Abstract
Germline mutations may cause human disease by various mechanisms. Missense and other in-frame mutations may be deleterious because the mutant proteins are not correctly targeted, do not function correctly, or both. We studied a child with mycobacterial disease caused by homozygosity for a novel in-frame microinsertion in IFNGR2. In cells transfected with the mutant allele, most of the interferon γ receptor 2 (IFN-γR2) protein was retained within the cell, and that expressed on the cell surface had an abnormally high molecular weight (MW). The misfolding mutation was not gain-of-glycosylation, as it created no new N-glycosylation site. The mutant IFNGR2 allele was null, as the patient's cells did not respond to IFN-γ. Based on the well-established relationship between protein N-glycosylation and protein quality control processes, we tested 29 compounds affecting maturation by N-glycosylation in the secretory pathway. Remarkably, up to 13 of these compounds reduced the MW of surface-expressed mutant IFN-γR2 molecules and restored cellular responsiveness to IFN-γ. Modifiers of N-glycosylation may therefore complement human cells carrying in-frame and misfolding, but not necessarily gain-of-glycosylation, mutations in genes encoding proteins subject to trafficking via the secretory pathway. Some of these compounds are available for clinical use, paving the way for clinical trials of chemical complementation for various human genetic traits.
Mendelian susceptibility to mycobacterial diseases (MSMD) is a primary immunodeficiency that selectively weakens host resistance to mycobacteria, such as bacille Calmette-Guérin (BCG) vaccines and environmental mycobacteria (1, 2). There are six known MSMD-causing genes (IL12B, IL12RB1, NEMO, IFNGR1, IFNGR2, and STAT1), all of which are involved in IFN-γ–mediated immunity. Mutations in IL12B, IL12RB1, and NEMO impair the IL-12–dependent production of IFN-γ. In contrast, mutations in IFNGR1, IFNGR2, and STAT1 impair cellular responses to IFN-γ. These genes display high levels of allelic heterogeneity, resulting in the definition of at least 13 different disorders (3–5). Four forms of IFN-γR2 deficiency have been described at the cellular level, although all known patients with IFN-γR2 deficiency present only one of the three types of recessive MSMD. The first type was first identified in 1998 in a patient with complete IFN-γR2 deficiency, homozygous for a loss-of-expression mutation (6). Patients with complete IFN-γR2 deficiency caused by surface-expressed, nonfunctional IFN-γR2 molecules were later reported (7, 8). Finally, a child homozygous for a hypomorphic IFNGR2 mutation and residual IFN-γ responsiveness was reported, defining partial IFN-γR2 deficiency (9). Dominant IFN-γR2 deficiency has not yet been reported in any symptomatic patient, but it was recently shown that cells from a healthy heterozygous carrier display weak IFN-γ responses, suggesting that there may be patients with novel, disease-causing, dominant mutations in IFNGR2 (10). In any event, patients with complete IFN-γR2 deficiency have a much more severe prognosis than patients with partial deficiency (5).
We studied a new patient with MSMD in whom we diagnosed complete IFN-γR2 deficiency. She was homozygous for an in-frame IFNGR2 mutation (382-387dup), potentially associated with a lack of production, a lack of surface expression, or the surface expression of nonfunctional receptors. Most of the mutant 382-387dup IFN-γR2 protein was of low molecular weight (MW) and failed to mature normally. However, mature, Endoglycosidase H (Endo-H)–resistant proteins of high MW, reflecting excessive N-glycosylation, were also found. Unlike the T168N mutation previously reported in IFNGR2 (7, 8), this mutation was not gain-of-glycosylation, in that it did not create a new N-glycosylation site. The in-frame 382-387dup IFNGR2 allele thus encoded misfolded proteins that were abnormally targeted, abnormally N-glycosylated, or both. The link between protein quality control and protein N-glycosylation in the secretory pathway is well established (see Supplemental discussion, available at http://www.jem.org/cgi/content/full/jem.20071987/DC1) (11–19). In particular, glucosidases I and II, in addition to their role in the N-glycosylation process, also contribute to the folding of glycoproteins in the endoplasmic reticulum (ER), their concomitant quality control by calnexin-calreticulin, and their subsequent trafficking (12–18). ER–mannosidase I is also involved in glycoprotein folding, quality control, and trafficking (see Fig. 2 B) (12–18). The potential genetic implications of the connection between quality control and N-glycosylation for the chemical complementation of in-frame mutations leading to misfolded proteins have not been investigated. We investigated whether modifiers of N-glycosylation, including compounds available for clinical use for other purposes, could complement our IFN-γR2–deficient patients' cells in vitro and ex vivo.
Figure 2.
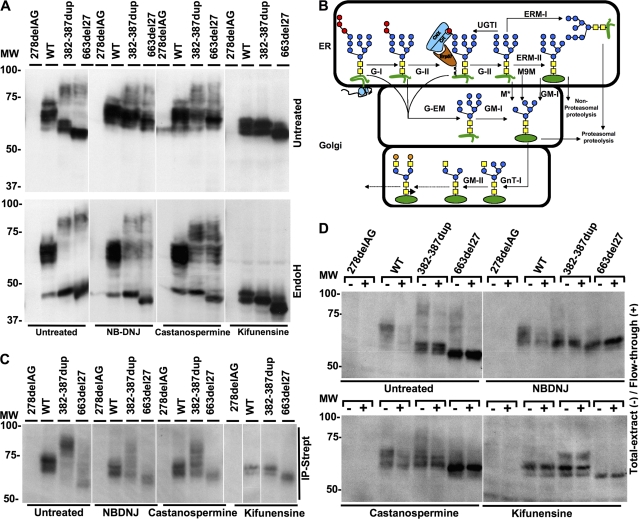
Biochemical properties of IFN-γR2 under various chemical treatments. (A) Hek-293 cells were transfected with 278delAG, WT, 382-387dup, and 663del27 IFN-γR2–tagged V5 constructs. They were then incubated alone or with 1 μM kifunensine, 1.5 mM NB-DNJ, or 2 mM castanospermine for 48 h. Whole-cell extracts were generated, and left untreated or digested with Endo-H overnight. They were then subjected to SDS-PAGE and immunoblotting with an anti-V5 antibody. (B) Maturation of N-linked oligosaccharides: the triglucosylated polymannose oligosaccharides (GlcNac, yellow squares; mannose, blue circles; glucose, red circles; galactose, orange circles) transferred to the asparagine residues of proteins (green) from dolichol phosphate during the translation of mRNA on ribosomes. This oligosaccharide was then matured by incubation with a series of enzymes, including glucosidase I or II (G-I or G-II), glucosyl transferase (UGTI), erp57, calnexin (CNX), calreticulin (Crt), ER–mannosidase I or II (ERM-I or ERM-II), M9-mannase (M9M), Golgi–mannosidase I or II (GM-I or GM-II), and glucosyl transferase I (GnT-I). Alternative pathways involving mannosidases (M*) or Golgi-endomannosidase (G-EM) are able to bypass the classical pathway. Some pathways for unfolded proteins lead to proteasomal or nonproteasomal proteolysis. (C) IFN-γR2–deficient SV40-transformed fibroblasts were transformed with the 278delAG, WT, 382-387dup, and 663del27 IFN-γR2–tagged V5 constructs, either without prior treatment or 48 h after treatment with 1.5 mM NB-DNJ, 2 mM castanospermine, or 1 μM kifunensine. Cell surfaces were biotinylated, and precipitates (IP-Strept) were analyzed by Western blotting with HRP-conjugated anti-V5 antibody. (D) IFN-γR2–deficient SV40-transformed fibroblasts were transformed with the 278delAG, WT, 382-387dup, or 663del27 IFN-γR2–tagged V5 constructs, either without prior treatment or 48 h after treatment with 1.5 mM NB-DNJ, 2 mM castanospermine, or 1 μM kifunensine. Cell surfaces were biotinylated, and total extract (−) or flow through (+) from the biotinylated cells was analyzed by Western blotting with HRP-conjugated anti-V5 antibody. White lines indicate that intervening lanes have been spliced out.
RESULTS AND DISCUSSION
A novel loss-of-function IFNGR2 allele (382-387dup)
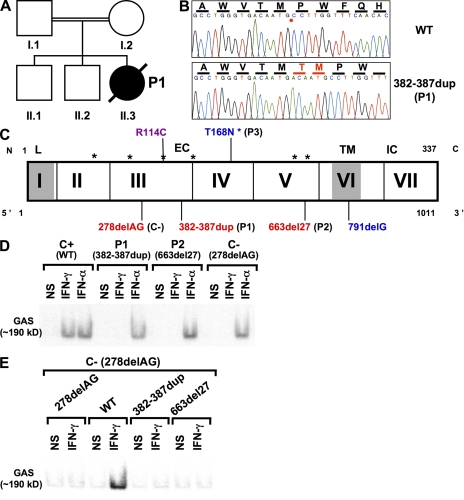
Patient 1 (P1) with MSMD was homozygous for a previously unidentified in-frame microduplication of six nucleotides (382–387) in IFNGR2 (designated 382-387dup; (Fig. 1 A). This mutation is predicted to result in the duplication of amino acids T128 and M129 in the protein (WVTMPW→WVTMTMPW; Fig. 1, B and C). This duplication neither creates nor deletes known consensus sites for posttranslational modifications; in particular, it is not a gain-of-glycosylation mutation, as it creates no consensus site for N-glycosylation, unlike the previously reported T168N mutation in IFNGR2 (7). It was not found in 50 and 77 unrelated healthy individuals of European and Arabian descent, respectively. The parents and one sibling of this patient are heterozygous for the mutation, which was not found in the other sibling. No IFN-γ–activated sequence (GAS)–binding proteins were detected in EMSAs with EBV-transformed B cells from P1 stimulated with IFN-γ (Fig. 1 D). Similarly, no such proteins were detected in cells from two previously described children homozygous for other mutations: an in-frame 27-bp microdeletion of nucleotides 663–689 (designated 663del27 in P2, another in-frame IFNGR2 mutation) (7) and the 278delAG-null frameshift deletion (negative control, C−, lacking IFN-γR2 expression, used as a negative control; Fig. 1, C and D) (6). IFN-γR2–deficient EBV-transformed B cells homozygous for the 278delAG allele (6) were complemented by the WT allele but not by any of the three mutant IFNGR2 alleles (Fig. 1 E). Thus, P1, like P2, was homozygous for an in-frame IFNGR2 mutation causing complete functional IFN-γR2 deficiency (7), an allele conferring a complete lack of cellular responses to IFN-γ.
Figure 1.
Clinical phenotype, IFNGR2 genotype, and cellular phenotype of patients with MSMD. (A) Clinical phenotypes of consanguineous patients. Healthy individuals are shown in white. P1, with M. avium, is shown in black. (B) Electrophoregram showing the mutations in exon 3 of the IFNGR2 gene in P1 (382-387dup) and the WT sequence of a control. The position of the insertion is indicated by the red asterisk on the WT sequence, and the additional amino acids present in the mutant are shown in red. (C) Novel mutation in the IFNGR2 gene (382-387dup). The IFNGR2 coding region is represented by vertical bars between exons designated by Roman numerals. The leader sequence (L, 1–22), extracellular domain (EC, 23–248), transmembrane domain (TM, 249–272), and intracellular domain (IC, 273–337) are indicated. Consensus sites for N-glycosylation are indicated by asterisks. Mutations marked in red cause complete IFN-γR2 deficiency with no detectable expression of IFN-γR2 at the cell surface. The mutations indicated in blue cause complete IFN-γR2 deficiency, with detectable surface expression of a nonfunctional IFN-γR2 (reference 7). The mutation marked in purple causes partial, as opposed to complete, IFN-γR2 deficiency. (D) Response of EBV-transformed B cells to IFN-γ and IFN-α, as determined by EMSA analysis of nuclear proteins binding a GAS probe, from a positive control (C+), two patients (P1, bearing mutation 382-387dup, and P2, bearing mutation 663del27), and a negative control (C−, homozygous for the 278delAG IFNGR2 allele), in response to 105 IU/ml IFN-γ or IFN-α treatment for 30 min and in the absence of such treatment. (E) GAS probe–binding nuclear protein from EBV-transformed B cells, IFN-γR2–deficient cells (278delAG) transiently transfected with vectors encoding WT, 278delAG, 663del27, and 382-387dup IFN-γR2 without (NS) or with 105 IU/ml IFN-γ, as determined by EMSA.
The 382-387dup and 663del27 IFNGR2 alleles encode misfolded proteins
We investigated the subcellular distribution of the mutant IFN-γR2 molecules by confocal microscopy in IFN-γR2–deficient SV40-transformed fibroblasts transformed with WT, 382-387dup, and 663del27 IFN-γR2-eGFP constructs. WT IFN-γR2 was predominantly located in large vesicles in the cytoplasm, displaying the perinuclear distribution typical of the secretory pathway. In contrast, the 382-387dup and 663del27 IFN-γR2 molecules were disseminated in smaller cytoplasmic vesicles, suggesting that they were abnormally trafficked as misfolded proteins (Fig. S1 A, available at http://www.jem.org/cgi/content/full/jem.20071987/DC1). We then explored the intracellular trafficking of mutant IFN-γR2 from P1 and P2 by transfecting Hek293 cells with IFNGR2 alleles encoding V5-tagged molecules and detecting IFN-γR2 in the lysates of these cells by Western blotting (Fig. 2 A; and Fig. S2, A and B). No IFN-γR2–specific antibody suitable for such experiments on the patients' cells (or cells overexpressing IFNGR2 alleles) is currently available. Both mutant proteins segregated into two major groups of low (∼55) and high (∼80) MW. This maturation profile is typical of misfolded proteins and results from insufficient glycosylation (giving the lower MW) and impaired maturation upstream in the secretory pathway (lack of trimming of the glycan moieties, resulting in an abnormally high MW). In both cases, most proteins were of low MW and failed to mature normally, as shown by their sensitivity to Endo-H (Fig. 2 A) (20). The high MW of mature, Endo-H–resistant proteins reflected insufficient maturation of the glycosylated proteins, as shown by their digestion with PNGase-F. Polyubiquitinylation, which has been reported to be responsible for the high MW of other misfolded proteins (21), was not involved in this case, because digestion with PNGase-F resulted in the complete normalization of MW (Fig. S1 B). Thus, the two in-frame loss-of-function IFNGR2 alleles (382-387dup in P1 and 663del27 in P2) encoded misfolded proteins that were abnormally N-glycosylated.
Maturation of the 382-387dup IFN-γR2 protein with NB-DNJ, castanospermine, or kifunensine
Glucosidases I and II are involved in the folding of glycoproteins in the ER, their concomitant quality control by calnexin-calreticulin, and their subsequent trafficking (12–18). ER–mannosidase I is also involved in glycoprotein folding, quality control, and trafficking (Fig. 2 B) (12–18). We therefore hypothesized that NB-DNJ (Zavesca) and castanospermine (22), which inhibit glucosidases I and II, and kifunensine (22), an inhibitor of ER–mannosidase I, might improve the glycosylation or trafficking of the nonnative IFN-γR2 molecules (Fig. 2 B; and Table S1, available at http://www.jem.org/cgi/content/full/jem.20071987/DC1). NB-DNJ and castanospermine had no detectable impact on WT IFN-γR2 glycoproteins (MW of ∼60), but most of the mutant molecules, including Endo-H–resistant proteins of high MW, displayed a shift in MW to ∼60 (Fig. 2 A). In contrast, kifunensine impaired the maturation of both WT and mutant molecules, which became sensitive to Endo-H (Fig. 2 A). We assessed the surface expression of IFN-γR2 by biotinylating surface-expressed proteins in IFN-γR2–deficient fibroblasts (6) transfected with various V5-tagged IFN-γR2 constructs, and assessing IFN-γR2 production in total extracts, the streptavidin-bound fraction, and flow through by Western blotting with a V5-specific antibody (Fig. 2, C and D). Most WT molecules with a MW >60 were expressed at the cell surface, except in the presence of kifunensine. In contrast, only a small fraction of mutant molecules was expressed at the surface, even after treatment with NB-DNJ, castanospermine, or kifunensine. However, after treatment with any of these three drugs, mutant IFN-γR2 molecules with a MW of ∼60, corresponding to the MW of WT molecules, appeared on the cell surface (Fig. 2 C). The maturation of the 382-387dup and 663del27 IFN-γR2 molecules in the presence of NB-DNJ, castanospermine, or kifunensine thus resulted in the expression of a detectable fraction of receptors at the cell surface with a MW similar to that of WT proteins under the same conditions, with 663del27 molecules being somewhat lighter owing to the deletion of nine residues. It is unclear whether the surface-expressed mutant IFN-γR2 molecules of normal MW detected in the presence of these compounds were generated by the maturation of IFN-γR2 molecules of low MW that would otherwise have been retained in the secretory pathway, or by the maturation of high MW molecules that had reached the cell surface. The time-course experiments revealed that NB-DNJ exerted its effects on MW after 9 h, whereas castanospermine and kifunensine exerted their effects only after 24 h (Fig. S2, A and B). The exact MW of the IFN-γR2 WT molecules responding to IFN-γ is unknown. We can only predict that the mutant IFN-γR2 molecules with a MW closer to that of the WT in the presence of glycosylation inhibitors are the most likely to be active. Finally, the stability and natural degradation of WT and mutant IFN-γR2 molecules were independent of the proteasome, as shown by experiments with the inhibitor MG132 (Fig. S3). Moreover, mutant and WT proteins behaved similarly in the presence of cycloheximide, a protein synthesis inhibitor (Fig. S3).
Functional complementation of the 382-387dup IFNGR2 allele in EBV-transformed B cells, SV40-transformed fibroblasts, and blood cells
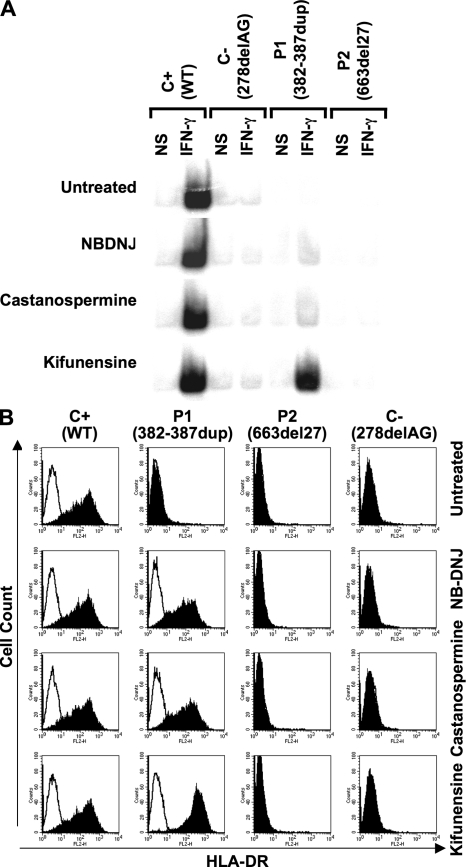
We therefore investigated whether cells from P1 or P2 could be functionally complemented by culturing EBV-transformed B cells with NB-DNJ, castanospermine, or kifunensine for 16 h, and then stimulating them with IFN-γ for 30 min (Fig. 3 A). WT cells responded to IFN-γ in the presence of each of these compounds, as shown by EMSA. Cells from a negative control (C−) (6) and cells from P2 (663del27) did not respond to IFN-γ, even in the presence of these drugs. However, cells from P1 (382-387dup) regained responsiveness to IFN-γ upon treatment with NB-DNJ or castanospermine. Surprisingly, they responded to an even greater extent when treated with kifunensine, with responses reaching normal levels (Fig. 3 A). This functional complementation did not result from the trimming of extended IFN-γR2 glycans impairing IFN-γ binding to IFN-γR1, as the patient's cells had normal numbers of IFN-γ binding sites of normal affinity (unpublished data). When EBV-transformed B cells from P1 were treated with NB-DNJ, castanospermine, or kifunensine for 16 h and were stimulated with various doses of IFN-γ for 30 min, they responded to as little as 100 IU/ml IFN-γ (Fig. S4 A, available at http://www.jem.org/cgi/content/full/jem.20071987/DC1). We then assayed a more distal event, using flow cytometry to test the induction of HLA-DR on the surface of SV40-transformed fibroblasts (Fig. 3 B). Cells from P1, unlike those from all other patients, responded normally to IFN-γ after treatment with any of the three molecules tested. Kifunensine was particularly efficient, as it was effective at concentrations <1 μM and no toxicity was observed, even at millimolar concentrations. In such conditions, fibroblasts from P1 responded to as little as 100 IU/ml IFN-γ (Fig. S4 B). Finally, we stimulated freshly prepared PBMCs with live BCG, BCG plus IFN-γ, or IFN-γ alone, and determined IL-12p40 and p70 levels by ELISA (23), in the presence or absence of the only commercially available compound, NB-DNJ (Zavesca). NB-DNJ restored the responsiveness to BCG plus IFN-γ and to IFN-γ alone with PBMCs from P1 (Fig. S5 and Fig. S6). Unfortunately, our patient died before we could test kifunensine. Thus, IFN-γR2–deficient cells from P1, whether immortalized lymphoid and fibroblastic cell lines or freshly prepared blood cells, were complemented by modifiers of glycosylation.
Figure 3.
Chemical complementation of the cellular phenotype with multiple drugs. (A) Response of EBV-transformed B cells to IFN-γ, as determined by EMSA analysis of GAS probe–binding nuclear proteins from a positive control (C+), a negative control (C−, homozygous for the 278delAG IFNGR2 allele), and two patients (P1, bearing mutation 382-387dup, and P2, bearing mutation 663del27) in response to 105 IU/ml IFN-γ treatment for 30 min, 16 h after incubation with 1.5 mM NB-DNJ, 2 mM castanospermine, or 1 μM kifunensine. (B) SV40-transformed fibroblasts from a positive control (C+), two patients (P1, bearing mutation 382-387dup, and P2, bearing mutation 663del27), and a negative control (C−, bearing the 278delAG mutation) were incubated for 72 h in complete culture medium with (shaded histogram) or without (open histogram) 2.4 × 104 IU/ml IFN-γ, with or without 1.5 mM NB-DNJ, 2 mM castanospermine, or 1 or 0.375 μM kifunensine. They were analyzed 48 h later. The surface expression of HLA-DR molecules was determined by flow cytometry using a specific antibody.
Complementation of the 382-387dup IFNGR2 allele with multiple modifiers of N-glycosylation
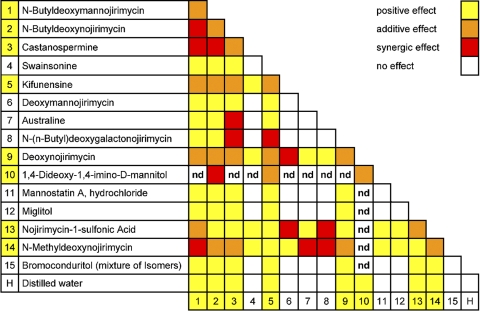
We assessed the specificity of enzyme inhibition by transfecting human cells defective for the glucosidase I or glucosidase II gene with various tagged IFN-γR2 constructs (24–26). The 382-387dup IFN-γR2 molecules were not of normal MW (i.e., 60) in cells genetically deficient for either glucosidase II or glucosidase I (these cells are functionally deficient for both enzymes, as they cannot produce glucosidase II substrates; unpublished data). NB-DNJ and castanospermine therefore complemented the patient's cells by inhibiting another, as yet unknown, enzyme, and possibly, but not necessarily, by also inhibiting glucosidase I and/or II. We tested this hypothesis using most of the commercially available compounds known to inhibit the various steps of glycosylation (Fig. 2 B and Table S1). We incubated SV40-transformed fibroblasts separately with each of the 29 drugs and IFN-γ for 72 h. Cell-surface HLA-DR expression was assessed by flow cytometry (Fig. S7, available at http://www.jem.org/cgi/content/full/jem.20071987/DC1). None of the compounds impaired the response of control cells or restored IFN-γ responsiveness in fibroblasts from P2 and C−. However, 13 compounds restored IFN-γ responsiveness in cells from P1 (Fig. S7). Six drugs fully complemented cells from P1 when tested at the recommended inhibitory concentrations (NB-DNJ, castanospermine, NM-DNJ, NB-DMJ, kifunensine, and 2-5-DM; Fig. S7). Eight other compounds had a detectable but weaker effect. In contrast, 16 inhibitors did not complement the patient's cells (these compounds included tunicamycin, an inhibitor of N-glycosylation that blocks assembly of the lipid-linked oligosaccharide precursor). The inefficacy of australine, DMJ, and swainsonine suggested that the inhibition of glucosidase I, ER–mannosidase I, or Golgi–mannosidase II alone was insufficient to complement cells from P1 (Fig. 2 B and Table S1). We tested combinations of two compounds, each used at its minimal effective concentration (Fig. 4 and Table S1). No inhibitory effect was detected, but additive effects were observed for several combinations and synergic effects were observed in several cases (Fig. S8). Interestingly, no complementation was observed if australine was used with bromoconduritol or miglitol (Fig. 4), suggesting that NB-DNJ and castanospermine also inhibited another, as yet unknown, enzyme in the N-glycosylation machinery. Further evidence to support this hypothesis is provided by the synergy conferred by NB-DGJ. Nonetheless, australine and castanospermine acted in synergy, suggesting that a potent and combined inhibition of glucosidase I and II is efficient. Paradoxically, kifunensine and swainsonine complemented the cells when used together, whereas DMJ alone did not (Fig. 4). The complementation of cells from P1 may thus reflect the combined inhibition of several enzymes, such as glucosidases I and II, ER–mannosidase I, and other, as yet unknown, enzymes.
Figure 4.
Observation of the effects of pairs of chemical compounds. SV40-transformed fibroblasts from P1, bearing mutation 382-387dup, were incubated for 16 h in complete culture medium supplemented with 2.4 × 104 IU/ml IFN-γ. The following compounds were added, in pairs, to the culture medium: 1, 0.375 mM NB-DMJ; 2, 0.375 mM NB-DNJ; 3, 0.5 mM castanospermine; 4, 0.962 mM swainsonine; 5, 0.125 μM kifunensine; 6, 0.483 mM DMJ; 7, 0.338 mM australine; 8, 1.75 mM NB-DGJ; 9, 2.36 mM DNJ; 10, 3.86 mM DIM; 11, 0.15 mM mannostatin A; 12, 0.93 mM miglitol; 13, 0.37 mM NJ1-S; 14, 1.4 mM NM-DNJ; 15, 0.92 mM bromoconduritol (mixture of isomers); and H, distilled water. The surface expression of HLA-DR molecules was determined by flow cytometry, using a specific antibody, 72 h later.
Concluding remarks
Chemical chaperones, such as DMSO and glycerol, have been reported to complement some cell lines in vitro, albeit with low specificity and high toxicity, preventing their use in clinical trials (27–30). None of these compounds or the proteasome inhibitor tested (MG132) complemented the cell lines tested in our study (unpublished data). Curcumin was reported to complement delF508-CFTR in one study (28) but not in another (30), and it did not complement the cell lines studied in this report (unpublished data). Pharmacological chaperones, designed for the specific complementation of target proteins, are less toxic and therefore more promising, but their specificity is not compatible with broad application (29, 31, 32). Our report of modifiers of N-glycosylation complementing misfolding alleles is consistent with the well-established role of glycans in protein folding and quality control in the ER (12–16, 18). We could not assess whether the unfolded protein responses were triggered by the mutant proteins, as X-box binding protein 1–specific antibodies gave negative results in Western blots of HEK epithelial cells transfected with the different constructs, whereas we detected X-box binding protein 1 in similar amounts in WT and mutant SV40–transformed fibroblastic cells (unpublished data). Unlike chemical and pharmacological chaperones, inhibitors of glycosylation do not interact directly. Instead, they have an indirect effect via the inhibition of selected glycosylation enzymes. Our data identify no simple, specific mechanism of complementation, but they do show convincingly that multiple modifiers of N-glycosylation, alone or in combination, can efficiently complement the IFNGR2 mutation, strongly suggesting that the key process involved is the modification of N-glycosylation. Deciphering the exact molecular mechanism of action of these compounds is, however, beyond the scope of this report.
Our successful complementation of misfolding mutations by this novel class of chemical chaperone–like molecules opens up new avenues of research. Several modifiers of N-glycosylation complemented a misfolding mutation in IFNGR2. They might complement mutations in other genes. Moreover, unlike most other previously reported chemical and pharmacological chaperones, which were active in transfected or tumor cells overproducing the mutant protein, these compounds complemented germline cells in vitro and ex vivo. Our findings raise hopes that chemical modifiers of N-glycosylation could be curative in some patients bearing mutations in genes encoding proteins subject to trafficking via the secretory pathway: not only patients with gain-of-glycosylation mutations (7, 8), but also patients bearing other types of misfolding mutation. Kifunensine is a good candidate, as it has a wide window of efficacy without toxicity in vitro; unlike chaperones, which work at millimolar concentrations, it worked at below micromolar concentrations. Derivatives of castanospermine are also potentially useful (33). Nevertheless, modifiers of glycosylation should be tested carefully, as they may result in an acquired form of congenital disorders of glycosylation (26). In this context, NB-DNJ (Zavesca, also known as Miglustat) is especially promising, as this molecule has displayed no overt toxicity in human clinical trials (34) and is currently used in patients with type I Gaucher's disease because of its ability to inhibit glucosylceramide synthase at concentrations lower than those used in our study (35–37). It will be essential to define the concentrations of modifiers of N-glycosylation that rescue ad hoc mutant proteins without compromising cell viability in vitro and in vivo.
MATERIALS AND METHODS
Affected individuals.
We studied three children from three unrelated families with severe mycobacterial disease. All three families were consanguineous. P1 developed disseminated Mycobacterium avium disease at the age of 2 yr. She tested negative for HIV, CMV, and EBV in serological tests. Despite antimycobacterial treatment with rifamycin, moxifloxacin, hydroxychloroquine, clofazimine, and ethambutol, P1 died at the age of 5 yr because of dissemination and uncontrolled M. avium infection. Neither of her two brothers has ever developed mycobacterial infection. P2 developed disseminated M. avium infection, which responded to antibiotics, at the age of 3 yr. He has received continuous antibiotic treatment for the last 7 yr. P3 developed disseminated BCG vaccine infection at the age of 1 mo; he is now 2.5 yr old and is still on antibiotic treatment. P2 and P3 have been previously described (7). This study was approved by the local institutional review committee (Comité Consultatif de Protection des Personnes dans la Recherche Biomédicale), and informed consent was obtained from all families.
Cell culture and transfection.
EBV-transformed B cells and SV40-transformed fibroblasts were cultured in RPMI 1640 supplemented with 10% heat-inactivated bovine fetal serum (complete medium; Invitrogen). Transfection was performed as previously described (7).
Cytokines, enzymes, inhibitors, and antibodies.
We used recombinant nonglycosylated human IFN-γ (Imukin), recombinant IFN-α2b (R&D Systems), anti-V5 antibody (1:5,000; Invitrogen), Endo-H (New England Biolabs, Inc.), PNGase-F (New England Biolabs, Inc.), NB-DNJ (Sigma-Aldrich), castanospermine (Sigma-Aldrich), MG132 (Sigma-Aldrich), cycloheximide (Sigma-Aldrich), streptavidin-agarose (Invitrogen), PE-conjugated mouse anti–HLA-DR antibody (Becton Dickinson), and horseradish peroxidase (HRP)–labeled anti–mouse Ig antibody (1:10,000; GE Healthcare), NB-DNJ, and australine (Qbiogene). All other drugs were obtained from Toronto Research Chemicals Inc. All chemical compounds were dissolved or suspended in water.
Expression vectors.
We used AmpliTaq DNA polymerase (Applied Biosystems) to amplify cDNA fragments encoding human IFNγ-R2 and other genes. PCR products were digested with BglII and XmaI and inserted into peGFP-N1 (Clontech Laboratories, Inc.) or the pcDNA3 (Invitrogen) vector, with or without a V5/His Tag, by a directional topoisomerase-based method. We used a Stratagene kit, according to the manufacturer's instructions, for direct mutagenesis.
Cell-surface biotinylation.
2 d after transfection, SV40-transformed fibroblasts were labeled by incubation for 30 min at 4°C in PBS (pH 8), with or without sulfo-NHS-LC-Biotin (Thermo Fisher Scientific). They were then washed twice in PBS. The cross-linking reaction was stopped by adding 50 mM NH4Cl in PBS. Cells were harvested by centrifugation in an Eppendorf tube (1.5 ml) and were washed three times with PBS. Biotinylated SV40-transformed fibroblasts were lysed with the appropriate buffer for immunoprecipitation (as recommended by the manufacturer) and incubated overnight with streptavidin-agarose. Immunoprecipitates were analyzed by SDS-PAGE, using an anti-V5-antibody and the enhanced chemiluminescence system for detection.
EMSA.
EBV-transformed B cells were analyzed by EMSA, as previously described (7).
Endo-H digestions and SDS-PAGE.
Endo-H digestions were analyzed by SDS-PAGE, as previously described (7).
Flow cytometry.
The HLA-DR expression profile of SV40-transformed fibroblasts was investigated by flow cytometry, as previously described (7).
PBMC cultures and activation by BCG.
PBMCs were obtained from whole blood by centrifugal separation through a Ficoll gradient. We cultured 2 × 105 cells in triplicate in 200 μl RPMI 1640, supplemented with 10% FCS per well, in a 96-well plate. The plate was incubated for 48 h at 37°C under an atmosphere of 5% CO2/95% air, and was treated with NB-DNJ or castanospermine at 37°C, with medium alone, with live BCG (M. bovis BCG, Pasteur substrain) at a multiplicity of infection of 20 (BCG/leukocytes) and with BCG plus 5,000 IU/ml IFN-γ, as previously described (7). Final results were standardized per million PBMCs, and are expressed as pg/ml/106 PBMCs.
Cytokine ELISA.
Cytokine concentrations were analyzed by ELISA using the human Quantikine IL-12p40 and IL-12p70 kits from R&D Systems and the human Pelipair IFN-γ kit from Sanquin, according to the manufacturers' instructions. Optical density was determined as previously described (7).
Online supplemental material.
Fig. S1 depicts subcellular distribution of WT, 382-387dup, and 663del27 IFN-γR2 in SV40-transformed fibroblasts, as shown by indirect immunofluorescence on confocal microscopy with IFN-γR2–eGFP constructs. This figure also shows that the high MW of the mutant IFN-γR2 proteins results from abnormal glycosylation. Fig. S2 shows biochemical properties of IFN-γR2 molecules, in the presence of NB-DNJ, castanospermine, or kifunensine, in a time-course experiment. Fig. S3 depicts biochemical properties of IFN-γR2 molecules, in the presence of inhibitors of the proteasome or of protein synthesis, in a time-course experiment. Fig. S4 shows an IFN-γ or IFN-α response of EBV-transformed B cells and SV40-transformed fibroblasts from a positive control, the patient, and a negative control in the presence of NB-DNJ, castanospermine, or kifunensine. This figure also shows that the IFN-α response of EBV-transformed B cells is not altered by NB-DNJ or castanospermine treatment. Fig. S5 shows IFN-γ responses of PBMCs from a positive control and the patient in the presence of NB-DNJ and castanospermine, as assessed by quantifying the production of IL-12p40 and IL-12p70. Fig. S6 depicts IFN-γ responses of PBMCs from positive controls in the presence of NB-DNJ and castanospermine and from negative controls, as assessed by quantifying the production of IL-12p40. Fig. S7 depicts IFN-γ responses of SV40-transformed fibroblasts from a positive control, the patient, and a negative control in the presence of 13 inhibitors of N-glycosylation, as assessed by measurements of HLA-DR induction. Fig. S8 shows examples of the additive and synergistic effects of the different drugs on the patient's IFN-γ response, as assessed by measuring HLA-DR induction in SV40-transformed cells. Supplemental discussion contains information about N-glycosylation and quality control of proteins undergoing trafficking in the secretory pathway. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20071987/DC1.
Supplementary Material
Acknowledgments
We thank Laurent Abel for helpful discussions and critical reading, Philippe Even and Jean-Jacques Leboyer for the international patent application, Tony Leclerc for technical assistance, and Catherine Bidalled and Martine Courat for secretarial assistance. We thank Valérie Tolyan-Vogt, Nadia Chuzhanova, David Cooper, Garfa Meriem, Maryvonne Legros, Emmanuelle Jouanguy, Vanessa Sancho Shimizu, Lucile Janniere, and all members of the Laboratory of Human Genetics of Infectious Diseases for discussions, and Sergio Rosenzweig and Steven Holland for cell lines.
This work was supported by grants from BNP-Paribas and the European Union (QLK2-CT-2002-00846), and fellowships from Pasteur-Necker and the Fondation pour la Recherche Médicale. Professor J.-L. Casanova is an International Scholar of the Howard Hughes Medical Institute.
An international patent application was filed in the name of Institut Necker, with the designated inventors as G. Vogt and J.-L. Casanova. The authors have no other conflicting financial interests.
A. Chapgier, J. Feinberg, and S. Boisson Dupuis contributed equally to this paper.
References
- 1.Casanova, J.L., and L. Abel. 2002. Genetic dissection of immunity to mycobacteria: the human model. Annu. Rev. Immunol. 20:581–620. [DOI] [PubMed] [Google Scholar]
- 2.Alcais, A., C. Fieschi, L. Abel, and J.L. Casanova. 2005. Tuberculosis in children and adults: two distinct genetic diseases. J. Exp. Med. 202:1617–1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Filipe-Santos, O., J. Bustamante, M.H. Haverkamp, E. Vinolo, C.L. Ku, A. Puel, D.M. Frucht, K. Christel, H. von Bernuth, E. Jouanguy, et al. 2006. X-linked susceptibility to mycobacteria is caused by mutations in NEMO impairing CD40-dependent IL-12 production. J. Exp. Med. 203:1745–1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chapgier, A., S. Boisson-Dupuis, E. Jouanguy, G. Vogt, J. Feinberg, A. Prochnicka-Chalufour, A. Casrouge, K. Yang, C. Soudais, C. Fieschi, et al. 2006. Novel STAT1 alleles in otherwise healthy patients with mycobacterial disease. PLoS Genet. 2:e131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Filipe-Santos, O., J. Bustamante, A. Chapgier, G. Vogt, L. de Beaucoudrey, J. Feinberg, E. Jouanguy, S. Boisson-Dupuis, C. Fieschi, C. Picard, and J.L. Casanova. 2006. Inborn errors of IL-12/23- and IFN-gamma-mediated immunity: molecular, cellular, and clinical features. Semin. Immunol. 18:347–361. [DOI] [PubMed] [Google Scholar]
- 6.Dorman, S.E., and S.M. Holland. 1998. Mutation in the signal-transducing chain of the interferon-gamma receptor and susceptibility to mycobacterial infection. J. Clin. Invest. 101:2364–2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vogt, G., A. Chapgier, K. Yang, N. Chuzhanova, J. Feinberg, C. Fieschi, S. Boisson-Dupuis, A. Alcais, O. Filipe-Santos, J. Bustamante, et al. 2005. Gains of glycosylation comprise an unexpectedly large group of pathogenic mutations. Nat. Genet. 37:692–700. [DOI] [PubMed] [Google Scholar]
- 8.Vogt, G., B. Vogt, N. Chuzhanova, K. Julenius, D.N. Cooper, and J.L. Casanova. 2007. Gain-of-glycosylation mutations. Curr. Opin. Genet. Dev. 17:245–251. [DOI] [PubMed] [Google Scholar]
- 9.Doffinger, R., E. Jouanguy, S. Dupuis, M.C. Fondaneche, J.L. Stephan, J.F. Emile, S. Lamhamedi-Cherradi, F. Altare, A. Pallier, G. Barcenas-Morales, et al. 2000. Partial interferon-gamma receptor signaling chain deficiency in a patient with bacille Calmette-Guerin and Mycobacterium abscessus infection. J. Infect. Dis. 181:379–384. [DOI] [PubMed] [Google Scholar]
- 10.Rosenzweig, S.D., S.E. Dorman, G. Uzel, S. Shaw, A. Scurlock, M.R. Brown, R.H. Buckley, and S.M. Holland. 2004. A novel mutation in IFN-gamma receptor 2 with dominant negative activity: biological consequences of homozygous and heterozygous states. J. Immunol. 173:4000–4008. [DOI] [PubMed] [Google Scholar]
- 11.Herscovics, A. 1999. Importance of glycosidases in mammalian glycoprotein biosynthesis. Biochim. Biophys. Acta. 1473:96–107. [DOI] [PubMed] [Google Scholar]
- 12.Cabral, C.M., Y. Liu, and R.N. Sifers. 2001. Dissecting glycoprotein quality control in the secretory pathway. Trends Biochem. Sci. 26:619–624. [DOI] [PubMed] [Google Scholar]
- 13.Ellgaard, L., and A. Helenius. 2003. Quality control in the endoplasmic reticulum. Nat. Rev. Mol. Cell Biol. 4:181–191. [DOI] [PubMed] [Google Scholar]
- 14.Hebert, D.N., S.C. Garman, and M. Molinari. 2005. The glycan code of the endoplasmic reticulum: asparagine-linked carbohydrates as protein maturation and quality-control tags. Trends Cell Biol. 15:364–370. [DOI] [PubMed] [Google Scholar]
- 15.Ohtsubo, K., and J.D. Marth. 2006. Glycosylation in cellular mechanisms of health and disease. Cell. 126:855–867. [DOI] [PubMed] [Google Scholar]
- 16.Parodi, A.J. 2000. Protein glucosylation and its role in protein folding. Annu. Rev. Biochem. 69:69–93. [DOI] [PubMed] [Google Scholar]
- 17.Helenius, A., and M. Aebi. 2004. Roles of N-linked glycans in the endoplasmic reticulum. Annu. Rev. Biochem. 73:1019–1049. [DOI] [PubMed] [Google Scholar]
- 18.Parodi, A.J. 2000. Role of N-oligosaccharide endoplasmic reticulum processing reactions in glycoprotein folding and degradation. Biochem. J. 348:1–13. [PMC free article] [PubMed] [Google Scholar]
- 19.Trombetta, E.S., and A.J. Parodi. 2003. Quality control and protein folding in the secretory pathway. Annu. Rev. Cell Dev. Biol. 19:649–676. [DOI] [PubMed] [Google Scholar]
- 20.Maley, F., R.B. Trimble, A.L. Tarentino, and T.H. Plummer Jr. 1989. Characterization of glycoproteins and their associated oligosaccharides through the use of endoglycosidases. Anal. Biochem. 180:195–204. [DOI] [PubMed] [Google Scholar]
- 21.Hershko, A., and A. Ciechanover. 1998. The ubiquitin system. Annu. Rev. Biochem. 67:425–479. [DOI] [PubMed] [Google Scholar]
- 22.Elbein, A.D. 1991. Glycosidase inhibitors: inhibitors of N-linked oligosaccharide processing. FASEB J. 5:3055–3063. [DOI] [PubMed] [Google Scholar]
- 23.Feinberg, J., C. Fieschi, R. Doffinger, M. Feinberg, T. Leclerc, S. Boisson-Dupuis, C. Picard, J. Bustamante, A. Chapgier, O. Filipe-Santos, et al. 2004. Bacillus Calmette Guerin triggers the IL-12/IFN-gamma axis by an IRAK-4- and NEMO-dependent, non-cognate interaction between monocytes, NK, and T lymphocytes. Eur. J. Immunol. 34:3276–3284. [DOI] [PubMed] [Google Scholar]
- 24.Volker, C., C.M. De Praeter, B. Hardt, W. Breuer, B. Kalz-Fuller, R.N. Van Coster, and E. Bause. 2002. Processing of N-linked carbohydrate chains in a patient with glucosidase I deficiency (CDG type IIb). Glycobiology. 12:473–483. [DOI] [PubMed] [Google Scholar]
- 25.Li, A., S. Davila, L. Furu, Q. Qian, X. Tian, P.S. Kamath, B.F. King, V.E. Torres, and S. Somlo. 2003. Mutations in PRKCSH cause isolated autosomal dominant polycystic liver disease. Am. J. Hum. Genet. 72:691–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Freeze, H.H. 2006. Genetic defects in the human glycome. Nat. Rev. Genet. 7:537–551. [DOI] [PubMed] [Google Scholar]
- 27.Soti, C., and P. Csermely. 2006. Pharmacological modulation of the heat shock response. Handb. Exp. Pharmacol. 2006:417–436. [DOI] [PubMed] [Google Scholar]
- 28.Egan, M.E., M. Pearson, S.A. Weiner, V. Rajendran, D. Rubin, J. Glockner-Pagel, S. Canny, K. Du, G.L. Lukacs, and M.J. Caplan. 2004. Curcumin, a major constituent of turmeric, corrects cystic fibrosis defects. Science. 304:600–602. [DOI] [PubMed] [Google Scholar]
- 29.Ulloa-Aguirre, A., J.A. Janovick, S.P. Brothers, and P.M. Conn. 2004. Pharmacologic rescue of conformationally-defective proteins: implications for the treatment of human disease. Traffic. 5:821–837. [DOI] [PubMed] [Google Scholar]
- 30.Grubb, B.R., S.E. Gabriel, A. Mengos, M. Gentzsch, S.H. Randell, A.M. Van Heeckeren, M.R. Knowles, M.L. Drumm, J.R. Riordan, and R.C. Boucher. 2006. SERCA pump inhibitors do not correct biosynthetic arrest of deltaF508 CFTR in cystic fibrosis. Am. J. Respir. Cell Mol. Biol. 34:355–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bernier, V., M. Lagace, D.G. Bichet, and M. Bouvier. 2004. Pharmacological chaperones: potential treatment for conformational diseases. Trends Endocrinol. Metab. 15:222–228. [DOI] [PubMed] [Google Scholar]
- 32.Cohen, F.E., and J.W. Kelly. 2003. Therapeutic approaches to protein-misfolding diseases. Nature. 426:905–909. [DOI] [PubMed] [Google Scholar]
- 33.Torres, G. 1995. BUCAST: another new antiviral. GMHC Treat. Issues. 9:4–5. [PubMed] [Google Scholar]
- 34.Tierney, M., J. Pottage, H. Kessler, M. Fischl, D. Richman, T. Merigan, W. Powderly, S. Smith, A. Karim, J. Sherman, et al. 1995. The tolerability and pharmacokinetics of N-butyl-deoxynojirimycin in patients with advanced HIV disease (ACTG 100). The AIDS Clinical Trials Group (ACTG) of the National Institute of Allergy and Infectious Diseases. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 10:549–553. [PubMed] [Google Scholar]
- 35.Weinreb, N.J., J.A. Barranger, J. Charrow, G.A. Grabowski, H.J. Mankin, and P. Mistry. 2005. Guidance on the use of miglustat for treating patients with type 1 Gaucher disease. Am. J. Hematol. 80:223–229. [DOI] [PubMed] [Google Scholar]
- 36.Cox, T., R. Lachmann, C. Hollak, J. Aerts, S. van Weely, M. Hrebicek, F. Platt, T. Butters, R. Dwek, C. Moyses, et al. 2000. Novel oral treatment of Gaucher's disease with N-butyldeoxynojirimycin (OGT 918) to decrease substrate biosynthesis. Lancet. 355:1481–1485. [DOI] [PubMed] [Google Scholar]
- 37.Dwek, R.A., T.D. Butters, F.M. Platt, and N. Zitzmann. 2002. Targeting glycosylation as a therapeutic approach. Nat. Rev. Drug Discov. 1:65–75. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.