Abstract
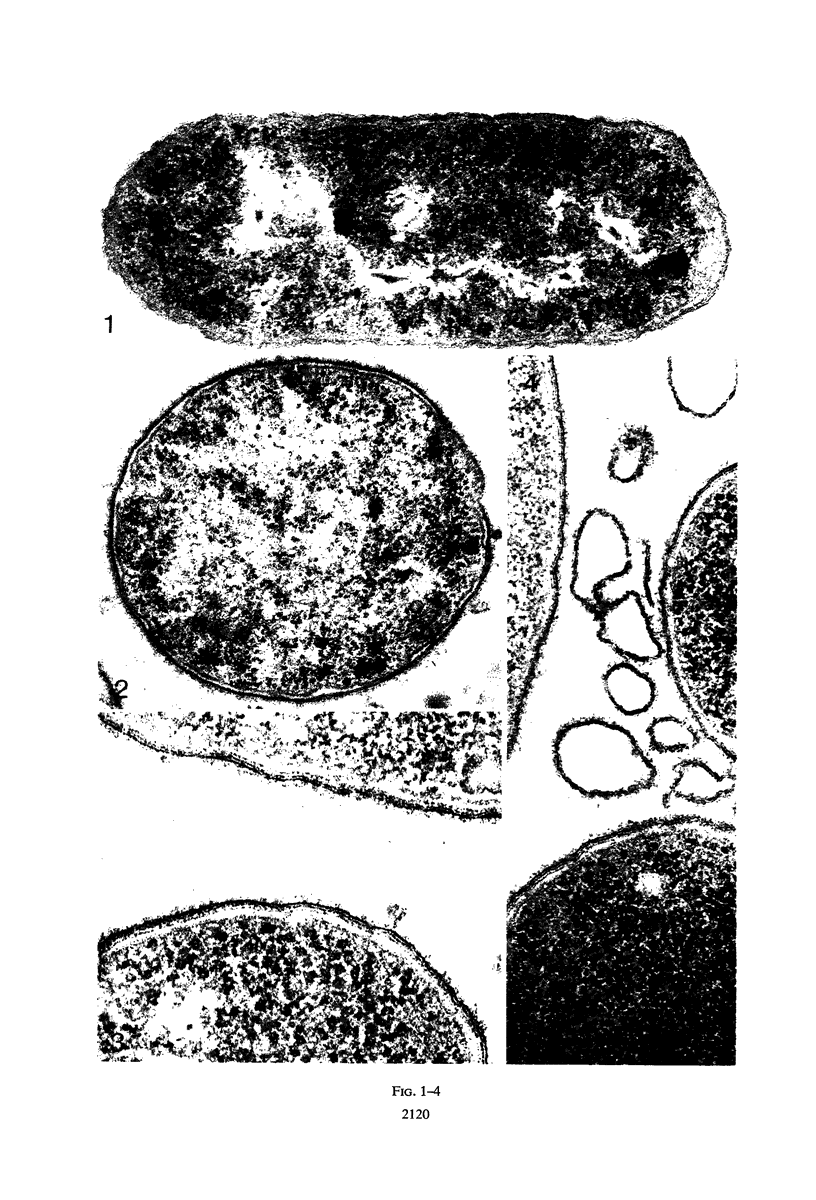
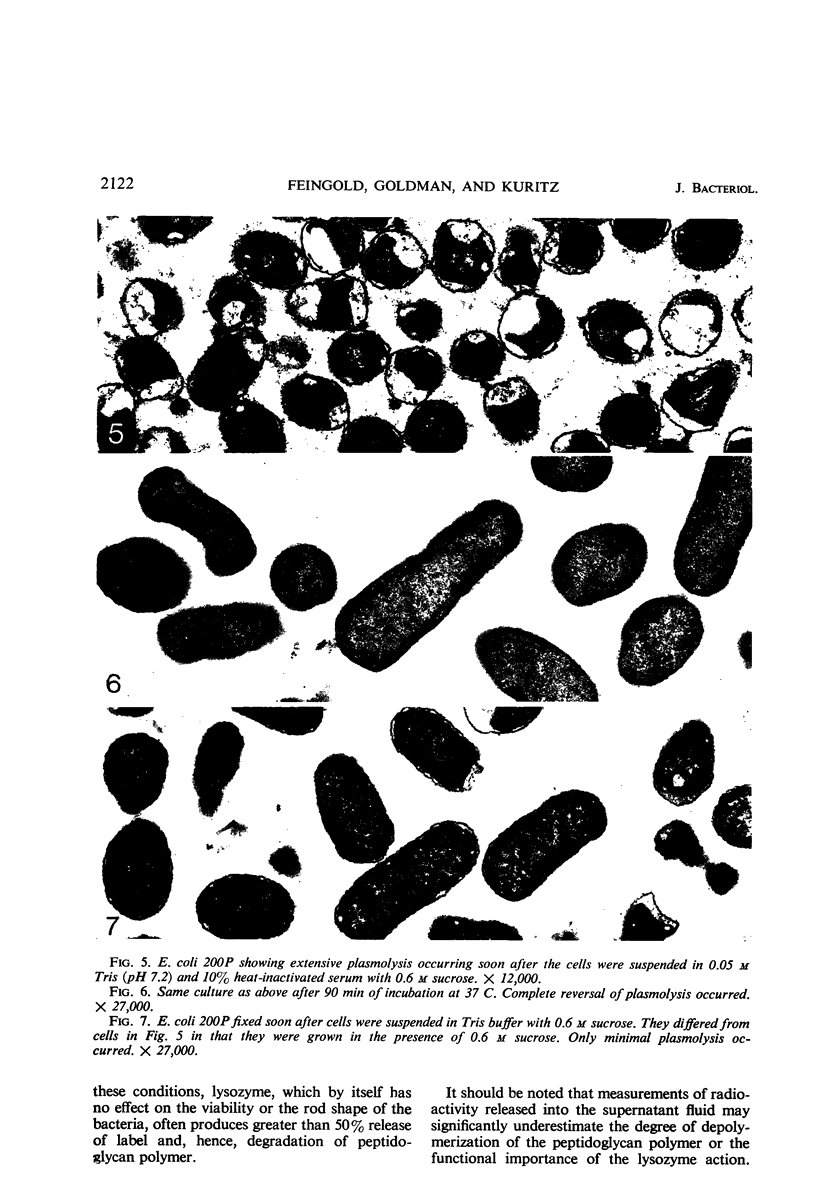
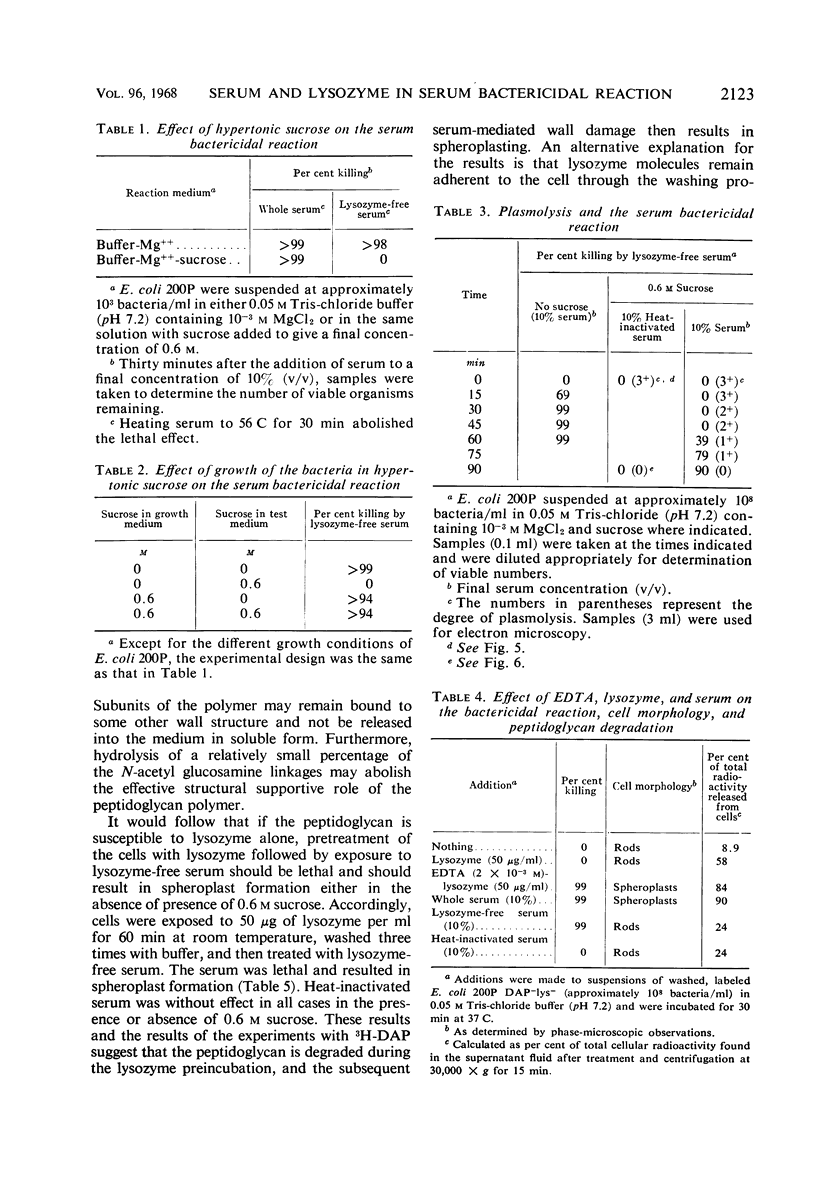
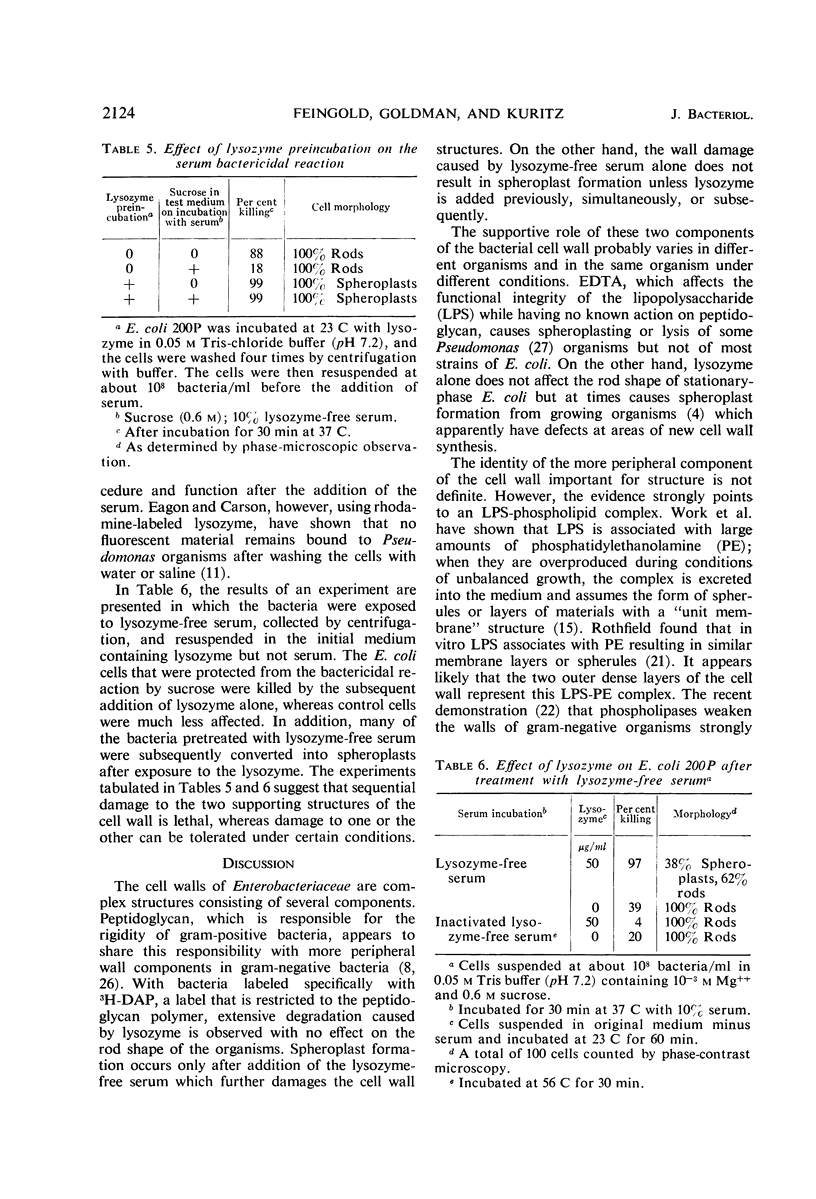
The mechanism of the lethal action of human serum on a rough strain of Escherichia coli was investigated by use of serum with and without lysozyme, in medium of low and high osmotic pressure, with cells radioactively labeled in the peptidoglycan polymer, and by electron microscopy. The results suggested that there are two separate components in the bacterial cell wall that afford structural support for the cell. Lysozyme attacked one of these, the peptidoglycan polymer. Serum damaged the other, which is probably the peripherally located lipopolysaccharide-phospholipid complex. The cell wall damage caused by lysozyme-free serum promptly resulted in cell death under usual conditions. In plasmolyzed cells, however, the wall damage was not lethal, presumably because the membrane of the plasmolyzed cell was protected from secondary lethal changes which otherwise occur.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bayer M. E. Response of Cell Walls of Escherichia coli to a Sudden Reduction of the Environmental Osmotic Pressure. J Bacteriol. 1967 Mar;93(3):1104–1112. doi: 10.1128/jb.93.3.1104-1112.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayer M. E. The cell wall of Escherichia coli: early effects of penicillin treatment and deprivation of diaminopimelic acid. J Gen Microbiol. 1967 Feb;46(2):237–246. doi: 10.1099/00221287-46-2-237. [DOI] [PubMed] [Google Scholar]
- Birdsell D. C., Cota-Robles E. H. Production and ultrastructure of lysozyme and ethylenediaminetetraacetate-lysozyme spheroplasts of Escherichia coli. J Bacteriol. 1967 Jan;93(1):427–437. doi: 10.1128/jb.93.1.427-437.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bladen H. A., Evans R. T., Mergenhagen S. E. Lesions in Escherichia coli membranes after action of antibody and complement. J Bacteriol. 1966 Jun;91(6):2377–2381. doi: 10.1128/jb.91.6.2377-2381.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bladen H. A., Gewurz H., Mergenhagen S. E. Interactions of the complement system with the surface and endotoxic lipopolysaccharide of Veillonella alcalescens. J Exp Med. 1967 May 1;125(5):767–786. doi: 10.1084/jem.125.5.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson K. J., Eagon R. G. Lysozyme sensitivity of the cell wall of Pseudomonas aeruginosa. Further evidence for the role of the non-peptidoglycan components in cell wall rigidity. Can J Microbiol. 1966 Feb;12(1):105–108. doi: 10.1139/m66-015. [DOI] [PubMed] [Google Scholar]
- DEPETRIS S. ULTRASTRUCTURE OF THE CELL WALL OF ESCHERICHIA COLI. J Ultrastruct Res. 1965 Apr;12:247–262. doi: 10.1016/s0022-5320(65)80098-3. [DOI] [PubMed] [Google Scholar]
- Davis S. D., Gemsa D., Wedgwood R. J. Kinetics of the transformation of Gram-negative rods to spheroplasts and ghosts by serum. J Immunol. 1966 Apr;96(4):570–577. [PubMed] [Google Scholar]
- EAGON R. G., CARSON K. J. LYSIS OF CELL WALLS AND INTACT CELLS OF PSEUDOMONAS AERUGINOSA BY ETHYLENEDIAMINE TETRAACETIC ACID AND BY LYSOZYME. Can J Microbiol. 1965 Apr;11:193–201. doi: 10.1139/m65-025. [DOI] [PubMed] [Google Scholar]
- Feingold D. S., Goldman J. N., Kuritz H. M. Locus of the lethal event in the serum bactericidal reaction. J Bacteriol. 1968 Dec;96(6):2127–2131. doi: 10.1128/jb.96.6.2127-2131.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn A. A., Milne C. M. A kinetic study of the bacteriolytic and bactericidal action of human serum. Immunology. 1967 Jun;12(6):639–653. [PMC free article] [PubMed] [Google Scholar]
- Knox K. W., Cullen J., Work E. An extracellular lipopolysaccharide-phospholipid-protein complex produced by Escherichia coli grown under lysine-limiting conditions. Biochem J. 1967 Apr;103(1):192–201. doi: 10.1042/bj1030192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox K. W., Vesk M., Work E. Relation between excreted lipopolysaccharide complexes and surface structures of a lysine-limited culture of Escherichia coli. J Bacteriol. 1966 Oct;92(4):1206–1217. doi: 10.1128/jb.92.4.1206-1217.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MURRAY R. G., STEED P., ELSON H. E. THE LOCATION OF THE MUCOPEPTIDE IN SECTIONS OF THE CELL WALL OF ESCHERICHIA COLI AND OTHER GRAM-NEGATIVE BACTERIA. Can J Microbiol. 1965 Jun;11:547–560. doi: 10.1139/m65-072. [DOI] [PubMed] [Google Scholar]
- Marquis R. E. Osmotic stability of bacterial protoplasts related to molecular size of stabilizing solutes. Biochem Biophys Res Commun. 1965 Sep 8;20(5):580–585. doi: 10.1016/0006-291x(65)90438-9. [DOI] [PubMed] [Google Scholar]
- ROTHER K., ROTHER U., PETERSEN K. F., GEMSA D., MITZE F. IMMUNE BACTERICIDAL ACTIVITY OF COMPLEMENT. SEPARATION AND DESCRIPTION OF INTERMEDIATE STEPS. J Immunol. 1964 Aug;93:319–330. [PubMed] [Google Scholar]
- Rothfield L., Horne R. W. Reassociation of purified lipopolysaccharide and phospholipid of the bacterial cell envelope: electron microscopic and monolayer studies. J Bacteriol. 1967 May;93(5):1705–1721. doi: 10.1128/jb.93.5.1705-1721.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slein M. W., Logan G. F., Jr Lysis of Escherichia coli by ethylenediaminetetraacetate and phospholipases as measured by beta-galactosidase activity. J Bacteriol. 1967 Oct;94(4):934–941. doi: 10.1128/jb.94.4.934-941.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. K., Becker E. L. Serum complement and the enzymatic degradation of erythrocyte phospholipid. J Immunol. 1968 Mar;100(3):459–474. [PubMed] [Google Scholar]
- Spitznagel J. K. Normal serum cytotoxicity for P32-labeled smooth Enterobacteriaceae. II. Fate of macromolecular and lipid phosphorus of damaged cells. J Bacteriol. 1966 Jan;91(1):148–152. doi: 10.1128/jb.91.1.148-152.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitznagel J. K., Wilson L. A. Normal serum cytotoxicity for P32-labeled smooth Enterobacteriaceae. I. Loss of label, death, and ultrastructural damage. J Bacteriol. 1966 Jan;91(1):393–400. doi: 10.1128/jb.91.1.393-400.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinbaum G., Rich R., Fischman D. A. Enzyme-induced formation of spheres from cells and envelopes of Escherichia coli. J Bacteriol. 1967 May;93(5):1693–1698. doi: 10.1128/jb.93.5.1693-1698.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolin M. J. Lysis of Vibrio succinogenes by ethylenediamine-tetraacetic acid or lysozyme. J Bacteriol. 1966 May;91(5):1781–1786. doi: 10.1128/jb.91.5.1781-1786.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]