Abstract
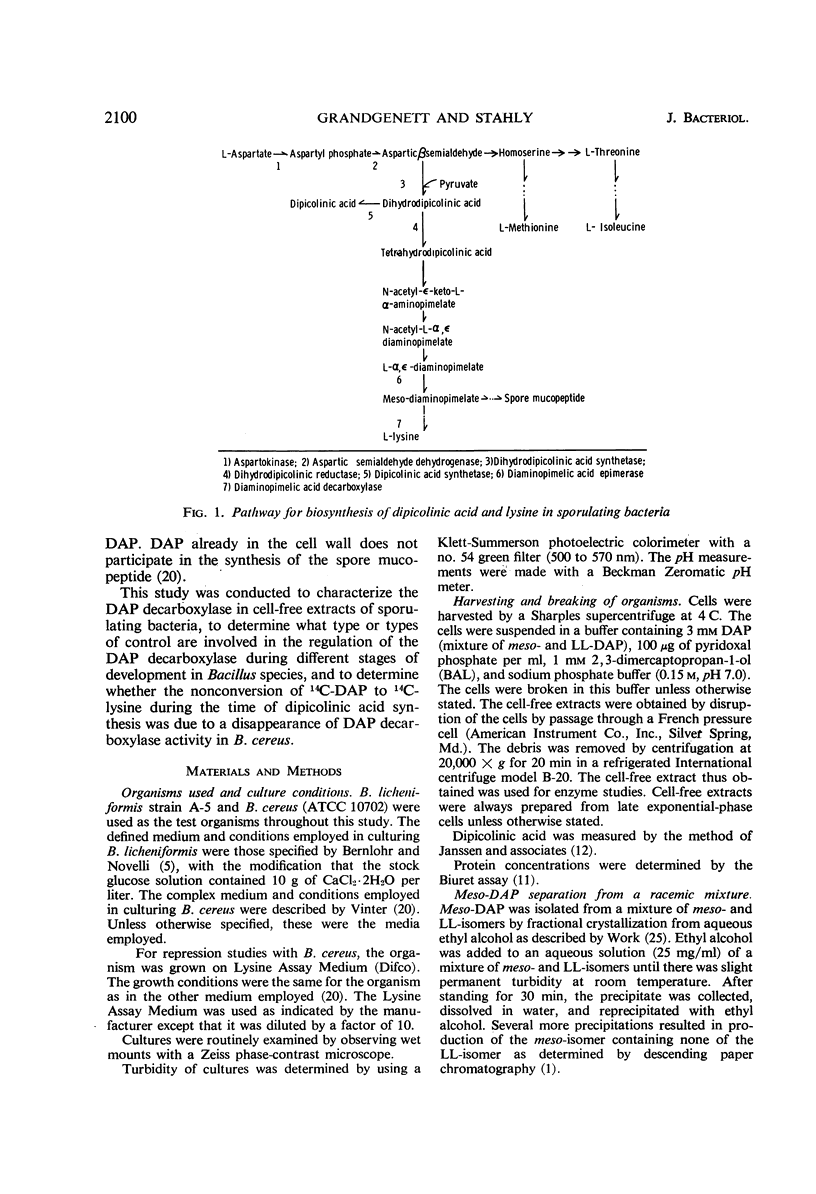
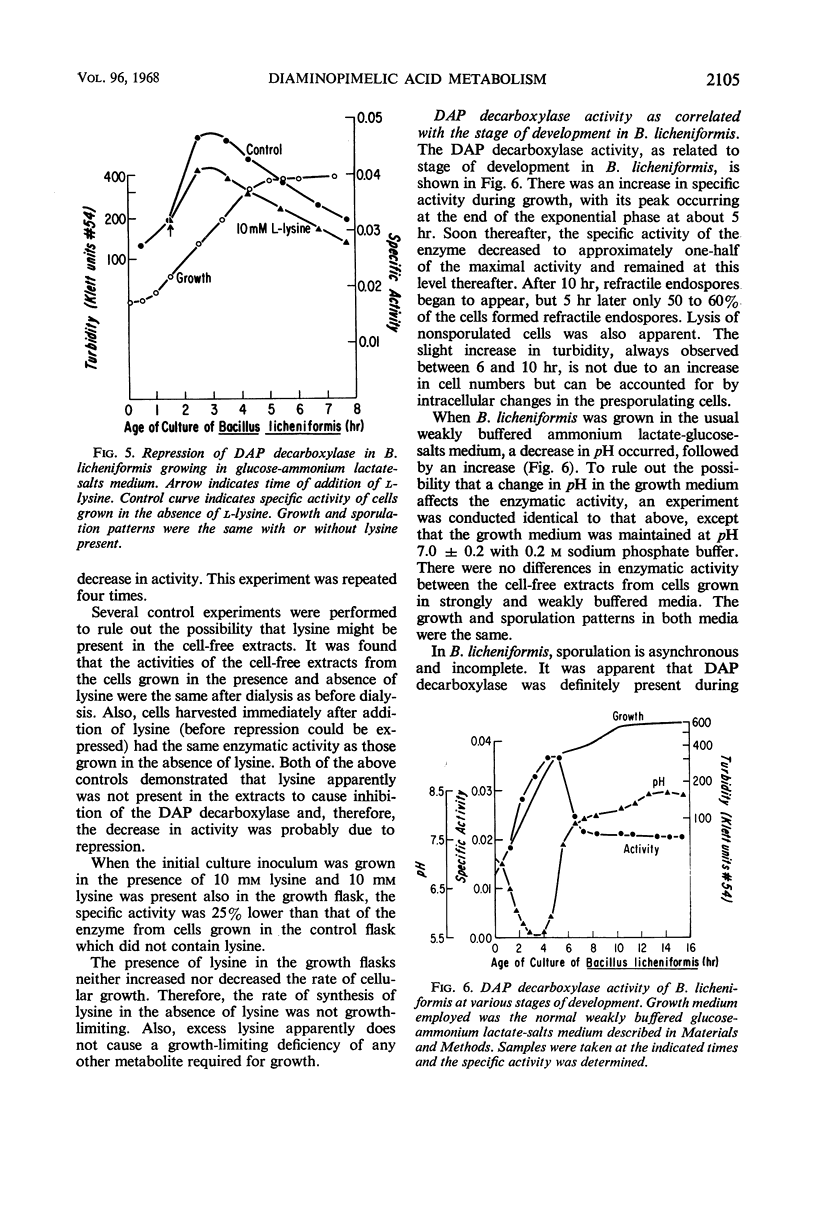
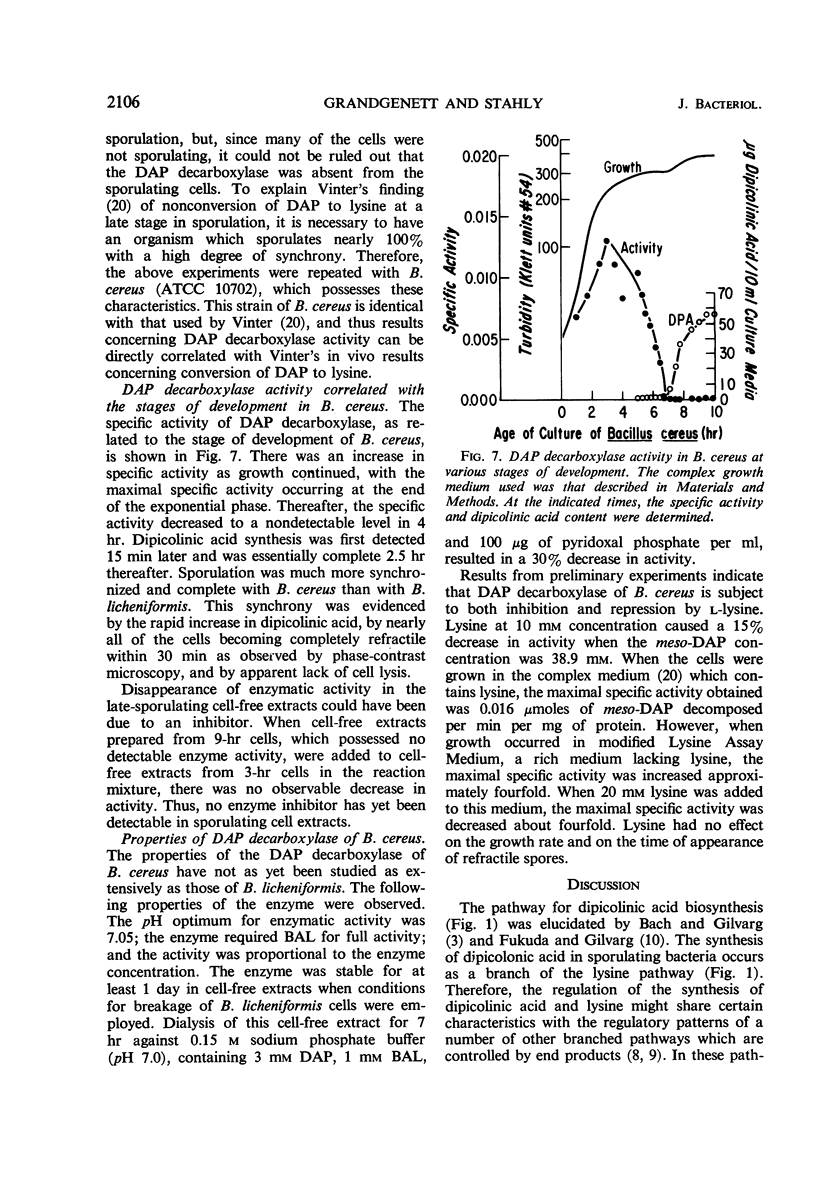
The meso-diaminopimelate (DAP) decarboxylase of Bacillus licheniformis, a pyridoxal phosphate-requiring enzyme, was stabilized in vitro by 0.15 m sodium phosphate buffer (pH 7.0) containing 1 mm 2,3-dimercaptopropan-1-ol, 100 μg of pyridoxal phosphate per ml, and 3 mm DAP. When the meso-DAP concentration was varied, the enzyme in cell-free extracts of B. licheniformis exhibited Michaelis-Menten kinetics. Pyridoxal phosphate was the only pyridoxine derivative which acted as a cofactor. The enzyme was subject to both inhibition and repression by l-lysine. The inhibitory effect of lysine was on the Km (meso-DAP). A maximum repression of about 20% was obtained. No significant inhibition or activation was produced by cadaverine, dipicolinic acid, phenylalanine, pyruvate, ethylenediamine-tetraacetate, adenosine triphosphate, adenosine diphosphate, or adenosine monophosphate. When B. licheniformis was grown in an ammonium lactate-glucose-salts medium, an increase in DAP decarboxylase specific activity occurred during cellular growth with a maximal specific activity at the end of the exponential phase. As soon as growth ceased, the specific activity of the enzyme decreased to approximately one-half of the maximal specific activity and remained at this level thereafter. When B. cereus was grown in complex media, there was an increase in DAP decarboxylase specific activity up to the end of the exponential phase. Thereafter, the specific activity decreased to a nondetectable level in 4 hr. Dipicolinic acid synthesis was first detected 15 min later and was essentially complete after an additional 2.5 hr. The significance of the disappearance of DAP decarboxylase in B. cereus was discussed with regard to control of dipicolinic acid and spore mucopeptide biosynthesis.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANTIA M., HOARE D. S., WORK E. The stereoisomers of alpha epsilon-diaminopimelic acid. III. Properties and distribution of diaminopimelic acid racemase, an enzyme causing interconversion of the LL and meso isomers. Biochem J. 1957 Mar;65(3):448–459. doi: 10.1042/bj0650448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronson A. I., Henderson E., Tincher A. Participation of the lysine pathway in dipicolinic acid synthesis in Bacillus cereus T. Biochem Biophys Res Commun. 1967 Feb 21;26(4):454–460. doi: 10.1016/0006-291x(67)90568-2. [DOI] [PubMed] [Google Scholar]
- BERNLOHR R. W. POSTLOGARITHMIC PHASE METABOLISM OF SPORULATING MICROORGANISMS. I. PROTEASE OF BACILLUS LICHENIFORMIS. J Biol Chem. 1964 Feb;239:538–543. [PubMed] [Google Scholar]
- Bach M. L., Gilvarg C. Biosynthesis of dipicolinic acid in sporulating Bacillus megaterium. J Biol Chem. 1966 Oct 10;241(19):4563–4564. [PubMed] [Google Scholar]
- Bechet J., Wiame J. M. Indication of a specific regulatory binding protein for ornithinetranscarbamylase in Saccharomyces cerevisiae. Biochem Biophys Res Commun. 1965 Nov 8;21(3):226–234. doi: 10.1016/0006-291x(65)90276-7. [DOI] [PubMed] [Google Scholar]
- Chasin L. A., Szulmajster J. Biosynthesis of dipicolinic acid in Bacillus subtilis. Biochem Biophys Res Commun. 1967 Dec 15;29(5):648–654. doi: 10.1016/0006-291x(67)90265-3. [DOI] [PubMed] [Google Scholar]
- DATTA P., GEST H. ALTERNATIVE PATTERNS OF END-PRODUCT CONTROL IN BIOSYNTHESIS OF AMINO-ACIDS OF THE ASPARTIC FAMILY. Nature. 1964 Sep 19;203:1259–1261. doi: 10.1038/2031259a0. [DOI] [PubMed] [Google Scholar]
- Fukuda A., Gilvarg C. The relationship of dipicolinate and lysine biosynthesis in Bacillus megaterium. J Biol Chem. 1968 Jul 25;243(14):3871–3876. [PubMed] [Google Scholar]
- JANSSEN F. W., LUND A. J., ANDERSON L. E. Colorimetric assay for dipicolinic acid in bacterial spores. Science. 1958 Jan 3;127(3288):26–27. doi: 10.1126/science.127.3288.26. [DOI] [PubMed] [Google Scholar]
- Levisohn S., Aronson A. I. Regulation of extracellular protease production in Bacillus cereus. J Bacteriol. 1967 Mar;93(3):1023–1030. doi: 10.1128/jb.93.3.1023-1030.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PATTE J. C., LOVINY T., COHEN G. N. [Repression of meso-alpha, epsilon-diaminopimelic acid decarboxylase by L-lysine in Escherichia coli]. Biochim Biophys Acta. 1962 Apr 9;58:359–360. doi: 10.1016/0006-3002(62)91024-7. [DOI] [PubMed] [Google Scholar]
- POWELL J. F., STRANGE R. E. Alpha-Epsilon-Diaminopimelic acid metabolism and sporulation in Bacillus sphaericus. Biochem J. 1957 Apr;65(4):700–708. doi: 10.1042/bj0650700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundharadas G., Gilvarg C. Biosynthesis of alpha,epsilon-diaminopimelic acid in Bacillus megaterium. J Biol Chem. 1967 Sep 10;242(17):3983–3984. [PubMed] [Google Scholar]
- VINTER V. Spores of microorganisms. Chloramphenicol-sensitive and penicillin-resistant incorporation of 14C-diaminopimelic acid into sporulating cells of Bacillus cereus. Experientia. 1963 Jun 15;19:307–308. doi: 10.1007/BF02150422. [DOI] [PubMed] [Google Scholar]
- WHITE P. J., KELLY B. PURIFICATION AND PROPERTIES OF DIAMINOPIMELATE DECARBOXYLASE FROM ESCHERICHIA COLI. Biochem J. 1965 Jul;96:75–84. doi: 10.1042/bj0960075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WORK E. Reaction of ninhydrin in acid solution with straight-chain amino acids containing two amino groups and its application to the estimation of alpha epsilon-diaminopimelic acid. Biochem J. 1957 Nov;67(3):416–423. doi: 10.1042/bj0670416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White P. J., Kelly B., Suffling A., Work E. Variation of activity of bacterial diaminopimelate decarboxylase under different conditions of growth. Biochem J. 1964 Jun;91(3):600–610. doi: 10.1042/bj0910600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yugari Y., Gilvarg C. The condensation step in diaminopimelate synthesis. J Biol Chem. 1965 Dec;240(12):4710–4716. [PubMed] [Google Scholar]



