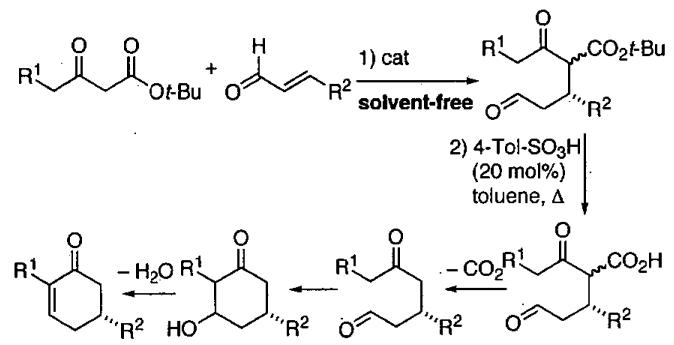
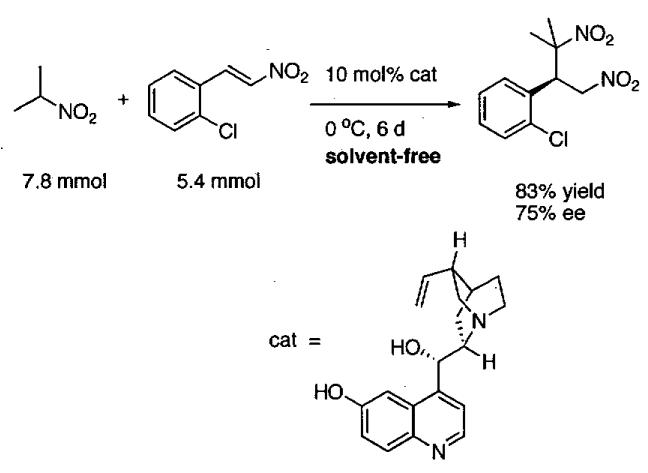
1 Introduction and Scope
In an age when organic chemists have shown that even the most complex natural and unnatural products can be synthesized,1-3 the emphasis of synthetic chemistry is shifting to how they can be assembled in a truly practical fashion.4-6 A pressing challenge facing organic chemists, therefore, is to advance new processes that are not only efficient, selective, and high yielding, but that are also environmentally friendly.7,8 Historically, the metric of reaction success has been the yield. Although yields will remain imperative, alternative measures are the ‘greenness’ of a reaction, or E factor,9 and the volume productivity.10 The E factor, introduced by Sheldon,9 is defined as the ratio of weight waste to weight product while the volume productivity is the grams product per liter of reaction medium. The E factor for many pharmaceuticals has been estimated to exceed 100.11,12 The largest contributors to the magnitude of E factor are organic solvents, many of which are ecologically harmful and require expensive remediation.
As global environmental legislation becomes stricter, consideration of environmental concerns must be addressed at early stages of product development. By doing so, project cost reduction of 50% or more have been realized.13 Although steps toward sustainability can be made by reusing solvents, rarely is recycling accomplished with complete efficiency. An alternative strategy to reduce the E factor of reactions and their impact on the environment is to conduct them under solvent-free conditions.11,14-16 Among the benefits of solvent-free processes are cost savings, decreased energy consumption, reduced reaction times, and a large reduction in reactor size and capital investment. As a result, introduction of solvent-free reactions, including solvent-free polymerizations,14,17-20 radical additions,14,21-24 ionic reactions,25-31 solid state reactions15,32-34 and photochemical reactions15,35-37 has increased steadily in recent years. Solvent-free catalytic enantioselective reactions, however, have received much less attention.
The paucity of catalytic asymmetric solvent-free reactions is not unexpected. Catalyst efficiency and enantioselectivity is frequently highly sensitive to the nature of the solvent.38 Examples in which a catalyst can generate enantiomeric products with high levels of enantioselectivity in different solvents have been reported.39 Moreover, enantioselectivities are often dependent on catalyst concentrations. Consequently, two of the most important variables for catalyst optimization, solvent properties and concentration, are eliminated under solvent-free conditions. Furthermore, under solvent-free conditions the reaction medium will change as reagents and substrates are converted to products. The impact of such changes is currently unpredictable, complicating reaction development. We believe that these limitations have dissuaded many investigators from considering solvent-free catalytic asymmetric reactions. The potential environmental benefits and the economic incentive, however, have created significant demand for such processes.
In response to these challenges, an increasing number of research groups are developing asymmetric catalysts for use under solvent-free or highly concentrated reaction conditions. The results of these studies have been mixed. In some cases, catalysts that demonstrate excellent enantioselectivity and activity under standard solvent conditions exhibit lower selectivity in the absence of solvent. In contrast, other catalysts react with excellent levels of enantioselectivity and greatly increased activity, enabling significant reductions in catalyst loading under solvent-free conditions.
The potential advantages of asymmetric catalysis under solvent-free and highly concentrated reaction conditions have inspired this review. In turn, it is hoped that a comprehensive compilation of reports in this area will stimulate further investigations into solvent-free catalytic asymmetric reactions.
A significant body of research has been published concerning solvent-free organic synthesis and the subject has been reviewed.10,11,14,15,40 Solvent-free asymmetric catalysis, however, is a relatively new area and we are not aware of reviews devoted to this topic. This review will cover small molecule catalyzed enantioselective reactions under solvent-free or highly concentrated reaction conditions up to the end of 2006. Additionally, we will include reactions of enantioenriched substrates with enantioselective catalysts in which the diastereoselectivity is catalyst controlled (not substrate controlled). We define solvent-free reactions as those employing less than five equivalents of one reagent with respect to the substrate. The definition of highly concentrated reaction conditions used herein is those that utilize less than 5 equivalents of solvent with respect to the substrate.
Despite amazing advances in chemistry database coverage, technology, and search engines, there are no straightforward ways to search the literature for ‘highly concentrated catalytic asymmetric reactions’. Even reactions performed in the absence of solvent do not always contain the key words ‘solvent-free.’ The citations included herein come from extensive literature searches, scouring experimental sections and supporting information, and personal knowledge. Nonetheless, our apologies are extended to those who have made contributions in this area but whose work we missed.
2 Solvent-Free and Highly Concentrated Reactions in Asymmetric Catalysis
2.1 Epoxide Opening Reactions
The catalytic asymmetric ring opening (ARO) of epoxides by nucleophiles can provide access to a variety of functionalized chiral building blocks that are of enormous utility in enantioselective organic synthesis.41-45 In several cases, epoxide opening reactions are also exemplary environmentally friendly processes in which all or nearly all of the atoms in the starting materials are present in the products, therefore, little or no waste is generated. Such processes have been classified as highly atom economical.46,47
The Jacobsen group has been actively investigating transition metal (salen)M(III)-based catalysts for the desymmetrization of meso epoxides and the kinetic resolution of racemic epoxides via ARO reactions.42 An important feature of (salen)M(III) catalysts is that a wide variety of complexes with diverse substitution patterns are easily synthesized, facilitating catalyst screening and reaction optimization.
The mechanisms of (salen)M catalyzed epoxide opening reactions have also been studied in detail and have provided significant insight into the operation of these highly efficient and enantioselective catalysts. The mechanistic understanding garnered by the Jacobsen group has allowed expedient optimization of both catalyst activity and enantioselectivity in epoxide opening reactions. Most of the mechanistic studies have been conducted in common solvents, and it is assumed that the reaction mechanisms do not change significantly under solvent-free conditions.42,48-53
2.1.1 Desymmetrization of meso-Epoxides
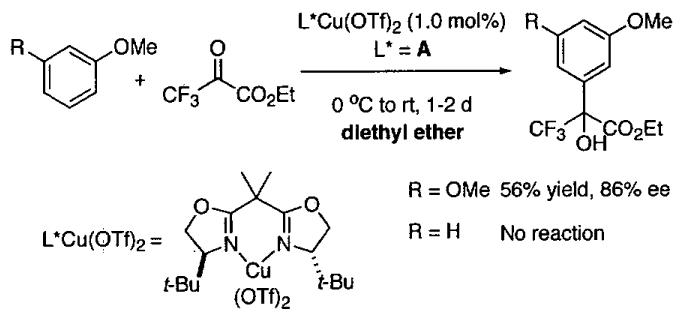
In pioneering studies, Jacobsen and coworkers reported the (salen)Cr(III) catalyzed opening of meso epoxides and the kinetic resolution of racemic terminal epoxides with trimethylsilyl azide.49,51,54-67 The tetra-tert-Bu derived (salen)M complexes with the ligand illustrated in Figure 1 have proven so useful in solvent-free asymmetric catalysis68 that (salen)M will refer to this ligand structure throughout this review unless otherwise mentioned.
Figure 1.
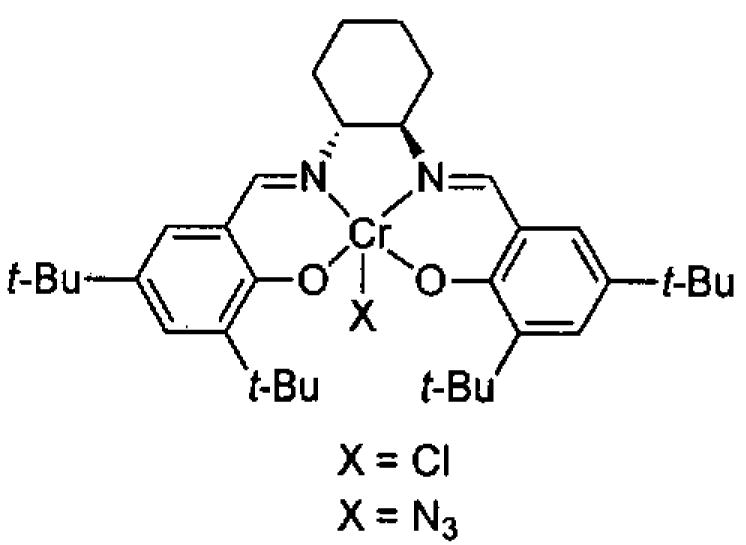
Jacobsen's (salen)CrCl precatalyst and (salen)CrN3 catalyst for epoxide opening with trimethylsilyl azide. In this review, (salen)M will refer to a metal bound to this salen ligand unless otherwise stated.
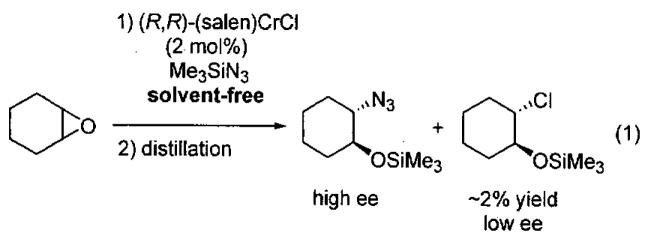
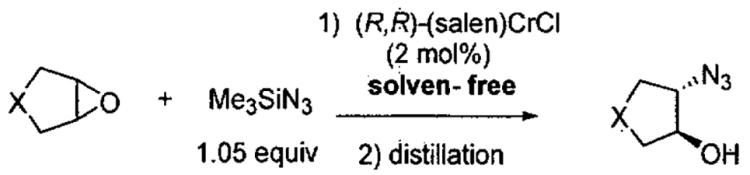
Initial studies of ARO of meso epoxides with (salen)CrCl precatalyst (Figure 1) were conducted in diethyl ether solvent.54 A few representative examples are shown in Table 1 that demonstrate both the enantioselectivity and overall efficiency of this process. It was also reported that reactions could be conducted in the absence of solvent (Table 2).54 Under solvent-free conditions, cyclic 5 and 6-membered epoxides underwent ring opening with 2 mol% (salen)CrCl and only a slight excess of Me3SiN3. After complete consumption of the epoxide, the reaction mixture was distilled under reduced pressure providing the ring opened product in high purity. The catalyst can be easily recovered and reused for several cycles without loss of activity. For example, Table 2 illustrates the use of a single catalyst dose in three cycles with cyclohexene oxide (cycles 1-3). In cycles four and five, cyclopentene oxide and 4,5-epoxycyclohexene were employed as substrates. In each case, isolated yields were ≥ 75% and enantioselectivities rivaled those outlined in reactions conducted with diethyl ether as solvent (Table 1).
Table 1.
ARO of meso epoxides in diethyl ether with (R,R)-(salen)CrCl (Figure 1) as precatalyst.
 | ||||
|---|---|---|---|---|
| entry | X | time(h) | isolated yield (%) |
ee(%) |
| 1 | CH2CH2 | 18 | 80 | 88 |
| 2 | CH2 | 18 | 80 | 94 |
| 3 | CH=CH | 46 | 72 | 81 |
Table 2.
ARO of meso epoxides under solvent-free conditions with (salen)CrCl (Figure 1) as precatalyst illustrating catalyst recyclability.
 | ||||
|---|---|---|---|---|
| cycle | X | time(h) | isolated yield (%) |
ee(%) |
| 1 | CH2CH2 | 18 | 86 | 84 |
| 2 | CH2CH2 | 21 | 88 | 87 |
| 3 | CH2CH2 | 20 | 91 | 88 |
| 4 | CH2 | 4 | 81 | 94 |
| 5 | CH=CH | 18 | 75 | 83 |
2.1.2 Mechanistic Investigations and Implications for Solvent-Free Epoxide Opening
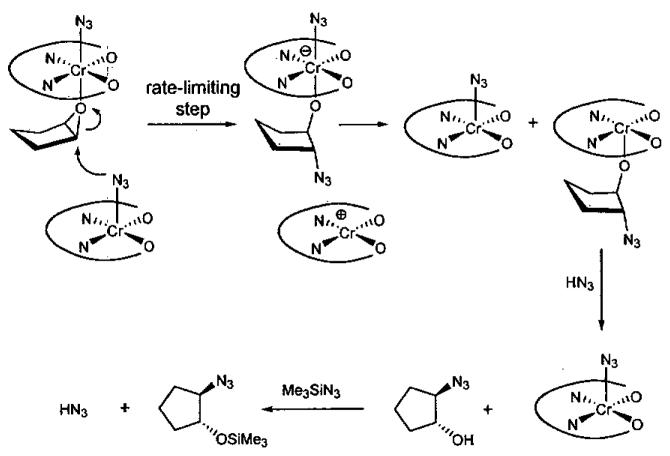
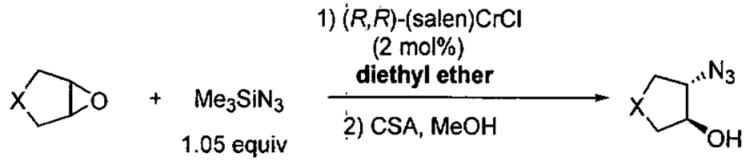
Insight into the mechanism of ARO with (salen)CrCl was first gained through product analysis,54 where it was found that a chlorohydrin byproduct was generated in the initial stages of the reaction from epoxide opening by the chromium-bound chloride (Eq 1). Elemental analysis of the recovered catalyst after completion of the reaction indicated the absence of chloride and a ratio of Cr : N of 1 : 5. The IR spectrum of the recovered catalyst contained a strong stretch at 2058 cm−1, consistent with the formation a chromium azide, Cr-N3. In support of this proposal, (salen)Cr(N3)(THF) has been isolated and characterized crystallographically.51 Spectroscopic data support (salen)Cr(N3)(epoxide) as the resting state of the active catalyst in the ARO, hinting that the catalyst activates the epoxide as well as the azide. Also of note, the reaction does not proceed in the absence of trace water, which is necessary to generate HN3 from hydrolysis of Me3SiN3.
The dual role of the catalyst was confirmed by kinetic analysis, which indicated a second order catalyst dependency, inverse order in epoxide, and zero order in azide. The key steps of the proposed mechanism are illustrated in Figure 2.
Figure 2.
Key steps in the proposed mechanism of the ARO illustrating the bifunctional nature of the (salen)CrN3 catalyst.
The second order dependence on (salen)CrN3 concentration has important practical implications. For example, decreasing the catalyst loading by 10-fold is predicted to result in a 100-fold decrease in the rate of the reaction. This drawback can be partially offset under solvent-free conditions, where the concentrations of the reagents and catalysts are maximized.
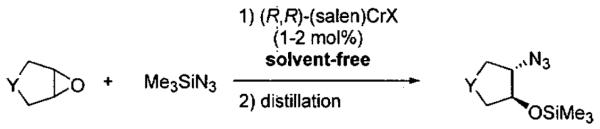
Guided by the results of the mechanistic studies, Jacobsen and coworkers proceeded to optimize the ARO reaction. To eliminate the chlorohydrin byproduct (Eq 1) formed in conversion of the precatalyst (salen)CrCl to the catalyst (salen)CrN3, (salen)CrN3 was prepared synthetically.55 A comparison of product ee from (salen)CrCl and (salen)CrN3 was conducted under solvent-free conditions.69 Direct application of (salen)CrN3 resulted in slightly higher product ee's and yields than use of the precatalyst (salen)CrCl (Table 3). Also noteworthy is that these reactions were performed using 25–50 mmol of epoxide, and generated important synthetic intermediates.
Table 3.
Comparison of (salen)CrCl and (salen)CrN3 in the solvent-free asymmetric opening of meso epoxides.
 | |||
|---|---|---|---|
| % ee (% yield)a |
|||
| entry | Y | (salen)CrCl | (salen)CrN3 |
| 1 | CH2 | 93 (97) | 94 (99) |
| 2 | (CH2)2 | 85(96) | 88 (99) |
| 3 | O | 97 (96) | 97 (99) |
| 4 | NCOCF3 | 95 (87) | 95 (96) |
Scale: 25 - 50 mmol epoxide.
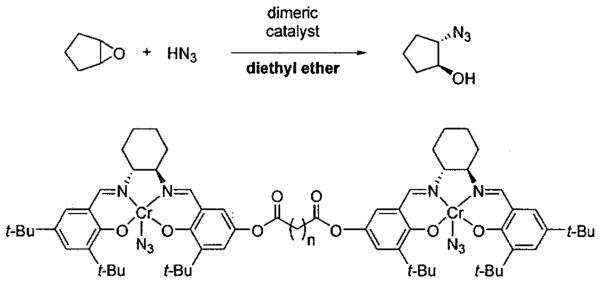
To overcome the second order catalyst dependency of the ARO, tethered dimeric catalysts were used in diethyl ether to enforce the cooperative catalyst behavior (Table 4).48 A kinetic study was performed and analysis of the rate data indicated that both intra-and intermolecular epoxide opening pathways were in operation, and their ratio was dependent on the tether length. Dimers with n = 2 and 4 proceeded largely through intermolecular pathways, while catalyst with n = 5 exhibited the greatest intramolecular rate (Table 4). As the tether length is further increased, the ratio of intramolecular to intermolecular pathway decreases, presumably due to the increase in entropic cost with longer tethers.48
Table 4.
Dimeric catalysts with tether lengths between n = 2 to 10 used in the ARO of cyclopentene oxide. The rate constant kintra is for the cooperative intramolecular pathway.
 | |||
|---|---|---|---|
| n | ee (%) | kintra (min−1 × 10−2) | kintra (M−1 min−1) |
| 2 | 90 | 4.4 | 15.7 |
| 4 | 90 | 5.4 | 15.1 |
| 5 | 93 | 42.9 | 27.4 |
| 6 | 93 | 31.7 | 15.8 |
| 7 | 93 | 20.9 | 7.9 |
| 8 | 94 | 14.7 | 10.5 |
| 10 | 92 | 3.8 | 4.4 |
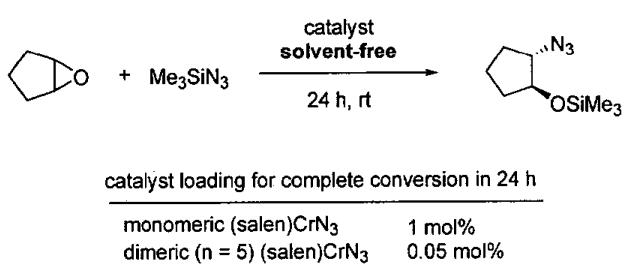
Based on these results, dimeric catalyst with n = 5 was compared to monomeric analogs in the solvent-free ARO (Figure 3). At 0.05 mol%, the dimeric catalyst promoted the ARO of cyclopentene oxide with Me3SiN3 in 24 h at room temperature, whereas the monomeric catalyst required a loading of 1 mol% to effect complete conversion under the same conditions.
Figure 3.
Comparison of catalyst loading with monomeric (salen)CrN3 and dimeric (n = 5) (salen)CrN3 (from Table 4).
The ARO of meso epoxides with trimethylsilyl azide is an important example of the design of environmentally friendly catalysts and reaction conditions. Not only can the reactions be conducted under solvent-free conditions, the catalyst can be recycled and the products isolated by distillation. Furthermore, the highly enantioenriched products are very useful in the synthesis of biologically active molecules.44,55,70-72
2.1.3 ARO of meso Epoxides with Benzoic Acid
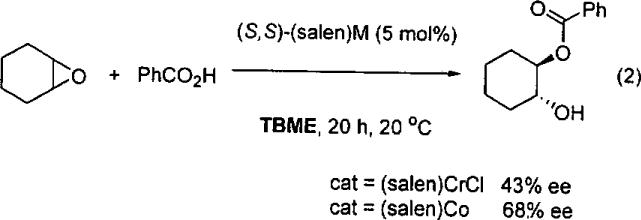
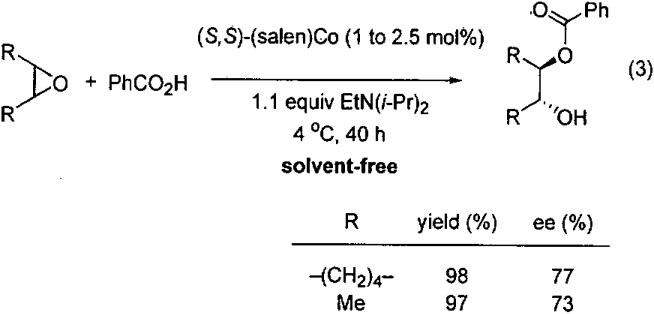
In search of catalysts to promote the ARO of meso epoxides with different nucleophiles, the Jacobsen group screened first row (salen)M complexes in the presence of benzoic acid.58 It was found that the (salen)CrCl complex (5 mol%) promoted the ARO with 43% ee. In contrast, the cobalt analog, (salen)Co(II), promoted the ARO to give the ester product in 68% ee (Eq 2). When the reaction was conducted in the presence of EtN(i-Pr)2 the product ee increased, most likely due to the greater solubility of the benzoic acid in the tert-butyl methyl ether (TBME) in the presence of the amine.
The (salen)Co(II) complex was then employed in the solvent-free ARO with benzoic acid (Eq 3). ARO with cyclohexene oxide was conducted on multigram scale in the presence of 1 mol% catalyst, providing the product with 77% ee and 98% yield. While the product ee was moderate, a single recrystallization afforded the product with 98% ee in 75% isolated yield. Use of cis-2-butene oxide as substrate under solvent-free conditions furnished the product with 73% ee in 97% yield.
The products of the ARO of meso epoxides with benzoic acid can be readily hydrolyzed to chiral diols, which are useful ligands in asymmetric catalysis and valuable building blocks in synthesis. The active catalyst in this reaction, as well as others introduced in the next sections, is generated by oxidation of the (salen)Co(II) to (salen)Co(III) by oxygen.
While the ARO of meso epoxides represents elegant research that formed the basis of several other synthetically important reactions, it is limited to meso epoxides. These substrates form a relatively small subset of epoxides. As will be outlined in the next sections, the kinetic resolution of epoxides enables the isolation of a broader range of chiral building blocks with high ee.
2.2 Reactions with Racemic Epoxides
2.2.1 Kinetic Resolution of Epoxides with Me3SiN3
The stereospecific nucleophilic ring opening of enantioenriched epoxides allows access to a wealth of chiral building blocks. As such, much effort has been invested in the direct enantioselective synthesis of epoxides and considerable progress has been made.73-78 Nonetheless, certain classes of epoxides remain difficult to prepare enantioselectively. Highly enantioenriched terminal epoxides, which are probably the most synthetically challenging, are also among the most useful due to the high stereoselectivity with which they undergo nucleophilic ring opening.
Difficulties with direct enantioselective methods for the synthesis of epoxides have led researchers to examine methods for kinetic resolution of these substrates. The well-known drawback of resolution is that the maximum yield is 50%. This disadvantage, however, can be relatively minor when compared to the benefits of kinetic resolutions.79-81 If the desired compound is the resolved starting material, it is relatively easy to control its ee simply by allowing reaction conversion to exceed 50%. Even in cases where the catalyst is only moderately enantioselective, starting material of high enantiopurity can be obtained. For example, if the relative rate of the fast to slow reacting substrate (krel = kfast/kslow) is 10, the slower reacting starting material can be recovered in 95% ee and up to 34% yield. Of course, if krel is higher, the reaction can be quenched at lower conversion, providing greater yield of the recovered starting material.
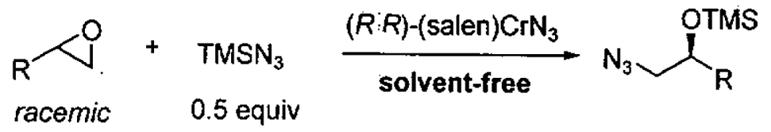
Initial studies by the Jacobsen group in the kinetic resolution of terminal epoxides employed (salen)CrN3.54 Thus, reaction of racemic propylene oxide with 0.5 equivalents of Me3SiN3 in the presence of 1 mol% (R,R)-(salen)CrN3 at 0 °C in the absence of solvent gave a mixture of the azide product and propylene oxide. Removal of the volatile epoxide and distillation of the product furnished the azidoalcohol in near quantitative yield with 97% ee (Table 5, entry 1). The relative rate of the fast reacting enantiomer to the slow reacting enantiomer (krel) for the resolution of propylene oxide was determined to be > 200. As outlined in Table 5, other substrates also exhibited very high krel values (44 - 280) and the ring opened products could be isolated with excellent ee's.56
Table 5.
Kinetic resolution of racemic terminal epoxides with Me3SiN3 catalyzed by (R,R)-(salen)CrN3 under solvent-free conditions.
 | |||||
|---|---|---|---|---|---|
| entry | R | cat. mol% | yield (%)b | ee (%) | krel |
| 1 | CH3 | 1.0 | 98 | 97 | 230 |
| 2 | CH2CH3 | 2.0 | 83 | 97 | 140 |
| 3 | (CH2)3CH3 | 2.0 | 89 | 97 | 160 |
| 4 | CH2OTBDMS | 3.0 | 96 | 96 | 150 |
| 5 | CH2O(1-naphthyl) | 5.0 | 74 | 93 | 48 |
| 6 | CH2C6H5 | 2.0 | 94 | 93 | 71 |
| 7 | c-C6H11 | 2.0 | 84c | 97 | 140 |
| 8 | (CH2)2CH=CH2 | 2.0 | 94 | 98 | 280 |
| 9 | CH(OEt)2 | 2.0 | 96 | 89 | 44 |
| 10 | CH2CN | 2.0 | 80 | 92 | 45 |
Reactions were run without solvent for 18-50 h at 0-2 °C.
Isolated yield of the azido sily ether based on TMSN3.
Isolated yield of the azido alcohol.
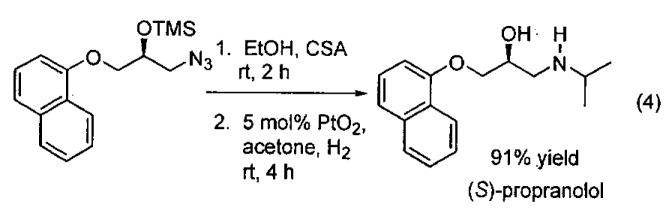
The azidoalcohols synthesized in the KR reaction are very useful chiral building blocks for the synthesis of biologically important molecules, as exemplified by the synthesis of (S)-propranolol, a widely used anti-hypertensive agent (Eq 4), and the synthesis of an enantioenriched amino alcohol used in the synthesis of (R)-9-[2-(phosphonomethoxy)propyl]adenine (PMPA), which displays prophylactic activity against SIV infections (Figure 4).56
Figure 4.
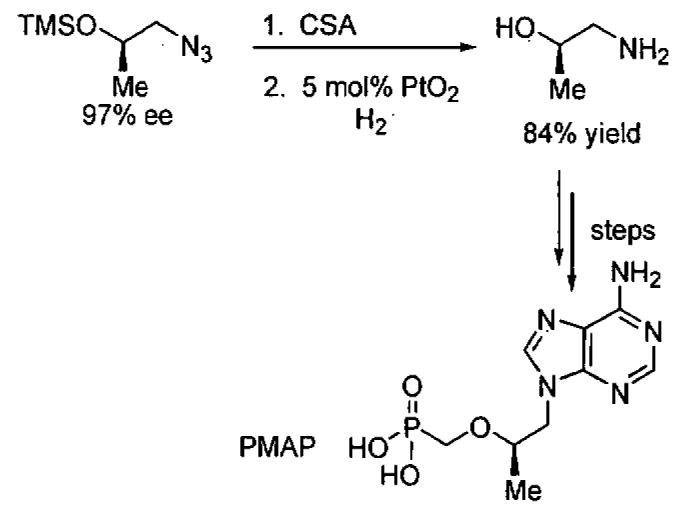
Application of enantioenriched azidoalcohol to the synthesis of (R)-9-[2-(phosphonomethoxy) propyl]adenine (PMPA).
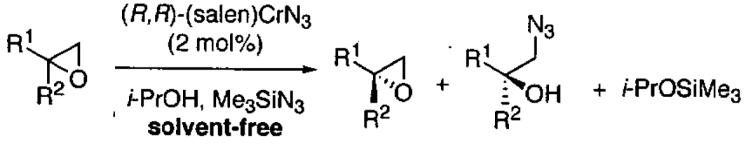
Terminal epoxides are excellent substrates for kinetic resolution reactions employing (salen)M-based catalysts. In contrast, chiral 2,2-disubstituted epoxides are less reactive and fail to undergo ARO with (salen)Co-based catalysts. These more sterically hindered epoxides do react with azide in the presence of (salen)CrN3. Most of the substrates examined exhibited excellent relative rates and afforded product in ≥ 97% ee and ≥ 42% yield when TBME was employed as solvent (not shown).82 Certain substrates, however, gave better results when ARO was conducted under solvent-free conditions (Table 6).
Table 6.
Kinetic resolution of racemic 2,2-disubstituted epoxides under solvent-free conditions.
 | ||||
|---|---|---|---|---|
| Me3SIN3 (equiv) |
i-PrOH (equiv) |
yield (%) | ee (%) | epoxide product |
| 0.55 | 0.55 | 46 | 85 |  |
| 0.70 | 0.70 | 48 | 80 |  |
2.2.2 Dynamic Kinetic Resolution of Epichlorohydrin
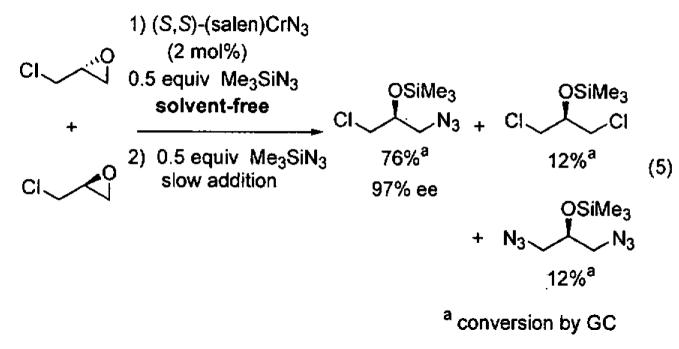
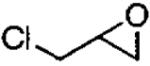
Kinetic resolutions, such as those outlined above, can be very effective for the preparation of organic compounds, particularly if the starting materials are readily available, krel is high, and the resolved starting material and product are easily separated.79 Dynamic kinetic resolutions (DKR)83,84 are more desirable, however, because it is possible to obtain highly enantioenriched product in significantly greater than the maximum yield in a kinetic resolution (50%). A rare example of a solvent-free DKR is the ARO of racemic epichlorohydrin, which provided the densely functionalized azidoalcohol in 76% yield and 97% ee after optimization (Eq 5).57 In the initial investigations of this reaction, full conversion to the azidoalcohol was observed, but the ee was low (40%). It was hypothesized that ARO of the slow reacting epichlorohydrin enantiomer was competitive with its racemization. To increase the rate of racemization relative to ARO, the Me3SiN3 was added in two portions. The first 0.5 equivalents was combined with the epichlorohydrin to initiate the ARO. After the first 0.5 equivalents of Me3SiN3 had reacted, most of the slow reacting epoxide remained. The second dose of Me3SiN3 was added slowly over 16 h to reduce the rate of the ARO with respect to the racemization.
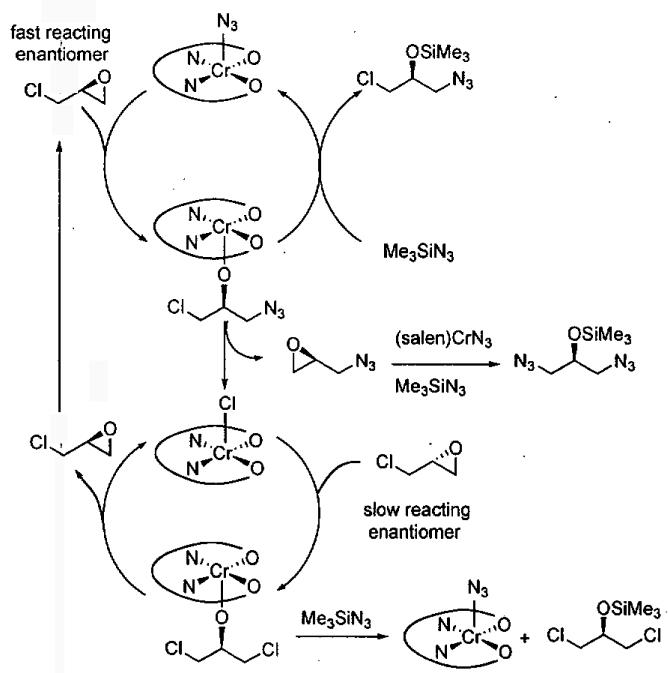
Analysis of the reaction products provided insight into the DKR (Eq 5). A mechanism that accounts for the formation of the 1,3-dichloro and 1,3-diazido byproducts is illustrated in Figure 5. It has been shown that the ARO with (salen)CrN3 results in the formation of an alkoxide intermediate (upper cycle).48 This unsymmetrical alkoxide can react with Me3SiN3 to produce the desired azidoalcohol and regenerate the catalyst via the upper cycle. It can also undergo ring closure to generate the azidoepoxide and (salen)CrCl. Ring opening of the azidoepoxide by (salen)CrN3 leads to the 1,3-diazide byproduct. The (salen)CrCl initiates the racemization of epichlorohydrin in the lower cycle. Ring opening of epichlorohydrin by (salen)CrCl generates a 1,3-dichloro alkoxide intermediate that can close to give either enantiomer of epichlorohydrin or undergo reaction with Me3SiN3 to regenerate (salen)CrN3 and the 1,3-dichloro product. As outlined in the proposed mechanism, the formation of the byproducts is intimately tied to the DKR process and has been difficult to suppress while maintaining high ee.57
Figure 5.
Proposed mechanism for the dynamic kinetic resolution of epichlorohydrin.
The densely functionalized product of the DKR is a useful chiral building block for the synthesis of enantioenriched biologically active compounds, such as U-100592, a promising antibacterial.57
2.2.3 Hydrolytic Kinetic Resolution of Epoxides
The kinetic resolution of terminal epoxides with (salen)CrN3 and Me3SiN3 is an excellent method to prepare useful enantioenriched 1-azido-2-alcohols, however, it has several drawbacks. One disadvantage is that it is difficult to prepare resolved epoxides with this method. Also, Me3SiN3 is too costly to make the epoxide resolution economically feasible for all but high value epoxides. Finally, there is some concern about the thermal stability of the azidoalcohol byproduct, especially on large-scale resolutions. For these reasons, the search for more practical methods for the solvent-free and highly concentrated kinetic resolution of epoxides was undertaken.
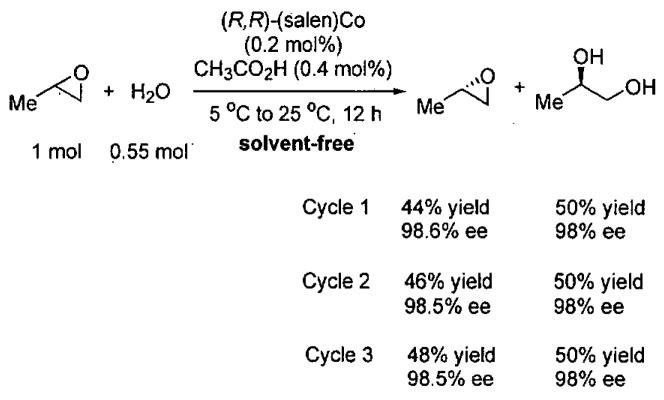
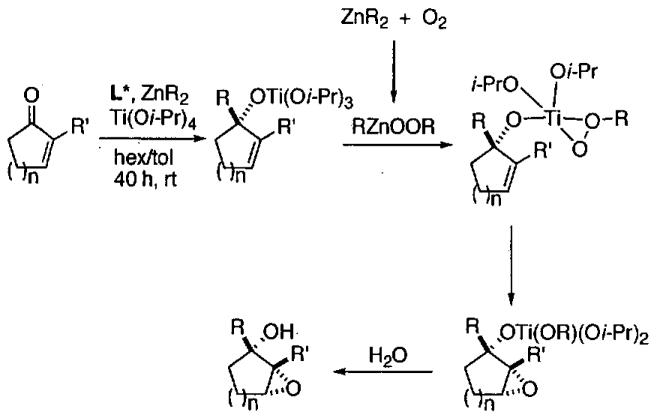
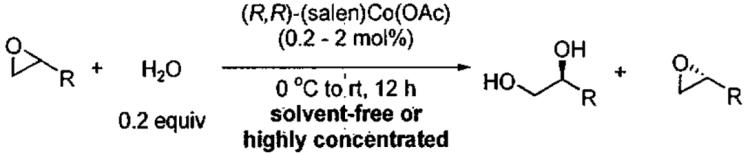
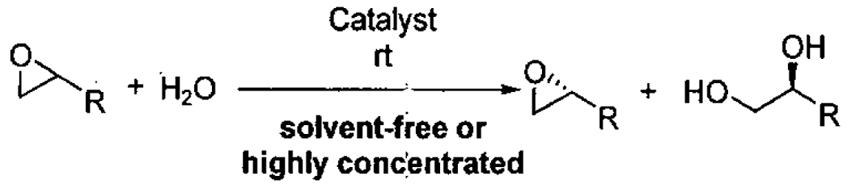
In 1997, Jacobsen and co-workers reported a major break through in the isolation of terminal epoxides with high enantioselectivity. It was found that water could serve as a nucleophile in the presence of catalytic (salen)Co(OAc) to open epoxides (Figure 6), giving rise to the hydrolytic kinetic resolution (HKR).59
Figure 6.
HKR of propylene oxide illustrating catalyst recycling.
The HKR provides direct access to both unreacted epoxide and 1,2-diol products in high enantiomeric excess and yield. Many HKR processes are conducted under solvent-free conditions by combining the catalyst with slightly more than 0.5 equivalents of water if the epoxide is the desired product. If the diol is desired, just under 0.5 equivalents of water is needed. Due to the low water solubility of epoxides with hydrophobic substituents, many reactions are conducted by combining the epoxide with a suitable solvent (1 : 1 v/v) for the HKR. Under these conditions, a minimal amount of solvent is employed and the reaction mixture is highly concentrated.
As an example of the HKR, the resolution of propylene oxide is outlined in Figure 6.59 Preformed (salen)Co(OAc) (0.2 mol%) and propylene oxide were combined, cooled to 0 °C and water added. The reaction was stirred for 14 h at room temperature, and then the reaction mixture was distilled. The volatile epoxide was distilled at atmospheric pressure and the diol at 65 °C and 0.25 torr, allowing facile separation of the mixture. The HKR of propylene oxide represents a special case, because both the epoxide and diol product are relatively volatile and can be distilled from the catalyst. The remaining catalyst residue is reactivated by addition of acetic acid and can be recycled without loss of activity or enantioselectivity for three cycles (Figure 6). It is also noteworthy that the HKR of propylene oxide has been performed on large scale (> 200 kg).42
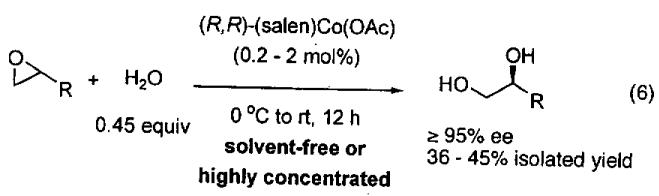
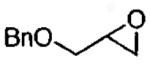
Many terminal epoxides have been tested in HKR and the reaction has been found to be incredibly general and tolerant of functionality. The results of extensive studies will be succinctly summarized here. Table 7 contains krel values. Recovered resolved epoxide ee's and yields are given in the text. Aliphatic epoxides with small to sterically demanding substituents (Me to t-Bu) are excellent substrates for HKR (Table 7, enties 1 - 7). The resolved epoxides could be isolated in 82–92% of the theoretical yield (41–46% yield based on racemic epoxide) in ≥ 99% ee. While some substrates underwent HKR under solvent-free conditions, lipophilic epoxides with low solubility in water gave better results with a small amount of solvent (highly concentrated conditions).66
Table 7.
Measured krel values for a series of epoxides under solvent-free and highly concentrated reaction conditions.
 | ||||
|---|---|---|---|---|
| entry | epoxide substituent | conv. (%)c | diol ee (%) | krel |
| Aliphatic Epoxides | ||||
| 1a | CH3 | 19 | 99.5 | 500 |
| 2a | (CH2)3CH3 | 19 | 99.2 | 310 |
| 3b | (CH2)11CH3 | 18 | 99.5 | 490 |
| 4b | (CH2)2CH=CH2 | 20 | 99.4 | 420 |
| 5b | CH2Ph | 20 | 97.4 | 96 |
| 6b | c-C6H11 | 19 | 99.6 | 630 |
| 7b | t-C4H9 | 16 | 97.0 | 79 |
| Halogenated Epoxides | ||||
| 8b | CH2Cl | 20 | 98.7 | 190 |
| 9b | CH2Br | 20 | 96 | 49 |
| 10a | CH2F | 17 | 98 | 120 |
| 11a | CF3 | 18 | 99.6 | 620 |
| Epoxides Bearing Ether and Carbonyl Functionality | ||||
| 12b | CH2OBn | 20 | 97 | 83 |
| 13b | CH2OTBS | 18 | 99 | 250 |
| 14b | CH2OPh | 18 | 98 | 120 |
| 15b | CH2O(1-naphthyl) | 20 | 99 | 250 |
| 16b | CH2CH2OBn | 19 | 97 | 82 |
| 17b | oxiranyld | 20 | 98 | 130 |
| 18b | CH2OCOn-C3H7 | 54 | 99.4 | 68 |
| 19b | CH2CO2Et | 20 | 98 | 130 |
| 20b | CH2NHBoc | 18 | 74 | 7.8 |
| 21b | CO2CH3 | 19 | 98 | 120 |
| 22b | COCH3 | 18 | 97 | 81 |
| 23b | COCH2CH3 | 18 | 96 | 60 |
| Aryl, Vinyl, and Alkynyl Epoxides | ||||
| 24b | C6H5 | 20 | 98 | 130 |
| 25b | 4-ClC6H4 | 18 | 97 | 81 |
| 26b | 3-ClC6H4 | 17 | 98 | 120 |
| 27b | 3-(CH3O)C6H4 | 19 | 98 | 120 |
| 28b | 3-(NO2)C6H4 | 19 | 99 | 280 |
| 29b | 2-ClC6H4 | 18 | 98 | 120 |
| 30b | CH=CH2 | 18 | 98 | 120 |
| 31b | CCTBS | 19 | 99.4 | 420 |
Solvent-free.
Highly concentrated with epoxide : THF = 1 : 1 v/v ratio.
Isolated yield of 1,2-diol.
The substrate was d,l-butadiene diepoxide.
Halogenated epoxides (Table 7, entries 8 - 11), ether containing epoxides (Table 7, entries 12 - 16), and carbonyl containing epoxides (bearing esters, ketones, and carbamates) (Table 7, entries 17 - 23) were all excellent substrates for HKR. Terminal epoxides bearing unsaturated C-C bonds, such as styryl, vinyl and alkynyl derivatives (Table 7, entries 24 - 31) proved to be useful substrates in HKR, affording valuable epoxides with ≥ 99% ee in 36 – 44% yield (based on racemic epoxide).
Isolation of the product of a kinetic resolution, however, is more complicated because the product ee decreases with increasing reaction conversion. Nonetheless, with high krel values, products of very high ee can be isolated at yields approaching 50%. As outlined above, with krel of 10, starting material of 95% ee can be obtained in up to 34% yield. To obtain product with identical ee and yield, a krel value of 63 is necessary!66
The krel values of a variety of epoxides were determined in the HKR as outlined in Table 7. In a kinetic resolution, the ee's of both the starting material and the product change with conversion. Thus, to describe a kinetic resolution with product ee's is not as informative as characterization by krel values. In the determination of krel values in Table 7, 0.2 equivalents of water were employed.
The use of the HKR under solvent-free and highly concentrated conditions to isolate enantioenriched diols is illustrated in Eq 6. In almost all cases, diol ee's exceeded 95% and isolated yields ranged from 36 – 45% (based on racemic epoxide).
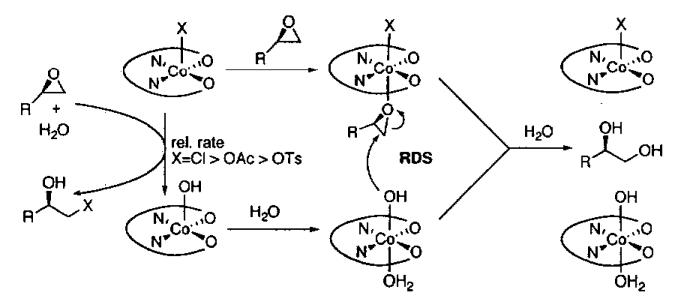
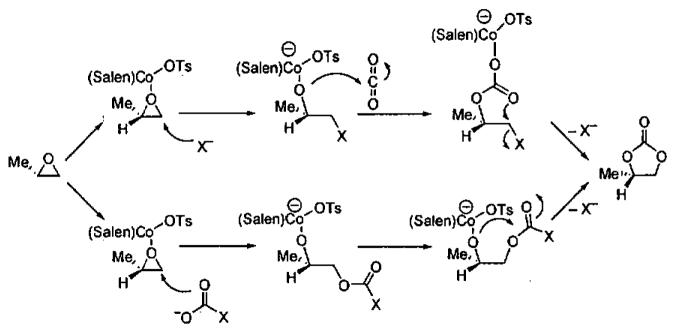
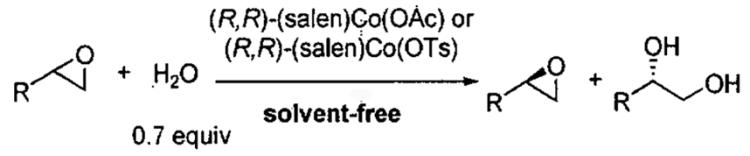
Detailed investigations into the mechanism of the HKR by Nielsen, Stevenson, Blackmond, and Jacobsen50 have not only provided insight into cooperative interactions between (salen)Co(III) complexes, but have lead to an improved catalyst for the solvent-free process. When (salen)CoCl is employed, it rapidly reacts with epoxide and water to generate (salen)Co(OH), which also serves as a Lewis acid to activate the epoxide. In contrast, with less nucleophilic counterions, such as acetate and tosylate, the reaction of the counterion with the epoxide is slower (Figure 7). The more Lewis acidic complexes (salen)Co(OAc) and (salen)Co(OTs) not only activate the epoxide more strongly, increasing its reactivity, but also exhibit greater binding constants for epoxide coordination. Thus, while the catalyst does play a dual role in the epoxide ring-opening reactions, the optimal characteristics of each (salen)Co(III) component are not identical. By way of comparison, when the precatalyst has been converted entirely to Co-OH, the HKR is up to 30 times slower than when (salen)Co-OH and Co-OTs are both present. Under ideal conditions, the ratio of (salen)Co(OH) to (salen)Co(OTs) is 1 : 1. Comparison of the improved catalyst (salen)Co(OTs) to (salen)Co(OAc) in the HKR of several epoxides is illustrated in Table 8.
Figure 7.
Mechanism of the HKR illustrating the dual roles of the (salen)Co(III) centers.
Table 8.
Comparison of the HKR with (salen)Co(OAc) and (salen)Co(OTs) with various epoxides.
 | ||||
|---|---|---|---|---|
| epoxide | catalyst | catalyst (mol%)a |
time (h) |
yield (%)b |
 |
(salen)Co(OAc) | 0.5 | 16 | 43 |
| (salen)Co(OTs) | 0.15 | 3 | 44 | |
| (saten)Co(OTs) | 0.05 | 16 | 45 | |
 |
(salen)Co(OAc)c | 2.0 | 48 | 40 |
| (salen)Co(OTs)c | 1.2 | 48 | 39 | |
 |
(salen)Co(OAc) | 0.5 | 16 | 43 |
| (salen)Co(OTs) | 0.2 | 16 | 42 | |
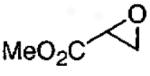 |
(salen)Co(OAc) | 2.0 | 24 | 43 |
| (salen)Co(OTs) | 0.5 | 16 | 42 | |
 |
(salen)Co(OAc) | 0.5 | 16 | 47 |
| (salen)Co(OTs) | 0.06 | 16 | 45 | |
Catalyst loading based on racemic epoxide.
Isolated yield of >99% ee epoxide based on racemate (theoretical maximum 50%).
Reaction at 0-4 °C.
In summary, HKR with (salen)Co(III) displays an extraordinary scope, as a wide assortment of sterically and electronically varied epoxides can be resolved to 99% ee. The HKR has several appealing features from a practical standpoint, including the use of H2O as a reagent, low loadings of a recyclable and commercially available catalyst, high yield and selectivity, low reagent cost, and low reagent toxicity. The ability to conduct reactions under solvent-free and highly concentrated reaction conditions results in high volumetric productivity and low waste generation. These characteristics make HKR attractive for large-scale applications.
2.2.4 Oligomeric (salen)Co(III) Catalysts for the HKR
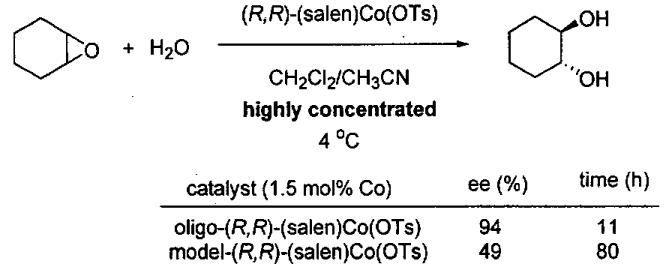
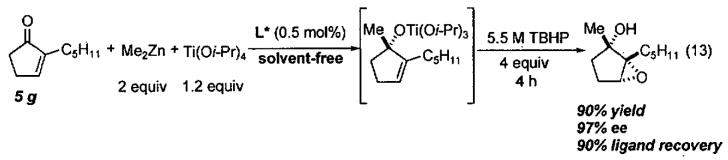
Like the ARO of meso epoxides by (salen)CrN3 catalysts, the HKR with (salen)Co(III) catalysts proceeds by a cooperative bimetallic mechanism wherein one molecule of catalysts activates the epoxide and the second activates the water in the form of a (salen)Co(OH). As outlined previously, such second order behavior in catalyst concentration results in dramatically increased reaction times as the catalyst loading is reduced. The catalyst loading of monomeric (salen)Co(III) used in the HKR of terminal epoxides, while relatively low (0.2 – 2 mol%), remains the primary cost determinant in the HKR. To circumvent this limitation, several cyclic oligomeric catalysts were prepared and examined in the HKR of racemic epoxides under solvent-free and highly concentrated conditions.
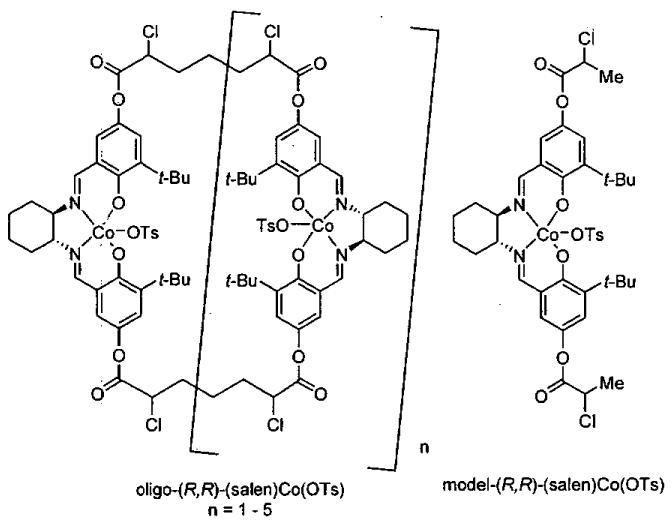
Initial cyclic oligomeric catalyst structures are illustrated in Figure 8 along with a monomeric model system prepared for the purpose of comparison. The stereocenters on the tethers are random and give rise to diastereomeric catalyst mixtures. The reactivity of the oligomeric catalyst mixture was compared to the monomeric model system from Figure 8 in the challenging ARO of cyclohexene oxide under highly concentrated reaction conditions (Figure 9).85 With 1.5 mol% loading of cobalt metal centers, the oligomeric catalyst reached completion in 11 h while the monomeric model system required 80 h. The enhanced reactivity of the oligomeric catalyst was attributed to the increase in effective molarity of the cobalt centers due to the tethering of the (salen)Co(III) units. Surprisingly, the oligomeric catalyst also exhibited much higher enantioselectivity (94%) than the monomeric model catalyst (49% ee). The remarkable increase in enantioselectivity of the oligomeric catalyst is likely due to the constrained orientation of the two reacting cobalt centers in the oligomeric catalyst system.
Figure 8.
Structures of oligomeric Co(III) catalysts and a monomeric model system for the ARO of epoxides.
Figure 9.
Comparison of the ARO of cyclohexene oxide with oligomeric and monomeric model catalysts from Figure 8.
The positive effects of tethering on enantioselectivity and reactivity were encouraging with cyclohexene oxide, leading to examination of four racemic substrates in the HKR under solvent-free or highly concentrated conditions (Table 9).85 The practicality of the oligomeric catalyst in Figure 8 is highlighted in entry 1. With 0.5 mol of epichlorohydrin and 0.3 mol of water, the HKR was performed at room temperature with only 50 mg of the oligomeric catalyst. After 11 h, the epoxide was vacuum transferred from the reaction mixture and filtered through a pad of MgSO4 to remove the excess water, furnishing 23 g of epoxide (45% yield, >99% ee). As outlined in Table 9, use of the oligomeric catalyst allowed a 10- to 50-fold decrease in the cobalt loading with a simultaneous reduction of up to a factor of 16 in reaction time.
Table 9.
Catalyst comparison in the HKR of terminal epoxides with the oligomeric catalyst (Figure 8) and the standard (R,R)-(salen)Co(OAc).
 | |||||
|---|---|---|---|---|---|
| entry | isolated product | catalyst | Co (mol%)a | time (h)% | yieldf (% ee) |
| epoxidesg | |||||
| 1b | 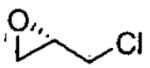 |
monomer oligomer |
0.5 0.01 |
18 11 |
42(99) 45(99) |
| 2c |  |
monomer oligomer |
0.85 0.05 |
68 24 |
26(99) 36 (99) |
| 3d |  |
monomer oligomer |
0.8 0.08 |
72 24 |
44(99) 37(99) |
| 4e |  |
monomer oligomer |
2.0 0.1 |
48 24 |
41(99) 41(99) |
| diolsg | |||||
| 5d | 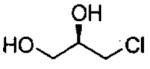 |
monomer oligomer |
2.0 0.01 |
24 1.5 |
50(96) 40(97) |
| 6d | 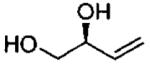 |
monomer oligomer |
0.64 0.03 |
20 4 |
49(94) 46(97) |
| 7d | 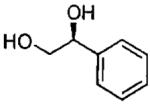 |
monomer oligomer |
0.8 0.08 |
12 4 |
41(98) 43(96) |
| 8d | 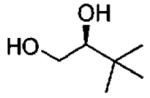 |
monomer oligomer |
2.0 0.05 |
20 18 |
40(95) 49(95) |
Loading on a Co basis relative to racemic epoxide.
Solvent-free.
Highly concentrated reaction using butyronitrile as solvent.
Highly concentrated reaction using 1:1 CH2Cl2: CH3CN as solvent.
Highly concentrated reaction using 1,2-hexanediol as solvent.
Isolated yield based on racemic epoxide.
For reactions in which resolved epoxide was targeted, 0.55-0.60 equiv H2O was used for the resolution. For recovery of diol, 0.45 equiv H2O was employed.
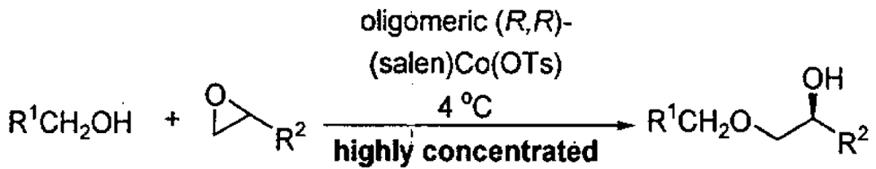
The oligomeric catalyst was also employed in the kinetic resolution of epoxides, using alcohols as the reacting partner (Table 10). Under highly concentrated conditions, a variety of primary alcohols participated in epoxide opening reactions to afford monoprotected alcohols with high levels of regio- and enantioselectivity (94–99% ee). In most cases, yields were high, catalyst loading was low, and reaction times were 3 – 24 h. These results represented the first kinetic resolution of epoxides using alcohols as nucleophiles.85
Table 10.
Kinetic resolution of epoxides with alcohols using the oligomeric catalyst from Figure 8.
 | |||||
|---|---|---|---|---|---|
| entrya | R1 | R2 | Co (mol%)b | yield (%)c,d | ee (%) |
| 1 | Ph | (CH2)3CH3 | 2.0 | 87 | 98 |
| 2 | CH2TMS | (CH2)3CH3 | 0.2 | 97 | 99 |
| 3 | H | (CH2)3CH3 | 0.1 | 96 | 94 |
| 4 | 2-C6H4-Br | (CH2)3CH3 | 0.1 | 99 | 99 |
| 5 | 4-C6H4-OMe | (CH2)3CH3 | 2.0 | 62 | 94 |
| 6 | 2-C6H4-NO2 | (CH2)3CH3 | 0.5 | 98 | 99 |
| 7 | CH=CH2 | (CH2)3CH3 | 0.5 | 87 | 97 |
| 8 | Ph | CH2Cl | 2.0 | 91 | 98 |
| 9 | Ph | CH2O(allyl) | 2.0 | 95 | 98 |
Reactions were carried out with 5M substrates in CH3CN.
Catalyst loading on a per Co bases relative to alcohol.
Isolated yields based on RCH2OH.
Reaction times 3 - 24 h.
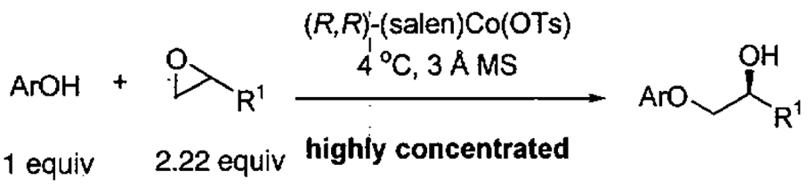
Kinetic resolution of terminal epoxides using phenol derivatives as nucleophiles provides α-aryloxy alcohols with high ee's. A comparison of the reactivity and enantioselectivity of the oligomeric (salen)Co(OTs) catalyst with the parent (salen)Co(OTs) was performed and is outlined in Table 11.85 The oligomeric catalyst is significantly more reactive, as judged by entries 2 and 3, and in some cases is also more enantioselective.
Table 11.
Comparison of the oligomeric catalyst from Figure 8 and the (salen)Co(OTs) in the kinetic resolution of epoxides with phenol derivatives.
 | ||||||
|---|---|---|---|---|---|---|
| entry | Ar | R1 | (R,R)-cat. | Co (mol%)c | yield (%)d,e | ee (%) |
| 1 | Ph | CH2Cl | (salen)Co(OTs)a | 4.0 | 96 | 99 |
| oligomerb | 0.25 | 99 | 99 | |||
| 2 | 2-C6H4-(Oallyl) | CH2Cl | (salen)Co(OTs)a | 4.4 | 48 | 84 |
| oligomerb | 0.25 | 99 | 98 | |||
| 3 | Ph | Ph | (salen)Co(OTs)a | 4.0 | f | n.d. |
| oligomerb | 1.0 | 60 | 97 | |||
| 4 | Ph | c-hexyl | (salen)Co(OTs)a | 8.0 | 89 | 94 |
| oligomerb | 0.5 | 99 | 98 | |||
| 5 | 2-C6H4-Cl | n-Bu | (salen)Co(OTs)a | 4.0 | 80g | 68 |
| oligomerb | 0.8 | 98 | 99 | |||
5M substrates in TBME.
5M substrates in CH3CN.
Catalyst loading on a per Co basis relative to ArOH.
Isolated yields based on ArOH.
Reaction times 4 - 24 h unless otherwise noted.
After 10 days 63% conversion, 2:3 mixture favoring internal attack.
72 h reaction time.
Kinetic experiments with racemic 1,2-epoxyhexane, 2-chlorophenol, and the oligomeric catalyst indicated that the ring-opening was first order in cobalt catalyst, consistent with an intramolecular ring-opening. Such behavior can be contrasted with the second order dependence observed for the monomeric (salen)Co(OTs). These results indicate that the epoxide opening with the oligomeric catalyst occurs in an intramolecular fashion via a cooperative mechanism involving at least two cobalt centers.
The primary drawback of the oligomeric catalyst system in Figure 8 is its hydrophobic character, which resulted in limited solubility in the epoxide-water mixtures of the HKR. The solubility properties of the chlorinated oligomer required solvent to solubilize the catalyst, complicating the otherwise simple isolation of the volatile epoxide. This difficulty inspired continued investigation into oligomeric catalysts with greater solubility in the HKR reaction medium.
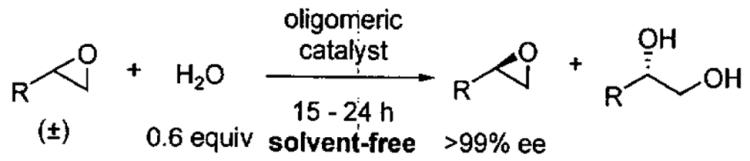
In designing the next generation of oligomeric catalysts, the Jacobsen group focused on the tether to modulate the solubility and the counterion to increase reactivity.86,87 By using tethers containing ether linkages and void of chloro groups, oligomeric catalyst with increased solubility in the epoxide/water mixture of the HKR were identified (Figure 10). To evaluate the reactivity of the new tethered catalyst, the HKR of methyl glycidate was studied (Table 12, entry 1). Under solvent-free conditions with 0.03 mol% catalyst, the reaction reached completion in 8 h. Resolved methyl glycidate with > 99% ee was isolated. In contrast, under identical conditions with the monomeric (salen)Co(OAc) the epoxide ee was 8% after 8 h.
Figure 10.
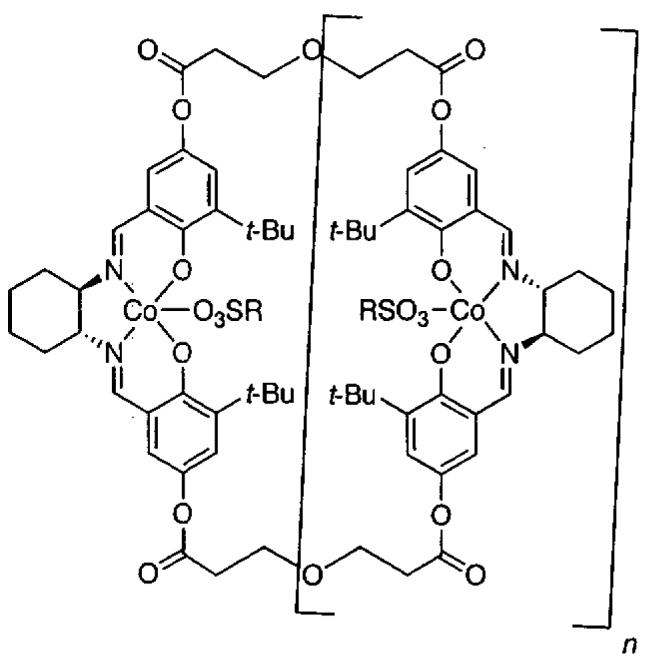
Next generation soluble oligomeric catalyst for the HKR of terminal epoxides (R = CF3, 3-NO2-C6H4).
Table 12.
HKR of terminal epoxides catalyzed by oligomeric (salen)Co(O3SR) from Figure 10.
 | ||||
|---|---|---|---|---|
| entry | epoxide | (salen)Co(O3SR) R = |
catalyst loading (mol%) |
yielda (%) |
| 1 | 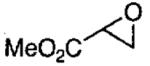 |
CF3 | 0.015 | 44 |
| 2 | CF3 | 0:0025 | 43 | |
| 3 | 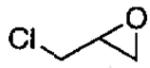 |
CF3 | 0.001 | 44 |
| 4 |  |
CF3 | 0.0003 | 40 |
| 5 |  |
3-NO2-C6H4 | 0.04 | 40 |
Isolated yields based on racemic epoxides (theoretical maximum = 50%).
The HKR of propylene oxide, an important substrate, was also extremely efficient and easily scalable (Table 12, entry 4). Under solvent-free conditions, 1.5 mol of epoxide was resolved with 3.6 mg catalyst (41 ppm by mass, 3 ppm on a molar basis) furnishing 35 g of isolated epoxide with > 99% ee.86
The mixture of oligomers (Figure 10, n = 1 – 3) was separated to determine their individual reactivity. Surprisingly, a substantial difference in reactivity was observed with n = 3, which was significantly more reactive and enantioselective than the dimer or tetramer.87
The use of oligomeric catalysts has allowed circumvention of many of the pitfalls associated with systems that are second order in catalyst. By tethering (salen)Co(III) units, high effective concentrations of cobalt centers are achieved at low cobalt loading.
2.2.5 Supported (salen)Co(III) Catalyst for the HKR
The homogenization of (salen)Co(III) complexes has been examined to facilitate separation of the catalyst from the reaction mixture and as a method to maintain a high effective molar concentration of cobalt centers to promote the cooperative bimetallic mechanism.49,88 The resulting supported catalysts have been employed under highly concentrated conditions and have exhibited high values of krel. While the catalyst has been recycled several times, or even used in continuous flow systems,49 their application has been limited to small scale kinetic resolutions and do not present advantages over the oligomeric systems outlined above. They are not covered further here.
2.2.6 Asymmetric Ring-Opening of Epoxides Using Carbon Dioxide
In the nucleophilic ring opening of epoxides promoted by (salen)M complexes, there is ample evidence that the epoxide is activated by coordinating to the Lewis acidic (salen)M complex. In the systems that have been studied, the nucleophile is also activated by the (salen)M complex in the form of (salen)M-Nu. As illustrated below, however, this is not always the case.
An interesting partner for the asymmetric epoxide opening is carbon dioxide. This handy C1 building block does not readily react with epoxides in the presence of Lewis acids. It was discovered that, in the presence of certain nucleophiles, epoxide opening does occur, ultimately generating cyclic carbonates. The synthesis of five-membered cyclic carbonates via the coupling of CO2 and epoxides is an environmentally benign process and CO2 is a safe and plentiful C1 building block.89
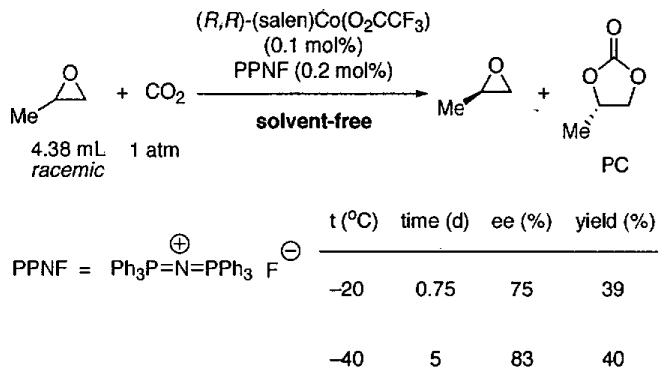
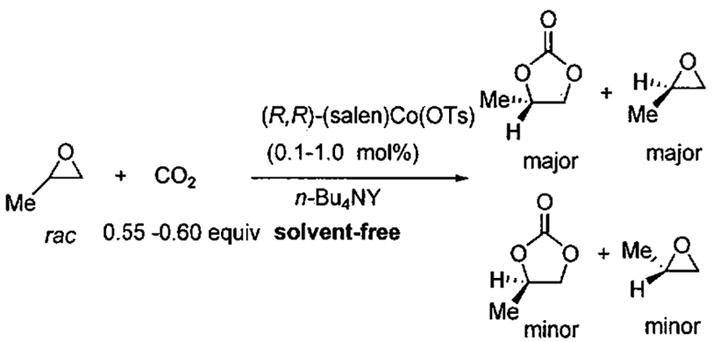
Based on the possible mechanisms of epoxide opening catalyzed by Lewis acids and nucleophilic cocatalysts (Figure 11), Lu et al. designed a system for generating optically active cyclic carbonates from racemic epoxides.90 Using Jacobsen's chiral (salen)Co(III) catalysts42 in combination with catalytic tetrabutylammonium halides (n-Bu4NX), these researchers devised a convenient solvent-free kinetic resolution of propylene oxide to provide enantioenriched propylene carbonate (PC). Chiral (salen)CoX complexes with a sterically bulky axial X-group, such as p-toluene sulfonate, were essential for maximizing enantioselectivity in the kinetic resolution. Additionally, the anion of the quaternary ammonium co-catalyst has a significant impact on propylene carbonate enantiomeric purity.
Figure 11.
Two possible mechanisms for epoxide opening with carbon dioxide.
In the absence of the halide cocatalyst, no reaction was observed (Table 13, entry 1). Lu et al found that reaction of neat rac-propylene oxide (0.5 mol) with 0.55 – 0.60 equiv of CO2 in the presence of 0.1 mol% of (salen)Co(OTs) complex and 0.1 mol % of n-Bu4NBr as nucleophile produced optically active propylene carbonate (PC) of moderate ee in the kinetic resolution (Table 13, entry 2).90 The relative rate of the fast vs. slow reacting epoxide was just over 5. The best results were obtained with n-Bu4NCl (Table 13, entries 3 – 8).
Table 13.
Catalytic kinetic resolution of rac-propylene oxide by CO2 coupling.
 | |||||||
|---|---|---|---|---|---|---|---|
| entry | cocatalyst n-Bu4NY (equiv) |
T (°C) | time (h) | conv (%) | krel | TOF (h−1) | PC ee (%) |
| 1 | none | 25 | 10.0 | 0 | - | 0 | - |
| 2 | n-Bu4NBr (1) | 25 | 2.5 | 50.8 | 5.2 | 203 | 51.6 |
| 3 | n-Bu4NCl (1) | 25 | 4.0 | 50.5 | 6.4 | 126 | 56.7 |
| 4 | n-Bu4NCl (2) | 25 | 2.5 | 46.8 | 6.0 | 187 | 57.2 |
| 5 | n-Bu4NCl (10) | 25 | 2.0 | 48.1 | 5.8 | 240 | 55.9 |
| 6 | n-Bu4NCl (2) | 45 | 1.5 | 47.4 | 2.8 | 316 | 35.2 |
| 7 | n-Bu4NCl (2) | 15 | 6.0 | 43.9 | 7.2 | 73 | 63.5 |
| 8 | n-Bu4NCl (2) | 0 | 15.0 | 40.0 | 9.0 | 27 | 70.2 |
While the krel values measured in the epoxide resolution with carbon dioxide are below what is generally considered useful, these investigations are important because they address a long-standing challenge in organic chemistry: the feasibility of using CO2 as a C1 source in asymmetric catalysis. The (salen)Co(OTs)/n-Bu4NCl based catalyst system is highly efficient at catalyzing this reaction. The low relative rates of the fast and slow reacting enantiomers have inspired the search for better catalysts. Using the same (salen)CoX, Berkessel and Brandenburg91 examined the use of different counterions X and cocatalysts under solvent-free conditions. They observed that the counterion X had a large impact on catalyst activity, but not enantioselectivity and that the counterion of the cocatalyst influenced the activity and selectivity. After extensive screening, a catalyst system was found that operated under much lower pressure of carbon dioxide (Figure 12). Under optimal conditions, the selectivity factor was 18.7.
Figure 12.
Kinetic resolution of propylene oxide with formation of enantioenriched propylene carbonate (PC)
The results outlined in Section 2.2 illustrate how a series of (salen)M-based catalysts have been applied to the ARO and kinetic resolution of epoxides. In general, these transformations work well under standard solvent conditions. Under solvent-free and highly concentrated reaction conditions, little or no loss in enantioselectivity is observed with respect to those reactions conducted in solvent. It is found that the (salen)M-based catalysts are more active under solvent-free and highly concentrated reaction conditions. Given the breadth of the reactions outlined above, one must wonder if the (salen)M-based catalysts are unique in their ability to exhibit high levels of enantioselectivity under standard solvent conditions, highly concentrated conditions, and solvent-free conditions; or if these results are due primarily to the efforts of a few researchers interested in conducting reactions in a non-traditional fashion to develop more practical asymmetric processes. We believe that the latter rational is more likely and that many other catalysts may display similar levels of enantioselectivity and activity under highly concentrated and solvent-free conditions.
2.3 Asymmetric Hetero-Diels-Alder Reaction
The catalytic asymmetric hetero-Diels–Alder (HDA) reaction is one of the most important asymmetric C–C bond-forming processes in heterocyclic chemistry.92-94 It provides a highly effective method for preparing optically active six-membered ring compounds such as dihydropyrans and dihydropyranones, and has been extensively utilized in the synthesis of natural products. Many main-group, transition metal, lanthanide, and even organocatalysts have been developed that promote the enantioselective HDA reaction.95,96 The asymmetric HDA reaction has been frequently and successfully examined under solvent-free and highly concentrated reaction conditions. The successful adaptation of the HDA from solvent to solvent-free reaction conditions likely stems from the concerted nature of the cycloaddition transition state, which exhibits only minimal dependency on the reaction medium.
2.3.1 Asymmetric Hetero-Diels-Alder Reaction of Danishefsky's Diene and Aldehydes
The formal HDA reaction between 1-methoxy-3-(trimethylsilyloxy)buta-1,3-diene (Danishefsky's diene)97 and aldehydes provides a powerful method to access dihydropyranones, a class of heterocycles with broad utility in organic synthesis. A number of chiral catalysts have been reported for HDA reactions under standard solvent conditions. For example, Keck and co-workers reported that a catalyst generated from (R)- or (S)-BINOL and Ti(O-iPr)4 in a 2:1 ratio in the presence of 4 Å molecular sieves and 3-4 equiv of CF3COOH in Et2O was able to promote the reaction of Danishefsky's diene with aldehydes to yield dihydropyranones with good to excellent ee's (55-97%).98
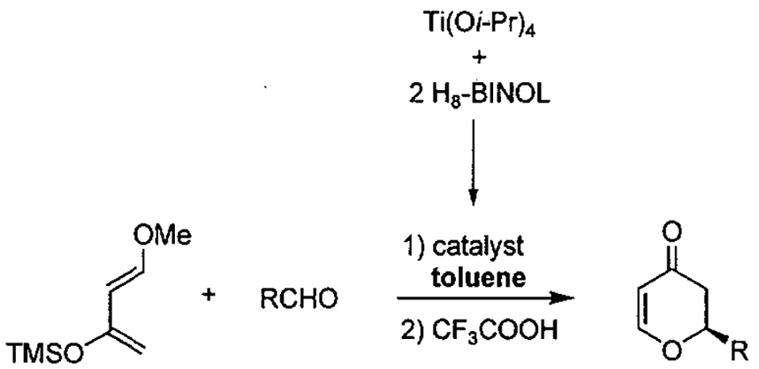
In 2000, Jiang and co-workers99 also reported that asymmetric HDA reactions of Danishefsky's diene with aldehydes gave high enantioselectivity using chiral H8-BINOL-Ti(O-iPr)4 complexes as catalysts and toluene as the solvent (Table 14). The yield and enantioselectivity were found to be dependent on the catalyst loading. When the amount of catalyst was reduced from 20 mol% to 10 mol%, the enantioselectivity and yield of the reaction decreased considerably (Table 14, entries 1 vs. 2, 97% ee to 73% ee, yield 92% to 75%).
Table 14.
Asymmetric HDA reactions of Danishefsky's diene and aldehydes with toluene solvent.
 | |||||
|---|---|---|---|---|---|
| entrya | R | temp(°C) | time (h) | yield (%) | ee (%) |
| 1 | Ph | 0 | 24 | 92 | 97 |
| 2b | Ph | 0 | 12 | 75 | 73 |
| 3 | Ph | 23-25 | 6 | 92 | 93 |
| 4 | 4-C6H4-OMe | 0 | 24 | 52 | >99 |
| 5 | 4-C6H4-Me | 0 | 24 | 60 | 99 |
| 6 | 4-C6H4-F | 0 | 24 | 54 | 99 |
All reactions were carried out using 20 mol% of catalyst in toluene.
10 mol% catalyst used.
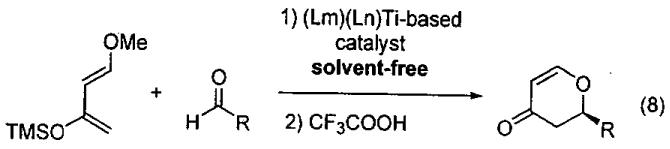
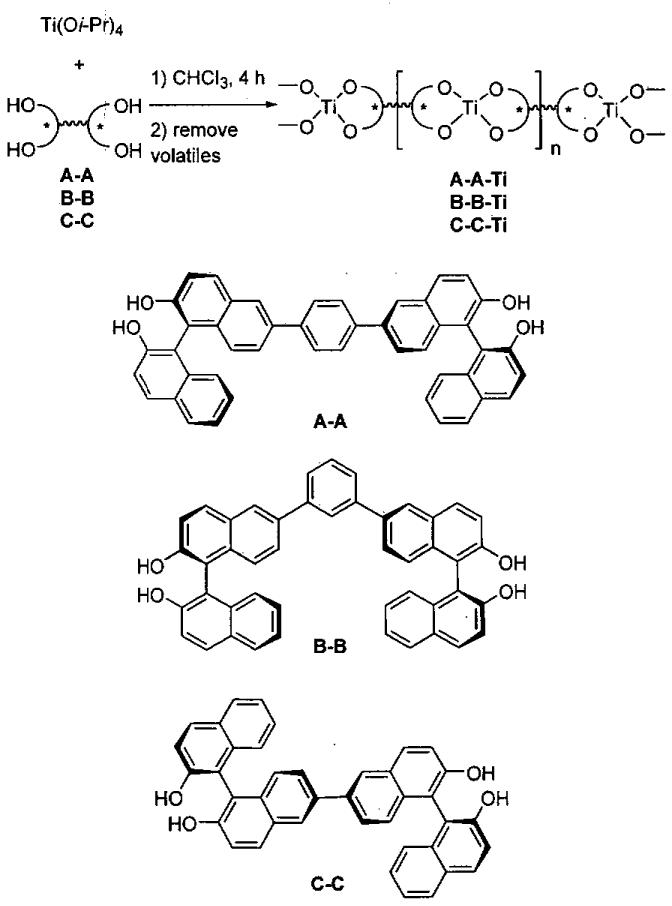
In related chemistry, Ding and co-workers discovered highly efficient catalysts for solvent-free enantioselective HDA reaction by high-throughput screening of a combinatorial library of chiral ligands attached to titanium (Eq 7).100,101
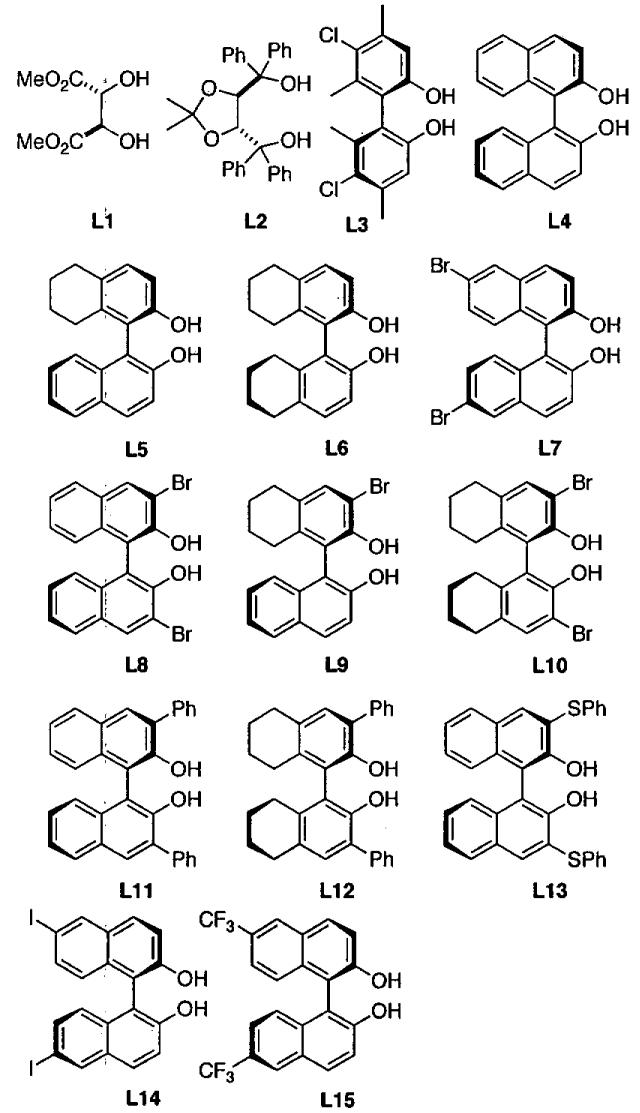
Using titanium tetraisopropoxide and two ligands from the collection in Figure 13 in a Lm : Ln : Ti ratio of 1 : 1 : 1, a series of (Lm)(Ln)Ti-based catalysts were generated. In mixing all possible combinations with L1-L13, a library of 104 catalysts can be generated. Most of the ligand combinations above will result in a mixture of titanium compounds under thermodynamic control. It is anticipated that the titanium centers will bind identical ligands giving (Lm)2Ti- and (Ln)2Ti-based catalysts or two different ligands as in (Lm)(Ln)Ti-derivatives. Furthermore, the resulting complexes may exhibit monomer-dimer-oligomer equilibria, complicating analysis of the enantioselective reactions.
Figure 13.
Ligands used to prepare (Lm)(Ln)Ti-based catalysts and (Lm)Zn-based catalysts for the solvent-free HDA.
Initial screening of in situ prepared (Lm)(Ln)Ti-based complexes in the HDA reaction with Danishesky's diene and benzaldehyde (Eq 7) in diethyl ether indicated ligands L4 - L7 generated catalysts that exhibited > 75% ee. The most promising ligand combinations were then examined under solvent-free conditions at lower catalyst loadings (Eq 8). The combinations (L5)2Ti and (L5)(L6)Ti each exhibited 99% ee and then were both used with a series of aldehydes to define the scope of the catalysts, as outlined in Table 15.
Table 15.
Solvent-free asymmetric HDA reaction of aldehyde with Danishefsky's diene as illustrated in Eq 8.
| aldehydes | (L5)2Ti |
(L5)(L6)Ti |
||||||
|---|---|---|---|---|---|---|---|---|
| loading (%) | time (h) | yield (%) | ee (%) | loading (%) | time (h) | yield (%) | ee (%) | |
| benzaldehyde | 0.05 | 24 | >99 | 99.3 | 0.05 | 24 | 82 | 99.4 |
| p-anisaldehyde | 0.05 | 48 | >99 | 90.8 | 0.05 | 48 | >99 | 98.0 |
| m-anisaldehyde | 0.05 | 48 | 81 | 96.6 | 0.05 | 48 | 82.6 | 99.8 |
| o-anisaldehyde | 0.05 | 48 | 95 | 75.1 | 0.05 | 48 | >99 | 95.1 |
| 3-phenylpropionaldehyde | 0.05 | 96 | >99 | 97.9 | 0.05 | 96 | >99 | 98.3 |
| trans-cinnamaldehyde | 0.1 | 96 | 82 | 98.4 | 0.05 | 96 | 56.6 | 96.6 |
| furfural | 0.05 | 48 | >99 | 99.2 | 0.05 | 48 | >99 | 99.7 |
| furfural | 0.01 | 96 | 37 | 94.7 | 0.01 | 96 | >99 | 97.7 |
| furfural | - | - | - | - | 0.005 | 144 | 63 | 96.2 |
| m-tolualdehyde | 0.1 | 48 | 95 | 98.5 | 0.05 | 48 | 92 | 99.5 |
| 1-naphthaldehyde | 0.05 | 48 | 55 | 85.6 | 0.05 | 48 | 65 | 98.5 |
| p-cyanobenzaldehyde | 0.1 | 48 | >99 | 92.9 | 0.05 | 48 | 98.4 | 97.9 |
| m-bromobenzaldehyde | 0.1 | 48 | >99 | 97.4 | 0.05 | 48 | 98.3 | 97.6 |
| p-bromobenzaldehyde | 0.05 | 48 | >99 | 98.0 | 0.05 | 48 | >99 | 98.4 |
| p-chlorobenzaldehyde | 0.05 | 48 | >99 | 91.2 | 0.05 | 48 | >99 | 99.1 |
| p-nitrobenzaldehyde | 0.05 | 48 | >99 | 97.3 | 0.05 | 24 | >99 | 99.4 |
Inspection of the results in Table 15 indicates that the reaction is particularly successful with aromatic aldehydes. It is noteworthy that in the cycloaddition with furfural, only 0.005 mol% of the (L5)(L6)Ti-based catalyst was needed to promote the reaction smoothly and furnish the product in 63% yield with 96.3% ee. This is one of the lowest catalyst loadings reported in early transition metal Lewis acid catalyzed processes! Comparison of the results in Table 14 and Table 15 obtained with two very similar catalysts, indicate the potential benefits of solvent-free and highly concentrated reaction conditions.
The dihydropyranones generated in this HDA reaction are very useful building blocks that have been employed in numerous syntheses. This study is particularly impressive given the unprecedented low catalyst loadings (0.1 – 0.005 mol%) and high enantioselectivities observed under solvent-free reaction conditions.
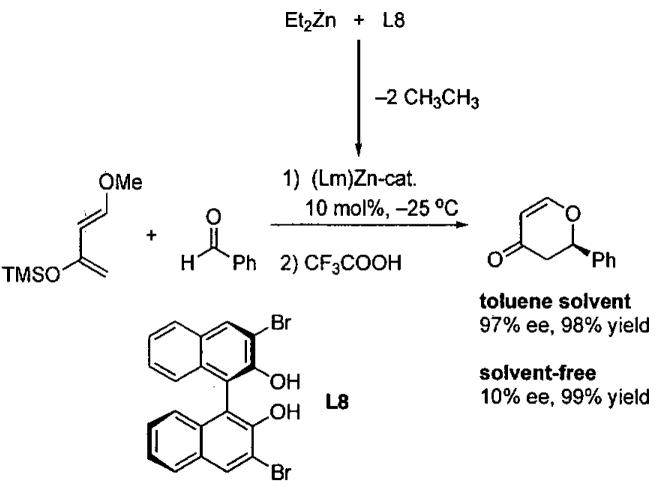
In a related study, the Ding group reported a highly enantioselective HDA reaction of Danishefsky's diene with aldehydes catalyzed by zinc-based chiral Lewis acids using toluene solvent.102 Most of the chiral ligands examined in this chemistry are shown in Figure 13. The catalyst was generated in situ by combining 10 mol% of diethylzinc with an equal amount of one of the chiral ligands in Figure 13. The catalyst prepared from 3,3'-dibromo-1,1'-bi-2-naphthol (L8) exhibited the highest enantioselectivity (Figure 14). When the reactions were carried out under solvent-free conditions, however, the product ee dropped from 97% to 10%, even though the yield was 99%. This result illustrates the dramatic role played by the solvent in this example, especially when compared with the titanium system in Eq 8 and Table 15.100
Figure 14.
Comparison of toluene and the solvent-free HDA reaction with ligand L8 from Figure 13.
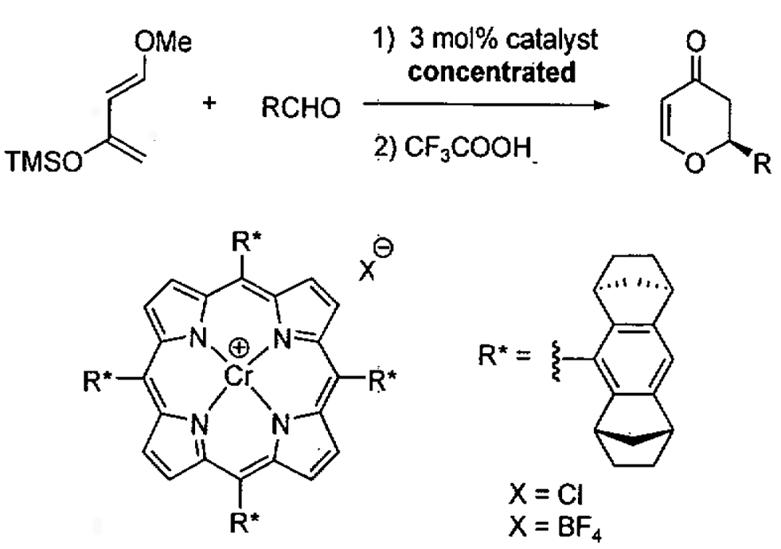
Many porphyrin complexes are unsaturated and can promote Lewis acid catalyzed processes such as the HDA reaction. Using Halterman's chiral porphyrin,103,104 Berkessel and co-workers reported that chiral Cr(III) porphyrins complexes are efficient and highly enantioselective catalysts for HDA between aldehydes and Danishefsky's diene under highly concentrated conditions (Table 16).105
Table 16.
Asymmetric HDA catalyzed by chiral porphyrin complexes.
 | |||||
|---|---|---|---|---|---|
| entrya | R | catalyst counterion (X−) |
temp (°C) | yield (%) | ee (%) |
| 1 | Ph | Cl− | −18 | 85 | 95 |
| 2 | Ph | BF4− | −18 | 92 | 88 |
| 3 | c-hexyl | Cl− | −18 | 76 | 88 |
| 4 | n-hexyl | Cl− | −18 | 75 | 92 |
| 5 | 2-furyl | Cl− | −18 | 70 | 97 |
| 6 | (E)-CH=CHPh | Cl− | 0 | 55 | 74 |
| 7 | 2-pyridyl | Cl− | −18 | 70 | 78 |
The reactions were performed at 2.0M substrate concentration in MTBE.
As illustrated, high enantioselectivities were observed with a variety of aldehydes. Interestingly, cinnamaldehyde underwent reaction at the carbonyl rather than the C-C double bond. Heterosubstituted aldehydes proved to be excellent substrates for this system as shown with furfural and 2-pyridinecarboxaldehyde (Table 16, entries 5 and 7). It is surprising that the pyridyl group does not interfere with the activation of the carbonyl group in 2-pyridinecarboxaldehyde. It is hypothesized that sever steric hindrance about the metal center does not allow tight binding of the pyridine nitrogen.
The porphyrin-based catalysts will also promote the HDA reaction with monooxygenated dienes with moderate enantioselectivities.105 The results of the HDA reaction above are impressive, given that porphyrin complexes have not been widely embraced by scientists working in asymmetric catalysis. This is because many consider the chirality to be overly distant from the Lewis acidic metal center.
2.3.2 Hetero-Diels-Alder Reaction Catalyzed by (salen)Cr(X) Complexes
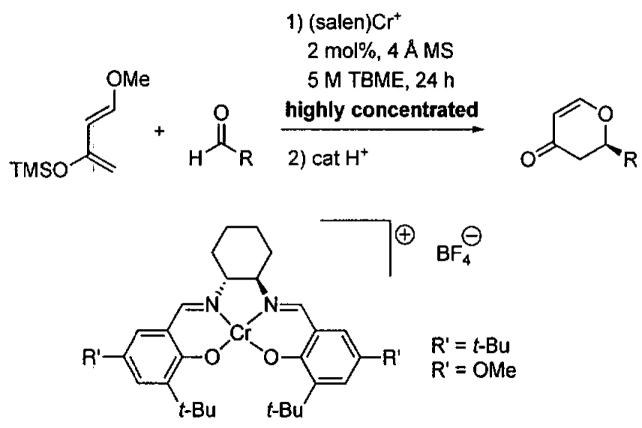
Schaus, Brånalt, and Jacobsen optimized the asymmetric HDA reaction catalyzed by (salen)Cr-based catalysts under highly concentrated conditions and provided insight into the reaction mechanism.106 Beginning with an initial substrate concentration of 1.0 M, the reaction achieved just over 90% conversion in 8 h with (salen)CrCl. Under highly concentrated conditions (5.0 M), complete conversion was attained after only 4 h with slightly higher ee (60%). The catalyst enantioselectivity was found to be highly dependent on the nature of the counterion, with the non-coordinating BF4− derivatives proving to be the most effective (Figure 15). Similar levels of enantioselectivity were observed with ligands R' = t-Bu and OMe (Table 17). In two cases, reactions were successfully conducted on a 10 mmol scale under highly concentrated reaction conditions.
Figure 15.
Enantioselective HDA reaction with cationic (salen)Cr(III) catalysts under highly concentrated conditions.
Table 17.
Results of the asymmetric HDA reaction catalyzed by (salen)Cr+ BF4 complexes.
| (salen)Cr+ R1 = t-Bu |
(salen)Cr+ R1 = OMe |
|||||
|---|---|---|---|---|---|---|
| entry | R | temp (°C) |
ee (%) | yield (%) | yield (%) | ee (%) |
| 1 | Ph | −30 | 87 | 85 | 98 | 65 |
| 2 | c-C6H11 | −20 | 93 | 71 | 76 | 85 |
| 3 | n-C5H11 | −40 | 83 | 86 | 85 | 62 |
| 4 | 2-furyl | −10 | 76 | 89 | 80 | 68 |
| 5 | (E)-CH=CHPh | 0 | 70 | 65 | 96 | 73 |
| 6 | CH2OCH2(4-C6H4-Br) | −30 | 79 | 67 | 94a | 84 |
| 7 | CH2O2C(2-C6H4-Cl) | −20 | 83a | 92 | 86 | 72 |
Reactions were run on 10.0 mmol scale
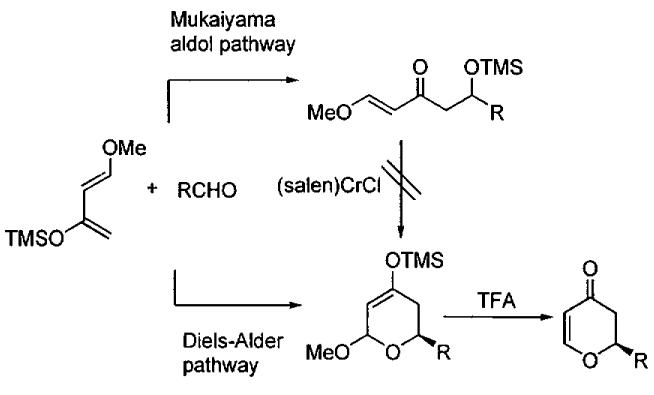
Lewis acid catalyzed HDA typically proceed through either a Mukaiyama aldol mechanism (stepwise mechanism) or a [4+2] Diels–Alder cycloaddition (concerted mechanism). Examination of the 1H NMR spectrum of the crude product indicated exclusive formation of cycloaddition product, a sign that the reaction likely proceeds through a concerted cycloaddition pathway. To test the possible intermediacy of a Mukaiyama aldol condensation adduct, the silyl ether product was independently synthesized and subjected to the conditions of the Cr(salen)-catalyzed HDA reaction (Figure 16). No detectable cyclization product was observed after 6 h at room temperature, suggesting that product formation proceeds via a concerted [4+2] mechanism.106
Figure 16.
Addition of (salen)CrCl to the independently prepared Mukaiyama aldol product did not lead to cyclization, suggesting that the reaction proceeds through a concerted [4+2] cycloaddition pathway.
2.3.3 Organocatalytic Hetero-Diels-Alder Reaction Catalyzed by TADDOL-Derivatives
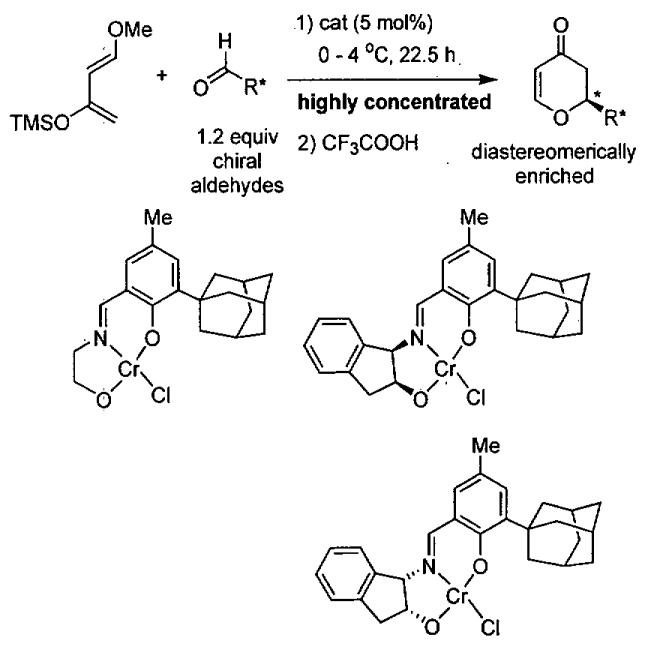
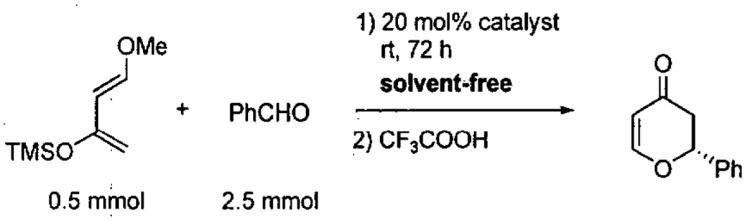
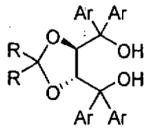
In 2003, Rawal and coworkers reported a seminal work involving the asymmetric HDA reaction catalyzed by chiral Brønsted acids, such as the chiral diol TADDOL (TADDOL = α,α,α',α'-tetraaryl-1,3-dioxolan-4,5-dimethanol).107 This work has served as the inspiration for numerous related asymmetric reactions promoted by chiral organocatalysts.108-113 In one such example, Wu, Ding, and coworkers conducted experimental and theoretical studies on the asymmetric HDA promoted by TADDOL derivatives under solvent-free conditions.114 As outlined in Table 18, reactions were typically conducted with 20 mol% TADDOL for 72 h at room temperature before treatment with TFA (trifluoroacetic acid). The ee and yield of the HDA product was found to be highly dependent on the structure of the TADDOL derivative employed.
Table 18.
HDA reaction catalyzed by chiral TADDOL derivatives.
 | ||||
|---|---|---|---|---|
| yield (%) | ee (%) | |||
 |
a: Ar = Ph | R = CH3 | 18 | 12.1 |
| b: Ar = 1-naphthyl | R = CH3 | 77 | 76.3 | |
| c: Ar = 2-naphthyl | R = CH3 | 36 | 18.7 | |
| d: Ar = 1-naphthyl | R-R = (CH2)4 | 50 | 69.1 | |
| e: Ar = 2-naphthyl | R-R = (CH2)4 | 60 | 4.0 | |
| f: Ar = 1-naphthyl | R-R = (CH2)5 | 58 | 77.0 | |
| g: Ar = 2-naphthyl | R-R = (CH2)5 | 58 | 21.2 | |
To differentiate between the two common mechanisms for the HDA reaction (Figure 16), a 1H NMR spectrum of the crude product was examined before treatment with acid. The absence of the Mukaiyama aldol product suggested that the reaction proceeded through the concerted [4+2] cycloaddition pathway.114 To further investigate the mechanism of the asymmetric HDA reaction catalyzed by TADDOL derivatives, calculations were performed using the ONIOM (our own N-layered integrated molecular orbital + molecular mechanics) (B3LYP/6-31G* : PM3) method. These studies are consistent with an intramolecular hydrogen bond between the two hydroxyl groups of the TADDOL and activation of the benzaldehyde via a single hydrogen bond (Figure 17).
Figure 17.
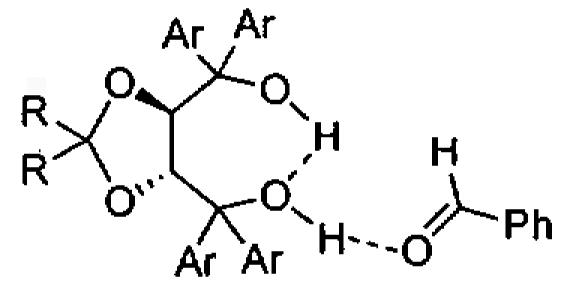
Proposed model for activation of benzaldehyde by TADDOL in the organocatalytic HDA reaction.
2.3.4 Diastereoselective Hetero-Diels-Alder Reaction
Typically, enantioselective syntheses of natural products begin with simple chiral building blocks that are elaborated in a diastereoselective fashion to construct the molecular skeleton and to install additional stereogenic centers. Unfortunately, diastereoselective reactions under substrate control can be problematic if they lead to significant amounts of the undesired stereoisomer. An underappreciated approach to controlling formation of new stereocenters in chiral substrates is to employ enantioselective catalysts to enhance or even override the inherent diastereoselectivity of the substrate. Such an approach has been employed in the doubly diastereoselective HDA reaction under highly concentrated conditions (Figure 18).
Figure 18.
HDA of enantioenriched aldehydes with achiral and chiral Cr(III) Schiff base complexes (see Table 19).
Using achiral and enantiomeric Cr(III)-Schiff base catalysts under concentrated reaction conditions, the HDA between Danishefsky's diene and enantioenriched aldehydes was examined (Figure 18).115 While the achiral catalyst gave poor diastereoselectivity, the chiral catalysts exhibited high levels of stereocontrol with unhindered chiral aldehydes. For example, with the (S)-lactaldehyde derivative, the achiral catalyst promoted the cycloaddition reaction with modest diastereoselectivity (Table 19, entry 1, 1 : 2 dr). In contrast, use of the resolved catalysts provided the products with dr between 1 : 12 and 15 : 1. Similar results were obtained with other unhindered chiral aldehydes (entries 2 and 3). In general, bulkier substrates gave lower dr with the chiral catalysts (Table 19). Importantly, the ee of several of the products was determined to be ≥ 97%, indicating that no epimerization of the aldehyde occurred under the Lewis acid catalyzed HDA reaction conditions.
Table 19.
Results of HDA of enantioenriched aldehydes with achiral and chiral Cr(III) Schiff base complexes from Figure 18.
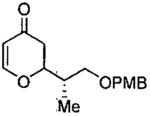
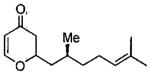
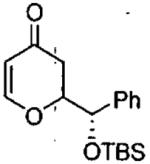
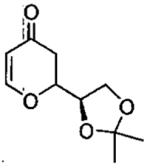
| entry | aldehyde | product | cat. | yield (%) | dr |
|---|---|---|---|---|---|
| 1 |  |
 |
achiral (1R,2S) (1S,2R) |
81 96 97 |
1 : 2.0 1 : 12 15 : 1 |
| 2 | 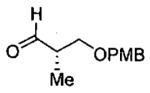 |
 |
achiral (1R,2S) (1S,2R) |
50 90 86 |
1 : 1.1 1: 11 9.3 : 1 |
| 3 | 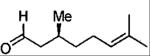 |
 |
achiral (1R,2S) (1S,2R) |
85 98 99 |
1:1.3 16 : 1 1 : 11 |
| 4 | 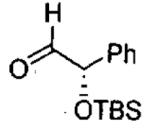 |
 |
achiral (1R,2S) (1S,2R) |
58 58 54 |
1.7 : 1 3.6 : 1 1 : 2.6 |
| 5 | 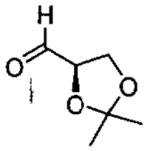 |
 |
achiral (1R,2S) (1S,2R) |
68 76 84 |
1 : 4.5 1 : 1.2 1 : 33 |
The challenging aldehydes in entries 4 and 5 of Table 19 underwent cycloaddition reactions with slightly higher diastereoselectivities with enantioenriched (salen)Cr+BF4− catalysts.115
These catalyst-controlled doubly diastereoselective HDA reactions selectively provide each of the four possible stereoisomers of the dihydropyranone products with good to excellent dr by judicious choice of aldehydes and catalyst enantiomers.115
2.3.5 Asymmetric Hetero-Diels-Alder Reaction of Danishefsky's Diene with N-Aryl Imines
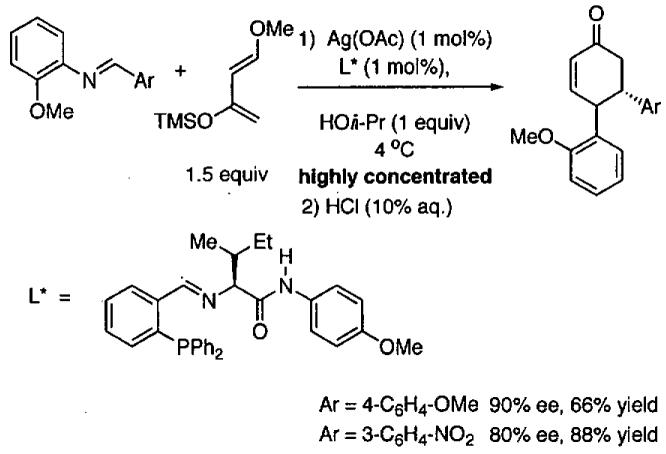
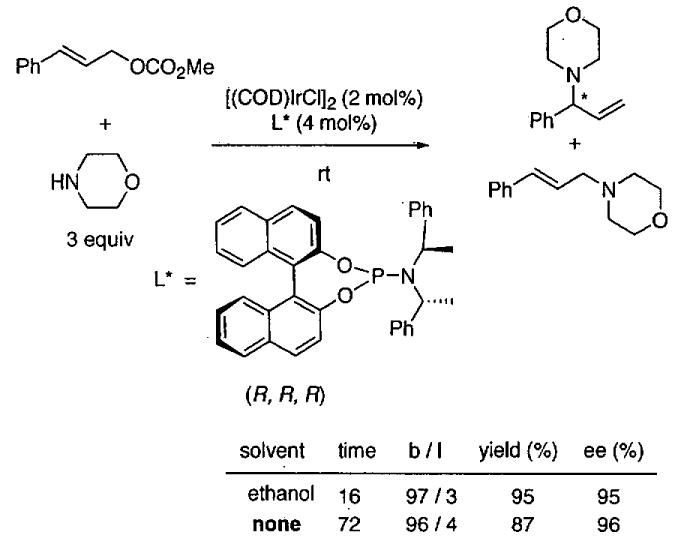
The HDA of Danishefsky's diene with imines provides access to chiral nitrogen-containing heterocycles, which are useful intermediates in the synthesis of functionalized enantioenriched piperidones.116-118 To develop catalysts prepared from readily available starting materials and used at low catalyst loading, Josephsohn, Snapper, and Hoveyda developed a highly enantioselective silver catalyst.119 After screening a parallel library of amino acid-based ligands, metals, and additives the system in Figure 19 was discovered. Use of 1 mol% each of Ag(OAc) and L* in the presence of 1 equiv of 2-propanol under highly concentrated conditions resulted in formation of the HDA in 80-90% yield after workup with acid. For comparison, reactions under standard solvent conditions in THF gave higher yields (86-92%) with these substrates and both were formed with 91% enantioselectivity. While the role of the 2-propanol is not understood, it is necessary for high TOF and enantioselectivity. Presumably a variety of other aryl imines would give similar results under the highly concentrated conditions in Figure 19.
Figure 19.
Asymmetric HDA reaction with a silver catalyst under highly concentrated conditions.
2.3.6 Asymmetric Hetero-Diels-Alder Reaction of Brassard's Diene and Aldehydes
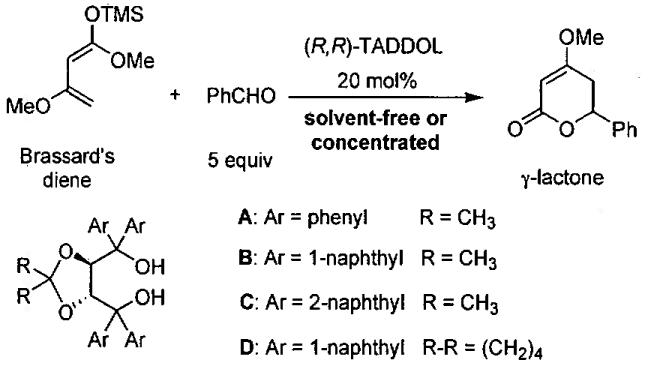
The asymmetric HDA reaction of electron-rich 1,3-dimethoxy-1-(trimethylsiloxy)butadiene (Brassard's diene)120 with aldehydes provides a useful route to synthetically important enantioenriched γ-lactones. Based on Rawal's discovery that TADDOL derivatives catalyze the asymmetric HDA between aldehydes and Danishefsky's diene,107 Ding and coworkers extended their earlier work to include asymmetric HDA with Brassard's diene.121 As outlined in Figure 20, four TADDOL derivatives (A – D) were examined in the hydrogen-bond promoted HDA reaction. The results of screening these catalysts with benzaldehyde and Brassard's diene are illustrated in Table 20. Initially, the reaction was carried out at room temperature with 0.5 mmol of diene, 2.5 mmol of benzaldehyde, and 20 mol% of catalyst (R,R)-A (Figure 20) under solvent-free conditions (entry 1). The reaction proceeded enantioselectively forming the cycloaddition product in 30% yield and 7% ee. Employing TADDOL derivative B at −30 °C, the ee improved to 71% with 70% yield. When the catalyst loading was reduced to 10 mol%, the product ee was unchanged although the yield decreased to 50% (entry 4). In contrast to the results with A and B, the use of TADDOL derivative C gave racemic product (entry 5), emphasizing the important role of the TADDOL aryl groups in the asymmetric induction. Changes in the ketal backbone were found to result in small differences in the product ee (compare entries 4 and 6).
Figure 20.
Enantioselective HDA reaction between Brassard's diene and benzaldehyde catalyzed by TADDOL derivatives.
Table 20.
Results from the enantioselective HDA reaction between Brassard's diene and benzaldehyde (0.5 mmol) catalyzed by TADDOL derivatives A - D (Figure 20).
| entry | catalyst | solvent (mL)a | temp (°C) | time (h) | yield (%) | ee (%) |
|---|---|---|---|---|---|---|
| 1 | A | none | rt | 12 | 30 | 7 |
| 2 | B | none | rt | 12 | 40 | 50 |
| 3 | B | none | −30 | 24 | 70 | 71 |
| 4b | B | none | −30 | 24 | 50 | 72 |
| 5b | C | none | −30 | 24 | 40 | 0 |
| 6b | D | none | −30 | 24 | 37 | 74 |
| 7 | B | toluene (0.05) | −30 | 24 | 70 | 75 |
| 8 | B | toluene (0.1) | −30 | 24 | 68 | 76 |
| 9 | B | toluene (0.2) | −60 | 48 | 67 | 83 |
| 10 | B | toluene (0.4) | −60 | 48 | 50 | 86 |
| 11 | B | toluene (0.2) | −78 | 48 | 26 | 89 |
All the reactions were carried out with 2.5 mmol of benzaldehyde and 0.5 mmoll of diene.
10 mol% catalyst was used.
In their work, Ding and co-workers experienced one of the limitations of solvent-free chemistry; the sharp increase in reaction medium viscosity with decreasing temperature. To circumvent this obstacle, small amounts of a low melting solvent, toluene in this case, were added to the reaction vessel to reduce the solution viscosity. Under these concentrated conditions, the reaction temperature could be reduced to −60 °C, which afforded the product in 83–86% ee without a significant reduction in yield. At −78 °C a slight increase in ee was observed, but the yield dropped sharply.
The TADDOL derivative B was effective for the reactions of a variety of aromatic aldehydes to give the corresponding 6-substituted 4-methoxy-5,6-dihydropyran-2-ones in 45–85% yield with 68–91% ee (Table 21). With solid aldehyde substrates, additional toluene was added to ensure that the reaction mixtures were homogeneous.
Table 21.
Reaction of Brassard's diene with various aldehydes catalyzed by TADDOL B (Figure 20).
 | |||||
|---|---|---|---|---|---|
| entry | Ar | toluene (mL)a | temp. (°C) | yield (%) | ee (%) |
| 1b | Ph | 0.2 | −60 | 67 | 83 (S) |
| 2b | furyl | 0.2 | −60 | 80 | 87 (S) |
| 3 | 2-C6H4-Me | 0.2 | −30 | 54 | 68 (R) |
| 4 | 4-C6H4-Cl | 0.2 | −30 | 85 | 76 (R) |
| 5 | 4-C6H4-Br | 0.2 | −30 | 72 | 78 (R) |
| 6 | 3-C6H4-Br | 0.2 | −60 | 67 | 89 (R) |
| 7 | 2-C6H4-Br | 0.2 | −60 | 75 | 82 (R) |
| 8 | 3-C6H4-OMe | 0.2 | −60 | 45 | 91 (n.d.) |
All the reactions were carried out with 2.5 mmol of benzaldehyde and 0.5 mmol of diene.
The catalyst employed in this case was (R,R)-B.
A comparison between the titanium- and zinc-based catalysts in Eq 8 and Figure 14, respectively, which were successfully used in the HDA with Danishefsky's diene, and the TADDOL derived organocatalyst B was made using Brassard's diene. In this case, the organocatalyst proved significantly more enantioselective and higher yielding than the metal containing catalysts under the reaction conditions outlined above.121
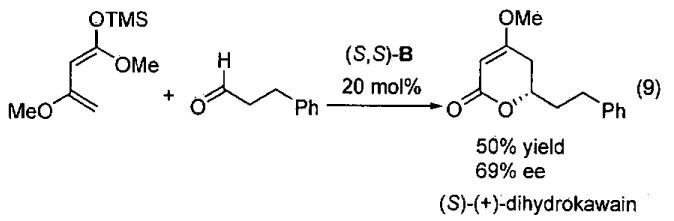
The TADDOL derivative B was also employed in the synthesis of (S)-(+)-dihydrokawain. Thus, reaction of 3-phenylpropionaldehyde with Brassard's diene in the presence of the chiral diol led to formation of the natural product in one step in 50% yield with 69% ee (Eq.9).
2.3.7 Asymmetric Hetero-Diels-Alder Reaction of Mono-Oxygenated Dienes and Aldehydes
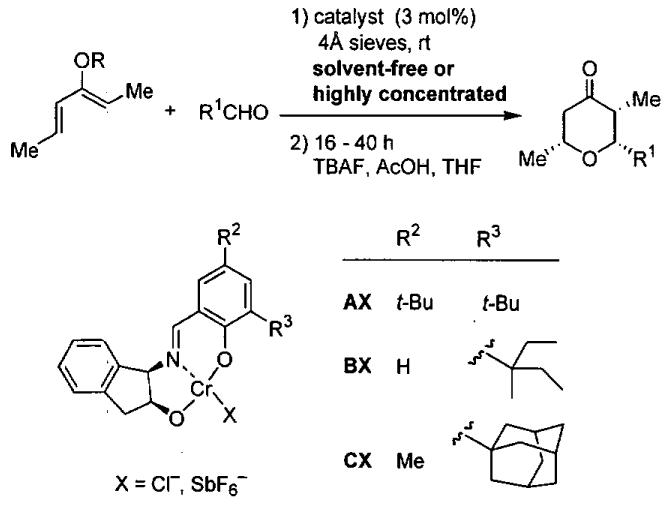
In contrast to the HDA reactions outlined above, which employ the electron rich Danishefsky and Brassard dienes, asymmetric HDA reactions with less electron-rich dienes were not explored until 1999 when Dossetter, Jamison, and Jacobsen studied HDA reactions with monooxygenated dienes catalyzed by Cr(III) species.122 Employing tridentate complexes A•Cl and A•SbF6 in the HDA with (2Z,4E)-triethylsilyloxy-2,4-hexadiene and two aldehydes (R1 = Ph, CH2OTBS) generated tetrahydropyranones after desilylation (Figure 21). While the ee's were moderate (80 and 57% ee, respectively) only the endo product was formed, leading to the all-cis stereochemistry of the tetrahydropyranone product (Table 22, entries 1 and 2). Increasing the size of the catalyst's R3 substituent from t-Bu to 1-ethyl-1-methylpropyl, and 1-adamantyl increased the product ee's to 98% (compare entries 2-5, Table 22). A similar increase in ee with benzaldehyde was not possible under solvent-free conditions (entries 6 and 7), inspiring the investigators to examine solvent additives.122
Figure 21.
Asymmetric HDA with chiral tridentate Schiff base complexes of Cr(III) and monooxygenated dienes.
Table 22.
Results from HDA reaction in Figure 21.
| entry | diene OR |
aldehyde R1 |
solvent | catalyst | yield (%) | ee (%) |
|---|---|---|---|---|---|---|
| 1 | OTES | Ph | none | A•SbF6 | 50 | 80 |
| 2 | OTES | CH2OTBS | none | A•SbF6 | n.d. | 57 |
| 3 | OTES | CH2OTBS | none | B•SbF6 | n.d. | 85 |
| 4 | OTES | CH2OTBS | none | C•Cl | 88 | 98 |
| 5 | OTES | CH2OTBS | none | C•SbF6 | 93 | 98 |
| 6 | OTES | Ph | none | C•Cl | n.d. | 65 |
| 7 | OTES | Ph | none | C•SbF6 | n.d. | 81 |
| 8 | OTES | Ph | conc. | C•SbF6 | 72 (80)a | 90 |
| 9 | OTES | CH2OTBS | conc. | C•Cl | 90 | 99 |
| 10 | OTES | CH2OTBS | conc. | C•SbF6 | 97 | >99 |
| 11 | OTES | CH2OBn | conc. | C•SbF6 | 89 | 94 |
| 12 | OTES | n-C5H11 | none | C•SbF6 | 85 | 98 |
| 13 | OTES | (CH2)4CH=CH2 | none | C•SbF6 | 78 | 98 |
| 14 | OTES | CH2CH2Ph | conc. | C•SbF6 | 78 (84)a | 98 |
| 15 | OTES | CH2CH2NHBoc | conc. | C•SbF6 | 28 (31)a | 96 |
| 16 | OTES | 2-furyl | conc. | C•SbF6 | 77 (86)a | 95 |
| 17 | OTMS | n-C5H11 | none | C•SbF6 | 81 | 98 |
| 18 | OTBS | n-C5H11 | none | C•SbF6 | 93 | 96 |
| 19 | OTIPS | n-C5H11 | none | C•SbF6 | 77 | 94 |
Reaction conversion after 40 h in parenthesis.
Screening various solvents under highly concentrated conditions led to the discovery that addition of acetone resulted in a significant increase in the product ee to 90% (entry 8). Addition of a small amount of acetone to the reactions was found to be general, as illustrated in Table 22; however, with some substrates (entries 12, 13, 17 – 19), the solvent-free conditions resulted in excellent enantioselectivities. It was also noted that the nature of the trialkylsilyl substituent had little impact on the ee or yield of the cycloaddition products (entries 12 and 17 – 19).
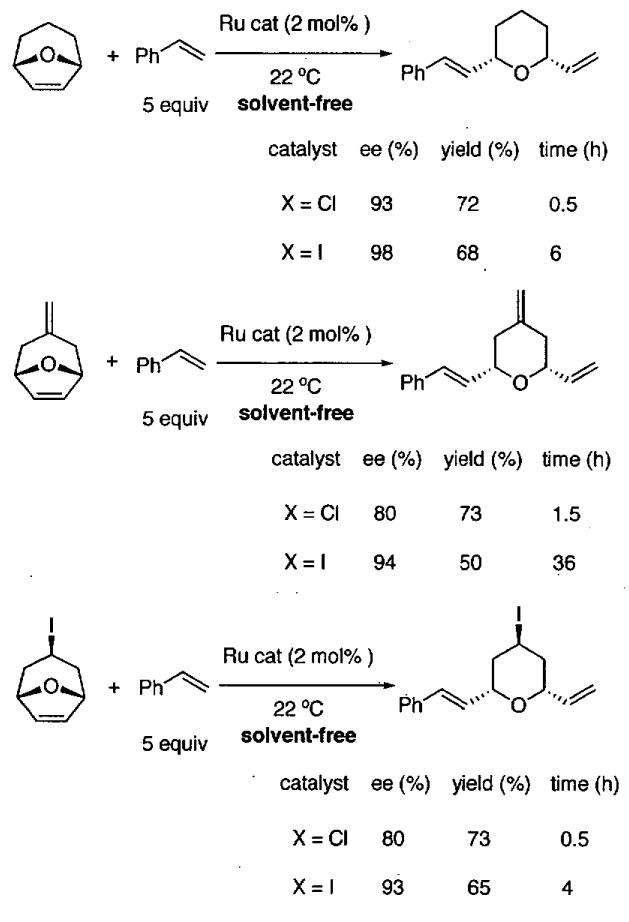
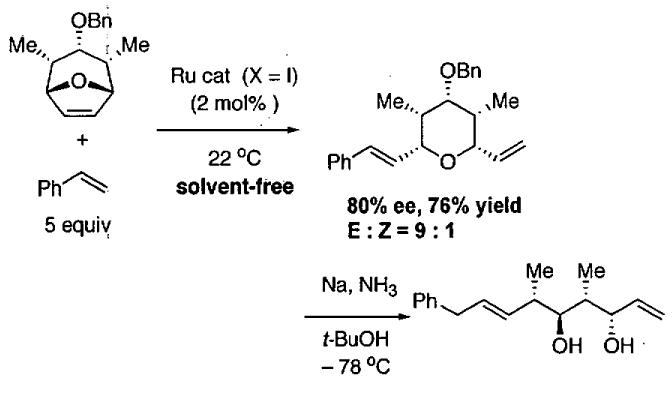
Given the successful HDA reactions between (2Z,4E)-triethylsilyloxy-2,4-hexadiene and various aldehydes catalyzed by C•SbF6 (Table 22), the authors examined the ability of C•C1 to promote HDA reactions with other dienes. As shown in Table 23, reaction of (2E)-2-triethylsilyloxy-l,3-pentadiene resulted in formation of the product in 78% yield with 98% ee (entry 1). Similar results were obtained with the isomeric pentadiene (entry 2). Use of 1-methoxybutadiene proceeded smoothly to furnish the product with >99% ee (91% yield, entry 3).
Table 23.
Catalytic asymmetric HDA reaction with dienes and aldehydes catalyzed by 3 mol% C•Cl from Figure 21.
| entry | diene | product | conditionsa,b | yield (%) | ee (%) | config. |
|---|---|---|---|---|---|---|
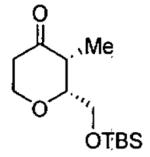
| 1 | 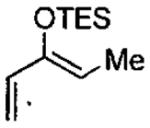 |
 |
200 μL acetone highly concentrated |
78 | 98 | (2R,6R) |
| 2 | 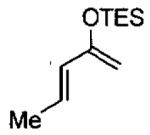 |
 |
sovent-free | 50 | 91 | (2R,3R) |
| 3 | 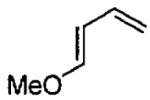 |
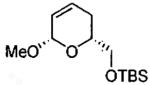 |
sovent-free | 91 | >99 | (2S,6R) |
Reactions were carried out with 1:1 diene : aldehyde on a 1.0 mmol scale with 3 mol% catalyst (1R,2S)-C•Cl and powdered 4 Å molecular sieves.
The products were isolated by treatment with either TFA in CH2Cl2 at 0 °C or with TBAF, AcOH, THF at 0°C.
These new classes of solvent-free and highly concentrated HDA reactions provide straightforward methods to enantiomerically enriched dihydropyran derivatives. Furthermore, up to three stereocenters can be established in the cycloaddition reaction. The resultant products can be elaborated to provide tetrahydropyran derivatives with five defined stereocenters after functionalization of the double bond.
2.3.7.1 Application of the HDA to the Synthesis of FR901464
The natural product FR901464 (Figure 22) was isolated by Fujisawa Pharmaceutical Co. and discovered to possess interesting biological properties, including promising antitumor activity.123-126 The synthesis of this complex target molecule was accomplished via two enantioselective HDA reactions under solvent-free and highly concentrated conditions employing the Cr(III) catalyst C•SbF6 (Figure 21) discussed above.122,127
Figure 22.
Structure of the natural product FR901464.
The key step in the synthesis of the central fragment is a HDA reaction catalyzed by (1R,2S)-C•SbF6 under solvent-free conditions as illustrated in Figure 23. The HDA proceeded in excellent yield and enantioselectivity, establishing 3 of the 4 stereocenters of the central ring in a single reaction. This intermediate was elaborated to the azide, a precursor to the coupling partner (Figure 23).127
Figure 23.
Synthesis of the central fragment of FR901464 via an enantioselective HDA reaction. The catalyst is from Figure 21.
Likewise, the right hand fragment was prepared under highly concentrated conditions via the HDA reaction using the same catalyst (Figure 24). Transformation of this intermediate to the epoxide was accomplished over several steps.
Figure 24.
Synthesis of the right hand fragment of FR901464 via an enantioselective HDA reaction. The catalyst is from Figure 21.
The efficient assembly of the fragments in Figure 23 and Figure 24 allowed the rapid construction of FR901464. The flexibility of this synthesis enabled preparation and biological evaluation of FR901464 analogs.127 The fact that this synthetic strategy employed solvent-free and highly concentrated catalytic asymmetric reactions in the generation of the key intermediates suggests that such approaches will also be applicable to other complex molecule syntheses.
The level of interest in the catalytic asymmetric HDA reactions under solvent-free and highly concentrated reaction conditions can be judged by the large number of successful reports dedicated to catalyst development for this process. The practicality and utility of these methods is highlighted by the efficient synthesis of FR901464 and its analogs.
2.4 Asymmetric Metathesis Reactions
Metathesis reactions have rapidly become one of the most useful tools in synthetic organic chemistry. With the advent very active and enantioselective catalysts for these reactions, researchers are beginning to compare catalyst performance under solution and solvent-free conditions. The resuts of these studies, highlighted below, show that significantly better performance is often observed in the absence of solvent.128,129
2.4.1 Asymmetric Ring Closing Metathesis Reactions
Ring closing metathesis reactions are well established as powerful methods for the synthesis of natural and unnatural products.130-133 Asymmetric ring closing metathesis (ARCM) reactions are emerging as a useful method to prepare small carbocyclic and heterocyclic compounds. Of particular interest are nitrogen containing heterocycles, which are commonly found in biologically active molecules and are relatively difficult to prepare enantioselectively.
In studies directed toward the synthesis of chiral N-heterocycIes, Hoveyda, Schrock, and their coworkers examined the desymmetrization of acyclic amino trienes by ARCM under solvent-free conditions.134 Using the chiral molybdenum catalyst A in Figure 25 (2.5 - 4 mol %), three amines were cyclized with excellent levels of enantioselectivity and high yields (Table 24). Importantly, this method delivers medium-ring unsaturated amines, including the challenging eight-membered derivative (entry 3). Interestingly, monitoring the reaction progress at 22 °C indicated that homodimers were the first-formed species from entries 2 and 3 (Table 24). On continued reaction, however, the oligomeric intermediates were almost entirely converted into cyclic products due to the reversibility of the olefin metathesis.135-137 The enantioenriched cyclized amine products, with their hindered trisubstituted double bonds, do not readily undergo ring opening.
Figure 25.
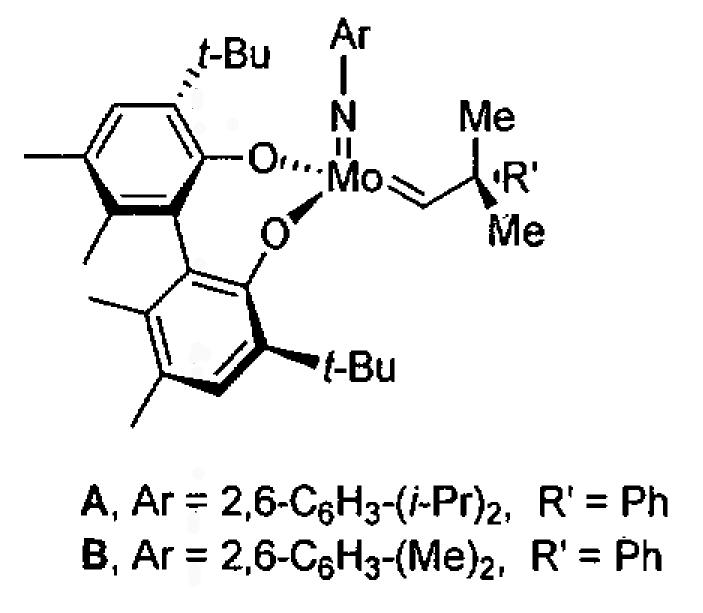
Catalysts for the ARCM.
Table 24.
Desymmetrization of acylic amines using ARCM under solvent-free conditions with catalyst A (Figure 25).
| entry | substrate | product | cat (mol%) |
time (h) |
yield (%) |
ee (%) |
|---|---|---|---|---|---|---|
| 1 |  |
 |
2.5 | 0.2 | 76 | >96 |
| 2 |  |
 |
3.5 | 3.5 | 95 | 95 |
| 3 |  |
 |
4 | 7 | >98 | 97 |
A related catalyst (B, Figure 25) has been used in the enantioselective desymmetrization of two allyl ethers. Under solvent-free conditions, B catalyzed the ARCM in 5 min affording highly enantioenriched dihydrofurans in high yields (Figure 26).138
Figure 26.
Enantioselective desymmetrization of allyl ethers with catalyst B (Figure 25).
The solvent-free desymmetrization of acyclic amines and ethers via ARCM is a unique and potentially useful entry into biologically important heterocycles. Given that there is little change in the solvent polarity in the ARCM reaction, it seems likely that other substrates that behave well under solvent conditions may also exhibit similar enantioselectivities under solvent-free and highly concentrated reaction conditions. Exceptions involve use of solid or semisolid substrates in which the catalysts have limited solubility. In such cases, addition of solvent is necessary for optimal catalyst performance.139
2.4.2 Asymmetric Ring-Opening Metathesis/Cross Metathesis Reactions
Enantioenriched 2,6-disubstituted pyrans are precursors to a variety of natural products of biological importance.140-142 In an effort to synthesize these valuable building blocks, Hoveyda and coworkers143 investigated the asymmetric ring-opening metathesis/cross-metathesis reaction (AROM/CM) of oxabicyclic olefins with the ruthenium catalysts in Figure 27.
Figure 27.
Catalysts for the AROM/CM reaction.
A comparison of standard solvent vs. solvent-free reactions was undertaken. When reactions in Figure 28 were conducted with 5 equiv of the cross-metathesis partner styrene in solution, less than 2% conversion was observed after 24 h. In contrast, in the absence of solvent reactions of oxabicycles proceeded to high conversion allowing products to be isolated in good yields. Although the iodide-based catalyst was less reactive than the chloride derivative, it was more enantioselective, providing pyran with > 90% ee.
Figure 28.
Catalyzed AROM/CM reactions with ruthenium catalysts in Figure 27.
This method was then applied to the preparation of a useful polypropionate precursors (Figure 29) using the iodide-based catalyst from Figure 27.144,145
Figure 29.
Application of the AROM/CM to a key intermediate in the total synthesis of natural polypropionates.
Not only does the catalyst perform better in the absence of solvent in several instances, the catalysts can be isolated more easily and recycled in subsequent reactions.143
2.5 Asymmetric Carbonyl-Ene Reaction
The thermal pericyclic ene reaction represents a typical concerted pathway involving six electrons with a suprafacial orbital interaction. Like the HDA reaction, the carbonyl-ene reaction is concerted and less susceptible to solvent effects. This characteristic suggests that the asymmetric carbonyl-ene reaction might be a good candidate for adaptation to solvent-free reaction conditions, which has proven to be the case. Under standard solvent conditions, asymmetric versions have been achieved with various chiral Lewis acids including Al, Ti, Ni, Pt, Pd, Yb, and Cu complexes.146-157 The catalyst loadings, however, are high, typically ranging from 1 to 10 mol%. Examination of this reaction under solvent-free conditions might allow for a reduction in the catalyst loading without sacrificing TOF.
With the idea of reducing the catalyst loading, Ding and coworkers developed a highly concentrated and solvent-free enantioselective carbonyl-ene reaction (Eq 10).158 Using an approach similar to the one they developed for the titanium-catalyzed HDA reaction (Eq 8), they employed a similar set of chiral BINOL and diol derivatives (Figure 13). Upon combination of two equivalents of ligand with titanium tetraisopropoxide, a library of (Lm)(Ln)Ti-based catalysts was generated. The initial screening indicated that catalysts prepared from BINOL derivatives, substituted at the 3,3'-positions, resulted in reduced enantioselectivities relative to the parent BINOL ligand, as did partially hydrogenated BINOL ligands. Examination of electron withdrawing bromo groups at the 6,6'-positions (L7, Figure 13) resulted in an increase in the enantioselectivity and inspired the synthesis of appropriately substituted BINOL ligands 6,6'-I2-BINOL (L14) and 6,6'-(CF3)2-BINOL (L15) (Figure 13). Application of the homo- and heterocombinations of L14 and L15 was performed under highly concentrated reaction conditions (the solvent volume was about 13% of the reaction mixture) with very low catalyst loadings (0.1–0.01 mol%, Eq 10). As illustrated in Table 25, α-hydroxy esters of very high ee were obtained from α-methyl styrene derivatives.
Table 25.
| entry | olefin | cat. | mol% | t (h) | yield (%) | ee (%) |
|---|---|---|---|---|---|---|
| 1 | A | (L14)2Ti | 0.1 | 48 | 98 | 98.2 |
| 2 | A | (L14)(L15)Ti | 0.1 | 48 | 85 | 97.6 |
| 3 | A | (L14)2Ti | 0.01 | 72 | 76 | 97.2 |
| 4 | A | (L14)(L15)Ti | 0.01 | 72 | 49 | 97.9 |
| 5 | B | (L14)2Ti | 0.1 | 48 | 89 | 99.4 |
| 6 | B | (L14)(L15)Ti | 0.1 | 36 | 96 | 98.2 |
| 7 | C | (L14)2Ti | 0.1 | 48 | 83 | 98.4 |
| 8 | C | (L14)(L15)Ti | 0.1 | 48 | 96 | 98.4 |
| 9 | D | (L14)2Ti | 0.1 | 36 | 92 | 97.1 |
| 10 | D | (L14)(L15)Ti | 0.1 | 36 | >99 | 97.0 |
Three cyclic derivatives were also examined and shown to give excellent enantioselectivities under highly concentrated conditions, as shown in Table 26. It is noteworthy that several of the reactions in Table 25 and Table 26 could be carried out on gram scale. This is particularly important in the case of olefin E (Table 26), because the product is a key intermediate in the synthesis of Trocade, a collagenase-selective inhibitor.159
Table 26.
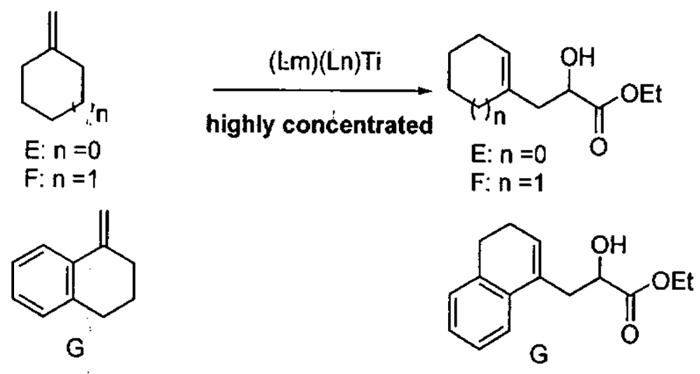 | ||||||
|---|---|---|---|---|---|---|
| entry | olefin | cat. | mol% | t (h) | yield (%) | ee (%) |
| 1 | E | (L14)2Ti | 0.1 | 24 | >99 | 91.6 |
| 2 | F | (L14)(L15)Ti | 0.01 | 42 | 42 | 91.8 |
| 3 | G | (L14)2Ti | 0.1 | 48 | 97 | 92.2 |
| 4 | G | (L14)(L15)Ti | 0.1 | 48 | 94 | 96.3 |
The utility of this methodology was demonstrated by conducting the reaction of ethyl glyoxylate with α-methylstyrene on a 0.1 mol scale, employing 0.05 mol% (L14)(L15)Ti at 0 °C (Eq 11). The carbonyl-ene reaction product was obtained in > 99% yield and 95% ee and was transformed into the functionalized γ-butyrolactone by iodolactonization in excellent yield.
This carbonyl-ene reaction introduced by the Ding group has several advantages: the catalyst is easily generated, the reaction is conducted under highly concentrated conditions, and the catalyst loading is extremely low. Furthermore, the products are furnished with very high enantioselectivities and yields, which make this protocol attractive for the preparation of enantiomerically enriched α-hydroxy esters.
2.5.1 Self-Supported (BINOLate)Ti-Based Catalysts for the Carbonyl-Ene Reaction
An emerging area of interest in asymmetric catalysis is the application of polymeric self-supported catalysts.160-162 One method of preparation of self-supported catalysts involves condensation of a metal complex with a polyfunctional ligand that contains two independent binding sites.163 This type of approach was taken by Ding and coworkers in the development of a chiral polymeric self-supported titanium complex for the asymmetric carbonyl-ene reaction. Using the linked bis(BINOL) ligands, the catalysts were prepared by condensation of ligand A-A, B-B, and C-C with Ti(O-iPr)4 (1:1 ratio) in CHCl3 (Figure 30). The mixtures were stirred at rt for 4 h and then the solvent was removed under reduced pressure.
Figure 30.
Condensation of bis(BINOL) ligands A-A, B-B, and C-C with Ti(Oi-Pr)4 (1:1). The titanium centers may contain coordinated isopropanol ligands.
The resulting solids A-A-Ti, B-B-Ti, and C-C-Ti (1 mol%) were subjected to the carbonyl-ene reaction of ethyl glyoxylate with α-methylstyrene under solvent-free or highly concentrated conditions with a small amount of toluene (Table 27). As showed in Table 27, the carbonyl-ene reaction proceeded readily at rt to generate corresponding α-hydroxy esters in high yield and ee. The nature of the linkers tethering the two BINOL units was found to have a significant impact on the catalyst enantioselectivity. Catalyst B-B-Ti, having a meta-phenylene linker, showed poor catalytic activity and enantioselectivity (entries 7 and 8). In contrast, catalyst C-C-Ti exhibited enhanced enantioselectivity and catalytic activity, affording product in > 99% yield and 96.5% ee (entry 9). Under solvent-free conditions, the catalysts exhibited similar or slightly decreased yield and enantioselectivity (Table 27).
Table 27.
Enantioselective carbonyl-ene reaction with catalysts A-A-Ti, B-B-Ti, and C-C-Ti from Figure 30.
 | ||||||||
|---|---|---|---|---|---|---|---|---|
| entry | catalyst | solvent additive |
4 Å MS | stirring | temp. (°C) |
time (h) |
yield (%) |
ee (%) |
| 1 | A-A-Ti | toluene | no | yes | rt | 48 | 91 | 94.4 |
| 2 | A-A-Ti | toluene | no | yes | 0 | 120 | 85 | 95.4 |
| 3 | A-A-Ti | toluene | no | no | 0 | 120 | 96 | 95.6 |
| 4 | A-A-Ti | toluene | yes | yes | rt | 120 | 93 | 92.1 |
| 5 | A-A-Ti | toluene | yes | yes | 0 | 120 | 65 | 92.5 |
| 6 | A-A-Ti | none | no | no | 0 | 120 | 75 | 94.4 |
| 7 | B-B-Ti | toluene | no | yes | rt | 48 | 32 | 9.8 |
| 8 | B-B-Ti | toluene | no | yes | 0 | 120 | 9 | 24.2 |
| 9 | C-C-Ti | toluene | no | yes | rt | 30 | >99 | 96.5 |
| 10 | C-C-Ti | toluene | no | yes | 0 | 96 | 95 | 91.5 |
| 11 | C-C-Ti | toluene | yes | yes | rt | 96 | 85 | 92.4 |
| 12 | C-C-Ti | toluene | yes | yes | 0 | 96 | 90 | 95.5 |
| 13 | C-C-Ti | none | no | yes | 0 | 96 | 90 | 92.7 |
These investigations clearly indicate that solvent-free and highly concentrated asymmetric reactions can even be conducted under heterogeneous conditions and afford products with high levels of enantioselectivity and yields. Applications such as this hint that it may be possible to couple solvent-free chemistry with heterogeneous supported catalysts that can be recycled, providing systems that minimize the environmental impact on several fronts.
2.6 Asymmetric Addition of Organometallic Reagents to Aldehydes and Ketones
2.6.1 Enantioselective Alkylation of Aldehydes with Dialkylzinc Reagents
Enantioenriched secondary alcohols are very useful chiral building blocks in asymmetric synthesis that have been prepared by asymmetric reduction of ketones164 and asymmetric addition of alkyl groups to aldehydes.165-167 Of these two methods, the later results in simultaneous formation of a C-C bond and a stereocenter, making it more synthetically efficient. The asymmetric addition of alkyl groups to aldehydes has been one of the most studied reactions in asymmetric catalysis, with literally hundreds of catalysts that will promote this reaction with high enantioselectivity.168-176 Despite the widespread attention that this reaction has received, it has seldom been examined under solvent-free or highly concentrated conditions.
In an early report of solvent-free asymmetric catalysis, Soai and coworkers examined the asymmetric addition of alkylzinc reagents to aldehydes.177 In the presence of N,N-dialkylnorephedrines as chiral ligands, the addition of diethylzinc to benzaldehyde was examined (Table 28). It was noted that the ligand loading could be decreased from 10 mol% to 3.4 mol% with no loss in enantioselectivity. The reaction under solvent-free conditions reached completion in 6 h at −28 °C, whereas when toluene was used at this temperature, little conversion was observed. Thus, under solvent-free conditions, reactions times were dramatically reduced with no loss of enantioselectivity.
Table 28.
Catalyst Evaluation in the Asymmetric addition of diethylzinc to benzaldehyde under solvent-free conditions.
 | ||||||
|---|---|---|---|---|---|---|
| entry | R | mol% cat | temp. (°C) | time (h) | yield (%) | ee (%) |
| 1 | n-Bu | 10 | 0 | 2 | 99 | 87 |
| 2 | n-Bu | 5 | 0 | 2 | 97 | 87 |
| 3 | n-Bu | 3.4 | 0 | 2 | 93 | 87 |
| 4 | n-Pr | 5 | 0 | 2 | 99 | 85 |
| 5 | −(CH2)4− | 5 | 0 | 2 | 97 | 86 |
| 6 | n-Bu | 5 | −10 | 4 | 98 | 89 |
| 7 | n-Bu | 5 | −28 | 6 | 99 | 87 |
A variety of aryl and alkyl substituted aldehydes were examined under solvent-free conditions with 3-5 equivalents of Et2Zn and 5 mol% of the enantioenriched amino alcohol (Table 29). The reactions were homogeneous and proceeded in 2 h at 0 °C in a highly enantioselective manner to afford secondary alcohols with 84–91% ee.
Table 29.
Asymmetric addition of diethylzinc to aryl and saturated aldehydes under solvent-free conditions.
 | |||
|---|---|---|---|
| entry | R | yield (%) | ee (%) |
| 1 | 4-C6H4-Me | 99 | 90 |
| 2 | 1-Naphthyl | 80 | 91 |
| 3 | 2-Naphthyl | 98 | 86 |
| 4 | 4-C6H4-Cl | 99 | 85 |
| 5 | 4-C6H4-OMe | 98 | 89 |
| 6 | c-C6H11 | 96 | 88 |
| 7 | CH2CH2Ph | 92 | 84 |
Soai and coworkers have also examined the asymmetric addition of diethylzinc to N-diphenylphosphinoylimines using chiral amino alcohol ligands. While enantioselectivities up to 97% were obtained under solvent-free conditions, the reactions were stoichiometric in chiral ligands and are not further discussed here.178
The results outlined above indicate that the asymmetric alkylation of aldehydes may be an ideal class of reactions for examination under solvent-free conditions. Given the efficiency of the addition, it might be possible to substantially reduce the catalyst loading without loss in enantioselectivity in this synthetically important transformation.
2.6.2 Catalytic Asymmetric Acetylide Additions to Aldehydes
In 2001, Anand and Carreira reported the solvent-free catalytic asymmetric addition of acetylides to aldehydes to provide enantioenriched propargylic alcohols, which are valuable precursors to a wide range of chiral materials.179 Under standard conditions with toluene as solvent, a variety of aliphatic aldehydes underwent addition providing propargylic alcohols with high levels of enantioselectivity (> 90%) and good to excellent yields. Employing the solvent-free conditions, dry Zn(OTf)2 was combined with the amino alcohol (22 mol%), alkyne (1.05 equiv), triethylamine (50 mol%) and the aldehyde (Figure 31). The reaction was stirred for 6 h at 60 °C before workup and isolation of the product. As outlined in Figure 31, enantioselectivities were excellent, although slightly diminished from reactions conducted with toluene as solvent. Isolated yields of the propargylic alcohols were higher using solvent-free conditions.
Figure 31.
Catalytic asymmetric addition of alkynes to aldehydes under solvent-free conditions.
Although only two examples of solvent-free additions were outlined in this report, this chemistry represents a significant breakthrough in that it is not only catalytic in chiral ligand, but it is also catalytic in zinc. Presumably this novel methodology could be extended to other aldehydes and alkynes.
2.6.3 Asymmetric Addition of Alkyl Groups to Ketones
In contrast to the asymmetric addition of dialkylzinc reagents to aldehydes, which is promoted by a multitude of catalysts, the analogous addition to ketones has proven quite challenging.180-191 The Walsh group has recently developed a catalyst for this process that exhibits high enantioselectivities with a broad range of ketone substrates in toluene and hexanes solvent (Figure 32). The resulting enantioenriched tertiary alcohols are useful chiral building blocks, and have been used in natural product synthesis.192
Figure 32.
Asymmetric addition of alkyl groups to ketones.
As outlined in Figure 32, the asymmetric addition of alkyl groups to ketones using a solvent mixture of toluene and hexanes required 2-10 mol% of the chiral bis(sulfonamide) diol ligand to achieve the highest levels of enantioselectivity. While 2 mol% ligand loading is low for early transition metal catalysts, many substrates required 10 mol% L* to maintain high enantioselectivities and reasonable reaction times. It was hypothesized that reducing the amount of solvent employed in the asymmetric addition would have several benefits. First, the corresponding increase in the concentration of the catalyst and reagents could result in greater catalyst turnover frequency, possibly allowing a reduction in catalyst loadings. Second, decreasing the quantity of solvent would make scale up more attractive by cutting waste generation and reducing costs.
In the initial examination of highly concentrated and solvent-free reactions, Jeon, Li and Walsh193 employed diethylzinc in the asymmetric addition to 3'-methylacetophenone (Table 30). Under standard conditions with toluene and hexanes solvents and 2 mol% catalyst, the reaction required 24 h providing 78% isolated yield of the tertiary alcohol with 99% ee (entry 1).194 In the absence of solvent, the same addition reaction was complete in much less time (4 h) with half the catalyst loading (1 mol%) and essentially the same enantioselectivity and yield (Table 30, entry 2). Similar results were obtained under highly concentrated conditions, when two equiv of toluene (relative to ketone) were added to the reaction mixture (Table 30, entry 3). Given these observations, the possibility of further decreasing the catalyst loading to as low as 0.1 mol% was investigated. In these cases, the addition product was obtained in slightly lower yields and with a small decrease in enantioselectivity (up to 3%). As anticipated, the reactions also required longer times at the lower catalyst loadings (Table 30, entries 4-6). The results in Table 30 indicate that low catalyst loadings can be successfully employed, in part because the catalyst exhibits a high degree of ligand acceleration195 over the uncatalyzed background reaction of diethylzinc with ketone substrates.
Table 30.
Diethylzinc additions to 3'-methylacetophenone under solvent-free and highly concentrated reaction conditions.
 | |||||
|---|---|---|---|---|---|
| entry | solvent | L* (mol %) | time (h) | yield (%) | ee (%) |
| 1 | tol/hex | 2 | 24 | 78 | >99 |
| 2 | none | 1 | 4 | 79 | 99 |
| 3 | 2 equiv tola | 1 | 7 | 78 | 99 |
| 4 | none | 0.5 | 20 | 80 | 98 |
| 5 | none | 0.25 | 21 | 62 | 97 |
| 6 | none | 0.1 | 24 | 58 | 96 |
Two equiv toluene relative to ketone substrate.
Table 31 shows the scope, limitations, and comparison of solvent-free, highly concentrated, and standard solvent conditions in diethylzinc additions to ketones. In several cases, the difference in these methods is quite large. For example, in toluene and hexanes, ethyl addition to 4'-methoxyacetophenone required 111 h at 10 mol% catalyst loading (Table 31, entry 3). Under the solvent-free conditions, the reaction was significantly faster, reaching completion in 12 h at 1 mol% catalyst loading. Unfortunately, both the yield and ee of the tertiary alcohol decreased to 50% and 81%, respectively. By conducting the addition with two equiv of toluene relative to ketone, the enantioselectivity increased to 89%. Valerophenone exhibited a decrease in reaction time from over 4 days at 2 mol% ligand under standard conditions to 1 day at 1 mol% ligand under solvent-free and highly concentrated conditions. The enantioselectivity dropped, however, to 80% (Table 31, entry 5). These initial results highlight an important difference between standard, highly concentrated, and solvent-free conditions in this system: in the latter cases, the enantioselectivities are more sensitive to substrate structure.
Table 31.
Diethylzinc additions to ketones under solvent-free, highly concentrated, and standard conditions.
 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| solvent-free or highly concentrated conditions |
standard conditions |
||||||||
| entry | substrates | L* (moi%) | t (h) | y (%) | ee (%) | L*(mol%) | t (h) | y (%) | ee (%) (config.) |
 |
|||||||||
| 1 | X = H | 1 0.5 |
4 21 |
75 78 |
97a 96a |
2 | 29 | 71 | 96 (S) |
| 2 | X = 3-CF3 | 0.5 | 17 | 77 | 96a | 2 | 14 | 56 | 98 |
| 3 | X = 4-OMe | 1 1 |
12 15 |
50 72 |
81a 89b |
10 | 111 | 85 | 94 |
| 4 |  |
0.5 0.5 |
12 24 |
74 76 |
98a 98b |
2 | 27 | 90 | 97 |
| 5 | 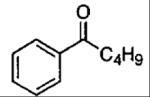 |
1 1 |
24 24 |
78 85 |
80a 80b |
2 | 102 | 79 | 88 (R) |
| 6 |  |
1 | 15 | 71 | 90b | 2 | 26 | 80 | 90 |
| 7 |  |
1 | 22 | 53 | 93b | 2 | 46 | 56 | 96 |
| 8 |  |
1 | 22 | 36 | 96b | 10 | 40 | 76 | 98 |
Solvent-free conditions.
2 Equiv toluene was added to the reaction.
One important class of substrates for these asymmetric addition reactions is enones. The addition products are tertiary allylic alcohols that can be functionalized at the double bond through hydroxyl directed reactions. As illustrated in Table 31, enantioselectivities > 90% were observed with a variety of enones at 1 mol% catalyst loading with 2 equiv of toluene. Surprisingly, 2,4,4-trimethylcyclohexenone gave only 36% yield of the addition product, with the remainder of the material isolated as byproducts. This is in contrast to the standard conditions, where the product was isolated with 76% yield (Table 31, entry 8). The increased quantity of byproducts under the highly concentrated reaction conditions appears to be substrate dependent.
It is well known that dimethylzinc adds to aldehydes around 20 times slower than diethylzinc with amino alcohol-based catalysts,196 thus experiments to compare the reactivity of dimethyl- and diethylzinc under solvent-free conditions were performed. As shown in Table 32, excellent results were obtained in the solvent-free dimethylzinc addition reactions. In these reactions, 2 equiv of the dimethylzinc and 1.2 equiv of Ti(O-iPr)4 were generally employed. Propiophenone and 3'-chloropropiophenone gave excellent yields (83-95%) and enantioselectivities (92-94%) using 1.0 or 0.5 mol% L* (Table 32, entries 1 and 2). The enantioselectivity in the methyl addition to propiophenone decreased to 80% at 0.25 mol% catalyst loading, however. It was hypothesized that the background reaction promoted by Ti(Oi-Pr)4 could be playing a role in the erosion of enantioselectivity at 0.25 mol% catalyst. A substoichiometric amount of Ti(Oi-Pr)4 was, therefore, employed under otherwise identical conditions. When the Ti(Oi-Pr)4 was reduced from 1.2 equiv to 0.4 equiv at 0.25 mol% catalyst loading, the enantioselectivity was restored to 92% (Table 32, entry 1).
Table 32.
Dimethylzinc additions under solvent-free and standard reaction conditions.
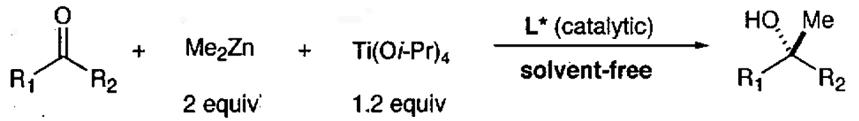 | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| solvent-free conditions |
standard conditions |
||||||||
| entry | substrates | L* (mol %) | t (h) | y (%) | ee (%) | L* (mol %) | t (h) | y (%) | ee (%) (config.) |
| 1 |  |
1 0.5 0.25 0.25 |
15 48 60 72 |
85 83 85 87 |
92 92 80 92a |
2 | 45 | 83 | 94 (R) |
| 2 | 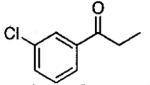 |
1 0.5 |
45 60 |
95 95 |
94 94 |
2 | 46 | 90 | 96 |
| 3 |  |
1 0.5 |
43 45 |
95 93 |
83 77 |
2 | 48 | 81 | 85 |
| 4 |  |
1 0.5 |
45 44 |
75 83 |
96 95 |
10 | 40 | 84 | 99 |
| 5 |  |
1 0.5 |
22 44 |
77 .78 |
96 97 |
10 | 40 | 62 | 99 |
| 6 | 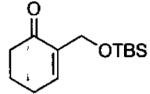 |
1 0.5 0.25 |
24 44 70 |
90 83 84 |
97 97 92 |
10 | 40 | 81 | 99 |
| 7 |  |
1 | 60 | 90 | 96 | 10 | 60 | 84 | 98 |
| 8 |  |
1 | 24 | 43 | 99 | 10 | 38 | 20 | 99 |
0.4 equiv Ti(Oi-Pr)4 was used.
Valerophenone provided an opportunity to directly compare the rates of addition of diethyl- and dimethylzinc with the catalyst in the absence of solvent. Under solvent-free conditions, diethylzinc was found to react about 2 times faster than dimethylzinc, as seen by comparison of entry 3 in Table 32.
Unlike diethylzinc addition to 2,4,4-trimethylcyclohexenone, which generated mostly byproducts, dimethylzinc addition proceeded smoothly in 75-83% yield with >95% enantioselectivity (entry 4). Other cyclic enones also proved to be excellent substrates for the solvent-free dimethylzinc additions (Table 32, entries 5-7). Of particular note are the results of addition to 2-pentyl-2-cyclopenten-1-one (entry 5) and the TBS protected enone (entry 6). For these substrates, the catalyst loading could be lowered 20-fold with similar or better enantioselectivity, yield, and reaction time as compared to the standard conditions. Furthermore, although the yield of addition to 2-benzylidene cyclohexanone remained low in the solvent-free reactions, it was double that of the standard solution phase reaction (Table 32, entry 8).
Addition of functionalized organozinc reagents to ketones, under standard conditions with 10 mol% loading of ligand L*, resulted in high enantioselectivities but long reaction times (48-120 h).185 With the goal to reduce the catalyst loading and reaction times, the addition of functionalized diorganozinc reagents to ketones was examined under solvent-free and highly concentrated reaction conditions.
Using the methods of Knochel for the preparation of functionalized organozinc reagents,197-203 a dialkylzinc reagent bearing a TBS-protected alcohol was employed in the solvent-free addition to 3'-methylacetophenone. Employing solvent-free conditions with 1 mol% catalyst, the enantioselectivities were 80% or less, significantly below the 98% enantioselectivity recorded for the standard conditions at 10 mol% catalyst (Table 33, entry 1).185 Addition of 2 equiv of toluene, however, restored the enantioselectivity to 97%. Similarly, addition of the bromoalkyl to this substrate exhibited higher yields and enantioselectivity under highly concentrated conditions than with the solvent-free conditions (Table 33, entry 2). Higher enantioselectivities under the concentrated reaction conditions relative to the solvent-free conditions was found to be general for functionalized organozinc additions (Table 33, entries 1-3). Surprisingly, using 2-acetonaphthone, the enantioselectivities with the functionalized organozinc reagents were higher under the concentrated conditions than under the standard conditions. In Table 33, entry 3, reduction of the amount of titanium tetraisopropoxide from 1.2 to 0.6 equiv at 0.25 mol% catalyst resulted in an increase in the enantioselectivity from 76 to 90%. Additions to trans-4-phenyl-3-buten-2-one at 1 mol% catalyst loading under concentrated conditions exhibited similar enantioselectivities compared to the standard conditions with significantly reduced reaction times (Table 33, entries 5-7). In general, addition of 2 equiv toluene allowed a 10-fold reduction in catalyst loading and reduced reaction times while maintaining high enantioselectivities. The origin of the beneficial effect of toluene is not understood. The results in Table 33 suggest that the highly concentrated nature of these reactions makes them particularly sensitive to the small changes in the composition of the reaction mixture.
Table 33.
Comparison of solvent-free, highly concentrated, and standard reaction conditions for the asymmetric addition of functionalized organozinc reagents to ketones.
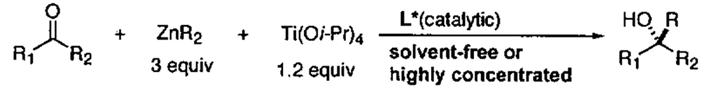 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| entry | substrates | ZnR2 | solvent-free or highly concentrated conditions |
standard conditions |
||||||
| L* (moi%) | t (h) | y (%) | ee (%) | L* (mol%) | t (h) | y (%) | ee (%) | |||
| 1 |  |
Zn((CH2)4OTBS)2 | 1 0.5 0.25 1 |
48 70 82 40 |
68 53 44 68 |
79a 80a 69a 97b |
10 | 72 | 89 | 98 |
| 2 | Zn((CH2)5Br)2 | 1 0.5 1 |
45 50 46 |
66 41 71 |
92a 92a 97b |
10 | 72 | 89 | 96 | |
| 3 |  |
Zn((CH2)5Br)2 | 1 0.5 0.25 0.25 1 |
40 76 84 90 38 |
55 47 30 30 72 |
94a 94a 76a 90a,c 97b |
10 | 72 | 55 | 94 |
| 4 | Zn((CH2)3CHMe2)2 | 1 | 18 | 56 | 94b | 10 | 72 | 75 | 90 | |
| 5 |  |
Zn((CH2)3CHMe2)2 | 1 | 27 | 63 | 93b | 10 | 72 | 86 | 93 |
| 6 | Zn((CH2)4OTBS)2 | 1 | 21 | 76 | 87b | 10 | 120 | 65 | 90 | |
| 7 | Zn((CH2)5Br)2 | 1 | 36 | 65 | 89b | 10 | 48 | 48 | 90 | |
Solvent-free conditions.
Concentrated reaction conditions (2 equiv toluene was added to the reaction).
0.6 Equiv Ti(Oi-Pr)4 was used.
The scalability of the solvent-free procedure for the enantioselective addition of alkyl groups to ketones was examined (Eq 12).193 The reaction of 5 g propiophenone with dimethylzinc was conducted with 0.5 mol% L* under solvent-free conditions for 48 h to provide 5 g (90% yield) of the addition product with 92% ee. The ligand was recovered in 84% yield. These results highlight the potential scalability of this solvent-free process.
2.6.3.1 Tandem One-Pot Asymmetric Addition/Diastereoselective Epoxidation Sequence
Jeon and Walsh recently reported a one-pot procedure whereby the asymmetric addition to enones could be followed by a directed epoxidation to afford syn epoxy alcohols with three contiguous stereocenters.191 In this chemistry, the asymmetric addition was conducted under standard conditions, and the resulting tertiary allylic alkoxide product was exposed to dioxygen to initiate the directed epoxidation. A proposed mechanism of this reaction (Figure 33) involves insertion of dioxygen into the Zn-C bond to generate a zinc peroxy species. Transmetalation of the peroxide to the titanium allylic alkoxide followed by oxygen atom transfer and work up affords the epoxy alcohol product. In support of this proposal, TBHP (tert-butyl hydroperoxide) can be substituted for dioxygen in the directed epoxidation step.204 Presumably, the TBHP protonates the dialkylzinc to generate a similar zinc peroxide. An advantage of TBHP over dioxygen in the epoxidation is that it is easier to control the rate of addition of the TBHP.
Figure 33.
One-pot asymmetric addition/diastereoselective epoxidation reaction with dioxygen.
A One-pot asymmetric addition/diastereoselective epoxidation protocol employing the solvent-free reaction conditions was developed based on the addition of dimethylzinc to enones (Table 32). After conducting the addition reaction under the solvent-free reaction conditions, the reaction mixture was cooled to 0 °C, and commercially available 5.5 M TBHP in decane was cautiously added (Table 34).205 After 4 h, the reaction was complete, and the epoxy alcohols were isolated in high yields (87-95%, Table 34). It is noteworthy that these yields are significantly higher than those obtained under standard conditions.191
Table 34.
One-pot asymmetric addition/diastereoselective epoxidation of cyclic enones with TBHP.
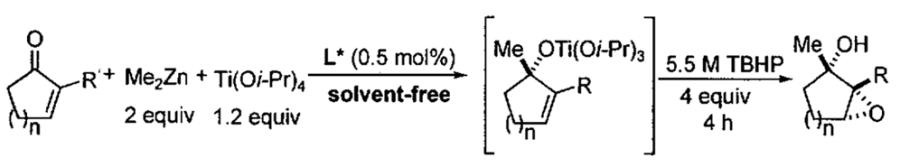 | |||||
|---|---|---|---|---|---|
| entry | substrates | time (h) |
products | yield (%) | |
| addition | epoxidation | ||||
| 1 | 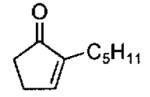 |
45 | 4 | 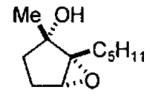 |
87 |
| 2 | 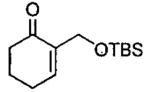 |
44 | 4 | 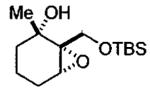 |
95 |
| 3 | 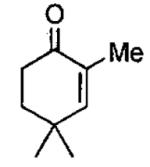 |
44 | 4 |  |
95 |
Inspired by these results in Table 34, the one-pot asymmetric addition/diastereoselective epoxidation was examined on a larger scale with dimethylzinc to 2-pentyl-2-cyclopenten-1-one using 5 g of the enone, 2 equiv dimethylzinc, and 0.5 mol% ligand (Eq 13). Upon completion of the addition, the reaction was cooled to −10 °C, and 5.5 M TBHP (4 equiv) was carefully added. After 4 h, the reaction was quenched and the epoxy alcohol was isolated in 90% yield (5.45 g) with 90% ligand recovery. The 90% yield of the syn epoxy alcohol obtained in this large-scale reaction represents a significant improvement over the 67% yield originally reported for the asymmetric addition/diastereoselective epoxidation under standard conditions.194
As outlined in this study of solvent-free and highly concentrated asymmetric additions to ketones, catalyst loading can be reduced 4- to 40-fold while maintaining similar yields and high levels of enantioselectivity compared to reactions conducted with solvents. This work represents the first examples of solvent-free asymmetric additions to ketones in tandem with a diastereoselective epoxidation reaction to provide syn epoxy alcohols with high levels of enantio- and diastereoselectivity. Furthermore, the results suggest that both the asymmetric alkylation of ketones and the asymmetric addition/diastereoselective epoxidation are amenable to scale up. The solvent-free and highly concentrated asymmetric additions outlined above represent the best methods to date to synthesize enantioenriched tertiary alcohols and tertiary epoxy alcohols.
2.6.4 Asymmetric Allylation of Ketones
The catalytic asymmetric allylation of aldehydes and ketones has proven to be a very useful transformation in organic synthesis.176,206 Although highly enantioselective catalysts for the allylation of aldehydes were reported in the early 1990's,207,208 highly enantioselective catalysts for ketone allylation have proven more challenging. New catalysts for this reaction, however, are beginning to emerge.176,206,209-218
Waltz, Gavenonis, and Walsh introduced the first general and highly enantioselective catalyst for the asymmetric allylation of ketones (Eq 14).219,220 The catalyst, based on titanium tetraisopropoxide, BINOL, and isopropanol required high loadings to achieve maximum enantioselectivity (Table 35, entry 1, 20 mol %). In an effort to reduce the amount of solvent employed, the allylation in entry 1 (96% ee) was conducted in the absence of dichloromethane solvent (Eq 14). The enantioselectivity of the product dropped to 85% at 20 mol% catalyst (Table 35, entries 1 vs. 5) and even further at lower loadings (entries 6 and 7). Under the highly concentrated conditions, the enantioselectivity fell below the useful level with this catalyst system.221
Table 35.
Catalytic asymmetric allylation of 3'-methylacetophenone from Eq 14.
| entry | solvent | Ti(Oi-Pr)4 (mol %) |
BiNOL (mol %) |
IPA (equiv) |
% ee (% yield) |
|---|---|---|---|---|---|
| 1 | CH2Cl2 | 20 | 20 | 20 | 96 (82) |
| 2 | 20 | 20 | 3 | 94 (91) | |
| 3 | 10 | 10 | 20 | 88 (94) | |
| 4 | 10 | 10 | 3 | 81 (86) | |
| 5 | no CH2Cl2 | 20 | 20 | 20 | 85 (91) |
| 6 | concentrated | 10 | 10 | 3 | 81 (88) |
| 7 | 5 | 5 | 3 | 79 (84) | |
It is known that the ratio of titanium to BINOL can impact the enantioselectivity in the asymmetric allylation of aldehydes.207,208,222 Wooten, Kim, and Walsh subsequently demonstrated that under highly concentrated conditions (no dichloromethane), increasing the ratio of Ti : BINOL from 1 : 1 to 1 : 2 in Eq 14 resulted in an increase in the enantioselectivity from 88% to 95% while maintaining high yield (compare entries 1 and 2, Table 36). On the basis of this observation, catalyst optimization was continued maintaining a 1 : 2 ratio of Ti : BINOL. Reduction of the titanium to 5, 2, and 1 mol% led to an erosion of enantioselectivity, which became large below 5 mol % (Table 36, entries 3-5).
Table 36.
Optimization of the asymmetric allylation of 3-methylacetophenone (Eq 14) by variation of the Ti: BINOL and the mol% catalyst loading.
| entry | Ti(Oi-Pr)4 (mol %) |
BINOL (mol %) |
IPA (equiv) |
% ee (% yield) |
|---|---|---|---|---|
| 1 | 10 | 10 | 3 | 88 (88) |
| 2 | 10 | 20 | 3 | 95 (93) |
| 3 | 5 | 10 | 3 | 90 (90) |
| 4 | 2 | 4 | 3 | 55 (97) |
| 5 | 1 | 2 | 3 | 28 (95) |
The substrte scope was explored with a 1 : 2 ratio of Ti : BINOL and 3 equiv of IPA under highly concentrated conditions (Table 37). Substituted acetophenone derivatives, such as 3'-(trifluoromethyl) and 4'-methoxyacetophenone, underwent allylation at 10 mol% catalyst loading with similar enantioselectivities (89% and 88%, entries 3 and 5). For comparison, 3'-methylacetophenone underwent allylation under the same conditions generating the product in 95% ee (Table 37, entry 1). Catalyst reduction to 5 mol% in the allylation of acetophenone derivatives resulted in a decrease in enantioselectivities to 90%, 79%, and 82% (entries 2, 4, and 6, respectively). The enone in entries 7 and 8 proved to be an excellent substrate, affording enantioselectivities of 91% and 85% at 10 and 5 mol% catalyst, respectively.
Table 37.
Substrate scope in the asymmetric allylation of ketones with 1 : 2 Ti: BINOL ratio under highly concentrated conditions.
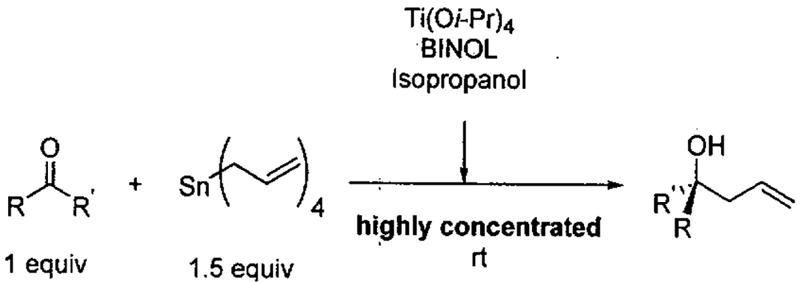 | |||||
|---|---|---|---|---|---|
| entry | substrate | Ti:BINOL (mol %) |
IPA (equiv) |
ee (%) | yield (%) |
| 1 |  |
10 : 20 | 3 | 95 | 93 |
| 2 | 5 : 10 | 3 | 90 | 90 | |
| 3 | 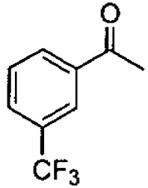 |
10 : 20 | 3 | 89 | 87 |
| 4 | 5:10 | 3 | 79 | 85 | |
| 5 | 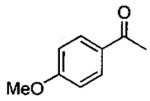 |
10 : 20 | 3 | 88 | 99 |
| 6 | 5 : 10 | 3 | 82 | 92 | |
| 7 | 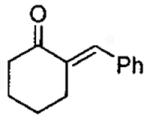 |
10 : 20 | 3 | 91 | 96 |
| 8 | 5 : 10 | 3 | 85 | 99 | |
| 9 | 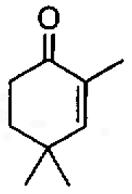 |
10 : 20 | 3 | 91 | 80 |
| 10 | 5 : 10 | 3 | 86 | 85 | |
| 11 |  |
10 : 20 | 3 | 87 | 99 |
| 12 | 5 : 10 | 3 | 79 | 99 | |
Allylation of the endocyclic α,β-unsaturated enone provided the corresponding homoallylic alcohol in 91% and 86% enantioselectivity at 10 and 5 mol% catalyst (entries 9 and 10). The cyclic ketone α-tetralone reacted with slightly lower enantioselectivity (87% and 79%, entries 11 and 12). the results listed in Table 37 demonstrate the potential range of substrates that can be transformed into homoallylic alcohols with high levels of enantioselectivity using this new catalyst under highly concentrated conditions.
2.6.4.1 Tandem One-Pot Asymmetric Allylation/Diastereoselective Epoxidation Sequence
The synthetic efficiency of a transformation can be increased via tandem reactions, which enable rapid increases in molecular complexity with minimal isolation and purification.223 Thus, Wooten, Kim and Walsh conducted the asymmetric allylation of enones under highly concentrated conditions followed by a chemo- and diastereoselective directed epoxidation of the allylic double bond (Table 38).221 In this reaction, the titanium allylation catalyst also catalyzes the diastereoselective directed epoxidation reaction. In this way, the ketone allylation under highly concentrated conditions was followed by addition of 1 equiv of anhydrous TBHP (5.5 M) (Table 38). The epoxidation proceeded smoothly at room temperature to provide syn epoxy alcohols as a single diastereomer in 84 – 87% yield (Table 38). No loss of ee was observed in the generation of the epoxy alcohols in Table 38 compared to the simple allylation products (Table 37). Starting from achiral precursors, this one-pot sequence results in the generation of three contiguous stereocenters with excellent enantio-, diastereo-, and chemoselectivity and with high yields.221
Table 38.
Tandem asymmetric allylation under concentrated conditions followed by a chemo- and diastereoselective epoxidation.
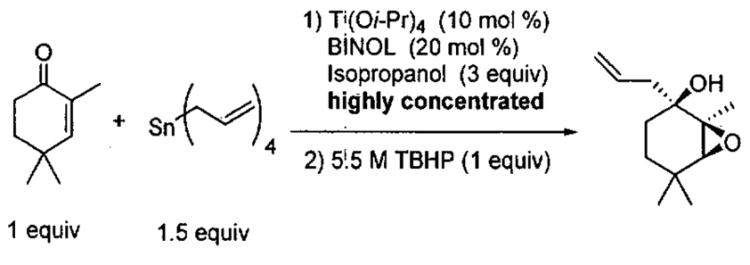 | ||||
|---|---|---|---|---|
| entry | substrate | product | ee (%) | yield (%) |
| 1 |  |
 |
91 | 84 |
| 2 |  |
 |
91 | 87 |
| 3 |  |
 |
90 | 85 |
There are several noteworthy features about this system. Although the adaptation of the original highly enantioselective 1 : 1 titanium tetraisopropoxide : BINOL allylation catalyst to highly concentrated conditions resulted in significant loss in enantioselectivity (Table 35), a new catalyst was developed that exhibited high enantioselectivity under highly concentrated conditions. The new catalyst has a 1 : 2 ratio of titanium tetraisopropoxide : BINOL. Under highly concentrated conditions, the loading of titanium tetraisopropoxide and BINOL were reduced with respect to the original catalyst system in dichloromethane. Although elimination of the dichloromethane is significant the use of tin reagents will need to be circumvented to increase the environmentally friendliness of this process. Nonetheless, the enantioenriched products accessible with these methods are particularly useful intermediates in synthesis.
2.7 Lewis Acid Catalyzed Asymmetric Michael Reactions
A driving force in asymmetric catalysis is the development of catalysts that are efficient, highly enantioselective, scalable, and applicable to the synthesis of natural and unnatural products. One system that comes very close to meeting these requirements is Shibasaki's aluminum lithium BINOLate-based (ALB) catalyst (Figure 34).224,225 At low catalyst loading (0.1 mol%) (R)-ALB promoted the asymmetric conjugate addition of dimethyl malonate to 2-cyclohexenone in the presence of catalytic KOt-Bu with 98% enantioselectivity as illustrated in Figure 34.226 Crystallization furnished the product in 91% yield with an ee of > 99%. The reaction was performed on a 6 mol scale with only 123 mL of THF solvent. The highly concentrated conditions allow the researchers to perform the asymmetric addition in a 2 L round-bottomed flask with very high volume productivity and atom efficiency.
Figure 34.
Highly efficient enantioselective Michael reaction catalyzed by (R)-ALB under highly concentrated conditions.
The efficient and highly enantioselective generation of the Michael addition product allowed Shibasaki and coworkers227 to synthesize (−)-strychnine, one of the most complex natural products for its weight. The Michael addition product is transformed into the E-ring of this alkaloid in an elegant synthesis (Figure 35).
Figure 35.
The asymmetric addition product was the starting material for the synthesis of (−)-strychnine.
The asymmetric Michael reaction catalyzed by the heterobimetallic ALB catalyst is an exemplary in many respects and certainly attests to the potential utility of highly concentrated reaction conditions on large scales.
2.8 Asymmetric Friedel-Crafts with Aromatic Ethers
The catalytic asymmetric Friedel-Crafts reaction has received considerable attention in recent years, resulting in significant advances.228 The products of these reactions contain benzylic carbon stereocenters, which are found in thousands of natural products and have achieved a privileged status in medicinal chemistry.229
Bis(oxazoline) ligands230 have been shown to give high enantioselectivities under normal solvent conditions.231,232 Jørgensen and coworkers had examined copper(II)-based bis(oxazoline) catalysts (10 mol%) in the asymmetric Friedel-Crafts reaction of 3'-methoxyanisole with ethyl 3,3,3-trifluoropyruvate in anhydrous diethyl ether. The pyruvate derivative is likely activated by chelation to the Lewis acidic copper catalyst through the two carbonyl oxygens before attack by the nucleophilic aryl ether. The electrophilic aromatic substitution product was isolated in 56% yield with 86% ee (Figure 36).232 The less activated anisole, however, did not undergo reaction under these conditions.
Figure 36.
Asymmetric Friedel-Crafts reaction with diethyl ether solvent and a copper-based catalyst.
To overcome the low reactivity of anisole Liu, Wang, Chen and their coworkers examined a series of bis(oxazoline) ligands (A-F, Figure 37) and metal salts in this reaction under solvent-free conditions.233 The results of the initial screening are outlined in Table 39. In contrast to the reactions conducted in diethyl ether (Figure 36), by using a slightly less bulky catalyst under solvent-free reaction conditions anisole reacted with 3,3,3-trifluoropyruvate to furnish product in very high yield and enantioselectivity (up to 90% yield and 88% ee). As illustrated in Table 39, the highest yields at 1.0 mol% catalyst were obtained using copper salts Cu(OTf)2 and Cu(ClO4)2 in combination with ligand A. This represents a 10-fold reduction in catalyst loading from previous studies under solvent conditions (Figure 36).232 Use of ligand F did not result in formation of product in diethyl ether (Figure 36) or under solvent-free conditions (entry 14), indicating the impact of subtle differences in the ligand structure on reactivity. These authors also found that lowering the catalyst loading to 0.1 and 0.01 mol% had little effect on the enantioselectivity or reactivity (compare entries 1, 16, and 17). The reaction of 1 gram anisole with catalyst derived from Cu(OTf)2 and ligand A was conducted at 0 °C resulting in formation of the product with 90% ee and in 70% yield. It was noted that the reaction was very exothermic and that caution should be used with increasing scale. This warning, of course, applies to all reactions conducted under solvent-free and highly concentrated conditions.
Figure 37.
Bis(oxazoline) ligands A – F used in the solvent-free asymmetric Friedel-Crafts reaction (Table 39 and Table 40).
Table 39.
Catalyst screening in the asymmetric Friedel-Crafts alkylation of anisole with ligands A-F of Figure 37.
 | ||||||
|---|---|---|---|---|---|---|
| entry | ligand | metal salt | mol% | time (h) | yield (%) | ee (%) |
| 1 | A | Cu(OTf)2 | 1.0 | 10 | 90 | 88 |
| 2 | A | Cu(ClO4)2 | 1.0 | 18 | 95 | 81 |
| 3 | A | Cu(OAc)2 | 1.0 | 24 | trace | - |
| 4 | A | CuCl2 | 1.0 | 24 | trace | - |
| 5 | A | Cu(OTf) | 1.0 | 24 | trace | - |
| 6 | A | Zn(OTf)2 | 1.0 | 24 | 45 | 81 |
| 7 | A | Mg(OTf)2 | 1.0 | 24 | trace | - |
| 8 | A | Sc(OTf)3 | 1.0 | 24 | trace | - |
| 9 | A | FeCl3 | 1.0 | 24 | <5 | - |
| 10 | B | Cu(OTf)2 | 1.0 | 15 | 80 | 80 |
| 11 | C | Cu(OTf)2 | 1.0 | 20 | 78 | 22 |
| 12 | D | Cu(OTf)2 | 1.0 | 17 | 85 | 79 |
| 13 | E | Cu(OTf)2 | 1.0 | 24 | trace | - |
| 14 | F | Cu(OTf)2 | 1.0 | 24 | trace | - |
| 15 | - | Cu(OTf)2 | 1.0 | 24 | 7 | - |
| 16 | A | Cu(OTf)2 | 0.1 | 20 | 85 | 86 |
| 17 | A | Cu(OTf)2 | 0.01 | 20 | 70 | 85 |
A series of aryl ethers were employed in the Friedel-Crafts asymmetric alkylation catalyzed by 1 mol% L*Cu(OTf)2 (L* = A, Figure 37), As illustrated in Table 40, yields ranged from 55 - 98% and enantioselectivities from 90 – 93%. In the case of solid substrates, reactions could be performed under highly concentrated conditions with CH2Cl2 solvent. The stereochemistry of the product in entry 7 was determined to have the (S)-configuration by X-ray crystallography.
Table 40.
Scope of the asymmetric Friedel-Crafts alkylation of aryl ethers with Cu(OTf)2 and ligand A of Figure 37 under solvent-free and highly concentrated reaction conditions.
 | |||||||
|---|---|---|---|---|---|---|---|
| entry | substrate | temp(°C) | time (h) | product | yield (%) | ee (%) | |
| 1 |  |
R = H | 0 | 10 | 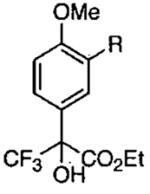 |
90 | 90 |
| 2 | R = Me | −20 | 30 | 80 | 90 | ||
| 3 | R = Ph | −20 | 28 | 55 | 90 | ||
| 4 |  |
R′ = n-Bu | −20 | 40 | 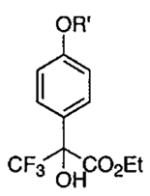 |
88 | 92 |
| 5 | R′ = Ph | 0 | 72 | 62 | 92 | ||
| 6a | R′ = CH2-2-C6H4-Br | 15 | 18 | 96 | 92 | ||
| 7 | R′ = CH2-4-C6H4-Br | 25 | 36 | 85 | 91 | ||
| 8 | R′ = CH2-Ph | 0 | 16 | 98 | 90 | ||
| 9 | R′ = CH2-3-C6H4-Cl | 0 | 28 | 78 | 93 | ||
| 10 |  |
R = H | −20 | 30 |  |
62 | 93 |
| 11 | R = Me | −20 | 30 | 75 | 90 | ||
| 12 | R = Ph | −20 | 48 | 70 | 93 | ||
highly concentrated with CH2Cl2.
The solvent-free and highly concentrated Friedel-Crafts reaction outlined above enables access to densely functionalized and highly enantioenriched fluorinated products.
2.9 Asymmetric Pauson-Khand-Type Reaction
Carbonylative alkene-alkyne coupling (the Pauson-Khand reaction when a cobalt carbonyl complex is used) has been extensively investigated, because it provides access to various synthetically useful cyclopentenones. Recent advancements in Pauson-Khand type reactions include catalytic and enantioselective versions.234
Shibata and coworkers reported an intramolecular catalytic carbonylative alkene-alkyne coupling using a rhodium(I) catalyst and aldehydes as a CO source.235,236 In the absence of solvent, the authors found that the Pauson-Khand type reaction using an achiral catalyst was faster when cinnamaldehyde was used as the CO source than when CO was employed. Based on these results, they prepared a chiral catalyst in situ from [(COD)RhCl]2 (5 mol%) and Tol-BINAP (2,2'-bis(di-p-tolylphosphino)-1,1'-binaphthyl, 10 mol%). In the presence of a large excess of cinnamaldehyde (20 equiv), the enyne coupling proceeded smoothly to give the cyclized product in good yield and 82% ee (Table 41, entry 1). When CO gas was used under solvent-free conditions the reaction was accelerated with the sacrifice of both yield and enantioselectivity (Table 41, entry 2). For comparison, the asymmetric carbonylative coupling was also examined in xylene in the presence of CO gas, although the yield was moderate and ee dropped dramatically (Table 41, entry 3). Unfortunately, the enantioselective reaction was not examined with fewer equivalents of cinnamaldehyde in this study.
Table 41.
Enantioselective carbonylative coupling of an enyne under solvent-free conditions.
 | |||||
|---|---|---|---|---|---|
| entry | CO source (equiv) | solvent | time (h) | yield (%) | ee (%) |
| 1 | cinnamaldehyde (20) | none | 4 | 89 | 82 |
| 2 | CO gas | none | 4 | 23 | 60 |
| 3 | CO gas | xylene | 36 | 51 | 10 |
2.10 Asymmetric Hydroformylation
The asymmetric hydroformylation of olefins is a direct route to optically active aldehydes, which are valuable precursors for a variety of pharmaceuticals and agrochemicals. Although homogeneous hydroformylation is the largest volume transition metal-catalyzed process, catalytic enantioselective versions are still underdeveloped.237 Much of the work in asymmetric hydroformylation has centered on vinyl arene substrates, which generate 2-aryl propionic acid derivatives, a class on nonsteroidal anti-inflammatory medications such as (S-Naproxen.238 Among the catalysts successfully applied to asymmetric hydroformylation reaction is the rhodium (R,S)-BINAPHOS-based catalyst (Figure 38), which promoted the hydroformylation of styrene in 96% ee.239,240 On the basis of research performed at Union Carbide with bis(phosphite) ligands,241,242 Colbey, Klosin, Whiteker, and their coworkers examined use of bis(phosphite) ligands with stereochemically dynamic biaryls backbones,243 as represented by (S,S)-Kelliphite (Figure 38).
Figure 38.
Useful ligands for rhodium catalyzed hydroformylation, (R,S)-BINAPHOS and (S,S)-Kelliphos.
These authors examined the catalytic asymmetric hydroformylation of allyl cyanide as a potential entry into important enantioenriched building blocks useful in the synthesis of biologically active compounds (Figure 39). Initially, a series of bis(phosphite) ligands were examined in the asymmetric hydroformylation in toluene. (S,S)-Kelliphite was identified as the most enantioselective over a series of related phosphite ligands with different backbones.244 A comparison of rhodium-based (R,S)-BINAPHOS and (S,S)-Kelliphos catalysts was undertaken at high substrate : catalyst ratios (2,000 – 10,000 :1 ) under solvent-free conditions (Eq 15). Reaction rates were found to be the highest in neat allyl cyanide. Kelliphite was about 7 times more active than BINAPHOS at 10,000 : 1 allyl cyanide : catalyst as shown in Table 42 (entries 3 vs. 6). The Kelliphite reaction was complete in 16 h with an average TOF of 635 per hour. Interestingly, the b/l ratio (b/l = branched : linear) increased at the highest substrate to catalyst ratio with Kelliphite. The hydroformylation was scaled up to 75 mL of allyl cyanide in a 300 mL pressure vessel under conditions similar to those in Eq 15 (1,500 :1 substrate : catalyst). Reaction completion was reached in 5 h with enantioselectivity of 80% and b/l of 20. It was found that the crude product could be readily used to prepare the enantioenriched precursors in Figure 39.
Figure 39.
Catalytic asymmetric hydroformylation of allyl cyanide provides key enantioenriched building blocks for the synthesis of potent antagonists.
Table 42.
Comparison of the asymmetric hydroformylation of allyl cyanide (Eq 15) with rhodium catalysts based on (R,S)-BINAPHOS and (S,S)-Kelliphos under solvent-free conditions.
| entry | ligand | nitrile / Rh | conv. (%) | regioselectivity (b/l) | ee (%) |
|---|---|---|---|---|---|
| 1 | (R,S)-BINAPhos | 2,000 | 64.0 | 2.8 | 74.7 |
| 2 | (R,S)-BINAPhos | 4,000 | 37.1 | 2.9 | 75.1 |
| 3 | (R,S)-BINAPhos | 10,000 | 14.8 | 2.7 | 77.2 |
| 4 | (S,S)-Kelliphite | 2,000 | 100 | 17.8 | 77.8 |
| 5 | (S,S)-Kelliphite | 4,000 | 100 | 17.3 | 78.8 |
| 6 | (S,S)-Kelliphite | 10,000 | 100 | 18.5 | 78.8 |
Similar results were obtained in the asymmetric hydroformylation reaction of vinyl acetate with Kelliphite under similar conditions (Eq 16). In this case, the enantioselectivity was 82%, the b/l ratio was 86, and the minimum average TOF was 200/h.237
These studies clearly indicate the benefits of catalytic asymmetric solvent-free processes as reactions were conducted on large laboratory scale (up to 75 mL substrate) in a 300 mL reaction vessel with very high volume efficiency. Further, this reaction is very atom economical, as all the atoms of the substrates are incorporated into the product.
Although the results outlined above are impressive, industrial experts contend that the reaction does not meet the standard required by industry, because 1) the reaction rates are too low at the temperatures needed to obtain good selectivity, 2) difficulties simultaneously controlling both regio- and enantioselectivity, and 3) lack of a ligand that exhibits broad substrate scope.245 Thus, new generation of asymmetric hydroformylation catalysts are needed that can be performed at higher temperature while maintaining selectivity.
In this study, Klosin and coworkers examined a series of ligands, including (R,S)-BINAPHOS240 and (S,S)-Kelliphite (Figure 38), (2R,4R)-Chiraphite, Landis' diazaphospholane,246 and a DuPHOS analog,247,248 (R,R)-Ph-BPE (Figure 40).249 Under solvent-free hydroformylation conditions, rhodium complexes of the ligands in Figure 38 and Figure 40 were employed at 80 °C, 150 psi and substrate : catalyst ratio of 30,000 : 1 (Figure 41). The average TOF of catalyst derived from (R,R)-Ph-BPE was 4,467/h, which was similar to (R,S)-BINAPHOS and (S,S)-Kelliphite. Catalysts containing (2R,4R)-Chiraphite and the diazaphospholane were even faster (although less selective). The results of these experiments are outlined in Table 43. It was found that rhodium complex of (R,R)-Ph-BPE gave the highest enantioselectivity with styrene (92%) and allyl cyanide (90%) while the diazaphospholane gave the best enantioselectivity with vinyl acetate (95%).
Figure 40.
Structures of ligands used in the asymmetric hydroformylation reaction: (2R,4R)-Chiraphite, Landis' diazaphospholane, and (R,R)-Ph-BPE.
Figure 41.
Simultanious asymmetric hydroformylation of 3 olefins under solvent-free conditions. The total volume of olefins was 4.43 mL. The results are shown in Table 43.
Table 43.
Asymmetric hydroformylation reactions of styrene, allyl cyanide, and vinyl acetate with different ligands under solvent-free conditions.
| entry | ligand | conv. (%) | b/l | ee (%) | config. |
|---|---|---|---|---|---|
 | |||||
| 1 | (2R,4R)-Chiraphite | 32 | 10.8 | 51 | R |
| 2 | (R,S)-BINAPHOS | 35 | 4.6 | 81 | R |
| 3 | (S,S)-Kelliphite | 32 | 9.2 | 3 | S |
| 4 | diazaphospholane | 73 | 5.7 | 80 | R |
| 5 | (R,R)-Ph-BPE | 33 | 45 | 92 | R |
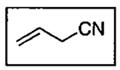 | |||||
| 6 | (2R,4R)-Chiraphite | 74 | 5.8 | 13 | R |
| 7 | (R,S)-BINAPHOS | 58 | 2.1 | 68 | R |
| 8 | (S,S)-Kelliphite | 99 | 10.1 | 66 | S |
| 9 | diazaphospholane | 100 | 3.9 | 80 | R |
| 10 | (R,R)-Ph-BPE | 67 | 7.6 | 90 | R |
 | |||||
| 11 | (2R,4R)-Chiraphite | 34 | 204 | 50 | R |
| 12 | (R,S)-BINAPHOS | 23 | 7.1 | 58 | S |
| 13 | (S,S)-Kelliphite | 32 | 100 | 75 | R |
| 14 | diazaphospholane | 92 | 47 | 95 | S |
| 15 | (R,R)-Ph-BPE | 34 | 263 | 82 | S |
On the basis of these results, the researchers concluded that (R,R)-Ph-BPE and diazaphospholane achieved sufficient TOF (> 4,000/h at 80 °C), yield, and enantioselectivity to be used in large-scale industrial processes.245
2.10.1 Heterogeneous Asymmetric Hydroformylation Reactions
Despite the many advantages of homogeneous asymmetric catalysts over their heterogeneous counterparts, there are two major drawbacks of soluble catalysts: separation of catalyst from products and use of organic solvents to suppress catalyst precipitation. Thus, the heterogenization of homogeneous catalysts on organic polymers has been investigated with great intensity.250-255 Many examples employing supported catalysts suspended in organic solvents have been reported, however, use of supported catalysts under solvent-free conditions in flow reactors is relatively unexplored.
In this context, Shibahara, Nozaki, and Hiyama reported the solvent-free vapor-phase asymmetric hydroformylation of the volatile olefins cis-2-butene and 3,3,3-trifluoropropene using a batchwise reactor and a continuous vapor-flow column reactor. The catalyst used in these reactions was a highly cross-linked polystyrene-supported (R,S)-BINAPHOS-Rh(I) complex, which is illustrated with its homogeneous derivative in Figure 42.256
Figure 42.
The homogeneous and highly cross-linked Rh(I) complexes of (R,S)-BINAPHOS.
They found that the initial pressure of hydrogen, carbon monoxide, and olefin greatly impacted the TON of the catalyst and the product ee. The results of vapor phase asymmetric hydroformylation of cis-2-butene in a batchwise reactor are summarized in Table 44. Starting with syngas (24 atm, H2/CO 1/1), cis-2-butene (3 atm), and the supported catalyst (Figure 42), (S)-2-methylbutanal was generated in 82% ee after 2 h (Table 44, entry 1). The product ee decreased with increasing reaction time, however (entry 2). The ee erosion could be partially offset by increasing the pressure of cis-2-butene or using liquefied olefin (compare entries 2 - 4). The activity and selectivity of the supported catalyst were comparable to those of the homogeneous analog, although TON and TOF were lower (Table 44, entry 5).257
Table 44.
Asymmetric hydroformylation of cis-2-butene in a batchwise reactor with the heterogeneous and homogeneous catalysts in Figure 42.
 | |||||||
|---|---|---|---|---|---|---|---|
| entry |
cis-2-butene (atm) |
solvent | syngas (atm) |
time (h) |
TON | TOF (h−1) |
ee (%) (config.) |
| 1 | 3.0 | none | 24 | 2 | 20 | 10 | 82 (S) |
| 2 | 3.5 | none | 21 | 6 | 52 | 9 | 18 (S) |
| 3 | 2.6 | none | 32 | 6 | 28 | 5 | 75 (S) |
| 4 | liquefied | none | 33 | 12 | 156 | 13 | 79 (S) |
| 5 | solution | benzene | 32 | 8 | 184 | 23 | 82 (S) |
Asymmetric hydroformylation of 3,3,3-trifluoropropene was performed with the same catalyst in a continuous flow system with TOF of 9 h−1, and iso/n (iso/normal) ratio of 95/5, and 90% ee at 40 °C under H2/CO pressure of 50 atm (Table 45, entries 1 and 2). High selectivities were achieved, but the TOF remained at a low level compared with the homogeneous reaction (Table 45, entry 3).256
Table 45.
Continuous flow asymmetric hydroformylation of 3,3,3-trifluoropropene with the heterogeneous and homogeneous catalysts in Figure 42.
 | |||||||
|---|---|---|---|---|---|---|---|
| entry | total pressure (atm) |
solvent | time (h) |
TON | TOF (h−1) |
iso/n | ee (%) (config.) |
| 1 | 20 | none | 4 | 22 | 5.5 | 88/12 | 82 (S) |
| 2 | 50 | none | 0.5 | 4.5 | 9 | 95/5 | 90 (S) |
| 3 | solution | benzene | 18 | 1152 | 64 | 95/5 | 93 (S) |
The asymmetric hydroformylation reactions discussed here demonstrated the successful use of the polymer-supported catalyst under solvent-free conditions. Easy separation of the catalyst from the products has been achieved, increasing the attractiveness of this method.258
2.11 Asymmetric Hydrogenation Reaction
Transition metal-catalyzed enantioselective hydrogenation has been established as one of the most efficient strategies for the synthesis of enantioenriched molecules. While most effort has focused on the design of chiral ligands, which is a key issue for obtaining high activity and selectivity,259-261 much less emphasis has been placed on the optimization of reactions under more environmentally friendly solvent-free conditions.
Great advances have been made in the asymmetric reduction of ketones via hydrogenations and transfer hydrogenation. The pioneering work of Noyori and coworkers has spawned numerous investigations into the application of bifunctional catalysts to ketone reduction as well as C-C bond-forming reactions.262,263 To our knowledge, however, application of these catalysts under solvent-free or highly concentrated conditions has been limited.
Abdur-Rashid, Lough, and Morris examined several catalysts in the asymmetric reduction of ketones and imines under highly concentrated and solvent-free conditions.264 Reactions were performed neat with liquid substrates and a small amount of benzene was added to solid substrates. Precatalysts A, B, and C (Figure 43) were employed in the hydrogenation of ketones and imines. The results are shown in Table 46. Catlayst formed from A exhibited higher levels of enantioselectivity than B, although both gave only moderate enantioselectivity. With acetophenone catalyst derived from A gave 88% enantioselectivity. Related catalysts gave lower enantioselectivities.265 In the asymmetric hydrogenation of imines, catalyst derived from C gave up to 92% enantioselectivity with the n-Bu imine of acetophenone and was about an order of magnitude more active than the BINAP catalysts formed from A and B.
Figure 43.
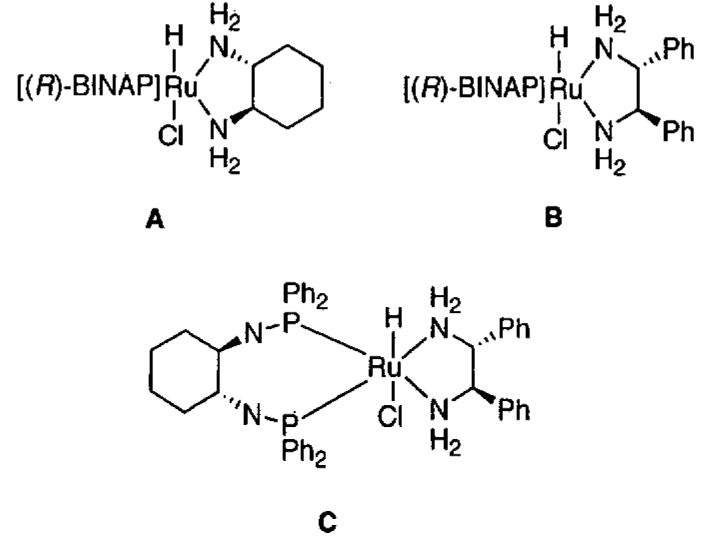
Precatalysts used in the asymmetric hydrogenation of ketones and imines (Table 46).
Table 46.
Results from the catalytic asymmetric hydrogenaiton of imines under solvent-free and highly concentrated conditions with precatalysts A-C (Figure 43).
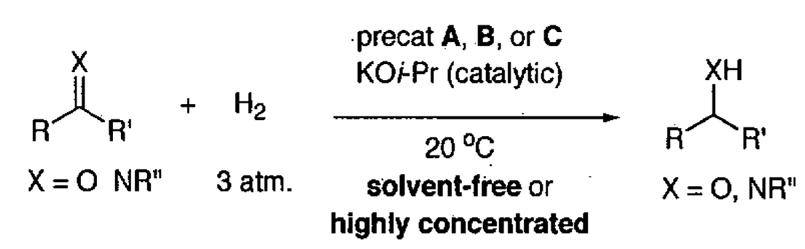 | |||||
|---|---|---|---|---|---|
| substrate | precat. | S : Ca | conv (%) | time (h) | ee (%) |
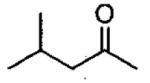 |
A | 5,000 | 100 | <12 | 52 |
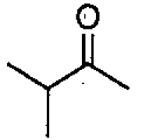 |
A | 3,600 | 100 | <12 | 46 |
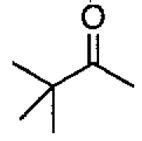 |
A | 5,000 | 87 | 48 | 50 |
 |
A | 5,000 | 100 | <12 | 88 |
| B | 5,000 | 100 | <12 | 73 | |
 |
A | 5,000 | 100 | <12 | 60 |
| B | 5,000 | 100 | <12 | 64 | |
 |
A | 500 | 100 | 36 | 71 |
| B | 500 | 90 | 72 | 70 | |
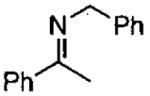 |
A | 500 | 100 | 36 | 60 |
| B | 500 | 90 | 24 | 50 | |
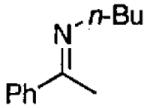 |
A | 500 | 12 | 60 | 60 |
| B | 500 | 17 | 60 | 60 | |
| C | 1,500 | 91 | 60 | 92 | |
S : C = substrate : catalyst
In a hydrogenation that goes by a more traditional mechanism involving metal hydride intermediates, Zhang et al. reported the synthesis and resolution of a bulky and electron-rich derivative of SEGPhos (SEGPhos', Figure 44) and its application in Ru-catalyzed asymmetric hydrogenation reaction.266
Figure 44.
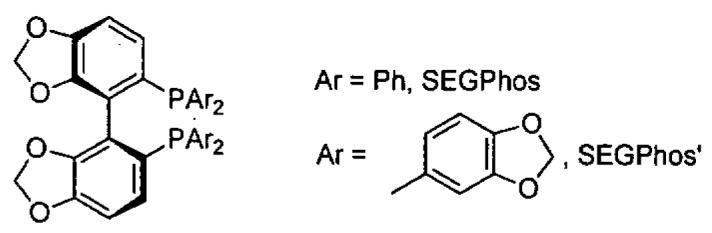
Structure of SEGPhos and a derivative, SEGPhos'.
The solvent-free catalytic enantioselective hydrogenation of ethyl acetoacetate with extremely low catalyst loading (20,000:1, substrate/catalyst) was reported by Zhang et al. in the presence of SEGPhos and SEGPhos'. The reaction was performed with 30 atm of H2 at 110 °C for 3.5 h. Under the solvent-free conditions, the reaction was accelerated with only a small decrease in enantioselectivity (Table 47, entries 1 – 4). Interestingly, addition of catalytic sulfuric acid (H2SO4/[Ru] = 1.5:1) elevated both the reaction rate and the enantioselectivity, resulting in 100 % conversion, and 97.5% ee (Table 47, entries 5 and 6).
Table 47.
Comparison of the asymmetric hydrogenation of ethyl acetoacetate with SEGPhos and SEGPhos' in ethanol solvent and under solvent-free conditions.
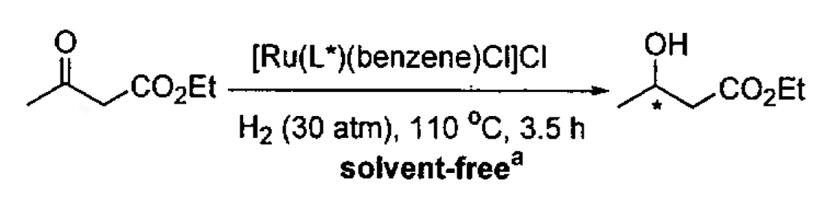 | |||||
|---|---|---|---|---|---|
| entry | solvent | additive | L* | conversion (%) | ee (%) |
| 1 | EtOH | none | SEGPhos′ | 35.9 | 96.0 |
| 2 | EtOH | none | SEGPhos | 35.7 | 96.6 |
| 3 | none | none | SEGPhos′ | 56.8 | 91.6 |
| 4 | none | none | SEGPhos | 62.5 | 88.4 |
| 5a | none | H2SO4 | SEGPhos′ | 100 | 96.8 |
| 6b | none | H2SO4 | SEGPhos | 100 | 97.5 |
All reactions were carried out with a ratio of 20,000:1 (substrate/catalyst).
Additive : Ru = 1.5:1.
The application of SEGPhos and SEGPhos' to the enantioselective solvent-free hydrogenation outlined above suggests that related hydrogenation catalysts may also exhibit high levels of enantioselectivity with very low catalyst loading under these conditions. Given the industrial importance of asymmetric reductions and the economic advantages of solvent-free reactions, it is anticipated that the examination of asymmetric hydrogenation catalysts under solvent-free conditions will become standard in the near future.
2.12 Asymmetric Hydrosilylation of Ketones
The enantioselective hydrosilylation of ketones is an excellent method for the synthesis of enantioenriched secondary alcohols. This approach to the preparation of chiral secondary alcohols has the advantage of very mild reaction conditions relative to other reduction methods. Further, the catalysts are often based on nitrogen containing ligands, many of which can be prepared from naturally occurring enantiopure precursors.267
In research to design ligands for the asymmetric hydrosilylation of ketones, Martens et al. reported the synthesis of a series of optically active nitrogen-based chelating ligands A – D (Table 48).268 These ligands (10 mol%) were employed in the rhodium catalyzed enantioselective reduction of acetophenone with diphenylsilane and a [(COD)RhCl]2 : L* ratio of 1 : 20 under solvent-free conditions (Table 48, entries 1, 3, 5 and 7). The addition of a small amount of toluene reduced both yield and enantioselectivity (Table 48, entries 2, 4 and 6), except with the SPh derivative ligand D, which gave better results in the presence of toluene (Table 48, entry 8).
Table 48.
Enantioselective hydrosilylation of acetophenone with chelating chiral nitrogen-based ligands.
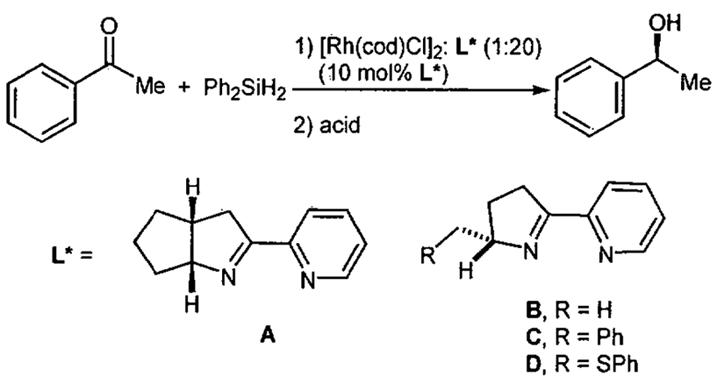 | |||||
|---|---|---|---|---|---|
| entry | ligand | solvent | yield (%) | ee (%) | config. |
| 1 | A | none | 53 | 28 | S |
| 2 | A | toluene | 38 | 40 | S |
| 3 | B | none | 86 | 54 | S |
| 4 | B | toluene | 32 | 25 | S |
| 5 | C | none | 60 | 39 | S |
| 6 | C | toluene | 40 | 38 | S |
| 7 | D | none | 36 | 31 | S |
| 8 | D | toluene | 44 | 41 | S |
Given the importance of asymmetric hydrosilylation reactions, and in view of the modest enantioselectivities observed under solvent-free conditions in this study, it is anticipated that further investigations will be forthcoming in this area. Highly enantioselective catalysts that are compatible with a range of functional groups are in great demand and will find applications in complex molecule synthesis.
2.13 Asymmetric Allylic Amination
Enantioenriched amines are important precursors in synthetic and medicinal chemistry. On convergent strategy for the generation of chiral amines is by enantioselective C-N bond-forming reactions. Using ligands introduced by Feringa arid coworkers269,270 Ohmura and Hartwig developed a regio- and enantioselective allylic amination reaction beginning with achiral allylic esters (Figure 45).271 Most of the reactions in this report were conducted in THF solvent. One example is given using 3 equiv morpholine and cinnamyl acetate. In ethanol, the iridium phosphoramidite-based catalyst promoted the reaction giving a ratio of chiral amine to achiral amine of 97 : 3. The enantioselectivity was 95%. Similar results were obtained under solvent-free conditions.
Figure 45.
Asymmetric allylic amination reactions.
Allylic amines of this type can be easily functionalized to 1,3-amino alcohols and amino acids. It is likely that the solvent-free conditions could be applied related substrates with similar levels of success.
2.14 Asymmetric Conjugate Addition of Cyanide to α,β-Unsaturated Imides
Conjugate addition reactions to α,β-unsaturated carbonyl compounds are one of the most important and useful strategies in organic chemistry. These reactions provide access to difunctional intermediates that are readily converted to a variety of useful chiral building blocks, including β-substituted-γ-aminobutyric acids and α-substituted-β-amino acids.272-276 Jacobsen and co-workers reported the application of (salen)Al(III) catalysts to the conjugate addition of hydrogen cyanide to α,β-unsaturated imides with high enantioselectivity (Eq 17).277 HCN was generated in situ from TMSCN and 2-propanol. Employing (salen)Al-Cl complex as the catalyst under standard solvent conditions, these investigators found that the conjugate addition of HCN occurred with high enantioselectivity (90%), but the catalyst turned over only 1-2 times. Much better results were acquired when reactions were conducted in highly concentrated toluene solutions. Under these conditions, the product was generated with 90% isolated yield and 97% ee (Method A). A significant substrate-dependence on reactivity was observed, as shown in Table 49. In general, good results were obtained with imide substrates bearing aliphatic β-substituents. Slower-reacting imides required elevated temperature and catalyst loading (method B, entries 7 and 8). Imide derivatives bearing unsaturated β–substituents (R = aryl, vinyl, alkynyl) proved unreactive, even under forcing conditions.
Table 49.
Conjugate addition of TMSCN to α,β-unsaturated imides.
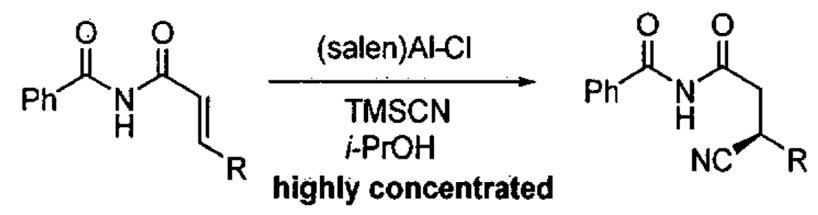 | |||||
|---|---|---|---|---|---|
| entry | R | methoda | time (h) | yield (%) | ee (%) |
| 1 | Me | A | 26 | 92 | 98 |
| 2 | Et | A | 26 | 95 | 97 |
| 3 | n-Pr | A | 26 | 90 | 97 |
| 4 | i-Pr | A | 26 | 91 | 94 |
| 5 | i-Bu | A | 26 | 93 | 96 |
| 6 | (CH2)3CH=CH2 | A | 48 | 96 | 95 |
| 7 | i-Bu | B | 48 | 90 | 97 |
| 8 | CH2OBn | B | 48 | 70 | 87 |
Reactions were carried out on a 0.5 mmol scale. Method A: 10 mol% (S,S)-(salen)Al-Cl, 2.5 equiv of TMSCN, 2.5 equiv 2-propanol, 0.2 mL of toluene, 24 °C. Method B: 15 mol% (S,S)-(salen)Al-Cl, 4 equiv of TMSCN, 4 equiv 2-propanol, 0.4 mL of toluene, 24 °C.
Spectroscopic studies revealed that the chloride complex (salen)Al-Cl is converted to two distinct species, (salen)Al-CN and (salen)Al(imidate), under the reaction conditions. Initial rate studies reveal a second-order kinetic dependence on catalyst concentration. These preliminary data are consistent with a bimetallic, dual activation mechanism involving cyanide delivery from the complex (salen)Al-CN to the electrophile bound as the imidate complex, (salen)Al(imidate).
The conjugate addition of cyanide outlined above can be applied to the synthesis of pregabalin [(S)-3-isobutyl-γ-aminobutyric acid], a promising anticonvulsant drug for the treatment for neuropathic pain (Figure 46). In general, enantioenriched β-substituted-γ-amino acids are difficult to synthesize. For example, the manufacturing process for pregabalin entails a non-enantioselective 1,4-cyanation, followed by late-stage classical resolution. Using the chiral (salen)Al-Cl catalyst, the required cyanide adduct was prepared in 96% ee on gram scale (Figure 46). The overall yield from commercially available materials was thus 62% over six steps.
Figure 46.
Synthesis of pregabalin using the catalytic asymmetric conjugate addition of cyanide.
Cyanide adducts prepared by this method can also be applied toward the synthesis of enantioenriched α-substituted-β-amino acid derivatives (Figure 47).
Figure 47.
Synthesis of an enantioenriched α-substituted-β-amino acid.
2.15 Asymmetric Organocatalytic Reactions
The discovery of the intramolecular aldol cyclizations of triketones was a milestone in asymmetric catalysis, the full impact of which would not be known for some 30 years.111,278-280 Reported independently by the group of Hajos and Parrish281 and the team of Eder, Sauer, and Weichert282,283 the reaction involved use of catalytic amounts of (S)-proline284-286 to form enantioenriched enediones, which have been used in the synthesis of natural products.287-292 These early works initiated the explosive growth in the area of organocatalysis. Although the application of enantioselective organocatalysts in synthesis is likely more environmently friendly than metal-based catalysts, few examples have been reported extending these catalysts to solvent-free reaction conditions. It is quite possible, however, that optimization of organocatalysts under solvent-free and highly concentrated reaction conditions will circumvent the high catalyst loadings frequently required for maximum enantioselectivity and short reaction times. Reported below are examples of organocatalytic reactions performed without solvent (also see Section 2.3.3 and 2.3.6).
2.15.1 Asymmetric Hajos-Parrish Reaction
Swaminathan et al. carried out the proline-catalyzed reaction of carbonyl compounds to afford enantioenriched bicyclic diketones under solvent-free conditions.293,294 The first type of reaction examined began with racemic diketo aldehyde (Figure 48). Reaction of this substrate with catalytic (S)-proline in DMSO solvent resulted in formation of the spirocycle in 70% yield with 22% ee. In contrast, when the reaction was conducted with catalytic proline in the absence of solvent, the product was formed in 49% yield with 43% ee. These numbers should be interpreted with care. First, the product ee's were determined by optical rotation, which is subject to a significantly larger experimental error than GC or HPLC with chiral columns. Second, this reaction appears to be a kinetic resolution, in which case the product ee is dependent on conversion. Increasing conversion in a kinetic resolution results in decreased product ee. Nonetheless, this is an interesting lead that is potentially useful in synthesis.
Figure 48.
(S)-Proline catalyzed annulation employing racemic tricarbonyl substrate.
The second type of substrates examined was symmetric tricarbonyl compounds. In this case, a desymmetrization reaction was performed to generate the Hajos-Parrish ketone (n = 1) and the Wieland-Miescher ketone (n = 2) (Figure 49).295,296
Figure 49.
Desymmetrization of the trione in the presence of catalytic (S)-proline under solvent-free conditions provides valuable diones with moderate ee's.
Given the widespread interest in the development of organocatalytic reactions and catalysts, it is certain that additional applications will continue to be introduced that employ solvent-free and highly concentrated reaction conditions.
2.15.2 Enantioselective Aldol Reaction
In interesting approach to asymmetric organocatalysis was taken by Rodríguez, Tantanen, and Bolm297 in the intermolecular proline-catalyzed aldol reaction.298-300 This important C-C bond forming reaction is typically conducted in polar solvents, most frequently dimethyl sulfoxide. Bolm and coworkers mixed ketones (1.1 equiv) and aldehydes (1.0 equiv) and 10 mol% (S)-proline in a ball mill. Ball milling is a mechanochemical method whereby chemically inert balls, in the present case composed of zirconium oxide, are placed inside a cylinder with the reagents. As the cylinder is rotated, the friction and impact of the tumbling balls grinds, mixes, and heats the contents. The results of this study are illustrated in Table 50 where method A is ball milling and method B is stirring.
Table 50.
Comparison of the enantioselective aldol reaction catalyzed by (S)-proline under solvent-free conditions using ball milling (method A) and stirring (method B).
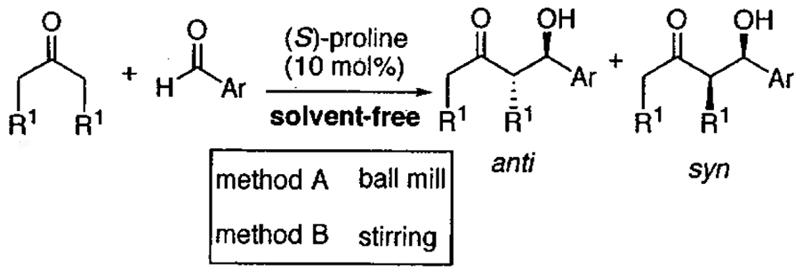 | ||||||
|---|---|---|---|---|---|---|
| entry | product | method | time (h) |
yield (%) |
anti / syn | ee of anti (%) |
| 1 |  |
A | 7 | 97 | 93:7 | 97 |
| 2 | B | 36 | 89 | 91:9 | 97 | |
| 3 | 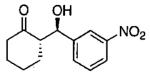 |
A | 7 | 94 | 88:12 | >99 |
| 4 | B | 16 | 89 | 82:18 | 98 | |
| 5 | 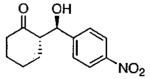 |
A | 5.5 | 99 | 89:11 | 94 |
| 6 | B | 96 | 98 | 87:13 | 94 | |
| 7 | 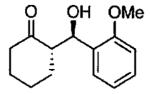 |
A | 36 | 65 | 66:34 | 63 |
| 8 | B | 96 | 64 | 71:29 | 67 | |
| 9 | 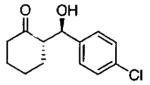 |
A | 20 | 87 | 74:26 | 75 |
| 10 | B | 72 | 85 | 78:22 | 67 | |
| 11 | 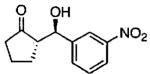 |
A | 5 | 93 | 50:50 | 90 |
| 12 | B | 36 | 93 | 46:54 | 75 | |
| 13 |  |
A | 5 | 53 | 57:43 | 95 |
| 14 | B | 24 | 90 | 52:48 | 87 | |
| 15 |  |
A | 19 | 73 | — | 56 |
| 16 | B | 36 | 69 | — | 54 | |
As illustrated in Table 50, impressive results were obtained using ball milling (method A). Enantioselectivities above 90% were measured for several substrates and poor to good control over the diastereoselectivity was observed. Comparison of ball milling with magnetic stirring under solvent-free conditions (method B) indicates that reactions times were considerably reduced with ball milling. Overall, the trends observed employing these two solvent-free methods were similar to those found in organic solvents298-300 and in water.301,302
2.15.3 Enantioselective Morita-Baylis-Hillman Reaction
Another important C-C bond-forming reaction is the Morita-Baylis-Hillman (MBH) reaction. Recently, asymmetric versions of this reaction have been developed, including those catalyzed by organocatalysts.303,304 The products of the MBH reaction are enantioenriched allylic alcohols, which are useful building blocks in enantioselective synthesis.
Berkessel, Roland, and Neudörf recently indroduce bis(urea) and bis(thiourea)-based catalysts derived isophoronediamine, which is produced on a multiton scale and readily resolved (Figure 50).305 These hydrogen bond donors activate the carbonyl group of the α,β-unsaturated carbonyl compound and the cocatalyst DABCO undergoes conjugate addition to generate an intermediate enolate, which is then stabilized by hydrogen bonding to the catalyst.306,307 Initial screening of several derivatives indicated that catalysts A – D (20 mol%) performed the best in this reaction in the presence of DABCO (20 mol%). These reactions were conducted under solvent-free conditions with 4 equiv of 2-cyclohexenone. Although the enantioselectivities were high, the conversions after 72 h were low except in the case of catalyst C. For the purpose of comparison, reactions were also conducted in the presence of solvent under similar conditions. Solvents with hydrogen bond accepting functionality, such as methanol, DMF, tetrahydrofuran, dioxane, and their mixtures with water deactivated the catalysts. The reaction in toluene with catalyst C, DABCO (20 mol% each), and 2 equiv 2-cyclohexenone, however, resulted in 96% ee with only slightly reduced conversion (70%). The reaction with aldehydes other than cyclohexanecarboxaldehyde, however, exhibited significantly reduced conversions in the presence of solvent. The substrate scope was, therefore, determined under the solvent-free conditions of Figure 50.
Figure 50.
MBR reaction catalyzed by bis(urea) and bis(thiourea) organocatalysts.
As shown in Table 51, aliphatic aldehydes gave better enantioselectivities than their aromatic counterparts with each of the three α,β-unsaturated carbonyl substrates employed. The results of this study are promising and it is likely that introduction of new catalysts will result in generation of synthetically valuable allylic alcohols in the MBH reaction with higher enantioselectivities.
Table 51.
Solvent-free MBH reaction catalyzed by organocatalysts A – C and DABCO (20 mol% each) from Figure 50.
| entrya | product | cat | yield (%) | ee (%) |
|---|---|---|---|---|
| 1 |  |
A | 65b | 77 |
| 2 | C | quantc | 60 | |
| 3 | 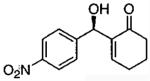 |
A | quantc | 38 |
| 4 | C | 65c | 34 | |
| 5 | 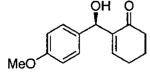 |
A | 35c | 79 |
| 6 | C | 79c | 50 | |
| 7 | 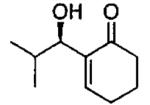 |
A | 62c | 90 |
| 8 | 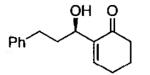 |
A | 28c | 79 |
| 9 | C | 52b | 69 | |
| 10 | 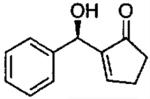 |
C | quantb | rac |
| 11 | 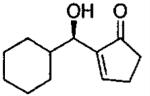 |
C | 22b | 58 |
| 12 |  |
C | quantb | rac |
| 13 |  |
B | 47c | 22 |
The reaction was carried out with 1 equiv of aldehyde and 4 equiv of enone or acrylate in the presence of 20 mol% catalyst and DABCO at 10°C for 72 h.
GC yield.
Isolated yield.
2.15.4 Enantioselective Organocatalytic Conjugate Additions
As the chemical community gains experience in the development and applications of enantioselective organocatalysts, the application of these catalysts to tandem reactions will become more common. One example of such a process is the asymmetric conjugate addition of β-ketoesters to α,β-unsaturated aldehydes catalyzed by a pyrrolidine derivative (Figure 51).308-311 Initial screening indicated that the catalyst enantioselectivity and reaction yield were insensitive to changes in the solvent, inspiring the researchers to conduct the reaction in the absence of solvent. Under the solvent-free conditions, the product was formed with 90% yield and 94% ee.312
Figure 51.
Organocatalytic Michael addition with and without solvent.
To access enantioenriched 2-cyclohexenone derivatives, the solvent-free asymmetric conjugate addition of β-ketoesters to α,β-unsaturated aldehydes was incorporated into a one-pot procedure for the preparation of these synthetically valuable chiral building blocks. Thus, after the asymmetric Michael addition step, 20 mol% of a sulfonic acid was added with toluene solvent and the reaction was heated to reflux. As depicted in Figure 52, the next steps of the reaction are proposed to proceed by acid catalyzed formation of the carboxylic acid, decarboxylation, and finally acid catalyzed intramolecular aldol and elimination of water.
Figure 52.
Reaction pathway for the one-pot organocatalytic synthesis of 2-cyclohexenone derivatives. The catalyst for the first step is shown in Figure 51.
The substrate scope of the one-pot procedure is outlined in Table 52. With the exception of 2-butenal, enantioselectivities were excellent (92 – 96%) and yields were good to very high (56 – 98%).
Table 52.
One-pot enantioselective synthesis of 2-cyclohexenone derivatives.
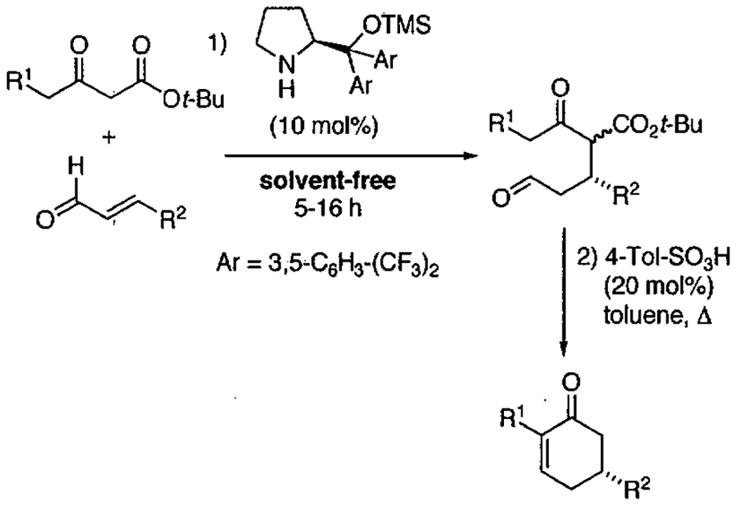 | ||||
|---|---|---|---|---|
| entry | R1 | R2 | yield (%) | ee (%) |
| 1 | H | Me | 93 | 80 |
| 2 | H | Et | 98 | 94 |
| 3 | H | i-Pr | 56 | 96 |
| 4 | H | n-Bu | 69 | 92 |
| 5 | H | Ph | 63 | 94 |
| 6 | H | 4-C6H4-F | 65 | 95 |
| 7 | H | 3-C6H4-Me | 72 | 94 |
| 8 | Me | Et | 82 | 91 |
The asymmetric Michael reaction was also used in the synthesis of cis-2-methyl-4-phenylpiperidine derivative by decarboxylation and double reductive amination with benzyl amine in 46% overall yield (Figure 53).312
Figure 53.
Synthesis of a piperidine derivative based on the catalytic asymmetric solvent-free Michael addition.
Other related catalytic asymmetric Michael reactions have been explored and found to be quite useful for the construction of a variety of enantioenriched products.110,313-316 One example is the organocatalytic conjugate addition of nitroalkanes to nitroolefins developed by Wang and coworkers using a modified Cinchona alkaloid catalyst (10 mol%).317 Although no solvent was used in this study, the all but one reaction was conducted with a large excess (> 50 equiv) of nitroalkane. A single large-scale reaction, however, was performed with only a slight excess of 2-nitropropane and illustrates the potential of this approach (Figure 54). The addition took place over 6 d at 0 °C and afforded the product of 75% ee in 83% yield. In comparison, the reaction with a large excess of 2-nitropropane led to product with slightly higher ee (81%) but lower yield (83%) and longer reaction time (10 d). It was noted that both hydroxyl groups on the alkaloid catalysts were necessary to obtain high enantioselectivity. The 1,3-dinitro products could serve as a useful entry into enantioenriched 1,3-diamines after reduction. These initial results are promising and further investigations into this solvent-free process are warranted.
Figure 54.
Michael addition of 2-nitropropane with a nitroalkene catalyzed by a modified Cinchona alkaloid under solvent-free conditions.
2.15.5 α-Amination of Ketones
The organocatalytic reactions above involve asymmetric C-C bond-forming reactions. Related C-N bond-forming reactions have also received attention. One example reported by Jørgensen and coworkers is the proline catalyzed asymmetric α-amination of ketones with azodicarboxylates.318 As illustrated in Table 53, reaction of butanone with diethyl azodicarboxylate (DEAD) in the presence of 20 mol% (S)-proline at room temperature proceeded in the absence of solvent with 92% enantioselectivity (entry 1). Under the solvent-free conditions the catalyst loading could be lowered to 5 mol% with no significant increase in reaction time or drop in enantioselectivity. For the purpose of comparison, the best conditions with solvent resulted in 95% ee, but with 20 mol% catalyst (entry 4).
Table 53.
Catalytic asymmetric α-amination of butanone with (S)-proline.
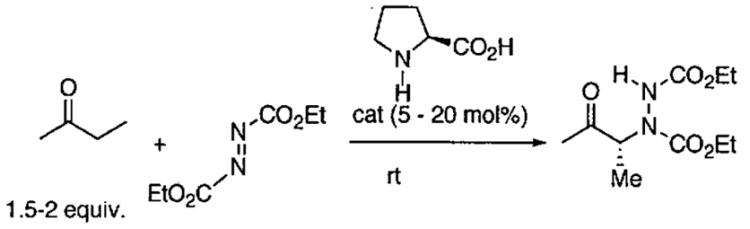 | ||||
|---|---|---|---|---|
| entry | cat (mol%) | solvent | time (h) | ee (%) |
| 1 | 20 | none | 65 | 92 |
| 2 | 10 | none | 65 | 93 |
| 3 | 5 | none | 65 | 93 |
| 4 | 20 | CH3CN | 52 | 96 |
3 Outlook
This review describes progress in the area of catalytic asymmetric reactions under solvent-free and highly concentrated conditions. Despite the enormous potential economic and environmental benefits, this area has been largely neglected by practitioners of asymmetric catalysis and synthetic chemistry. The research outlined herein, however, clearly indicates that a wide variety of structurally unrelated catalysts exhibit high enantioselectivities and yields in the absence of solvent. In several cases, the reactions under solvent-free and highly concentrated conditions constitute the best conditions reported.
Lastly, it is important to bear in mind that reactions conducted in the absence of solvent can rapidly generate heat. A greater degree of care must be exercised when reactions are performed at high concentrations to avoid run away reactions.
4 Acknowledgments
PJW is grateful to the National Institutes of Health, National Institute General Medical Sciences (GM58101), and the National Science Foundation (CHE-0615210) for continued support of our research. HL acknowledges Novartis for graduate fellowships and CA thanks Fulbright-García Robles Research Scholarship for visiting scholar. We are grateful to the members of our research group for their input into this review.
Abbreviations
- ALB
Aluminum lithium BINOLate based catalyst
- ARCM
Asymmetric ring closing metathesis
- ARO
Asymmetric ring opening
- AROM/CM
Asymmetric ring opening metathesis/cross metathesis
- b/l
Branched / linear
- BINAP
2,2′-Bis(diphenylphosphino)-1,1′-binaphthyl
- BINAPHOS
(R)-2-(Diphenylphosphino)-1,1'-binaphthalen-2'-yl (S)-1,1'-binaphthalene-2,2'-diyl phosphite
- BINOL
1,1′-Binaphthalenyl-2,2′-diol
- Chiraphite
6,6′-(((1R,3R)-1,3-dimethyl-1,3-propanediyl)bis(oxy))bis-(4,8-bis(1,1-dimethylethyl)-2,10-dimethoxy-dibenzo(d,f)(1,3,2)-dioxaphosphepin
- CSA
(S)-Camphorsulfonic acid
- DABCO
1,4-Diazabicyclo[2.2.2]octane
- DMSO
Dimethyl sulfoxide
- DuPHOS
1-Bis[(2R,5R)-2,5-dialkylphospholano]-benzene
- H8-BINOL
5,5',6,6',7,7',8,8'-Octahydro-1,1'-bi-2-naphthol
- HDA reaction
Hetero-Diels-Alder reaction
- HKR
Hydrolytic kinetic resolution
- Kelliphite
(S,S)-6,6'-((1,1'-Biphenyl)-2,2'-diylbis-(oxy))bis(4,8-bis(1,1-dimethylethyl)-l,2,10,ll-tetramethyldibenzo(d,f)(1,3,2)dioxaphosphepin
- MBH reaction
Morita-Baylis-Hillman reaction
- ONIOM
our own N-layered integrated molecular orbital + molecular mechanics
- PMPA
(R)-9-[2-(Phosphonomethoxy)propyl]adenine
- Ph-BPE
(R,R)-1,2-bis(2,5-dialkylphospholano)ethane
- salen
Bis(salicylidene)ethylenediamine
- TADDOL
2,2 Dimethyl-α,α,α′,α′-tetraary1-1,3-dioxolane-4,5 dimethanol
- TBAF
Tetra-n-butylammonium fluoride
- TBHP
tert-Butyl hydroperoxide
- TBME
tert-Butyl methy lether
- TFA
Trifluoroacetic acid
- Tol-BINAP
2,2′-Bis[bis(p-tolyl)phosphino]-1,1′-binaphthyl
- SEGPhos
(4,4′-Bi-1,3-benzodioxole)-5,5′-diylbis(diarylphosphine)
5 References
- 1.Corey EJ, Cheng X-M. The Logic of Chemical Synthesis. Wiley; New York: 1989. [Google Scholar]
- 2.Nicolaou KC, Sorensen EJ. Classics in Total Synthesis. VCH; Weinheim, Germany; 1996. [Google Scholar]
- 3.Nicolaou KC, Snyder SA. Classics in Total Synthesis II: More Targets, Strategies, Methods. Wiley-VCH; Weinheim, Germany: 2003. [Google Scholar]
- 4.Smith AB, III, Beauchamp TJ, LaMarche MJ, Kaufman MD, Qiu Y, Arimoto H, Jones DR, Kobayashi K. J. Am. Chem. Soc. 2000;122:8654. [Google Scholar]
- 5.Smith AB, III, Freeze BS, Xian M, Hirose T. Org. Lett. 2005;7:1825. doi: 10.1021/ol050455z. [DOI] [PubMed] [Google Scholar]
- 6.Mickel SJ, Niederer D, Daeffler R, Osmani A, Kuesters E, Schmid E, Schaer K, Gamboni R, Chen W, Loeser E, Kinder FR, Jr., Konigsberger K, Prasad K, Ramsey TM, Repic O, Wang R-M, Florence G, Lyothier I, Paterson I. Org. Proc. Res. Dev. 2004;8:122. [Google Scholar]
- 7.DeSimone JM. Science. 2002;297:799. doi: 10.1126/science.1069622. [DOI] [PubMed] [Google Scholar]
- 8.Anastas PT, Williamson TC. Green Chemistry: Frontiers in Benign Chemical Syntheses and Processes. Oxford Science Publications; New York: 1998. [Google Scholar]
- 9.Sheldon RA. Chem. Ind. 1992:903. [Google Scholar]
- 10.Jacobsen EN, Finney NS. Chem. Biol. 1994;1:85. doi: 10.1016/1074-5521(94)90045-0. [DOI] [PubMed] [Google Scholar]
- 11.Cave GWV, Raston CL, Scott JL. Chem. Commun. 2001:2159. [PubMed] [Google Scholar]
- 12.Sheldon RA. Pure Appl. Chem. 2000;72:1233. [Google Scholar]
- 13.Butters M, Catterick D, Craig A, Curzons A, Dale D, Gillmore A, Green SP, Marziano I, Sherlock J-P, White W. Chem. Rev. 2006;106:3002. doi: 10.1021/cr050982w. [DOI] [PubMed] [Google Scholar]
- 14.Metzger JO. Angew. Chem., Int. Ed. 1998;37:2975. doi: 10.1002/(SICI)1521-3773(19981116)37:21<2975::AID-ANIE2975>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 15.Tanaka K, Toda F. Chem. Rev. 2000;100:1025. doi: 10.1021/cr940089p. [DOI] [PubMed] [Google Scholar]
- 16.Tanaka K. Solvent-Free Organic Synthesis. Wiley-VCH; Weinheim, Germany: 2003. [Google Scholar]
- 17.Wang Y, Teng X, Wang J-S, Yang H. Nano Lett. 2003;3:789. [Google Scholar]
- 18.Mahapatro A, Kumar A, Kalra B, Gross RA. Macromolecules. 2004;37:35. [Google Scholar]
- 19.Doll KM, Shogren RL, Willett JL, Swift G. J. Polym. Sci., Part A: Polym. Chem. 2006;44:4259. [Google Scholar]
- 20.Komiya K, Fukuoka S, Aminaka M, Hasegawa K, Hachiya H, Okamoto H, Watanabe T, Yoneda H, Fukawa J, Dozono T. Green Chemistry, Designing Chemistry for the Environment. American Chemical Society; Washington, DC: 1996. [Google Scholar]
- 21.Metzger JO, Mahler R. Angew. Chem., Int. Ed. 1995;34:902. [Google Scholar]
- 22.Metzger JO. Organic Synthesis Highlights V. 2003:82. [Google Scholar]
- 23.Biermann U, Metzger JO. Top. Catal. 2004;27:119. [Google Scholar]
- 24.Metzger JO, Mahler R, Francke G. Liebigs Ann. Recl. 1997:2303. [Google Scholar]
- 25.Peng J, Deng Y. Tetrahedron Lett. 2001;42:5917. [Google Scholar]
- 26.Togo H, Hirai T. Synlett. 2003:702. [Google Scholar]
- 27.Huang J, Jiang T, Gao H, Han B, Liu Z, Wu W, Chang Y, Zhao G. Angew. Chem. Int. Ed. 2004;43:1397. doi: 10.1002/anie.200352682. [DOI] [PubMed] [Google Scholar]
- 28.Ji S-J, Jiang Z-Q, Lu J, Loh T-P. Synlett. 2004:831. [Google Scholar]
- 29.Duan Z, Gu Y, Deng Y. Synth. Commun. 2005;35:1939. [Google Scholar]
- 30.Wang H, Cui P, Zou G, Yang F, Tang J. Tetrahedron. 2006;62:3985. [Google Scholar]
- 31.Bram G, Decodts G, Bensaid Y, Farnoux CC, Galons H, Miocque M. Synthesis. 1985:543. [Google Scholar]
- 32.Kuroda R, Imai Y, Sato T. Chirality. 2001;13:588. doi: 10.1002/chir.1182. [DOI] [PubMed] [Google Scholar]
- 33.Parkin IP. Transition Met. Chem. 2002;27:569. [Google Scholar]
- 34.Ravinder V, Rani PU, Balaswamy G. Indian J. Heterocycl. Chem. 2004;14:73. [Google Scholar]
- 35.Wang Y-M, Wen Z, Chen X-M, Du D-M, Matsuura T, Meng J-B. J. Heterocycl. Chem. 1998;35:313. [Google Scholar]
- 36.Nagendrappa G. Resonance. 2002;7:64. [Google Scholar]
- 37.Shailaja J, Karthikeyan S, Ramamurthy V. Tetrahedron Lett. 2002;43:9335. [Google Scholar]
- 38.Jacobsen EN, Pfaltz A, Yamamoto H. Comprehensive Asymmetric Catalysis. Springer; Berlin: 1999. [Google Scholar]
- 39.Zhou J, Ye M-C, Huang Z-Z, Tang Y. J. Org. Chem. 2004;69:1309. doi: 10.1021/jo035552p. [DOI] [PubMed] [Google Scholar]
- 40.Loupy A. Modern Solvents in Organic Synthesis. 1999;206 [Google Scholar]
- 41.Pastor IM, Yus M. Curr. Org. Chem. 2005;9:1. [Google Scholar]
- 42.Jacobsen EN. Acc. Chem. Res. 2000;33:421. doi: 10.1021/ar960061v. [DOI] [PubMed] [Google Scholar]
- 43.Sekine A, Ohshima T, Shibasaki M. Tetrahedron. 2002;58:75. [Google Scholar]
- 44.Martinez LE, Nugent WA, Jacobsen EN. J. Org. Chem. 1996;61:7963. doi: 10.1021/jo961241l. [DOI] [PubMed] [Google Scholar]
- 45.Jacobsen EN, Wu MH. Comprehensive Asymmetric Catalysis I-III. 1999;3:1309. [Google Scholar]
- 46.Trost BM. Science. 1991;254:1471. doi: 10.1126/science.1962206. [DOI] [PubMed] [Google Scholar]
- 47.Trost BM. Angew. Chem., Int. Ed. 1995;34:259. [Google Scholar]
- 48.Konsler RG, Karl J, Jacobsen EN. J. Am. Chem. Soc. 1998;120:10780. [Google Scholar]
- 49.Annis DA, Jacobsen EN. J. Am. Chem. Soc. 1999;121:4147. [Google Scholar]
- 50.Nielsen LPC, Stevenson CP, Blackmond DG, Jacobsen EN. J. Am. Chem. Soc. 2004;126:1360. doi: 10.1021/ja038590z. [DOI] [PubMed] [Google Scholar]
- 51.Hansen KB, Leighton JL, Jacobsen EN. J. Am. Chem. Soc. 1996;118:10924. [Google Scholar]
- 52.Schaus SE, Jacobsen EN. Org. Lett. 2000;2:1001. doi: 10.1021/ol005721h. [DOI] [PubMed] [Google Scholar]
- 53.Larrow JF, Jacobsen EN. Top. Organomet. Chem. 2004;6:123. [Google Scholar]
- 54.Martinez LE, Leighton JL, Carsten DH, Jacobsen EN. J. Am. Chem. Soc. 1995;117:5897. [Google Scholar]
- 55.Leighton JL, Jacobsen EN. J. Org. Chem. 1996;61:389. [Google Scholar]
- 56.Larrow JF, Schaus SE, Jacobsen EN. J. Am. Chem. Soc. 1996;118:7420. [Google Scholar]
- 57.Schaus SE, Jacobsen EN. Tetrahedron Lett. 1996;37:7937. [Google Scholar]
- 58.Jacobsen EN, Kakiuchi F, Konsler RG, Larrow JF, Tokunaga M. Tetrahedron Lett. 1997;38:773. [Google Scholar]
- 59.Tokunaga M, Larrow JF, Kakiuchi F, Jacobsen EN. Science. 1997;277:936. doi: 10.1126/science.277.5328.936. [DOI] [PubMed] [Google Scholar]
- 60.Brandes BD, Jacobsen EN. Tetrahedron: Asymmetry. 1997;8:3927. [Google Scholar]
- 61.Wu MH, Jacobsen EN. J. Org. Chem. 1998;63:5252. doi: 10.1021/jo981332d. [DOI] [PubMed] [Google Scholar]
- 62.Bruns S, Haufe G. Tetrahedron: Asymmetry. 1999;10:1563. [Google Scholar]
- 63.Ready JM, Jacobsen EN. J. Am. Chem. Soc. 1999;121:6086. doi: 10.1021/ja005867b. [DOI] [PubMed] [Google Scholar]
- 64.Peukert S, Jacobsen EN. Org. Lett. 1999;1:1245. doi: 10.1021/ol990920q. [DOI] [PubMed] [Google Scholar]
- 65.Brandes BD, Jacobsen EN. Synlett. 2001:1013. [Google Scholar]
- 66.Schaus SE, Brandes BD, Larrow JF, Tokunaga M, Hansen KB, Gould AE, Furrow ME, Jacobsen EN. J. Am. Chem. Soc. 2002;124:1307. doi: 10.1021/ja016737l. [DOI] [PubMed] [Google Scholar]
- 67.Kim SK, Jacobsen EN. Angew. Chem. Int. Ed. 2004;43:3952. doi: 10.1002/anie.200460369. [DOI] [PubMed] [Google Scholar]
- 68.Yoon TP, Jacobsen EN. Science. 2003;299:1691. doi: 10.1126/science.1083622. [DOI] [PubMed] [Google Scholar]
- 69.Schaus SE, Larrow JF, Jacobsen EN. J. Org. Chem. 1997;62:4197. [Google Scholar]
- 70.Annis DA, Helluin O, Jacobsen EN. Angew. Chem. Int. Ed. 1998;37:1907. [Google Scholar]
- 71.Kassab DJ, Ganem B. J. Org. Chem. 1999;64:1782. doi: 10.1021/jo990167e. [DOI] [PubMed] [Google Scholar]
- 72.Wu MH, Jacobesen EN. Tetrahedron Lett. 1997;38:1693. [Google Scholar]
- 73.Collman JP, Zhang X, Lee VJ, Uffelman ES, Brauman JI. Science. 1993;261:1404. doi: 10.1126/science.8367724. [DOI] [PubMed] [Google Scholar]
- 74.Denmark SE, Wu Z. Synlett. 1999:847. [Google Scholar]
- 75.Katsuki T. Curr. Org. Chem. 2001;5:663. [Google Scholar]
- 76.Aggarwal VK, Winn CL. Acc. Chem. Res. 2004;37:611. doi: 10.1021/ar030045f. [DOI] [PubMed] [Google Scholar]
- 77.Yang D. Acc. Chem. Res. 2004;37:497. doi: 10.1021/ar030065h. [DOI] [PubMed] [Google Scholar]
- 78.McGarrigle EM, Gilheany DG. Chem. Rev. 2005;105:1563. doi: 10.1021/cr0306945. [DOI] [PubMed] [Google Scholar]
- 79.Keith JM, Larrow JF, Jacobsen EN. Adv. Synth. Catal. 2001;343:5. [Google Scholar]
- 80.Hoveyda AH, Didiuk MT. Curr. Org. Chem. 1998;2:489. [Google Scholar]
- 81.Cook GR. Curr. Org. Chem. 2000;4:869. [Google Scholar]
- 82.Lebel H, Jacobsen EN. Tetrahedron Lett. 1999;40:7303. [Google Scholar]
- 83.El Gihani MT, Williams JMJ. Curr. Opin. Chem. Biol. 1999;3:11. doi: 10.1016/s1367-5931(99)80003-9. [DOI] [PubMed] [Google Scholar]
- 84.Ward RS. Tetrahedron: Asymmetry. 1995;6:1475. [Google Scholar]
- 85.Ready JM, Jacobsen EN. J. Am. Chem. Soc. 2001;123:2687. doi: 10.1021/ja005867b. [DOI] [PubMed] [Google Scholar]
- 86.White DE, Jacobsen EN. Tetrahedron: Asymmetry. 2003;14:3633. [Google Scholar]
- 87.Ready JM, Jacobsen EN. Angew. Chem. Int. Ed. 2002;41:1374. doi: 10.1002/1521-3773(20020415)41:8<1374::aid-anie1374>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 88.Zheng X, Jones CW, Weck M. Chem. Eur. J. 2006;12:576. doi: 10.1002/chem.200500786. [DOI] [PubMed] [Google Scholar]
- 89.Darensbourg DJ, Holtcamp MW. Coord. Chem. Rev. 1996;153:155. [Google Scholar]
- 90.Lu X-B, Liang B, Zhang Y-J, Tian Y-Z, Wang Y-M, Bai C-X, Wang H, Zhang R. J. Am. Chem. Soc. 2004;126:3732. doi: 10.1021/ja049734s. [DOI] [PubMed] [Google Scholar]
- 91.Berkessel A, Brandenburg M. Org. Lett. 2006;8:4401. doi: 10.1021/ol061501d. [DOI] [PubMed] [Google Scholar]
- 92.Jorgensen KA. Angew. Chem. Int. Ed. 2000;39:3558. [Google Scholar]
- 93.Osborn HMI, Coisson D. Mini-Rev. Org. Chem. 2004;1:41. [Google Scholar]
- 94.Waldmann H. Synthesis. 1994:535. [Google Scholar]
- 95.Zamojski A. Chemtracts: Org. Chem. 1994;7:337. [Google Scholar]
- 96.Johannsen M, Yao S, Graven A, Jorgensen KA. Pure Appl. Chem. 1998;70:1117. [Google Scholar]
- 97.Danishefsky S, Kerwin JF, Jr., Kobayashi S. J. Am. Chem. Soc. 1982;104:358. [Google Scholar]
- 98.Keck GE, Li X-Y, Krishnamurthy D. J. Org. Chem. 1995;60:5998. [Google Scholar]
- 99.Wang B, Feng X, Cui X, Liu H, Jiang Y. Chem. Commun. 2000:1605. [Google Scholar]
- 100.Long J, Hu JY, Shen XQ, Ji BM, Ding KL. J. Am. Chem. Soc. 2002;124:10. doi: 10.1021/ja0172518. [DOI] [PubMed] [Google Scholar]
- 101.Bianchini C, Giambastiani G. Chemtracts. 2002;15:672. [Google Scholar]
- 102.Du H, Long J, Hu J, Li X, Ding K. Org. Lett. 2002;4:4349. doi: 10.1021/ol027034r. [DOI] [PubMed] [Google Scholar]
- 103.Halterman RL, Jan ST. J. Org. Chem. 1991;56:5253. [Google Scholar]
- 104.Halterman RL, Jan S-T, Nimmons HL, Standlee DJ, Khan MA. Tetrahedron. 1997;53:11257. [Google Scholar]
- 105.Berkessel A, Ertuerk E, Laporte C. Adv. Synth. Catal. 2006;348:223. [Google Scholar]
- 106.Schaus SE, Brnalt J, Jacobsen EN. J. Org. Chem. 1998;63:403. doi: 10.1021/jo981332d. [DOI] [PubMed] [Google Scholar]
- 107.Huang Y, Unni AK, Thadani AN, Rawal VH. Nature. 2003;424:146. doi: 10.1038/424146a. [DOI] [PubMed] [Google Scholar]
- 108.Schreiner PR. Chem. Soc. Rev. 2003;32:289. doi: 10.1039/b107298f. [DOI] [PubMed] [Google Scholar]
- 109.Houk KN, List B. Acc. Chem. Res. 2004;37:487. doi: 10.1021/ar0300571. [DOI] [PubMed] [Google Scholar]
- 110.Dalko PI, Moisan L. Angew. Chem. Int. Ed. 2004;43:5138. doi: 10.1002/anie.200400650. [DOI] [PubMed] [Google Scholar]
- 111.Seayad J, List B. Org. Bio. Chem. 2005;3:719. doi: 10.1039/b415217b. [DOI] [PubMed] [Google Scholar]
- 112.Bolm C, Rantanen T, Schiffers I, Zani L. Angew. Chem. Int. Ed. 2005;44:1758. doi: 10.1002/anie.200500154. [DOI] [PubMed] [Google Scholar]
- 113.Pihko PM. Angew. Chem. Int. Ed. 2004;43:2062. doi: 10.1002/anie.200301732. [DOI] [PubMed] [Google Scholar]
- 114.Zhang X, Du H, Wang Z, Wu Y-D, Ding K. J. Org. Chem. 2006;71:2862. doi: 10.1021/jo060129c. [DOI] [PubMed] [Google Scholar]
- 115.Joly GD, Jacobsen EN. Org. Lett. 2002;4:1795. doi: 10.1021/ol0258785. [DOI] [PubMed] [Google Scholar]
- 116.Yao S, Johannsen M, Hazell RG, Jorgensen KA. Angew. Chem. Int. Ed. 1998;37:3121. doi: 10.1002/(SICI)1521-3773(19981204)37:22<3121::AID-ANIE3121>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 117.Kobayashi S, Komiyama S, Ishitani H. Angew. Chem. Int. Ed. 1998;37:979. doi: 10.1002/(SICI)1521-3773(19980420)37:7<979::AID-ANIE979>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 118.Kobayashi S, Kusakabe K.-i., Komiyama S, Ishitani H. J. Org. Chem. 1999;64:4220. [Google Scholar]
- 119.Josephsohn NS, Snapper ML, Hoveyda AH. J. Am. Chem. Soc. 2003;125:4018. doi: 10.1021/ja030033p. [DOI] [PubMed] [Google Scholar]
- 120.Savard J, Brassard P. Tetrahedron Lett. 1979;20:4911. [Google Scholar]
- 121.Du H, Zhao D, Ding K. Chem. Eur. J. 2004;10:5964. doi: 10.1002/chem.200400515. [DOI] [PubMed] [Google Scholar]
- 122.Dossetter AG, Jamison TF, Jacobsen EN. Angew. Chem. Int. Ed. 1999;38:2398. doi: 10.1002/(sici)1521-3773(19990816)38:16<2398::aid-anie2398>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 123.Nakajima H, Sato B, Fujita T, Takase S, Terano H, Okuhara M. J. Antibiot. 1996;49:1196. doi: 10.7164/antibiotics.49.1196. [DOI] [PubMed] [Google Scholar]
- 124.Taunton J, Hassig CA, Schreiber SL. Science. 1996;272:408. doi: 10.1126/science.272.5260.408. [DOI] [PubMed] [Google Scholar]
- 125.Nakajima H, Hori Y, Terano H, Okuhara M, Manda T, Matsumoto S, Shimomura K. J. Antibiot. 1996;49:1204. doi: 10.7164/antibiotics.49.1204. [DOI] [PubMed] [Google Scholar]
- 126.Nakajima H, Kim YB, Terano H, Yoshida M, Horinouchi S. Exp. Cell Res. 1998;241:126. doi: 10.1006/excr.1998.4027. [DOI] [PubMed] [Google Scholar]
- 127.Thompson CF, Jamison TF, Jacobsen EN. J. Am. Chem. Soc. 2001;123:9974. doi: 10.1021/ja016615t. [DOI] [PubMed] [Google Scholar]
- 128.Schrock RR, Hoveyda AH. Angew. Chem. Int. Ed. 2003;42:4592. doi: 10.1002/anie.200300576. [DOI] [PubMed] [Google Scholar]
- 129.Grubbs RH. Handbook of Metathesis. VCH-Wiley; Wienheim: 2003. [Google Scholar]
- 130.Schrock RR. Tetrahedron. 1999;55:8141. [Google Scholar]
- 131.Dragutan V, Dragutan I, Balaban AT. Platinum Met. Rev. 2000;44:112. [Google Scholar]
- 132.Ramachandran PV, Reddy MVR, Brown HC. Pure Appl. Chem. 2003;75:1263. [Google Scholar]
- 133.Rivkin A, Cho YS, Gabarda AE, Yoshimura F, Danishefsky SJ. J. Nat. Prod. 2004;67:139. doi: 10.1021/np030540k. [DOI] [PubMed] [Google Scholar]
- 134.Dolman SJ, Sattely ES, Hoveyda AH, Schrock RR. J. Am. Chem. Soc. 2002;124:6991. doi: 10.1021/ja012534l. [DOI] [PubMed] [Google Scholar]
- 135.Xu Z, Johannes CW, Houri AF, La DS, Cogan DA, Hofilena GE, Hoveyda AH. J. Am. Chem. Soc. 1997;119:10302. [Google Scholar]
- 136.Smith AB, III, Adams CM, Kozmin SA. J. Am. Chem. Soc. 2001;123:990. doi: 10.1021/ja003745d. [DOI] [PubMed] [Google Scholar]
- 137.Marsella MJ, Maynard HD, Grubbs RH. Angew. Chem. Int. Ed. 1997;36:1101. [Google Scholar]
- 138.La DS, Alexander JB, Cefalo DR, Graf DD, Hoveyda AH, Schrock RR. J. Am. Chem. Soc. 1998;120:9720. [Google Scholar]
- 139.Sattely ES, Cortez GA, Moebius DC, Schrock RR, Hoveyda A,H. J. Am. Chem. Soc. 2005;127:8526. doi: 10.1021/ja051330s. [DOI] [PubMed] [Google Scholar]
- 140.Evans DA, Carter PH, Carreira EM, Charette AB, Prunet JA, Lautens M. J. Am. Chem. Soc. 1999;121:7540. [Google Scholar]
- 141.Hornberger KR, Hamblett CL, Leighton JL. J. Am. Chem. Soc. 2000;122:12894. [Google Scholar]
- 142.Hoye TR, Hu M. J. Am. Chem. Soc. 2003;125:9576. doi: 10.1021/ja035579q. [DOI] [PubMed] [Google Scholar]
- 143.Gillingham DG, Kataoka O, Garber SB, Hoveyda AH. J. Am. Chem. Soc. 2004;126:12288. doi: 10.1021/ja0458672. [DOI] [PubMed] [Google Scholar]
- 144.Danishefsky SJ, Selnick HG, Zelle RE, DeNinno MP. J. Am. Chem. Soc. 1988;110:4368. [Google Scholar]
- 145.Ziegler FE, Becker MR. J. Org. Chem. 1990;55:2800. [Google Scholar]
- 146.Mikami K, Terada M, Narisawa S, Nakai T. Synlett. 1992:255. [Google Scholar]
- 147.Berrisford DJ, Bolm C. Angew. Chem., Int. Ed. 1995;34:1717. [Google Scholar]
- 148.Mikami K, Yajima T, Siree N, Terada M, Suzuki Y, Takanishi Y, Takezoe H. Synlett. 1999:1895. doi: 10.1002/(sici)1521-3773(19990816)38:16<2353::aid-anie2353>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 149.Mikami K, Koizumi Y, Osawa A, Terada M, Takayama H, Nakagawa K, Okano T. Synlett. 1999:1899. [Google Scholar]
- 150.Braddock DC, Brown JM. Tetrahedron: Asymmetry. 2000;11:3591. [Google Scholar]
- 151.Mikami K. Pure Appl. Chem. 1996;68:639. [Google Scholar]
- 152.Kezuka S, Ikeno T, Yamada T. Org. Lett. 2001;3:1937. doi: 10.1021/ol015980m. [DOI] [PubMed] [Google Scholar]
- 153.Yamada YMA, Ichinohe M, Takahashi H, Ikegami S. Tetrahedron Lett. 2002;43:3431. [Google Scholar]
- 154.Alcaide B, Almendros P, Pardo C, Rodriguez-Ranera C, Rodriguez-Vicente A. J. Org. Chem. 2003;68:3106. doi: 10.1021/jo0340509. [DOI] [PubMed] [Google Scholar]
- 155.Yang D, Yang M, Zhu N-Y. Org. Lett. 2003;5:3749. doi: 10.1021/ol035486d. [DOI] [PubMed] [Google Scholar]
- 156.Ooi T, Ohmatsu K, Uraguchi D, Maruoka K. Tetrahedron Lett. 2004;45:4481. [Google Scholar]
- 157.Evans DA, Wu J. J. Am. Chem. Soc. 2005;127:8006. doi: 10.1021/ja0522130. [DOI] [PubMed] [Google Scholar]
- 158.Yuan Y, Zhang X, Ding KL. Angew. Chem. Int. Ed. 2003;42:5478. doi: 10.1002/anie.200352535. [DOI] [PubMed] [Google Scholar]
- 159.Brown PH,H. EP Appl9611814.9. 1996 [Google Scholar]
- 160.Fan Q-H, Li Y-M, Chan ASC. Chem. Rev. 2002;102:3385. doi: 10.1021/cr010341a. [DOI] [PubMed] [Google Scholar]
- 161.Li X, Lu G, Kwok WH, Chan ASC. J. Am. Chem. Soc. 2002;124:12636. doi: 10.1021/ja025541y. [DOI] [PubMed] [Google Scholar]
- 162.Dai L-X. Angew. Chem. Int. Ed. 2004;43:5726. doi: 10.1002/anie.200460301. [DOI] [PubMed] [Google Scholar]
- 163.Guo H, Wang X, Ding K. Tetrahedron Lett. 2004;45:2009. [Google Scholar]
- 164.Noyori R, Okhuma T. Angew. Chem. Int. Ed. 2001;40:40. [PubMed] [Google Scholar]
- 165.Pu L, Yu HB. Chem. Rev. 2001;101:757. doi: 10.1021/cr000411y. [DOI] [PubMed] [Google Scholar]
- 166.Pu L. Tetrahedron. 2003;59:9873. [Google Scholar]
- 167.Soai K, Niwa S. Chem. Rev. 1992;92:833. [Google Scholar]
- 168.Knochel P, Singer RD. Chem. Rev. 1993;93:2117. [Google Scholar]
- 169.Duthaler RO, Hafner A. Angew. Chem., Int. Ed. 1997;36:43. [Google Scholar]
- 170.Walsh P,J. Acc. Chem. Res. 2003;36:739. doi: 10.1021/ar0300219. [DOI] [PubMed] [Google Scholar]
- 171.Rousch WR, Hawkins JM, Grubbs RH. Chemtracts: Org. Chem. 1988;1:21. [Google Scholar]
- 172.Noyori R, Suga S, Oka H, Kitamura M. Chemical Record. 2001;1:85. doi: 10.1002/tcr.1. [DOI] [PubMed] [Google Scholar]
- 173.Lu G, Li Y-M, Li X-S, Chan ASC. Coord. Chem. Rev. 2005;249:1736. [Google Scholar]
- 174.Yus M, Ramon DJ. Pure Appl. Chem. 2005;77:2111. [Google Scholar]
- 175.Wingstrand E, Lundgren S, Penhoat M, Moberg C. Pure Appl. Chem. 2006;78:409. [Google Scholar]
- 176.Denmark SE, Fu J. Chem. Rev. 2003;103:2763. doi: 10.1021/cr020050h. [DOI] [PubMed] [Google Scholar]
- 177.Sato I, Saito T, Soai K. Chem. Commun. 2000:2471. [Google Scholar]
- 178.Sato I, Kodaka R, Soai K. J. Chem. Soc. Perkin Trans. 2001;1:2912. [Google Scholar]
- 179.Anand NK, Carreira EM. J. Am. Chem. Soc. 2001;123:9687. doi: 10.1021/ja016378u. [DOI] [PubMed] [Google Scholar]
- 180.Garcia C, LaRochelle LK, Walsh PJ. J. Am. Chem. Soc. 2002;124:10970. doi: 10.1021/ja026568k. [DOI] [PubMed] [Google Scholar]
- 181.Betancort JM, Garcia C, Walsh PJ. Synlett. 2004:749. [Google Scholar]
- 182.Li H, Walsh PJ. J. Am. Chem. Soc. 2004;126:6538. doi: 10.1021/ja049206g. [DOI] [PubMed] [Google Scholar]
- 183.Li H, Garcia C, Walsh PJ. Proc. Natl. Acad. Sci. U. S. A. 2004;101:5425. doi: 10.1073/pnas.0307119101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Li H, Walsh PJ. J. Am. Chem. Soc. 2005;127:8355. doi: 10.1021/ja0425740. [DOI] [PubMed] [Google Scholar]
- 185.Jeon S-J, Li H, Garcia C, LaRochelle LK, Walsh PJ. J. Org. Chem. 2005;70:448. doi: 10.1021/jo048683e. [DOI] [PubMed] [Google Scholar]
- 186.Garcia C, Walsh PJ. Org. Lett. 2003;5:3641. doi: 10.1021/ol0352963. [DOI] [PubMed] [Google Scholar]
- 187.Ramon DJ, Yus M. Angew. Chem. Int. Ed. 2004;43:284. doi: 10.1002/anie.200301696. [DOI] [PubMed] [Google Scholar]
- 188.Ramon DJ, Yus M. Tetrahedron Lett. 1998;39:1239. [Google Scholar]
- 189.Prieto O, Ramon DJ, Yus M. Tetrahedron: Asymmetry. 2003;14:1955. [Google Scholar]
- 190.Lu G, Li X, Jia X, Chan WL, Chan ASC. Angew. Chem. Int. Ed. 2003;42:5057. doi: 10.1002/anie.200352013. [DOI] [PubMed] [Google Scholar]
- 191.Jeon S-J, Walsh PJ. J. Am. Chem. Soc. 2003;125:9544. doi: 10.1021/ja036302t. [DOI] [PubMed] [Google Scholar]
- 192.Yus M, Ramon DJ, Prieto O. Eur. J. Org. Chem. 2003:2745. [Google Scholar]
- 193.Jeon S-J, Li H, Walsh PJ. J. Am. Chem. Soc. 2005;127:16416. doi: 10.1021/ja052200m. [DOI] [PubMed] [Google Scholar]
- 194.Jeon S-J, Li H, Walsh PJ. J. Am. Chem. Soc. 2005;127:16416. doi: 10.1021/ja052200m. [DOI] [PubMed] [Google Scholar]
- 195.Berrisford DJ, Bolm C, Sharpless KB. Angew. Chem., Int. Ed. 1995;34:1059. [Google Scholar]
- 196.Kitamura M, Okada S, Suga S, Noyori R. J. Am. Chem. Soc. 1989;111:4028. [Google Scholar]
- 197.Knochel P, Rozema MJ, Tucker CE, Retherford C, Furlong M, Rao SA. Pure Appl. Chem. 1992;64:361. [Google Scholar]
- 198.Langer F, Waas J, Knochel P. Tetrahedron Lett. 1993;34:5261. [Google Scholar]
- 199.Langer F, Devasagayaraj A, Chavant PY, Knochel P. Synlett. 1994:410. [Google Scholar]
- 200.Lutz C, Knochel P. J. Org. Chem. 1997;62:7895. [Google Scholar]
- 201.Rozema MJ, Sidduri A, Knochel P. J. Org. Chem. 1992;57:1956. [Google Scholar]
- 202.Knochel P. Chemtracts: Org. Chem. 1995;8:205. [Google Scholar]
- 203.Langer F, Schwink L, Devasagayaraj A, Chavant P-Y, Knochel P. J. Org. Chem. 1996;61:8229. doi: 10.1021/jo961129n. [DOI] [PubMed] [Google Scholar]
- 204.Kelly AR, Lurain AE, Walsh PJ. J. Am. Chem. Soc. 2005;127:14668. doi: 10.1021/ja051291k. [DOI] [PubMed] [Google Scholar]
- 205.Lippard SJ. Chem. Eng. News. 2000;78:64. [Google Scholar]
- 206.Yanagisawa A. Comprehensive Asymmetric Catalysis I-III. Vol. 2. Springer; Berlin: 1999. [Google Scholar]
- 207.Costa AL, Piazza MG, Tagliavini E, Trombini C, Umani-Ronchi A. J. Am. Chem. Soc. 1993;115:7001. [Google Scholar]
- 208.Keck GE, Tarbet KH, Geraci LS. J. Am. Chem. Soc. 1993;115:8467. [Google Scholar]
- 209.Lou S, Moquist PN, Schaus SE. J. Am. Chem. Soc. 2006;128:12660. doi: 10.1021/ja0651308. [DOI] [PubMed] [Google Scholar]
- 210.Casolari S, D'Addario D, Tagliavini E. Org. Lett. 1999;1:1061. [Google Scholar]
- 211.Wadamoto M, Yamamoto H. J. Am. Chem. Soc. 2005;127:14556. doi: 10.1021/ja0553351. [DOI] [PubMed] [Google Scholar]
- 212.Wada R, Oisaki K, Kanai M, Shibasaki M. J. Am. Chem. Soc. 2004;126:8910. doi: 10.1021/ja047200l. [DOI] [PubMed] [Google Scholar]
- 213.Teo Y-C, Goh J-D, Loh T-P. Org. Lett. 2005;7:2743. doi: 10.1021/ol051018n. [DOI] [PubMed] [Google Scholar]
- 214.Lu J, Hong M-L, Ji S-J, Teo Y-C, Loh T-P. Chem. Commun. 2005:4217. doi: 10.1039/b507768k. [DOI] [PubMed] [Google Scholar]
- 215.Prieto O, Woodward S. J. Organomet. Chem. 2006;691:1515. [Google Scholar]
- 216.Kim JG, Camp EH, Walsh PJ. Org. Lett. 2006;8:4413. doi: 10.1021/ol061417y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Hanawa H, Kii S, Maruoka K. Adv. Synth. Catal. 2001;343:57. [Google Scholar]
- 218.Yasuda M, Kitahara N, Fujibayashi T, Baba A. Chem. Lett. 1998;27:743. [Google Scholar]
- 219.Kim JG, Waltz KM, Garcia IF, Kwiatkowski D, Walsh PJ. J. Am. Chem. Soc. 2004;126:12580. doi: 10.1021/ja047758t. [DOI] [PubMed] [Google Scholar]
- 220.Waltz KM, Gavenonis J, Walsh PJ. Angew. Chem. Int. Ed. 2002;41:3697. doi: 10.1002/1521-3773(20021004)41:19<3697::AID-ANIE3697>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 221.Wooten AJ, Kim JG, Walsh PJ. Org. Lett. 2007;9:381. doi: 10.1021/ol062264h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Keck GE, Geraci LS. Tetrahedron Lett. 1993;34:7827. [Google Scholar]
- 223.Ho TL. Tandem Organic Reactions. Wiley; New York: 1992. [Google Scholar]
- 224.Shimizu S, Ohori K, Arai T, Sasai H, Shibasaki M. J. Org. Chem. 1998;63:7547. doi: 10.1021/jo981069g. [DOI] [PubMed] [Google Scholar]
- 225.Arai T, Sasai H, Aoe K.-i., Okamura K, Date T, Shibasaki M. Angew. Chem., Int. Ed. 1996;35:104. [Google Scholar]
- 226.Xu Y, Ohori K, Ohshima T, Shibasaki M. Tetrahedron. 2002;58:2585. [Google Scholar]
- 227.Ohshima T, Xu Y, Takita R, Shimizu S, Zhong D, Shibasaki M. J. Am. Chem. Soc. 2002;124:14546. doi: 10.1021/ja028457r. [DOI] [PubMed] [Google Scholar]
- 228.Bandini M, Melloni A, Umani-Ronchi A. Angew. Chem. Int. Ed. 2004;43:550. doi: 10.1002/anie.200301679. [DOI] [PubMed] [Google Scholar]
- 229.Paras NA, MacMillan DWC. J. Am. Chem. Soc. 2002;124:7894. doi: 10.1021/ja025981p. [DOI] [PubMed] [Google Scholar]
- 230.Desimoni G, Faita G, Jorgensen KA. Chem. Rev. 2006;106:3561. doi: 10.1021/cr0505324. [DOI] [PubMed] [Google Scholar]
- 231.Gathergood N, Zhuang W, Jorgensen KA. J. Am. Chem. Soc. 2000;122:12517. [Google Scholar]
- 232.Zhuang W, Gathergood N, Hazell RG, Jorgensen KA. J. Org. Chem. 2001;66:1009. doi: 10.1021/jo001176m. [DOI] [PubMed] [Google Scholar]
- 233.Zhao J-L, Liu L, Sui Y, Liu Y-L, Wang D, Chen Y-J. Org. Lett. 2006;8:6127. doi: 10.1021/ol0626037. [DOI] [PubMed] [Google Scholar]
- 234.Gibson SE, Stevenazzi A. Angew. Chem. Int. Ed. 2003;42:1800. doi: 10.1002/anie.200200547. [DOI] [PubMed] [Google Scholar]
- 235.Shibata T, Toshida N, Takagi K. Org. Lett. 2002;1619;4 doi: 10.1021/ol025836g. [DOI] [PubMed] [Google Scholar]
- 236.Shibata T, Toshida N, Takagi K. J. Org. Chem. 2002;67:7446. doi: 10.1021/jo0262661. [DOI] [PubMed] [Google Scholar]
- 237.Cobley CJ, Klosin J, Qin C, Whiteker GT. Org. Lett. 2004;6:3277. doi: 10.1021/ol0487938. [DOI] [PubMed] [Google Scholar]
- 238.Claver C, van Leeuwen PWNM. Rhodium Catalyzed Hydroformylation. Kluwer Academic Publishers; 2000. [Google Scholar]
- 239.Sakai N, Mano S, Nozaki K, Takaya H. J. Am. Chem. Soc. 1993;115:7033. [Google Scholar]
- 240.Nozaki K, Sakai N, Nanno T, Higashijima T, Mano S, Horiuchi T, Takaya H. J. Am. Chem. Soc. 1997;119:4413. [Google Scholar]
- 241.Whiteker GT, Briggs JR, Babin JE, Barner BA. In: Catalysis of Organic Reactions. Morrell DG, editor. Marcel Dekker; New York: 2003. [Google Scholar]
- 242.Babin JE, Whiteker GT. PCT Int. Appl. 1993 WO 9303839. [Google Scholar]
- 243.Walsh PJ, Lurain AE, Balsells J. Chem. Rev. 2003;103:3297. doi: 10.1021/cr0000630. [DOI] [PubMed] [Google Scholar]
- 244.Cobley CJ, Gardner K, Klosin J, Praquin C, Hill C, Whiteker GT, Zanotti-Gerosa A, Petersen JL, Abboud KA. J. Org. Chem. 2004;69:4031. doi: 10.1021/jo040128p. [DOI] [PubMed] [Google Scholar]
- 245.Axtell AT, Cobley CJ, Klosin J, Whiteker GT, Zanotti-Gerosa A, Abboud KA. Angew. Chem. Int. Ed. 2005;44:5834. doi: 10.1002/anie.200501478. [DOI] [PubMed] [Google Scholar]
- 246.Clark TP, Landis CR, Freed SL, Klosin J, Abboud KA. J. Am. Chem. Soc. 2005;127:5040. doi: 10.1021/ja050148o. [DOI] [PubMed] [Google Scholar]
- 247.Burk MJ, Feaster JE, Nugent WA, Harlow RL. J. Am. Chem. Soc. 1993;115:10125. [Google Scholar]
- 248.Burk MJ. Acc. Chem. Res. 2000;33:363. doi: 10.1021/ar990085c. [DOI] [PubMed] [Google Scholar]
- 249.Pilkington CJ, Zanotti-Gerosa A. Org. Lett. 2003;5:1273. doi: 10.1021/ol0341952. [DOI] [PubMed] [Google Scholar]
- 250.Parrinello G, Stille JK. J. Am. Chem. Soc. 1987;109:7122. [Google Scholar]
- 251.Nozaki K, Itoi Y, Shibahara F, Shirakawa E, Ohta T, Takaya H, Hiyama T. J. Am. Chem. Soc. 1998;120:4051. [Google Scholar]
- 252.Nozaki K, Shibahara F, Hiyama T. Chem. Lett. 2000:694. [Google Scholar]
- 253.Shibahara F, Nozaki K, Matsuo T, Hiyama T. Bioorg. Med. Chem. Lett. 2002;12:1825. doi: 10.1016/s0960-894x(02)00267-6. [DOI] [PubMed] [Google Scholar]
- 254.Li C. Cat. Rev. - Sci. Eng. 2004;46:419. [Google Scholar]
- 255.Kinoshita S, Shibahara F, Nozaki K. Green Chem. 2005;7:256. [Google Scholar]
- 256.Shibahara F, Nozaki K, Hiyama T. J. Am. Chem. Soc. 2003;125:8555. doi: 10.1021/ja034447u. [DOI] [PubMed] [Google Scholar]
- 257.Nozaki K, Shibahara F, Hiyama T. Chem. Lett. 2000:694. [Google Scholar]
- 258.Nozaki K, Shibahara F, Itoi Y, Shirakawa E, Ohta T, Takaya H, Hiyama T. Bull. Chem. Soc. Jpn. 1999;72:1911. [Google Scholar]
- 259.Tang W, Zhang X. Chem. Rev. 2003;103:3029. doi: 10.1021/cr020049i. [DOI] [PubMed] [Google Scholar]
- 260.Knowles WS. Adv. Synth. Catal. 2003;345:3. [Google Scholar]
- 261.Noyori R. Angew. Chem. Int. Ed. 2002;41:2008. [PubMed] [Google Scholar]
- 262.Ikariya T, Murata K, Noyori R. Org. Bio. Chem. 2006;4:393. doi: 10.1039/b513564h. [DOI] [PubMed] [Google Scholar]
- 263.Haack K-J, Hashiguchi S, Fujii A, Ikariya T, Noyori R. Angew. Chem., Int. Ed. 1997;36:285. [Google Scholar]
- 264.Abdur-Rashid K, Lough AJ, Morris RH. Organometallics. 2001;20:1047. [Google Scholar]
- 265.Clapham SE, Guo R, Zimmer-De Iuliis M, Rasool N, Lough A, Morris RH. Organometallics. 2006;25:5477. [Google Scholar]
- 266.Wan X, Sun Y, Luo Y, Dao L, Zhang Z. J. Org. Chem. 2005;70:1070. doi: 10.1021/jo048466d. [DOI] [PubMed] [Google Scholar]
- 267.Riant O, Mostefaï N, Courmarcel J. Synthesis. 2004:2943. [Google Scholar]
- 268.Graf v. Keyserlingk N, Martens J. Tetrahedron: Asymmetry. 2001;12:2213. [Google Scholar]
- 269.Feringa BL, Pineschi M, Arnold LA, Imbos R, De Vries AHM. Angew. Chem., Int. Ed. 1997;36:2620. [Google Scholar]
- 270.de Vries AHM, Meetsma A, Feringa BL. Angew. Chem., Int. Ed. 1996;35:2374. [Google Scholar]
- 271.Ohmura T, Hartwig JF. J. Am. Chem. Soc. 2002;124:15164. doi: 10.1021/ja028614m. [DOI] [PubMed] [Google Scholar]
- 272.Dahuron N, Langlois N. Synlett. 1996:51. [Google Scholar]
- 273.Bull SD, Davies SG, Garner AC, O'Shea MD. J. Chem. Soc, Perkin Trans. 2001;1:3281. [Google Scholar]
- 274.Mita T, Sasaki K, Kanai M, Shibasaki M. J. Am. Chem. Soc. 2005;127:514. doi: 10.1021/ja043424s. [DOI] [PubMed] [Google Scholar]
- 275.Esquivias J, Gomez Arrayas R, Carretero JC. J. Org. Chem. 2005;70:7451. doi: 10.1021/jo0511602. [DOI] [PubMed] [Google Scholar]
- 276.Sammis GM, Danjo H, Jacobsen EN. J. Am. Chem. Soc. 2004;126:9928. doi: 10.1021/ja046653n. [DOI] [PubMed] [Google Scholar]
- 277.Sammis GM, Jacobsen EN. J. Am. Chem. Soc. 2003;125:4442. doi: 10.1021/ja034635k. [DOI] [PubMed] [Google Scholar]
- 278.Berkessel A, Groerger H. Asymmetric Organocatalysis. Wiley-VCH Verlag GmbH; Weinheim, Germany: 2005. [Google Scholar]
- 279.List B. Chem. Commun. 2006:819. doi: 10.1039/b514296m. [DOI] [PubMed] [Google Scholar]
- 280.Pihko PM. Angew. Chem. Int. Ed. 2006;45:544. doi: 10.1002/anie.200502425. [DOI] [PubMed] [Google Scholar]
- 281.Hajos ZG, Parrish DR. J. Org. Chem. 1974;39:1615. [Google Scholar]
- 282.Eder U, Sauer G, Wiechert R. Angew. Chem., Int. Ed. 1971;10:496. doi: 10.1002/anie.197504171. [DOI] [PubMed] [Google Scholar]
- 283.Eder U, Wiechert R, Sauer G. In: Ger. Offen. Schering A-G, editor. p. 1971. De. [Google Scholar]
- 284.List B. Tetrahedron. 2002;58:5573. [Google Scholar]
- 285.Paraskar AS. Synlett. 2003:582. [Google Scholar]
- 286.Allemann C, Gordillo R, Clemente FR, Cheong PH-Y, Houk KN. Acc. Chem. Res. 2004;37:558. doi: 10.1021/ar0300524. [DOI] [PubMed] [Google Scholar]
- 287.Danishefsky S, Cain P. J. Am. Chem. Soc. 1976;98:4975. doi: 10.1021/ja00432a044. [DOI] [PubMed] [Google Scholar]
- 288.Cohen N. Acc. Chem. Res. 1976;9:412. [Google Scholar]
- 289.Smith AB, III, Kingery-Wood J, Leenay TL, Nolen EG, Sunazuka T. J. Am. Chem. Soc. 1992;114:1438. [Google Scholar]
- 290.Nagamitsu T, Sunazuka T, Obata R, Tomoda H, Tanaka H, Harigaya Y, Omura S, Smith AB., III J. Org. Chem. 1995;60:8126. [Google Scholar]
- 291.Danishefsky SJ, Masters JJ, Young WB, Link JT, Snyder LB, Magee TV, Jung D,K, Isaacs RCA, Bornmann WG, et al. J. Am. Chem. Soc. 1996;118:2843. [Google Scholar]
- 292.Pemp A, Seifert K. Tetrahedron Lett. 1997;38:2081. [Google Scholar]
- 293.Rajagopal D, Rajagopalan K, Swaminathan S. Tetrahedron: Asymmetry. 1996;7:2189. [Google Scholar]
- 294.Rajagopal D, Narayanan R, Swaminathan S. Proc. Indian Acad. Sci. Chem. Sci. 2001;113:197. [Google Scholar]
- 295.Buchschacher P, Fuerst A. Org. Synth. 1985;63:37. [Google Scholar]
- 296.Hajos ZG, Parrish DR. Org. Synth. 1985;63:26. [Google Scholar]
- 297.Rodriguez B, Rantanen T, Bolm C. Angew. Chem. Int. Ed. 2006;45:6924. doi: 10.1002/anie.200602820. [DOI] [PubMed] [Google Scholar]
- 298.Pihko PM, Laurikainen KM, Usano A, Nyberg AI, Kaavi JA. Tetrahedron. 2006;62:317. [Google Scholar]
- 299.List B, Lerner RA, Barbas CF., III J. Am. Chem. Soc. 2000;122:2395. [Google Scholar]
- 300.List B. Acc. Chem. Res. 2004;37:548. doi: 10.1021/ar0300571. [DOI] [PubMed] [Google Scholar]
- 301.Mase N, Nakai Y, Ohara N, Yoda H, Takabe K, Tanaka F, Barbas CF., III J. Am. Chem. Soc. 2006;128:734. doi: 10.1021/ja0573312. [DOI] [PubMed] [Google Scholar]
- 302.Hayashi Y, Sumiya T, Takahashi J, Gotoh H, Urushima T, Shoji M. Angew. Chem. Int. Ed. 2006;45:958. doi: 10.1002/anie.200502488. [DOI] [PubMed] [Google Scholar]
- 303.Langer P. Angew. Chem. Int. Ed. 2000;39:3049. doi: 10.1002/1521-3773(20000901)39:17<3049::aid-anie3049>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 304.Basavaiah D, Rao AJ, Satyanarayana T. Chem. Rev. 2003;103:811. doi: 10.1021/cr010043d. [DOI] [PubMed] [Google Scholar]
- 305.Berkessel A, Roland K, Neudoerfl JM. Org. Lett. 2006;8:4195. doi: 10.1021/ol061298m. [DOI] [PubMed] [Google Scholar]
- 306.Takemoto Y. Org. Bio. Chem. 2005;3:4299. doi: 10.1039/b511216h. [DOI] [PubMed] [Google Scholar]
- 307.Taylor MS, Jacobsen EN. Angew. Chem. Int. Ed. 2006;45:1520. doi: 10.1002/anie.200503132. [DOI] [PubMed] [Google Scholar]
- 308.Marigo M, Bertelsen S, Landa A, Jorgensen KA. J. Am. Chem. Soc. 2006;128:5475. doi: 10.1021/ja058490o. [DOI] [PubMed] [Google Scholar]
- 309.Brandau S, Landa A, Franzen J, Marigo M, Jorgensen KA. Angew. Chem. Int. Ed. 2006;45:4305. doi: 10.1002/anie.200601025. [DOI] [PubMed] [Google Scholar]
- 310.Marigo M, Schulte T, Franzen J, Jorgensen KA. J. Am. Chem. Soc. 2005;127:15710. doi: 10.1021/ja055291w. [DOI] [PubMed] [Google Scholar]
- 311.Huang Y, Walji AM, Larsen CH, MacMillan DWC. J. Am. Chem. Soc. 2005;127:15051. doi: 10.1021/ja055545d. [DOI] [PubMed] [Google Scholar]
- 312.Carlone A, Marigo M, North C, Landa A, Jorgensen KA. Chem. Commun. 2006:4928. doi: 10.1039/b611366d. [DOI] [PubMed] [Google Scholar]
- 313.Dalko PI, Moisan L. Angew. Chem. Int. Ed. 2001;40:3726. doi: 10.1002/1521-3773(20011015)40:20<3726::aid-anie3726>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 314.Christoffers J, Baro A. Angew. Chem. Int. Ed. 2003;42:1688. doi: 10.1002/anie.200201614. [DOI] [PubMed] [Google Scholar]
- 315.Berner OM, Tedeschi L, Enders D. Eur. J. Org. Chem. 2002:1877. [Google Scholar]
- 316.Sibi MP, Manyem S. Tetrahedron. 2000;56:8033. [Google Scholar]
- 317.Wang J, Li H, Zu L, Jiang W, Wang W. Adv. Synth. Catal. 2006;348:2047. [Google Scholar]
- 318.Kumaragurubaran N, Juhl K, Zhuang W, Bogevig A, Jorgensen KA. J. Am. Chem. Soc. 2002;124:6254. doi: 10.1021/ja026412k. [DOI] [PubMed] [Google Scholar]