Abstract
Tumor suppressor p53 is activated by several stimuli, including DNA damage and oncogenic stress. Previous studies (Takaoka, A., S. Hayakawa, H. Yanai, D. Stoiber, H. Negishi, H. Kikuchi, S. Sasaki, K. Imai, T. Shibue, K. Honda, and T. Taniguchi. 2003. Nature. 424:516–523) have shown that p53 is also induced in response to viral infections as a downstream transcriptional target of type I interferon (IFN) signaling. Moreover, many viruses, including SV40, human papillomavirus, Kaposi's sarcoma herpesvirus, adenoviruses, and even RNA viruses such as polioviruses, have evolved mechanisms designated to abrogate p53 responses. We describe a novel p53 function in the activation of the IFN pathway. We observed that infected mouse and human cells with functional p53 exhibited markedly decreased viral replication early after infection. This early inhibition of viral replication was mediated both in vitro and in vivo by a p53-dependent enhancement of IFN signaling, specifically the induction of genes containing IFN-stimulated response elements. Of note, p53 also contributed to an increase in IFN release from infected cells. We established that this p53-dependent enhancement of IFN signaling is dependent to a great extent on the ability of p53 to activate the transcription of IFN regulatory factor 9, a central component of the IFN-stimulated gene factor 3 complex. Our results demonstrate that p53 contributes to innate immunity by enhancing IFN-dependent antiviral activity independent of its functions as a proapoptotic and tumor suppressor gene.
Viral infection of mammalian cells leads to transactivation of the IFN-β promoter, triggered by the recognition of viral products by cellular sensors such as Toll-like receptors and the RNA helicases retinoic acid–inducible gene I (RIG-I) and melanoma differentiation–associated gene 5 (1). Type I IFN release from infected cells, mainly IFN-α and IFN-β, plays a key role in innate immunity against a wide range of viruses. IFN establishes an antiviral state in bystander cells by binding to the type I IFN receptor, formed by the subunits IFNAR1 and IFNAR2 (2). This, in turn, triggers the activation of the JAK–STAT signaling pathway, and the phosphorylation and translocation of the transcription factors STAT-1, STAT-2, and IFN regulatory factor (IRF) 9 to the nucleus, where they form a heterotrimeric complex designated as IFN-stimulated gene factor 3 (ISGF3) (3). ISGF3 binds to the IFN-stimulated response elements (ISREs) present in the promoters of IFN-stimulated genes (ISGs), leading to their induction and the activation of antiviral responses (2).
It has been previously shown that one of the many genes induced by IFN is the tumor suppressor p53, because of the presence of active ISREs in its promoter (4). Widely known as “the guardian of the genome,” tumor suppressor p53 is activated in response to several types of cellular stress, including DNA damage and oncogene expression. Its well-studied functions include inducing cell cycle arrest and apoptosis, which act to prevent the emergence of transformed cells (5). Since the first report that placed p53 downstream of type I IFN signaling, several studies have indicated that p53 is also activated indirectly by type I IFN through other IFN-inducible proteins, such as promyelocytic leukemia protein, STAT-1, or IFIXα-1 (6–8). Also, it has been recently reported that cells that conserve p53 functions show an increase in p53-dependent apoptosis in response to viral infection, which is associated with reduced viral replication (4, 9). Moreover, p53−/− mice are more permissive to viral infection, presumably because of the lack of a p53 apoptotic response (9). These findings, and the fact that not only oncogenic viruses but also RNA viruses have evolved mechanisms designed to abrogate p53 responses (6), suggest that p53 may have a broader role in antiviral defense. In agreement with this hypothesis, recent reports established that some IFN-related genes such as IRF5 or ISG15 are in fact p53 direct target genes, although the role of these interactions in antiviral defense has not yet been explored (10, 11).
To further characterize the role of p53 in antiviral immune response, we investigated the antiviral effects of WT p53 in response to low viral loads, which mimic the early stages of infection. We report a novel mechanism of p53-dependent enhancement of IFN signaling and production, both in vitro and in vivo, mediated by its transcriptional up-regulation of IRF9.
RESULTS AND DISCUSSION
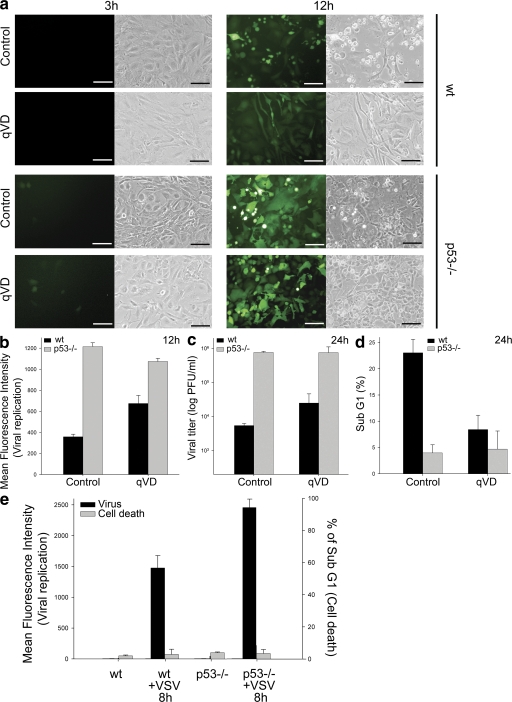
To investigate the mechanisms by which p53 might impair viral replication in addition to its known proapoptotic function, we infected WT and p53−/− mouse embryo fibroblasts (MEFs) with recombinant vesicular stomatitis virus (VSV) expressing GFP (VSV-GFP), in the presence or absence of a broad-spectrum caspase inhibitor (12). Virus titers were >100-fold higher in the absence of p53, which was consistent with previous reports (4, 9). However, p53 still markedly reduced viral replication in the presence of caspase inhibitors (Fig. 1, a–c), which reduced p53-dependent cell death to levels close to those observed with p53−/− MEFs (Fig. 1 d).
Figure 1.
p53 prevents viral replication in the absence of apoptosis. (a) Representative fluorescent and clear-field pictures of WT and p53−/− MEFs infected with VSV-GFP (MOI of 0.01 for 3 or 12 h). Immediately after infection, plates were treated or not with caspase inhibitor (20 μM qVD-OPH-109). Bars, 30 μm. (b) Quantitation by FACS analysis of the green fluorescence of cells in panel a 12 h after infection. (c) Viral titration of supernatants from MEFs infected with VSV-GFP for 24 h. The viral titers are represented as log (PFU/ml). (d) FACS analysis of MEFs infected with VSV for 24 h and stained with PI. (e) Mean fluorescence intensity and the percentage of Sub G1 events (representing cell death) of PI-stained WT and p53−/− HCT116 cells infected with VSV-GFP for 8 h. Values in all graphs represent the mean of three biological samples, and the error bars represent standard deviations.
To confirm these results, we used HCT116 colon cancer cells and HCT116p53−/− cells, in which p53 was inactivated by somatic gene targeting (13). As shown in Fig. 1 e, early viral replication was greater in the absence of p53 despite the fact that apoptosis was not increased either in the presence or absence of p53 over that of uninfected cells. The inhibitory effect of p53 on early virus replication suggested that basal levels of p53 are sufficient for the antiviral effects observed. We performed analogous experiments with EJp53, a p53-null bladder cancer cell with a tetracycline (tet)-regulatable p53 expression system (14). When tet is removed from the culture media, p53 is up-regulated at physiological levels, and cell cycle arrest is induced without significant induction of apoptosis (14, 15). We infected these cells with VSV-GFP and also observed significantly lower viral replication in cells expressing p53 (Fig. S1 a, available at http://www.jem.org/cgi/content/full/jem.20080383/DC1), in the absence of apoptosis (not depicted). To confirm that inhibition of viral replication in EJp53 cells was dependent on p53 functions unrelated to cell cycle arrest, we used EJp16, an EJ cell line with a tet-regulatable p16 expression system (16). As shown in Fig. S1 b, induced p16 expression did not interfere with virus replication under conditions in which these cells exhibited a pattern of cell cycle arrest similar to that induced by p53 (Fig. S1 c). Analysis of p53 and p16 protein levels confirmed a direct relationship between p53 expression and the dampening of viral replication (Fig. S1 d).
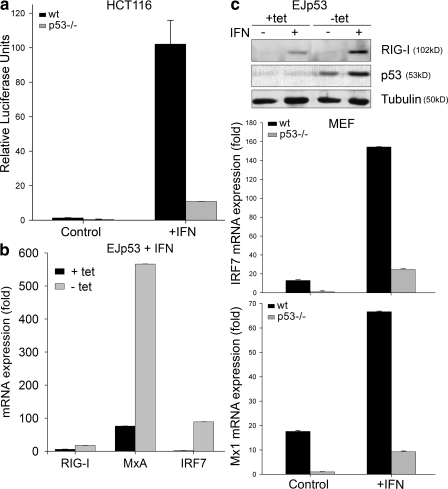
The type I IFN response is a major component of antiviral innate immunity (1). IFN establishes an antiviral state in bystander cells that results in the phosphorylation and/or translocation of the transcription factors STAT-1, STAT-2, and IRF9 to the nucleus, where they form a heterotrimeric complex termed ISGF3 (3). ISGF3 binds to ISREs on the promoters of ISGs, leading to their induction and the activation of different antiviral mechanisms (2). To test whether the apoptosis-independent antiviral functions of p53 were mediated by an effect on IFN signaling, we examined whether p53 could up-regulate ISRE-inducible genes. Fig. 2 a demonstrates a 10-fold higher increase in the transactivation of an ISRE luciferase reporter by IFN in cells containing WT p53 compared with p53-null HCT116 cells. To confirm the effects of p53 on ISRE-dependent ISGs, we examined the expression of RIG-I, MxA, and IRF7 in EJp53. As shown in Fig. 2 b, p53 expression was associated with higher basal levels and enhanced activation of each of these genes in response to IFN treatment, as confirmed at the protein level for RIG-I (Fig. 2 c, top). Of note, IFN treatment also stabilized induced p53, which was consistent with previous reports (7, 8). Basal transcript levels of IRF7 and Mx1 were also increased in WT MEFs compared with p53−/− MEFs, and their expression levels were preferentially increased in WT MEFs treated with IFN (Fig. 2 c, bottom). These findings suggest that basal p53 levels are sufficient to induce an elevation in ISG expression and prime the IFN-dependent antiviral state.
Figure 2.
p53 enhances type I IFN signaling. (a) Levels of luciferase expression in WT and p53−/− HCT116 cells transfected with a pFOS-ISRE luciferase reporter (and the internal Renilla luciferase expression vector pRL-CMV to normalize transfection efficiency). 24 h after transfection, cells were treated or not with 500 U/ml IFN for 12 h, and luciferase intensity was then measured. Values represent the mean of three biological samples, and error bars represent standard deviations. (b) Real-time PCR of EJp53 cells cultured in the presence or absence of tet for 1 d and treated with 500 U/ml IFN for 6 h. Results show RIG-I, MxA, and IRF7 mRNA expression levels in each condition compared with that of TATA binding protein (TBP; control). Results are mean values of triplicate experiments expressed as the fold difference between IFN-treated and untreated EJp53 cells. (c, top) Western blots of lysates of EJp53 cultured in the presence of tet or 24 h after tet removal, and treated with 500 U/ml IFN for 24 h. (bottom) Real-time PCR of WT and p53−/− MEFs. Results show IRF7 and Mx1 mRNA expression levels in each condition compared with that of TBP (control). Results are mean values of triplicate experiments, and error bars represent standard deviations.
To further investigate the contribution of p53 to the IFN response, we explored whether p53 expression influenced the levels of IFN secreted by infected cells. For this purpose, we used Sendai virus (SeV) strain Cantell, which induces strong type I IFN production (17). Supernatants from induced EJp53 cells contained higher levels of IFN in response to SeV infection than uninduced cells (Fig. S2 a, available at http://www.jem.org/cgi/content/full/jem.20080383/DC1). Pretreatment of Vero cells with supernatants from p53-expressing EJp53 infected with SeV was also more effective at preventing replication of recombinant Newcastle disease virus (NDV) expressing GFP (NDV-GFP), a virus that is extremely sensitive to IFN (Fig. S2 b) (18), confirming the p53-dependent increase of IFN production in response to viral infection.
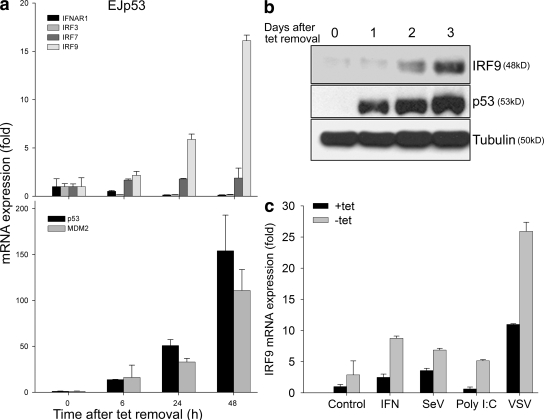
As p53 enhances IFN activity by increasing expression of ISGs, we reasoned that one or more IFN-related genes might be direct p53 transcriptional targets. IRF7 and IRF9 were considered as possible candidates because of their dual role in IFN signaling and production (19–21). IRF7 mRNA showed only a modest increase in response to p53 expression (Fig. 3 a, top). However, IRF9 mRNA increased with kinetics similar to that observed with MDM2, a known p53 transcriptional target (Fig. 3 a, bottom). Neither IFNAR1 nor IRF3 showed increased expression levels in response to p53 expression. The p53-dependent increase of IRF9 was also observed at the protein level (Fig. 3 b). Finally, the induction of IRF9 in response to viral infection, the IFN inducer Poly (I:C), or IFN treatment was significantly higher in the presence of p53 (Fig. 3 c).
Figure 3.
p53 enhances IRF9 expression. (a) Real-time PCR of EJp53 cells cultured in the presence or absence of tet for 6–48 h. Results show IFNAR1, IRF3, IRF7, and IRF9 (top), and p53 and MDM2 (bottom) mRNA expression levels in each condition compared with that of TBP (control). Results are mean values of triplicate experiments, and error bars represent standard deviations. (b) Western blot of lysates of EJp53 cells 0–3 d after tet removal. (c) Real-time PCR of EJp53 cells cultured in the presence or absence of tet for 24 h and treated with 500 U/ml IFN, SeV (MOI of 0.1), 100 ng/ml Poly (I:C), or VSV-GFP (MOI of 0.01). Results show IRF9 mRNA expression levels in each condition compared with that of TBP (control). Results are mean values of triplicate experiments, and error bars represent standard deviations.
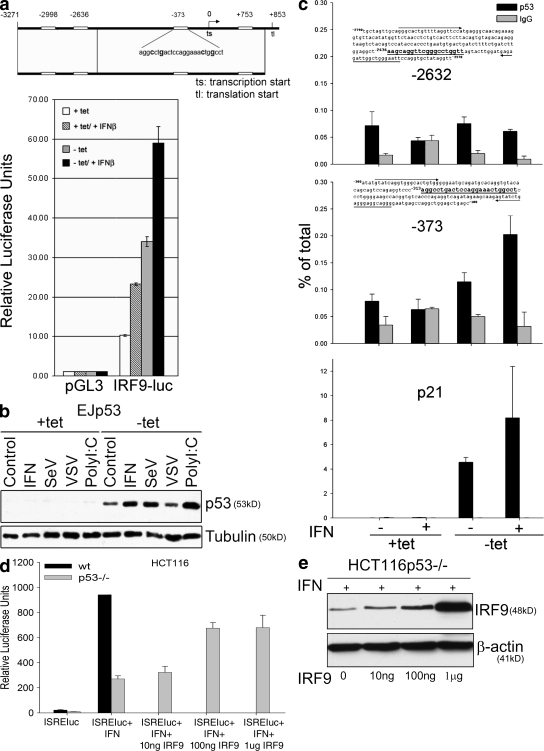
To assess whether IRF9 was a direct target of p53, we searched for p53 binding sites within the IRF9 promoter. We identified several putative sites (Fig. 4 a) and generated a luciferase reporter construct driven by the IRF9 promoter. As shown in Fig. 4 a, this construct strongly induced luciferase activity in response to either p53 or IFN. Moreover, a combination of both stimuli induced a synergistic response of the reporter. Chromatin immunoprecipitation (ChIP) analysis showed that the specific p53 binding to region −373 in the endogenous IRF9 promoter was enriched in chromatin immunoprecipitates using a p53 antibody, especially after IFN treatment (Fig. 4 c). This may be explained by the elevation in the p53 protein level caused by IFN signaling–dependent p53 stabilization (Fig. 2 c and Fig. 4 b). Because the transactivation of ISRE-dependent genes and the known positive feedback of IFN production relies on the formation of the ISGF3 complex, in which IRF9 plays a pivotal role (22), these results help to provide a mechanistic explanation for the observed p53-dependent enhancement of IFN signaling and production.
Figure 4.
IRF9 is a direct p53 target gene. (a) Putative p53 recognition sites located in the promoter region of the IRF9 gene (open boxes; the −373 site is illustrated with invariant residues bolded). This genomic region was cloned into the pGL3 firefly luciferase reporter vector (IRF9-luc). EJp53 cells were cotransfected with either pGL3 or IRF9-luc together with a Renilla luciferase construct to control for transfection efficiency. Cells were cultured in the presence or absence of tet for 24 h, treated with 1,000 U/ml IFN-β for 8 h, and assessed for dual luciferase activity. Luciferase activity for IRF9-luc is shown relative to pGL3 basal activity. Values represent the mean of three experiments, and error bars show standard deviations. (b) Western blot showing p53 levels in lysates of EJp53 in the presence of tet or 24 h after tet removal. (c) p53 DNA binding activity at the IRF9 promoter. EJp53 cells were cultured as described in Materials and methods, and ChIP assays were performed using an anti-p53 antibody (p53) or an equivalent amount of anti–rabbit IgG (IgG). Real-time PCR was performed for the IRF9 promoter regions indicated, as well as the p21 promoter, which served as a positive control. Quantification of the amount of promoter-specific immunoprecipitated DNA relative to that present in total chromatin was determined using the ΔCT method. (d) ISRE-luc reporter assay in HCT116 WT and p53−/− cells. Cells were transfected with the ISRE-luc reporter and treated with IFN using the conditions described in Materials and methods. In the p53−/− cells, the reporter was cotransfected with increasing amounts of exogenous IRF9 using the expression plasmid pCAGGs-hIRF9. Results are presented as the mean of three biological samples. The error bars represent standard deviations. (e) Protein levels of exogenous IRF9 in HCT116p53−/− cells.
We next performed a rescue assay by transfection of exogenous IRF9 into HCT116p53−/− cells to test whether the p53-dependent activation of IFN signaling was entirely attributable to IRF9. As shown in Fig. 4 d, expression of exogenous IRF9 in p53−/− cells significantly increased the transactivation of the ISRE reporter in a dose-dependent manner (Fig. 4 e). However, the maximum level achieved did not reach that observed in HCT116 cells expressing endogenous p53. These results support the conclusion that the p53-dependent transcriptional activation of IRF9 contributes importantly to the enhancement of IFN signaling but that p53 may have other targets as well.
p53 has been reported to possess activities independent of its transcriptional functions (5). To determine whether our newly identified role for p53 in enhancing IFN signaling also involved a nontranscriptional activity, we tested the ability of the p53 DNA binding domain mutants Y220C and V143A to enhance IFN signaling. Both of these p53 mutants occur frequently in human tumors, and both have been shown to lack p53 transcriptional activity (23, 24). Both mutants failed to transactivate the ISRE luciferase reporter in response to IFN treatment (Fig. S3 a, available at http://www.jem.org/cgi/content/full/jem.20080383/DC1) despite much higher levels of expression than that of endogenous WT p53, which strongly enhanced activation of this reporter in WT HCT116 cells (Fig. S3 c). As controls, neither p53 mutant activated the p21 promoter luciferase construct in response to doxorubicin, a DNA-damaging agent (25), or the IRF9 reporter in response to IFN (Fig. S3 b). All of these results establish that transcriptionally active WT p53 is required to enhance IFN signaling.
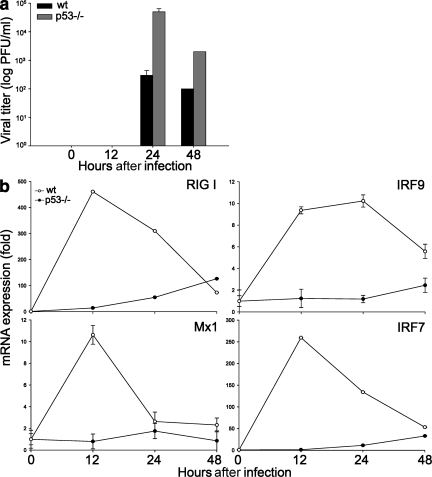
Previous evidence that p53−/− mice are more sensitive to viral infection was attributed to the absence of p53-dependent apoptosis in infected cells (9). To test whether p53−/− mice also exhibited an impaired IFN response, we infected WT and p53−/− mice with SeV strain Cantell, and measured viral titers and IFN response in lungs. As shown in Fig. 5 a, virus was not detectable at 12 h after infection, but by 24 h, the viral titer was >100-hundred fold higher in mice lacking p53. Of note, we observed a rapid increase in expression levels of ISGs, including IRF9, IRF7, Mx1, and RIG-I, in the infected lung tissue of WT mice, whereas this response was almost completely absent in p53−/− mice (Fig. 5 b). These results establish that p53 enhances IFN antiviral immunity in vivo, as well as in vitro by promoting the expression of ISGs. The levels of apoptosis in infected lungs did not vary significantly in WT and p53−/− mice at these early time points, arguing further that p53-induced cell death is not critical for its antiviral functions during the initial period after in vivo infection (unpublished data).
Figure 5.
p53 enhances IFN-mediated antiviral protection in vivo. (a) Viral titration of supernatants from the homogenized lungs of WT and p53−/− MEFs infected intranasally with 2 × 104 PFU of SeV strain Cantell for 0–48 h. The viral titers are represented as log (PFU/g of tissue). (b) Real-time PCR of lungs of WT and p53−/− mice. Results show RIG-I, Mx1, IRF9, and IRF7 mRNA expression levels in each condition compared with that of TBP (control). Results are mean values of three biological samples, and error bars represent standard deviations.
Our present studies demonstrate that p53 contributes to the up-regulation of ISRE-dependent genes by type I IFN through a mechanism involving transcriptional activation of IRF9 and the enhancement of IFN signaling. Moreover, our evidence that only WT transcriptionally active p53 was able to transactivate the IRF9 promoter and enhance the IFN activation of ISRE-dependent ISGs argues strongly against any nontranscriptional role. We showed further that p53 enhances not only IFN signaling but also IFN production after viral infection, which would help to establish the IFN-dependent antiviral state in bystander cells. This link between IFN signaling and production could also be caused by a p53-mediated increase in IRF9. As part of the ISGF3 complex, IRF9 contributes to IFN production by inducing IRF7, which primes IFN release by activating IFN-α production (19, 20). Consistent with this model, we observed a p53-dependent up-regulation of IRF7 in vitro and in vivo.
IRF5 has been previously shown to be a direct p53 target gene (10), although there is no evidence of cross talk between p53 and IRF5 in antiviral defense (26). It has also been reported that p53 directly transactivates ISG15 (11), which regulates several genes involved in IFN production, including PKR and RIG-I (27). However, ISG15 has been shown to be up-regulated by p53 in response to double-stranded RNA but not by IFN treatment or viral infection with NDV or SeV (11), thus making ISG15 an unlikely effector of the p53-dependent increase in IFN production. A recent paper also indicated a p53-dependent stabilization of STAT-1 in response to DNA damage (28), reinforcing the idea of a strong role for p53 in the induction of antiviral genes by type I IFN. These recent findings, and the fact that IRF9 overexpression could not completely restore the p53-dependent activation of the ISRE reporter in our rescue assays, suggest that p53 could also be acting to enhance IFN signaling through other IFN-related target genes. Therefore, further studies are necessary to determine whether p53 exerts direct control over the expression of other key elements of the IFN pathway.
A study by Dharel et al. recently reported that p53 mediates inhibition of hepatitis C virus replicons through cross talk between p53 and IFN signaling (29). The authors used a tumor cell line, Huh7, harboring a Y220C p53 mutant, which is commonly present in human tumors with p53-inactivating mutations (23, 24). They reported that this mutant retained transcriptional activity. However, this same mutant was previously shown (23) and demonstrated by us to lack transcriptional activity. Moreover, as shown in Fig. S3, it failed to activate ISRE and IRF9 reporters. Dharel et al. provided evidence that p53 bound to IRF9. However, the mechanism linking such binding to an enhancement of the IFN response was reported by them to be unclear. Our studies provide strong evidence that the mechanism by which p53 enhances IFN-regulated gene expression and antiviral responses requires transcriptionally active p53 to up-regulate IRF9 expression and enhance IFN signaling.
Our findings indicate that p53 not only enhances apoptosis in response to viral infection but also plays an important role in the IFN-dependent antiviral response. To reconcile these different roles of p53 in antiviral defense, we propose a two-phase model. First, p53 enhances IFN production and signaling by contributing to a positive feedback loop through the transcriptional induction of IRF9 and, probably, other IFN-related genes. This would presumably be important in the early stages to counteract viral infection through the induction of ISGs. In a second phase, p53 could help to thwart viral replication by eliminating infected cells through induction of apoptosis. Our study may also have relevance regarding the clinical use of oncolytic viruses in tumor therapy. For example, p53 mutant tumor cells might be more permissive than WT cells for viral replication because of the lack of optimal IFN production and signaling. Moreover, our results provide a rationale to devise therapeutic strategies that enhance p53 expression to control viral infections.
MATERIALS AND METHODS
Cell lines and viruses.
HCT116, 293T, Vero, and BHK-21 cells, as well as MEFs, were maintained in DMEM supplemented with 10% FBS and 50 U/ml penicillin-streptomycin. EJp53 and EJp16 were cultured as previously described (14, 30). VSV-GFP (a gift from S. Woo, Mount Sinai School of Medicine, New York, NY) was grown and titrated in BHK-21 cells, as previously described (31). NDV and SeV were grown as previously described (18, 32).
Plasmids, antibodies, and reagents.
pHISG54-RFP/CAT (33), pGL-FOS, and pGL-FOS-ISREluc (a gift from T. Ouchi, Northwestern University, Evanston, IL) were transfected with FuGENE (Roche), according to the manufacturer's instructions. The caspase inhibitor Q-VD-OPH was purchased from MP Biomedicals. Universal type I IFN was purchased from PBL Biomedical Laboratories. Doxorubicin was purchased from Sigma-Aldrich. Immunoblots were performed using antibodies against RIG-I (Prosci Inc.), p16 (BD Biosciences), p48/IRF9 (Santa Cruz Biotechnology, Inc.), β-tubulin (Sigma-Aldrich), β-actin (Sigma-Aldrich), and p53 (1801 mAb; reference 15). To obtain the pCAGGs-hIRF9 construct, hIRF9 was amplified by RT-PCR from RNA obtained from human Hep-2 cells. Primers used for RT-PCR were 5′-CGCGGAATTCACCATGGCATCAGGCAGGGCACGC-3′ (hIRF9/EcoRI/5′) and 5′-CGCGCTCGAGCTACACCAGGGACAGAATGGCTGC-3′ (hIRF9/XhoI/3′). The PCR product was cloned into the mammalian expression plasmid pCAGGs (34) by using the restriction endonucleases EcoRI and XhoI. IRF9 expression was verified by sequencing and Western blotting. To generate the pEGFP-C1-hIRF9 construct, hIRF9 was amplified from pCAGGs hIRF9 and cloned into the expression vector pEGFP-C1 (Clontech Laboratories, Inc.) by restriction endonucleases XhoI and EcoRI. GFP-IFR9 expression was confirmed by sequencing and Western blotting. The p53 Y220C and p53 V143A point mutants were generated by site-directed mutagenesis using the Change-IT Multiple Mutation Site Directed Mutagenesis Kit (USB), according to the manufacturer's instructions. As a template, we used the pFLAG-p53 plasmid (a gift from J. Manfredi, Mount Sinai School of Medicine, New York, NY). The primers used to generate the mutants were 5′-GTGGTGCCCTCTGAGCCGCCTG-3′ (p53YY220CFw) and 5′-GACCTGCCCTGCGCAGCTGTGG-3′ (p53V143A Fw). The pGL3-hIRF9 promoter luciferase construct was done by using 5′ RACE (Ambion) to sequence upstream of the transcription start site. We then cloned this region (−3271 bp) plus 817 bp downstream into pGL3 (Promega) using 5′-NheI and 3′-XhoI sites. The pGL3-p21 promoter construct was previously described (35).
FACS analysis.
Fluorescent-stained cells were transferred to polystyrene tubes with cell-strainer caps (BD Biosciences) and subjected to FACS (FACScan; Becton Dickinson) using CellQuest 3.2 software (Becton Dickinson) for acquisition and analysis. For cell-cycle analysis, cells were stained with propidium iodide (PI) using the CycleTEST Plus DNA reagent kit (Becton Dickinson), according to the manufacturer's instructions, and then subjected to FACS analysis.
Quantitative RT-PCR (qRT-PCR) analysis.
For qRT-PCR analysis, RNA was isolated from cells by TRIzol (Invitrogen) according to manufacturer's instructions. 100 ng RNA and SYBR green (Roche) were used in an ABI7900 HT instrument (Applied Biosystems), according to manufacturer's instructions. Table S1 (available at http://www.jem.org/cgi/content/full/jem.20080383/DC1) shows the primers used.
Reporter assays.
293T cells stably expressing the pHISG54-RFP/CAT reporter plasmid (a gift from B. Beitzel, U.S. Army Medical Research Institute of Infectious Diseases, Frederick, MD) were treated with the supernatants indicated in each experiment to test IFN presence. RFP expression was visualized by epifluorescence. For luciferase reporter assays, cells were cotransfected with the reporter plasmids and pRL-CMV (Promega) expressing Renilla luciferase as an internal control. 24 h after transfection, cells were treated with 500 U/ml IFN for 12 h more. Cell lysates were collected and procesed with a luciferase kit assay (Promega) according to the manufacturer's instructions.
NDV-GFP bioassay.
Supernatants from virus-infected cells were inactivated for 10 min under UV light and added to fresh Vero cells. 16 h later, cells were infected with NDV-GFP (multiplicity of infection [MOI] of 2). 24 h after infection, GFP expression was monitored by epifluorescence. As positive controls, we used Vero cells treated with the amounts of IFN-β indicated in the figures.
ChIP assay.
ChIP assays were performed according to the manufacturer's instruction, using the ChIP assay kit (Millipore). ChIP DNA extracted from the cell lysates was next used as a template for PCR.
In vivo viral infections.
WT and p53-null mice of a C57BL/6 pure background (B6;129-Trp53tm1Brd; Taconic) (36) were infected by applying the virus (2 × 104 PFU of SeV strain Cantell diluted in PBS) directly to the nostrils. Lungs were extracted and homogenized mechanically in a PBS solution. Supernatants were used to titrate SeV in 293T HISG54-RFP/CAT by counting red fluorescent cells in serial dilutions of such supernatants. Pellets were used for qRT-PCR. All animal protocols were approved by the Institutional Animal Care and Use Committee of the Mount Sinai School of Medicine.
Online supplemental material.
Fig. S1 shows a p53-dependent antiviral effect in the EJp53-inducible cell line. As shown by comparison with EJp16 cells, the p53-dependent antiviral effect is not caused by its ability to trigger cell cycle arrest. Fig. S2 shows that p53 also induces IFN production in response to viral infection. Fig. S3 shows a lack of activation of the ISRE reporter by the p53 point mutants Y220C and V143A compared with WT p53. Neither of these mutants was also able to transactivate the p21 promoter and IRF9 promoter reporter constructs, indicating a lack of transcriptional activity. Table S1 shows primers used in qRT-PCR. Online supplemental material is available at http://www.jem.org/cgi/content/full/jem.20080383/DC1.
Supplementary Material
Acknowledgments
We thank E. Rodríguez, G. Akiri, A. Mahale, and L. Grumolato for helpful comments and discussions about the manuscript; R. Cadagan, S. Yao, and R. Qiao for excellent technical support; and S. Woo, J. Manfredi, and T. Ouchi for providing us with reagents. We specially thank B. Beitzel for providing us with the 293T HISG54-RFP/CAT reporter cells.
C. Muñoz-Fontela is a recipient of a postdoctoral fellowship from the Spanish Ministry of Education and Science. S. Macip is a recipient of a postdoctoral fellowship from the National Cancer Institute (T32CA78207). This work was supported by National Institutes of Health grants CA80058 and CA85214 (to S.A. Aaronson), and CA127247 and CA097216 (to S.W. Lee). L. Martínez-Sobrido and A. García-Sastre were supported by grant U19AI62623 from the National Institute of Allergy and Infectious Disease–funded Center to Investigate Viral Immunity and Antagonism.
The authors have no conflicting financial interests.
S. Macip's present address is Dept. of Biochemistry, University of Leicester, Leicester LE1 9HN, England, UK.
References
- 1.García-Sastre, A., and C.A. Biron. 2006. Type 1 interferons and the virus-host relationship: a lesson in détente. Science. 312:879–882. [DOI] [PubMed] [Google Scholar]
- 2.Platanias, L.C., and E.N. Fish. 1999. Signaling pathways activated by interferons. Exp. Hematol. 27:1583–1592. [DOI] [PubMed] [Google Scholar]
- 3.Stark, G.R., I.M. Kerr, B.R. Williams, R.H. Silverman, and R.D. Schreiber. 1998. How cells respond to interferons. Annu. Rev. Biochem. 67:227–264. [DOI] [PubMed] [Google Scholar]
- 4.Takaoka, A., S. Hayakawa, H. Yanai, D. Stoiber, H. Negishi, H. Kikuchi, S. Sasaki, K. Imai, T. Shibue, K. Honda, and T. Taniguchi. 2003. Integration of interferon-alpha/beta signalling to p53 responses in tumour suppression and antiviral defence. Nature. 424:516–523. [DOI] [PubMed] [Google Scholar]
- 5.Fuster, J.J., S.M. Sanz-González, U.M. Moll, and V. Andrés. 2007. Classic and novel roles of p53: prospects for anticancer therapy. Trends Mol. Med. 13:192–199. [DOI] [PubMed] [Google Scholar]
- 6.Pampin, M., Y. Simonin, B. Blondel, Y. Percherancier, and M.K. Chelbi-Alix. 2006. Cross talk between PML and p53 during poliovirus infection: implications for antiviral defense. J. Virol. 80:8582–8592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Townsend, P.A., T.M. Scarabelli, S.M. Davidson, R.A. Knight, D.S. Latchman, and A. Stephanou. 2004. STAT-1 interacts with p53 to enhance DNA damage-induced apoptosis. J. Biol. Chem. 279:5811–5820. [DOI] [PubMed] [Google Scholar]
- 8.Ding, Y., J.F. Lee, H. Lu, M.H. Lee, and D.H. Yan. 2006. Interferon-inducible protein IFIXalpha1 functions as a negative regulator of HDM2. Mol. Cell. Biol. 26:1979–1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Muñoz-Fontela, C., M.A. Garcia, I. Garcia-Cao, M. Collado, J. Arroyo, M. Esteban, M. Serrano, and C. Rivas. 2005. Resistance to viral infection of super p53 mice. Oncogene. 24:3059–3062. [DOI] [PubMed] [Google Scholar]
- 10.Mori, T., Y. Anazawa, M. Iiizumi, S. Fukuda, Y. Nakamura, and H. Arakawa. 2002. Identification of the interferon regulatory factor 5 gene (IRF-5) as a direct target for p53. Oncogene. 21:2914–2918. [DOI] [PubMed] [Google Scholar]
- 11.Hummer, B.T., X.L. Li, and B.A. Hassel. 2001. Role for p53 in gene induction by double-stranded RNA. J. Virol. 75:7774–7777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Caserta, T.M., A.N. Smith, A.D. Gultice, M.A. Reedy, and T.L. Brown. 2003. Q-VD-OPh, a broad spectrum caspase inhibitor with potent antiapoptotic properties. Apoptosis. 8:345–352. [DOI] [PubMed] [Google Scholar]
- 13.Zhang, L., J. Yu, B.H. Park, K.W. Kinzler, and B. Vogelstein. 2000. Role of BAX in the apoptotic response to anticancer agents. Science. 290:989–992. [DOI] [PubMed] [Google Scholar]
- 14.Sugrue, M.M., D.Y. Shin, S.W. Lee, and S.A. Aaronson. 1997. Wild-type p53 triggers a rapid senescence program in human tumor cells lacking functional p53. Proc. Natl. Acad. Sci. USA. 94:9648–9653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Macip, S., M. Igarashi, P. Berggren, J. Yu, S.W. Lee, and S.A. Aaronson. 2003. Influence of induced reactive oxygen species in p53-mediated cell fate decisions. Mol. Cell. Biol. 23:8576–8585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Macip, S., M. Igarashi, L. Fang, A. Chen, Z.Q. Pan, S.W. Lee, and S.A. Aaronson. 2002. Inhibition of p21-mediated ROS accumulation can rescue p21-induced senescence. EMBO J. 21:2180–2188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnston, M.D. 1981. The characteristics required for Sendai virus preparation to induce high levels of interferon in human lymphoblastoid cells. J. Gen. Virol. 56:175–184. [DOI] [PubMed] [Google Scholar]
- 18.Park, M.S., A. García-Sastre, J.F. Cros, C.F. Basler, and P. Palese. 2003. Newcastle disease virus V protein is a determinant of host range restriction. J. Virol. 77:9522–9532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marié, I., J.E. Durbin, and D.E. Levy. 1998. Differential viral induction of distinct interferon-alpha genes by positive feedback through interferon regulatory factor-7. EMBO J. 17:6660–6669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sato, M., N. Hata, M. Asagiri, T. Nakaya, T. Taniguchi, and N. Tanaka. 1998. Positive feedback regulation of type I IFN genes by the IFN-inducible transcription factor IRF-7. FEBS Lett. 441:106–110. [DOI] [PubMed] [Google Scholar]
- 21.Yoneyama, M., W. Suhara, Y. Fukuhara, M. Sato, K. Ozato, and T. Fujita. 1996. Autocrine amplification of type I interferon gene expression mediated by interferon stimulated gene factor 3 (ISGF3). J. Biochem. 120:160–169. [DOI] [PubMed] [Google Scholar]
- 22.Bluyssen, A.R., J.E. Durbin, and D.E. Levy. 1996. ISGF3 gamma p48, a specificity switch for interferon activated transcription factors. Cytokine Growth Factor Rev. 7:11–17. [DOI] [PubMed] [Google Scholar]
- 23.Müller, M., S. Wilder, D. Bannasch, D. Israeli, K. Lehlbach, M. Li-Weber, S.L. Friedman, P.R. Galle, W. Stremmel, M. Oren, and P.H. Krammer. 1998. p53 activates the CD95 (APO-1/Fas) gene in response to DNA damage by anticancer drugs. J. Exp. Med. 188:2033–2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wong, K.B., B.S. DeDecker, S.M. Freund, M.R. Proctor, M. Bycroft, and A.R. Fersht. 1999. Hot-spot mutants of p53 core domain evince characteristic local structural changes. Proc. Natl. Acad. Sci. USA. 96:8438–8442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ellis, C.N., M.B. Ellis, and W.S. Blakemore. 1987. Effect of adriamycin on heart mitochondrial DNA. Biochem. J. 245:309–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yanai, H., H.M. Chen, T. Inuzuka, S. Kondo, T.W. Mak, A. Takaoka, K. Honda, and T. Taniguchi. 2007. Role of IFN regulatory factor 5 transcription factor in antiviral immunity and tumor suppression. Proc. Natl. Acad. Sci. USA. 104:3402–3407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao, C., C. Denison, J.M. Huibregtse, S. Gygi, and R.M. Krug. 2005. Human ISG15 conjugation targets both IFN-induced and constitutively expressed proteins functioning in diverse cellular pathways. Proc. Natl. Acad. Sci. USA. 102:10200–10205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Youlyouz-Marfak, I., N. Gachard, C. Le Clorennec, I. Najjar, F. Baran-Marszak, L. Reminieras, E. May, G.W. Bornkamm, R. Fagard, and J. Feuillard. 2008. Identification of a novel p53-dependent activation pathway of STAT1 by antitumour genotoxic agents. Cell Death Differ. 15:376–385. [DOI] [PubMed] [Google Scholar]
- 29.Dharel, N., N. Kato, R. Muroyama, H. Taniguchi, M. Otsuka, Y. Wang, A. Jazag, R.-X. Shao, J.-H. Chang, M.K. Adler, et al. 2008. Potential contribution of tumor suppressor p53 in the host defense against hepatitis C virus. Hepatology. 47:1136–1149. [DOI] [PubMed] [Google Scholar]
- 30.Fang, L., M. Igarashi, J. Leung, M.M. Sugrue, S.W. Lee, and S.A. Aaronson. 1999. p21Waf1/Cip1/Sdi1 induces permanent growth arrest with markers of replicative senescence in human tumor cells lacking functional p53. Oncogene. 18:2789–2797. [DOI] [PubMed] [Google Scholar]
- 31.Ebert, O., K. Shinozaki, T.G. Huang, M.J. Savontaus, A. García-Sastre, and S.L. Woo. 2003. Oncolytic vesicular stomatitis virus for treatment of orthotopic hepatocellular carcinoma in immune-competent rats. Cancer Res. 63:3605–3611. [PubMed] [Google Scholar]
- 32.Basler, C.F., A. Mikulasova, L. Martinez-Sobrido, J. Paragas, E. Mühlberger, M. Bray, H.D. Klenk, P. Palese, and A. García-Sastre. 2003. The Ebola virus VP35 protein inhibits activation of interferon regulatory factor 3. J. Virol. 77:7945–7956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martínez-Sobrido, L., E.I. Zúñiga, D. Rosario, A. García-Sastre, and J.C. de la Torre. 2006. Inhibition of the type I interferon response by the nucleoprotein of the prototypic arenavirus lymphocytic choriomeningitis virus. J. Virol. 80:9192–9199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Niwa, H., K. Yamamura, and J. Miyazaki. 1991. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene. 108:193–199. [DOI] [PubMed] [Google Scholar]
- 35.Kwak, J.C., P.P. Ongusaha, T. Ouchi, and S.W. Lee. 2003. IFI16 as a negative regulator in the regulation of p53 and p21(Waf1). J. Biol. Chem. 278:40899–40904. [DOI] [PubMed] [Google Scholar]
- 36.Donehower, L.A., M. Harvey, B.L. Slagle, M.J. McArthur, C.A. Montgomery Jr., J.S. Butel, and A. Bradley. 1992. Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature. 356:215–221. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.