Abstract
Multiple sclerosis (MS) is a chronic inflammatory disease that results in demyelination in the central nervous system, and a defect in the regulatory function of CD4+CD25high T cells has been implicated in the pathogenesis of the disease. Here, we reanalyzed the function of this T cell subset in patients with MS, but we depleted cells expressing IL-7 receptor α-chain (CD127), a marker recently described as present on activated T cells but not Tregs. Similar to other studies, we observed a marked defect in the suppressive function of unseparated CD4+CD25high T cells isolated from MS patients. However, when CD127high cells were removed from the CD4+CD25high population, patient and control cells inhibited T cell proliferation and cytokine production equally. Likewise, when the CD25 gate used to sort the cells was stringent enough to eliminate CD127high cells, CD4+CD25high T cells from patients with MS and healthy individuals had similar regulatory function. Additional analysis indicated that the CD127high cells within the CD4+CD25high T cell population from patients with MS appeared more proliferative and secreted more IFN-γ and IL-2 than the same cells from healthy individuals. Taken together, we conclude that CD4+CD25highCD127low Tregs from MS patients and healthy individuals exhibit similar suppressive functions. The decreased inhibitory function of unfractioned CD4+CD25high cells previously observed might be due to abnormal activation of CD127high T cells in patients with MS.
Introduction
In the adaptive immune system, the balance between the efficient recognition of pathogens and the control of autoimmune diseases is assumed by deletion of autoreactive clones and mechanisms of peripheral tolerance in which Tregs have a key role (1, 2). Such a role for Tregs was first described by Sakaguchi and colleagues, opening the way for the description of different types of Tregs (3, 4). The same group identified the transcription factor FOXP3 as the hallmark of regulatory function (5–7). However, FOXP3 cannot be used to isolate living Tregs because of its intracellular expression. In addition, FOXP3 can also be expressed by activated cells (8–10). Natural Tregs also express IL-2 receptor α-chain (IL-2Rα chain, also known as CD25), a cell surface marker commonly used to distinguish among regulatory (CD25high), activated (CD25int), and naive (CD25low) T cells (11, 12) in humans. However, despite CD25 being a useful marker, the level of CD25 expression alone does not enable a precise estimation of the content of Tregs within a biological sample. Recently, Seddiki et al. (13) and Liu et al. (14) showed that, in humans, low expression of IL-7Rα chain (CD127) combined with high expression of CD25 enables better isolation and purification of Treg populations among CD4+CD25+ T cells. In functional assays, CD4+CD25highCD127low T cells are highly suppressive. Furthermore, expression of CD127 negatively correlates with FOXP3 content, since FOXP3 interacts with the CD127 promoter, contributing to the low expression of CD127 in CD4+CD25high Tregs (14).
MS is a chronic inflammatory and demyelinating disease of the central nervous system. This disorder is thought to be initiated by autoreactive T cells recognizing peptides from myelin sheath proteins (15, 16). However, there is no compelling evidence that the frequency of autoreactive cells is increased in MS versus age-matched controls (17). In an initial study, the frequency of CD4+CD25high T cells was found to be normal, but the authors did not assess functional suppression (18). Several studies have sought to prove the hypothesis of a reduced suppressive function of this T cell subset in MS (19–21). Viglietta et al. (21) reported a decrease in the regulatory function of CD4+CD25high T cells from the peripheral blood of patients with relapsing-remitting MS (RR-MS) compared with healthy individuals (HIs). In addition, the levels of FOXP3 have also been reported as decreased, both at the single cell level and in the CD4+CD25+ population (22, 23). Hence, a defect in the control of the in vitro proliferative response of MS patient CD4+ T cells to myelin proteins has also been reported (19, 20). However, in all of these studies, a single-step CD25 enrichment protocol was used to isolate the T cell populations tested in a coculture system in which the regulatory potency assessment was based on the inhibition of CD4+CD25– cell growth following polyclonal stimulation.
In this study, we took advantage of new CD4+CD25high markers to revisit CD4 T cell regulatory function in MS patients. For this purpose, we used CD127-depleted cells to more precisely characterize the regulatory properties of CD4+CD25high T cells from MS patients compared with HIs. Based on a study of 34 patients and 25 healthy volunteers, we now report that the regulatory function of the CD4+CD25highCD127low T cells is similar in MS patients and HIs. We also show that the isolated CD4+CD25highCD127high T cell subset of MS patients may proliferate more and produce more mitogenic lymphokines in coculture assays, resulting in an apparent peripheral defect of CD4+CD25high regulation in MS patients.
Results
Suppressive function of the top 4% of sorted CD4+CD25high T cells from MS patients.
We first studied the frequency of CD4+CD25high T cells in MS patients and HIs using flow cytometry. Figure 1A shows that the mean frequency of CD4+CD25high cells within the CD4+ T cell population was 2.5% ± 1.4% for HIs and 3.5% ± 1.2% for MS patients (P = NS), confirming that there is no difference in the frequency of CD4+CD25high T cells between MS patients and HIs.
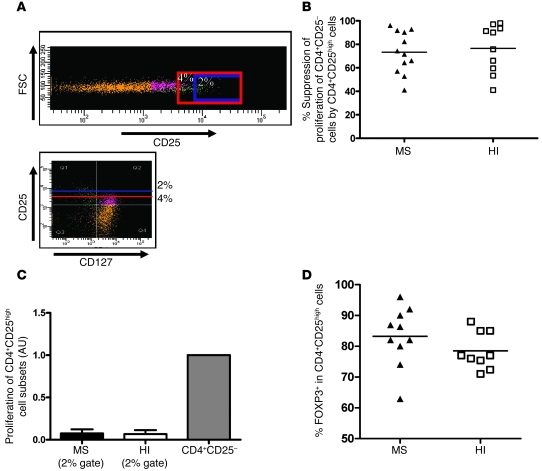
Figure 1. Frequency, proliferation, and suppressive activity of the top 4% of sorted CD4+CD25high T cells from MS patients and HIs.
(A) Comparison of the percentage of CD4+CD25high T cells from MS patients and controls. The cut-off for high-staining CD25 was placed at 6 × 103 of mean fluorescence intensity. No statistical difference can be shown between the groups (patients, n = 21; HI, n = 17). (B) Regulatory properties of CD4+CD25high cells were examined in 11 untreated patients with RR-MS and 11 healthy controls. Cocultures of CD4+CD25– and CD4+CD25high cells were performed at a 1:1 ratio and under anti-CD3 stimulation. Proliferation was measured by incorporation of 3H-thymidine after 5 days of incubation. The percentage of suppression of responding cell proliferation (CD4+CD25–) by CD4+CD25high cells was determined as 1 – (proliferation of coculture / proliferation of responder population alone) × 100, where proliferation is expressed as cpm. CD4+CD25high T cells from MS patients exhibited less suppressive activity when the gate for sorting was positioned as shown in Supplemental Figure 1 (P < 0.05, Mann-Whitney U test). Mean values in A and B are indicated by horizontal lines. (C) Comparison of the proliferation of CD4+CD25high T cells in MS patients and HIs relative to the proliferation of the CD4+CD25– T cell subset. Proliferation of CD4+CD25high cells was not significantly different between patients (n = 11) and HIs (n = 11). When the top 4% of CD4+CD25high T cells were sorted, the cells were not fully anergic, suggesting the presence of activated T cells. Data represent mean ± SD.
Next, in order to confirm the reported suppressive defect of the CD4+CD25high T cell population, we sorted these cells from the peripheral blood of patients (n = 11) and HIs (n = 11) using a high-speed FACS sorter and compared their inhibitory properties. The gate was set up to include 4% of the CD4+ T cells (based on a reference umbilical cord population) (Supplemental Figure 1; supplemental material available online with this article; doi: 10.1172/JCI35365DS1). A regulatory function assay was then performed based on the capacity of the cells to inhibit polyclonal proliferation of autologous CD4+CD25– cells. Figure 1B shows the results obtained under these conditions, when CD4+CD25high T cells sorted from MS patients were added to the coculture system, indicating an apparent defective regulatory function as compared with HIs (39.0% ± 28.4% suppression in MS patients versus 64.7% ± 33% in HIs; P = 0.048, Mann-Whitney U test). However, Figure 1C shows that under these sorting conditions, the isolated CD4+CD25high cells of both MS patients and normal individuals were not fully anergic, with a proliferation of 32.7% in MS patients and 26.3% in HIs (the proliferation of CD4+CD25– T cells from each group was used as the reference). The fact that the cells were not fully anergic under this gating condition led us to explore the possibility that contaminating cells were interfering with the proliferation assay. To do this, Tregs were sorted either by additionally taking into account their expression of CD127 or by using more stringent gating to select cells at the extreme end of CD25 positivity.
Similar suppressive activity of the top 2% of sorted CD4+CD25high T cells in MS patients and HIs.
Because contrasting expression of CD25 and CD127 markers has been reported (13, 14), we sorted the CD4+CD25high T cells using a more stringent threshold for CD25 expression (CD25high; less than 2% of the CD4+ T cells) to compare their inhibitory properties in patients and controls. Figure 2A shows that the gating stringency was indeed associated with a disappearance of CD127high T cells among the purified CD4+CD25high T cells. Figure 2B shows that when sorting the top 2%, the suppression of CD4+CD25– T cell proliferation was roughly similar between the 2 groups (73.3% ± 17.8% in MS patients, n = 12, and 76.5% ± 20.5% in healthy controls, n = 10). Furthermore, in this sorting condition, the purified CD4+CD25high T cells were fully anergic (Figure 2C). Finally, we analyzed the intracellular FOXP3 expression in this CD4+CD25high subset. No significant difference was noted between MS patients (n = 10) and HIs (n = 9), with a mean expression of 83.2% ± 9.5% in patients and 78.5% ± 6% in HIs when gating only on the top 2% of CD4+CD25high cells (Figure 2D).
Figure 2. Suppressive activity and proliferation of the top 2% of sorted CD4+CD25high cells from MS patients and healthy controls.
(A) CD4+ lymphocytes obtained from the peripheral blood of MS patients and healthy controls were stained with Pe-Cy7–conjugated anti-CD3, FITC-conjugated anti-CD8, Alexa Fluor 647–conjugated anti-CD25, and PE-conjugated anti-CD127. Sorting was performed on the CD4+CD25highCD127low and CD4+CD25highCD127high populations. Sorting was also performed on the CD4+CD25high population with 2 different gates. In the example provided for 1 MS patient, CD4+CD25+ T cells appear in orange, CD4+CD25+CD127low T cells appear in green, and CD127high cells appear in violet. The presence of CD127high activated cells can be observed in the CD4+CD25high sorted T cells when the gate is not stringent enough (4% gating stringency, area above the red line), while in the case of a 2% gating stringency (area above the blue line), only a few CD127high cells remain within the CD4+CD25high T cell subset. FSC, forward scatter. (B) CD4+CD25– responder cells were stimulated with anti-CD3 antibody (0.1 μg/ml) in coculture with the top 2% of CD4+CD25high sorted cells. Data are the mean of duplicate wells. Regulatory properties of CD4+CD25high cells are comparable in both HIs (n = 10) and patients (n = 12). (C) Comparison of the proliferation of the top 2% of sorted CD4+CD25high T cells in MS patients and HIs relative to the proliferation of the CD4+CD25– T cell subset. The proliferation of CD4+CD25high T cells was minimal and almost null in both patients and controls. Data are mean ± SD. (D) Intracellular FOXP3 staining was performed on the top 2% of sorted CD4+CD25high T cells in 10 patients and 9 HIs. In B and D, the mean values for each group are indicated by horizontal lines.
Thus, these experiments further support the idea that in MS patients, the presence of contaminating CD127high cells within the CD4+CD25high T cell subset may explain an apparent alteration in their regulatory function.
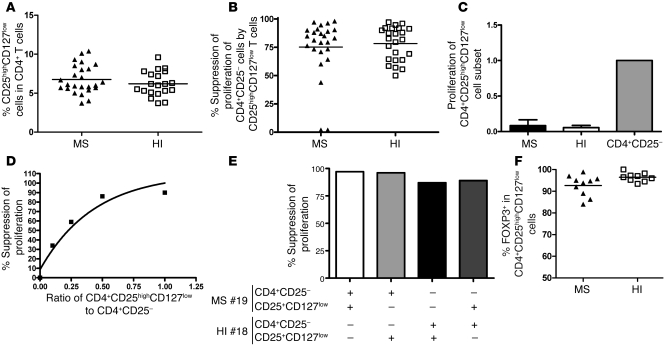
Similar frequency and suppressive activity of CD4+CD25highCD127low cells in MS patients and HIs.
Because CD25 is not a specific marker of Tregs (activated and memory T cells can also express CD25), we used expression of CD127 to discriminate activated and memory T cells (CD4+CD25highCD127high T cells) from regulatory cells (CD4+CD25highCD127low T cells) among the CD4+CD25high subset (13). We compared the regulatory properties of CD127-depleted CD25high T cells from MS patients and age-matched individuals. T cells were thus labeled with anti-CD127, and the CD4+CD25highCD127low cells were sorted (Figure 2A). Figure 3A shows that there was no difference in the percentage of CD25highCD127low cells within the CD4+ T cell populations of MS patients compared with HIs, with a mean frequency of 6.8% ± 1.8% for MS patients and 6.2% ± 1.6% for HIs (P = NS, Mann-Whitney U test). The cells were then tested for their ability to suppress the proliferation of CD4+CD25– cells in response to irradiated autologous PBMC and anti-CD3 activation over 5 days. Under these conditions, no significant difference in suppression of autologous CD4+CD25– proliferation was observed between the CD4+CD25highCD127low cells of RR-MS patients (n = 25) and those of healthy controls (n = 23) (Figure 3B). Proliferation of CD4+CD25– T cells was inhibited by a mean of 75.3% ± 20.9% in MS patients and 78.3% ± 15.0% in age-matched HIs. Thus, an improvement in the suppressive function of this cell subset was observed in both MS patients and HIs, as compared with the use of CD25 alone as a marker of Tregs. The proliferation of these cell subsets was then tested and compared with their CD4+CD25– counterparts. Figure 3C shows that in these new conditions, the basal proliferation of the CD4+CD25highCD127low T cells was very low or absent (a representative example is shown in Supplemental Figure 2) and clearly differed from that of the top 4% of sorted CD4+CD25high cells. In addition, this lack of proliferation was totally reversed by the addition of IL-2 (data not shown), suggesting a state of anergy. The suppressive activity was dose dependent in that dilution of the CD4+CD25highCD127low T cells (over a range of 1:1 to 1:10) decreased their suppressive function (Figure 3D). Finally, CD4+CD25highCD127low Tregs from MS patients were able to suppress effector CD4+CD25– cells from HIs to the same extent as the CD4+CD25highCD127low cells from HIs on CD4+CD25– T cells from MS patients (Figure 3E).
Figure 3. Frequency, proliferation, and suppressive activity of CD4+CD25highCD127low cells from MS patients and healthy controls.
(A) Percentages of CD25highCD127low cells within the total CD4+ T cell population were determined by flow cytometry analysis of PBMCs. No statistical difference was found between the groups (25 patients and 20 HIs). (B) Regulatory properties of CD4+CD25highCD127low cells were comparable in both HIs (n = 24) and patients (n = 25). (C) Comparison of the proliferation of CD4+CD25highCD127low T cells in MS patients and HIs normalized against the proliferation of the CD4+CD25– T cell subset. The proliferation of CD4+CD25highCD127low T cells was minimal and approaching 0 in both patients and controls. (D) Example in 1 patient of variations of the suppression of proliferation by CD4+CD25highCD127low cells in the cocultures at varying ratios of CD4+CD25highCD127low to CD4+CD25–. Decreasing the number of CD4+CD25highCD127low T cells resulted in less suppressor activity. (E) CD4+CD25highCD127low T cells from 1 patient with MS inhibited proliferation of responder T cells isolated from either the autologous individual or the healthy control. Conversely, Tregs from 1 HI were cocultured with responder T cells from the same subject or those from the MS patient. MS#19, MS patient 19; HI#18, HI subject 18. (F) Intracellular FOXP3 staining was performed on CD4+CD25highCD127low T cells in 10 patients and 9 HIs. In A, B, and F, mean values are indicated by horizontal lines.
Finally, we analyzed intracellular FOXP3 expression in the CD4+CD25highCD127low cells. No significant difference was noted between patients (n = 10) and HIs (n = 9), with a mean expression of 92.6% ± 4.8% in MS patients and 96.4% ± 2% in HIs (Figure 3F).
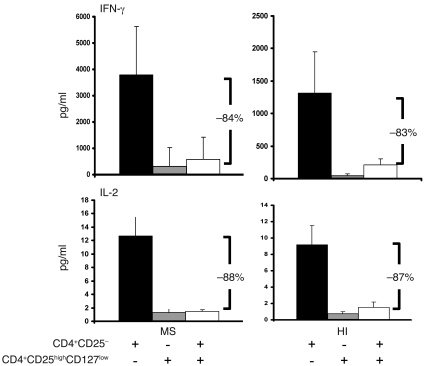
Further, when considering the production of IFN-γ or IL-2 by CD4+CD25– responder cells after 3 days of polyclonal stimulation in the presence of irradiated autologous PBMCs, the same suppressive capacity of CD4+CD25highCD127low T cells was observed in patients and controls (n = 4; Figure 4). An 84% and an 83% reduction in IFN-γ production was observed in MS patients and HIs, respectively. Similarly, the reduction in IL-2 production was 88% and 87% in MS patients and HIs, respectively.
Figure 4. Suppression of cytokine production by CD4+CD25highCD127low T cells in patients and controls.
Cytokines (IL-2 and IFN-γ) were measured in supernatants taken from each well 3 days after the initiation of coculture (CD4+CD25– T cells/CD4+CD25highCD127low T cells at a 1:1 ratio) using a multiplex fluorescent bead immunoassay. Three days after initiation of the coculture, the same percentage suppression of IFN-γ (top panels) and IL-2 (bottom panels) production by CD4+CD25highCD127low was observed in the cocultures from 4 patients and 4 HIs. Data are mean ± SD.
Taken together, these data indicate that CD4+CD25highCD127low Tregs from MS patients do not display a defect in their suppressive properties and that activated CD127high cells within the CD4+CD25high T cell population interfere with the proliferation assay.
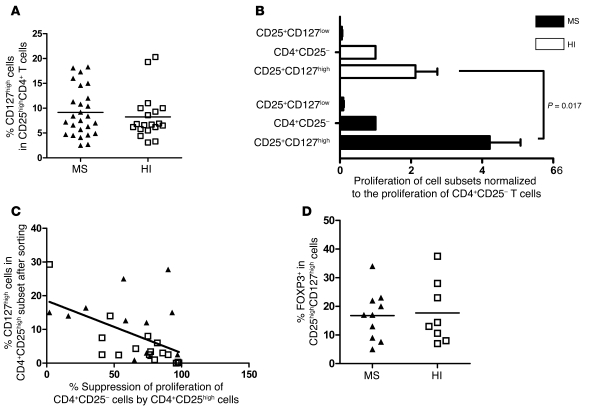
Significantly higher proliferation of activated CD4+CD25highCD127high T cells in MS patients versus HIs.
In order to know whether the apparently impaired suppressive function of the CD4+CD25high cell subset (not depleted of CD127high cells) from MS patients (Figure 1B) could be due to the presence of activated CD127high cells, we first compared the frequency of CD4+CD25highCD127high cells between MS patients and HIs. No difference was observed between the 2 groups (9.2% ± 4.8% in MS patients and 8.2% ± 4.6% in controls) (Figure 5A). Next, the proliferation of the CD4+CD25highCD127high T cells of both MS patients (n = 20) and HIs (n = 20) was compared with that of CD4+CD25highCD127low T cells. The data were normalized against the proliferation of CD4+CD25– T cells in order to compare individuals, as the absolute values of proliferation under CD3 stimulation can be variable. This type of presentation has been reported before (14). The raw data expressed in cpm are provided in Supplemental Figures 3–5. Figure 5B shows no difference in proliferation of the CD4+CD25highCD127low subset between MS patients and HIs, with a very low proliferation in both groups. On the contrary, the proliferation of CD4+CD25highCD127high T cells in MS patients was 1.9-fold higher (4.2 ± 3.9 relative to the proliferation of CD4+CD25– T cells) than in controls (2.2 ± 2.8; P = 0.017, Mann-Whitney U test; Figure 5B). This observation further supports the possibility that the CD127high T cells within the CD4+CD25high subset might interfere in the apparent defective regulation of CD4+CD25high T cells in MS patients. This CD127high contamination can be prevented using a selective sorting of CD127low cells or more stringent gating sorting conditions of CD4+CD25high cells (Figure 2A). The contribution of these activated cells to the apparent alteration in regulatory properties of the CD4+CD25high cells was also demonstrated by the significant inverse correlation observed between the suppressive function of CD4+CD25high cells and the presence of CD127high T cells within this cell subset (Pearson correlation coefficient of r = –0.51; P = 0.006, linear regression test; Figure 5C). Finally, we analyzed intracellular FOXP3 expression in the CD4+CD25highCD127high cells. No significant difference was noted between MS patients (n = 10) and HIs (n = 9), with a mean expression of 17.7% ± 10.8% in patients and 16.8% ± 8.6% in controls (Figure 5D).
Figure 5. Comparison of the proliferation of CD4+CD25highCD127low T cells and CD4+CD25highCD127high T cells.
(A) CD4+ lymphocytes obtained from the peripheral blood of MS patients and healthy controls were stained with Pe-Cy7–conjugated anti-CD3, FITC-conjugated anti-CD8, Alexa Fluor 647–conjugated anti-CD25, and PE-conjugated anti-CD127. No statistically significant difference was observed in the frequency of CD4+CD25highCD127high T cells between MS patients (n = 25) and healthy controls (n = 19). Mean value is indicated for each group. (B) CD4+CD25highCD127low T cells or CD4+CD25highCD127high T cells were cocultured with irradiated autologous PBMCs and stimulated with anti-CD3 antibody. CD25highCD127low cells were isolated from 25 patients and 23 HIs. CD25highCD127high cells were isolated from 20 patients and 20 HIs. A significant difference was observed in the proliferation of CD25highCD127high T cells between MS patients and HIs (P = 0.017, Mann-Whitney U test). Bar graphs indicate the mean ± SD. (C) Suppression of proliferation of CD4+CD25– cells by CD4+CD25high cells was calculated in 13 patients and 15 HIs. The percentage of CD127high cells present in the sorted CD4+CD25high T cells was estimated in the same manner. A correlation was found between this percentage and the regulatory properties of CD4+CD25high cells with a Pearson coefficient of r = –0.50 (P = 0.006, linear regression test). Black triangles represent data obtained from MS patients, and white squares represent data from HIs. (D) Intracellular FOXP3 staining was performed on CD4+CD25highCD127high T cells in 10 patients and 9 HIs. In B and D, horizontal lines indicate the mean.
Cytokine secretion by the CD4+CD25highCD127high, CD4+CD25–, and CD4+CD25highCD127low populations in MS patients and HIs.
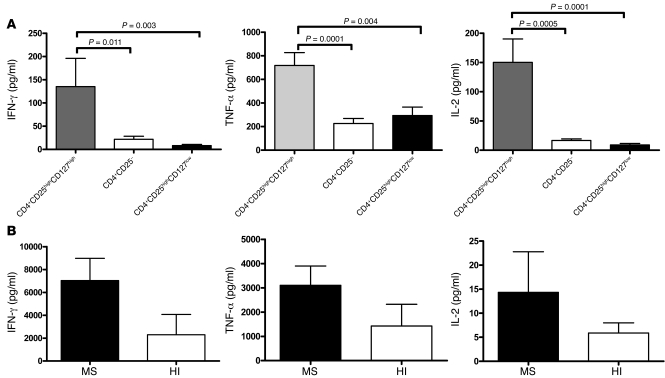
The cytokines TNF-α, IFN-γ, and IL-2 were also measured in the supernatants of the proliferation assays from the CD4+CD25highCD127high, CD4+CD25highCD127low, and CD4+CD25– cell populations and their cocultures after 24 hours of proliferation. No significant difference was observed between patients (n = 10) and HIs (n = 10) for the 3 cell subsets (data not shown) after 24 hours. However, CD4+CD25highCD127high cells from MS patients secreted significantly higher levels of cytokines than CD4+CD25highCD127low and CD4+CD25– T cells (Figure 6A; P = 0.011, P = 0.0001, and P = 0.0005 when compared with CD4+CD25– for IFN-γ, TNF-α, and IL-2, respectively; and P = 0.0031, P = 0.0039, and P < 0.0001 when compared with CD4+CD25highCD127low for IFN-γ, TNF-α, and IL-2, respectively; Mann-Whitney U test), suggesting that these cells had a proinflammatory potential. In addition, CD4+CD25highCD127low T cells did not produce IL-2 or IFN-γ when stimulated by anti-CD3, as would be expected from their regulatory phenotype.
Figure 6. Cytokines secreted by the CD4+CD25highCD127high, CD4+CD25–, and CD4+CD25highCD127low populations in MS patients and HIs.
(A) The supernatants from each well of the proliferation assays were removed 24 hours after the beginning of the incubation. The cytokines TNF-α, IFN-γ, and IL-2 were measured from 10 MS patients in the following 3 cell subsets: CD4+CD25highCD127high, CD4+CD25–, and CD4+CD25highCD127low. Mann-Whitney U tests were performed to compare the 3 cell subsets. (B) Comparison of cytokine production by CD4+CD25highCD127high T cells under CD3 polyclonal stimulation between MS patients (n = 4) and HIs (n = 4). Supernatants were removed 3 days after the beginning of the incubation. The results are the mean ± SD from 4 patients and 4 healthy controls.
Hence, to study their production of cytokines, CD4+CD25highCD127high cells from MS patients (n = 4) and HIs (n = 4) were cultured under CD3 polyclonal stimulation in the presence of irradiated PBMCs for 3 days. IFN-γ and TNF-α were subsequently measured using a multiplex fluorescent bead assay. As shown in Figure 6B, 6,580 ± 2,093 pg/ml of IFN-γ was detected in the cultures of MS patient cells as compared with 2,567 ± 1,092 pg/ml for HIs. Concerning TNF-α production, the cells from MS patients produced 3,107 ± 346 pg/ml compared with 1,571 ± 629 pg/ml for HIs. IL-2 production was also increased in MS patients as compared with HIs (14 ± 3 versus 6 ± 2 pg/ml, respectively). The CD4+CD25highCD127high cells from MS patients produced nearly 2-fold more proinflammatory cytokines compared with the same cells from HIs. While statistical significance was not achieved, a trend toward significance was observed (IFN-γ, P = 0.06, Mann-Whitney U test; TNF-α, P = 0.09; IL-2, P = 0.1) despite the very low number of patients and controls studied (n = 4).
FOXP3 content of CD4+CD25high and CD4+CD25highCD127low populations in MS patients and HIs.
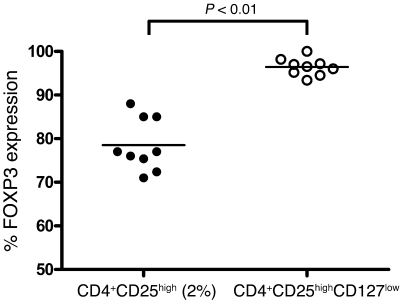
We compared intracellular FOXP3 expression between CD4+CD25high and CD4+CD25highCD127low cell subsets in patients (n = 10) and controls (n = 9). The use of anti-CD127 antibody provided a purified CD4+CD25+CD127low population expressing more FOXP3 in MS patients and HIs (92.6% ± 4.8% and 96.4% ± 2%, respectively), as compared with 83.2% ± 9.5% in patients and 78.5% ± 6% in HIs when gating only on the top 2% of the CD4+CD25high population (P < 0.0001, Mann-Whitney U test; Figure 7).
Figure 7. Comparison of FOXP3 expression in CD4+CD25high T cells and CD4+CD25highCD127low cells.
Intracellular FOXP3 staining was performed in 9 HIs. A significant difference was observed between the top 2% of sorted CD4+CD25high and CD4+CD25highCD127low T cells (P < 0.0001, Mann-Whitney U test). The horizontal lines indicate the mean values for each group.
Discussion
CD4+CD25+FOXP3+ regulatory T lymphocytes have been shown to play a key role in controlling potentially harmful responses to self-determinants in mice (24) and humans (1). Recently, a possible defect in the function of CD4+CD25high cells has been reported in patients with RR-MS (1, 19–21). In this paper, we revisited this observation by analyzing the properties of subpopulations of CD4+CD25high T cells, taking into consideration the recent findings that activated/memory cells expressing CD127 are also present within the CD4+CD25+ T cell population and can potentially interfere in the classical functional assays for measuring CD4+CD25high Treg suppressive properties (13, 14, 25). To our knowledge, this is the first study using CD127 to discriminate Tregs in MS patients and showing that this CD4+CD25highCD127low T cell subset actually has the same regulatory potency in patients and in age-matched control subjects. The frequency of the CD4+CD25high T cells also appeared to be similar between MS patients and HIs, as reported previously (18). However, several studies have reported a defective suppressive function in CD4+CD25high T cells from MS patients under polyclonal (21) or antigen-based stimulation (19, 20), suggesting that this defect might be involved in the physiopathology of MS (26, 27). At the time of these studies, CD127low staining was not available for the CD4+CD25high Tregs. Our investigations suggest that CD4+CD25high Treg function may not be altered in MS, since CD4+CD25highCD127low T cells display a normal suppressive function. Rather, a discrete population of CD4+CD25highCD127high cells in patients is likely to interfere with the coculture assay by a trend for hyperproliferation and for producing more proinflammatory cytokines able to enhance CD25– T cell proliferation. Our data shed new light on the heterogeneity of the CD4+CD25high T cell population and suggest a possible role for CD4+CD25highCD127high cells in MS. These findings are indirectly supported by recent genomic studies in MS suggesting alterations in IL2RA and IL7RA genes (28–30).
As expected, we first confirmed a defective suppressive function of the CD4+CD25high T cells in MS patients (39% in MS patients versus 69% in age-matched HIs, P < 0.05). However, because CD25 is not specific for Tregs but is also expressed by activated T cells (1, 31–33), it was important to take into consideration other markers that distinguish activated/memory cells not endowed with regulatory function. Another difficulty in assessing Treg function by studying CD4+CD25+ cells comes from the fact that the CD4+CD25high population is difficult to distinguish from the CD4+CD25int population (thought to contain activated T cells) because there is no clear and stereotyped cut-off between high and intermediate CD25 expression in humans (1, 2). In fact, our data show that changing the gating stringency when sorting these cells dramatically affects the suppressive capacity of the CD4+CD25high T cells in the proliferation assay, probably by introducing activated T cells in the coculture assay. Using too low a stringency sorting threshold may thus result in an apparent defect in regulatory function in the CD4+CD25high T cell subset in MS patients as in HIs, but not in the same proportion (see Figure 1C and Figure 2B for the difference obtained in percentage of suppression when using a 2% or a 4% gating stringency both in patients or controls). Indeed, the same cells obtained from MS patients using a more stringent sorting threshold did not present abnormal regulatory function. It is thus difficult to compare the results obtained in different studies when Tregs are purified based solely on their expression of CD4 and CD25.
Recently, CD127 has been shown to be negatively correlated with FOXP3 expression in CD4+CD25high T cells, enabling improved sorting of viable Tregs (13, 14). Thus, we used anti-CD127 to discriminate the properties of CD127high-depleted cells among the CD4+CD25high T cell subset in MS patients and HIs and found that the suppressive function of the CD4+CD25highCD127low cells was similar between the 2 groups. Furthermore, 94% of the CD4+CD25highCD127low T cells expressed FOXP3 protein compared with 82% in CD4+CD25high cells (P < 0.0001, Mann-Whitney U test; Figure 7), indicating that the cells sorted using the CD127 marker are a purer population than those obtained using only CD25. The comparable high FOXP3+ score in the CD127-depleted CD25high cells and the comparable regulatory function observed in MS patients and HIs also suggests that MS patients have no defect in their Tregs. Hence, when comparing the production of IFN-γ by CD4+CD25– cells under polyclonal stimulation in the presence or absence of CD4+CD25highCD127low cells, a similar suppressive property was found for this Treg subset, confirming the data observed with the proliferation assays. This observation of a similar regulatory function between MS patients and HIs suggests that contaminating CD127high T cells interfere in the suppression assays. The presence of activated CD127high T cells within the CD4+CD25high T cells of patients with MS could explain the discrepancy observed between the 2 groups despite a similar frequency of CD4+CD25highCD127high cells in MS patients and HIs. An enhanced proliferation of this T cell subset was observed compared with the proliferation of CD25– cells, suggesting that this subpopulation may exhibit an abnormal activation state in MS patients. This would at least partly explain the defect in suppressive function observed with the CD4+CD25high T cells. In addition, in our experiments the CD4+CD25highCD127high T cells exhibited a proinflammatory profile as measured by the levels of IL-2, IFN-γ, and TNF-α produced in the supernatants of the proliferation assays as compared with the responder CD4+CD25– cell subset. Hence, when compared with HIs, this subset of cells from MS patients seemed to produce more proinflammatory cytokines (IFN-γ, TNF-α, and IL-2). The extent to which this increased production of T cell mitogenic cytokines (such as IL-2 and IFN-γ; ref. 34) may play a role in the proliferation of the CD25– T cell subset within the coculture system requires further investigation. Recently, CD4+CD25highCD127high cells have also been suggested to play a role in allograft rejection in humans. Grafts infiltrated by CD4+CD25highCD127high T cells contained allospecific CD4+ T cells and also secreted effector cytokines such as TNF-α and IFN-γ (35). Altogether, it is possible that this CD4+CD25highCD127high T cell subset plays a role in MS or other inflammatory processes. Our observations also indirectly corroborate the results of 3 recent studies on risk alleles associated with MS, which revealed an alteration of 2 genes, IL2RA and IL7RA (28–30). Recently, Venken and colleagues also used CD127 to discriminate memory and naive Tregs in MS patients (36). They still found a defective suppressive function of the Tregs in MS patients, even after a sorting strategy based on CD127low cells. However, they also used the counterpart CD4+CD25–CD127high cells as responders. Our data suggest that these cells are different between MS patients and HIs, since we show here that CD4+CD25highCD127high cells may be hyperproliferative and produce more proinflammatory cytokines, also resulting in an apparent defect in regulation.
To conclude, using 2 different parameters (suppression of proliferation and cytokine production), our work suggests that the inhibitory function of the regulatory CD4+CD25high T cell subset may not actually be altered in MS when contaminating CD127high cells are removed. In addition, the counterpart CD4+CD25highCD127high T cell subpopulation in MS patients may proliferate more or produce more mitogenic cytokines, at least partly explaining the previous observation of a decreased suppressive property of CD4+CD25high cells in MS patients. This CD4+CD25highCD127high T cell subset will be the subject of future investigations, since it might play a role in the inflammatory process observed in MS.
Methods
Patients.
Thirty-four patients with definite MS according to the McDonald criteria (37) were enrolled in the study. All patients presented RR-MS and did not receive disease-modifying therapy (including corticosteroid boluses) for at least 3 months prior to testing. The patients were between the ages of 19 and 60 (mean, 35.9 ± 9.5 years), and their Kurtzke Expanded Disability Status Scale (EDSS) scores (38) were between 0 and 6.5 (mean, 1.75 ± 1.6). The disease duration ranged from 6 months to 17 years (mean, 4.5 ± 4.9 years). MS patients 1–25 were used for suppression and proliferation experiments concerning the different T cell subsets studied, while MS patients 25–34 were used for experiments concerning cytokine production. The characteristics of all patients are available in Supplemental Table 1.
Twenty-five HIs, matched for age with MS patients, between the ages of 21 and 60 (mean, 34.5 ± 8.7 years) and with no history of autoimmune diseases or recent infection episodes, were also enrolled in this study. All patients and HIs gave written informed consent prior to the study.
Isolation of CD4+CD25–, CD4+CD25high, CD4+CD25highCD127low, and CD4+CD25highCD127 high T cell populations.
PBMCs were isolated from 100 ml of EDTA whole blood by density centrifugation over Ficoll-Paque (Eurobio). Freshly-isolated cells (1.5 × 108) from each experiment were incubated for 20 minutes in PBS with 30 μl FITC-conjugated anti-CD8, 30 μl PE-conjugated anti-CD127, 20 μl PE-Cy7–conjugated anti-CD3 (BD Biosciences), and 5 μl Alexa Fluor 647–conjugated anti-CD25 (anti-CD25 from Immunotech coupled to the fluorochrome using a molecular probe kit from Invitrogen). Human CD4+CD25high, CD4+CD25–, CD4+CD25highCD127low, and CD4+CD25highCD127high T cells were then separated from PBMCs using a high-speed cell sorter (FACSAria; BD Biosciences). Purity was systematically greater than 98% for CD4+CD25– T cells and greater than 95% for CD4+CD25highCD127low cells. For sorting, parameters were set once and automatic compensations were performed using FACSDiva software (BD Biosciences). Because we performed anti-CD3 staining for the sorting procedure, we checked that this staining had no influence on further experiments. We compared the proliferation of CD4+CD25– and CD4+CD25highCD127low cells as well as their coculture when the sorting was performed with or without anti-CD3. We did not find any difference in terms of proliferation or suppression (data not shown).
Proliferation assay.
All experiments were performed on fresh peripheral blood lymphocytes. To assess the functional activity of the different subsets of cells (CD4+CD25high and CD4+CD25highCD127low), 2 × 104 responder cells (CD4+CD25– T cells) were cocultured for 5 days with 2 × 104 CD4+CD25high cells or CD4+CD25highCD127low cells (ratio 1:1) and 1 × 105 autologous irradiated PBMCs in complete RPMI 1640 medium supplemented with HEPES, l-glutamine, penicillin, streptomycin, sodium pyruvate, nonessential aminoacids, and 10% human AB serum. All experiments were run in duplicate in 96-well plates bound with anti-CD3 (Orthoclone OKT3; Janssen-Cilag) at 1 μg/ml as described previously (39). Anti-CD3 antibody was used as a polyclonal T cell stimulus. Cultures were incubated at 37°C in a humidified atmosphere. After 5 days of culture, the cells were pulsed with 1 μCi per well of 3H-thymidine (Amersham Biosciences) for 16 hours. The cells were then harvested and counted in a scintillation counter. 3H-thymidine incorporation was measured as cpm. The percentage of suppression of the responding cell proliferation was determined as 1 – (proliferation of coculture / proliferation of responder population alone) × 100, where proliferation was expressed as cpm.
In order to compare the proliferation of each cell subpopulation and among individuals, and because of variability in proliferation from one patient to another, the proliferation of the T cell subsets (CD4+CD25high, CD4+CD25highCD127low, and CD4+CD25highCD127high) was normalized to the proliferation of the CD4+CD25– subset in each patient or control. In each case, baseline proliferation in absolute cpm was checked, and several representative examples are provided. All data concerning the proliferation of each subpopulation of T cells for each patient and HI are available in Supplemental Figures 1–4.
Measurement of cytokine production.
Cytokines (IL-2, TNF-α, and IFN-γ) were measured in supernatants taken from each well 24 hours after the initiation of coculture using a multiplex fluorescent bead immunoassay (Lincoplex) together with a Luminex device. To assess the suppression of IL-2 and IFN-γ production by CD4+CD25– T cells, these cytokines were measured after 3 days in the cocultured supernatants of CD4+CD25highCD127low and CD4+CD25– responder cells (ratio 1:1) and 1 × 105 autologous irradiated PBMCs under the CD3 polyclonal stimulation as described above.
For comparison of cytokine production by CD4+CD25highCD127high T cells from patients and controls, CD4+CD25highCD127high T cells were cocultured with irradiated PBMCs as described above together with anti-CD3. Supernatants were removed 3 days after the beginning of the assay, and IFN-γ, TNF-α, and IL-2 were measured using the multiplex fluorescent bead immunoassay as above. For this experiment, 4 more patients and HIs were randomly included in the study according to the criteria set out in the patients section.
FACS analysis.
PBMCs (106 per sample) were stained with PE-Cy7–conjugated anti-CD3, FITC-conjugated anti-CD8, PE-Cy5–conjugated anti-CD25, and PE-conjugated anti-CD127 (all from BD Biosciences), followed by intracellular staining with human FOXP3 (APC conjugate) staining assay kit (Imgenex).
Statistics.
Statistical analyses were performed using GraphPad Prism 4.0. Parametric statistical analysis (mean and SEM) was performed using standard methods. Significant differences were calculated using the nonparametric Mann-Whitney U test, and linear regression analysis was performed using the Pearson correlation test. For all tests, P values of less than 0.05 were considered significant.
Supplementary Material
Acknowledgments
We would like to thank Joanna Ashton-Chess for her help in manuscript editing. This work was supported by a grant from the Collège des Enseignants de Neurologie et les Journées de Neurologie de Langue Française (to L. Michel) and a grant from the Association pour la Recherche sur la Sclérose en Plaques (to L. Berthelot).
Footnotes
Nonstandard abbreviations used: HI, healthy individual; RR-MS, relapsing-remitting MS.
Conflict of interest: The authors have declared that no conflict of interest exists.
Citation for this article: J. Clin. Invest. 118:3411–3419 (2008). doi:10.1172/JCI35365
Sophie Brouard, Jean-Paul Soulillou, and David-Axel Laplaud are co-senior authors.
Laure Michel, Laureline Berthelot, and Ségolène Pettré contributed equally to this work.
References
- 1.Baecher-Allan C., Hafler D.A. Human regulatory T cells and their role in autoimmune disease. Immunol. Rev. 2006;212:203–216. doi: 10.1111/j.0105-2896.2006.00417.x. [DOI] [PubMed] [Google Scholar]
- 2.Tang Q., Bluestone J.A. Regulatory T-cell physiology and application to treat autoimmunity. Immunol. Rev. 2006;212:217–237. doi: 10.1111/j.0105-2896.2006.00421.x. [DOI] [PubMed] [Google Scholar]
- 3.Asano M., Toda M., Sakaguchi N., Sakaguchi S. Autoimmune disease as a consequence of developmental abnormality of a T cell subpopulation. J. Exp. Med. 1996;184:387–396. doi: 10.1084/jem.184.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sakaguchi S., Sakaguchi N., Asano M., Itoh M., Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J. Immunol. 1995;155:1151–1164. [PubMed] [Google Scholar]
- 5.Fontenot J.D., Gavin M.A., Rudensky A.Y. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat. Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 6.Khattri R., Cox T., Yasayko S.A., Ramsdell F. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nat. Immunol. 2003;4:337–342. doi: 10.1038/ni909. [DOI] [PubMed] [Google Scholar]
- 7.Hori S., Nomura T., Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 8.Allan S.E., et al. The role of 2 FOXP3 isoforms in the generation of human CD4+ Tregs. J. Clin. Invest. 2005;115:3276–3284. doi: 10.1172/JCI24685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morgan M.E., et al. Expression of FOXP3 mRNA is not confined to CD4+CD25+ T regulatory cells in humans. Hum. Immunol. 2005;66:13–20. doi: 10.1016/j.humimm.2004.05.016. [DOI] [PubMed] [Google Scholar]
- 10.Ziegler S.F. FOXP3: not just for regulatory T cells anymore. Eur. J. Immunol. 2007;37:21–23. doi: 10.1002/eji.200636929. [DOI] [PubMed] [Google Scholar]
- 11.Dieckmann D., Plottner H., Berchtold S., Berger T., Schuler G. Ex vivo isolation and characterization of CD4(+)CD25(+) T cells with regulatory properties from human blood. J. Exp. Med. 2001;193:1303–1310. doi: 10.1084/jem.193.11.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thornton A.M., Shevach E.M. Suppressor effector function of CD4+CD25+ immunoregulatory T cells is antigen nonspecific. J. Immunol. 2000;164:183–190. doi: 10.4049/jimmunol.164.1.183. [DOI] [PubMed] [Google Scholar]
- 13.Seddiki N., et al. Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells. J. Exp. Med. 2006;203:1693–1700. doi: 10.1084/jem.20060468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu W., et al. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J. Exp. Med. 2006;203:1701–1711. doi: 10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hohlfeld R., Wekerle H. Autoimmune concepts of multiple sclerosis as a basis for selective immunotherapy: from pipe dreams to (therapeutic) pipelines. Proc. Natl. Acad. Sci. U. S. A. 2004;101(Suppl. 2):14599–14606. doi: 10.1073/pnas.0404874101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sospedra M., Martin R. Immunology of multiple sclerosis. Annu. Rev. Immunol. 2005;23:683–747. doi: 10.1146/annurev.immunol.23.021704.115707. [DOI] [PubMed] [Google Scholar]
- 17.Hellings N., et al. T-cell reactivity to multiple myelin antigens in multiple sclerosis patients and healthy controls. J. Neurosci. Res. 2001;63:290–302. doi: 10.1002/1097-4547(20010201)63:3<290::AID-JNR1023>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 18.Putheti P., Pettersson A., Soderstrom M., Link H., Huang Y.M. Circulating CD4+CD25+ T regulatory cells are not altered in multiple sclerosis and unaffected by disease-modulating drugs. J. Clin. Immunol. 2004;24:155–161. doi: 10.1023/B:JOCI.0000019780.93817.82. [DOI] [PubMed] [Google Scholar]
- 19.Haas J., et al. Reduced suppressive effect of CD4+CD25high regulatory T cells on the T cell immune response against myelin oligodendrocyte glycoprotein in patients with multiple sclerosis. Eur. J. Immunol. 2005;35:3343–3352. doi: 10.1002/eji.200526065. [DOI] [PubMed] [Google Scholar]
- 20.Kumar M., et al. CD4+CD25+FoxP3+ T lymphocytes fail to suppress myelin basic protein-induced proliferation in patients with multiple sclerosis. J. Neuroimmunol. 2006;180:178–184. doi: 10.1016/j.jneuroim.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 21.Viglietta V., Baecher-Allan C., Weiner H.L., Hafler D.A. Loss of functional suppression by CD4+CD25+ regulatory T cells in patients with multiple sclerosis. J. Exp. Med. 2004;199:971–979. doi: 10.1084/jem.20031579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Venken K., et al. Compromised CD4+ CD25(high) regulatory T-cell function in patients with relapsing-remitting multiple sclerosis is correlated with a reduced frequency of FOXP3-positive cells and reduced FOXP3 expression at the single-cell level. Immunology. 2008;123:79–89. doi: 10.1111/j.1365-2567.2007.02690.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huan J., et al. Decreased FOXP3 levels in multiple sclerosis patients. J. Neurosci. Res. 2005;81:45–52. doi: 10.1002/jnr.20522. [DOI] [PubMed] [Google Scholar]
- 24.Sakaguchi S., et al. Foxp3+ CD25+ CD4+ natural regulatory T cells in dominant self-tolerance and autoimmune disease. Immunol. Rev. 2006;212:8–27. doi: 10.1111/j.0105-2896.2006.00427.x. [DOI] [PubMed] [Google Scholar]
- 25.Mazzucchelli R., Durum S.K. Interleukin-7 receptor expression: intelligent design. Nat. Rev. Immunol. 2007;7:144–154. doi: 10.1038/nri2023. [DOI] [PubMed] [Google Scholar]
- 26.Roncarolo M.G., Battaglia M. Regulatory T-cell immunotherapy for tolerance to self antigens and alloantigens in humans. Nat. Rev. Immunol. 2007;7:585–598. doi: 10.1038/nri2138. [DOI] [PubMed] [Google Scholar]
- 27.McFarland H.F., Martin R. Multiple sclerosis: a complicated picture of autoimmunity. Nat. Immunol. 2007;8:913–919. doi: 10.1038/ni1507. [DOI] [PubMed] [Google Scholar]
- 28.Hafler D.A., et al. Risk alleles for multiple sclerosis identified by a genomewide study. N. Engl. J. Med. 2007;357:851–862. doi: 10.1056/NEJMoa073493. [DOI] [PubMed] [Google Scholar]
- 29.Lundmark F., et al. Variation in interleukin 7 receptor alpha chain (IL7R) influences risk of multiple sclerosis. Nat. Genet. 2007;39:1108–1113. doi: 10.1038/ng2106. [DOI] [PubMed] [Google Scholar]
- 30.Gregory S.G., et al. Interleukin 7 receptor alpha chain (IL7R) shows allelic and functional association with multiple sclerosis. Nat. Genet. 2007;39:1083–1091. doi: 10.1038/ng2103. [DOI] [PubMed] [Google Scholar]
- 31.Baecher-Allan C., Brown J.A., Freeman G.J., Hafler D.A. CD4+CD25high regulatory cells in human peripheral blood. J. Immunol. 2001;167:1245–1253. doi: 10.4049/jimmunol.167.3.1245. [DOI] [PubMed] [Google Scholar]
- 32.Baecher-Allan C.M., Hafler D.A. Functional analysis of highly defined, FACS-isolated populations of human regulatory CD4+CD25+ T cells. Clin. Immunol. 2005;117:192; discussion 193. doi: 10.1016/j.clim.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 33.Beissert S., Schwarz A., Schwarz T. Regulatory T cells. J. Invest. Dermatol. 2006;126:15–24. doi: 10.1038/sj.jid.5700004. [DOI] [PubMed] [Google Scholar]
- 34.Josien R., et al. Recombinant IFN-gamma abrogates allograft tolerance induced by donor-specific blood transfusion by restoring alloantibody production. Eur. J. Immunol. 1999;29:317–326. doi: 10.1002/(SICI)1521-4141(199901)29:01<317::AID-IMMU317>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 35.Codarri L., et al. Expansion and tissue infiltration of an allospecific CD4+CD25+CD45RO+IL-7R{alpha}high cell population in solid organ transplant recipients. J. Exp. Med. 2007;204:1533–1541. doi: 10.1084/jem.20062120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Venken K., et al. Natural naive CD4+CD25+CD127low regulatory T cell (Treg) development and function are disturbed in multiple sclerosis patients: recovery of memory Treg homeostasis during disease progression. J. Immunol. 2008;180:6411–6420. doi: 10.4049/jimmunol.180.9.6411. [DOI] [PubMed] [Google Scholar]
- 37.McDonald W.I., et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann. Neurol. 2001;50:121–127. doi: 10.1002/ana.1032. [DOI] [PubMed] [Google Scholar]
- 38.Kurtzke J.F. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS). Neurology. 1983;33:1444–1452. doi: 10.1212/wnl.33.11.1444. [DOI] [PubMed] [Google Scholar]
- 39.Braudeau C., et al. Variation in numbers of CD4(+)CD25(high)FOXP3(+) T cells with normal immuno-regulatory properties in long-term graft outcome. Transpl. Int. 2007;20:845–855. doi: 10.1111/j.1432-2277.2007.00537.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.