multiple itgα4low TCEs survived and increased in size over a 3-month period, an itgα4high TCE dramatically decreased in size over this period (Fig. 2E). itgα4low TCEs were also detectable 8 months after transfer (data not shown).
TCEs may disappear because of competition with a secondary TCE. However, in the majority of mice in which TCEs failed to grow (5 of 7), no new TCEs were detected (data not shown). When new TCEs were detected (in 2 of 7 mice), new TCEs were itgα4low. In sum, these data indicate that the itgα4 phenotype is correlated with the growth dynamics of TCEs with itgα4high TCEs frequently disappearing over 2–4 months.
itgα4high and itgα4low TCEs Have Different Localization in Vivo.
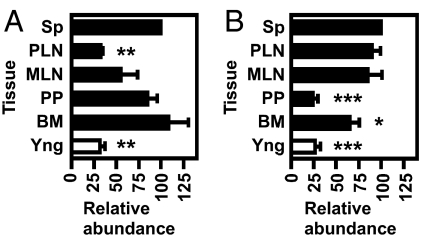
itgα4 is important in trafficking to sites of inflammation, lymph nodes, Peyer's patches, and bone marrow (11, 12). We hypothesized that itgα4high and itgα4low TCEs would therefore be located at different sites in vivo. To test this, we measured the size of TCEs in spleen, peripheral lymph nodes (PLNs) that were not gut-associated, mesenteric lymph nodes (MLNs), Peyer's patches (PPs), and bone marrow (BM) from individual aged mice. PLNs, MLNs and PPs were analyzed separately given their distinct developmental and trafficking requirements (13, 14). Given the variable size of TCEs, the abundance of a TCE in different tissues was measured relative to that observed in the spleen (set as 100% for each TCE). itgα4high and itgα4low TCEs were present in spleen, MLNs and BM (Fig. 3). However, itgα4high TCEs were absent from PLNs (Fig. 3A), whereas itgα4low TCEs were absent from PP (Fig. 3B). TCEs were ruled to be absent from a given tissue if the percent of CD8+ Vβ+ cells in that tissue was not increased above the normal percent of CD8+ Vβ+ cells in a normal, young mouse (“Yng” values in Fig. 3).
Fig. 3.
itgα4high and itgα4low TCEs have differential localization in vivo. (A and B) Tissues from aged mice with itgα4high (A) or itgα4low (B) TCEs were harvested and analyzed for the percentage of CD8 T cells that used the Vβ of each TCE. Data indicate the relative size of the TCE in each tissue compared with TCE size in the spleen, calculated by (percentage of CD8+Vβ+ in tissue)/(percentage of CD8+Vβ+ in spleen) × 100. Young controls were included in all experiments, and the percentage of CD8+Vβ+ events in these animals were plotted as “Yng,” calculated by (percentage of CD8+Vβ+ in young spleen)/(percentage of CD8+Vβ+ in spleen of mouse with TCE) × 100. Data represent mean ± SEM from itgα4high (A, n = 3) or itgα4low (B, n = 5) TCEs. Sp, spleen; PLN, peripheral lymph nodes; MLN, mesenteric lymph nodes; PP, Peyer's patches; BM, bone marrow. Asterisks indicate samples with statistically significant differences from abundance of TCE in spleen, as determined by a paired t test. (A) PLN, P = 0.0012; Yng, P = 0.007. (B) PP, P = 0.0002; BM, P = 0.0286; Yng, P = 0.0002.
itgα4high and itgα4low TCEs Have Differences in Proliferative Capacity.
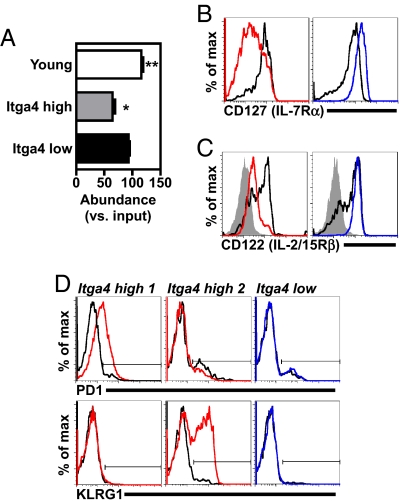
TCEs are heterogeneous based on their response to activation in vitro (3). To find out whether itgα4 expression correlated with this variable, TCEs were stimulated in vitro and analyzed for their relative ability to proliferate and survive in bulk culture. Whereas itgα4low TCEs were maintained at levels similar to those present at the start of the culture, itgα4high TCEs became under-represented after mitogenic stimulation (Fig. 4A). itgα4high TCEs did not show a defect in short-term survival when cultured in low concentrations of IL-7 (Fig. S3). Based on these experiments, itgα4high TCEs were at a selective disadvantage relative to other CD8 T cells after a strong mitogenic stimulus.
Fig. 4.
itgα4high TCEs have an impaired response to stimulation in vitro and features of chronic antigen stimulation. (A) itgα4high TCEs have an impaired response to mitogenic stimulation in vitro. Splenocytes were harvested from mice with itgα4high (n = 3) or itgα4low (n = 4) TCEs and cultured with PMA and ionomycin (to induce proliferation). After 50 h, cells were analyzed for the percentage of CD8 T cells that expressed the Vβ used for each individual TCE. Data represent mean ± SEM, plotting (percentage of CD8+Vβ+ cells in PMA and ionomycin)/(percentage of CD8+Vβ+ cells at time 0) × 100. Samples were subjected to two-tailed, paired t test analysis, to determine samples that were significantly different from 100. The asterisk indicates statistically significant differences from 100 (Young, P = 0.0023; itgα4high, P = 0.0172). (B–D) itgα4high TCEs have markers of chronic antigen stimulation. Cell surface expressions of IL-7Rα (B), IL-2/15Rβ (C) and PD1 and KLRG1 (D) are shown. itgα4high and itgα4low TCEs and young CD8 MP T cells were analyzed in parallel, and all plots were gated on live cells that were CD8+ CD44+ MP T cells that expressed the Vβ used by the TCE. Data compare itgα4high TCEs (red), itgα4low TCEs (blue), young CD8 MP T cells (black), and young, naïve CD8 T cells [solid gray, defined as CD8+ CD44low T cells that expressed the Vβ used by TCE (C)]. (D) Data include two different itgα4high TCEs. The solid horizontal line indicates the gate that contains positive cells.
itgα4high TCEs Have a Phenotype Consistent with Chronic Antigen Stimulation.
The impaired response of itgα4high TCEs to stimulation and their frequent CD8low phenotype are properties of CD8 T cells chronically stimulated by antigen (15–17). When we analyzed itgα4high TCEs for phenotypic changes characteristic of chronic stimulation, itgα4high TCEs had two notable changes: (i) decreased expression of the cytokine receptors IL-7Rα and IL-2/15Rβ, both typically expressed at higher levels on CD8 memory T cells (Fig. 4 B and C) and (ii) increased expression of various inhibitory receptors, including PD1 and KLRG1 (Fig. 4D) that are not normally expressed on nonactivated CD8 memory T cells. Different itgα4high TCEs had distinct expression patterns of inhibitory receptors (e.g., PD1+ KLRG1−, PD1− KLRG1+, and PD1− KLRG1−) (Fig. 4D; data not shown). A minor fraction of each itgα4high TCE had a nonactivated phenotype (e.g., IL-7Rαhigh PD1negative) (Fig. 4B). itgα4low TCEs expressed cell surface proteins consistent with a nonactivated CD8 memory T cell (CD44high IL-2/15Rβhigh IL-7Rαhigh CD62Lmid/high) (Fig. 4 B and C and Table S5) similar to previous reports (18, 19).
Discussion
Here, we show that mice develop two major types of age-associated TCEs with highly divergent properties, and that these two types of TCEs can be distinguished by their itgα4 profile. Although both TCEs can similarly perturb the overall CD8 T cell repertoire, these TCEs differ by multiple criteria: (i) stability and growth in vivo; (ii) in vivo localization; (iii) in vitro proliferative capacity; (iv) cell surface phenotype; and (v) the age of mice which contain these TCEs.
itgα4high TCEs have multiple characteristics observed in conditions of ongoing antigen stimulation: (i) they are poorly responsive to stimulation in vitro, a characteristic reminiscent of anergic T cells (20); (ii) they frequently express low levels of CD8, a phenomenon observed in the context of self-reactivity, recent stimulation, and activation in the presence of IL-4 (15, 16, 21–23); (iii) they bear low levels of receptors for the cytokines IL-7 and IL-2/15, a phenotype observed in conditions of chronic viral infection (24), as well as in a subset of MHC class I-dependent CD8 MP T cells (25); and (iv) some of the itgα4high TCEs express inhibitory receptors, PD1 or KLRG1, a phenotype observed in the context of chronic viral infection (26, 27). Based on microarray data, itgα4high TCEs may also express other inhibitory receptors (e.g., CTLA-4). Curiously, neither itgα4high nor itgα4low TCEs express multiple Ly49 proteins (Table S5).
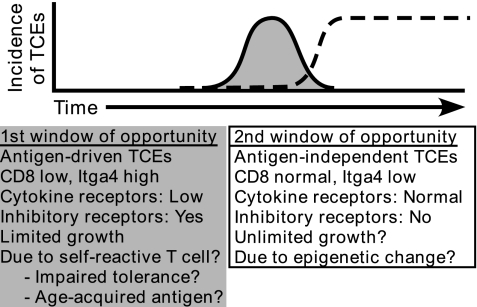
Based on these properties, we hypothesize that itgα4high TCEs result from a transient immune response against self or environmental antigens, and that this response results from age-dependent alterations in tolerance and/or antigen expression (Fig. 5). This outcome may result from: (i) impaired tolerance (e.g., insufficient negative selection or a hole in the regulatory T cell pool); (ii) the sporadic development of malignancies, which could elicit a T cell response against tumor antigens; or (iii) the emergence of other neo-self antigens, particularly proteins whose expression significantly increases with age. Age dependent neo-self antigens may derive from endogenous retroviruses (28, 29). Whatever the basis for this phenomenon, our data suggest that there is something unique in the aging environment that permits the development of itgα4high TCEs in a discrete window of time. Notably, the paucity of itgα4high TCEs in older mice does not appear to be because of the premature death of mice with itgα4high TCEs, an important consideration inherent in studies of aging.
Fig. 5.
Revised model for the development and properties of TCEs. itgα4high (gray) and itgα4low TCEs (black dashed line) occur in distinct temporal windows. Although TCEs are drawn sequentially, at this time, these events appear independent. See Discussion for additional details.
We postulate that itgα4high CD8low TCEs have limited growth capacity because of reduced expression of cytokine receptors and increased expression of inhibitory receptors. One candidate that might coordinate these molecular alterations is the transcriptional regulator Special A-T rich binding protein 1 (SATB1), whose expression was decreased 30-fold in the CD8low itgα4high TCE compared to polyclonal CD8 MP T cells from the same mouse (Fig. S4). SATB1 has been reported to be decreased in two independent studies of T cell anergy (30, 31). Although we hypothesize that itgα4high TCEs may have diminished proliferation in vivo, it is also possible that these TCEs aggressively proliferate yet are actively removed from the body through sites such as the liver or the gut (tissues not analyzed here). Measurements of in vivo proliferation and analysis of nonlymphoid tissues would be required to further define the in vivo factors that limit itgα4high TCEs.
In contrast to itgα4high TCEs, itgα4low TCEs appear to represent a more stable type of TCE reminiscent of an antigen-independent CD8 memory T cell. We hypothesize that itgα4low TCEs have a proliferative advantage because of cell-intrinsic alterations, independent of antigen stimulation (Fig. 5). We propose that itgα4low TCEs were the type of TCE that grew on transfer into β2-microglobulin-deficient mice and were capable of continued growth over 4 years of serial adoptive transfer (C.-C. Ku, personal communication) (18). Consistent with this hypothesis, preliminary data indicate that an itgα4low TCE can survive for >20 months after adoptive transfer and come to dominate the CD8 T cell pool (unpublished data).
Mechanistically, our lead candidate for a molecular basis for itgα4low TCEs is altered epigenetic regulation of an important growth regulatory gene(s), resulting from either inappropriate DNA methylation or histone deacetylation. One candidate is the transcription factor Helios that was decreased by an average of 10-fold in three independent itgα4low TCEs compared to polyclonal CD8 MP T cells from the same mice (Fig. S4). Helios is in the Ikaros-family of transcription factors that are known to regulate chromatin modifications and cellular proliferation and transformation (32, 33). We are currently testing whether reduced Helios is sufficient to confer an increased rate of proliferation in CD8 memory T cells.
The divergent itgα4 phenotype of TCEs raises many questions. First, does altered itgα4 expression contribute to the differential homeostasis of these TCE subtypes? Although itgα4 is important in trafficking, itgα4 engagement can also result in signal transduction, e.g., costimulatory signals (34). Second, does itgα4 phenotype reflect different origins for TCE subtypes? For example, the site of T cell priming influences the trafficking patterns and receptors of T cells (35, 36). Whereas itgα4 is typically up-regulated after activation (37, 38), itgα4 may be down-regulated in certain conditions (39–42). Third, does itgα4 identify different types of TCEs in humans or in mice subjected to experimental manipulations (such as infection)? At this time, preliminary data indicate that human TCEs possess diverse itgα4 phenotypes, comparable to mouse TCEs (J. Rhiannon, personal communication).
In conclusion, we have identified two distinct types of TCEs in mice that are readily distinguishable by itgα4 phenotype and differ from each other in many ways. These studies clearly demonstrate two independent paths by which CD8 memory T cell homeostasis can be altered in aged individuals. Notably, these studies reveal that the aged environment is not a static entity but instead encompasses a series of dynamic changes that influence immune function.
Materials and Methods
Mice.
C57BL/10SnJ and B10.BR (B10.BR-H2k H2-T18a/SgSnJ) mice were used for microarray analyses. C57BL/6J and B6.PL (B6.PL-Thy1a/CyJ) mice were used for all subsequent studies. Mice were obtained either from The Jackson Laboratory and aged at the National Jewish Research and Medical Center (all strains) or from the National Institute on Aging (NIA) aged rodent colony (aged C57BL/6J mice). Mice obtained from the NIA colony were primarily used to confirm that mice from an independent aging colony also developed itgα4high and itgα4low TCEs. For the analysis of heterogeneity among TCEs, young mice were 3–5.5 months old and aged mice were 11.5–35 months old (with 65 mice, 16 months of age or older). All mice were maintained in a pathogen-free environment and used in accordance with institutional and federal guidelines. A small number of mice were treated with selamectin as a preventative measure to limit possible pinworm infection. These mice never tested positive for pinworm and were only treated given their proximity to a small number of pinworm positive animals.
Identification and Purification of TCEs.
TCEs were defined by analysis of TCR Vβ chain usage in peripheral blood CD8 T cells, with antibodies against Vβ 2, 3, 4, 5x, 6, 8x, 9, and 14. In each screen, 3–10 young mice were included to standardize staining (56–60% of CD8 T cells express one of the above Vβs). TCEs were defined as an increased percentage of CD8 T cells bearing a particular Vβ that was at least 3 SD above the mean Vβ use in young mice. TCEs were only considered further if they occupied >5% of the CD8 T cell repertoire, defined by (percent of CD8+ cells bearing the Vβ+ in the aged mouse with the TCE) − (percent of CD8 T cells bearing the same Vβ in young mice). The 5% cutoff allowed us to exclude small TCEs but retain a large number of TCEs for further analysis.
Purification of TCEs.
Mice with large TCEs were identified, and spleen and lymph nodes (typically inguinal, brachial, axillary, lumbar and mesenteric) were collected. Samples were stained with antibodies to MHC class II, CD8α, IL-2/15 receptor β chain (IL-2/15Rβ, also known as CD122), and the Vβ expressed by the TCE. To isolate the TCE as well as polyclonal CD8 MP T cells from the same mouse, samples were purified as CD8+ IL-2/15Rβ+ MHC class IInegative events that were Vβ+ (TCE) or Vβneg events (polyclonal CD8 MP T cells). Samples were stained and sorted at 4°C to limit activation. Sorts were done on a MoFlo (Dako).
itgα4 Phenotype.
itgα4 phenotypes were based on analysis of young and aged mice on the same day, processed in parallel, by using the following sequential criteria: (i) itgα4mid events were gated based on the major population present in young samples (always >75% of CD8+ events); (ii) itgα4low events were gated based on an isotype control antibody for itgα4 (this gate always contained >96% of isotype control stained events); and (iii) itgα4high events were gated based on aged mice in which there was a pronounced itgα4high population (routinely CD8low in the majority of mice).
TCEs were assigned an itgα4 phenotype as follows. First, it was assumed that TCEs were clonal, and that within each affected Vβ, there were residual nonclonal cells that expressed the same Vβ. The minimum size estimate of TCE within the affected Vβ was defined as (percent of CD8 T cells bearing a particular Vβ within the mouse with the TCE) − (percent of CD8 T cells bearing that Vβ within young mice)/(percent of CD8 T cells bearing that Vβ within the mouse with TCE). This method provided a conservative estimate of TCE size because it is assumed that there was no decrease in the number of nonclonal CD8 T cells expressing the Vβ in question. Second, we calculated the percentage of cells that were itgα4high, itgα4mid, and itgα4low within the affected Vβ. Third, we assessed the relative size of the TCE within the Vβ compared with each itgα4 population. If the estimated minimum size of the TCE could only be contained within a single itgα4 population, the TCE was given this phenotype (itgα4high, itgα4mid or itgα4low). If a TCE could not be given a single itgα4 phenotype, it was defined as ambiguous. The vast majority of ambiguous TCEs were too small, relative to other Vβ+ nonclonal cells, to determine whether the TCE was itgα4high, itgα4mid, or itgα4low (i.e., the estimated size of the TCE could be present in >1 itgα4 population). A handful of TCEs were heterogeneous for itgα4 (i.e., the estimated size of the TCE was larger than any single itgα4 population). Given the clonal nature of TCEs, this last situation likely reflects multiple, distinct TCEs within the affected Vβ.
TCE Transfer.
Spleens from aged B6 mice with TCEs were harvested and 20 × 106 splenocytes were transferred into 7-week-old B6.PL recipients (2–4 recipients per TCE) by intravenous injection. Three months after transfer, spleens were harvested, and the percent of transferred cells (defined as CD8+ Thy1.2+) expressing the original TCE Vβ was determined.
Flow Cytometry and Antibodies.
Flow cytometry was performed by using a FacScan (BD Biosciences), a FACSCalibur (BD Biosciences), or a CyAn (Dako). Antibodies were from BD PharMingen, eBioscience, BioLegend, or grown in our laboratory (details in SI Text).
RNA Purification and Microarray Analysis.
After purification of cells, total RNA was harvested. Amplified RNA samples were labeled with biotin, and microarray analysis was performed by using Affymetrix mouse genome 430 2.0 microarrays (details in SI Text).
Stimulation and Cell Culture.
Bulk splenocytes from aged mice with TCEs were cultured (1 × 106 cells/ml) in flat-bottom, 96-well plates, in complete media supplemented with 10 nM phorbol 12-myristate 13-acetate (PMA) and 1 μM ionomycin. Cells were harvested 50 h later and analyzed for the percent of CD8+ Vβ+ cells expressing the affected Vβ. All TCE samples were done in triplicate, and values represent the mean of these replicates.
Software and Statistical Analysis.
Microarray data were analyzed by using GeneSpring 6.2 (Silicon Genetics). Data analysis and plotting were done with Microsoft Excel and Prism 4.0c (GraphPad). Flow cytometric data were analyzed by using FlowJo (TreeStar, Inc.), with data displayed as high-resolution zebra plots showing outliers, with log10 scales (from 100 to 104). Samples collected on CyAn were subjected to compensation after collection. Statistical analyses were performed by using Prism 4.0c with paired t tests (for in vivo localization data). For statistical analysis of in vitro growth to stimulation, the null hypothesis was that samples would have no selective advantage or disadvantage between the two conditions (i.e., relative abundance would be 100). Samples were then subjected to two-tailed, paired t test analyses (with one group containing values of 100 as null hypothesis). Asterisks indicate samples that were significantly different from 100 (either increased or decreased).
Supplementary Material
Acknowledgments.
We thank Tibor Vass for invaluable assistance with our aging mouse colony, Josh Loomis and the Flow Cytometry Core at the National Jewish Research and Medical Center for assistance with cell sorting, the Microarray Core Facility at the University of Colorado, Health Sciences Center for assistance with microarray analysis, Karl Andreasen and Drs. Megan MacLeod, Melissa McCoy, Amy McKee, and Rick Willis for technical assistance, Dr. Linda van Dyk for insightful discussion and technical assistance, Drs. Chia-Chi Ku and Julia Rhiannon for providing unpublished data, and members of the Marrack/Kappler laboratory for helpful discussion. This work was funded by U.S. Public Health Service Grants AI-17143, AI-18785, AI-22295, and AI-52225. E.T.C. is supported by an NRSA postdoctoral fellowship from the National Institutes of Health, J.W. is supported by the Howard Hughes Medical Institute, and J.W.K. and P.M. are investigators at the Howard Hughes Medical Institute.
Footnotes
The authors declare no conflict of interest.
Data deposition: The data reported in this paper have been deposited in the Gene Expression Omnibus, www.ncbi.nlm.nih.gov/geo (accession nos. GSE11677 and GSM 296650–296657).
This article contains supporting information online at www.pnas.org/cgi/content/full/0805465105/DCSupplemental.
References
- 1.Kaech SM, Wherry EJ, Ahmed R. Effector and memory T-cell differentiation: Implications for vaccine development. Nat Rev Immunol. 2002;2:251–262. doi: 10.1038/nri778. [DOI] [PubMed] [Google Scholar]
- 2.Surh CD, Boyman O, Purton JF, Sprent J. Homeostasis of memory T cells. Immunol Rev. 2006;211:154–163. doi: 10.1111/j.0105-2896.2006.00401.x. [DOI] [PubMed] [Google Scholar]
- 3.Callahan JE, Kappler JW, Marrack P. Unexpected expansions of CD8-bearing cells in old mice. J Immunol. 1993;151:6657–6669. [PubMed] [Google Scholar]
- 4.Posnett DN, Sinha R, Kabak S, Russo C. Clonal populations of T cells in normal elderly humans: The T cell equivalent to “benign monoclonal gammapathy.”. J Exp Med. 1994;179:609–618. doi: 10.1084/jem.179.2.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fitzgerald JE, et al. Analysis of clonal CD8+ T cell expansions in normal individuals and patients with rheumatoid arthritis. J Immunol. 1995;154:3538–3547. [PubMed] [Google Scholar]
- 6.Messaoudi I, Lemaoult J, Guevara-Patino JA, Metzner BM, Nikolich-Zugich J. Age-related CD8 T cell clonal expansions constrict CD8 T cell repertoire and have the potential to impair immune defense. J Exp Med. 2004;200:1347–1358. doi: 10.1084/jem.20040437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saurwein-Teissl M, et al. Lack of antibody production following immunization in old age: Association with CD8(+)CD28(-) T cell clonal expansions and an imbalance in the production of Th1 and Th2 cytokines. J Immunol. 2002;168:5893–5899. doi: 10.4049/jimmunol.168.11.5893. [DOI] [PubMed] [Google Scholar]
- 8.Chamberlain WD, Falta MT, Kotzin BL. Functional subsets within clonally expanded CD8(+) memory T cells in elderly humans. Clin Immunol. 2000;94:160–172. doi: 10.1006/clim.1999.4832. [DOI] [PubMed] [Google Scholar]
- 9.LeMaoult J, et al. Age-related dysregulation in CD8 T cell homeostasis: Kinetics of a diversity loss. J Immunol. 2000;165:2367–2373. doi: 10.4049/jimmunol.165.5.2367. [DOI] [PubMed] [Google Scholar]
- 10.Messaoudi I, Warner J, Nikolich-Zugich D, Fischer M, Nikolich-Zugich J. Molecular, cellular, and antigen requirements for development of age-associated T cell clonal expansions in vivo. J Immunol. 2006;176:301–308. doi: 10.4049/jimmunol.176.1.301. [DOI] [PubMed] [Google Scholar]
- 11.Mora JR, von Andrian UH. T-cell homing specificity and plasticity: New concepts and future challenges. Trends Immunol. 2006;27:235–243. doi: 10.1016/j.it.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 12.Mazo IB, et al. Bone marrow is a major reservoir and site of recruitment for central memory CD8+ T cells. Immunity. 2005;22:259–270. doi: 10.1016/j.immuni.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 13.Wagner N, et al. L-selectin and beta7 integrin synergistically mediate lymphocyte migration to mesenteric lymph nodes. Eur J Immunol. 1998;28:3832–3839. doi: 10.1002/(SICI)1521-4141(199811)28:11<3832::AID-IMMU3832>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 14.Mowat AM. Anatomical basis of tolerance and immunity to intestinal antigens. Nat Rev Immunol. 2003;3:331–341. doi: 10.1038/nri1057. [DOI] [PubMed] [Google Scholar]
- 15.Kisielow P, Bluthmann H, Staerz UD, Steinmetz M, von Boehmer H. Tolerance in T-cell-receptor transgenic mice involves deletion of nonmature CD4+8+ thymocytes. Nature. 1988;333:742–746. doi: 10.1038/333742a0. [DOI] [PubMed] [Google Scholar]
- 16.von Boehmer H, Kirberg J, Rocha B. An unusual lineage of alpha/beta T cells that contains autoreactive cells. J Exp Med. 1991;174:1001–1008. doi: 10.1084/jem.174.5.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dillon SR, MacKay VL, Fink PJ. A functionally compromised intermediate in extrathymic CD8+ T cell deletion. Immunity. 1995;3:321–333. doi: 10.1016/1074-7613(95)90117-5. [DOI] [PubMed] [Google Scholar]
- 18.Ku CC, Kappler J, Marrack P. The growth of the very large CD8+ T cell clones in older mice is controlled by cytokines. J Immunol. 2001;166:2186–2193. doi: 10.4049/jimmunol.166.4.2186. [DOI] [PubMed] [Google Scholar]
- 19.Messaoudi I, Warner J, Nikolich-Zugich J. Age-related CD8+ T cell clonal expansions express elevated levels of CD122 and CD127 and display defects in perceiving homeostatic signals. J Immunol. 2006;177:2784–2792. doi: 10.4049/jimmunol.177.5.2784. [DOI] [PubMed] [Google Scholar]
- 20.Schwartz RH. T cell anergy. Annu Rev Immunol. 2003;21:305–334. doi: 10.1146/annurev.immunol.21.120601.141110. [DOI] [PubMed] [Google Scholar]
- 21.Maile R, et al. Peripheral “CD8 tuning” dynamically modulates the size and responsiveness of an antigen-specific T cell pool in vivo. J Immunol. 2005;174:619–627. doi: 10.4049/jimmunol.174.2.619. [DOI] [PubMed] [Google Scholar]
- 22.Gold MC, et al. Murine cytomegalovirus interference with antigen presentation has little effect on the size or the effector memory phenotype of the CD8 T cell response. J Immunol. 2004;172:6944–6953. doi: 10.4049/jimmunol.172.11.6944. [DOI] [PubMed] [Google Scholar]
- 23.Kienzle N, Baz A, Kelso A. Profiling the CD8low phenotype, an alternative career choice for CD8 T cells during primary differentiation. Immunol Cell Biol. 2004;82:75–83. doi: 10.1111/j.1440-1711.2004.01210.x. [DOI] [PubMed] [Google Scholar]
- 24.Wherry EJ, Barber DL, Kaech SM, Blattman JN, Ahmed R. Antigen-independent memory CD8 T cells do not develop during chronic viral infection. Proc Natl Acad Sci USA. 2004;101:16004–16009. doi: 10.1073/pnas.0407192101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boyman O, Cho JH, Tan JT, Surh CD, Sprent J. A major histocompatibility complex class I-dependent subset of memory phenotype CD8+ cells. J Exp Med. 2006;203:1817–1825. doi: 10.1084/jem.20052495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thimme R, et al. Increased expression of the NK cell receptor KLRG1 by virus-specific CD8 T cells during persistent antigen stimulation. J Virol. 2005;79:12112–12116. doi: 10.1128/JVI.79.18.12112-12116.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barber DL, et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 28.McCubrey J, Risser R. Genetic interactions in the spontaneous production of endogenous murine leukemia virus in low leukemic mouse strains. J Exp Med. 1982;156:337–349. doi: 10.1084/jem.156.2.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barbot W, Dupressoir A, Lazar V, Heidmann T. Epigenetic regulation of an IAP retrotransposon in the aging mouse: Progressive demethylation and de-silencing of the element by its repetitive induction. Nucleic Acids Res. 2002;30:2365–2373. doi: 10.1093/nar/30.11.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lechner O, et al. Fingerprints of anergic T cells. Curr Biol. 2001;11:587–595. doi: 10.1016/s0960-9822(01)00160-9. [DOI] [PubMed] [Google Scholar]
- 31.Kurella S, et al. Transcriptional modulation of TCR, Notch and Wnt signaling pathways in SEB-anergized CD4+ T cells. Genes Immun. 2005;6:596–608. doi: 10.1038/sj.gene.6364245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Georgopoulos K. Haematopoietic cell-fate decisions, chromatin regulation and ikaros. Nat Rev Immunol. 2002;2:162–174. doi: 10.1038/nri747. [DOI] [PubMed] [Google Scholar]
- 33.Zhang Z, et al. Expression of a non-DNA-binding isoform of Helios induces T-cell lymphoma in mice. Blood. 2007;109:2190–2197. doi: 10.1182/blood-2005-01-031930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Davis LS, Oppenheimer-Marks N, Bednarczyk JL, McIntyre BW, Lipsky PE. Fibronectin promotes proliferation of naive and memory T cells by signaling through both the VLA-4 and VLA-5 integrin molecules. J Immunol. 1990;145:785–793. [PubMed] [Google Scholar]
- 35.Mora JR, et al. Selective imprinting of gut-homing T cells by Peyer's patch dendritic cells. Nature. 2003;424:88–93. doi: 10.1038/nature01726. [DOI] [PubMed] [Google Scholar]
- 36.Mullins DW, et al. Route of immunization with peptide-pulsed dendritic cells controls the distribution of memory and effector T cells in lymphoid tissues and determines the pattern of regional tumor control. J Exp Med. 2003;198:1023–1034. doi: 10.1084/jem.20021348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hemler ME, et al. Characterization of the cell surface heterodimer VLA-4 and related peptides. J Biol Chem. 1987;262:11478–11485. [PubMed] [Google Scholar]
- 38.Andersson EC, Christensen JP, Marker O, Thomsen AR. Changes in cell adhesion molecule expression on T cells associated with systemic virus infection. J Immunol. 1994;152:1237–1245. [PubMed] [Google Scholar]
- 39.Calabresi PA, Pelfrey CM, Tranquill LR, Maloni H, McFarland HF. VLA-4 expression on peripheral blood lymphocytes is down-regulated after treatment of multiple sclerosis with interferon beta. Neurology. 1997;49:1111–1116. doi: 10.1212/wnl.49.4.1111. [DOI] [PubMed] [Google Scholar]
- 40.Chabot S, Williams G, Yong VW. Microglial production of TNF-alpha is induced by activated T lymphocytes. Involvement of VLA-4 and inhibition by interferonbeta-1b. J Clin Invest. 1997;100:604–612. doi: 10.1172/JCI119571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bartholdy C, Stryhn A, Hansen NJ, Buus S, Thomsen AR. Incomplete effector/memory differentiation of antigen-primed CD8+ T cells in gene gun DNA-vaccinated mice. Eur J Immunol. 2003;33:1941–1948. doi: 10.1002/eji.200323928. [DOI] [PubMed] [Google Scholar]
- 42.Sasaki K, et al. Preferential expression of very late antigen-4 on type 1 CTL cells plays a critical role in trafficking into central nervous system tumors. Cancer Res. 2007;67:6451–6458. doi: 10.1158/0008-5472.CAN-06-3280. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.