Abstract
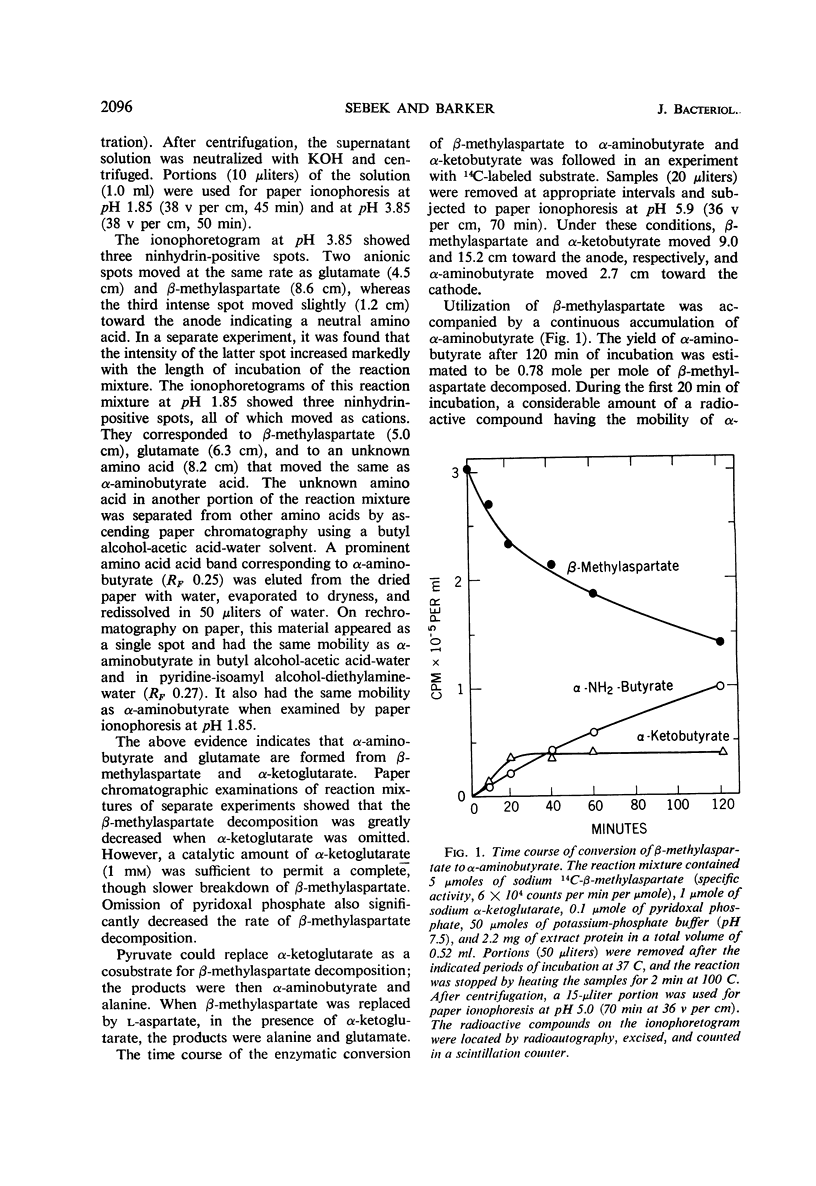
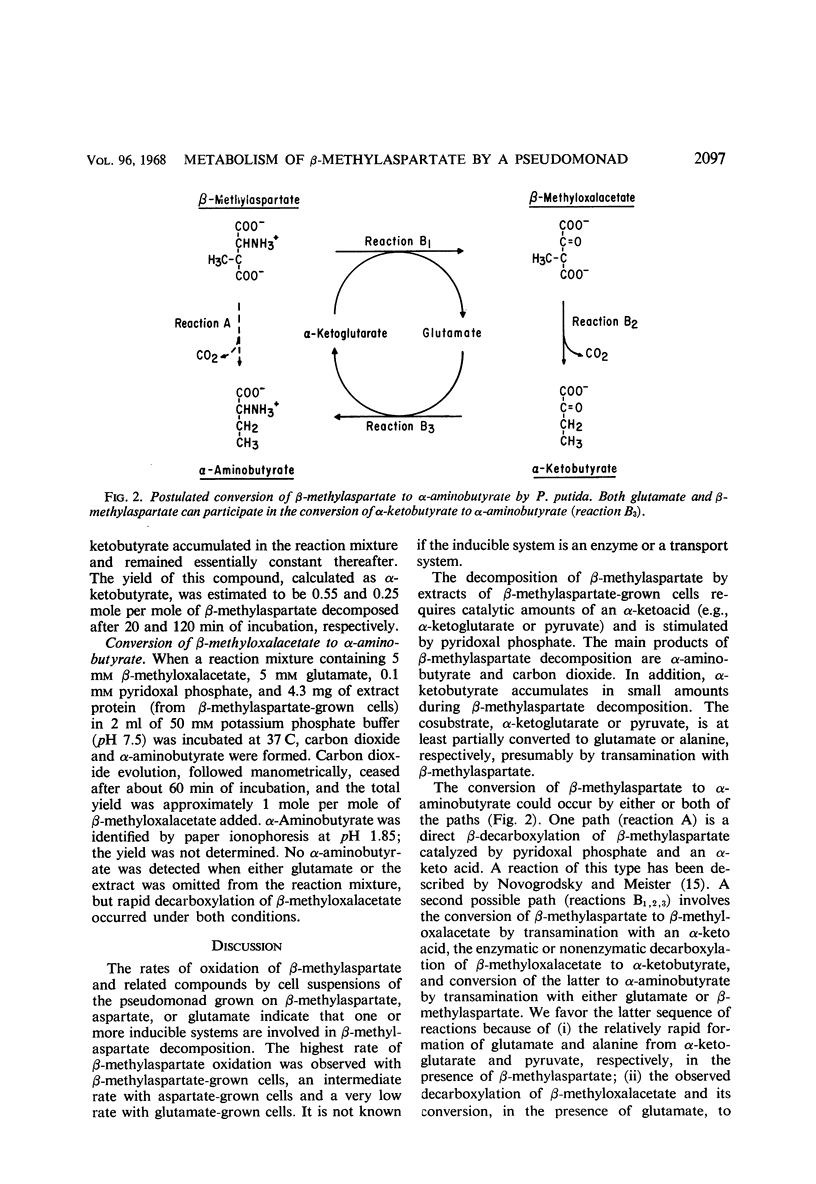
A bacterium was isolated from soil which utilizes threo-β-methyl-l-aspartate, certain other amino acids, and a variety of organic substances as single energy sources. It is, or closely resembles, Pseudomonas putida biotype B. The ability of this organism to rapidly decompose such amino acids is dependent on inducible enzyme systems. Dialyzed cell-free extracts of this bacterium metabolize β-methylaspartate only when catalytic amounts of α-ketoglutarate, or pyruvate, and pyridoxal phosphate are also present. The main products formed from β-methylaspartate under these conditions are α-aminobutyrate, carbon dioxide, and α-ketobutyrate. When l-aspartate is substituted for β-methylaspartate in this system, it is converted mainly to alanine and carbon dioxide. β-Methyloxalacetate is decarboxylated, and the resulting α-ketobutyrate is converted enzymatically in the presence of glutamate to α-aminobutyrate which accumulates. The added keto acids are converted, in part, to the corresponding amino acids probably by transamination. The data indicate that β-methylaspartate is converted to α-aminobutyrate, and aspartate to alanine, by a circuitous transamination-β-decarboxylation-transamination sequence rather than by a direct β-decarboxylation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ABRAMSKY T., SHEMIN D. THE FORMATION OF ISOLEUCINE FROM BETA-METHYLASPARTIC ACID IN ESCHERICHIA COLI W. J Biol Chem. 1965 Jul;240:2971–2975. [PubMed] [Google Scholar]
- ATFIELD G. N., MORRIS C. J. Analytical separations by highvoltage paper electrophoresis. Amino acids in protein hydrolysates. Biochem J. 1961 Dec;81:606–614. doi: 10.1042/bj0810606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARKER H. A., ROOZE V., SUZUKI F., IODICE A. A. THE GLUTAMATE MUTASE SYSTEM. ASSAYS AND PROPERTIES. J Biol Chem. 1964 Oct;239:3260–3266. [PubMed] [Google Scholar]
- BARKER H. A., SMYTH R. D., WAWSZKIEWICZ E. J., LEE M. N., WILSON R. M. Enzymic preparation and characterization of an alpha-L-beta-methylaspartic acid. Arch Biochem Biophys. 1958 Dec;78(2):468–476. doi: 10.1016/0003-9861(58)90371-0. [DOI] [PubMed] [Google Scholar]
- BARKER H. A., SMYTH R. D., WILSON R. M., WEISSBACH H. The purification and properties of beta-methylaspartase. J Biol Chem. 1959 Feb;234(2):320–328. [PubMed] [Google Scholar]
- BARKER H. A. Structure and function of cobamide coenzymes. Fed Proc. 1961 Dec;20:956–961. [PubMed] [Google Scholar]
- Barker H. A. Biochemical functions of corrinoid compounds. The sixth Hopkins memorial lecture. Biochem J. 1967 Oct;105(1):1–15. doi: 10.1042/bj1050001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MARDASHEW S. R. The metabolism of beta-methylaspartic acid in brain and liver. Clin Chim Acta. 1961 Mar;6:157–162. doi: 10.1016/0009-8981(61)90079-1. [DOI] [PubMed] [Google Scholar]
- NOVOGRODSKY A., MEISTER A. CONTROL OF ASPARTATE BETA-DECARBOXYLASE ACTIVITY BY TRANSAMINATION. J Biol Chem. 1964 Mar;239:879–888. [PubMed] [Google Scholar]
- Stanier R. Y., Palleroni N. J., Doudoroff M. The aerobic pseudomonads: a taxonomic study. J Gen Microbiol. 1966 May;43(2):159–271. doi: 10.1099/00221287-43-2-159. [DOI] [PubMed] [Google Scholar]



