Abstract
Tumor necrosis factor (TNF) has very potent antitumor activity, but it also provokes a systemic inflammatory response syndrome that leads to shock, organ failure, and death. Here, we demonstrate that interleukin (IL)-17, a proinflammatory cytokine known to be produced mainly by activated T cells, has a critical role in this process. Antiserum against IL-17 or deletion of Il17r protected mice against a lethal TNF challenge. Serum levels of TNF-induced IL-6 and nitric oxide metabolites were significantly reduced in mice deficient in the IL-17R. TNF-induced leukocyte influx in the small intestine was reduced, and there was no injury to the small intestine. Surprisingly, electron microscopy showed that IL-17 was constitutively present in Paneth cells of the crypts. Upon TNF challenge, the intracellular pool of IL-17 in these cells was drastically reduced, suggesting rapid release of IL-17 from the granules of Paneth cells. Our findings assign a novel role for IL-17 in an acute inflammation and identify Paneth cells as a source of the IL-17 that plays a role in this process. These data indicate that innate immune cytokine responses in the local mucosa may participate in rapidly amplifying responses to systemic inflammatory challenges.
TNF has a very powerful antitumor activity. Therapeutic administration of TNF to tumor-bearing animals or to human patients, however, is greatly limited by its toxicity, which is due to its strong proinflammatory nature. Indeed, injection of TNF leads to refractory hypotension, systemic inflammation, multi-organ failure, shock, and death, collectively known as systemic inflammatory response syndrome (SIRS) (1). Only a fundamental understanding of the mechanisms, molecules, and cells leading to TNF-induced SIRS will allow full exploitation of the potent antitumor activity of TNF in specific interventions against cancer. Our previous findings demonstrated that manipulation of several pathways protects the host against the toxicity of TNF without affecting its antitumor activity (2, 3).
IL-17 belongs to a family of proinflammatory cytokines (4). The role of IL-17 in host immune defense and in inflammation has been studied extensively in recent years. Numerous subtypes of IL-17–like ligands and IL-17R–like receptors have been described. The IL-17 family consists so far of six members, IL-17A to IL-17F. Their receptors, IL-17R and IL-17RB-E, form a family whose ligand specificity is only partially known (4). IL-17 is mainly produced by a subset of T cells implicated in autoimmune inflammation; these cells, designated Th17 cells, arise from a CD4 precurser pool and are distinct from Th1 or Th2 cells (5–7). Spontaneous development of Th17 causes autoimmune arthritis (8). IL-17–neutralizing antibodies or deletion of the gene encoding the IL-17 or IL-17R protects animals in models of autoimmune diseases, whereas transfer of Th17 or overexpression of IL-17 aggravates the disease (6, 9–13). IL-17 induces expression of inflammatory genes, such as il8, and synergizes with TNF (14). We investigated the role of the IL-17–IL-17R axis in a model of TNF-induced lethal shock. We demonstrate that inhibition of IL-17 or IL-17R strongly protects against TNF-induced gene induction, organ damage, and death. Furthermore, we found that intestinal Paneth cells produce and release a high level of IL-17 during inflammation.
RESULTS AND DISCUSSION
Neutralizing antibodies against IL-17 protect mice against a lethal TNF challenge
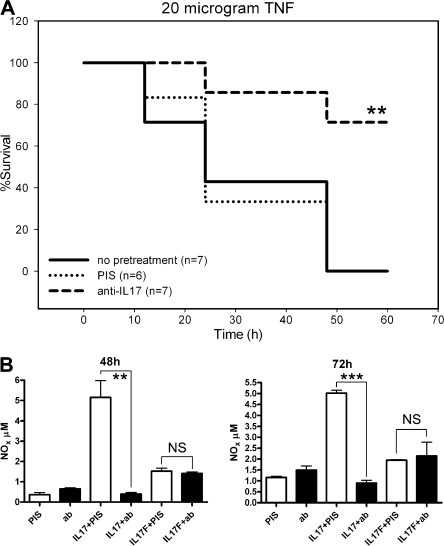
We investigated the role of IL-17 in TNF-induced shock using neutralizing antibodies. Mice were injected i.v. with 10 μg TNF 2 h after treatment with 100 μl of a rabbit anti–IL-17 antiserum. Control mice received an equal volume of rabbit preimmune serum. Mortality was monitored for up to 60 h, and body temperature was assessed 4 and 6 h after injection. In the first experiment, all five control mice died within 24 h after challenge, whereas all three mice treated with anti–IL-17 antiserum survived. Mice pretreated with anti–IL-17 were significantly protected against hypothermia (not depicted). In the second experiment, significant protection by the anti–IL-17 serum was again observed (Fig. 1 A). This antiserum specifically neutralized IL-17(A) but not IL-17F, as shown by an in vitro assay (Fig. 1 B). As IL-17 is produced mainly by a subset of T cells, its role is presumably restricted to inflammation associated with underlying T cell–mediated immunity, such as autoimmunity or allergy (6, 9, 10, 12, 15). The time course of IL-17 production is slow, reflecting the development of a specific T cell subset (6). However, in our model, SIRS developed a few hours after systemic administration of TNF, and mice died after 12–48 h. Also, nude and SCID mice were fully responsive to TNF-induced SIRS (unpublished data), which reveals no major role for specific T cells. Hence, the prevention of TNF-induced shock by anti–IL-17 antiserum suggests a potentially new role for IL-17. Our observation supports a recent report on the aggravating effect of IL-17 in another model of SIRS, caecal ligation and puncture (16).
Figure 1.
Anti–IL-17 antibody protects mice against TNF-induced lethal shock. (A) Mice were pretreated 2 h before TNF challenge with 100 μl anti–IL-17 serum (n = 7), 100 μl control rabbit serum (n = 6), or PBS (n = 7). Mortality was monitored for 60 h after challenge. No further deaths occurred. **, P = 0.0074, preimmune versus anti–IL-17 serum; **, P = 0.0072, PBS versus anti–IL-17 serum. (B) H4 cells were incubated with 25 ng/ml IL-17(A) or 25 ng/ml IL-17F with or without anti–IL-17 serum (1:400). **, P = 0.0044; ***, P = 0.0001.
IL-17R KO mice are protected against a lethal TNF challenge
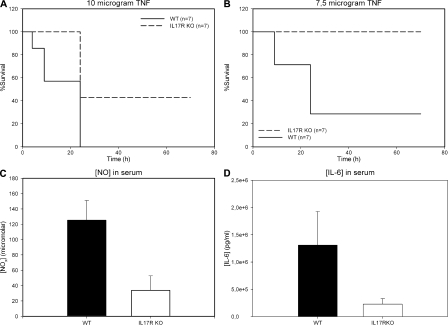
Mice made IL-17R deficient by targeted gene deletion (17) were moderately but significantly protected against 10 μg TNF, which causes 100% mortality in control WT mice (Fig. 2 A). Protection was much more pronounced when 7.5 μg TNF was used (Fig. 2 B). These results confirm our previous data on the use of antiserum against IL-17 and indicate that an intact IL-17–IL-17R axis plays a critical role in the lethality of TNF-induced shock. The partial dependency of the TNF effect on IL-17 indicates that IL-17 enhances or amplifies this effect, resulting in significant reduction of the lethal threshold of TNF. This is in agreement with the observed synergy between IL-17 and other proinflammatory cytokines such as TNF and IL-1 (14, 15).
Figure 2.
IL-17R KO mice are less susceptible to TNF-induced shock. TNF was injected i.v. in WT (n = 7) and IL-17R KO (n = 7) mice, and mortality was monitored. Blood samples were taken 3 h after the injection, and serum samples were tested for IL-6 and NOx. (A and B) Survival curves after 10 and 7.5 μg TNF, respectively. *, P = 0.00175 and **, P = 0.0075 compared with WT control. (C and D) Serum levels of NOx (***, P = 0.0002; n = 5) and IL-6 (**, P = 0.0017; n = 6) 3 h after injecting 7.5 μg TNF.
Reduced serum levels of IL-6 and nitric oxide (NO) metabolites and reduced tissue damage and inflammation in IL-17R KO mice
Serum levels of IL-6 and NO metabolites increase after injection of TNF, faithfully reflect the degree of TNF-induced shock, and correlate with lethality (3, 18). 3 h after injection of 7.5 μg TNF, NOx levels increased to 120 μM in WT mice but remained significantly lower in IL-17R KO mice (Fig. 2 C). Similarly, the increase in serum IL-6 concentration was large in WT mice but significantly less in IL-17R KO mice (Fig. 2 D). These results strongly support the mediating role of IL-17, together with its receptor, in TNF-induced shock.
TNF injected into mice or humans causes severe inflammation and tissue damage in the small bowel (19). 3 h after injection of 7.5 μg TNF, small bowel samples (jejunum) were collected and stained for histopathological and immunohistochemical (IHC) analyses. The intestinal epithelium of TNF-treated WT mice (n = 6) was extensively damaged, with partial loss of morphological structure. The villi were flattened and their tops were denuded or missing. Crypts contained mucus and debris (Fig. 3 A). In contrast, the morphology of all IL-17R KO intestines (n = 6) after TNF challenge was almost normal with little or no damage (Fig. 3 B). Staining the intestinal sections of WT mice for leukocytes with an anti-CD45 antibody 3 h after TNF injection demonstrated massive leukocyte influx in the villi, where the extensive tissue damage occurred (Fig. 3 C). Much fewer leukocytes were found in the IL-17R KO mice, correlating with the absence of tissue damage (Fig. 3, D and E). IL-17 induces expression of CXC chemokines, such as CXCL1, which account for chemotaxis of leukocytes (14) and activates neutrophils to release elastase and myeloperoxidase (MPO) in vivo (20). In line with this, by using anti-MPO antibody we observed neutrophil infiltrate in some WT mice, which was not seen in any of the IL-17R KO mice (not depicted).
Figure 3.
IL-17 R KO mice are protected against TNF-induced bowel tissue destruction. 7.5 μg TNF was injected i.v. in WT mice (n = 6) and IL-17R KO mice (n = 6), and jejunum was sampled 3 h later. (A and C) WT mice. (B and D) IL17R KO mice. (A and B) Standard hematoxylin and eosin staining showed more extensive tissue damage in the bowels of WT mice compared with KO mice. (C and D) Staining with an anti-CD45 antibody shows significant influx of leukocytes in WT mice (C) but not in IL17R KO mice (D). (E) Quantification of CD45+ cells in WT and IL17R KO mice 3 h after TNF injection in 10 defined fields (*, P = 0.0121). Bar, 10 μm.
Induction of IL-17 by TNF
Protection against TNF-induced shock by the anti–IL-17 antibody and by genetic deletion of the IL-17R indicates that IL-17 is produced endogenously and acts synergistically to enhance the actions of TNF. To test this hypothesis, we studied the time course of IL-17 abundance in the serum and small intestine where the pronounced tissue damage was observed after TNF administration. Serum samples were screened for IL-17 activity by Luminex assay; IL-17 remained below the detection limit (10 pg/ml) at all time points (not depicted). However, IHC examination using an antibody against mouse IL-17 revealed IL-17+ cells in the small intestine. Jejunum of untreated mice showed a constitutive level of IL-17 in cells located at the crypt bottom, which is surprising (Fig. 4 A). A few faint signals could occasionally be found in lamina propria, but the most prominent specific signal was found at the crypt bottom. The IL-17 signal clearly increased 1 h after TNF administration, peaked at 3 h, and remained high up to 9 h (not depicted). At 3 h after TNF administration, the overall signal intensity was dramatically increased and extended outward to the surface of the villi (Fig. 4 B). That the antibody is IL-17 specific was confirmed using the tissue of untreated IL-17 KO mice (provided by Y. Iwakura, Tokyo University, Tokyo, Japan) (Fig. 4 C). As lamina propria lymphocytes and γδT cells are the dominant cell types producing IL-17 (20–22), the increased IL-17 associated with the villi can be partly attributed to these cells. The negative control for each time point was devoid of any signal (Fig. 4, D and E, respectively). This local expression was characteristic of IL-17, as we did not detect any signal using antibody against IL-1β or IL-18 (not depicted). As the unexpected localization of IL-17 at the crypt bottom seemed to be associated with Paneth cells on morphological grounds, we further explored the identity of these cells using the IL-17 antibody and a specific anti-matrilysin antibody on serial sections (Fig. 4, H and I). Because γδT cells reside in the intraepithelial layer (23) and not at the crypt bottom (24), it is unlikely that the observed signal came from this cell type. The matrilysin signal was specific, as shown by the absence of signal in matrilysin-deficient mice (Fig. 4 F). Matrilysin has been shown to be specifically expressed in Paneth cells, where it activates α-defensins (25). We confirmed the expression of IL-17 in the jejunum by real-time PCR; 3 h after TNF challenge, the transcript level of IL-17 was significantly up-regulated, about threefold (Fig. 4 G).
Figure 4.
Expression of IL-17 in mouse jejunum after TNF injection. Small intestines were dissected from untreated mice (A, C, D, F, H, and I) and 3 h after i.v. injection with 7.5 μg TNF (B and E). WT mice (A, B, D, E, H, and I), matrilysin-deficient mice (F), and IL-17–deficient mice (C) were used. (A, B, C and H) IHC with anti–IL-17 antibody shows presence of IL-17 by TNF in Paneth cells at 0 h (A and H) and 3 h (B). No signal could be detected in IL-17–deficient mice (C). (D and E) Negative control with control antibody at 0 h (D) and 3 h (E). (F and I) IHC with anti-matrilysin antibody shows expression of matrilysin in Paneth cells of WT mice (I) and its absence in matrilysin KO mice (F). (G) Real-time PCR for IL-17 in samples isolated from jejunum at indicated times. *, P = 0.0458. Bar, 50 μm.
Identification of Paneth cells as the producers of IL-17
Co-localization of IL-17 and matrilysin strongly indicated that Paneth cells are the likely producers of IL-17, and so we set out to confirm this by electron microscopy (EM). We examined the localization of IL-17 within Paneth cells by using immunogold (Fig. 5 A). The Paneth cells were identified on the basis of typical ultrastructural morphology with large secretory granules and abundant endoplasmic reticulum. The gold particles were closely associated with secretory granules (Fig. 5 A, black arrow), demonstrating high constitutive levels of IL-17 in Paneth cells. Some signals were associated with microvilli (Fig. 5 A, white arrow), most likely due to secreted IL-17 adhering on the surface. We did not detect any γδT cells producing IL-17 because the sensitivity might have been too low or because their spatial distribution might have made it difficult to identify these cells in the ultrathin sections. Contrary to expectation, the level of IL-17 within the Paneth cells declined 3 h after injection with TNF (Fig. 5 B). The negative control using the control antibody was devoid of any signal, confirming the specificity of the observed signal (Fig. 5 C). This finding seemed to contradict the result of IHC using the same antibody, where an increase in the signal was detected after TNF injection. This seeming discrepancy might be due to TNF inducing rapid secretion of IL-17 stored in the granules. IHC may detect the cumulative level of secreted IL-17 around the neighboring cells beyond the immediately adjacent microvilli, whereas EM may not. Indeed, in IHC the strong signal was observed in the villi surface and occasionally in cells, presumably lamina propria lymphocytes and intraepithelial lymphocytes (Fig. 4 B). Therefore, it appears that the TNF-induced positive feedback loop involves two levels. IL-17 is first secreted from the abundant intracellular vesicles, and then mRNA for IL-17 is up-regulated to restore the intracellular reservoir. This kind of regulation fits very well with the function of Paneth cells in the first line of defense.
Figure 5.
Expression of IL-17 in Paneth cells. Small intestines were dissected from untreated mice (A and C) and 3 h after i.v. injection with 10 μg TNF (B) and examined by EM. Immunogold detection of IL-17 in secretory granules (black arrow) and in microvilli (white arrow) (A and B). Control antibody shows no signal (C). The antibodies used were the same as in Fig. 4. Bar, 1 μm.
Indeed, Paneth cells are very important in innate immunity against pathogens, expressing pattern recognition receptors, e.g., NOD2, and many antimicrobial peptides, such as lysozyme, soluble phospholipases, α-defensins, and cryptdin-related peptides (26). Paneth cells are involved in anti-parasitic immunity and also express several inflammatory molecules, such as TNF, GM-CSF, and inducible NO synthase (27, 28). Moreover, Paneth cells have a clear capacity to respond to inflammatory cytokines as shown in the induction of inducible NO synthase by TNF (28). The leukocyte-like Paneth cells, because they release TNF, are thought to be essential both in mucosal immunity against pathogens and in development of Crohn's disease. IL-17 expression seems to be increased in the colons of Crohn's patients (29), but it has never been associated with Paneth cells until now.
To date, IL-17–producing cells were identified only among leukocytes: Th17 cells, subset of CD8+ cells, NK cells, αβT cells, γδT cells, and neutrophils (30). Our data clearly demonstrate that IL-17 is produced in Paneth cells and released upon challenge with TNF, and that it seems to be involved in a positive amplification loop together with the major IL-17 receptor. This receptor is expressed on a variety of cell types, such as fibroblasts, epithelial cells, endothelial cells, and leukocytes (4). Thus, Paneth cells clearly respond to TNF by producing IL-17 and this leads to a fast, mucosa-specific reaction. Th17 cells and γδT cells also participate in these mucosa-specific immune responses (23). For instance, Th17 cells play a role in mucosal innate immunity by inducing antimicrobial peptides (31). Th17 cells, γδT cells, and Paneth cells might participate in a coordinated interplay between innate and adaptive immunity in a spatiotemporally regulated manner to ensure the host defense at the local mucosa. This is in agreement with the role of local mucosal tissue as the first immunological barrier to pathogens. A rapid local amplification mechanism needed for defense, however, may turn out detrimental in case of systemic inflammatory syndromes caused by bacterial endotoxins or by TNF.
Our finding sheds light on the role of Paneth cells as a ready source of IL-17, which amplifies signals in acute inflammation. It may also add a new dimension to the mechanism by which Paneth cells ensure mucosal immunity to gut pathogens.
MATERIALS AND METHODS
Mice.
C57BL/6J WT mice were purchased from Iffa Credo. IL-17R KO mice (IL-17R KO) (16) and Matrilysin-deficient mice were provided by J.J. Peschon (Amgen Washington, Seattle, WA) and L. Matrisian (Vanderbilt University, Nashville, TN), respectively. Mouse lines had been crossed back into a C57BL/6J background for over five generations. The mice were kept in specific pathogen-free conditions and used at matched ages. Animal studies were approved by the Institutional Review Board of the Radboud University Nijmegen and by the ethics committee of Ghent University.
Agents.
Recombinant mouse TNF (7.8 × 107 units/mg) was produced at Ghent University. Polyclonal rabbit antibodies were raised against recombinant mouse IL-17 (R&D Systems) by CFA immunization and repeated subcutaneous injections of IL-17 mixed with alum, as described previously (12). Titers were determined by a specific mIL-17 ELISA (R&D Systems). No cross-reactivity was observed with IL-1β, IL-1α, or TNF. To test subtype specificity, H4 cells were incubated with IL-17(A) and IL-17F with or without anti–IL-17 antibody. The conditioned medium was analyzed for the production of NO metabolite using Griess reagent.
Injections, monitoring, and sampling.
TNF was diluted in endotoxin-free PBS and injected i.v. into the lateral tail vein. Antiserum or control rabbit preimmune serum was injected i.p. Mortality was scored for 60 h. Serum and tissue samples were collected 0, 1, 3, and 9 h after injection for the initial time course study. For later study, we concentrated on a 3-h time point when the induction of IL-17 was maximal. Tissue samples were fixed briefly and embedded in paraffin by a standard protocol (Tissue Tek VIP; Sakura). For EM, samples were briefly immersed in hexadecene and frozen immediately in a high-pressure freezer (EM Pact; Leica). After freeze substitution (EM AFS; Leica) followed by infiltration in LR-White (hard grade; London Resin), samples were embedded in capsules. RNA samples were isolated by using RNeasy (QIAGEN).
Determination of IL-6, IL-17, and NOx in the serum.
IL-6 and NO metabolites were determined in serum and H4 supernatant as described previously (2). Serum levels of IL-17 were determined by a Luminex multi-analysis system (BioPlex; Bio-Rad Laboratories) according to the manufacturer's instructions. The sensitivity of the IL-6 assay, NO assay, and the multiplex kit was 1 pg/ml, 10 μM, and 10 pg/ml, respectively.
Tissue section, histology, and immunohistochemistry.
The following antibodies were used: anti–IL-17 antibody and control antibody (sc-6077 and sc-2028, respectively; Santa Cruz Biotechnology, Inc.), anti-CD45 antibody (BD Biosciences), anti-MPO antibody (Dako), and anti-matrilysin antibody (provided by B. Fingleton, Vanderbilt University, Nashville, TN). Biotin-conjugated IgGs, the Vectastain ABC kit (Vector Laboratories), AEC, and/or DAB were used for visualization. The slides were counterstained with Harris' hematoxylin. CD45+ cells were counted in 10 random fields in double-blinded fashion.
Real-time PCR.
RNA samples were reverse transcribed by using oligo-dT primers and MMLV reverse transcription. Primers were designed with Primer Express (Applied Biosystems) and purchased from Biolegio. Quantitative PCR was performed using the ABI/Prism 7000 sequence detection system (Applied Biosystems) for 10 ng cDNA with SYBR Green Master mix. The relative mRNA level was expressed as 2−ΔCt × 10,000 (ΔCt, relative cycle threshold compared with GAPDH).
Immunodetection of IL-17 by EM.
Ultrathin sections of gold interference color were cut on an ultramicrotome (Ultracut E; Reichert-Jung) and collected on formvar-coated copper slot grids. Grids were floated on blocking solution, followed by incubation with anti–IL-17 antibody or control antibody (sc-6077 or sc-2028; Santa Cruz Biotechnology, Inc.). The grids were labeled with the secondary antibody, anti–goat IgGs coupled with 12-nm gold (Jackson ImmunoResearch Laboratories). Sections were poststained in an LKB ultrastainer with uranyl acetate and with lead stain. Grids were viewed by using a JEOL 1010 TEM operating at 80 kV.
Statistical analysis.
Student's t test was used for statistical analysis. All p-values of <0.05 were considered significant.
Acknowledgments
We thank Dr. J.J. Peschon (Amgen Washington, Seattle, WA) for generously providing IL-17R KO mice, Dr. L. Matrisian (Vanderbilt University, Nashville, TN) for matrilysin-deficient mice, and Dr. M. Boots (Organon N.V., Oss, Netherlands) for providing recombinant murine IL-17A and anti–IL-17 serum. Dr. B. Fingleton (Vanderbilt University, Nashville, TN) is acknowledged for providing the anti-matrilysin antibody. Professor Yoichiro Iwakura of the Center for Experimental Medicine at the Institute for Medical Science of Tokyo University is acknowledged for providing an IL-17 KO intestine and Dr. M. Netea is thanked for his help in this matter. We also wish to thank Ms. B. Walgreen and Ms. I. van den Brink for excellent technical assistance.
This work was supported by IOP Genomics (IGE02032), by FWO Vlaanderen, Belgium, and the Interuniversity Attraction Poles, Belgium. I. Vanlaere is an aspirant of the FWO-Vlaanderen, Belgium. E. Lubberts was supported by a Veni Fellowship (grant no. 906-02-038) from the Netherlands Organization for Scientific Research (NWO). A. Cauwels is a postdoctoral fellow of the FWO-Vlaanderen, Belgium.
The authors have no other conflicting financial interests.
N. Takahashi and I. Vanlaere contributed equally to this paper.
References
- 1.Aggarwal, B.B., and K. Natarajan. 1996. Tumor necrosis factors: developments during the last decade. Eur. Cytokine Netw. 7:93–124. [PubMed] [Google Scholar]
- 2.Van Molle, W., B. Wielockx, T. Mahieu, M. Takada, T. Taniguchi, K. Sekikawa, and C. Libert. 2002. HSP70 protects against TNF-induced lethal inflammatory shock. Immunity. 16:685–695. [DOI] [PubMed] [Google Scholar]
- 3.Cauwels, A., W. Van Molle, B. Janssen, B. Everaerdt, P. Huang, W. Fiers, and P. Brouckaert. 2000. Protection against TNF-induced lethal shock by soluble guanylate cyclase inhibition requires functional inducible nitric oxide synthase. Immunity. 13:223–231. [DOI] [PubMed] [Google Scholar]
- 4.Kolls, J.K., and A. Linden. 2004. Interleukin-17 family members and inflammation. Immunity. 21:467–476. [DOI] [PubMed] [Google Scholar]
- 5.Park, H., Z. Li, X.O. Yang, S.H. Chang, R. Nurieva, Y.H. Wang, Y. Wang, L. Hood, Z. Zhu, Q. Tian, and C. Dong. 2005. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat. Immunol. 6:1133–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Langrish, C.L., Y. Chen, W.M. Blumenschein, J. Mattson, B. Basham, J.D. Sedgwick, T. McClanahan, R.A. Kastelein, and D.J. Cua. 2005. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J. Exp. Med. 201:233–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harrington, L.E., R.D. Hatton, P.R. Mangan, H. Turner, T.L. Murphy, K.M. Murphy, and C.T. Weaver. 2005. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat. Immunol. 6:1123–1132. [DOI] [PubMed] [Google Scholar]
- 8.Hirota, K., M. Hashimoto, H. Yoshitomi, S. Tanaka, T. Nomura, T. Yamaguchi, Y. Iwakura, N. Sakaguchi, and S. Sakaguchi. 2007. T cell self-reactivity forms a cytokine milieu for spontaneous development of IL-17+ Th cells that cause autoimmune arthritis. J. Exp. Med. 204:41–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang, Z., M. Zheng, J. Bindas, P. Schwarzenberger, and J.K. Kolls. 2006. Critical role of IL-17 receptor signaling in acute TNBS-induced colitis. Inflamm. Bowel Dis. 12:382–388. [DOI] [PubMed] [Google Scholar]
- 10.Nakae, S., Y. Komiyama, A. Nambu, K. Sudo, M. Iwase, I. Homma, K. Sekikawa, M. Asano, and Y. Iwakura. 2002. Antigen-specific T cell sensitization is impaired in IL-17-deficient mice, causing suppression of allergic cellular and humoral responses. Immunity. 17:375–387. [DOI] [PubMed] [Google Scholar]
- 11.Nakae, S., A. Nambu, K. Sudo, and Y. Iwakura. 2003. Suppression of immune induction of collagen-induced arthritis in IL-17-deficient mice. J. Immunol. 171:6173–6177. [DOI] [PubMed] [Google Scholar]
- 12.Lubberts, E., M.I. Koenders, B. Oppers-Walgreen, B.L. van den, C.J. Coenen-de Roo, L.A. Joosten, and W.B. van den Berg. 2004. Treatment with a neutralizing anti-murine interleukin-17 antibody after the onset of collagen-induced arthritis reduces joint inflammation, cartilage destruction, and bone erosion. Arthritis Rheum. 50:650–659. [DOI] [PubMed] [Google Scholar]
- 13.Sutton, C., C. Brereton, B. Keogh, K.H. Mills, and E.C. Lavelle. 2006. A crucial role for interleukin (IL)-1 in the induction of IL-17–producing T cells that mediate autoimmune encephalomyelitis. J. Exp. Med. 203:1685–1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jones, C.E., and K. Chan. 2002. Interleukin-17 stimulates the expression of interleukin-8, growth-related oncogene-alpha, and granulocyte-colony-stimulating factor by human airway epithelial cells. Am. J. Respir. Cell Mol. Biol. 26:748–753. [DOI] [PubMed] [Google Scholar]
- 15.Miossec, P. 2003. Interleukin-17 in rheumatoid arthritis: if T cells were to contribute to inflammation and destruction through synergy. Arthritis Rheum. 48:594–601. [DOI] [PubMed] [Google Scholar]
- 16.Flierl, M.A., D. Rittirsch, H. Gao, L.M. Hoesel, B.A. Nadeau, D.E. Day, F.S. Zetoune, J.V. Sarma, M.S. Huber-Lang, J.L. Ferrara, and P.A. Ward. 2008. Adverse functions of IL-17A in experimental sepsis. FASEB J. 22:2198–2205. [DOI] [PubMed] [Google Scholar]
- 17.Ye, P., F.H. Rodriguez, S. Kanaly, K.L. Stocking, J. Schurr, P. Schwarzenberger, P. Oliver, W. Huang, P. Zhang, J. Zhang, et al. 2001. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J. Exp. Med. 194:519–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Libert, C., N. Takahashi, A. Cauwels, P. Brouckaert, H. Bluethmann, and W. Fiers. 1994. Response of interleukin-6-deficient mice to tumor necrosis factor-induced metabolic changes and lethality. Eur. J. Immunol. 24:2237–2242. [DOI] [PubMed] [Google Scholar]
- 19.Tracey, K.J., B. Beutler, S.F. Lowry, J. Merryweather, S. Wolpe, I.W. Milsark, R.J. Hariri, T.J. Fahey III, A. Zentella, J.D. Albert, et al. 1986. Shock and tissue injury induced by recombinant human cachectin. Science. 234:470–474. [DOI] [PubMed] [Google Scholar]
- 20.Stark, M.A., Y. Huo, T.L. Burcin, M.A. Morris, T.S. Olson, and K. Ley. 2005. Phagocytosis of apoptotic neutrophils regulates granulopoiesis via IL-23 and IL-17. Immunity. 22:285–294. [DOI] [PubMed] [Google Scholar]
- 21.Lockhart, E., A.M. Green, and J.L. Flynn. 2006. IL-17 production is dominated by gammadelta T cells rather than CD4 T cells during Mycobacterium tuberculosis infection. J. Immunol. 177:4662–4669. [DOI] [PubMed] [Google Scholar]
- 22.Ivanov, I.I., B.S. McKenzie, L. Zhou, C.E. Tadokoro, A. Lepelley, J.J. Lafaille, D.J. Cua, and D.R. Littman. 2006. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 126:1121–1133. [DOI] [PubMed] [Google Scholar]
- 23.Chen, Y., K. Chou, E. Fuchs, W.L. Havran, and R. Boismenu. 2002. Protection of the intestinal mucosa by intraepithelial gamma delta T cells. Proc. Natl. Acad. Sci. USA. 99:14338–14343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garabedian, E.M., L.J. Roberts, M.S. McNevin, and J.I. Gordon. 1997. Examining the role of Paneth cells in the small intestine by lineage ablation in transgenic mice. J. Biol. Chem. 272:23729–23740. [DOI] [PubMed] [Google Scholar]
- 25.Wilson, C.L., A.J. Ouellette, D.P. Satchell, T. Ayabe, Y.S. Lopez-Boado, J.L. Stratman, S.J. Hultgren, L.M. Matrisian, and W.C. Parks. 1999. Regulation of intestinal alpha-defensin activation by the metalloproteinase matrilysin in innate host defense. Science. 286:113–117. [DOI] [PubMed] [Google Scholar]
- 26.Ayabe, T., T. Ashida, Y. Kohgo, and T. Kono. 2004. The role of Paneth cells and their antimicrobial peptides in innate host defense. Trends Microbiol. 12:394–398. [DOI] [PubMed] [Google Scholar]
- 27.Keshav, S., L. Lawson, L.P. Chung, M. Stein, V.H. Perry, and S. Gordon. 1990. Tumor necrosis factor mRNA localized to Paneth cells of normal murine intestinal epithelium by in situ hybridization. J. Exp. Med. 171:327–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bultinck, J., P. Sips, L. Vakaet, P. Brouckaert, and A. Cauwels. 2006. Systemic NO production during (septic) shock depends on parenchymal and not on hematopoietic cells: in vivo iNOS expression pattern in (septic) shock. FASEB J. 20:2363–2365. [DOI] [PubMed] [Google Scholar]
- 29.Fujino, S., A. Andoh, S. Bamba, A. Ogawa, K. Hata, Y. Araki, T. Bamba, and Y. Fujiyama. 2003. Increased expression of interleukin 17 in inflammatory bowel disease. Gut. 52:65–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weaver, C.T., R.D. Hatton, P.R. Mangan, and L.E. Harrington. 2007. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu. Rev. Immunol. 25:821–852. [DOI] [PubMed] [Google Scholar]
- 31.Liang, S.C., X.Y. Tan, D.P. Luxenberg, R. Karim, K. Dunussi-Joannopoulos, M. Collins, and L.A. Fouser. 2006. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J. Exp. Med. 203:2271–2279. [DOI] [PMC free article] [PubMed] [Google Scholar]