Abstract
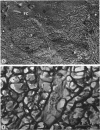
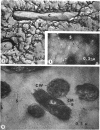
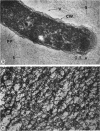
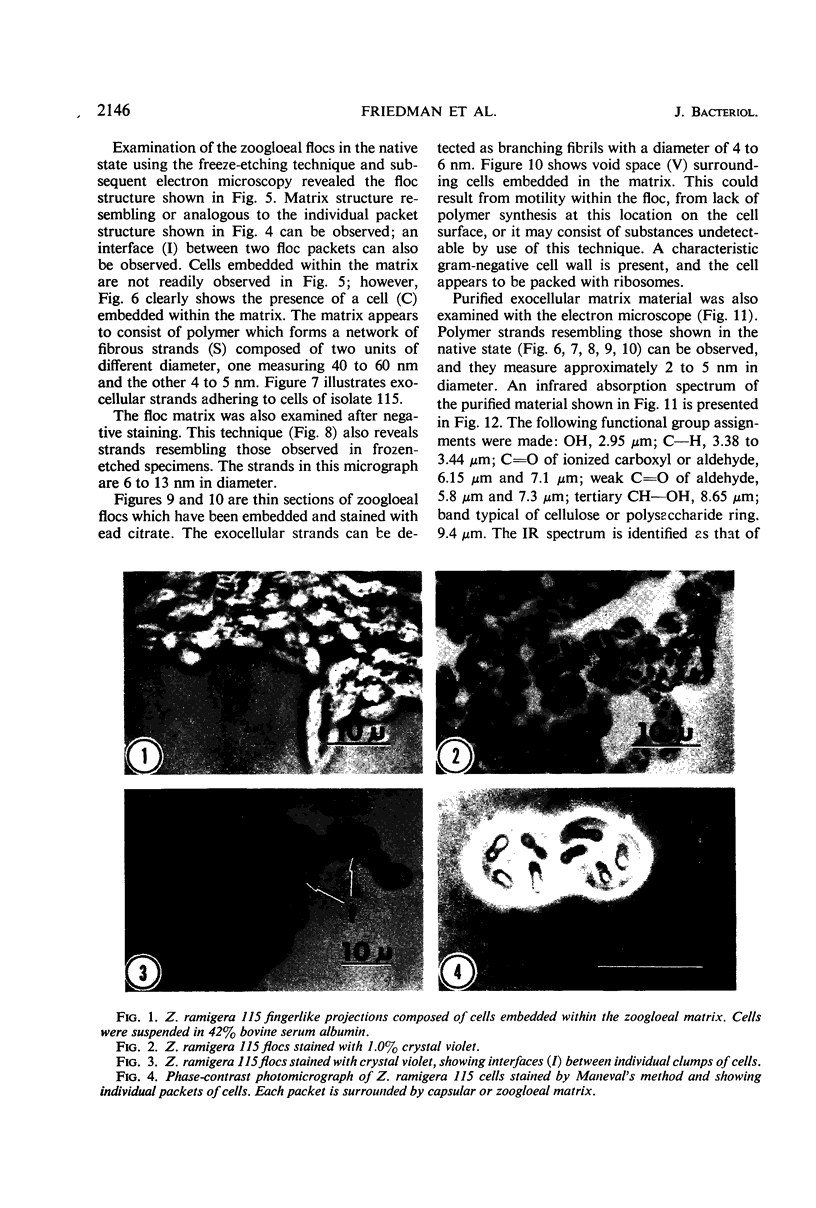
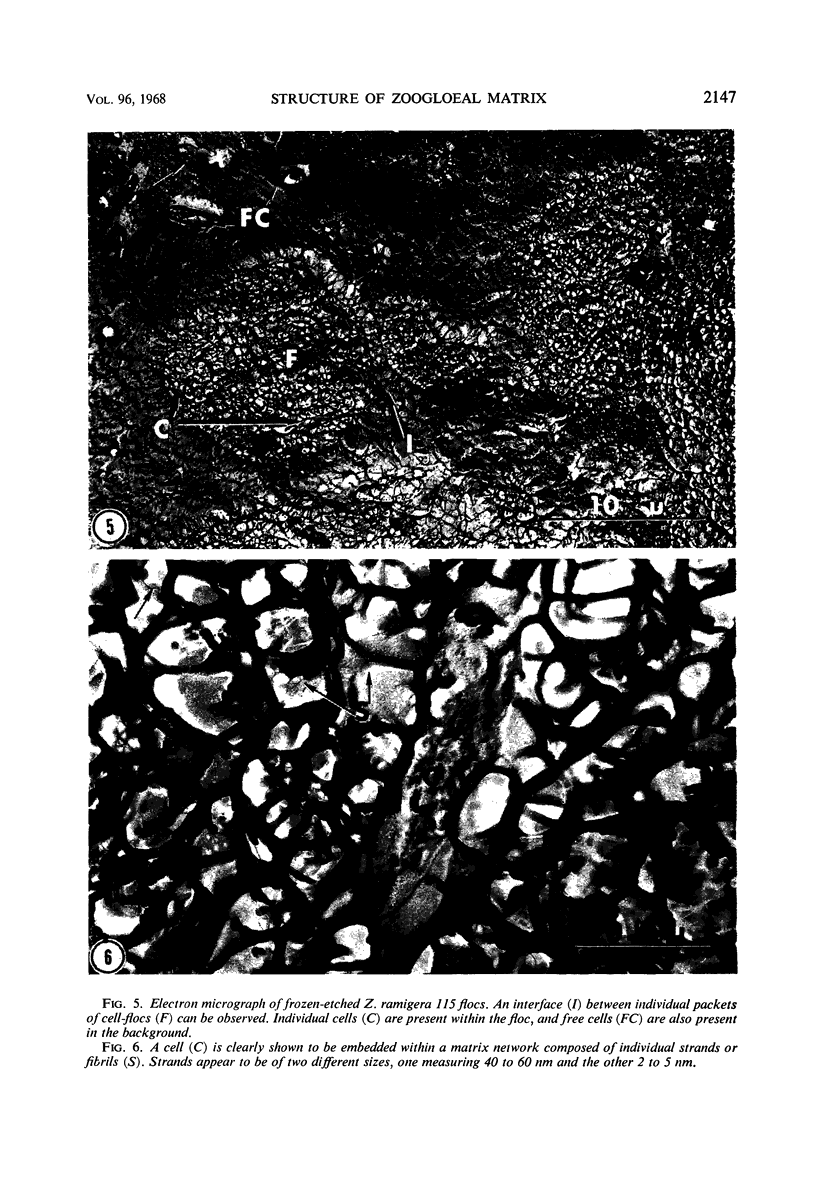
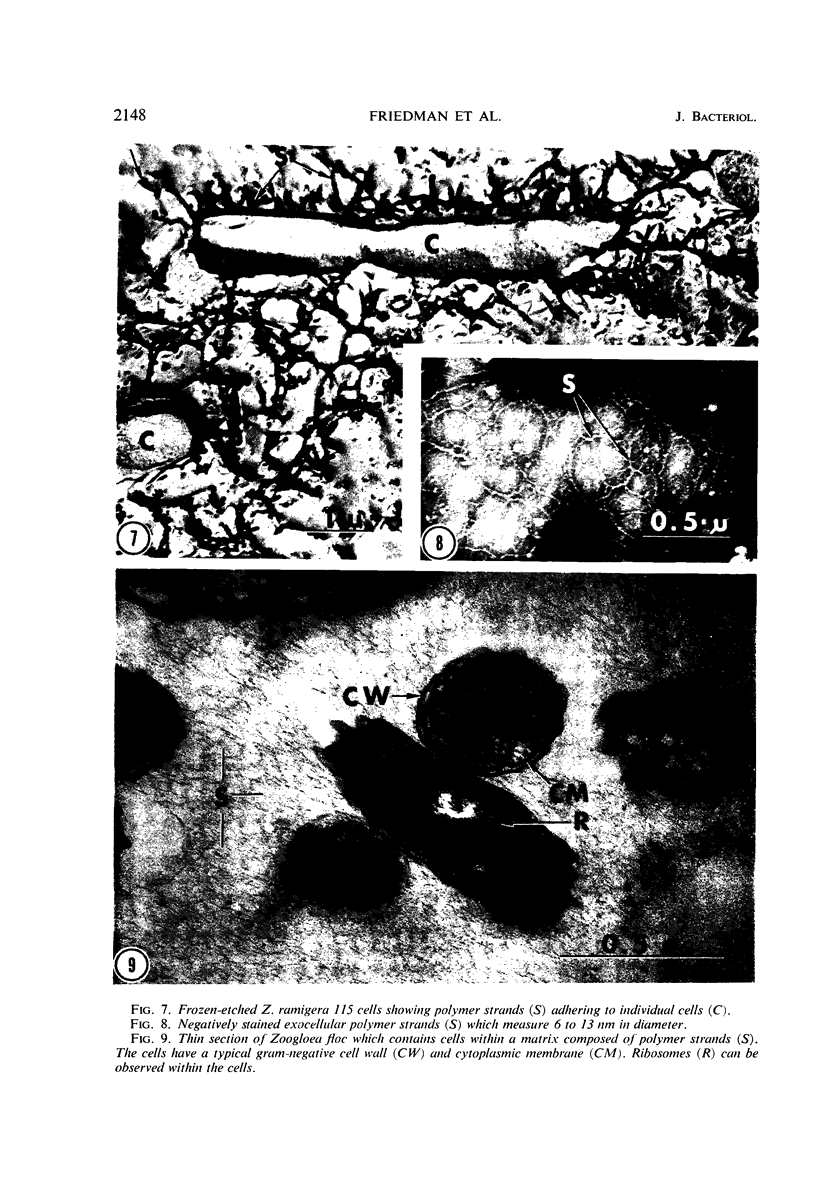
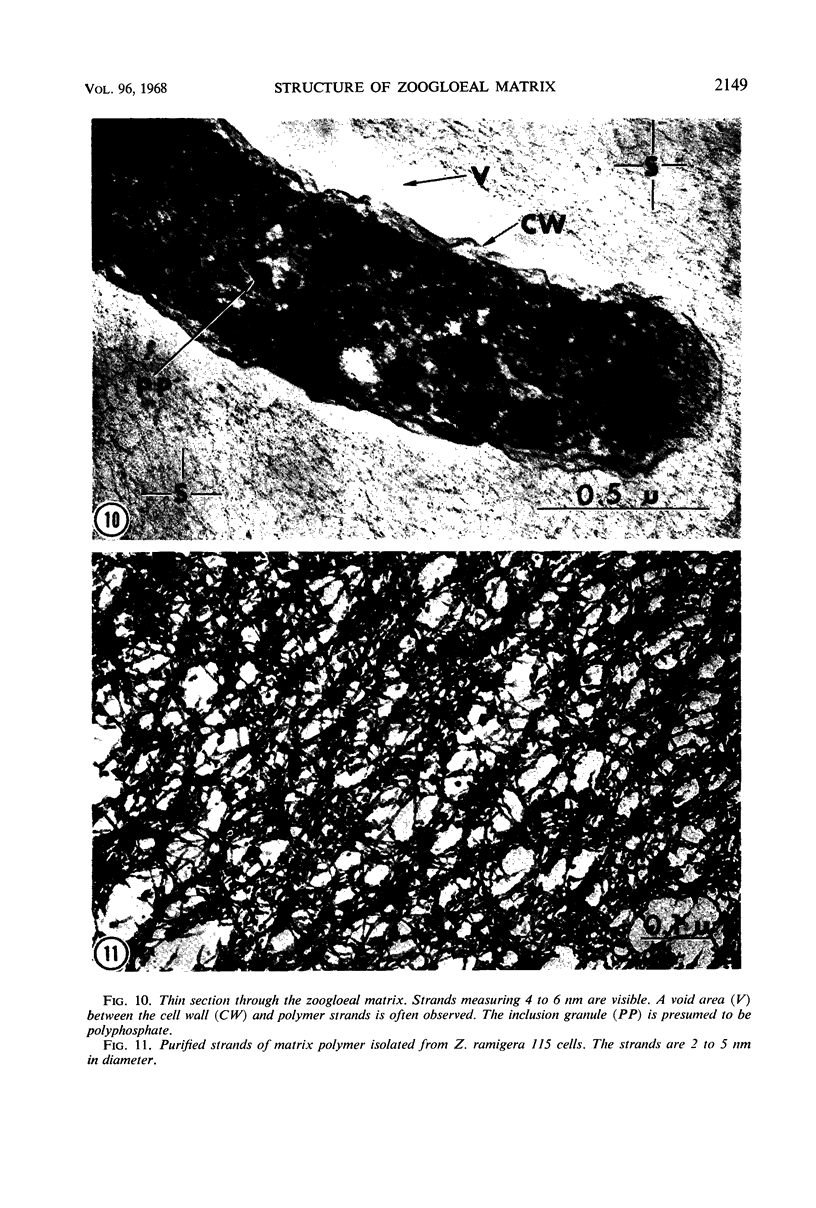
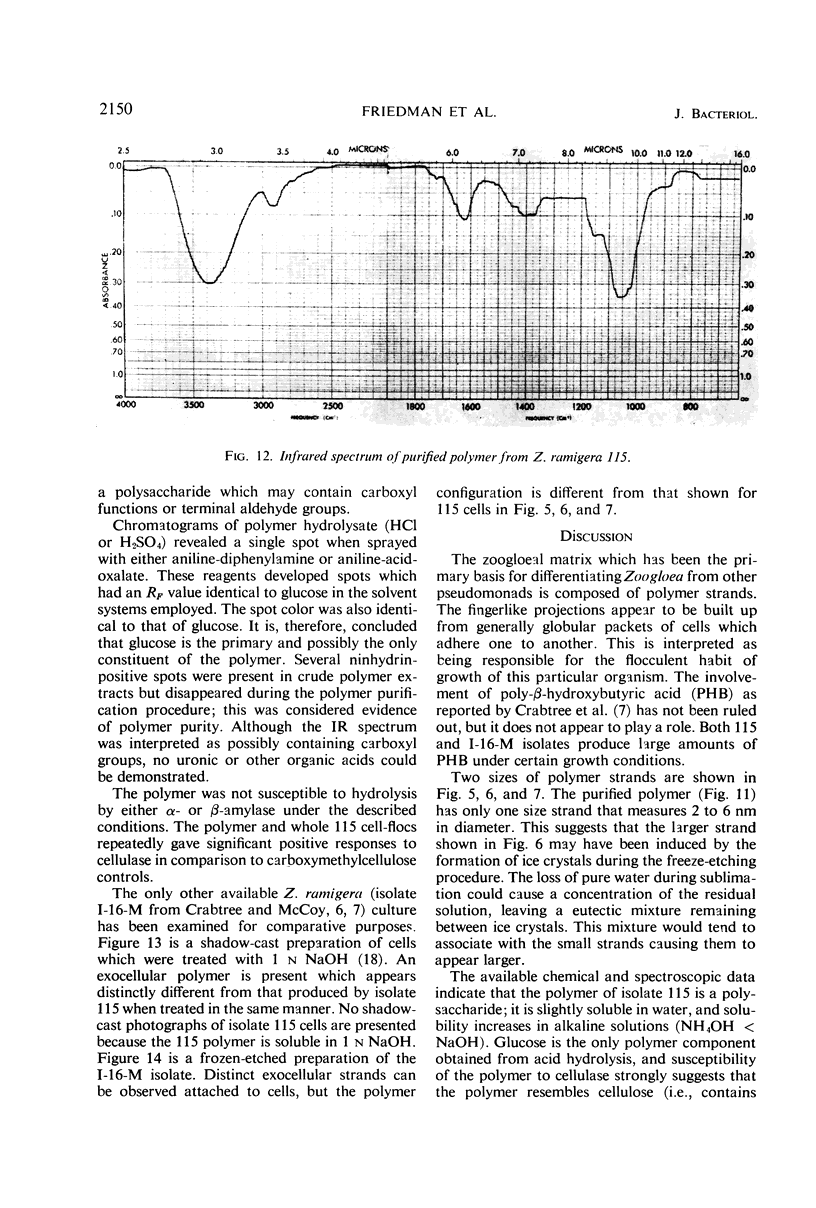
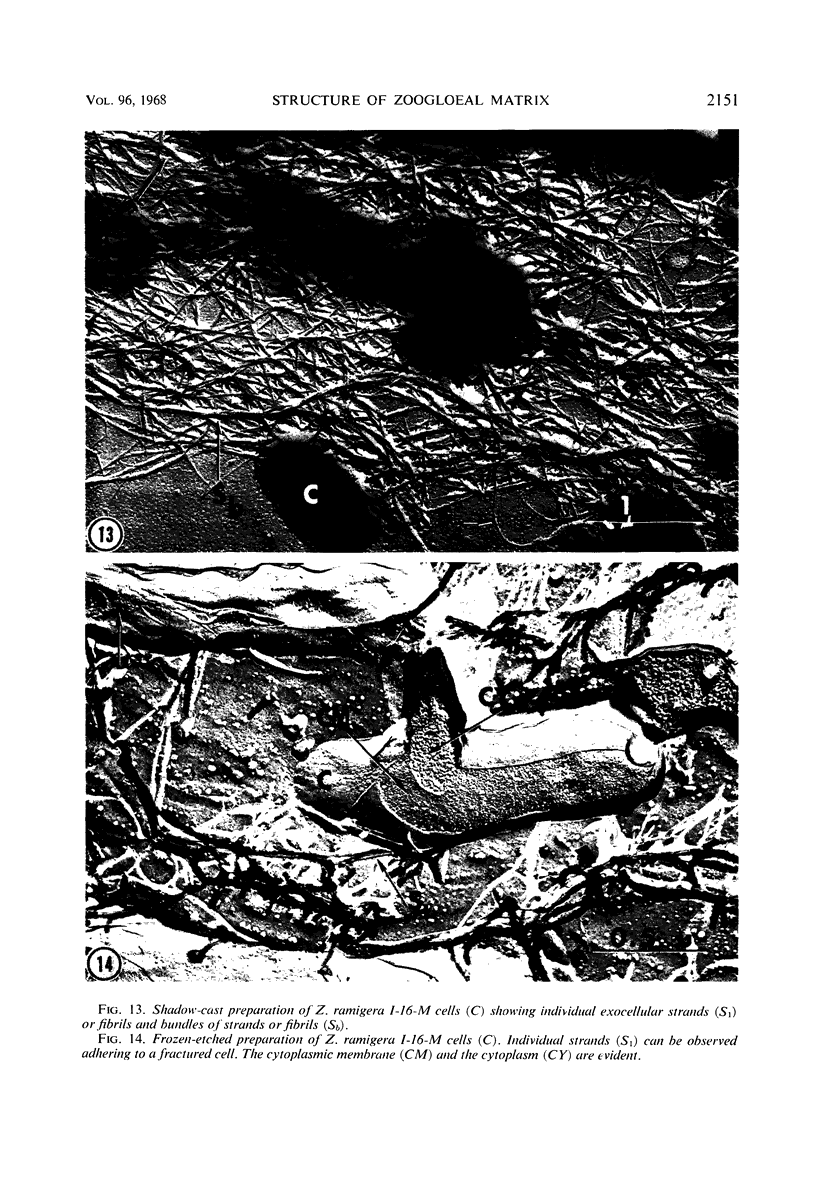
The fingerlike projections, which have been considered to be characteristic of the genus Zoogloea, appear to consist of generally globular packets of cells, each surrounded by capsular matrix. The individual packets which surround Z. ramigera 115 cells appear to adhere one to another by intermeshed fibrils that measure 2 to 5 nm in diameter. The fibril polymer appears to be polyglucose that is susceptible to cellulase. The polymer resembles cellulose in several respects, with the exception that it is soluble in 1 n NaOH. Although Z. ramigera I-16-M does not possess an observable zoogloeal matrix when viewed under a light microscope, chemical data indicate that it does produce cellulase-susceptible polymer. Fibrils can be observed under the electron microscope, which are resistant to 1 n NaOH and may be cellulose. Less polymer fibrils are observed around isolate I-16-M cells than around isolate 115 cells; the inability to observe the I-16-M material as a zoogloeal matrix under light microscopy may be due to lack of sufficient amount of polymer.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARER R., ROSS K. A. F. Refractometry of living cells. J Physiol. 1952 Oct;118(2):38P–39P. [PubMed] [Google Scholar]
- CRABTREE K., MCCOY E., BOYLE W. C., ROHLICH G. A. ISOLATION, IDENTIFICATION, AND METABOLIC ROLE OF THE SUDANOPHILIC GRANULES OF ZOOGLOEA RAMIGERA. Appl Microbiol. 1965 Mar;13:218–226. doi: 10.1128/am.13.2.218-226.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUGAN P. R., LUNDGREN D. G. Isolation of the floc-forming organism Zoogloea ramigera and its culture in complex and synthetic media. Appl Microbiol. 1960 Nov;8:357–361. doi: 10.1128/am.8.6.357-361.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman B. A., Dugan P. R. Identification of Zoogloea species and the relationship to zoogloeal matrix and floc formation. J Bacteriol. 1968 May;95(5):1903–1909. doi: 10.1128/jb.95.5.1903-1909.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUFT J. H. Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol. 1961 Feb;9:409–414. doi: 10.1083/jcb.9.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MOOR H. DIE GEFRIER-FIXATION LEBENDER ZELLEN UND IHRE ANWENDUNG IN DER ELEKTRONENMIKROSKOPIE. Z Zellforsch Mikrosk Anat. 1964 Apr 28;62:546–580. [PubMed] [Google Scholar]
- OHAD I., DANON I. O., HESTRIN S. Synthesis of cellulose by Acetobacter xylinum. V. Ultrastructure of polymer. J Cell Biol. 1962 Jan;12:31–46. doi: 10.1083/jcb.12.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfister R. M., Folger D. Device for linear gradient dehydration of specimens for electron microscopy. Appl Microbiol. 1968 May;16(5):812–814. doi: 10.1128/am.16.5.812-814.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unz R. F., Dondero N. C. The predominant bacteria in natural zoogloeal colonies. I. Isolation and identification. Can J Microbiol. 1967 Dec;13(12):1671–1682. doi: 10.1139/m67-217. [DOI] [PubMed] [Google Scholar]
- Unz R. F., Dondero N. C. The predominant bacteria in natural zoogloeal colonies. II. Physiology and nutrition. Can J Microbiol. 1967 Dec;13(12):1683–1694. doi: 10.1139/m67-218. [DOI] [PubMed] [Google Scholar]