Abstract
Src homology 2 domain–containing leukocyte phosphoprotein of 76 kD (SLP76), an adaptor that plays a critical role in platelet activation in vitro, contains three N-terminal tyrosine residues that are essential for its function. We demonstrate that mice containing complementary tyrosine to phenylalanine mutations in Y145 (Y145F) and Y112 and Y128 (Y112/128F) differentially regulate integrin and collagen receptor signaling. We show that mutation of Y145 leads to severe impairment of glycoprotein VI (GPVI)–mediated responses while preserving outside-in integrin signaling. Platelets from Y112/128F mice, although having mild defects in GPVI signaling, exhibit defective actin reorganization after GPVI or αIIbβ3 engagement. The in vivo consequences of these signaling defects correlate with the mild protection from thrombosis seen in Y112/128F mice and the near complete protection observed in Y145F mice. Using genetic complementation, we further demonstrate that all three phosphorylatable tyrosines are required within the same SLP76 molecule to support platelet activation by GPVI.
Platelet activation is critical for the termination of bleeding after injury. In diseased vessels, platelets contribute to thrombus formation, leading to vessel occlusion and tissue infarction. Two receptors promote platelet activation through distinct but complementary roles: glycoprotein VI (GPVI), which together with integrin α2β1 binds collagen, and integrin αIIbβ3, which recognizes several ligands, including fibrinogen and von Willebrand factor (1).
GPVI associates with the immunoreceptor tyrosine-based activation motif (ITAM)–containing FcRγ, which undergoes phosphorylation by Src family protein tyrosine kinases (PTKs) after GPVI cross-linking. Once phosphorylated, the FcRγ ITAM binds and activates Syk, initiating a signaling cascade that results in platelet spreading, secretion of soluble mediators, and aggregation (2). For platelet aggregate stabilization under the shear flow conditions of the blood vessels, activation of αIIbβ3 is required. In resting platelets, αIIbβ3 is present in a low-affinity state and is unable to bind soluble ligands. Agonist-initiated intracellular signals result in increased affinity of αIIbβ3, a process known as inside-out signaling. Ligand binding to activated αIIbβ3 then generates a second wave of PTK-initiated signals, known as outside-in signaling, that promotes firm platelet adhesion and spreading. The adaptor protein Src homology 2 (SH2) domain–containing leukocyte phosphoprotein of 76 kD (SLP76) is central to the signaling pathways induced by engagement of GPVI and αIIbβ3.
SLP76 was originally described as a regulator of TCR signaling (3, 4), and it functions as a scaffold to nucleate a multimolecular complex after TCR engagement (5). SLP76 was subsequently shown to be important for signaling in other hematopoietic lineages, including platelets. Platelets from SLP76−/− animals do not couple GPVI engagement with activation of the downstream signaling cascade, resulting in failed degranulation or aggregation in response to collagen (6, 7). SLP76−/− platelets show a marked reduction in spreading when adhered to a fibrinogen-coated surface, indicating that SLP76 is critical for signaling downstream of αIIbβ3 (8). These in vitro defects suggested that SLP76 was important for platelet function in vivo. However, it had not been feasible to test this question given the vascular development abnormalities of SLP76−/− mice that lead to significant hemodynamic compromise (9).
SLP76 is composed of four modular domains: a C-terminal SH2 domain, a proline-rich region, a sterile α motif domain, and an N-terminal acidic domain that contains three tyrosine phosphorylation motifs (Y112ESP, Y128ESP, and Y145EPP). Phosphorylated Y112 and Y128 are recognized by SH2 domain–containing proteins, including Vav (10–14) and noncatalytic region of tyrosine kinase (15, 16). Y145 is recognized by the Tec family kinases IL-2–inducible T cell kinase (Itk) and Bruton's tyrosine kinase (Btk) (17, 18). The critical role of these N-terminal tyrosines for SLP76 function was initially shown in T cells, where SLP76 with a mutation in all three tyrosines (Y3F) was unable to rescue TCR signaling in SLP76−/− Jurkat cells and failed to rescue T cell development in SLP76−/− mice (19–21). Retroviral transduction of SLP76-deficient bone marrow with the Y3F mutant followed by transplant into irradiated recipients resulted in the development of platelets with defects in GPVI and αIIbβ3 signaling (22). The precise mechanism by which these N-terminal tyrosines contribute to SLP76 function during platelet activation is not well understood.
In this paper, we explore the role of these tyrosines in platelet function in vitro and in vivo. Our data indicate that SLP76 Y145 is essential for normal thrombus formation in a model of arterial injury and that mutation of either Y145 or both Y112 and Y128 results in significant alterations in signaling downstream of GPVI and αIIbβ3. Generation of knock-in (KI) mice with mutations in SLP76 tyrosines allowed us to ask whether these phosphorylation sites act independently or in combination during SLP76 function. Using a genetic complementation approach, we find that all three phosphorylatable tyrosines are required within the same SLP76 molecule for platelet activation.
RESULTS
Mice with mutant SLP76 tyrosines are resistant to thrombus formation
Although we and others have documented severe signaling defects in platelets from SLP76-deficient mice (6, 23), it has been impossible to assess the consequences of SLP76 deficiency on platelet function in vivo, because mice lacking this adaptor protein manifest a severe vascular abnormality associated with cardiac hypertrophy, altered hemodynamics, and abnormal connections between blood and lymphatic vessels (9). This vascular defect is also present in mice in which an SLP76 Y3F mutant is expressed (22). Recently, we found that mice in which two of these tyrosines (Y112 and Y128) or an individual tyrosine (Y145) have been altered to phenylalanine are born at Mendelian ratios, have no overt vascular phenotype, and have normal platelet counts (24). Therefore, we used these animals to probe the importance of SLP76 and, in particular, its N-terminal tyrosines for in vivo platelet function.
WT or KI mice expressing SLP76 containing either combined mutations in Y112 and Y128 (Y112/128F mice) or a single mutation in Y145 (Y145F mice) were studied in a model of arterial thrombosis (Fig. 1). For these experiments, the carotid artery was exposed for thrombus induction by topical application of 10% FeCl3, a protocol that leads to local denudation of the vessel endothelium and exposure of collagen fibers (25). To monitor vessel occlusion, a Doppler flow probe was used to measure the rate of blood flow through the artery, with each experiment lasting 30 min after application of the irritant. A flow rate <0.1 ml/min for the duration of the study was scored as a stable occlusive thrombus. Animals in which blood flow stopped but then resumed were scored as having an unstable thrombus.
Figure 1.
Impaired thrombus formation in mice with mutations in SLP76 tyrosines. (A) Schematic of SLP76 KI mice. (B) Thrombus formation in Y112/128F (n = 12), Y145F (n = 13), WT littermate control (n = 16), or C57BL/6 (n = 8) mice after FeCl3-induced injury of the carotid artery. Representative flow traces for each genotype are shown, and arrows indicate when 10% FeCl3 was applied. The time to vessel occlusion in individual mice for each group is shown in the scattergram. Symbols above the 30-min threshold represent mice that had not occluded when the experiment was terminated. Horizontal bars show the mean occlusion time for WT animals. Unstable mice (U) formed a transient occlusion that resolved. Ratios of the number of animals that did not form occlusion to the number of animals that did were compared between WT and SLP76 KI mice using Fisher's exact test. (C) Platelets in whole blood were perfused over collagen-coated slides at variable shear rates for 4 min (n = 3). Representative phase-contrast images after perfusion are shown (top, shear rate = 400 s−1; bottom, shear rate = 1,300 s−1). Bar, 10 μm. (D and E) Thrombus volume (D) and thrombi surface area (E) were measured at a shear rate of 1,300 s−1 at five surface locations (mean ± SEM). Statistical analysis was performed using the Student's t test. *, P < 0.05; and **, P < 0.01 for differences between mutant and WT platelets.
In 100% of WT mice of either a pure C57BL/6 or a mixed C57BL6/129Sv background (littermate controls for KI animals), stable occlusive thrombi formed at 7.1 ± 0.6 or 7.5 ± 1.1 min after injury, respectively (Fig. 1 B). In contrast, 69% (9 out of 13) of Y145F mice never formed a thrombus after injury. Among the four mice that did occlude, the time to initial occlusion was delayed (14.9 ± 2.4 min), and two of these animals formed unstable thrombi that embolized soon after formation (Fig. 1 B). Y112/128F mice showed a milder defect in thrombus formation, with 25% (3 out of 12) failing to occlude blood flow.
To visualize platelet aggregation, we modeled thrombus formation ex vivo by examining platelet dynamics under physiological flow conditions. Platelets in whole blood were perfused over collagen at variable shear rates from 400 to 1,300 s−1 for 4 min. Blood from WT mice exhibited robust accumulation of densely packed platelets on collagen (Fig. 1 C). Y145F platelets adhered less well to collagen (33.3 ± 2.3% surface coverage by Y145F platelets vs. 50.3 ± 1.9% surface coverage by WT platelets), and the overall thrombus volume was markedly reduced (0.3 × 105 ± 0.06 × 105 μm3 compared with 105 ± 0.06 × 105 μm3 as measured from WT samples; Fig. 1, D and E). Platelets from the Y112/128F mice efficiently covered the collagen surface (Fig. 1 E); however, the overall thrombus volume (0.8 × 105 ± 0.1 × 105 μm3) tended to be smaller than that seen with WT platelets. Collectively, these data suggest that only Y145 is necessary for firm adhesion to collagen; however, mutation of either Y145 or combined mutations of Y112 and Y128 impairs signaling events necessary for the formation of tight platelet aggregates under flow. These data make it clear that other regions of SLP76 are important for platelet adhesion and aggregate formation on collagen, as the analysis of platelets isolated from SLP76−/− mice revealed a much more dramatic phenotype with very few platelets adhering to collagen (0.09 ± 0.01% surface coverage at 1,300 s−1) and with a complete absence of detectable aggregates (0.01 ± 0.001 μm3).
SLP76 Y145 is critical for GPVI-induced aggregation
To investigate the mechanism underlying the defect in thrombus formation in SLP76 KI mice, we tested whether Y145F or Y112/128F platelets aggregate in solution in response to collagen. Similar to SLP76−/− platelets, Y145F or Y112/128F platelets showed a marked defect in aggregation in response to low-dose collagen (2.5 μg/ml; Fig. 2 A). Raising the collagen concentration increased aggregation in cells from both KI animals; however, platelets from Y145F mice required a much higher collagen concentration (10 vs. 3.3 μg/ml) to reconstitute a normal response (Fig. 2, A and C). In contrast, SLP76−/− platelets manifested a barely detectable response, even to the highest dose of collagen stimulation, indicating that the Y145 mutation or the combined Y112/Y128 mutation results in a reduction but not a complete loss of SLP76 function.
Figure 2.
Y145F mutation inhibits GPVI-mediated platelet aggregation. (A–D) Platelets were stimulated with various doses of collagen (A) or CVX (B), and aggregation was assessed for 5 min. Representative aggregation tracings are shown. Quantification of the maximum percentage of aggregation in SLP76 mutant versus WT platelets in response to collagen (C) or CVX (D) are shown. Each point is the mean ± SEM of three to seven experiments, and the difference between Y145F and WT is significant. *, P < 0.05; **, P < 0.01. (E) WT or KI platelets were pretreated with the anti-α2 antibody Ha1/29 (red) or DMSO (black), and aggregation was measured (n = 2).
Because two receptors, GPVI and α2β1, function together to activate platelets in response to collagen (26), we investigated whether the defect in collagen-induced aggregation in Y145F and Y112/128F platelets is caused by impaired signals from one or both of these receptors. We measured aggregation in platelets stimulated with the GPVI agonist convulxin (CVX). Before proceeding with the analysis, we confirmed that the cell-surface expression of GPVI is equivalent between SLP76 KI and WT platelets (unpublished data). Y112/128F platelets exhibited a mild reduction in CVX-induced aggregation, with the difference compared with WT cells greatest at the lowest (0.2 nM) dose of agonist (Fig. 2, B and D). Strikingly, aggregation of Y145F platelets was completely abolished, with only a minor shape change in response to the highest concentrations (1 nM) of CVX. SLP76−/− platelets exhibited minimal responses to high-dose CVX (as high as 5 nM was tested). To ensure that the abnormal responses were restricted to SLP76-dependent pathways, we stimulated platelets from all mice with two G protein–coupled receptor agonists (ADP or thrombin) and observed normal aggregation responses (Fig. S1, available at http://www.jem.org/cgi/content/full/jem.20080240/DC1; and not depicted).
The fact that the Y145F platelets responded, albeit less well, to collagen but not to CVX suggests that the function of the integrin α2β1 may be retained in the setting of this SLP76 mutation. To test α2β1 function in platelets from the SLP76 KI mice, we used a blocking mAb directed against the α2 component of the integrin. Although blocking α2β1 on WT platelets resulted in slightly delayed aggregation to collagen (26), this same treatment completely inhibited the collagen response of Y145F platelets, even at high collagen concentrations (Fig. 2 E). Y112/128F platelets showed an intermediate phenotype in which inhibition of α2β1 significantly reduced but did not completely abrogate the collagen response. These results indicate that aggregation of SLP76 KI platelets to higher doses of collagen relies on α2β1. Thus, the abnormal aggregation phenotype of the mutant platelets appears to be caused by decreased signaling via GPVI in Y112/128F platelets and a complete lack of this response in Y145F platelets.
α Granule secretion and αIIbβ3 activation are reduced in Y112/128F and severely defective in Y145F platelets
After GPVI stimulation, platelet aggregation is accompanied by secretion of α granule contents. To further characterize the signaling capacity of GPVI in SLP76 KI platelets, we studied induced surface expression of P-selectin in response to GPVI activation. Consistent with previous results (7), SLP76−/− platelets failed to up-regulate P-selectin expression after GPVI stimulation, whereas WT cells showed a dose-dependent response (Fig. 3 A). Y112/128F platelets demonstrated a moderate decrease (40–50% of control) in induced P-selectin expression with a range of CVX concentrations, whereas secretion in Y145F cells was reduced >80%. All platelets responded to AYPGKF, a protease apoptosis response 4 receptor agonist that activates platelets in an SLP76-independent manner.
Figure 3.
Secretion and fibrinogen-binding defects in SLP76 KI platelets in response to CVX. (A) P-selectin surface expression was analyzed by flow cytometry after stimulation with 0.2 nM or 1 nM CVX, or 1 mM AYPGKF. Representative histograms are shown. (B) Platelets were treated as described in A and were stained with 100 μg/ml of Alexa Fluor 647–labeled fibrinogen. EDTA was added to control for nonspecific binding. Mean percentages ± SEM of P-selectin–positive (A) or fibrinogen-bound (B) cells after the indicated stimulations (n = 4–6 mice per genotype) are shown. *, P < 0.01.
Increased fibrinogen binding to αIIbβ3 is also critical for platelet aggregation. GPVI stimulation generates a signal that induces a conformational change in αIIbβ3, increasing its affinity for fibrinogen. To investigate the contribution of SLP76 tyrosines to GPVI-induced αIIbβ3 activation, platelets from WT or SLP76 KI mice were stimulated with CVX in the presence of soluble fluorophore-conjugated fibrinogen. Resting platelets from all mice bound fibrinogen poorly (Fig. 3 B). Similar to what we observed for P-selectin up-regulation, the combined Y112/128F mutation resulted in a smaller increase in fibrinogen binding relative to WT cells (9.6 ± 0.5– vs. 14.8 ± 0.9–fold induction relative to the resting state), a response that was absent in SLP76−/− platelets. The Y145F mutation dramatically reduced the ability of GPVI signaling to couple to increased fibrinogen binding (5.6 ± 2.6–fold induction relative to the resting state). This defect is not caused by diminished levels of the integrin, because all genotypes showed similar level of β3 surface expression (unpublished data). Furthermore, all cells bound fibrinogen similarly after activation with ADP, an agonist that induces αIIbβ3 activation in an SLP76-independent manner.
SLP76 tyrosines are critical for GPVI-induced phosphorylation of Btk and phospholipase C γ2 (PLCγ2)
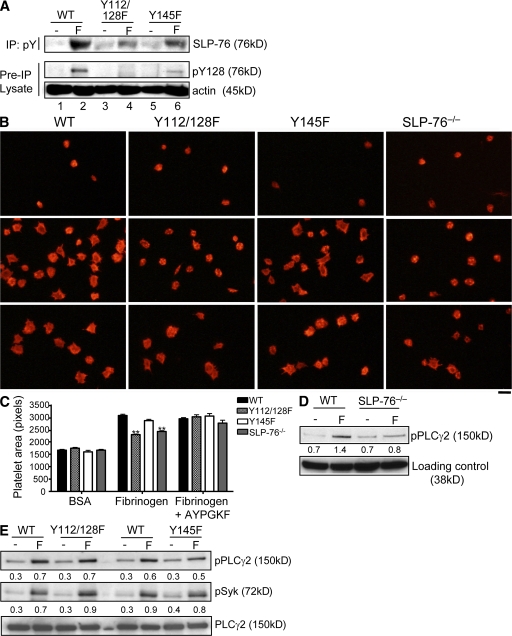
To investigate the molecular basis of the activation defect in SLP76 KI platelets, we measured tyrosine phosphorylation events occurring downstream of GPVI engagement. Stimulation of WT cells with CVX led to a dramatic increase in SLP76 phosphorylation using the panphosphotyrosine-specific antibody 4G10 (Fig. 4 A, lane 2). In SLP76 Y112/128F cells, phosphorylation of Y145 was greatly diminished (lane 4). Because residues 112, 128, and 145 are the only tyrosine phosphorylation sites detected by 4G10 antibody (27), these results indicate a dependency of intact Y112 and/or Y128 for optimal Y145 phosphorylation. Furthermore, Y145 and Y128 show cooperation in phosphorylation of Y128. Using a phospho-specific antibody to Y128, we found a decrease in Y128 phosphorylation in Y145F platelets (bottom blot, lane 2 vs. lane 6). In five separate experiments, quantification of Y128 phosphorylation revealed a decrease ranging from 30 to 50%. These data suggest that the diminished phosphorylation of SLP76 from the Y145F cells observed using 4G10 (top blot, lane 2 vs. lane 6) is likely caused by loss of Y145 and diminished phosphorylation of Y128. This reduction in the phosphorylation of specific tyrosine residues is not caused by diminished levels of SLP76 expression (top and bottom blots, lane 2 vs. lanes 4 and 6). The altered phosphorylation of the SLP76 mutants is also not caused by decreased PTK activity, as activation of Syk and phosphorylation of its substrate linker for activation of T cells (LAT) are not affected in the SLP76 KI platelets (Fig. 4 B).
Figure 4.
Optimal phosphorylation of SLP76, Btk, and PLCγ2 is dependent on synergy between Y112, Y128, and Y145 during GPVI signaling. Washed platelets from various mouse strains were left unstimulated (−) or stimulated with 0.8 nM CVX (+) for 90 s (A and C–F) or for 30 and 90 s (B) in the presence of apyrase. Equal amounts of platelet lysates were used for immunoblotting or IP. (A) Total SLP76 phosphorylation was measured by IP with an SLP76 antibody followed by blotting with 4G10 antibody, whereas phosphorylation of SLP76 Y128 was measured with a phospho-Y128 antibody. In both cases, blots were reprobed for total SLP76. (B) Platelet lysates were probed for phospho-Syk (Y525/526) or phospho-LAT (Y191) and reprobed with actin as a loading control. (C) PLCγ2 phosphorylation was detected by IP with 4G10, followed by immunoblotting with anti-PLCγ2 or by immunoblotting with phospho-PLCγ2 antibody (Y1217). (D) Platelets were stimulated with increasing doses of CVX, and lysates were analyzed for pPLCγ2. (E) Btk phosphorylation was assessed by IP with anti-Btk antibody and blotting with 4G10 antibody. Total Btk was used as a loading control. (F) Vav1 phosphorylation was assessed by IP with anti-Vav1 antibody and blotting with 4G10 antibody. Total Vav was used as a loading control. Quantification of the Western blot band intensities in unstimulated and stimulated samples (B and C) is shown relative to the corresponding loading control.
Activation of PLCγ2 is a critical SLP76-dependent biochemical signal after GPVI stimulation. To examine whether Y112 plus Y128 or Y145 are necessary for PLCγ2 activation, we examined the PLCγ2 phosphorylation status in platelets from the various mice after stimulation with a moderate dose of CVX. WT platelets showed significant PLCγ2 phosphorylation induction, whereas this response was not detected in the absence of SLP76 (Fig. 4 C). Both tyrosine mutants exhibited diminished PLCγ2 phosphorylation; however, Y145F cells consistently exhibited a more profound phenotype than that observed in Y112/128F cells (Fig. 4 C). Because a high CVX concentration (1 nM) induces a nearly normal aggregation response in Y112/128F but not in Y145F platelets, we investigated the dose dependency of CVX on GPVI-induced PLCγ2 phosphorylation in KI cells. When platelets are activated with escalating doses of CVX (0.4–1 nM), there is a dose-dependent increase in PLCγ2 phosphorylation in WT and mutant platelets (Fig. 4 D). However, although Y145F platelets exhibit defective PLCγ2 phosphorylation at all agonist doses, high CVX concentrations induce levels of phospho-PLCγ2 in Y112/128F platelets near those produced in WT cells.
Btk has been implicated as the major kinase responsible for PLCγ2 phosphorylation during GPVI signaling (28). Given that the proposed binding site for the Btk homologue Itk is Y145 of SLP76 and that its kinase activity depends on SLP76 N-terminal tyrosines in Jurkat T cells (29), we hypothesized that SLP76 tyrosines contribute to the regulation of PLCγ2 phosphorylation in platelets by facilitating the activation of Btk. We immunoprecipitated Btk from resting or stimulated platelets and measured its tyrosine phosphorylation status, an indicator of activation (30). In contrast to the prominent Btk phosphorylation in WT cells, SLP76−/− cells exhibited no enhancement of Btk phosphorylation in CVX-stimulated platelets. Y112/128F and Y145F platelets showed a significant reduction in inducible Btk phosphorylation (Fig. 4 E). These results suggest that SLP76, particularly its N-terminal tyrosines, potentiates GPVI-induced PLCγ2 activation by regulating phosphorylation of Btk.
Tyrosines 112 and 128 of SLP76 have been shown, in Jurkat T cells, to be the sites responsible for Vav association (12, 14, 31). Vav proteins have also been demonstrated to be necessary for optimal phosphorylation and activation of PLCγ2 after GPVI stimulation in platelets (32). Based on these observations, we reasoned that in Y112/128F mice, Vav/SLP76 binding would be abolished and Vav phosphorylation might also be reduced. We were surprised to find that the Vav1/SLP76 association and Vav1 phosphorylation were preserved in Y112/128 platelets (Fig. 4 F and not depicted). Interestingly, we observed that there was a near complete loss of Vav1 phosphorylation in Y145F platelets.
SLP76 tyrosines link GPVI signaling to actin assembly and platelet spreading
SLP76 has been implicated in GPVI-induced actin polymerization. Resting platelets adopt a spherical discoid shape (Fig. 5 B; and see Fig. 6 B). Upon GPVI stimulation, the cells rapidly spread, a process that is accompanied by actin polymerization (Fig. 5, A and B). Consistent with the role of SLP76 in GPVI signaling, actin assembly was shown to be markedly reduced in SLP76−/− platelets activated with the GPVI agonist collagen-related peptide (23). Furthermore, in the absence of SLP76, platelets fail to spread on a collagen matrix (7). To test the requirement for SLP76 tyrosines in these processes, we stimulated platelets with CVX and analyzed actin polymerization quantitatively using fluorescently labeled phalloidin, which binds to F-actin. GPVI stimulation in WT platelets induced a reproducible increase in F-actin content above resting levels (Fig. 5 A). In contrast, both Y112/128F and Y145F platelets showed only a mild change in F-actin staining (Fig. 5 A). These data demonstrate that GPVI-induced actin assembly in platelets is dependent on SLP76 Y112, Y128, and Y145 residues, consistent with actin polymerization defects observed in T cells from these mice after TCR ligation (24).
Figure 5.
Effect of SLP76 mutations on GPVI-induced F-actin assembly and platelet spreading on collagen. (A) Histograms with numbers indicating the mean fluorescence intensity of F-actin levels in unstimulated (gray) and CVX-treated (continuous line) platelets are shown. The data are representative of two experiments, each with two animals per genotype. (B) Platelet spreading on BSA (top) or collagen-coated (bottom) surfaces. Platelets were fixed, stained for F-actin, and analyzed by microscopy. Results shown are representative of five experiments. Spreading was quantified by a computer analysis of platelet surface areas (graph). Error bars represent the mean ± SD, where 35–100 platelets were analyzed. *, P < 0.0001 between Y112/128F, Y145F, and WT platelets. Bar, 10 μm.
Figure 6.
SLP76 Y112 and Y128 are necessary for platelet spreading on fibrinogen but dispensable for PLCγ2 phosphorylation downstream of αIIbβ3. (A, D, and E) Washed platelets from SLP76 mutant or WT mice were applied to fibrinogen-coated dishes for 30 min at 37°C. Nonadherent platelets were removed, and adherent cells were lysed. Resting samples were obtained by lysing platelets in suspension from BSA-coated dishes (−). Tyrosine phosphorylation of SLP76 was assessed by IP with 4G10 antibody, followed by immunoblotting with an anti-SLP76 antibody. Phosphorylation of SLP76 Y128 (A), PLCγ2 Y1217 (D and E), or Syk (E) was assessed using phospho-specific antibodies. Protein loading was measured by reblotting for actin (A), p38 (D), or PLCγ2 (E). Values below each band in D and E indicate the quantification of band intensity relative to the loading control and are representative of two and five experiments, respectively. (B) Platelet spreading on BSA (top), on fibrinogen alone (middle), or in the presence of 0.25 mM AYPGKF peptide (bottom). Cells were stained with rhodamine-phalloidin and analyzed by microscopy. Spreading was quantified by a computer analysis of platelet surface areas. Error bars represent the mean ± SD from a representative experiment (n = 3) where ≥143 platelets were analyzed. **, P < 0.0001 between Y112/128F and WT platelets and between SLP76−/− and WT platelets. Bar, 10 μm.
To extend these studies to a physiologically relevant platelet response, we examined whether Y112/128 or Y145 were required for cytoskeleton reorganization during platelet spreading on collagen. Platelets from WT or SLP76 KI mice were plated onto slides coated with fibrillar collagen, and morphological changes were examined by staining cells with rhodamine-phalloidin. WT platelets spread, forming extensive lamellipodia upon adhesion to a collagen surface (Fig. 5 B). There was a marked reduction in the formation of lamellipodia with Y112/128F or Y145F platelets, resulting in decreased spreading (Fig. 5 B). Full spreading is restored in the SLP76 mutants by the addition of ADP (unpublished data). These results demonstrate that SLP76 tyrosines link GPVI signaling to actin polymerization and platelet spreading.
Roles of SLP76 tyrosines in outside-in signaling
Fibrinogen binding to αIIbβ3 triggers outside-in signals that lead to actin reorganization to ensure efficient platelet aggregation and spreading. To investigate the role of SLP76 tyrosines for this function, we studied the requirements of Y112, Y128, and Y145 in signaling by αIIbβ3. To examine whether SLP76 tyrosines are phosphorylated in response to integrin engagement, we examined the phosphorylation status of the various mutants after the attachment of platelets to fibrinogen. αIIbβ3 induced an increase in WT SLP76 tyrosine phosphorylation as assessed by 4G10 (Fig. 6 A, lane 2). Phosphorylation of SLP76 Y112/Y128F was detectable but decreased, indicating that Y145 is phosphorylated after αIIbβ3 engagement (top blot, lane 4). WT but not Y112/128F cells also showed reactivity to anti-pY128 antibody, indicating that Y128 is also phosphorylated downstream of αIIbβ3 engagement (lanes 2 and 4). Notably, Y145F platelets showed reduced phosphorylation of Y128 (bottom blot, lane 6 vs. lane 2). These results indicate that SLP76 Y128 and Y145 are phosphorylated after αIIbβ3 stimulation and that there is an interdependence of phosphorylation events at Y145 and Y128, similar to that seen downstream of GPVI engagement.
To understand the biological implications of SLP76 tyrosine phosphorylation, we investigated the effects of SLP76 tyrosine mutations on αIIbβ3 signaling capacity by analyzing platelet spreading on fibrinogen. Platelets from WT or SLP76 mutant mice were incubated on a fibrinogen-coated surface, stained with rhodamine-phalloidin, and visualized by microscopy. WT platelets underwent spreading, forming filopodia and limited lamellipodia (Fig. 6 B, middle). In contrast, the morphology of Y112/128F cells resembled SLP76−/− platelets, with fewer spread cells that remained round, although a few filopodial extensions could be seen. Y145F platelets showed considerable variability in the spreading response, with most cells spreading normally on fibrinogen. Quantitative analysis of mean platelet surface areas revealed a significant difference only for Y112/128F versus control platelets (Fig. 6 C). KI platelets spread as well as WT cells when co-stimulated with the peptide AYPGKF (Fig. 6, B and C).
Platelet adhesion to a fibrinogen matrix is associated with PLCγ2 activation and subsequent calcium mobilization (33). Because SLP76 is critical for PLCγ2 activation after GPVI engagement, we assessed whether a defect in PLCγ2 regulation downstream of αIIbβ3 could underlie the defective spreading response observed in Y112/128F platelets. WT and SLP76 KI platelets were left unstimulated or were stimulated on a fibrinogen-coated surface and assessed for PLCγ2 phosphorylation by Western blotting using a phospho-specific PLCγ2 antibody. WT platelets exhibited a robust increase in the phosphorylation of PLCγ2, which was significantly reduced in SLP76−/− platelets (Fig. 6 D). Adhesion of Y112/128F or Y145F platelets to fibrinogen resulted in relatively normal levels of phospho-PLCγ2, with only a mild reduction seen in Y145F cells (Fig. 6 E). Given that PLCγ2 phosphorylation is normal in Y112/128F cells exhibiting impaired spreading, it appears that additional signals are required for platelet spreading on fibrinogen.
SLP76 tyrosine mutants do not complement each other in trans
Our original mutagenesis strategy involved altering both Y112 and Y128 to phenylalanine in one mouse and altering Y145 alone in a second mouse, because tyrosines 112 and 128 are found within a similar YESP motif, whereas tyrosine 145 is found within a YEPP motif. Studies in Jurkat T cells showed that Y112 and Y128 together are responsible for interactions with Vav and noncatalytic region of tyrosine kinase, whereas Y145 appears most important for its interaction with Tec family PTKs. As shown in this paper, platelets from Y112/128F and Y145F mice exhibit abnormalities in key SLP76-dependent signaling pathways. We wondered if the defects in the signaling pathways observed in platelets from the individual KI animals could be rescued by expression of the complementary mutation. We bred mice carrying the Y112/128F allele with mice carrying the Y145F allele to generate animals that were heterozygous for each mutation but null for WT SLP76. Experiments were then performed examining the GPVI signaling phenotype of the single- or double-mutant platelets.
As shown earlier, homozygous KI mice have diminished GPVI-induced P-selectin expression upon stimulation with CVX (Fig. 7 A). Coexpression of both mutant molecules in the same mouse failed to restore this GPVI-mediated response. Quantitative comparison of P-selectin up-regulation in the various mutant platelets revealed a 50% decrease in inducible expression in the double mutant compared with that of the Y112/128F cells. Because double-mutant platelets showed a response that was intermediate between platelets with two copies of Y112/128F and platelets with two copies of Y145F alleles, we tested whether this outcome could be caused by a dose effect of SLP76 expression. We generated mice expressing one null allele and one copy of either the Y145F or the Y112/128F allele and compared platelet responses from these mice with those of the single- or double-mutant cells. Quantification of cells staining positive for P-selectin after GPVI stimulation showed that platelets from the double mutant responded equivalently to those from mice with one null allele and one Y112/128F allele (Fig. S2, available at http://www.jem.org/cgi/content/full/jem.20080240/DC1).
Figure 7.
Coexpression of the complementary SLP76 tyrosine mutants does not reconstitute GPVI signaling. Platelets from homozygous KI Y112/128F or Y145F mice were compared with platelets isolated from animals expressing both Y112/128F and Y145F alleles in assays of GPVI activation, as described in 2–4. Double-mutant platelets do not show an improvement of GPVI-mediated responses compared with those of Y112/128F platelets in the assays of (A) α granule secretion, (B) soluble fibrinogen binding, (C) aggregation to 0.4 nM CVX (top) or 5 μg/ml collagen (bottom), or (D) phosphorylation of PLCγ2. Each panel depicts the representative results of two or three experiments.
Having established that Y145F and Y112/128F mutants do not cooperate with each other in trans to support degranulation, we asked if other GPVI-mediated responses could be complemented by expressing both mutants in the same mouse. Our findings indicate that the double-mutant platelets do not have improved GPVI-mediated responses compared with Y112/128F platelets when αIIbβ3 activation is assessed (Fig. 7 B), aggregation to CVX or collagen is measured (Fig. 7 C), or PLCγ2 phosphorylation is assayed (Fig. 7 D). These results suggest that phosphorylation of tyrosines on a single SLP76 molecule is required to support GPVI function.
DISCUSSION
Activation of platelets and their subsequent incorporation into a developing thrombus require the orchestration of numerous signaling pathways. SLP76 was shown to be central for platelet activation in vitro, but its role in vivo was unknown. In this study, we show that mice containing mutations in SLP76 tyrosines manifest significant alterations in signaling downstream of collagen receptor GPVI and integrin αIIbβ3 in vitro and are protected, to varying degrees, from thrombus formation in vivo.
Mobilization of intracellular Ca2+ and the duration of Ca2+ signaling have been shown to play critical roles in platelet secretion, integrin activation, and the formation of stable platelet aggregates in flowing blood (34). Y145F and Y112/128F KI platelets exhibit impaired secretion and aggregation responses and form smaller thrombi under flow. The reduction in PLCγ2 phosphorylation observed in KI platelets after GPVI stimulation suggests that compromised Ca2+ signaling could underlie these functional defects.
Based on studies in cell lines from several laboratories (for review see reference 35), a model emerged positing that Y112 and Y128 are both required for SLP76 to associate with Vav and that Tec family PTKs bind to Y145; however, there are other domains of SLP76 that may contribute to this interaction (17). We predicted that SLP76 isolated from the Y112/128F mice would not associate with Vav and that the interaction between Y145F SLP76 and Itk in T cells or Btk in platelets would be diminished. Therefore, we were surprised to find no evidence of diminished binding between the tyrosine mutants of SLP76 and their binding partners, either in T cells (24) or in platelets. Thus, we focused on whether the SLP76 partner proteins Btk and Vav were modified after GPVI stimulation.
We examined inducible Btk phosphorylation in platelets from WT and SLP76 mutant mice. The absence of SLP76 results in a marked reduction in GPVI-stimulated phosphorylation of Btk (Fig. 4), suggesting failed induction of its enzymatic activity (30), and inducible Btk phosphorylation was only marginally rescued by the expression of SLP76 Y145F. Thus, we speculate that one possible mechanism by which SLP76 tyrosines regulate PLCγ2 activation and intracellular Ca2+ is by affecting GPVI-induced Btk activity. If a major function of Y145F is to facilitate Btk activation, the signaling phenotype of Y145F platelets should be similar to Btk−/− or to mice that are deficient in both Btk and its family member Tec. Indeed, although Btk−/− platelets are not as defective as Y145F platelets in response to GPVI (28), Btk−/−Tec−/− platelets have a phenotype similar to that of Y145F mice.
In contrast to Y145F cells, platelets expressing Y112/Y128F show only mild defects in GPVI-mediated platelet activation. The fact that phosphorylation of Btk was also diminished in Y112/128F cells suggests that another biochemical event may be differentially preserved in these cells. We detected a selective loss of Vav phosphorylation in Y145F but not in Y112/128F platelets stimulated via GPVI. Vav plays a critical role in platelet activation, at least in part by regulating GPVI-induced PLCγ2 phosphorylation (32). These results were surprising, as we predicted that Vav phosphorylation may be preserved in Y145F platelets and absent in Y112/128F platelets, as seen in the T cell compartment of these mice (24). Although the cause of the reduced Vav phosphorylation in Y145F and not Y112/128F mice remains unknown, this defect likely contributes to the differential impairment of GPVI signaling observed in Y145F versus Y112/128F platelets.
The most severe phenotype of Y112/128F platelets was in outside-in signaling by αIIbβ3. Our preliminary studies indicate that Vav1 phosphorylation was diminished in Y112/128F downstream of αIIbβ3 engagement. Vav1−/−Vav3−/− platelets exhibit reduced spreading on fibrinogen (36), consistent with our findings. We speculate that SLP76 via Y112 and/or Y128 may help recruit Vav proteins to αIIbβ3, where they can be phosphorylated by Syk and facilitate actin reorganization. It is intriguing to speculate that defective platelet spreading might account for the smaller aggregate size in Y112/128F platelets compared with WT in an in vitro thrombus formation assay.
Comparing biochemical and functional readouts of GPVI and αIIbβ3 signaling, we find that the mutant that exhibits more profound GPVI signaling defects (Y145F) also shows a more severe thrombosis defect. These results agree with evidence indicating a significant role for GPVI signaling in collagen-mediated platelet activation in vivo (25, 37). There is also compelling evidence that αIIbβ3 is important for thrombus formation, because mice deficient in β3 integrin are protected against thrombosis (38). Studies of Y112/128F platelets indicate that although it is necessary for fibrinogen-dependent platelet spreading, the role of SLP76 in outside-in signaling by αIIbβ3 appears less important for thrombus formation. Further studies are necessary to evaluate the contributions of SLP76-dependent signals downstream of GPVI versus αIIbβ3 to platelet activation in vivo.
The generation of mice containing complementary mutants of SLP76 provided a unique opportunity to study SLP76 functions in vivo. Our simplistic prediction was that two SLP76 molecules with complementary phosphorylation sites would become part of a larger signaling network and complement each other in trans, thus rescuing the defective functional responses seen in platelets that express either Y112/128F or Y145F. We found that Y112/128F and Y145F mutants do not complement each other during GPVI signaling. One potential explanation for this lack of cooperation is that mutations in individual tyrosines disrupt phosphorylation of the adjacent sites, thus making complementation improbable. Indeed, our results suggest that phosphorylation of SLP76 tyrosines occurs in a coordinated fashion, demonstrating interdependence between Y128 and Y145 sites.
In contrast to the lack of complementation in platelets, coexpression of Y112/128F and Y145F mutants within the T cell compartment rescues the defective T cell development and function found in the individual Y112/128F or Y145F mice (24). One potential explanation for the difference between T cells and platelets is the mechanism by which ITAMs of the GPVI or TCR are coupled to an SLP76-nucleated complex. Although both SLP76 and LAT are essential for TCR signaling, SLP76 can support signaling downstream of GPVI in the absence of LAT (26). A recent report (39) demonstrated that LAT oligomerizes after TCR cross-linking, which could allow for the transactivation of the signaling proteins associated with LAT, such as SLP76. LAT clustering has not been shown in platelets. Alternatively, the number of ITAMs available to support signaling in T cells (10 ITAMs) versus platelets (2 ITAMs) may allow for the formation of a larger complex, which might be necessary for the complementation of these mutations. A similar requirement for cis-mediated adaptor function has been demonstrated for SLP65–B cell linker (a two ITAM–containing signaling complex), where two complementary mutations in tyrosines responsible for binding PLCγ2 were not able to complement each other to support BCR signaling (40). Thus, SLP76 family adapters might function to nucleate a single molecular scaffold, but if organized into larger oligomerized complexes and supported by multiple ITAMs, as they potentially are in T cells, multiple SLP76 molecules could cooperate in trans.
MATERIALS AND METHODS
Reagents.
Antibodies were purchased from the following vendors: 4G10 and Vav1 (Millipore); pSLP76 (Y128; BD Biosciences); mouse SLP76 (eBioscience); pPLCγ2 (Y1217), pSyk (Y525/526), pLAT (Y191), PLCγ2, and p38 (Cell Signaling Technology); and Syk, PLCγ2, Vav, and actin (Santa Cruz Biotechnology, Inc.). Btk antibody was a gift of M. Tomlinson and J. Bolen (University of Birmingham, Birmingham, England, UK). TrueBlot Ig immunoprecipitation (IP) beads and TrueBlot horseradish peroxidase–conjugated IgGs were obtained from eBioscience. All reagents were purchased from Sigma-Aldrich except for the following: type I bovine collagen (Chrono-Log Corp.); fibrinogen depleted of plasminogen, von Willebrand factor, and fibronectin (Enzyme Research Laboratories); CVX (ALEXIS Biochemicals); and AYPGKF peptide, which was synthesized by the University of Pennsylvania Protein Chemistry Laboratory.
Mice.
Y145F and Y112/128F mice were generated as previously described (24) and were backcrossed to C57BL/6 mice two to five times. Mice were viable and showed no gross phenotypic abnormalities. To generate compound or hemizygous KI mice, Y145F and Y112/128F homozygous mice were bred with each other or with SLP76−/− mice (6). For all experiments, littermates were used as WT controls. C57BL/6 mice were obtained from Taconic. All mice were housed under pathogen-free conditions at the University of Pennsylvania Animal Facility and were used in accordance with National Institutes of Health guidelines and approved protocols from the University of Pennsylvania Institutional Review Board committee.
Platelet preparation.
Whole blood was anticoagulated using heparin (aggregation studies) or acidic citrate dextrose, followed by dilution in Tyrode's buffer (137 mM NaCl, 2.9 mM KCl, 12 mM NaHCO3, 0.2 mM CaCl2, 0.2 mM MgCl2, 0.1% BSA, 0.1% glucose, and 5 mM Hepes [pH 7.4]). Platelet-rich plasma (PRP) was prepared by centrifugation at 960 g for 8 min. Purified platelets were obtained by centrifugation of PRP at 1,500 g for 10 min in the presence of 1 U/ml apyrase, 1 μM prostaglandin E1, and 1 mM aspirin. Platelet pellets were resuspended in Walsh buffer (137 mM NaCl, 2.7 mM KCl, 1 mM MgCl2, 5.6 mM glucose, 1 mg/ml BSA, 3.3 mM NaH2PO4, and 20 mM Hepes [pH 7.4]). For hematological analysis, blood was collected into EDTA-coated tubes (Sarstedt) and analyzed by a Hemavet machine (model HV950FS; Drew Scientific). For flow cytometry, blood was collected by retroorbital bleed into heparin-treated tubes (Thermo Fischer Scientific) and diluted in Tyrode's buffer containing 1 U/ml heparin.
Flow cytometry.
106 platelets, in the presence of 1 mM Ca2+, were stimulated with 0.2–1 nM CVX, 0.5–1 mM AYPGKF, or 0.1–20 μM ADP in the presence of CD49b-FITC and P-selectin–PE (BD Biosciences) or fibrinogen-A647 (Invitrogen) for 20 min at 37°C. To ensure specificity of fibrinogen binding, platelets were stimulated in the presence of 0.5 mM EDTA. For the measurement of GPVI surface expression, platelets were stained with anti–JAQ-1–FITC (Emfret Analytics) or anti–rat IgG2a (BD Biosciences) antibodies. For the measurement of F-actin levels, platelets (108 cells/ml) were stimulated with CVX for 2 min at 37°C. Cells were fixed with 1% paraformaldehyde (PFA), permeabilized with 1% Triton X-100, and stained with phalloidin-A594 (Invitrogen). Samples were analyzed on a FACSCalibur (BD Biosciences) using FlowJo software (Tree Star, Inc.).
Platelet aggregation.
Stimulation of PRP (2.5 × 108 cells/ml) with various concentrations of CVX, collagen, or ADP was performed in an aggregometer (model 460-VS; Chrono-Log) with stirring at 37°C. Aggregation was monitored by measuring changes in light transmission. For the inhibition studies, PRP was incubated with 30 μg/ml Ha1/29 blocking antibody (BD Biosciences) or DMSO for 30 min before the experiment.
Immunofluorescence.
20 × 106 purified platelets were added to slides coated with 10 mg/ml BSA, 50 μg/ml collagen, or 100 μg/ml fibrinogen and allowed to spread for 45 min at 37°C. In control experiments, platelets were allowed to spread in the presence of AYPGKF peptide. Slides were washed in PBS, and attached platelets were fixed with 2% PFA/PBS for 10 min at room temperature, permeabilized with 0.2% Triton X-100 for 5 min at room temperature, and incubated with rhodamine-phalloidin (Invitrogen) to stain F-actin. The quantification of platelet surface area was performed by determining the pixel number within each platelet using IP Laboratories Scientific Image Processing software (Scanalytics, Inc.).
IP and immunoblotting.
For GPVI stimulation, purified platelets were resuspended in Walsh buffer containing 2 U/ml apyrase and 10 μM indomethacin, and were stimulated with 0.8 nM CVX (unless otherwise indicated in the figures). Reactions were stopped by the addition of 2× lysis buffer (1% NP-40 containing 1:100 complete protease inhibitor cocktail [Roche], 1 mM PMSF, 2 mM NaVO3, 10 μM NaF, and 10 μM sodium pyrophosphate). For αIIbβ3 stimulation, platelets were added to dishes and coated with 10 mg/ml BSA or 100 μg/ml fibrinogen for 30 min at 37°C. Platelets adherent to fibrinogen were washed in PBS and solubilized on ice in 1× lysis buffer. Nonadherent cells from BSA plates were sedimented at 13,000 g for 10 s and lysed in 1× lysis buffer. Lysates were centrifuged at 13,000 g for 10 min. Protein concentration was quantified using BCA protein assay (Thermo Fisher Scientific), and 10–20 or 200–500 μg of lysates were used for Western blotting or IP, respectively. The bead pellets were washed once with 1× lysis buffer containing 0.36 M NaCl and twice with 1× lysis buffer before 1× sample buffer was added (Invitrogen). Proteins were resolved by SDS-PAGE.
Thrombus formation on collagen under flow.
Slides were coated with 300 μg/ml collagen and blocked with denatured BSA. Heparin-anticoagulated whole blood was perfused over the collagen-coated slide at room temperature for 4 min. Subsequently, slides were rinsed with Tyrode's buffer for 10 min. Phase-contrast images were recorded using a microscope (model TE-300; Nikon) at different axial positions corresponding to various shear rates using a 10× objective lens with a 1.5 numerical aperture. For analysis of surface coverage and thrombus volume, slides were stained with rhodamine-phalloidin and analyzed by confocal microscopy. Quantification of surface coverage was performed on a single frame using IP Laboratories software. Thrombus volume was calculated as the summation of partial volumes measured from the area occupied by platelet aggregates in each plane of Z-stacks using Volocity software (Improvision).
Thrombus formation in vivo.
To measure blood flow, a Doppler probe was placed around the isolated carotid artery of 8–16-wk-old mice anesthetized with sodium pentobarbitone. Vessel wall damage was induced by application of a 0.5 × 1–mm paper filter saturated with 10% FeCl3 for 3 min. After removal of the filter paper, the tissue was washed using saline solution for 1 min. The time to complete occlusion was calculated from the time the filter paper was removed to when blood flow reached 0–0.1 ml/min. The experiment was terminated at 30 min.
Statistical analysis.
Statistical significance was analyzed using the Student's t tests for independent samples, and P < 0.05 was considered significant. Where applicable, results are shown as the mean ± SEM. A comparison of the ratio of the number of mice that formed thrombotic occlusions to those that did not between WT and Y145F and Y112/128F mice was performed using Fisher's exact test.
Online supplemental material.
Fig. S1 shows that platelets from SLP76 KI mice aggregate normally in response to ADP. Fig. S2 shows that platelets from double-mutant (Y112/128F)/Y145F mice exhibit similar levels of α granule secretion after CVX stimulation as platelets from hemizygous mice expressing only one allele of Y112/128F (e.g., Y112/128F/−). Online supplemental material is available at available at http://www.jem.org/cgi/content/full/jem.20080240/DC1.
Supplementary Material
Acknowledgments
We thank Drs. T. Stalker and L. Zhu for technical help, Dr. H. Chen for reagents, and Drs. J. Smith and J. Maltzman for critical reading of the manuscript.
This work was supported by grants from the National Institutes of Health (to C.S. Abrams, L.F. Brass, M.L. Kahn, M.S. Jordan, and G.A. Koretzky) and the Arthritis Foundation (to M.S. Jordan), and a Howard Hughes Medical Institute Predoctoral Fellowship (to N.A. Bezman).
The authors have no conflicting financial interests.
Abbreviations used: Btk, Bruton's tyrosine kinase; CVX, convulxin; GPVI, glycoprotein VI; IP, immunoprecipitation; ITAM, immunoreceptor tyrosine-based activation motif; Itk, IL-2–inducible T cell kinase; KI, knock-in; LAT, linker for activation of T cells; PLCγ2, phospholipase C γ2; PRP, platelet-rich plasma; PTK, protein tyrosine kinase; SH2, Src homology 2; SLP76, SH2 domain–containing leukocyte phosphoprotein of 76 kD.
References
- 1.Kasirer-Friede, A., M.L. Kahn, and S.J. Shattil. 2007. Platelet integrins and immunoreceptors. Immunol. Rev. 218:247–264. [DOI] [PubMed] [Google Scholar]
- 2.Watson, S.P., J.M. Auger, O.J. McCarty, and A.C. Pearce. 2005. GPVI and integrin alphaIIb beta3 signaling in platelets. J. Thromb. Haemost. 3:1752–1762. [DOI] [PubMed] [Google Scholar]
- 3.Motto, D.G., S.E. Ross, J. Wu, L.R. Hendricks-Taylor, and G.A. Koretzky. 1996. Implication of the GRB2-associated phosphoprotein SLP-76 in T cell receptor–mediated interleukin 2 production. J. Exp. Med. 183:1937–1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yablonski, D., M.R. Kuhne, T. Kadlecek, and A. Weiss. 1998. Uncoupling of nonreceptor tyrosine kinases from PLC-gamma1 in an SLP-76-deficient T cell. Science. 281:413–416. [DOI] [PubMed] [Google Scholar]
- 5.Jordan, M.S., A.L. Singer, and G.A. Koretzky. 2003. Adaptors as central mediators of signal transduction in immune cells. Nat. Immunol. 4:110–116. [DOI] [PubMed] [Google Scholar]
- 6.Clements, J.L., J.R. Lee, B. Gross, B. Yang, J.D. Olson, A. Sandra, S.P. Watson, S.R. Lentz, and G.A. Koretzky. 1999. Fetal hemorrhage and platelet dysfunction in SLP-76-deficient mice. J. Clin. Invest. 103:19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Judd, B.A., P.S. Myung, A. Obergfell, E.E. Myers, A.M. Cheng, S.P. Watson, W.S. Pear, D. Allman, S.J. Shattil, and G.A. Koretzky. 2002. Differential requirement for LAT and SLP-76 in GPVI versus T cell receptor signaling. J. Exp. Med. 195:705–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Judd, B.A., P.S. Myung, L. Leng, A. Obergfell, W.S. Pear, S.J. Shattil, and G.A. Koretzky. 2000. Hematopoietic reconstitution of SLP-76 corrects hemostasis and platelet signaling through alpha IIb beta 3 and collagen receptors. Proc. Natl. Acad. Sci. USA. 97:12056–12061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abtahian, F., A. Guerriero, E. Sebzda, M.M. Lu, R. Zhou, A. Mocsai, E.E. Myers, B. Huang, D.G. Jackson, V.A. Ferrari, et al. 2003. Regulation of blood and lymphatic vascular separation by signaling proteins SLP-76 and Syk. Science. 299:247–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gross, B.S., J.R. Lee, J.L. Clements, M. Turner, V.L. Tybulewicz, P.R. Findell, G.A. Koretzky, and S.P. Watson. 1999. Tyrosine phosphorylation of SLP-76 is downstream of Syk following stimulation of the collagen receptor in platelets. J. Biol. Chem. 274:5963–5971. [DOI] [PubMed] [Google Scholar]
- 11.Onodera, H., D.G. Motto, G.A. Koretzky, and D.M. Rothstein. 1996. Differential regulation of activation-induced tyrosine phosphorylation and recruitment of SLP-76 to Vav by distinct isoforms of the CD45 protein-tyrosine phosphatase. J. Biol. Chem. 271:22225–22230. [DOI] [PubMed] [Google Scholar]
- 12.Raab, M., A.J. da Silva, P.R. Findell, and C.E. Rudd. 1997. Regulation of Vav-SLP-76 binding by ZAP-70 and its relevance to TCR zeta/CD3 induction of interleukin-2. Immunity. 6:155–164. [DOI] [PubMed] [Google Scholar]
- 13.Tuosto, L., F. Michel, and O. Acuto. 1996. p95vav associates with tyrosine-phosphorylated SLP-76 in antigen-stimulated T cells. J. Exp. Med. 184:1161–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu, J., D.G. Motto, G.A. Koretzky, and A. Weiss. 1996. Vav and SLP-76 interact and functionally cooperate in IL-2 gene activation. Immunity. 4:593–602. [DOI] [PubMed] [Google Scholar]
- 15.Bubeck Wardenburg, J., R. Pappu, J.Y. Bu, B. Mayer, J. Chernoff, D. Straus, and A.C. Chan. 1998. Regulation of PAK activation and the T cell cytoskeleton by the linker protein SLP-76. Immunity. 9:607–616. [DOI] [PubMed] [Google Scholar]
- 16.Wunderlich, L., A. Farago, J. Downward, and L. Buday. 1999. Association of Nck with tyrosine-phosphorylated SLP-76 in activated T lymphocytes. Eur. J. Immunol. 29:1068–1075. [DOI] [PubMed] [Google Scholar]
- 17.Bunnell, S.C., M. Diehn, M.B. Yaffe, P.R. Findell, L.C. Cantley, and L.J. Berg. 2000. Biochemical interactions integrating Itk with the T cell receptor-initiated signaling cascade. J. Biol. Chem. 275:2219–2230. [DOI] [PubMed] [Google Scholar]
- 18.Su, Y.W., Y. Zhang, J. Schweikert, G.A. Koretzky, M. Reth, and J. Wienands. 1999. Interaction of SLP adaptors with the SH2 domain of Tec family kinases. Eur. J. Immunol. 29:3702–3711. [DOI] [PubMed] [Google Scholar]
- 19.Kumar, L., V. Pivniouk, M.A. de la Fuente, D. Laouini, and R.S. Geha. 2002. Differential role of SLP-76 domains in T cell development and function. Proc. Natl. Acad. Sci. USA. 99:884–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Myung, P.S., G.S. Derimanov, M.S. Jordan, J.A. Punt, Q.H. Liu, B.A. Judd, E.E. Meyers, C.D. Sigmund, B.D. Freedman, and G.A. Koretzky. 2001. Differential requirement for SLP-76 domains in T cell development and function. Immunity. 15:1011–1026. [DOI] [PubMed] [Google Scholar]
- 21.Yablonski, D., T. Kadlecek, and A. Weiss. 2001. Identification of a phospholipase C-gamma1 (PLC-gamma1) SH3 domain-binding site in SLP-76 required for T-cell receptor-mediated activation of PLC-gamma1 and NFAT. Mol. Cell. Biol. 21:4208–4218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abtahian, F., N. Bezman, R. Clemens, E. Sebzda, L. Cheng, S.J. Shattil, M.L. Kahn, and G.A. Koretzky. 2006. Evidence for the requirement of ITAM domains but not SLP-76/Gads interaction for integrin signaling in hematopoietic cells. Mol. Cell. Biol. 26:6936–6949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Falet, H., K.L. Barkalow, V.I. Pivniouk, M.J. Barnes, R.S. Geha, and J.H. Hartwig. 2000. Roles of SLP-76, phosphoinositide 3-kinase, and gelsolin in the platelet shape changes initiated by the collagen receptor GPVI/FcR gamma-chain complex. Blood. 96:3786–3792. [PubMed] [Google Scholar]
- 24.Jordan, M.S., J.E. Smith, J.C. Burns, J.E. Austin, K.E. Nichols, A.C. Aschenbrenner, and G.A. Koretzky. 2008. Complementation in trans of altered thymocyte development in mice expressing mutant forms of the adaptor molecule SLP76. Immunity. 28:359–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dubois, C., L. Panicot-Dubois, G. Merrill-Skoloff, B. Furie, and B.C. Furie. 2006. Glycoprotein VI-dependent and -independent pathways of thrombus formation in vivo. Blood. 107:3902–3906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen, H., and M.L. Kahn. 2003. Reciprocal signaling by integrin and nonintegrin receptors during collagen activation of platelets. Mol. Cell. Biol. 23:4764–4777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jordan, M.S., J. Sadler, J.E. Austin, L.D. Finkelstein, A.L. Singer, P.L. Schwartzberg, and G.A. Koretzky. 2006. Functional hierarchy of the N-terminal tyrosines of SLP-76. J. Immunol. 176:2430–2438. [DOI] [PubMed] [Google Scholar]
- 28.Atkinson, B.T., W. Ellmeier, and S.P. Watson. 2003. Tec regulates platelet activation by GPVI in the absence of Btk. Blood. 102:3592–3599. [DOI] [PubMed] [Google Scholar]
- 29.Bogin, Y., C. Ainey, D. Beach, and D. Yablonski. 2007. SLP-76 mediates and maintains activation of the Tec family kinase ITK via the T cell antigen receptor-induced association between SLP-76 and ITK. Proc. Natl. Acad. Sci. USA. 104:6638–6643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Felices, M., M. Falk, Y. Kosaka, and L.J. Berg. 2007. Tec kinases in T cell and mast cell signaling. Adv. Immunol. 93:145–184. [DOI] [PubMed] [Google Scholar]
- 31.Fang, N., and G.A. Koretzky. 1999. SLP-76 and Vav function in separate, but overlapping pathways to augment interleukin-2 promoter activity. J. Biol. Chem. 274:16206–16212. [DOI] [PubMed] [Google Scholar]
- 32.Pearce, A.C., Y.A. Senis, D.D. Billadeau, M. Turner, S.P. Watson, and E. Vigorito. 2004. Vav1 and vav3 have critical but redundant roles in mediating platelet activation by collagen. J. Biol. Chem. 279:53955–53962. [DOI] [PubMed] [Google Scholar]
- 33.Wonerow, P., A.C. Pearce, D.J. Vaux, and S.P. Watson. 2003. A critical role for phospholipase Cgamma2 in alphaIIbbeta3-mediated platelet spreading. J. Biol. Chem. 278:37520–37529. [DOI] [PubMed] [Google Scholar]
- 34.Nesbitt, W.S., S. Giuliano, S. Kulkarni, S.M. Dopheide, I.S. Harper, and S.P. Jackson. 2003. Intercellular calcium communication regulates platelet aggregation and thrombus growth. J. Cell Biol. 160:1151–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koretzky, G.A., and P.S. Myung. 2001. Positive and negative regulation of T-cell activation by adaptor proteins. Nat. Rev. Immunol. 1:95–107. [DOI] [PubMed] [Google Scholar]
- 36.Pearce, A.C., O.J. McCarty, S.D. Calaminus, E. Vigorito, M. Turner, and S.P. Watson. 2007. Vav family proteins are required for optimal regulation of PLCgamma2 by integrin alphaIIbbeta3. Biochem. J. 401:753–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Munnix, I.C., A. Strehl, M.J. Kuijpers, J.M. Auger, P.E. van der Meijden, M.A. van Zandvoort, M.G. oude Egbrink, B. Nieswandt, and J.W. Heemskerk. 2005. The glycoprotein VI-phospholipase Cgamma2 signaling pathway controls thrombus formation induced by collagen and tissue factor in vitro and in vivo. Arterioscler. Thromb. Vasc. Biol. 25:2673–2678. [DOI] [PubMed] [Google Scholar]
- 38.Smyth, S.S., E.D. Reis, H. Vaananen, W. Zhang, and B.S. Coller. 2001. Variable protection of beta 3-integrin–deficient mice from thrombosis initiated by different mechanisms. Blood. 98:1055–1062. [DOI] [PubMed] [Google Scholar]
- 39.Houtman, J.C., H. Yamaguchi, M. Barda-Saad, A. Braiman, B. Bowden, E. Appella, P. Schuck, and L.E. Samelson. 2006. Oligomerization of signaling complexes by the multipoint binding of GRB2 to both LAT and SOS1. Nat. Struct. Mol. Biol. 13:798–805. [DOI] [PubMed] [Google Scholar]
- 40.Chiu, C.W., M. Dalton, M. Ishiai, T. Kurosaki, and A.C. Chan. 2002. BLNK: molecular scaffolding through ‘cis’-mediated organization of signaling proteins. EMBO J. 21:6461–6472. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.