Abstract
Immune tolerance to self-antigens is a complex process that utilizes multiple mechanisms working in concert to maintain homeostasis and prevent autoimmunity. We developed a system that revealed a population of self-specific CD8 T cells within the endogenous T cell repertoire. Immunization of ovalbumin (OVA)-expressing transgenic mice with recombinant viruses expressing OVA-peptide variants induced self-reactive T cells in vivo that matured into memory T cells able to respond to secondary infection. However, whereas the avidity of memory cells in normal mice increased dramatically with repeated immunizations, avidity maturation was limited for self-specific CD8 T cells. Despite decreased avidity, such memory cells afforded protection against infection, but did not induce overt autoimmunity. Further, up-regulation of self-antigen expression in dendritic cells using an inducible system promoted programmed death-1 expression, but not clonal expansion of preexisting memory cells. Thus, the self-reactive T cell repertoire is controlled by overlapping mechanisms influenced by antigen dose.
Many autoimmune diseases, such as type I diabetes and multiple sclerosis, are the consequence of damage mediated by autoreactive T cells. These cells arise as a result of the stochastic process of TCR gene rearrangement that is required to generate adequate diversity within the T cell pool (1). Although most newly generated T cells are specific for foreign antigens and are beneficial to the host, some display TCR with reactivity to self-proteins, and therefore must be silenced to maintain normal immunological homeostasis. The majority of tolerance induction occurs during T cell development in the thymus, in which T cells with strong reactivity to self-peptide–MHC complexes are eliminated through negative selection (2). Recent reports have demonstrated that the transcription factor autoimmune regulator (AIRE) mediates T cell deletion in the thymus through driving promiscuous expression of genes encoding many self-antigens, including those proteins believed to only be expressed in the periphery (3). However, this process is not entirely effective, and a fraction of cells with specificity for self-antigens escape from thymic negative selection.
The export of T cells from the thymus with potential reactivity to self-proteins requires the presence of additional tolerogenic mechanisms in the periphery to prevent autoimmunity. Past reports have clearly demonstrated that different mechanisms, such as TCR down-regulation, antigen-induced anergy, and regulation caused by FoxP3+CD4+CD25+ T cells, may play a role in preventing disease induced by autoreactive T cells (4–8). Additionally, tolerance can also be induced through cross-presentation of proteins by APC in the draining lymph nodes. This presentation in the absence of inflammatory signals results in T cell activation and subsequent removal through deletional tolerance (9–11). More recently, one study demonstrated that in addition to its role in thymic selection (3, 12), AIRE may also participate in maintaining peripheral tolerance. Thus, AIRE expression in the stromal cells of lymph nodes leads to antigen expression and subsequent deletion of T cells with potential self-reactivity (13). These mechanisms appear to operate for T cells with high avidity for self-antigens, whereas other populations of T cells with weaker reactivity to self-antigens may persist (14, 15). In the presence of the correct environmental cues, it is these T cells that may provide a potential population required for the initiation of autoimmunity.
Although the initiating factors for autoimmunity are largely unknown, one hypothesis is that infection with pathogens expressing antigenic homologues of self-proteins may promote activation of potentially autoreactive T cells. For example, pathogens such as group A streptococcus and Borrelia burgdorferi have been implicated in the initiation of myocarditis and arthritis, respectively (16, 17). Further, experimental systems have demonstrated this to occur in experimental murine models, including experimental autoimmune encephalitis (EAE), and suggest that infection with bacteria or viruses that express peptides with homology to normal self-proteins may initiate autoimmune diseases (18). Although the exact mechanism is unknown, molecular mimicry is thought to occur through the degeneracy of the T cell receptor, where a TCR specific for a given antigen may have weaker interactions for other epitopes, including self-proteins (19). This strategy has been used to generate T cells with specificities against various antigens expressed by tumor cells with the hopes of developing therapies for the treatment of various malignancies. This includes immunization with xenoantigens such as gp100 or prostate acid phosphatase for the treatment of melanoma or prostate cancer, respectively (20, 21). Alternatively, defined epitopes may be specifically mutated to generate altered peptide ligands with the hopes of enhancing T cell signaling and generating T cell responses to tumor antigens (22, 23).
In this study, we demonstrate the development of self-reactive CD8 T cells after immunization with viruses containing mutant epitopes of the chicken OVA epitope SIINFEKL in mice expressing OVA as a self-antigen. These self-reactive cells established a functional memory population in vivo, and are able to perform effector functions and respond to and protect against secondary infection. Surprisingly, despite very high frequencies, autoreactivity was not evident in vivo. The mechanism by which these cells are kept in check is related at least in part to the limiting of avidity maturation after reactivation. Thus, the self-reactive repertoire can exert beneficial effects in the absence of autoimmunity.
RESULTS
Single amino acid SIINFEKL variants elicit OVA-specific CD8 T cells in tolerant mice
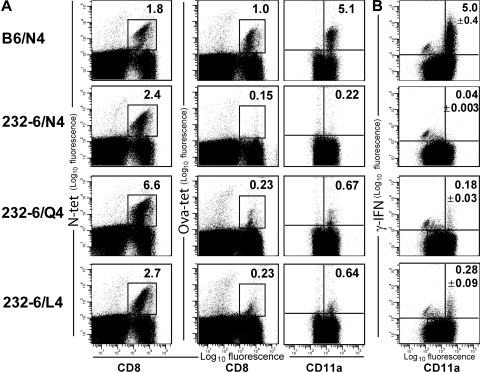
The ability of the immune system to prevent autoreactivity in the presence of T cells with specificities for self-proteins requires the presence of potent tolerogenic mechanisms. However, little work has addressed tolerance induction in the endogenous T cell repertoire. To evaluate how tolerance is maintained for endogenous CD8 T cells in vivo, we used the 232-6 transgenic mouse model, in which a truncated form of chicken OVA is expressed at low levels by the enterocytes of the small intestine (9). Whereas previous studies using these animals and OT-I TCR transgenic T cell transfer demonstrate that recognition of OVA as a self-protein resulted in deletional tolerance (9), transfer in the presence of inflammatory signals results in intestinal tissue damage and weight loss (9). Thus, to investigate how tolerance of endogenous CD8 T cells is maintained in this system, we attempted to elicit OVA-specific cells in 232-6 mice. Infection with WT vesicular stomatitis virus (VSV)-OVA (designated as N4 for asparagine at the fourth residue) elicited few, if any, SIINFEKL-specific CD8 T cells in 232-6 mice, but drove a robust response in WT mice (Fig. 1 A), indicating that tolerance was being maintained. To attempt to reveal populations of OVA-specific CD8 T cells in the 232-6 repertoire, we infected mice with rVSV expressing peptide variants of SIINFEKL in which the fourth position was altered from an asparagine to a glutamic acid (N260Q) or a leucine (N260L), termed Q4 and L4, respectively. The fourth position was chosen because it has been determined to be a contact residue for T cell recognition and is not involved in MHC binding (24). Remarkably, 232-6 mice infected with VSV expressing either the Q4 or L4 variants generated populations of CD8 T cells reactive with the SIINFEKL-H-2Kb tetramer (OVA-tet; Fig. 1 A). Although the frequencies of these populations of T cells were small compared with the response generated by N4 infection in C57BL/6 mice, the overall population was approximately threefold greater than 232-6 mice infected with the N4 variant. Furthermore, this population expressed high levels of CD11a, indicating an activated phenotype, and when evaluated as part of the overall CD8 population, it demonstrated a 5–8-fold increase in frequency compared with 232-6 mice infected with VSV-N4. As expected, all groups of mice infected with the rVSV developed CD8 T cell populations specific for the VSV nucleoprotein (N) epitope demonstrating that a productive VSV infection had occurred. These results indicated that immunization with Q4 or L4 variants of the SIINFEKL epitope generated CD8 T cells with the ability to recognize WT SIINFEKL peptide in transgenic animals that express OVA as a self-antigen.
Figure 1.
Q4 and L4 SIINFEKL variants elicit cross-reactive T cell responses in 232-6 mice. (A) C57BL/6 and 232-6 OVA transgenic mice were immunized with rVSV expressing the N4, Q4, or L4 variants of the SIINFEKL epitopes. 7 d later, splenocytes were evaluated for N-tet– (left) and OVA-tet–specific (middle) T cell populations. CD8+ T cells were also evaluated for the coexpression of OVA-tet and CD11a (right). (B) IFN-γ expression by OVA-reactive T cells. C57BL/6 and 232-6 mice were immunized with VSV-N4, Q4, and L4. 7 d later, splenocytes were restimulated in culture for 4 h with SIINFEKL peptide and evaluated for intracellular IFN-γ expression. The percentage of CD11ahiIFN-γ+ cells among CD8 T cell is indicated in the top right of the dot plot. Data are representative of three animals/group.
We also determined the functionality of the OVA-specific cells from rVSV-infected 232-6 mice. To do so, splenocytes from C57BL/6 and 232-6 mice infected with VSV-N4 or VSV-Q4/L4, respectively, were cultured in vitro with 0.01 μg/ml SIINFEKL peptide for 4 h and evaluated for IFN-γ production by intracellular staining (Fig. 1 B). Cells from C57BL/6 mice immunized with control virus that lacks OVA and cells from 232-6 mice infected with WT VSV-OVA did not produce detectable IFN-γ upon restimulation with SIINFEKL in vitro (Fig. 1 B and not depicted). In contrast, cells from C57BL/6 mice immunized with VSV-N4 generated a robust response, with ∼5% of the CD8 population producing IFN-γ. Cells from 232-6 mice immunized with VSV-Q4 or -L4 and restimulated with WT peptide also produced IFN-γ (Fig. 1 B). Approximately 0.2–0.4% of the cells produced IFN-γ, which correlated with the frequency of OVA-tet+ cells. Interestingly, based on mean fluorescence intensities, the amount of IFN-γ produced by Q4/L4 immunization in 232-6 mice, on a per cell basis, was also reduced compared with OVA-reactive cells from C57BL/6 mice, and this difference was also observed at higher peptide concentrations (1.0 μg/ml; unpublished data). Collectively, these data demonstrated that infection with Q4 and L4 SIINFEKL VSV variants resulted in the generation of an obvious, but small, population of OVA-specific CD8 T cells in transgenic mice that express OVA as a self-antigen.
Variant-induced, SIINFEKL-specific CD8 T cells develop functional memory populations
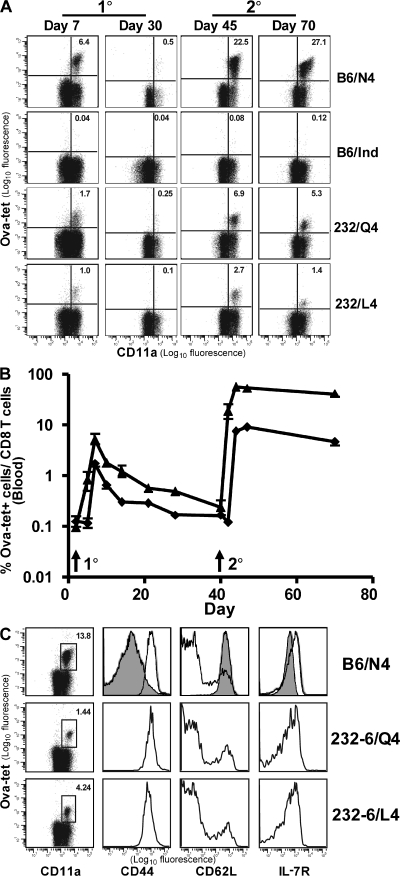
The presence of SIINFEKL-specific T cells in 232-6 OVA transgenic animals after VSV-Q4 or L4 immunization indicated that even in the presence of abundant self-antigen, a primary self-specific response could develop. However, it was possible that over time such cells would undergo deletion. Therefore, we examined the overall kinetics of the response and tested whether a self-specific memory population developed. The latter is of particular interest, considering the apparent increased sensitivity of memory cells to antigenic stimulation (25, 26). To this end, C57BL/6 and 232-6 mice were immunized with VSV-N4, -Q4, -L4, or the control VSV-Indiana, and the percentage of SIINFEKL-reactive cells in the blood was evaluated over time (Fig. 2, A and B). C57BL/6 mice immunized with VSV-N4 mounted a robust OVA-specific response that peaked 7 d after infection with ∼6% of the CD8 population staining brightly with OVA-tet. This was followed by a contraction phase such that by 40 d after infection, ∼0.5% of the CD8 population remained OVA-tet positive. 232-6 mice immunized with either VSV-Q4 or -L4 developed CD8 T cell responses specific for SIINFEKL with similar kinetics to that observed for C57BL/6 mice, although the overall magnitude of the response was reduced by ∼5-6-fold as compared with controls. By 40 d after infection, the memory population comprised 0.1–0.25% of the CD8 population.
Figure 2.
Kinetics and phenotype of OVA-reactive CD8 T cells in 232-6 and C57BL/6 mice. (A) C57BL/6 and 232-6 mice were immunized with control VSV-Indiana or VSV containing the N4, Q4, or L4 SIINFEKL epitopes. 40 d later, N4-, Q4-, and L4-immunized mice were challenged with 103 LM-OVA. All animals were bled periodically during the primary and secondary responses to monitor OVA-tetramer+ CD8 T cells. (B) Kinetic analysis of OVA-reactive CD8 T cells in C57BL/6 and 232-6 mice immunized with VSV-N4 (▴) or L4 (♦), respectively, and then rechallenged with LM-OVA as described in A (three animals/group). (C) Memory markers were evaluated on OVA-specific splenocytes 40 d after recall. Open histograms, CD11ahi, OVA-tet+ cells; dark histograms, CD11alo naive cells.
We next examined whether the OVA-tet+ memory cells could mount a secondary response to infection. Mice harboring memory cells raised by infection 40 d earlier with VSV-N4 or -L4 were infected with recombinant Listeria monocytogenes (LM)–expressing OVA that contains the WT (N4) epitope (LM-OVA; Fig. 2, A and B). In control mice, tet+ CD8 T cells increased by ∼50-fold, from 0.5 to ∼25% of the CD8 population, with the peak of expansion occurring at ∼3 d after recall. Remarkably, 40 d after primary VSV-Q4 or -L4 infection, 232-6 mice also mounted a robust recall response to LM-OVA challenge that was characterized by a 20–30-fold increase in OVA-reactive CD8 T cells. An appreciable lag in the response was observed in 232-6 mice compared with the response in B6 mice, which is likely caused by the reduced number of initial antigen-specific cells before challenge. In both the WT and 232-6 mice, the contraction of the OVA-specific response was limited compared with that of the primary response, and thus resulted in the generation of a substantial pool of secondary memory cells (Fig. 2 B). Thus, self-specific CD8 T cells persisted long term without a significant loss of cells. In addition, despite the presence of large numbers of OVA-reactive CD8 T cells during and after the recall response in 232-6 mice, the gross morphology of the intestinal mucosa remained normal and weight loss did not occur (unpublished data).
The previous experiment demonstrated that VSV-Q4 and -L4 immunization can produce a population of OVA-specific T cells with characteristics of memory cells, including the ability to persist long term and respond to secondary infection. To determine whether any phenotypic distinctions were evident between OVA-specific memory cells in 232-6 and C57BL/6 mice, we evaluated expression of CD11a, CD62L, and IL-7R. The phenotype of memory cells from either source was similar, as follows: CD11ahi, CD62Llo, and IL-7Rhi (Fig. 2 C). These data suggested that the 232-6–derived memory cells were not chronically stimulated with antigen because they expressed high levels of IL-7Rα (27). This was also true of the limited numbers of OVA-tet+ cells found in the intestinal mucosa (unpublished data). A subset of CD62Lhi central memory cells (28, 29) was also detected in both groups, indicating that a bias toward a particular memory subset did not occur as the result of self-antigen expression.
OVA-reactive CD8 T cells exhibit lytic activity in vivo
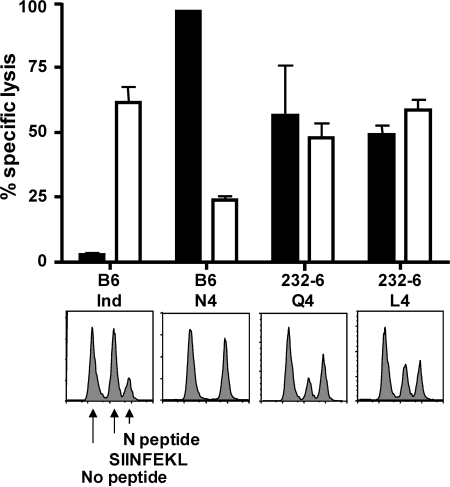
OT-I cells activated by infection induce intestinal epithelial cell damage in 232-6 mice (9) resulting in weight loss. The finding that the endogenous self-specific CD8 T cells resident in 232-6 mice did not cause obvious intestinal damage suggested a state of anergy. Although the primary OVA-tet+ cells produced cytokines (Fig. 1), it was possible that longer-term exposure to antigen-induced functional abnormalities. Therefore, we tested the in vivo lytic activity of the OVA-reactive cells at the peak of the recall response when autoimmune activity might be expected (Fig. 3). Previously infected WT or 232-6 mice were challenged with LM-OVA and, 5 d later, peptide-coated, CFSE-labeled target cells were injected. 4 h later, spleen cells were evaluated for the presence or absence of target cells. As expected, high levels of lytic activity against VSV nucleoprotein N peptide–coated cells, but little lysis of the unpulsed targets, was detected in all mice (Fig. 3). Conversely, SIINFEKL-specific lysis was not observed in those animals that were only immunized with VSV-Indiana, but robust activity was demonstrated in mice after immunization with the VSV-N4–positive control. Interestingly, 232-6 mice immunized with either VSV-Q4 or -L4 also demonstrated lysis of the SIINFEKL-pulsed target cells (Fig. 3). Although killing levels were less than that of the positive control, it should be noted that this result directly correlated with the reduced frequencies of OVA-reactive cells in 232-6 mice. Nevertheless, it remains possible that cells in the 232-6 mice exhibited reduced lytic activity on a per cell basis compared with those from WT mice.
Figure 3.
Q4- and L4-induced OVA-specific T cells demonstrate reduced lytic activity in vivo. C57BL/6 and 232-6 mice were infected with control VSV-Indiana or rVSV expressing the N4, Q4, or L4 variant SIINFEKL epitopes. 30 d later, animals were challenged with 103 LM-OVA. 5 d later, animals were injected with congenically disparate splenocytes labeled with CFSE (2, 0.2, and 0.02 μM) and pulsed with SIINFEKL (shaded) or N-peptide (open). Percentage of killing was evaluated 4 h later in the spleen. Error bars indicate the SEM of three animals/group.
Self-specific CD8 T cells protect against LM-OVA infection
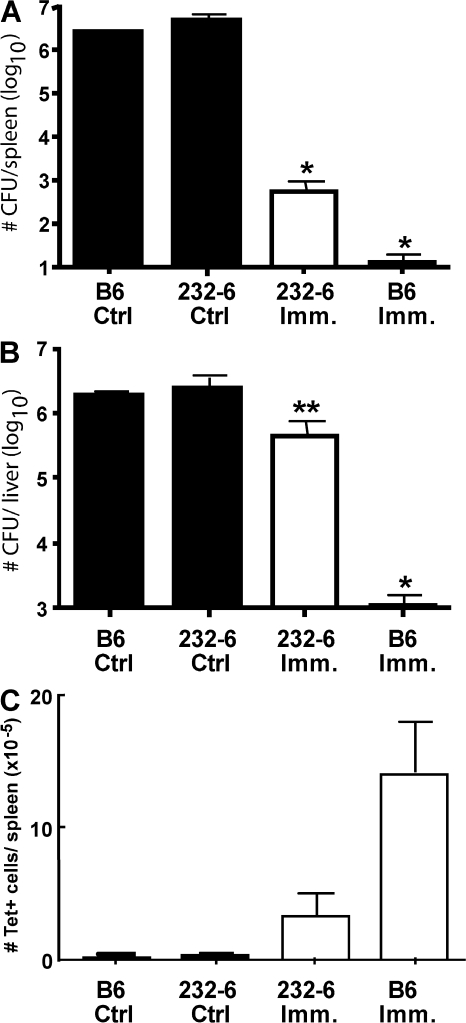
Because OVA-reactive cells in 232-6 mice performed effector functions, we next evaluated their ability to protect against high-dose LM-OVA infection (Fig. 4). C57BL/6 and 232-6 mice were immunized with VSV-N4 or -Q4, respectively. Both groups of mice were infected 30 d later with vaccinia virus (VV)–expressing OVA (VV-OVA) to expand the number of OVA-reactive cells while concurrently preventing responses to endogenous LM antigens. Immunized and nonimmunized animals were challenged with 2 × 105 LM-OVA, and 3 d later, bacterial burdens in the spleen and liver were enumerated. Naive C57BL/6 and 232-6 mice challenged with LM-OVA presented high bacterial counts in both the spleen and liver (Fig. 4, A and B). Conversely, immunized C57BL/6 mice were effectively protected, with virtually all LM-OVA cleared within the 3-d period. Importantly, 232-6 mice initially infected with VSV-Q4 and recalled with VV-OVA demonstrated a significant level of protection in the spleen and partial protection in the liver. Although less than that observed for C57BL/6 mice, 232-6 mice demonstrated a 3 log reduction in the spleen (P < 0.0001) and 1 log reduction for the liver (P = 0.037) when compared with uninfected control groups. Additional control studies in which WT LM (i.e., not expressing OVA) was used to challenge previously infected B6 or 232-6 mice, showed that the protection observed was antigen-specific and not the result of nonspecific innate immunity (unpublished data). Collectively, these results indicate that the OVA-specific T cells in 232-6 mice are able to protect against LM-OVA infection. Although the overall reduction in 232-6 mice is lower than that observed in C57BL/6 mice, this was likely attributable to the presence of reduced numbers of OVA-reactive cells in 232-6 mice (Fig. 4 C).
Figure 4.
OVA cross-reactive T cells protect against LM-OVA challenge. C57BL/6 and 232-6 mice were immunized with rVSV-N4 or -Q4, respectively, and recalled 30 d later with VV-OVA. 30 d after recall, mice were challenged with 2 × 105 LM-OVA, and after 3 d, the number of LM-OVA CFU in the spleen (A) and liver (B) were evaluated. (C) Splenic OVA-reactive CD8 T cells were also evaluated for each group by OVA-tet staining. Data are representative of three to five mice/group. *, P < 0.0001; **, P = 0.037. Error bars represent the SEM of three to five mice/group.
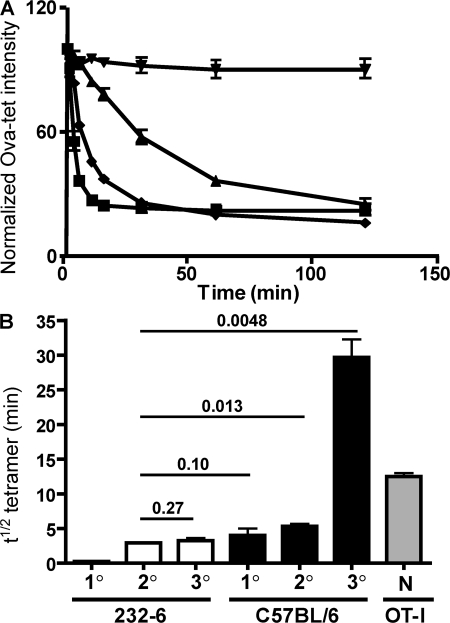
T cell avidity maturation is limited in 232-6 mice
The level of tetramer staining of primary OVA-specific CD8 T cells in 232-6 mice raised by VSV-Q4/L4 infection suggested that these cells were of lower avidity compared with control CD8 T cells. However, the level of tetramer staining for secondary memory cells in 232-6 mice appeared similar to that of OVA-specific cells from WT mice (Fig. 2 C), which is in agreement with a previous study showing that avidity maturation of the effector CD8 T cell population occurs after secondary infection (30). Nonetheless, tetramer staining by itself is an inaccurate measure of TCR avidity (31). To more precisely compare the avidity maturation of self-specific CD8 T cells in 232-6 and WT mice, we used a tetramer binding/decay assay that allows comparisons of apparent dissociation rates between populations by measuring the time required to reach 50% of maximal tetramer binding (T1/2) (31). Primary, secondary, and tertiary OVA-specific memory CD8 T cells from WT and 232-6 mice were analyzed. OVA-specific T cells from the primary response in C57BL/6 mice exhibited a T1/2 of ∼3.5 min (Fig. 5). This value increased to ∼5 min for secondary memory cells and further increased to a remarkable 30 min after tertiary immunization. By comparison, naive OT-I TCR Tg cells that are used extensively in autoimmunity models and cause intestinal disease in 232-6 mice (9), displayed a T1/2 of 13 min, a value intermediate between secondary and tertiary endogenous memory cells.
Figure 5.
OVA cross-reactive T cells undergo limited avidity maturation in vivo. (A) Tetramer decay on OVA-reactive T cells from tertiary immunization of 232-6 mice immunized initially with VSV-Q4 (▪), C57BL/6 mice immunized with VSV-N4 (♦), and naive OT-I T cells (▴). Also included are 232-6–derived T cells in the absence of Y3 Fab fragments (▾). (B) Avidity maturation of OVA-reactive T cells in 232-6 and C57BL/6 mice after multiple immunizations. Tetramer decay analysis was evaluated 7 d after primary immunization. 30 d after secondary recall with VV-OVA and 30 d after tertiary immunization with LM-OVA. Naive OT-I (N) were also tested by decay analysis. Error bars indicate the SEM of values from three animals/group. P values are shown for the indicated comparisons.
For primary OVA-reactive cells from 232-6 mice, a T1/2 of <1 min was determined. Nevertheless, avidity maturation did occur after secondary infection of 232-6 mice, with secondary memory cells exhibiting a T1/2 of ∼3 min, a value not statistically significantly different than that obtained for primary WT cells. However, in stark contrast to events in WT mice, tertiary immunization did not result in an increase of avidity in 232-6 mice, with the T1/2 remaining at ∼3 min, despite a substantial increase in total antigen-specific CD8 T cells (Fig. 5 B and not depicted). Collectively, these data suggested that self-antigen limits avidity maturation of the memory population by elimination or negative regulation of high-avidity clones in the naive repertoire.
Self-specific memory CD8 T cells retain the ability to recognize self-antigen
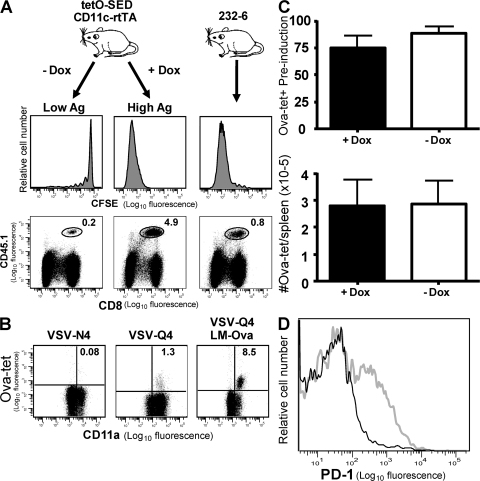
The previous experiments suggested that CD8 T cell tolerance in 232-6 mice was maintained by reduced TCR avidity, which limited avidity maturation of responding cells. Nevertheless, such cells mounted a robust response to antigen presented in the context of infection. Thus, it remained unclear why the OVA-specific cells in 232-6 mice did not exhibit apparent signs of antigen experience. Either these cells were refractory to self-antigen presentation or antigen levels were insufficient to drive activation. This may be of particular importance in situations where antigen levels increase over time, such as with developmental proteins or tumor-associated antigens. To evaluate this possibility, we developed a transgenic mouse line in which SIINFEKL expression could be regulated in CD11c+ cells upon treatment with doxycycline (CD11c-rtTA/tetO-pMinCMV-SED [SED-rtTA]). To compare antigen levels in SED-rtTA mice to the levels in 232-6 mice, we performed transfers with CFSE-labeled OT-I cells. In the absence of doxycycline treatment of SED-rtTA mice, adoptively transferred CFSE-labeled naive OT-I T cells underwent minimal division in the mesenteric lymph nodes after 5 d in vivo (Fig. 6 A). In contrast, treatment with doxycycline for 5 d before OT-I transfer resulted in robust expansion of OT-I cells to ∼5% of the total lymphocytes, confirming the inducibility of the system. Expansion of OT-I cells in 232-6 mice was substantially less than that observed in induced SED-rtTA mice, with OT-I cells comprising <1% of the population (Fig. 6 A). This result indicated that 232-6 mice expressed lower levels of antigen than doxycycline-treated SED-rtTA mice, which allowed us to test the effect of increased antigen expression by all CD11c+ cells.
Figure 6.
OVA cross-reactive memory T cells up-regulate PD-1 in response to self-antigen expression by dendritic cells. (A) Comparison of SED-rtTA and 232-6 transgenic mice. SED-rtTA mice were treated with or without 10 ng/ml of doxycycline for 7 d. Both groups of SED-rtTA and 232-6 mice were transferred with 5 × 105 CFSE-labeled OT-I T cells and evaluated for proliferation in the mesenteric lymph nodes 5 d later. CFSE dilution of transferred OT-I is indicated in the histograms (top) and the percentage of OT-I T cells of total lymphocytes is shown below. (B) SED-rtTA mice were immunized with VSV-N4 (left) or VSV-Q4 (middle) and evaluated for the presence of OVA-reactive CD8 T cells in the peripheral blood 7 d later. Mice immunized with VSV-Q4 and infected with LM-OVA 35 d later were evaluated for OVA-reactive cells 5 d later (right). (C) CD11c-inducible transgenic mice were immunized with VSV-Q4, and 30 d later, they were recalled with 103 LM-OVA. 30 d after recall, mice were bled and before and 5 d after treatment with or without 10 ng/ml of doxycycline. (top) Levels of OVA-specific T cells were normalized to preinduction levels. (bottom) Total numbers of OVA-specific T cells were enumerated in the spleen 5 d after induction. (D) PD-1 staining on CD8+, OVA-tetramer+ lymphocytes isolated from the spleen of VSV-Q4 and LM-OVA boosted SED-rtTA mice. Black histogram indicates PD-1 level in untreated and gray histogram indicates the level in doxycycline treated mice. Data are representative of three animals/group.
Even in the absence of doxycycline induction, SED-rtTA mice do not mount an endogenous OVA-specific CD8 T cell response, indicating that “leakage” in the system allows tolerance induction similar to that observed in 232-6 mice. Just as with 232-6 mice, however, infection with VSV-Q4 reveals a population of endogenous OVA-specific CD8 T cells that are able to respond to LM-OVA challenge (Fig. 6 B, middle). We then tested whether such cells could respond to the increased antigen levels present in doxycycline-induced SED-rtTA mice. 30 d after secondary infection, LM-OVA SED-rtTA mice were fed doxycycline for 7 d, and the OVA-reactive cells were then quantified in the peripheral blood. No change in the overall frequencies of OVA-reactive cells in the blood or in the total numbers of OVA-specific cells in the spleen was observed in doxycycline-fed mice compared with untreated mice (Fig. 6 C). These data indicated that elevated self-antigen expression did not result in overt expansion or deletion of the OVA-specific population. However, ∼40% of OVA-reactive T cells in doxycycline-treated, but not in untreated, mice expressed increased levels of the negative regulator PD-1, indicating that antigen-recognition had occurred (Fig. 6 D). Thus, with increased antigen levels, low-avidity self-reactive memory CD8 T cells were able to sense antigen, but, at least in the short-term, they were not deleted; instead, they were perhaps negatively regulated by PD-1–PDL1 interactions.
DISCUSSION
Although the T cell repertoire is believed to be largely purged of autoreactive cells via central tolerance during thymic selection, this mechanism is not fully effective, and cells expressing TCR specific for self-antigens are present in the periphery. However, the peripheral endogenous self-reactive repertoire has not been analyzed in detail, largely because of the paucity of such cells. Thus, TCR transgenic T cells with clonal single-avidity TCR have been used in many cases to study T cell selection and peripheral tolerance. These systems have revealed much regarding the mechanisms governing T cell tolerance induction; nevertheless, the results need to be considered in light of analyses of endogenous T cells. In our system, in which high-avidity CD8 T cell clones are absent or nonresponsive, as indicated by the lack of a response to virally expressed cognate antigen, infection with virus-encoded peptide variants revealed a population of self-specific, low-avidity CD8 T cells. In comparison, primarily low-avidity CD8 T cells specific for influenza virus hemagglutinin or nucleoprotein, are present in mice expressing hemagglutinin in the pancreas (Ins hemagglutinin mice) (14, 32) or nucleoprotein ubiquitously (33), respectively, suggesting that the escape of such clones from deletion or negative regulation is a hallmark of tolerance induction. However, in some cases high avidity self-reactive clones may also be retained, and, under certain circumstances, may be responsible for autoimmunity, but they can also be exploited for tumor immunotherapy (34, 35).
Our results also addressed the issue of CD8 T cell avidity maturation in the presence or absence of a constitutively expressed “neo-self” antigen. Whereas previous reports measured the sensitivity of TCR triggering by varying peptide concentrations to assess avidities, we used tetramer binding decay with anti-MHC blocking using Fab fragments to determine the half-life of peptide–MHC–TCR complexes (31). This technique has the advantage of providing a more specific measurement of peptide–MHC–TCR interactions without measuring potential differences in co-stimulation or restructuring of signaling components that might influence functional assays (36). Of considerable interest was the finding that although a significant increase in avidity occurred between primary and secondary responses (as previously suggested [30]), a much greater avidity increase was detected after tertiary immunization. Whether this is a general feature of tertiary immunization or is related to epitope specificity or immunization regimen will require further studies, but nevertheless, it indicated the utility of additional boosting to enhance vaccination. In the case of 232-6 mice, whereas primary OVA-specific CD8 T cells were of very low avidity, after secondary immunization, the avidity of the OVA-specific CD8 T cell population increased to the level of CD8 T cells in the primary response of normal mice. Tertiary immunization, however, did not result in a further avidity increase, indicating that either the high-avidity clones had been deleted centrally and/or peripherally or were somehow suppressed. Teleologically, the ability of the CD8 T cell repertoire to “focus” toward increasingly higher avidities in response to repeated infections or vaccinations is, at least in part, one basis for more effective protection. However, one potential consequence of increased TCR avidity is an enhanced risk of cross-reactivity with self-antigens. In 232-6 mice, an apparent “avidity ceiling” existed for OVA-reactive TCR that was likely based on the level of antigen needed intra- or extrathymically to drive negative selection and thereby maintain tolerance.
The level of self-antigen, the avidity of autoreactive T cells, and the susceptibility of the target organ to autoimmune damage all play important roles in whether autoimmunity is manifested. Indeed, the low avidity of self-specific CD8 T cells does not preclude the ability of these cells from recognizing self-antigen in vivo and either clearing tumors or inducing autoimmunity. For example, a recent study demonstrated that crossing transgenic mice expressing a fixed β-chain TCR (derived from the OT-I TCR) with RIP-mOVA animals expressing membrane-bound OVA in the pancreas results in loss of high-avidity, OVA-specific CD8 T cells, but does not prevent diabetes after infection with LM-OVA (37). Although the overall avidity of these cells is considered low compared with fixed β-chain cells that have not developed in the presence of antigen, comparisons with endogenously derived T cells have not been performed. One might speculate that because of expression of the β-chain, these low-avidity T cells may recognize antigen with a higher avidity than endogenous T cells that have developed under tolerogenic conditions. In addition, the pancreas may be more sensitive to autoimmune damage compared with, for example, the intestine, which may be more resilient, thereby requiring a more substantial autoreactive insult to exhibit disease. Thus, although avidity is important in the maintenance of tolerance, other mechanisms must also be involved.
Of what functional significance are low-avidity T cells? In our system, low-avidity, self-specific memory cells provided protection against infection, despite exhibiting undetectable autoreactivity. Additionally, in the aforementioned transgenic systems, low-avidity CD8 T cells are able to reject tumors (32, 38). Thus, low-avidity T cells may have protective capabilities, but retain a reduced capacity for autoreactivity. This scenario may be the result, at least in part, of regulation of reactivity by antigen dosage. In our system, self-specific memory cells showed no obvious signs of antigen recognition unless confronted with a pathogen expressing their cognate antigen or with DCs expressing high levels of antigen. In the former case, expansion occurred, whereas in the latter, up-regulation of programmed death-1 in the absence of expansion or deletion of the population was the outcome, at least in the relatively short time frame studied (7 d). It should be noted, however, that high-avidity naive cells responded with robust proliferation within this time period. These results suggested that inflammation, and likely increased antigen levels, were driving activation after infection, whereas in the absence of inflammation, PD-1 was up-regulated, thereby limiting expansion. The latter remains to be tested, but is in agreement with recent studies regarding negative regulation of T cell expansion (39). The ability of low avidity T cells to respond to antigen may play an important role in circumstances in which antigen levels increase in vivo, such as tumors. Indeed, this may impact the strategy and design of cancer vaccines. Overall, the findings point to a scenario in which high-avidity pathogenic autoreactive CD8 T cells are kept at bay, through either elimination or regulation, whereas the system retains low-avidity, self-specific cells that are able to contribute to immunity.
MATERIALS AND METHODS
Mice.
C57BL/6J mice were purchased from The Jackson Laboratory and were age and sex matched for each experiment. 232-6 transgenic mice expressing aa 138–338 of chicken OVA under the control of the intestinal fatty acid binding protein promoter were generated as previously described (9, 40). Transgenic mice with inducible SIINFEKL expression were generated by cloning in tandem the coding sequences for SIINFEKL (S), I-Ea 52–68 (E), and DSRed-2 (D) (Clontech Laboratories) into pBluescript SK containing the tetO-pMinCMV promoter, a gift from R. Zamoyska (University of Edinburgh, Edinburgh, Scotland) (41). Inducible regulation was controlled by cloning the reverse tetracycline controlled transactivator (rtTA-M2), generously provided by W. Hillen (Univsersitat Erlangen, Erlangen, Germany) downstream of the CD11c promoter (42). Each transgene was confirmed by sequence analysis, and founder animals were generated by microinjection into fertilized embryos at the University of Connecticut Health Center transgenic facility. Founder animals were intercrossed to obtain CDllc-rtTA/tetO-pMinCMV-SED transgenic mice (indicated as SED). Inducible antigen expression was achieved by treating CD11c-rtTA/tetO-SED mice with 10 ng/ml of doxycycline in the drinking water, along with 5% sucrose. All animals were housed and bred under pathogen-free conditions, and all studies were performed in compliance with the University of Connecticut Health Center Animal Care Committee.
Recombinant viruses.
rVSVs expressing WT and single amino acid variants of the chicken OVA H-2Kb-restricted epitope SIINFEKL were generated by cloning in-frame the genes for enhanced green fluorescent protein and a truncated region of OVA spanning aa 138–338 using polymerase chain reaction. The resulting fusion construct was then subcloned into the VSV-XN2 expression vector, provided by J. Rose (Yale University, New Haven, CT), and variants of OVA257-263 were generated using site-directed mutagenesis as described by the manufacturer (Invitrogen). Specifically, the fourth position of the SIINFEKL epitope was mutated from an aspargine (N4) to either an aspartic acid (Q4) or a leucine (L4). Constructs were confirmed by sequence analysis, and virus was produced as previously described (40).
Cell isolation and flow cytometric analysis.
Cells were isolated from the spleen, peripheral lymph nodes, mesenteric lymph nodes, intestinal epithelium, and lamina propria as previously described (43, 44). Staining was performed by resuspending cells at 106–107 cells/ml in PBS containing 0.2% BSA, 0.01% NaN3 (FACS Buffer). OVA and N-specific T cells were identified by staining with APC-labeled H-2Kb tetramers containing the SIINFEKL epitope (OVA-tet) or VSV-nucleoprotein peptide (N-tet) for 1 h at room temperature in the presence of anti-CD8 PerCP (clone 53.6.7), followed by additional staining with antibodies specific for CD11a, CD44, CD62L, IL-7R, or PD-1 at 4°C for an additional 30 min (all antibodies were purchased from BD Biosciences or eBioscience). Cells were washed and fixed with 3% paraformaldehyde/PBS, fluorescence intensities were measured with a FACSCalibur (BD Biosciences), and data were analyzed using FlowJo software (Tree Star, Inc.).
Tetramer decay analysis.
Avidity of T cell populations was evaluated by decay of tetramer binding, as previously described (31). Splenocytes from immunized animals were stained with OVA-tetramer for 1 h at room temperature in the presence of anti-CD8 mAb, followed by anti-CD11a mAb for an additional 30 min at 4°C. After extensive washing, anti-H-2Kb (Y-3) Fab fragments were added to a final concentration of 10 mM and aliquots of cells were removed at various time points and immediately fixed in 2% paraformaldehyde/PBS. The half-life of tetramer binding was determined by normalizing the mean fluorescence intensity of each time point to the zero time point control. The time taken to reach one half of the fluorescence intensity of the control (zero time point) was determined to be the relative avidity of each population.
Protection assay.
232-6 and C57BL/6 mice were immunized with 106 PFU rVSV encoding the tOVA-GFP fusion construct or Q4 and L4 variants i.v. 30 d later, animals were rechallenged with 5 × 106 PFU recombinant VV encoding chicken OVA (VV-OVA), which was provided by J. Yewdell (National Institutes of Health, Bethesda, MD). 30 d later, immunized and unimmunized animals were challenged with 2 × 105 CFU LM expressing chicken OVA (LM-OVA), as previously described (45). 3 d later, the spleen and liver were evaluated for the presence of LM by dissociation of tissues through a 40-μm filter with 1% saponin in PBS. Dilutions of each tissue were inoculated onto brain–heart infusion agar plates containing 1 μg/ml streptomycin, and the number of colonies was enumerated 48 h later.
In vivo killing assay.
In vivo killing was performed as previously described, with slight modifications (46). Splenocytes from CD45.1 B6 mice were stained with 2 μm, 0.2 μm, or 0.02 μm of CFSE, followed by incubation with 1 μg/ml of VSV N peptide (RGYVYQGL), SIINFEKL (N4) peptide, or PBS, respectively, for 30 min at 37°C. N4 is the WT SIINFEKL sequence. Equal numbers of each target population were mixed and injected i.v. into CD45.2 B6 mice that were naive or had been infected as indicated in the figure legend. 4 h later, recipient mice were evaluated for the presence of CFSE-labeled target cells in the spleen. Percent specific lysis was determined by the following equation: 100 − ([Immunized percentage of N4-pulsed target/Immunized percentage unpulsed target] / [Unimmunized percentage N4 pulsed target/Unimmunized unpulsed target] × 100).
Acknowledgments
The authors wish to thank Dr. Kimberly Schluns (MD Anderson Cancer Center, Houston, TX) for initial efforts in construction of the inducible transgenic mice and Quynh Mai-Pham and Kristina Gumpenberger for expert technical assistance.
This work was supported by National Institutes of Health grants DK57932 (L. Lefrancois), HL80508 (L. Puddington), and F32 AI062006 (M. Turner).
The authors have no conflicting financial interests.
Abbreviations used: AIRE, autoimmune regulator; LM, Listeria monocytogenes; VSV, vesicular stomatitis virus; VV, vaccinia virus.
References
- 1.Nemazee, D. 2000. Receptor selection in B and T lymphocytes. Annu. Rev. Immunol. 18:19–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Palmer, E. 2003. Negative selection–clearing out the bad apples from the T-cell repertoire. Nat. Rev. Immunol. 3:383–391. [DOI] [PubMed] [Google Scholar]
- 3.Anderson, M.S., E.S. Venanzi, L. Klein, Z. Chen, S.P. Berzins, S.J. Turley, H. von Boehmer, R. Bronson, A. Dierich, C. Benoist, and D. Mathis. 2002. Projection of an immunological self shadow within the thymus by the aire protein. Science. 298:1395–1401. [DOI] [PubMed] [Google Scholar]
- 4.Kurts, C., H. Kosaka, F.R. Carbone, J.F.A.P. Miller, and W.R. Heath. 1997. Class I-restricted cross-presentation of exogenous self-antigens leads to deletion of autoreactive CD8 T cells. J. Exp. Med. 186:239–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Walker, L.S., and A.K. Abbas. 2002. The enemy within: keeping self-reactive T cells at bay in the periphery. Nat. Rev. Immunol. 2:11–19. [DOI] [PubMed] [Google Scholar]
- 6.Probst, H.C., J. Lagnel, G. Kollias, and M. van den Broek. 2003. Inducible transgenic mice reveal resting dendritic cells as potent inducers of CD8(+) T cell tolerance. Immunity. 18:713–720. [DOI] [PubMed] [Google Scholar]
- 7.Redmond, W.L., and L.A. Sherman. 2005. Peripheral tolerance of CD8 T lymphocytes. Immunity. 22:275–284. [DOI] [PubMed] [Google Scholar]
- 8.Liston, A., and A.Y. Rudensky. 2007. Thymic development and peripheral homeostasis of regulatory T cells. Curr. Opin. Immunol. 19:176–185. [DOI] [PubMed] [Google Scholar]
- 9.Vezys, V., S. Olson, and L. Lefrançois. 2000. Expression of intestine-specific antigen reveals novel pathways of CD8 T cell tolerance induction. Immunity. 12:505–514. [DOI] [PubMed] [Google Scholar]
- 10.Adler, A.J., D.W. Marsh, G.S. Yochum, J.L. Guzzo, A. Nigam, W.G. Nelson, and D.M. Pardoll. 1998. CD4+ T cell tolerance to parenchymal self-antigens requires presentation by bone marrow–derived antigen-presenting cells. J. Exp. Med. 187:1555–1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kurts, C., W.R. Heath, F.R. Carbone, J. Allison, J.F.A.P. Miller, and H. Kosaka. 1996. Constitutive class I-restricted exogenous presentation of self antigens in vivo. J. Exp. Med. 184:923–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.DeVoss, J., Y. Hou, K. Johannes, W. Lu, G.I. Liou, J. Rinn, H. Chang, R.R. Caspi, L. Fong, and M.S. Anderson. 2006. Spontaneous autoimmunity prevented by thymic expression of a single self-antigen. J. Exp. Med. 203:2727–2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee, J.W., M. Epardaud, J. Sun, J.E. Becker, A.C. Cheng, A.R. Yonekura, J.K. Heath, and S.J. Turley. 2007. Peripheral antigen display by lymph node stroma promotes T cell tolerance to intestinal self. Nat. Immunol. 8:181–190. [DOI] [PubMed] [Google Scholar]
- 14.Nugent, C.T., D.J. Morgan, J.A. Biggs, A. Ko, I.M. Pilip, E.G. Pamer, and L.A. Sherman. 2000. Characterization of CD8+ T lymphocytes that persist after peripheral tolerance to a self antigen expressed in the pancreas. J. Immunol. 164:191–200. [DOI] [PubMed] [Google Scholar]
- 15.Theobald, M., J. Biggs, J. Hernandez, J. Lustgarten, C. Labadie, and L.A. Sherman. 1997. Tolerance to p53 by A2.1-restricted cytotoxic T lymphocytes. J. Exp. Med. 185:833–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huber, S.A., and M.W. Cunningham. 1996. Streptococcal M protein peptide with similarity to myosin induces CD4+ T cell-dependent myocarditis in MRL/++ mice and induces partial tolerance against coxsakieviral myocarditis. J. Immunol. 156:3528–3534. [PubMed] [Google Scholar]
- 17.Gross, D.M., T. Forsthuber, M. Tary-Lehmann, C. Etling, K. Ito, Z.A. Nagy, J.A. Field, A.C. Steere, and B.T. Huber. 1998. Identification of LFA-1 as a candidate autoantigen in treatment-resistant Lyme arthritis. Science. 281:703–706. [DOI] [PubMed] [Google Scholar]
- 18.Croxford, J.L., H.A. Anger, and S.D. Miller. 2005. Viral delivery of an epitope from Haemophilus influenzae induces central nervous system autoimmune disease by molecular mimicry. J. Immunol. 174:907–917. [DOI] [PubMed] [Google Scholar]
- 19.Kessels, H.W., K. E.de Visser, F.H. Tirion, M. Coccoris, A.M. Kruisbeek, and T.N. Schumacher. 2004. The impact of self-tolerance on the polyclonal CD8+ T cell repertoire. J. Immunol. 172:2324–2331. [DOI] [PubMed] [Google Scholar]
- 20.Overwijk, W.W., A. Tsung, K.R. Irvine, M.R. Parkhurst, T.J. Goletz, K. Tsung, M.W. Carroll, C. Liu, B. Moss, S.A. Rosenberg, and N.P. Restifo. 1998. gp100/pmel 17 is a murine tumor rejection antigen: induction of “self”-reactive, tumoricidal T cells using high-affinity, altered peptide ligand. J. Exp. Med. 188:277–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fong, L., D. Brockstedt, C. Benike, J.K. Breen, G. Strang, C.L. Ruegg, and E.G. Engleman. 2001. Dendritic cell-based xenoantigen vaccination for prostate cancer immunotherapy. J. Immunol. 167:7150–7156. [DOI] [PubMed] [Google Scholar]
- 22.Slansky, J.E., F.M. Rattis, L.F. Boyd, T. Fahmy, E.M. Jaffee, J.P. Schneck, D.H. Margulies, and D.M. Pardoll. 2000. Enhanced antigen-specific antitumor immunity with altered peptide ligands that stabilize the MHC-peptide-TCR complex. Immunity. 13:529–538. [DOI] [PubMed] [Google Scholar]
- 23.Fong, L., Y. Hou, A. Rivas, C. Benike, A. Yuen, G.A. Fisher, M.M. Davis, and E.G. Engleman. 2001. Altered peptide ligand vaccination with Flt3 ligand expanded dendritic cells for tumor immunotherapy. Proc. Natl. Acad. Sci. USA. 98:8809–8814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barnden, M.J., W.R. Heath, S. Rodda, and F.R. Carbone. 1994. Peptide antagonists that promote positive selection are inefficient at T cell activation and thymocyte deletion. Eur. J. Immunol. 24:2452–2456. [DOI] [PubMed] [Google Scholar]
- 25.Veiga-Fernandes, H., U. Walter, C. Bourgeois, A. McLean, and B. Rocha. 2000. Response of naive and memory CD8+ T cells to antigen stimulation in vivo. Nat. Immunol. 1:47–53. [DOI] [PubMed] [Google Scholar]
- 26.Veiga-Fernandes, H., and B. Rocha. 2004. High expression of active CDK6 in the cytoplasm of CD8 memory cells favors rapid division. Nat. Immunol. 5:31–37. [DOI] [PubMed] [Google Scholar]
- 27.Barber, D.L., E.J. Wherry, D. Masopust, B. Zhu, J.P. Allison, A.H. Sharpe, G.J. Freeman, and R. Ahmed. 2006. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 439:682–687. [DOI] [PubMed] [Google Scholar]
- 28.Sallusto, F., D. Lenig, R. Forster, M. Lipp, and A. Lanzavecchia. 1999. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 401:708–712. [DOI] [PubMed] [Google Scholar]
- 29.Masopust, D., V. Vezys, A.L. Marzo, and L. Lefrançois. 2001. Preferential localization of effector memory cells in nonlymphoid tissue. Science. 291:2413–2417. [DOI] [PubMed] [Google Scholar]
- 30.Busch, D.H., I. Pilip, and E.G. Pamer. 1998. Evolution of a complex T cell receptor repertoire during primary and recall bacterial infection. J. Exp. Med. 188:61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang, X.L., and J.D. Altman. 2003. Caveats in the design of MHC class I tetramer/antigen-specific T lymphocytes dissociation assays. J. Immunol. Methods. 280:25–35. [DOI] [PubMed] [Google Scholar]
- 32.Morgan, D.J., H.T. Kreuwel, S. Fleck, H.I. Levitsky, D.M. Pardoll, and L.A. Sherman. 1998. Activation of low avidity CTL specific for a self epitope results in tumor rejection but not autoimmunity. J. Immunol. 160:643–651. [PubMed] [Google Scholar]
- 33.de Visser, K.E., T.A. Cordaro, D. Kioussis, J.B. Haanen, T.N. Schumacher, and A.M. Kruisbeek. 2000. Tracing and characterization of the low-avidity self-specific T cell repertoire. Eur. J. Immunol. 30:1458–1468. [DOI] [PubMed] [Google Scholar]
- 34.Yee, C., P.A. Savage, P.P. Lee, M.M. Davis, and P.D. Greenberg. 1999. Isolation of high avidity melanoma-reactive CTL from heterogeneous populations using peptide-MHC tetramers. J. Immunol. 162:2227–2234. [PubMed] [Google Scholar]
- 35.McWilliams, J.A., S.M. McGurran, S.W. Dow, J.E. Slansky, and R.M. Kedl. 2006. A modified tyrosinase-related protein 2 epitope generates high-affinity tumor-specific T cells but does not mediate therapeutic efficacy in an intradermal tumor model. J. Immunol. 177:155–161. [DOI] [PubMed] [Google Scholar]
- 36.Slifka, M.K., and J.L. Whitton. 2001. Functional avidity maturation of CD8(+) T cells without selection of higher affinity TCR. Nat. Immunol. 2:711–717. [DOI] [PubMed] [Google Scholar]
- 37.Zehn, D., and M.J. Bevan. 2006. T cells with low avidity for a tissue-restricted antigen routinely evade central and peripheral tolerance and cause autoimmunity. Immunity. 25:261–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cordaro, T.A., K. E.de Visser, F.H. Tirion, T.N. Schumacher, and A.M. Kruisbeek. 2002. Can the low-avidity self-specific T cell repertoire be exploited for tumor rejection? J. Immunol. 168:651–660. [DOI] [PubMed] [Google Scholar]
- 39.Sharpe, A.H., E.J. Wherry, R. Ahmed, and G.J. Freeman. 2007. The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. Nat. Immunol. 8:239–245. [DOI] [PubMed] [Google Scholar]
- 40.Lawson, N.D., E.A. Stillman, M.A. Whitt, and J.K. Rose. 1995. Recombinant vesicular stomatitis viruses from DNA. Proc. Natl. Acad. Sci. USA. 92:4477–4481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Legname, G., B. Seddon, M. Lovatt, P. Tomlinson, N. Sarner, M. Tolaini, K. Williams, T. Norton, D. Kioussis, and R. Zamoyska. 2000. Inducible expression of a p56Lck transgene reveals a central role for Lck in the differentiation of CD4 SP thymocytes. Immunity. 12:537–546. [DOI] [PubMed] [Google Scholar]
- 42.Urlinger, S., U. Baron, M. Thellmann, M.T. Hasan, H. Bujard, and W. Hillen. 2000. Exploring the sequence space for tetracycline-dependent transcriptional activators: novel mutations yield expanded range and sensitivity. Proc. Natl. Acad. Sci. USA. 97:7963–7968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Goodman, T., and L. Lefrançois. 1988. Expression of the γ-δ T-cell receptor on intestinal CD8+ intraepithelial lymphocytes. Nature. 333:855–858. [DOI] [PubMed] [Google Scholar]
- 44.Laky, K., L. Lefrançois, and L. Puddington. 1997. Age-dependent intestinal lymphoproliferative disorder due to stem cell factor receptor deficiency: parameters in small and large intestine. J. Immunol. 158:1417–1427. [PubMed] [Google Scholar]
- 45.Lefrancois, L., A. Marzo, and K. Williams. 2003. Sustained response initiation is required for T cell clonal expansion but not for effector or memory development in vivo. J. Immunol. 171:2832–2839. [DOI] [PubMed] [Google Scholar]
- 46.Stoklasek, T.A., K.S. Schluns, and L. Lefrancois. 2006. Combined IL-15/IL-15Rα immunotherapy maximizes IL-15 activity in vivo. J. Immunol. 177:6072–6080. [DOI] [PMC free article] [PubMed] [Google Scholar]