Abstract
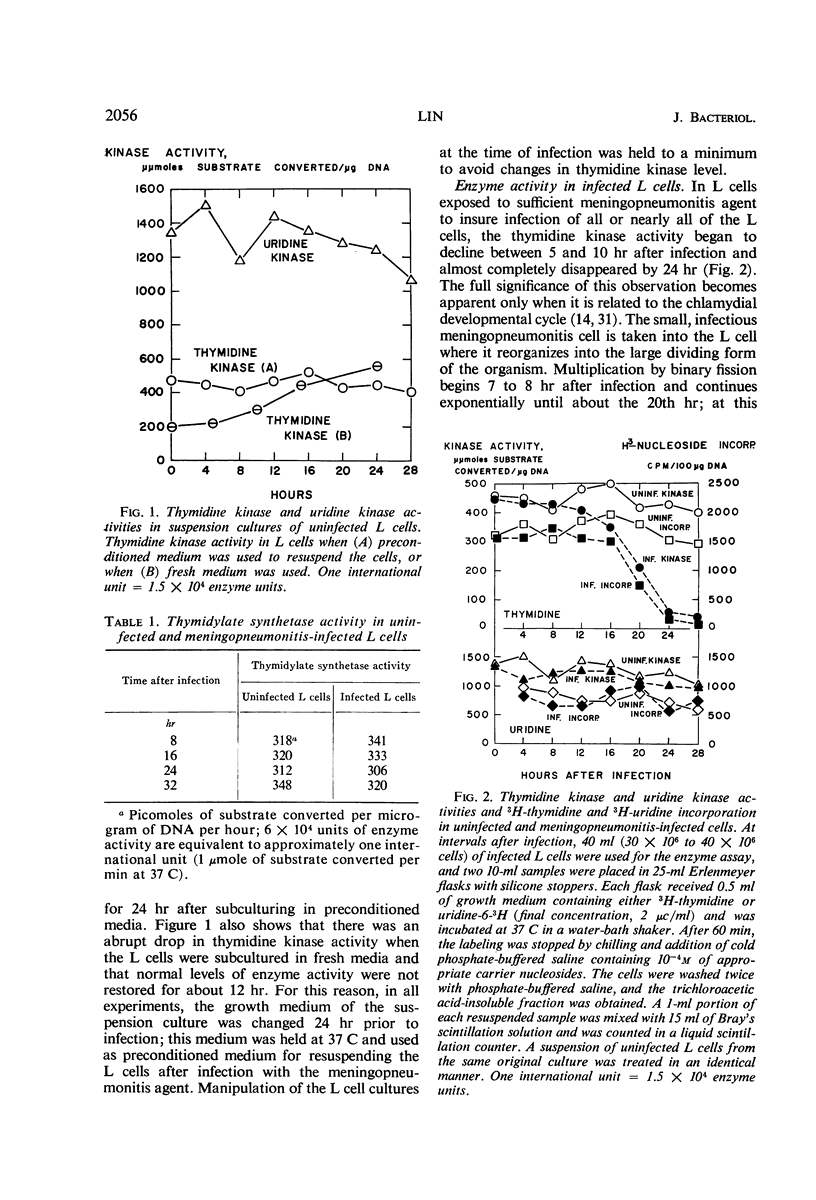
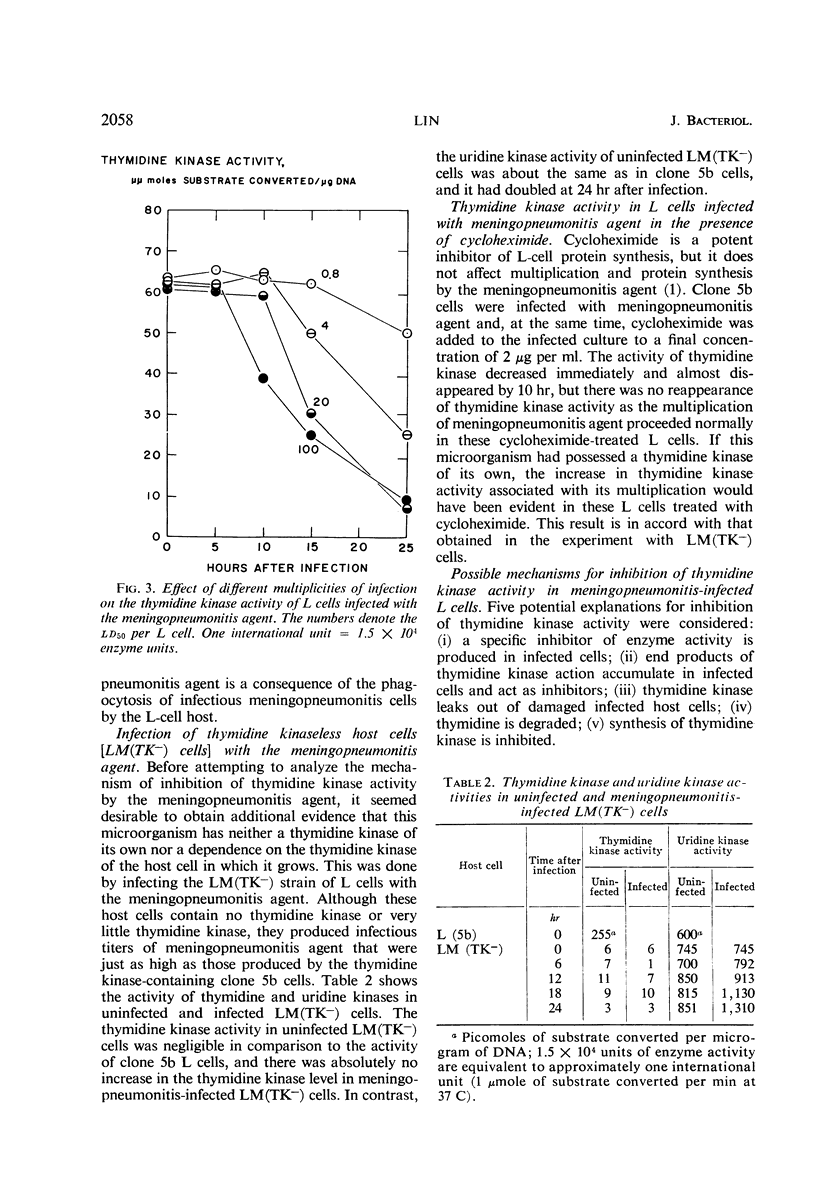
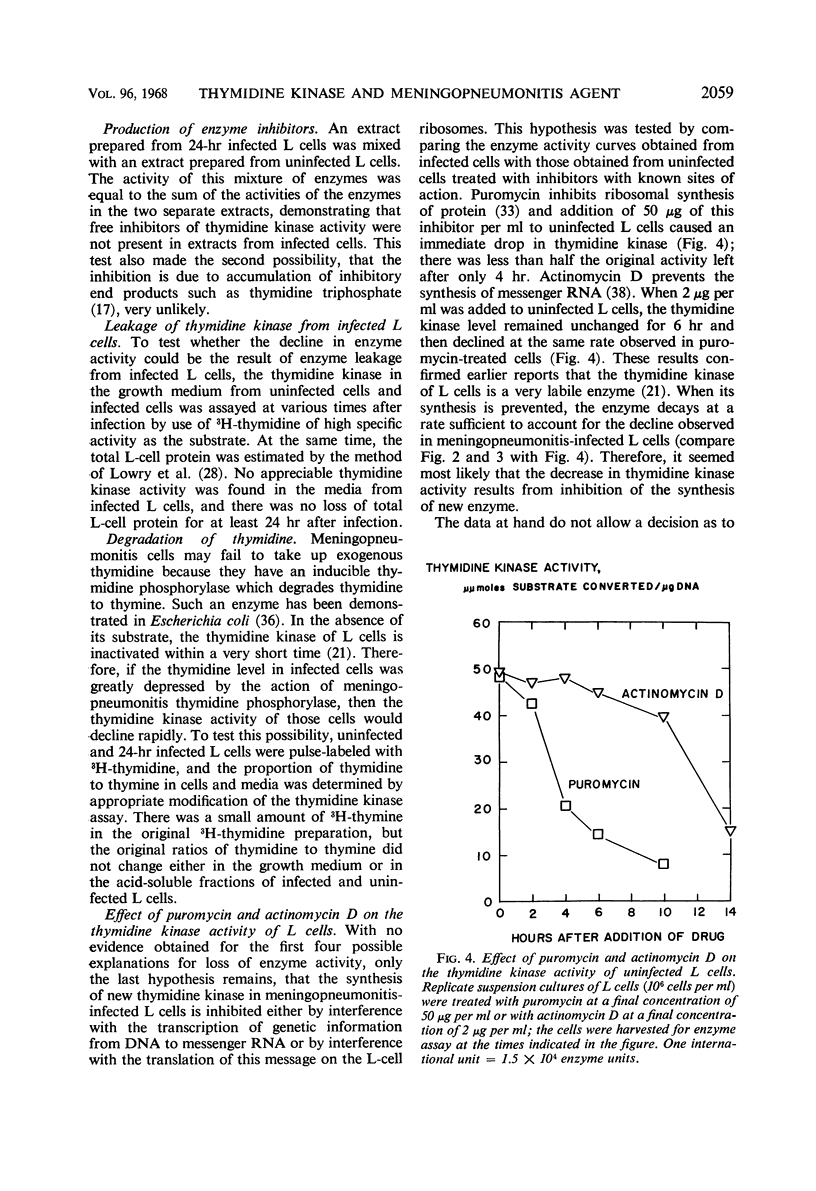
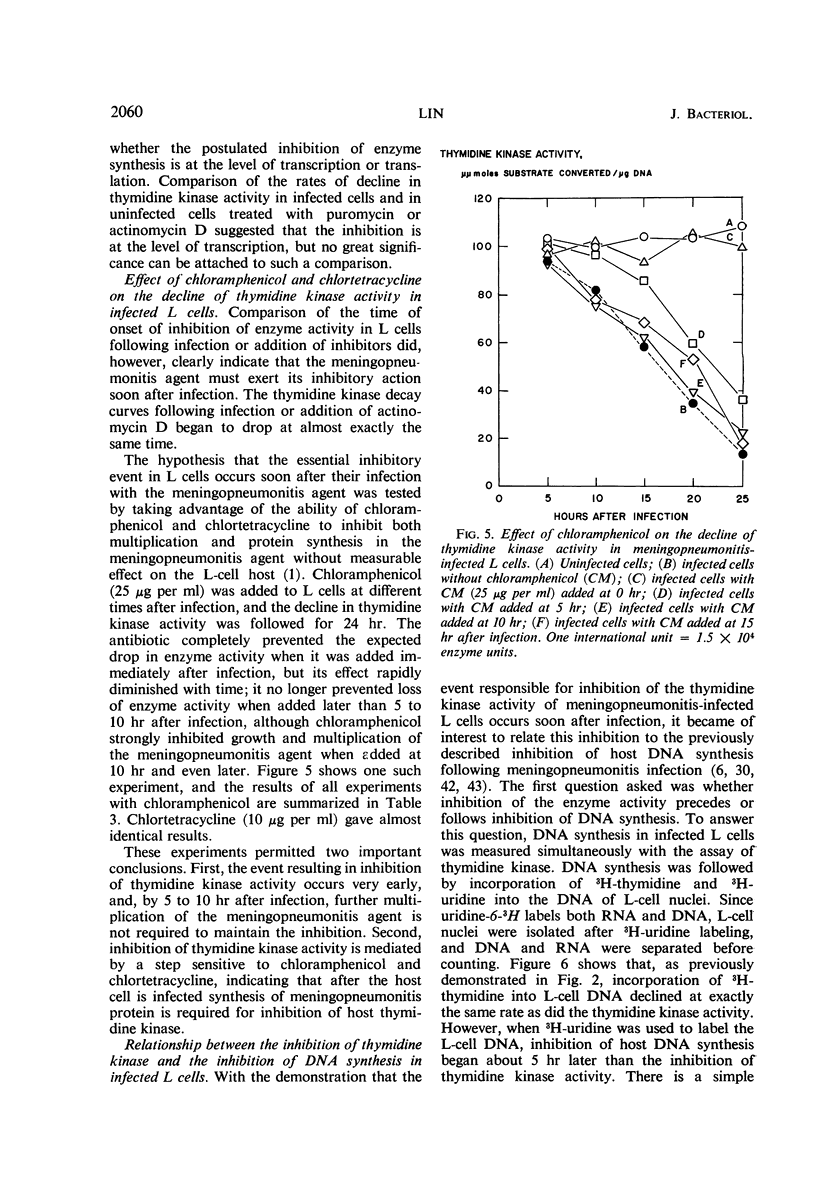
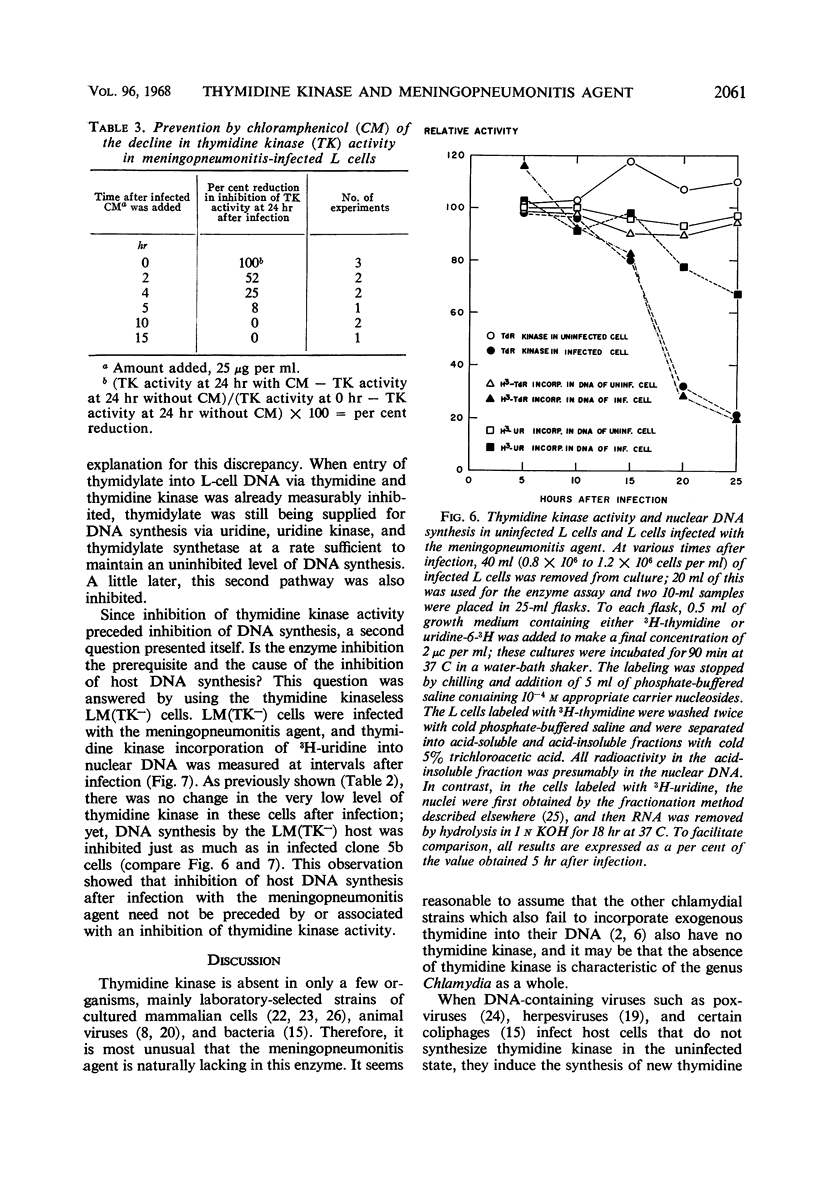
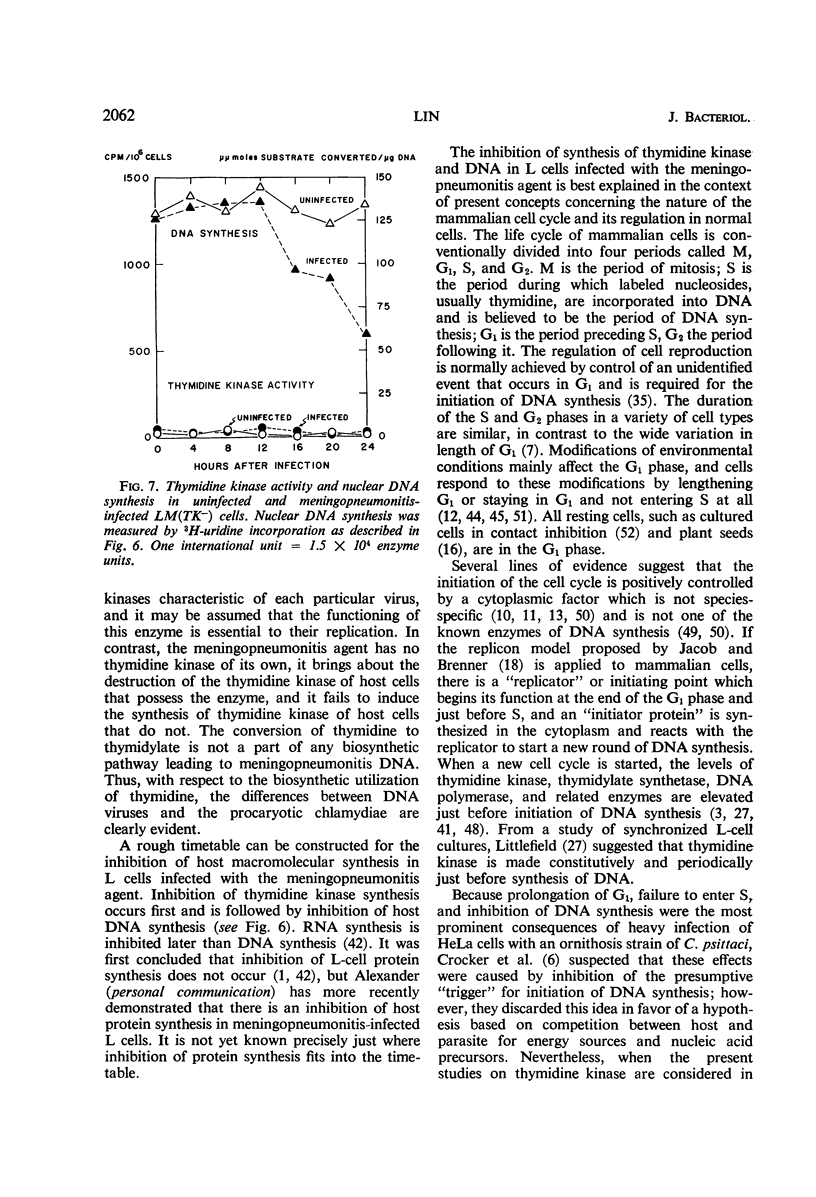
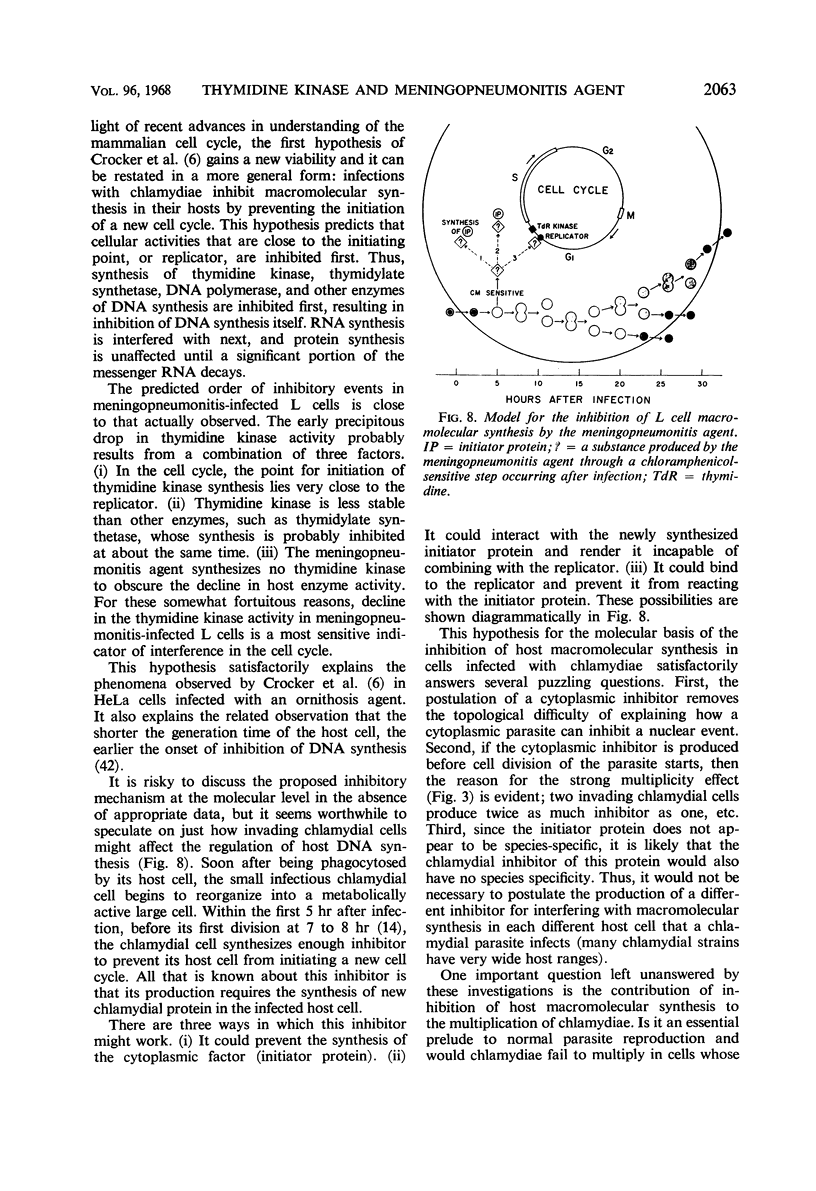
The activities of enzymes related to deoxyribonucleic acid (DNA) synthesis were studied in uninfected L cells and in L cells infected with Chlamydia psittaci (strain meningopneumonitis). The meningopneumonitis agent multiplied normally but failed to induce the synthesis of thymidine kinase in LM (TK−) cells which contain no thymidine kinase in the uninfected state. It was concluded that this microorganism has no thymidine kinase of its own and that it does not depend on the functioning of the host enzyme for synthesizing its DNA. Exposure of clone 5b L cells to the meningopneumonitis agent was followed by a decline in their thymidine kinase activity to nearly zero levels, whereas the levels of uridine kinase and thymidylate synthetase remained unchanged. Inhibition of thymidine kinase activity in L cells occurred soon after infection and required new protein synthesis by the meningopneumonitis agent. This inhibition occurred before inhibition of host DNA synthesis, but it was not an essential prelude to the latter inhibition. On the basis of this and previous investigations and in light of present knowledge of the mammalian cell cycle, it was postulated that the meningopneumonitis agent inhibits macromolecular synthesis in L cells by preventing the initiation of a new cell cycle.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander J. J. Separation of protein synthesis in meningopneumonitisgent from that in L cells by differential susceptibility to cycloheximide. J Bacteriol. 1968 Feb;95(2):327–332. doi: 10.1128/jb.95.2.327-332.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERNKOPF H., MASHIAH P., BECKER Y. Correlation between morphological and biochemical changes and the appearance of infectivity in FL cell cultures infected with trachoma agent. Ann N Y Acad Sci. 1962 Mar 5;98:62–81. doi: 10.1111/j.1749-6632.1962.tb30532.x. [DOI] [PubMed] [Google Scholar]
- BOLLUM F. J., POTTER V. R. Nucleic acid metabolism in regenerating rat liver. VI. Soluble enzymes which convert thymidine to thymidine phosphates and DNA. Cancer Res. 1959 Jun;19(5):561–565. [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CROCKER T. T., PELC S. R., NIELSEN B. I., EASTWOOD J. M., BANKS J. POPULATION DYNAMICS AND DEOXYRIBONUCLEIC ACID SYNTHESIS IN HELA CELLS INFECTED WITH AN ORNITHOSIS AGENT. J Infect Dis. 1965 Apr;115:105–122. doi: 10.1093/infdis/115.2.105. [DOI] [PubMed] [Google Scholar]
- DUBBS D. R., KIT S. ISOLATION AND PROPERTIES OF VACCINIA MUTANTS DEFICIENT IN THYMIDINE KINASE-INDUCING ACTIVITY. Virology. 1964 Feb;22:214–225. doi: 10.1016/0042-6822(64)90006-6. [DOI] [PubMed] [Google Scholar]
- DULBECCO R., VOGT M. Plaque formation and isolation of pure lines with poliomyelitis viruses. J Exp Med. 1954 Feb;99(2):167–182. doi: 10.1084/jem.99.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurdon J. B. On the origin and persistence of a cytoplasmic state inducing nuclear DNA synthesis in frogs' eggs. Proc Natl Acad Sci U S A. 1967 Aug;58(2):545–552. doi: 10.1073/pnas.58.2.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIGASHI N. ELECTRON MICROSCOPIC STUDIES ON THE MODE OF REPRODUCTION OF TRACHOMA VIRUS AND PSITTACOSIS VIRUS IN CELL CULTURES. Exp Mol Pathol. 1965 Feb;76:24–39. doi: 10.1016/0014-4800(65)90021-3. [DOI] [PubMed] [Google Scholar]
- Hahn G. M., Bagshaw M. A. Serum concentration: effects on cycle and x-ray sensitivity of mammalian cells. Science. 1966 Jan 28;151(3709):459–461. doi: 10.1126/science.151.3709.459. [DOI] [PubMed] [Google Scholar]
- Harris H. The reactivation of the red cell nucleus. J Cell Sci. 1967 Mar;2(1):23–32. doi: 10.1242/jcs.2.1.23. [DOI] [PubMed] [Google Scholar]
- Hiraga S., Igarashi K., Yura T. A deoxythymidine kinase-deficient mutant of Escherichia coli. I. Isolation and some properties. Biochim Biophys Acta. 1967 Aug 22;145(1):41–51. doi: 10.1016/0005-2787(67)90652-1. [DOI] [PubMed] [Google Scholar]
- Hotta Y., Stern H. MOLECULAR FACETS OF MITOTIC REGULATION, I. SYNTHESIS OF THYMIDINE KINASE. Proc Natl Acad Sci U S A. 1963 May;49(5):648–654. doi: 10.1073/pnas.49.5.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IVES D. H., MORSE P. A., Jr, POTTER V. R. Feedback inhibition of thymodine kinase by thymodine triphosphate. J Biol Chem. 1963 Apr;238:1467–1474. [PubMed] [Google Scholar]
- JACOB F., BRENNER S. [On the regulation of DNA synthesis in bacteria: the hypothesis of the replicon]. C R Hebd Seances Acad Sci. 1963 Jan 2;256:298–300. [PubMed] [Google Scholar]
- KIT S., DUBBS D. R. Acquisition of thymidine kinase activity by herpes simplex-infected mouse fibroblast cells. Biochem Biophys Res Commun. 1963 Apr 2;11:55–59. doi: 10.1016/0006-291x(63)90027-5. [DOI] [PubMed] [Google Scholar]
- KIT S., DUBBS D. R., PIEKARSKI L. J., HSU T. C. DELETION OF THYMIDINE KINASE ACTIVITY FROM L CELLS RESISTANT TO BROMODEOXYURIDINE. Exp Cell Res. 1963 Aug;31:297–312. doi: 10.1016/0014-4827(63)90007-7. [DOI] [PubMed] [Google Scholar]
- KIT S., DUBBS D. R. PROPERTIES OF DEOXYTHYMIDINE KINASE PARTIALLY PURIFIED FROM NONINFECTED MOUSE FIBROBLAST CELLS. Virology. 1965 May;26:16–27. doi: 10.1016/0042-6822(65)90021-8. [DOI] [PubMed] [Google Scholar]
- KIT S., PIEKARSKI L. J., DUBBS D. R. Induction of thymidine kinase by vaccinia-infected mouse fibroblasts. J Mol Biol. 1963 Jan;6:22–33. doi: 10.1016/s0022-2836(63)80078-9. [DOI] [PubMed] [Google Scholar]
- Kit S., Dubbs D. R., Frearson P. M. HeLa cells resistant to bromodeoxyuridine and deficient in thymidine kinase activity. Int J Cancer. 1966 Jan;1(1):19–30. doi: 10.1002/ijc.2910010105. [DOI] [PubMed] [Google Scholar]
- LITTLEFIELD J. W. STUDIES ON THYMIDINE KINASE IN CULTURED MOUSE FIBROBLASTS. Biochim Biophys Acta. 1965 Jan 11;95:14–22. doi: 10.1016/0005-2787(65)90206-6. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lin H. S. Stability of the nucleic acids of L cells after infection with the meningopneumonitis agent. J Bacteriol. 1968 Dec;96(6):2049–2093. doi: 10.1128/jb.96.6.2049-2053.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littlefield J. W. The periodic synthesis of thymidine kinase in mouse fibroblasts. Biochim Biophys Acta. 1966 Feb 21;114(2):398–403. doi: 10.1016/0005-2787(66)90319-4. [DOI] [PubMed] [Google Scholar]
- McCOY T. A., MAXWELL M., KRUSE P. F., Jr Amino acid requirements of the Novikoff hepatoma in vitro. Proc Soc Exp Biol Med. 1959 Jan;100(1):115–118. doi: 10.3181/00379727-100-24542. [DOI] [PubMed] [Google Scholar]
- Moore D. E., Moulder J. W. Autoradiographic study of deoxyribonucleic acid synthesis in L cells infected with the agent of meningopneumonitis. J Bacteriol. 1966 Oct;92(4):1128–1132. doi: 10.1128/jb.92.4.1128-1132.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulder J. W. The relation of the psittacosis group (Chlamydiae) to bacteria and viruses. Annu Rev Microbiol. 1966;20:107–130. doi: 10.1146/annurev.mi.20.100166.000543. [DOI] [PubMed] [Google Scholar]
- Nardone R. M., Todd J., Gonzalez P., Gaffney E. V. Nucleoside incorporation into strain L cells: inhibition by pleuropneumonia-like organisms. Science. 1965 Sep 3;149(3688):1100–1101. doi: 10.1126/science.149.3688.1100. [DOI] [PubMed] [Google Scholar]
- RACHMELER M., GERHART J., ROSNER J. Limited thymidine uptake in Escherichia coli due to an inducible thymidine phosphorylase. Biochim Biophys Acta. 1961 Apr 29;49:222–225. doi: 10.1016/0006-3002(61)90888-5. [DOI] [PubMed] [Google Scholar]
- Randall C. C., Gafford L. G., Gentry G. A., Lawson L. A. Lability of host-cell DNA in growing cell cultures due to Mycoplasma. Science. 1965 Sep 3;149(3688):1098–1099. doi: 10.1126/science.149.3688.1098. [DOI] [PubMed] [Google Scholar]
- Roberts D. An isotopic assay for thymidylate synthetase. Biochemistry. 1966 Nov;5(11):3546–3548. doi: 10.1021/bi00875a022. [DOI] [PubMed] [Google Scholar]
- SCHECHTER E. M., TRIBBY I. I., MOULDER J. W. NUCLEIC ACID METABOLISM IN L CELLS INFECTED WITH A MEMBER OF THE PSITTACOSIS GROUP. Science. 1964 Aug 21;145(3634):819–821. doi: 10.1126/science.145.3634.819. [DOI] [PubMed] [Google Scholar]
- STARR T. J., SHARON N. AUTORADIOGRAPHY WITH THE AGENTS OF PSITTACOSIS AND TRACHOMA. Proc Soc Exp Biol Med. 1963 Aug-Sep;113:912–914. doi: 10.3181/00379727-113-28529. [DOI] [PubMed] [Google Scholar]
- Sachsenmaier W., von Fournier D., Gürtler K. F. Periodic thymidine kinase production in synchronous plasmodia of Physarum polycephalum: inhibition by actinomycin and actidion. Biochem Biophys Res Commun. 1967 Jun 23;27(6):655–660. doi: 10.1016/s0006-291x(67)80085-8. [DOI] [PubMed] [Google Scholar]
- Schechter E. M. Synthesis of nucleic acid and protein in L cells infected with the agent of meningopneumonitis. J Bacteriol. 1966 May;91(5):2069–2080. doi: 10.1128/jb.91.5.2069-2080.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisken J. E., Morasca L., Kibby S. Effects of temperature on the kinetics of the mitotic cycle of mammalian cells in culture. Exp Cell Res. 1965 Aug;39(1):103–116. doi: 10.1016/0014-4827(65)90012-1. [DOI] [PubMed] [Google Scholar]
- Stubblefield E., Mueller G. C. Thymidine kinase activity in synchronized HeLa cell cultures. Biochem Biophys Res Commun. 1965 Aug 16;20(4):535–538. doi: 10.1016/0006-291x(65)90613-3. [DOI] [PubMed] [Google Scholar]
- Taylor E. W. Control of DNA synthesis in mammalian cells in culture. Exp Cell Res. 1965 Nov;40(2):316–332. doi: 10.1016/0014-4827(65)90265-x. [DOI] [PubMed] [Google Scholar]
- Thompson L. R., McCarthy B. J. Stimulation of nuclear DNA and RNA synthesis by cytoplasmic extracts in vitro. Biochem Biophys Res Commun. 1968 Jan 25;30(2):166–172. doi: 10.1016/0006-291x(68)90465-8. [DOI] [PubMed] [Google Scholar]
- Tobey R. A., Anderson E. C., Petersen D. F. The effect of thymidine on the duration of G1 in Chinese hamster cells. J Cell Biol. 1967 Oct;35(1):53–59. doi: 10.1083/jcb.35.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todaro G. J., Lazar G. K., Green H. The initiation of cell division in a contact-inhibited mammalian cell line. J Cell Physiol. 1965 Dec;66(3):325–333. doi: 10.1002/jcp.1030660310. [DOI] [PubMed] [Google Scholar]
- Tribby I. I., Moulder J. W. Availability of bases and nucleosides as precursors of nucleic acids in L cells and in the agent of meningopneumonitis. J Bacteriol. 1966 Jun;91(6):2362–2367. doi: 10.1128/jb.91.6.2362-2367.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]



