Abstract
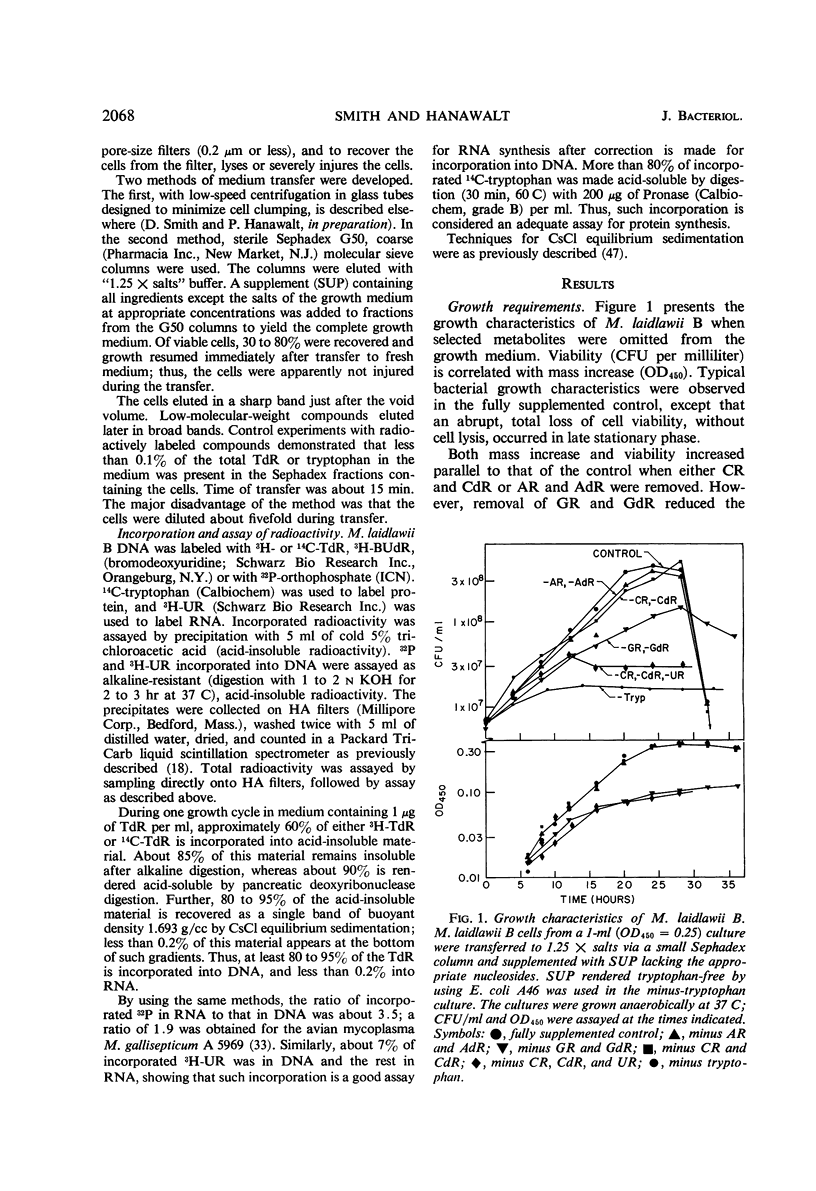
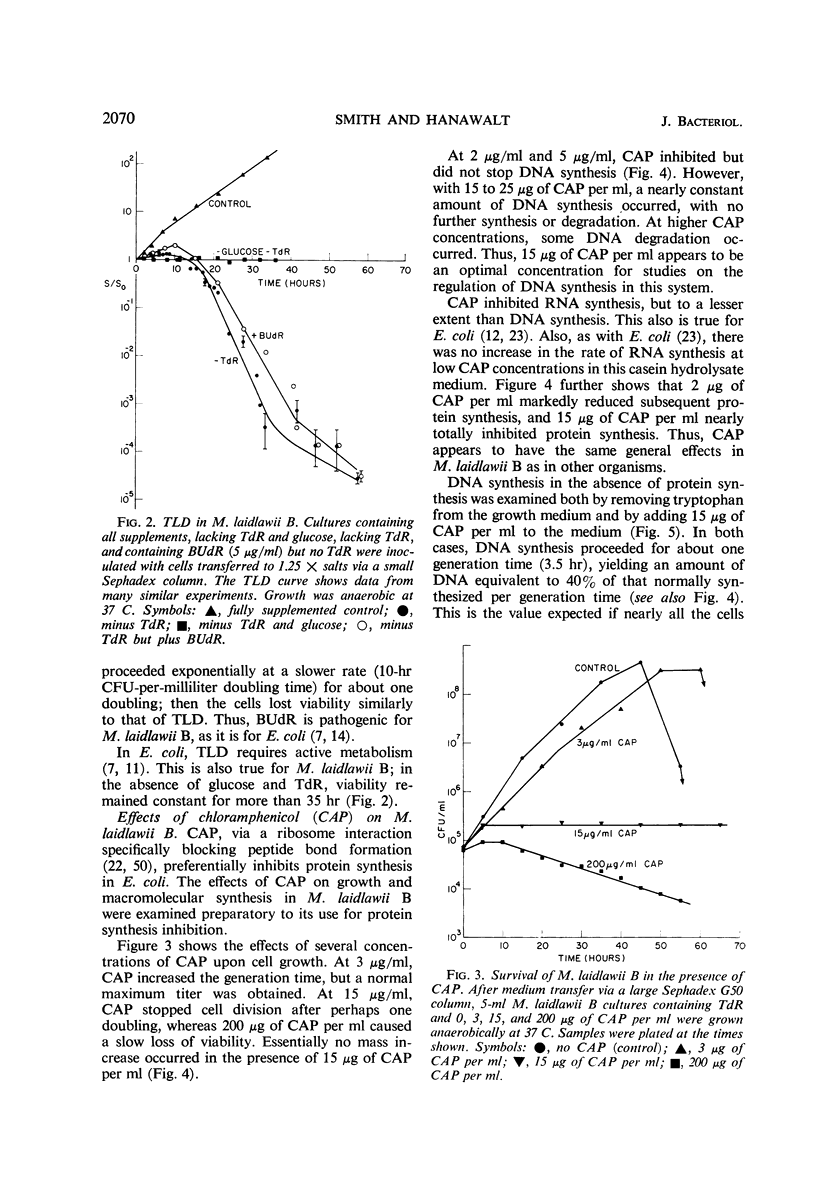
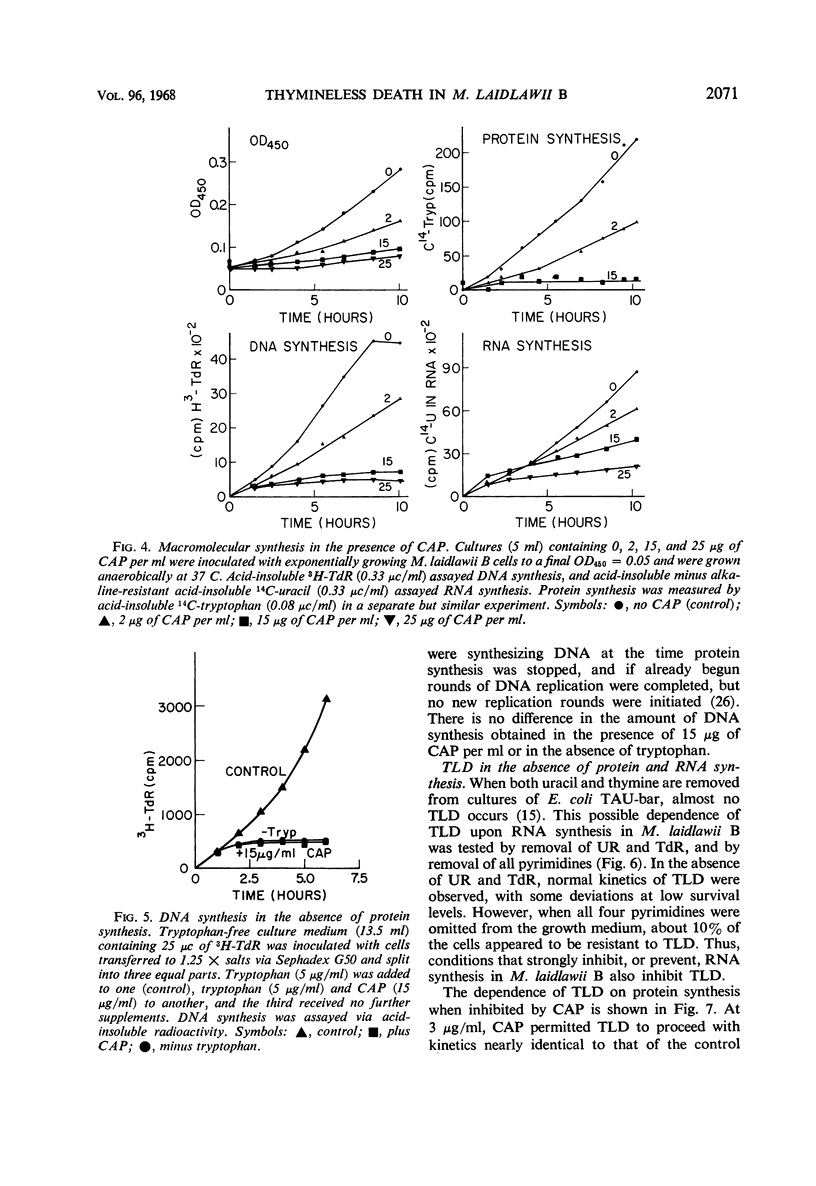
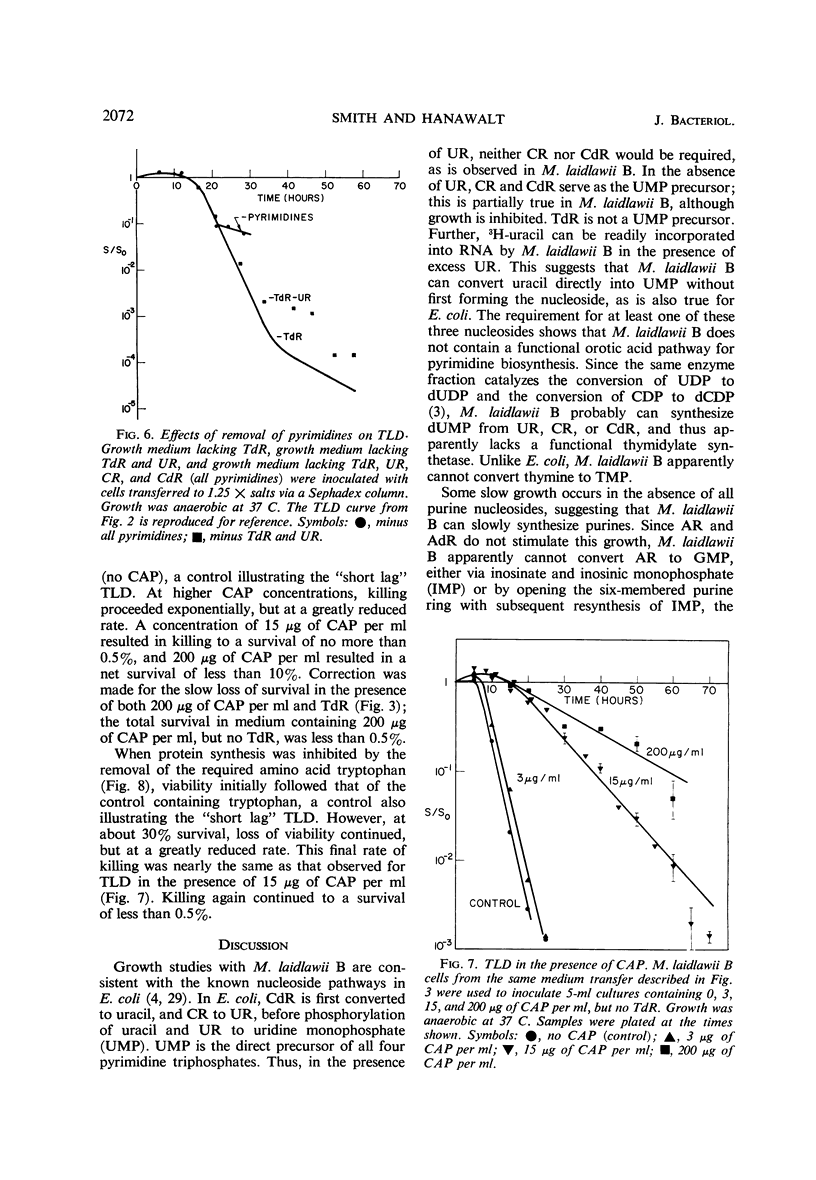
The relationships between macromolecular synthesis and viability have been studied in the pleuropneumonia-like organism Mycoplasma laidlawii B adapted to a semidefined grwoth medium. This organism exhibited an absolute growth requirement for the nucleosides uridine and thymidine, a partial requirement for guanosine and deoxyguanosine, but no requirement for adenosine, deoxyadenosine, cytosine, and deoxycytosine. Cytosine and deoxycytosine partially satisfied the requirement for uridine. Loss in viability resulted from thymidine deprivation, but not from a deficiency in other growth requirements. This phenomenon of thymineless death in a mycoplasma is similar in many respects to that reported in other bacterial systems. Chloramphenicol specifically inhibited protein synthesis and allowed deoxyribonucleic acid synthesis to proceed to only about 40% of that normally produced per generation period, while causing less inhibition of ribonucleic acid synthesis. Protein synthesis inhibition permitted thymineless death to a survival level of less than 0.5%, but ribonucleic acid synthesis inhibition resulted in a higher (10%) survival level. These results are consistent with previously noted aspects of thymineless death in Escherichia coli strains, which suggest that thymineless death is coupled to ribonucleic acid synthesis.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALLEN M. K., YANOFSKY C. A BIOCHEMICAL AND GENETIC STUDY OF REVERSION WITH THE A-GENE A-PROTEIN SYSTEM OF ESCHERICHIA COLI TRYPTOPHAN SYNTHETASE. Genetics. 1963 Aug;48:1065–1083. doi: 10.1093/genetics/48.8.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARNER H. D., COHEN S. S. The isolation and properties of amino acid requiring mutants of a thymineless bacterium. J Bacteriol. 1957 Sep;74(3):350–355. doi: 10.1128/jb.74.3.350-355.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. S., Barner H. D. STUDIES ON UNBALANCED GROWTH IN ESCHERICHIA COLI. Proc Natl Acad Sci U S A. 1954 Oct;40(10):885–893. doi: 10.1073/pnas.40.10.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings D. J., Mondale L. Thymineless death in Escherichia coli: strain specificity. J Bacteriol. 1967 Jun;93(6):1917–1924. doi: 10.1128/jb.93.6.1917-1924.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ENDO H., AYABE K., AMAKO K., TAKEYA K. INDUCIBLE PHAGE OF ESCHERICHIA COLI 15. Virology. 1965 Mar;25:469–471. doi: 10.1016/0042-6822(65)90067-x. [DOI] [PubMed] [Google Scholar]
- FREIFELDER D., MAALOE O. ENERGY REQUIREMENT FOR THYMINELESS DEATH IN CELLS OF ESCHERICHIA COLI. J Bacteriol. 1964 Oct;88:987–990. doi: 10.1128/jb.88.4.987-990.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freifelder D. Lack of a relation between deoxyribonucleic acid methylation and thymineless death in Escherichia coli. J Bacteriol. 1967 May;93(5):1732–1733. doi: 10.1128/jb.93.5.1732-1733.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GALLANT J., SUSKIND S. R. Ribonucleic acid synthesis and thymineless death. Biochim Biophys Acta. 1962 May 14;55:627–638. doi: 10.1016/0006-3002(62)90841-7. [DOI] [PubMed] [Google Scholar]
- HANAWALT P. C. Involvement of synthesis of RNA in thymineless death. Nature. 1963 Apr 20;198:286–286. doi: 10.1038/198286a0. [DOI] [PubMed] [Google Scholar]
- HANAWALT P. C., MAALOE O., CUMMINGS D. J., SCHAECHTER M. The normal DNA replication cycle. II. J Mol Biol. 1961 Apr;3:156–165. doi: 10.1016/s0022-2836(61)80042-9. [DOI] [PubMed] [Google Scholar]
- HANAWALT P. C., RAY D. S. ISOLATION OF THE GROWING POINT IN THE BACTERIAL CHROMOSOME. Proc Natl Acad Sci U S A. 1964 Jul;52:125–132. doi: 10.1073/pnas.52.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HANAWALT P., WAX R. TRANSCRIPTION OF A REPRESSED GENE: EVIDENCE THAT IT REQUIRES DNA REPLICATION. Science. 1964 Sep 4;145(3636):1061–1063. doi: 10.1126/science.145.3636.1061. [DOI] [PubMed] [Google Scholar]
- Hackett P., Jr, Hanawalt P. Selectivity for thymine over 5-bromouracil by a thymine-requiring bacterium. Biochim Biophys Acta. 1966 Aug 17;123(2):356–363. doi: 10.1016/0005-2787(66)90288-7. [DOI] [PubMed] [Google Scholar]
- Ishibashi M., Hirota Y. Hybridization between Escherichia coli K-12 and 15T- and thymineless death of their derivatives. J Bacteriol. 1965 Nov;90(5):1496–1497. doi: 10.1128/jb.90.5.1496-1497.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JULIAN G. R. (14C)LYSINE PEPTIDES SYNTHESIZED IN AN IN VITRO ESCHERICHIA COLI SYSTEM IN THE PRESENCE OF CHLORAMPHENICOL. J Mol Biol. 1965 May;12:9–16. doi: 10.1016/s0022-2836(65)80277-7. [DOI] [PubMed] [Google Scholar]
- KURLAND C. G., MAALOE O. Regulation of ribosomal and transfer RNA synthesis. J Mol Biol. 1962 Mar;4:193–210. doi: 10.1016/s0022-2836(62)80051-5. [DOI] [PubMed] [Google Scholar]
- Luzzati D. Effect of thymine starvation on messenger ribonucleic acid synthesis in Escherichia coli. J Bacteriol. 1966 Nov;92(5):1435–1446. doi: 10.1128/jb.92.5.1435-1446.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAALOE O., HANAWALT P. C. Thymine deficiency and the normal DNA replication cycle. I. J Mol Biol. 1961 Apr;3:144–155. doi: 10.1016/s0022-2836(61)80041-7. [DOI] [PubMed] [Google Scholar]
- MENNIGMANN H. D., SZYBALSKI W. Molecular mechanism of thymine-less death. Biochem Biophys Res Commun. 1962 Nov 27;9:398–404. doi: 10.1016/0006-291x(62)90023-2. [DOI] [PubMed] [Google Scholar]
- MOROWITZ H. J., TOURTELLOTTE M. E., GUILD W. R., CASTRO E., WOESE C. The chemical composition and submicroscopic morphology of Mycoplasma gallisepticum, avian PPLO 5969. J Mol Biol. 1962 Feb;4:93–103. doi: 10.1016/s0022-2836(62)80041-2. [DOI] [PubMed] [Google Scholar]
- MOYED H. S. False feedback inhibition: inhibition of tryptophan biosynthesis by 5-methyltryptophan. J Biol Chem. 1960 Apr;235:1098–1102. [PubMed] [Google Scholar]
- Mennigmann H. D. Electron microscopy of the anti-bacterial agent produced by Escherichia coli 15. J Gen Microbiol. 1965 Nov;41(2):151–154. doi: 10.1099/00221287-41-2-151. [DOI] [PubMed] [Google Scholar]
- OKAGAKI H., TSUBOTA Y., SIBATANI A. Unbalanced growth and bacterial death in thymine-deficient and ultraviolet irradiated Escherichia coli. J Bacteriol. 1960 Dec;80:762–771. doi: 10.1128/jb.80.6.762-771.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PETTIJOHN D., HANAWALT P. EVIDENCE FOR REPAIR-REPLICATION OF ULTRAVIOLET DAMAGED DNA IN BACTERIA. J Mol Biol. 1964 Aug;9:395–410. doi: 10.1016/s0022-2836(64)80216-3. [DOI] [PubMed] [Google Scholar]
- Pauling C., Hanawalt P. Nonconservative DNA replication in bacteria after thymine starvation. Proc Natl Acad Sci U S A. 1965 Dec;54(6):1728–1735. doi: 10.1073/pnas.54.6.1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritikin W. B., Romig W. R. Death of Bacillus subtilis Auxotrophs Due to Deprivation of Thymine, Tryptophan, or Uracil. J Bacteriol. 1966 Aug;92(2):291–296. doi: 10.1128/jb.92.2.291-296.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAZIN S. Nucleic acid precursor requirements of Mycoplasma laidlawii. J Gen Microbiol. 1962 Jun;28:243–250. doi: 10.1099/00221287-28-2-243. [DOI] [PubMed] [Google Scholar]
- RAZIN S., OLIVER O. Morphogenesis of Mycoplasma and bacterial L-form colonies. J Gen Microbiol. 1961 Feb;24:225–237. doi: 10.1099/00221287-24-2-225. [DOI] [PubMed] [Google Scholar]
- ROSENKRANZ H. S., CARR H. S., ROSE H. M. PHENETHYL ALCOHOL. II. EFFECT ON THYMINE-REQUIRING ESCHERICHIA COLI. J Bacteriol. 1965 May;89:1370–1373. doi: 10.1128/jb.89.5.1370-1373.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolfe R. On the mechanism of thymineless death in Bacillus subtilis. Proc Natl Acad Sci U S A. 1967 Jan;57(1):114–121. doi: 10.1073/pnas.57.1.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SICARD N., DEVORET R. [Effects of thymine dificiency on the K 12 T lysogenic and 15 T colicinogenic strains of Escherichia coli]. C R Hebd Seances Acad Sci. 1962 Sep 10;255:1417–1419. [PubMed] [Google Scholar]
- Schaiberger G. E., Giegel J., Sallman B. Functional activity of DNA and DNA polymerase during thymine starvation of Escherichia coli 15T. Biochem Biophys Res Commun. 1967 Jul 10;28(1):30–37. doi: 10.1016/0006-291x(67)90401-9. [DOI] [PubMed] [Google Scholar]
- Sicard N., Simonnet G., Astrachan L. Base composition of rapidly-labelled RNA in E. coli undergoing thymineless death. Biochem Biophys Res Commun. 1967 Mar 9;26(5):532–538. doi: 10.1016/0006-291x(67)90097-6. [DOI] [PubMed] [Google Scholar]
- Smith B. J., Burton K. The integrity of deoxyribonucleic acid extracted from Escherichia coli 15T after thymine-less death. Biochem J. 1965 Oct;97(1):240–246. doi: 10.1042/bj0970240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. W., Hanawalt P. C. Properties of the growing point region in the bacterial chromosome. Biochim Biophys Acta. 1967 Dec 19;149(2):519–531. doi: 10.1016/0005-2787(67)90180-3. [DOI] [PubMed] [Google Scholar]
- Soska J. Growth of Lactobacillus acidophilus in the absence of folic acid. J Bacteriol. 1966 May;91(5):1840–1847. doi: 10.1128/jb.91.5.1840-1847.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOLFE A. D., HAHN F. E. MODE OF ACTION OF CHLORAMPHENICOL. IX. EFFECTS OF CHLORAMPHENICOL UPON A RIBOSOMAL AMINO ACID POLYMERIZATION SYSTEM AND ITS BINDING TO BACTERIAL RIBOSOME. Biochim Biophys Acta. 1965 Jan 11;95:146–155. doi: 10.1016/0005-2787(65)90219-4. [DOI] [PubMed] [Google Scholar]
- Wachsman J. T., Kemp S., Kogg L. Thymineless death in Bacillus megaterium. J Bacteriol. 1964 May;87(5):1079–1086. doi: 10.1128/jb.87.5.1079-1086.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]


