Abstract
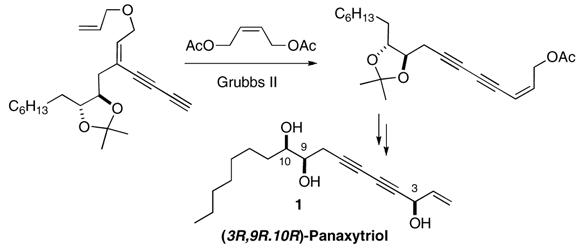 Enyne metathesis is unique for its capacity to carry out multiple bond formation in tandem fashion. Its combined use with metallotropic [1,3]-shift allowed for the development of a novel strategy for the total synthesis of a conjugated 1,3-diyne-containing natural product (3R,9R,10R)-panaxytriol.
Enyne metathesis is unique for its capacity to carry out multiple bond formation in tandem fashion. Its combined use with metallotropic [1,3]-shift allowed for the development of a novel strategy for the total synthesis of a conjugated 1,3-diyne-containing natural product (3R,9R,10R)-panaxytriol.
The most unique aspect of synthetic chemistry stems from its capacity to create molecules crucial to addressing problems ranging from fundamental science to human health. The practical synthesis1 of these target molecules is contingent upon the availability of effective synthetic methods, and thus the development of tandem reactions2 draws great deal of attention as it induces a significant increase in molecular complexity within a given step.
Recently, we have introduced a metathesis-based tandem reaction sequence, where an enyne ring-closing metathesis is juxtaposed with one or more metallotropic [1,3]-shift followed by another RCM step.3 In this communication, we describe a powerful tandem reaction sequence initiated by relay metathesis, 4 which is followed by metallotropic [1,3]-shift and cross metathesis,5 as a unique and efficient way for the synthesis of a 1,3-diyne-containing natural compound. 6
(3R,9R,10R)-panaxytriol 1 was isolated as one of the characteristic constituents of panax ginseng C. A. Meyer in 1983. 7 It exhibits inhibitory activity against a range of tumor cell types, including human gastric adenocarcinoma (MK-1),8 human breast carcinoma (Breast M25-SF),9 and mouse lymphoma (P388D1)10. The structure of panaxytriol was established as heptadec-1-ene-4,6-diyne-3,9,10-triol in 1989,11 and its absolute configuration was determined as 3R,9R,10R by circular dichroism (CD) analysis 12 and confirmed by total syntheses.13
Our strategy for the synthesis of 1 is outlined in Scheme 1. We envisioned that the main carbon framework of the target molecule could arise from a tandem reaction sequence of relay metathesis, metallotropic [1,3]-shift, and cross metathesis with enediyne 4 in the presence of an excess amount of external alkene 3. The intricate array of multiply unsaturated functional groups in 4 could be orchestrated by the recently developed regioselective Alder ene reaction of multiyne 5 14 with terminal alkene 6 followed by alkyne homologation via the Cadiot-Chodkiewicz reaction15 with bromoalkyne 7.
Scheme 1.
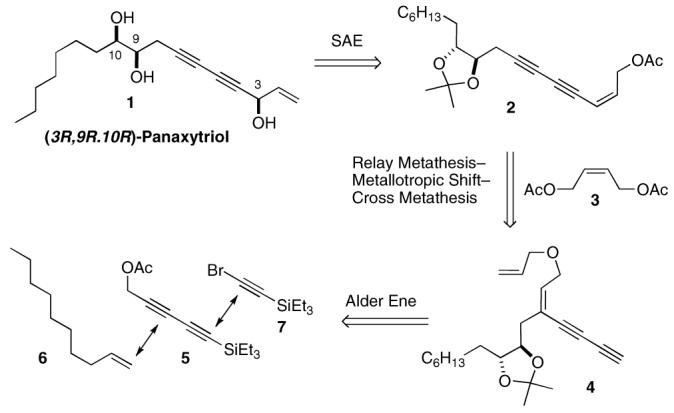
The eight-step synthesis of endiyne 4 was initiated by the Ru-catalyzed Alder ene reaction of silylated diyne 5 and 1-decene to provide enyne 8 in 81% yield (Scheme 2).16 The required (9R,10R)-diol was installed by the Sharpless asymmetric dihydroxylation,17 which selectively took place on the disubstituted trans-double bond of 8 in 95% yield. The resulting diol was then protected as its acetonide by treating with 2,2-dimethoxypropane (cat. PTSA, THF) to give 9 in 98% yield. In turn, 9 was converted to enyne 10 in 93% overall yield through deacetylation (DIBAL-H, THF, −78 °C), desilylation (TBAF, 10 mol % of AcOH, THF), and O-allylation (NaH, allyl bromide, DMF). Addition of a small amount of acetic acid to the reaction in the desilyation minimizes undesired side reactions that lead to extensive decomposition. For the etherification of the subsequent allylic alcohol, we found that pre-formation of alkoxide increased the extent of the undesired intramolecular addition of the alkoxide to the nearby triple bond. This undesired byproduct could be suppressed by adding sodium hydride to the mixture of the alcohol and allyl bromide. The elongation of enyne 10 to diyne 4 was achieved in 92% yield employing the Cadiot-Chodkiewicz reaction14 with silylated bromoalkyne 7 followed by desilylation (TBAF, 10 mol % of AcOH, THF).
Scheme 2.
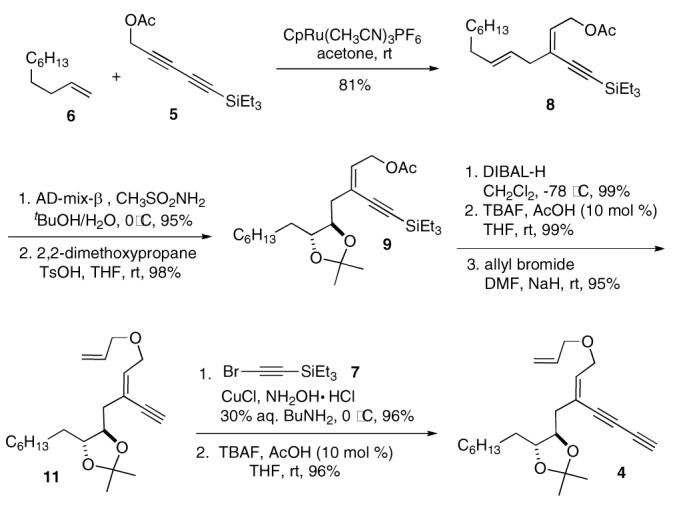
With the key substrate 4 in hand, we explored the tandem ring-closing metathesis, metallotropic [1,3]-shift, and cross metathesis. When 4 was treated with Grubbs second generation catalyst18 (Grubbs II, 10 mol %, CH2Cl2, 40 °C) in the presence of 2.0 equivalent of alkene 3, the expected product 2 was obtained in 61% yield as a mixture of Z/E-isomers (5:1)4a,19 together with ruthenium alkylidene 11′ (10%). The isolated complex 11′ could be turned over to 2 upon treatment with 3, which implies that this complex is a catalytically viable intermediate in the catalytic cycle. The yield of 11′ was increased up to 40% with stoichiometric amount of Grubbs complex. We speculate that the stability (low reactivity) of 11′ is the consequence of the low steric pressure of the alkynyl group and the hydrogen on the carbenic carbon, which ultimately lowers the rate of phosphine dissociation from the ruthenium center (Scheme 3).
Scheme 3.
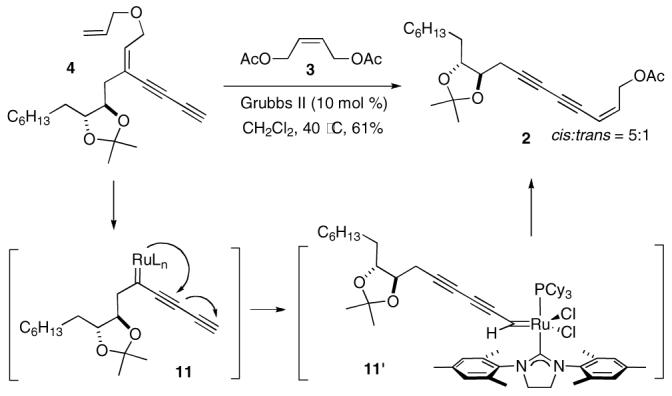
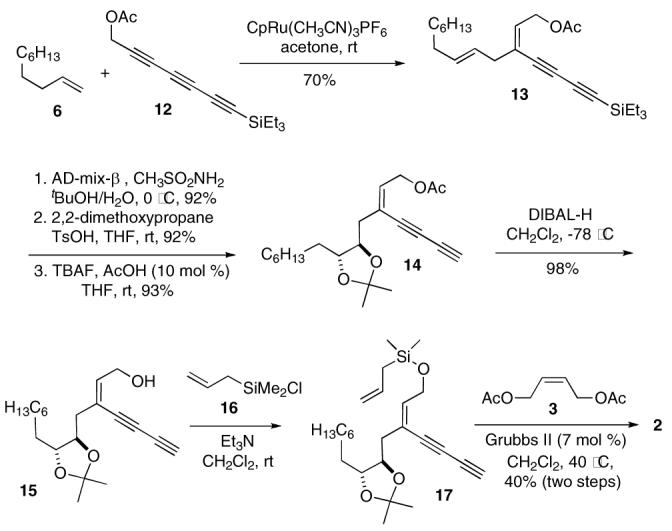
To make the synthetic sequence more convergent, the Alder ene reaction was carried out with triyne 12 and 1-decene 6, providing diyne 13 in 70% yield (Scheme 4).20 Through the standard sequence, 13 was elaborated to 15 via intermediate 14. Unfortunately, due to the facile formation of the cyclic ether in basic conditions, 21 the desired allyl ether 4 could not be prepared from 15, which, however, could be converted to the corresponding allyl silyl ether 17 under less basic conditions. Upon isolation, 17 was directly subjected to the metathesis conditions without purification due to its instability, yielding 2 in 40% overall yield. 22
Scheme 4.
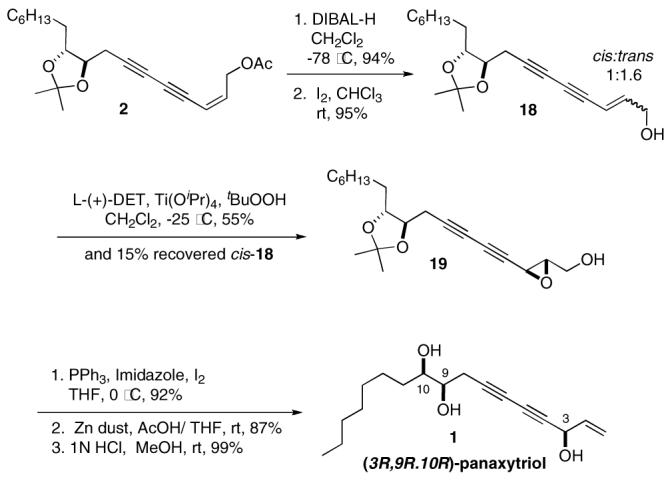
The completion of total synthesis of (3R,9R,10R)-panaxytriol 1 was achieved in 6 steps from 2 as shown in Scheme 5. Removal of the acyl group of 2 (cis : trans = 5:1) with DIBAL-H afforded allylic alcohol 18, which was converted to the required hydroxyl group at C3 through epoxidation followed by ring opening reaction. Trans-18 was founded to react much faster than the corresponding cis- isomer in the Sharpless asymmetric epoxidation (SAE) 23 leading to the formation of a 2.8:1 mixture of epoxide 19 with mostly recovered cis-18. To exploit the faster SAE reaction of trans-18, the C2–C3 double bond of 18 was isomerized with iodine,24 resulting in a 1.6:1 ratio of trans:cis isomers. The Sharpless asymmetric epoxidation of this mixture provided a 8.8:1 mixture of diasteromers 19 in 55% yield together with 15% of unreacted cis-18. The conversion of the primary alcohol to the corresponding iodoepoxide followed by its reductive ring opening with Zn dust 25 gave the (3R)-secondary allylic alcohol. Finally, deprotection of the acetonide provided (3R,9R,10R)-panaxytriol 1 the spectroscopic data of which are identical to those reported for natural 1.
Scheme 5.
In conclusion, we have developed a novel strategy for a total synthesis of (3R,9R,10R)-panaxytriol (1) based on the tandem sequence of relay metathesis–metallotropic [1,3]-shift–cross metathesis. This powerful multiple bond-forming reaction allowed an efficient synthesis of the target molecule in 15 steps with 15% overall yield, highlighting its utility for the synthesis of natural products with highly unsaturated carbon skeletons.
Supplementary Material
Acknowledgment
We thank NIH (CA106673) for financial support of this work as well as the NSF and NIH for NMR and Mass Spectrometry instrumentation.
Footnotes
Supporting Information Available General procedures and characterization data of new compounds. This material is available free of charge via the internet at http://pubs.acs.org
References
- 1.Atom-economy: Trost BM. Science. 1991;254:1471. doi: 10.1126/science.1962206. Step-economy: Trost BM. Acc. Chem. Res. 2002;35:695. doi: 10.1021/ar010068z. Wender PA, Bi FC, Gamber GG, Gosselin F, Hubbard RD, Scanio MJC, Sun R, Williams TJ, Zhang L. Pure Appl. Chem. 2002;74:25. Wender PA, Miller BL. Toward the Ideal Synthesis. Organic Synthesis: Theory and Application. 2:27–66.
- 2.(a) Ho T-L. Tandem organic reactions. Wiley; New York: 1992. [Google Scholar]; (b) Tietze LF. Chem. Rev. 1996;96:115. doi: 10.1021/cr950027e. [DOI] [PubMed] [Google Scholar]; (c) Wasilke J-C, Obrey SJ, Baker RT, Bazan GC. Chem. Rev. 2005;105:1001. doi: 10.1021/cr020018n. [DOI] [PubMed] [Google Scholar]; (d) Nicolaou KC, Edmonds DJ, Bulger PG. Angew. Chem. Int. Ed. 2006;45:7134. doi: 10.1002/anie.200601872. [DOI] [PubMed] [Google Scholar]
- 3.Kim M, Lee D. J. Am. Chem. Soc. 2005;127:18024. doi: 10.1021/ja057153c.Hansen EC, Lee D. Acc. Chem. Res. 2006;39:509. doi: 10.1021/ar050024g. For a review of metallotropic [1,3]-shift, see: Lee D, Kim M. Org. Biomol. Chem. 2007;5:3418. doi: 10.1039/b710379d.
- 4.(a) Hansen EC, Lee D. Org. Lett. 2004;6:2035. doi: 10.1021/ol049378i. [DOI] [PubMed] [Google Scholar]; (b) Hoye TR, Jeffrey CS, Tennakoon MA, Wang J, Zhao H. J. Am. Chem. Soc. 2004;126:10210. doi: 10.1021/ja046385t. [DOI] [PubMed] [Google Scholar]
- 5.A review on cross metathesis, see: Connon SJ, Blechert S. Angew. Chem., Int. Ed. 2003;42:1900. doi: 10.1002/anie.200200556.
- 6.A review of naturally occurring polyynes, see: Shi Shun ALK, Tykwinski RR. Angew. Chem. Int. Ed. 2006;45:1034. doi: 10.1002/anie.200502071.
- 7.Kitagawa I, Yoshikawa M, Yoshihara M, Hayashi T, Taniyama T. Yakugaku Zasshi. 1983;103:612. [PubMed] [Google Scholar]
- 8.Saita T, Katano M, Matsunaga H, Kouno I, Fujito H, Mori M. Bio. Pharm. Bull. 1995;18:933. doi: 10.1248/bpb.18.933. [DOI] [PubMed] [Google Scholar]
- 9.Matsunaga H, Saita T, Naguo F, Mori M, Katano M. Cancer Chemother. Pharmacol. 1995;35:291. doi: 10.1007/BF00689447. [DOI] [PubMed] [Google Scholar]
- 10.Kim JY, Lee KW, Kim SH, Wee JJ, Kim YS, Lee HJ. Planta Med. 2002;68:119. doi: 10.1055/s-2002-20240. [DOI] [PubMed] [Google Scholar]
- 11.Matsunaga H, Katano M, Yamamoto H, Mori M, Takata K. Chem. Pharm. Bull. 1989;37:1279. doi: 10.1248/cpb.37.1279. [DOI] [PubMed] [Google Scholar]
- 12.Kovayashi M, Mahmund T, Umezome T, Wang W, Murakami N, Kitagawa I. Tetrahedron. 1997;53:15691. [Google Scholar]
- 13.Total Syntheses: Lu W, Zheng G, Cai J. Synlett. 1998:737.Lu W, Zheng G, Gao D, Cai J. Tetrahedron. 1999;55:7157.Gurjar MK, Kumar VS, Rao BV. Tetrahedron. 1999;55:12563.Yadav JS, Maiti A. Tetrahedron. 2002;58:4955.Mayer SF, Steinreiber A, Orru RVA, Faber K. J. Org. Chem. 2002;67:9115. doi: 10.1021/jo020073w.Yun H, Danishefsky SJ. J. Org. Chem. 2003;68:4519. doi: 10.1021/jo0341665.Yun H, Chou T-C, Dong H, Tian Y, Li Y, Danishefsky SJ. J. Org. Chem. 2005;70:10375. doi: 10.1021/jo0515475.
- 14.Cho EJ, Lee D. J. Am. Chem. Soc. 2007;129:6692. doi: 10.1021/ja0719430. For reviews on ruthenium-catalyzed Alder ene reaction, see: Trost BM, Frederiksen MU, Rudd MT. Angew. Chem., Int. Ed. 2005;44:6630. doi: 10.1002/anie.200500136.Trost BM, Toste FD, Pinkerton AB. Chem. Rev. 2001;101:2067. doi: 10.1021/cr000666b.
- 15.Marino JP, Nguyen HN. J. Org. Chem. 2002;67:6841. doi: 10.1021/jo025745x. and Supporting Information (SI) (b) SI of ref 3a for a slightly modified reaction protocol.
- 16.The transformation of 5 and 6 to 8 was reported in ref 13a.
- 17.(a) Sharpless KB, Amberg W, Bennani YL, Crispino GA, Hartung J, Jeong K-S, Kwong H-L, Morikawa K, Wang Z-M, Xu D, Zhang X-L. J. Org. Chem. 1992;57:2768. [Google Scholar]; (b) Kolb HC, VanNieuwenhze MS, Sharpless KB. Chem. Rev. 1994;94:2483. [Google Scholar]
- 18.Scholl M, Ding S, Lee CW, Grubbs RH. Org. Lett. 1999;1:953. doi: 10.1021/ol990909q. [DOI] [PubMed] [Google Scholar]
- 19.Z-preference in cross metathesis of enynes and acrylonitrile, see: Crowe WE, Goldberg DR. J. Am. Chem. Soc. 1995;117:5162.Randl S, Gessler S, Wakamatsu H, Blechert S. Synlett. 2001:430.Kang B, Kim D.-h., Do Y, Chang S. Org. Lett. 2003;5:3041. doi: 10.1021/ol035014z.Kang B, Lee JM, Kwak J, Lee YS, Chang S. J. Org. Chem. 2004;69:7661. doi: 10.1021/jo048883q.Love JA, Morgan JP, Trnka TM, Grubbs RH. Angew. Chem. Int. Ed. 2002;41:4035. doi: 10.1002/1521-3773(20021104)41:21<4035::AID-ANIE4035>3.0.CO;2-I.
- 20.The transformation of 12 and 6 to 13 was reported previously in ref 13a.
- 21.For the addition of alkoxide to an alkyne followed by [3,3]-sigmatropic rearrangement, see: Li X, Ovaska TV. Org. Lett. 2007;9:3837. doi: 10.1021/ol701633z.Li X, Kyne RE, Ovaska TV. Org. Lett. 2006;8:5153. doi: 10.1021/ol0620848.Martinez I, Alford PE, Ovaska TV. Org. Lett. 2005;7:1133. doi: 10.1021/ol050144o.
- 22.To the best of our knowledge, this is the first example of using silyl ether as a relay tether.
- 23.(a) Katsuki T, Sharpless KB. J. Am. Chem. Soc. 1980;102:5974. [Google Scholar]; (b) Bellina F, Carpita A, Mannocci L, Rossi R. Eur. J. Org. Chem. 2004:2610. [Google Scholar]
- 24.Giacomelli G, Lardicci L, Saba A. J. C. S. Perkin I. 1978:314. [Google Scholar]
- 25.Bernet B, Vasella A. Helv. Chim. Acta. 1979;62:1990. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


