Abstract
Rab5-dependent endosome fusion is sensitive to the phosphoinositide 3-kinase inhibitor, wortmannin. It has been proposed that phosphoinositide 3-kinase activity may be required for activation of rab5 by influencing its nucleotide cycle such as to promote its active GTP state. In this report we demonstrate that endosome fusion remains sensitive to wortmannin despite preloading of endosomes with stimulatory levels of a GTPase-defective mutant rab5Q79L or of a xanthosine triphosphate-binding mutant, rab5D136N, in the presence of the nonhydrolysable analogue XTPγS. These results suggest that activation of rab5 cannot be the principal function of the wortmannin-sensitive factor on the endosome fusion pathway. This result is extrapolated to all GTPases by demonstrating that endosome fusion remains wortmannin sensitive despite prior incubation with the nonhydrolysable nucleotide analogue GTPγS. Consistent with these results, direct measurement of clathrin-coated vesicle-stimulated nucleotide dissociation from exogenous rab5 was insensitive to the presence of wortmannin. A large excess of rab5Q79L, beyond levels required for maximal stimulation of the fusion assay, afforded protection against wortmannin inhibition, and partial protection was also observed with an excess of wild-type rab5 independent of GTPγS.
INTRODUCTION
An established cell-free assay of lumenal mixing between early endosomal compartments is thought to principally reflect a homotypic membrane fusion event (Gruenberg and Howell, 1987). In common with other intracellular transport assays, it has been shown to require the fusion factors N-ethylmaleimide sensitive factor and α-SNAP (Diaz et al., 1989; Rodriguez et al., 1994). Specificity of the fusion reaction may be due, in part, to the small GTPase rab5, which has been shown to localize to early endosomes (Chavrier et al., 1991) and is required for the fusion reaction (Gorvel et al., 1991). GTPases other than rab5, including ADP ribosylation factor and trimeric G proteins, have also been shown to influence endosome fusion (Colombo et al., 1992; Lenhard et al., 1992), but as they are also known to influence other trafficking steps, their role may be less specific. We and others have recently shown that a phosphoinositide (PI) 3-kinase activity is required for efficient endosome fusion (Jones and Clague, 1995; Li et al., 1995; Spiro et al., 1996). On the basis of experiments with a constitutively active form of rab5 (Q79L mutant), it has been proposed that this requirement for PI 3-kinase activity reflects an event upstream of the activation of rab5 (Li et al., 1995).
The cycling of rab5 between cytosol and endosomes and the associated nucleotide state of the enzyme have been extensively studied. Upon translation, rab5 in its GDP-bound form complexes with rab escort protein (REP-1). The REP-1/rab5 complex then interacts with the heterodimeric rab geranylgeranyltransferase (GGTase), which transfers a geranylgeranyl group to cysteine residues at the rab5 C terminus (Alexandrov et al., 1994). This modification is vital for rab5 activity (Gorvel et al., 1991). After prenylation the GGTase dissociates, and the prenylated REP–1/rab5 complex can interact with endosomal membranes, whereupon rab5 is transferred to the organelle (Alexandrov et al., 1994). Subsequent to delivery to endosomes, a membrane-associated exchange factor facilitates the exchange of GDP for GTP (Ullrich et al., 1994), producing the active form of rab5 (Stenmark et al., 1994). Hydrolysis of GTP returns rab5 to the GDP form, which can be extracted from the membrane by guanine nucleotide dissociation inhibitor, a protein homologous to REP-1 (Ullrich et al., 1993). Subsequently, both association with and dissociation from endosomes of prenylated-rab5 can be mediated by guanine nucleotide dissociation inhibitor.
Elegant studies by Rybin et al. have shown that an in vitro endosome fusion assay could be configured such that it is dependent on a recombinant rab5 mutant, rab5D136N, that binds xanthosine 5′-triphosphate (XTP) rather than GTP (Rybin et al., 1996). Fusion was dependent on exchange of xanthosine 5′-diphosphate (XDP) for XTP but not XTP hydrolysis.
There is a growing catalogue of evidence for a role of PIs as mediators of membrane-trafficking events. Particular attention has focused on the generation of PI 3-phosphates mediated by PI 3-kinases since the Saccharomyces cerevisiae VPS34 gene, implicated in traffic from the trans-Golgi network (TGN) to the vacuole, was shown to be a PI 3-kinase (Schu et al., 1993). The study of PI 3-kinase function in mammalian membrane traffic has recently been facilitated by the availability of specific inhibitors of PI 3-kinases, wortmannin and LY294002 (Arcaro and Wymann, 1993; Vlahos et al., 1994). In addition to endosome fusion, a role for PI 3-kinase in a multitude of membrane trafficking steps has been demonstrated, including fluid phase endocytosis (Clague et al., 1995; Li et al., 1995), TGN to lysosomal transport (Brown et al., 1995; Davidson, 1995), and PDGF receptor trafficking (Joly et al., 1995). A number of mammalian PI 3-kinases that have different substrate preferences and are regulated differently have now been cloned and sequenced, but all display sensitivity to wortmannin (Zvelebil et al., 1996), such that the role of individual enzymes is not easy to assess.
Wortmannin and LY294002 inhibit endosome fusion (Jones and Clague, 1995; Li et al., 1995; Spiro et al., 1996) whilst insect cell cytosol overexpressing p110α PI 3-kinase catalytic subunit enhances endosome fusion relative to control cytosol (Li et al., 1995). Most interestingly, when a GTPase-deficient mutant rab5Q79L was included in an assay of fusion between endosomes derived from J774 macrophages, sensitivity to wortmannin was completely lost (Li et al., 1995). As the Q79L mutant of rab5 is deficient in GTP hydrolysis, it is trapped in the GTP bound form or “on state”; this suggests that a PI 3-kinase activity may directly or indirectly promote the activity of a factor that influences the nucleotide cycle of rab5 to increase the proportion of rab5 in the GTP bound form either by enhancing guanine nucleotide exchange or inhibiting GTPase activity. Thus the Q79L mutation would overcome this requirement and render the assay insensitive to PI 3-kinase inhibition.
In this paper we have extended the studies of Li et al. (1995), on the relationship between the wortmannin-sensitive factor and rab5 with respect to endosome fusion. Our results do not rule out a direct effect of wortmannin on the nucleotide cycle of rab5; however, they strongly suggest that this cannot be the primary reason for the inhibition of endosome fusion by wortmannin.
MATERIALS AND METHODS
Materials
Monoclonal rab5 antibody 4F11 was from Dr A. Wandinger-Ness (Department of Biochemistry, Molecular Biology and Cell Biology, Northwestern University, Chicago, IL). Wortmannin, geranylgeranyl pyrophosphate (GGPP), XDP, XTP, and GTPγS were from Sigma. Wortmannin was prepared immediately before use in appropriate buffer from a 1 mM stock solution in dimethylsulfoxide. XTPγS was prepared by the method of Goody et al. (1972). [3H]-GDP 25Ci/mmol was from DuPont NEN (Wilmington, DE).
Cell Culture
Reagents were from Life Technologies (Gaithersburg, MD). Baby hamster kidney cells (BHK-21) were grown on 10-cm diameter Petri dishes in Glasgow minimal essential medium (BHK 21) media supplemented with 5% vol/vol fetal bovine serum, 100 U/ml penicillin/streptomycin, 10% vol/vol tryptose phosphate broth.
Expression and Purification of Recombinant Proteins
REP-1 and heterodimeric rab-GGTase complex were purified from baculovirus-infected Sf9 cells as described previously (Alexandrov et al., 1994; Andres et al., 1993; Cremers et al., 1990). His6-tagged wild-type rab5, rab5Q79L, and rab5D136N plasmids were introduced into Escherichia coli strain BL21, and the proteins were purified as described by Nuoffer et al. (1995). GDP was replaced with XDP during rab5D136N purification.
Preparation of Posttranslationally Modified rab5/REP-1 Complex
The method was essentially as described previously (Alexandrov et al., 1994). Briefly, 10.9 μM of his6 rab5, his6 rab5D136N, or his6 rab5Q79L was incubated with 2 μM rabGGTase, 3 μM REP-1, and 60 μM GGPP for 15 min at 30°C in (20 mM HEPES-KOH, pH 7.2, 20 mM NaCl, 2 mM dithiothreitol (DTT), 2 mM MgCl2, 0.005% [wt/vol] Triton X-100). The samples were then diluted 120-fold in REP-1 complex buffer (25 mM Tris, pH 7.9, 50 mM KCl, 5 mM MgCl2, 1 mM DTT) and concentrated in a Microcon 10 microconcentrator (Amicon, ) before storage at −70°C.
Early Endosome Fusion Assay
The experiments were carried out essentially as described previously (Clague et al., 1994; Gorvel et al., 1991). Briefly, confluent BHK-21 cells grown on 10-cm diameter Petri dishes were washed with 37°C phosphate-buffered saline, pH 7.4. Subsequently, either 4 mg/ml avidin or 1.8 mg/ml biotinylated horseradish peroxidase (HRP) in Dulbecco’s phosphate-buffered saline supplemented with 1 mM CaCl2 and 1 mM MgCl2 were incubated with the cells for 9 min at 37°C. This incubation was followed by extensive washing at 4°C before removal of cells from the dish with a cell scraper and homogenization in homogenization buffer (3 mM imidazole/HCl pH 7.4, 250 mM sucrose, 1 μg/ml pepstatin, 2 μg/ml aprotinin, 2 μg/ml leupeptin) by several passages through a 23-gauge needle. Postnuclear supernatants were obtained by centrifugation at 1,500 × g for 15 min.
Postnuclear fractions containing avidin or biotin-HRP–loaded endosomes were then combined in a mixture (total volume 217 μl) containing 10 mM HEPES-KOH, pH 7.0, 1.2 mM MgCl2, 50 mM KOAc, 0.8 mM DTT, biotin-insulin (to quench any free avidin) and ATP-regenerating system, to which had been added the various factors used in this study. This mixture was then incubated at 37°C for the indicated times. Alternatively, postnuclear supernatants containing avidin or biotin-HRP–labeled early endosomes were aliquoted and separately preincubated for 10 min at 37°C with the described factors. Preincubations were performed in the absence of ATP-regenerating system and salts. In all procedures the appropriate buffer was used to substitute volume additions of added reagents. Further particulars of specific experiments are given in the figure legends. The samples were then placed on ice before mixing in fusion assays as above. Fusion assays were stopped by lysis on ice for 15 min with 0.25% wt/vol Triton X-100. Fusion results in the formation of an avidin–biotin–HRP complex, which was immunoprecipitated with anti-avidin antibodies bound to protein A sepharose. The relative amounts of immunoprecipitated HRP were quantified by determination of the HRP activity with o-dianisidine as substrate.
Rab5 Binding to Postnuclear Supernatant Membranes
BHK-21 postnuclear supernatants without internalized markers were prepared as above. REP-1/rab5Q79L or REP-1/wild type rab5 complex were centrifuged at 115,000 × g for 2 min at 2°C to remove aggregates. Postnuclear supernatants (60 μl) containing 330 μg of total protein and 100 μM GTP or 60 μM GTPγS were incubated in the absence or presence of 50–1000 nM REP-1/rab5Q79L or REP-1/wild-type rab5 complex, respectively, for 10 min before addition of 20× sample volume of ice-cold wash buffer (12.5 mM HEPES, pH 7.2, 75 mM KOAc, 1 mM MgOAc, 1 mM DTT, 5 mM MgCl2) and centrifugation at 150,000 × g for 10 min at 4°C. The pellets were washed with wash buffer, and the solutions were centrifuged at 115,000 × g for 5 min at 4°C. The supernatants were aspirated, and the pellets were resuspended in SDS electrophoresis sample buffer. Rab5 was determined by Western blotting.
Assay for Dissociation of [3H]GDP from rab5 Stimulated by Clathrin-coated Vesicles (CCVs)
Rat brain CCVs were purified by differential centrifugation as described by Maycox et al. (1992) and resuspended in 0.1 M 2-(N-morpholino)ethanesulfonic acid, pH 6.5, 0.5 mM MgCl2, 1 mM EGTA, 1 mM DTT. The assay method is essentially as described by Horiuchi et al. (1995). REP-1/[3H]GDP–rab5 complex was prepared by incubating purified his6 rab5 (0.2 μM) with [3H]GDP (5 μM) in 20 mM HEPES/KOH, pH 7.2, 10 mM EDTA, 5 mM MgCl2,1 mM DTT for 30 min at 30°C. The solution was placed on ice and adjusted to 20 mM MgCl2. [3H]GDP-rab5 (1.25 μM) was then incubated with GGTase (0.82 μM), REP-1 (1.24 μM), and GGPP (50 μM) in 20 mM HEPES/KOH, pH 7.2, 20 mM NaCl, 0.0048% (wt/vol) Triton X-100, 1 mM DTT for 20 min at 30°C before passing the solution through a Biospin 6 column (Bio-Rad, Richmond, CA) equilibrated with 20 mM HEPES/KOH, pH 7.2, 20 mM NaCl, 5 mM MgCl2, 1 mM DTT, 0.005% (wt/vol) Triton X-100.
REP-1/[3H]GDP–rab5 complex (100 nM, 20,000 cpm) was incubated at 30°C with CCVs (1 mg/ml) in 0.1 M 2-(N-morpholino)ethanesulfonic acid, pH 6.5, 1 mM EGTA, 5 mM MgCl2, 5 mM GTP, 1 mM DTT for the various times indicated in Figure 8. The reactions were stopped by addition of 6× sample volume of ice-cold 20 mM HEPES/KOH, pH 7.2, 100 mM NaCl, 25 mM MgCl2 and immediately loaded on presoaked 0.45 μM HA filters (Millipore, Bedford, MA) to which a vacuum was then applied. The filters were washed with 50 ml of the same buffer before scintillation counting.
RESULTS
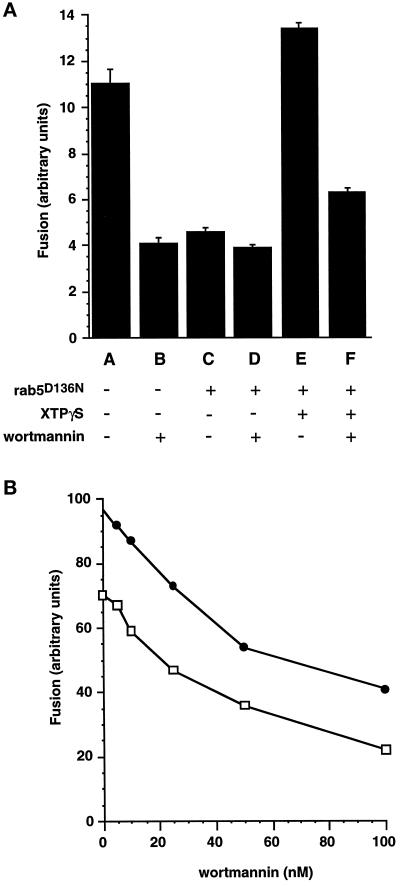
Geranylgeranylated Rab5D136N was preloaded onto avidin- and biotin-HRP–containing endosomes before they were combined in a fusion incubation. In the absence of XTP or XTPγS, addition of this mutant exerts a dominant negative effect on the endosome fusion assay (Figure 1a) as previously observed by Rybin et al. (1996). The degree of inhibition of endosome fusion is very similar to that obtained by application of the PI 3-kinase inhibitor wortmannin, and no further inhibition is observed by combining the mutant protein and wortmannin in the assay. Fusion activity in the presence of this XTP-binding mutant can be restored and stimulated beyond control levels by the addition of XTP (not shown) or the nonhydrolysable analogue XTPγS. In the presence of XTPγS, the major part of the fusion signal is therefore contingent on the presence of this nucleotide (Figure 1a), which will trap exogenously added rab5 in an activated state. The fusion signal after preincubation of components with REP-1/rab5D136N and XTPγS was still substantially inhibited by wortmannin (Figure 1a). This experiment is very suggestive that the influence of wortmannin on endosome fusion is exerted through a means other than a postulated effect on the rab5 nucleotide cycle. The dose response of the fusion assay to wortmannin was found to be very similar for endosome fusion under control conditions and for XTPγS-dependent fusion (Figure 1b). The concentration range over which wortmannin inhibition is observed (IC50<50 nM) is consistent with the well characterized inhibition of PI 3-kinases by this drug (Wymann et al., 1996).
Figure 1.
Wortmannin inhibition of XTPγS-REP-1d136n-dependent early endosome fusion. (a) Postnuclear supernatants containing avidin- or biotin-HRP–loaded early endosomes were aliquoted and separately preincubated at 37°C for 10 min with REP-1 complex buffer (A and B); 100 nM REP-1/rab5D136N (C and D); 100 nM REP-1/rab5D136N, 0.5 mM XTPγS (E and F). Correspondingly treated avidin- or biotin-HRP–containing fractions were then combined in a standard fusion assay and incubated at 37°C for 30 min in the presence (B, D, and F) or absence (A, C, and E) of 100 nM wortmannin. (b) Postnuclear supernatants containing avidin- or biotin-HRP–loaded early endosomes were aliquoted and separately preincubated for 10 min at 37°C with REP-1 complex buffer (□) or 100 nM REP-1/rab5D136N,0.5 mM XTPγS(•). Correspondingly treated avidin- or biotin-HRP–containing fractions were then combined in a standard fusion assay and incubated at 37°C for 30 min with the indicated concentrations of wortmannin.
We next repeated the endosome fusion assay under the same conditions except that the GTPase-deficient mutant rab5Q79L was loaded onto membranes (Figure 2). By virtue of its poor ability to hydrolyze GTP, this rab5 mutant is trapped in the active GTP state and, like XTPγS in the preceding experiment, is predicted to render the assay insensitive to factors that regulate the nucleotide cycle of rab5. The fusion assay was stimulated by addition of rab5Q79L, yet this stimulated fusion was substantially sensitive to wortmannin (Figure 2) with a similar dose response to fusion driven by endogenous rab5. This result is surprising because Li et al. (1995) have convincingly shown that rab5Q79L renders an assay of fusion between macrophage endosomes insensitive to wortmannin. In each of our experiments (Figures 1 and 2) however, it is noticeable that the activated mutant proteins, even in the presence of wortmannin, retain some stimulatory activity relative to control incubations.
Figure 2.
Early endosome fusion is stimulated by REP-1/rab5Q79L but remains sensitive to inhibition by wortmannin. Postnuclear supernatants containing 100 μM GTP and either avidin- or biotin-HRP–loaded early endosomes were aliquoted and separately incubated for 10 min at 37°C with REP-1 complex buffer (□) or 200 nM REP-1/rab5Q79L (•). Correspondingly treated avidin- or biotin-HRP–containing fractions were then combined in a standard fusion assay and incubated at 37°C for 30 min with the indicated concentrations of wortmannin.
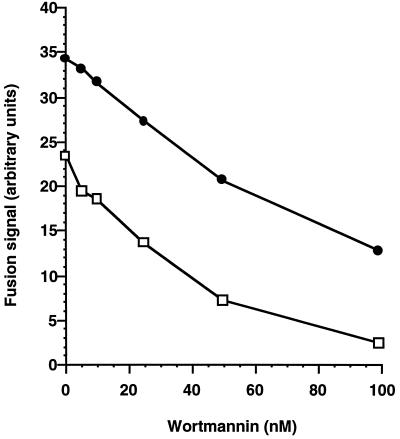
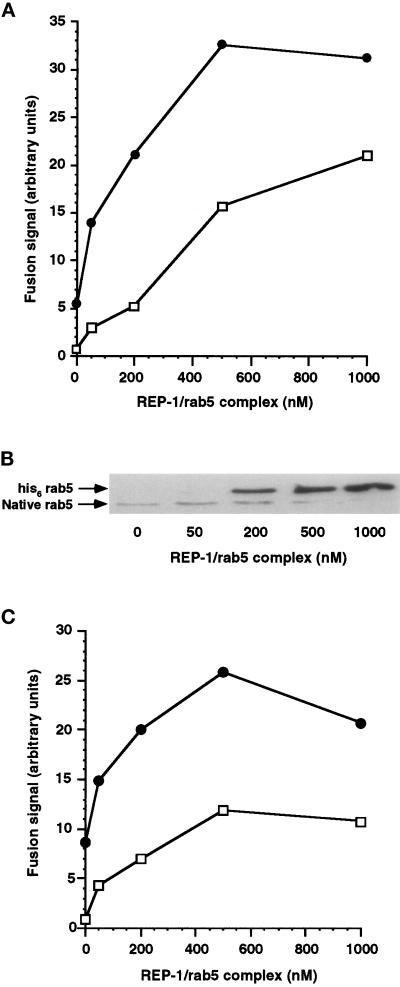
This observation prompted us to vary the concentration of exogenous REP/rab5Q79L complex we added to the system and thereby the amount of rab5Q79L delivered to membranes. We observed that at high concentrations of REP-1/rab5Q79L, we could reproduce the observation of Li et al., in that the fusion signal becomes insensitive to the presence of wortmannin (Figure 3a). It is noteworthy that the concentrations of rab5Q79L required for full protection from wortmannin are significantly higher than those required for maximal stimulation of the fusion assay. Figure 3b illustrates the association of exogenous rab5 with membranes at the end of the preincubation period. Even at the lowest concentrations used, the level of membrane-associated rab5Q79L is many fold that of endogenous levels of rab5. In BHK cells, about 20% of endogenous membrane-associated rab5 is in the GTP state at any one time (Stenmark et al., 1994). If the primary action of wortmannin was to reduce the number of rab5 molecules in the GTP state, for example by inhibiting nucleotide exchange, then we would expect full protection from wortmannin by loading an equal number of rab5Q79L molecules that will accumulate in the GTP form. In fact, Figure 3 shows that we can add back a much larger number of rab5Q79L molecules than the highest conceivable number of GTP-bound wild-type rab5 molecules present in control conditions and still retain significant sensitivity to wortmannin. Densitometric analysis of an enhanced chemiluminescence (ECL)-developed Western blot (Figure 3b) indicates a 5- to 10-fold excess of membrane-associated his6-rab5Q79L over endogenous wild-type rab5, at the lowest concentration of REP-1/rab5Q79L that we have used (50 nM). This result is inconsistent with the hypothesis that the primary effect of wortmannin is to influence the nucleotide state of rab5.
Figure 3.
Increasing membrane concentrations of rab5Q79L protects early endosome fusion from wortmannin inhibition. (a) Postnuclear supernatants containing avidin- or biotin-HRP–loaded early endosomes were separately preincubated for 10 min at 37°C with 100 μM GTP and 0, 50, 200, 500, or 1000 nM REP-1/rab5Q79L. Correspondingly treated avidin or biotin-HRP containing fractions were then combined in a standard fusion assay and incubated at 40 min at 37°C in the presence (□) or absence (•) of wortmannin (100 nM). (b) Postnuclear supernatants were incubated as in Figure 5a. The samples were centrifuged and 13 μg of the pelleted fraction were loaded on a 13% SDS-PAGE gel. Rab5 was detected by enhanced chemiluminescence Western blotting using a rab5 monoclonal antibody. The position of BHK-21 rab5 and his6-rab5Q79L is shown.
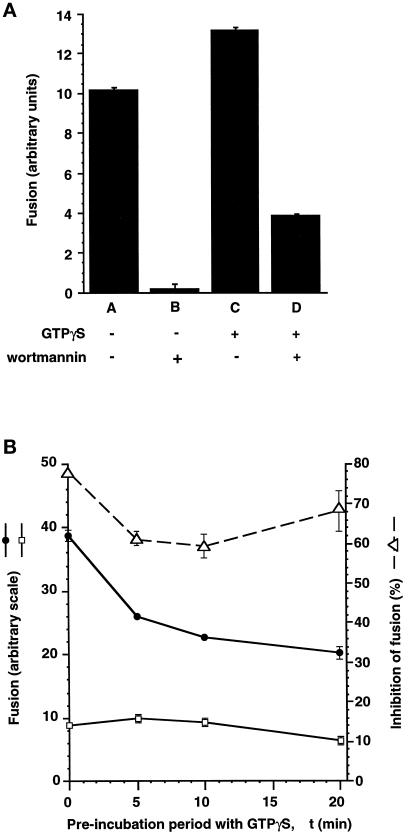
The rab5D136N mutation allowed us to selectively activate rab5-dependent fusion with XTPγS (Figure 1). We also conducted an experiment in which we used GTPγS to preactivate all GTPase proteins in the fusion incubation mixture including endogenous rab5 (Figure 4). We obtained similar results to those when rab5D136N alone is activated (Figure 1), i.e., the fusion assay remained substantially sensitive to wortmannin, despite the presumed entrapment of all GTPases in their active state. This result suggests that the requirement of endosome fusion for PI 3-kinase activity is not upstream of the activation of any GTPase.
Figure 4.
Early endosome fusion is stimulated by GTPγS but is still sensitive to inhibition by wortmannin. (a) Postnuclear supernatants containing avidin- or biotin-HRP–loaded early endosomes were separately preincubated for 10 min at 37°C without (A and B) or with (C and D) 40 μM GTPγS. Correspondingly treated avidin- or biotin-HRP–containing fractions were then combined in a standard fusion assay supplemented with buffer (A and C) or 100 nM wortmannin (B and D) and incubated for 30 min at 37°C. (b) Postnuclear supernatants containing avidin- or biotin-HRP–loaded early endosomes were separately preincubated for various periods of time (Δt) at 37°C with 40 μM GTPγS. Correspondingly treated avidin- or biotin-HRP–containing fractions were then combined in a standard fusion assay in the absence (•) or presence (□) of 100 nM wortmannin and incubated for 30 min at 37°C. The percentage inhibition of the fusion assay signal for each condition is also plotted (▵, dashed line).
In the way that we configured our standard assay, fractions were preincubated with nonhydrolysable nucleotide in the absence of wortmannin for 10 min before the fusion incubation. It could be argued that this is insufficient time for enough nucleotide exchange on the relevant GTPase to take place and that the subsequent addition of wortmannin imposes an absolute block on further exchange. Arguing against this, it is fair to say that most GTPases will undergo exchange on this time scale; for example >80% nucleotide exchange took place within 10 min for rab5D136N loaded onto BHK cell-derived membranes (Rybin et al., 1996). Furthermore, in the study of Li et al. (1995), wortmannin was added to the system before addition of rab5Q79L. As rab5 associates with membranes in the GDP state (Alexandrov et al., 1994; Ullrich et al., 1994), but nevertheless they observed rab5Q79L-dependent recovery from the wortmannin block, a significant amount of GTP association with rab5Q79L must have taken place in the presence of wortmannin, during the course of the assay. We conducted an experiment in which we systematically varied the point at which GTPγS was added, during a constant 20-min preincubation period (Figure 4b). We found no evidence that an increased time of preincubation with GTPγS led to increased protection from wortmannin inhibition (Figure 4b).
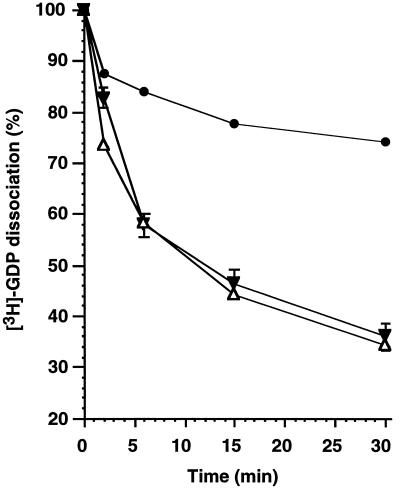
The rate-limiting event for nucleotide exchange is nucleotide dissociation. We prepared REP-1/[3H]GDP–rab5 complex and presented it to purified CCVs. We observed a CCV-dependent stimulation of nucleotide dissociation, similar to that previously observed by Horiuchi et al. (1995), which was independent of wortmannin (Figure 5).
Figure 5.
The stimulation of [3H]GDP dissociation from rab5 by CCVs is not affected by wortmannin. REP-1/[3H]GDP–rab5 (100 nM) was incubated in the absence (•) or presence (▵,▾) of 1 mg/ml CCVs for the indicated periods of time in the absence (▾) or presence (▵) of 200 nM wortmannin. At the end of the reaction the samples were loaded on filters and protein-bound radioactivity was quantified as described in MATERIALS AND METHODS. The data represent the mean values from two independent experiments of filter- associated counts expressed relative to counts measured before incubation.
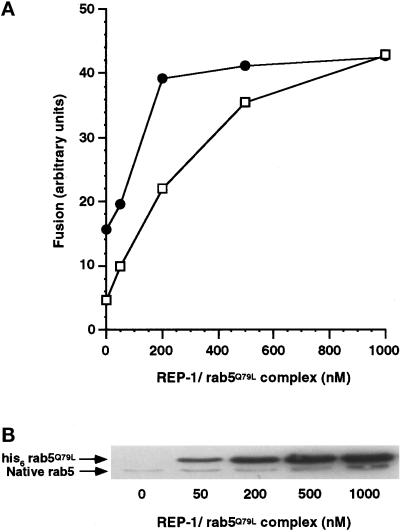
Incubation with GTPγS offers a modest stimulation of fusion (Figure 4). We reasoned that this may be mediated through rab5, similar to the observation with XTPγS (Figure 1), such that if we increased the level of wild-type rab5 in the system, GTPγS-dependent rescue from wortmannin treatment may be observed. This is analogous to increasing amounts of rab5Q79L in the system that we have shown leads to recovery from wortmannin inhibition in a concentration-dependent manner (Figure 3). Membrane loading of wild-type rab5 in the presence of GTPγS was less efficient than rab5Q79L but was nevertheless many fold higher than native BHK rab5 (Figure 6b). We found rab5-dependent stimulation of both control and wortmannin-treated signals in the presence of GTPγS (Figure 6a), although in a total of three experiments, we were never able to completely overcome wortmannin inhibition as observed for rab5Q79L (Figure 3). Surprisingly, we obtained very similar results when wild-type rab5 was added to the assay in the absence of GTPγS (Figure 6c), suggesting that the amount of rab5 bound to membranes is the crucial factor for overcoming wortmannin inhibition of endosome fusion.
Figure 6.
Increasing membrane concentrations of his6 rab5 with or without GTPγS partially protect early endosome fusion from wortmannin inhibition. (a) Postnuclear supernatants containing avidin- or biotin-HRP—loaded early endosomes were separately preincubated for 10 min at 37°C with 60 μM GTPγS and 0, 50, 200, 500, or 1000 nM REP-1/rab5. Correspondingly treated avidin or biotin-HRP—containing fractions were then combined in a standard fusion assay for 40 min at 37°C in the presence (□) or absence (•) of wortmannin (100 nM). (b) Postnuclear supernatants were incubated as in Figure 6a. The samples were centrifuged and 13 μg of the pelleted fractions were loaded on a 13% SDS-PAGE gel. Rab5 was detected by Western blotting using a rab5 monoclonal antibody. The position of BHK-21 rab5 and his6-tagged native rab5 is shown. (c) Postnuclear supernatants containing avidin- or biotin-HRP—loaded early endosomes were separately preincubated without added nucleotide for 10 min at 37°C with 0,50, 200, 500, or 1000 nM REP-1/rab.5 Correspondingly treated avidin or biotin-HRP containing fractions were then combined in a starndard fusion assay for 40 min at 37°C in the presence (□) or absence (•) of wortmannin (100 nM).
DISCUSSION
Effects on Nucleotide Hydrolysis Do Not Explain Wortmannin Inhibition
The demonstration that an XTP-binding mutant of rab5 (D136N) behaves as the wild-type protein, except in the matter of nucleotide preference, has provided an important tool for functional studies of this protein (Rybin et al., 1996). XTP is not produced naturally, so the addition of exogenous XTP achieves a specific activation of rab5 that cannot be achieved for the wild- type rab5 with GTP, because of the large number of other GTP-binding proteins in the system. We have delivered XDP-rab5D136N to the endosomal membrane after geranylgeranylation of the recombinant protein in a complex with REP-1 and subsequent presentation of REP-1/prenylated rab5 complex to membrane fractions. We have observed qualitatively similar effects on an in vitro assay of endosome fusion to those reported by Rybin et al. (1996). In the absence of XTP the mutant exerts a dominant negative effect on endosome fusion, and addition of XTP or the nonhydrolysable analogue XTPγS stimulates endosome fusion above control levels obtained in the absence of exogenous rab5 (Figure 1a). As the major component of the fusion signal is inhibited by exogenous rab5D136N in the absence of XTP, it is clear that the fusion signal produced by inclusion of XTPγS is mediated through rab5. We clearly show that rab5-dependent endosome fusion is inhibited by the PI 3-kinase inhibitor wortmannin, in agreement with previous results (Jones and Clague, 1995; Li et al., 1995; Spiro et al., 1996). It is important to note that in this experiment, exogenous rab5 and XTPγS are preincubated with postnuclear supernatant fractions in the absence of ATP before exposure with wortmannin. As we have used a nonhydrolysable analogue of XTP to elicit rab5-dependent fusion, the inhibitory action of wortmannin cannot be the result of stimulation of nucleotide hydrolysis by rab5. This result is confirmed by the fact that we also see wortmannin inhibition of fusion in the presence of a GTPase-deficient mutant of rab5 (Q79L) at similar concentrations (Figure 2). Finally, this result can be extrapolated to all GTP-binding proteins in the fusion incubation, as wortmannin is also shown to inhibit endosome fusion in the presence of GTPγS (Figure 4).
Effects on Nucleotide Exchange Do Not Explain Wortmannin Inhibition
Inhibition of endosome fusion by wortmannin could be due to a reduction in nucleotide exchange on rab5 (or another GTPase) that would lead to less protein in the GTP form. PI-3 kinase activity is known to stimulate the nucleotide exchange rate of the small GTPase rac (Hawkins et al., 1995) and of rab4 in adipocytes (Shibata et al., 1997). However, this attractive hypothesis is contraindicated by the fact that for experiments that were carried out with nonhydrolysable nucleotide analogues (Figures 1 and 4), fractions were preincubated with these analogues for 10 min at 37°C, allowing time for nucleotide exchange before combination in a fusion assay and exposure to wortmannin. Nevertheless, we still observed inhibition of fusion by wortmannin. Furthermore, the time of preincubation with GTPγS (up to 20 min) had no effect on the fusion signal observed with wortmannin (Figure 4b). We also made a direct measurement of the rate-limiting event in nucleotide exchange on rab5, i.e., nucleotide dissociation, in the presence of CCV- coated vesicles. Although it is not certain that this CCV-associated stimulatory activity is the same as that on early endosomes, the stimulated dissociation of nucleotide was unaffected by the presence of wortmannin (Figure 5), consistent with our other observations.
High Levels of rab5Q79L Mutant Overcome Wortmannin Inhibition
Li et al have shown previously that rab5Q79L mutant, which is deficient in GTP hydrolysis, can render an endosome fusion assay insensitive to the presence of wortmannin (Li et al., 1995). We were surprised by our observation that we could retain significant sensitivity to wortmannin even after addition of stimulatory levels of rab5Q79L (Figure 2) in apparent contradiction to the results of Li et al. We were able to resolve this contradiction by adding even higher levels of rab5Q79L to the assay, which rendered it insensitive to wortmannin (Figure 3a). It is important to note that there are significant differences in the experimental setup of these two studies, notably in the endosome-labeling procedure, the type of cells used, and the methods by which prenylated rab5 is produced and delivered to endosomes. Nevertheless, there is a convergence in the findings of both studies. The concentration of exogenous rab5 added to the assay by Li et al. corresponds to a final concentration of 700 nM, similar to that which we have found to be required for complete recovery (Figure 3). From our results (Figure 3), it is clear that endosome fusion does not absolutely require PI 3-kinase activity if there is enough GTP-bound rab5 in the system, but also that the requirement for this activity when only endogenous levels of rab5 are present is not simply to promote the GTP-bound state of rab5 (see preceding discussion). The levels of rab5Q79L that must be added to the system for protection from wortmannin far exceed the highest conceivable amount of native rab5 present that could be indirectly inactivated by wortmannin. Of course, this does not rule out the possibility that wortmannin may directly or indirectly influence the nucleotide cycle of rab5, but suggests that this cannot be its primary role in endosome fusion.
The fact that rab5Q79L can prevent a wortmannin block argues against the requirement for PI 3-kinase lying downstream of rab5 on the fusion pathway. We suggest that, taken all together, these results are most easily explained if PI 3-kinase activity generates or regulates an accessory factor that acts in concert with rab5. We have found conditions for which endosome fusion is effectively stimulated due to enhanced levels of active rab5, yet for which inhibition is still observed with a similar dose response to control conditions (Figures 1 and 2). This may be expected if PI 3-kinase activity is required at a different step on the fusion pathway to rab5 but will also occur if its product governs an equilibrium reaction with a high-power dependence on its concentration. For example, the creation of a lipid microdomain may depend on the cooperation of many lipid molecules of a particular type.
We have also shown that, in our hands, high levels of wild-type rab5 are able to promote fusion in the presence of wortmannin. The presence of GTPγS had no significant supplementary effect on wild-type rab5-mediated prevention of a wortmannin block. We were not able to obtain complete protection as in the case of rab5Q79L, but this may reflect the lower degree of membrane loading that we were able to achieve (compare Figures 3b and 6b). This observation is opposite to that made by Li et al. who saw no rescue from wortmannin inhibition by adding wild-type rab5 to their assay. Differences between the two assay systems that could underlie this discrepancy have already been outlined above.
The major function of rab proteins is believed to be the promotion of interaction between cognate v- and t-SNAREs, which mediate binding between compartments destined to fuse. The strongest evidence for this idea comes from studies of endoplasmic reticulum to Golgi transport in the yeast S. cerivisiae which have shown that mutations in the rab protein YPT1 prevent assembly of the relevant SNARE complex. It has recently been proposed that the role of YPT1 is to displace a negative regulator of SNARE complex assembly, SLY1, from interacting with the t-SNARE Sed5p, by virtue of a direct transient interaction with Sed5p (Lupashin and Waters, 1997). As most intracellular fusion events (from yeast to man) are presumed to use the same basic design, it is attractive to suppose that rab5 engages in similar interactions, although hard evidence is currently lacking. Furthermore, as phosphatidylinositols have been shown to be ubiquitous regulators of protein–protein interactions, it may be that PI 3-kinase activity is required to generate phosphorylated lipids that promote rab5 interaction with a t-SNARE or other effector protein. An excess of rab5 may overcome this requirement by a mass action effect. This is frequently observed for nonessential elements of a membrane transport reaction, e.g., deletions in a rab gene in yeast (YPT1) can be overcome by increasing the cellular concentration of v-SNAREs required at the same transport step (Dascher et al., 1991; Ossig et al., 1991).
Concluding Remarks
PI 3-kinase activity clearly influences rab5-dependent events in a number of cell lines. However, this is unlikely to be a conserved feature of rab protein-dependent mechanisms. Transport through the biosynthetic pathway is insensitive to wortmannin, yet dependent on rab proteins. Moreover, the rab9-dependent transport of cation-independent mannose 6-phosphate receptor from late endosomes to the TGN is insensitive to wortmannin (Nakajima and Pfeffer, 1997). In adipocytes, insulin stimulation of PI 3-kinase activity influences the subcellular localization of rab4 (Cormont et al., 1996) and the nucleotide exchange on this protein (Shibata et al., 1997), whereas insulin-induced rab5 movement is wortmannin insensitive.
Rab5 may be different from most other rab proteins by virtue of participating in signal transduction cascades initiated at the plasma membrane. Activated ras has been shown to increase fluid phase endocytosis, and this stimulation is abolished by a dominant negative mutant of rab5 (Li et al., 1997). Activated ras has also previously been shown to interact with PI 3-kinase (Rodriguez-Viciana et al., 1996). Interestingly, the tumor suppressor, tuberin, has recently been identified as a candidate protein for regulating rab5 activity (Xiao et al., 1997). The characterization of these interactions is an important task for the future, as reciprocal relationships between signal transduction and membrane trafficking emerge as a theme of study. Toward this goal, our results indicate that the effects of wortmannin on a rab5-dependent event cannot be accounted for by any effect on the nucleotide cycle of rab5.
ACKNOWLEDGMENTS
We thank Harald Stenmark, Miguel Seabra, Angela Wandinger-Ness, and Marino Zerial for generous gifts of reagents; Ian Prior for preparing clathrin coated vesicles; Andrea Beste for preparing XTPγS; Bob Burgoyne and Patrick Gaffet for comments on the manuscript. This work was supported by the Wellcome Trust.
Footnotes
Abbreviations used: CCV, clathrin-coated vesicle; GGTase, geranylgeranyltransferase; GGPP, geranylgeranyl pyrophosphate; HRP, horseradish peroxidase; PI, phosphoinositide; REP, rab escort protein; TGN, trans-Golgi network; XTP, xanthosine 5′-triphosphate.
REFERENCES
- Alexandrov K, Horiuchi H, Steele-Mortimer O, Seabra M C, Zerial M. Rab escort protein-1 is a multifunctional protein that accompanies newly prenylated rab proteins to their target membranes. EMBO J. 1994;13:5262–5273. doi: 10.1002/j.1460-2075.1994.tb06860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andres DA, Seabra MC, Brown MS, Armstrong SA, Smeland TE, Cremers FP M, Goldstein JL. cDNA cloning of component A of rab geranylgeranyltransferase and demonstration of its role as a rab escort protein. Cell. 1993;73:1091–1099. doi: 10.1016/0092-8674(93)90639-8. [DOI] [PubMed] [Google Scholar]
- Arcaro A, Wymann MP. Wortmannin is a potent phosphatidylinositol 3-kinase inhibitor: the role of phosphatidylinositol 3,4,5-triphosphate in neutrophil responses. Biochem J. 1993;296:297–301. doi: 10.1042/bj2960297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown WJ, DeWald DB, Emr SD, Plutner H, Balch WE. Role for phosphatidylinositol 3-kinase in the sorting and transport of newly synthesized lysosomal enzymes in mammalian cells. J Cell Biol. 1995;130:781–796. doi: 10.1083/jcb.130.4.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavrier P, Gorvel JP, Stelzer E, Simons K, Gruenberg J, Zerial M. Hypervariable C-terminal domain of rab proteins acts as a targetting signal. Nature. 1991;353:769–772. doi: 10.1038/353769a0. [DOI] [PubMed] [Google Scholar]
- Clague MJ, Thorpe C, Jones AT. Phosphatidylinositol 3-kinase regulation of fluid phase endocytosis. FEBS Lett. 1995;367:272–274. doi: 10.1016/0014-5793(95)00576-u. [DOI] [PubMed] [Google Scholar]
- Clague MJ, Urbe S, Aniento F, Gruenberg J. Vacuolar-ATPase activity is required for endosomal carrier vesicle formation. J Biol Chem. 1994;269:21–24. [PubMed] [Google Scholar]
- Colombo MI, Mayorga LS, Casey PJ, Stahl PD. Evidence of a role for heterotrimeric GTP-binding proteins in endosome fusion. Science. 1992;255:1695–1697. doi: 10.1126/science.1348148. [DOI] [PubMed] [Google Scholar]
- Cormont M, Van Obberghen E, Zerial M, Le Marchand-Brustel Y. Insulin induces a change in rab5 subcellular localization in adipocytes independently of phosphatidylinositol 3-kinase activation. Endocrinology. 1996;137:3408–3415. doi: 10.1210/endo.137.8.8754768. [DOI] [PubMed] [Google Scholar]
- Cremers FP M, van de Pol DJ R, van Kerkhoff LP M, Wieringa B, Ropers HH. Cloning of a gene that is rearranged in patients with choroideraemia. Nature. 1990;347:674–677. doi: 10.1038/347674a0. [DOI] [PubMed] [Google Scholar]
- Dascher C, Ossig R, Gallwitz D, Schmitt HD. Identification and structure of 4 yeast genes (SLY) that are able to suppress the functional loss of YPT1, a member of the ras superfamily. Mol Cell Biol. 1991;11:872–885. doi: 10.1128/mcb.11.2.872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson HW. Wortmannin causes mistargeting of procathepsin D. Evidence for the involvement of a phosphatidylinositol 3-kinase in vesicular transport to lysosomes. J Cell Biol. 1995;130:797–806. doi: 10.1083/jcb.130.4.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz R, Mayorga L, Weidman PJ, Rothman JE, Stahl PD. Vesicle fusion following receptor-mediated endocytosis requires a protein active in Golgi transport. Nature. 1989;339:398–400. doi: 10.1038/339398a0. [DOI] [PubMed] [Google Scholar]
- Goody RS, Eckstein F, Schirmer RH. The enzymatic synthesis of thiophosphate analogs of nucleotides. Biochim Biophys Acta. 1972;276:155–161. doi: 10.1016/0005-2744(72)90016-2. [DOI] [PubMed] [Google Scholar]
- Gorvel JP, Chavrier P, Zerial M, Gruenberg J. Rab 5 controls early endosome fusion in vitro. Cell. 1991;64:915–925. doi: 10.1016/0092-8674(91)90316-q. [DOI] [PubMed] [Google Scholar]
- Gruenberg J, Howell KE. An internalised transmembrane protein resides in a fusion competent endosome for less than 5 minutes. Proc Natl Acad Sci USA. 1987;84:5758–5762. doi: 10.1073/pnas.84.16.5758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins PT, Eguinoa A, Qiu R-G, Stokoe D, Cooke FT, Walters R, Wennstrom S, Claesson-Welsh L, Evans T, Symons M, Stephens L. PDGF stimulates an increase in GTP-rac via activation of phosphoinositide 3-kinase. Curr Biol. 1995;5:393–403. doi: 10.1016/s0960-9822(95)00080-7. [DOI] [PubMed] [Google Scholar]
- Horiuchi H, Giner A, Hoflack B, Zerial M. A GDP/GTP exchange stimulatory activity for the rab5-rabGDI complex on clathrin-coated vesicles from bovine brain. J Biol Chem. 1995;270:11257–11262. doi: 10.1074/jbc.270.19.11257. [DOI] [PubMed] [Google Scholar]
- Joly M, Kazlauskas A, Corvera S. Phosphatidylinositol 3-kinase activity is required at a postendocytic step in platelet-derived growth-factor receptor trafficking. J Biol Chem. 1995;270:13225–13230. doi: 10.1074/jbc.270.22.13225. [DOI] [PubMed] [Google Scholar]
- Jones AT, Clague MJ. Phosphatidylinositol 3-kinase activity is required for early endosome fusion. Biochem J. 1995;311:31–34. doi: 10.1042/bj3110031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenhard JM, Kahn RA, Stahl PD. Evidence for ADP-ribosylation factor (ARF) as a regulator of in vitro endosome-endosome fusion. J Biol Chem. 1992;267:13047–13052. [PubMed] [Google Scholar]
- Li G, D’Souza-Schorey C, Barbieri MA, Cooper JA, Stahl PD. Uncoupling of membrane ruffling and pinocytosis during ras signal transduction. J Biol Chem. 1997;272:10337–10340. [PubMed] [Google Scholar]
- Li GP, D’Souza-Schorey C, Barbieri MA, Roberts RL, Klippel A, Williams LT, Stahl PD. Evidence for phosphatidylinositol 3-kinase as a regulator of endocytosis via activation of rab5. Proc Natl Acad Sci USA. 1995;92:10207–10211. doi: 10.1073/pnas.92.22.10207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupashin VV, Waters GM. t-SNARE activation through transient interaction with a rab-like guanosine triphosphatase. Science. 1997;276:1255–1258. doi: 10.1126/science.276.5316.1255. [DOI] [PubMed] [Google Scholar]
- Maycox PR, Link E, Reetz A, Morris SA, Jahn R. Clathrin-coated vesicles in nervous tissue are involved primarily in synaptic vesicle recycling. J Cell Biol. 1992;118:1379–1388. doi: 10.1083/jcb.118.6.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima Y, Pfeffer SR. Phosphatidylinositol 3-kinase is not required for recycling of mannose 6-phosphate receptors from late endosomes to the trans-Golgi network. Mol Biol Cell. 1997;8:577–582. doi: 10.1091/mbc.8.4.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuoffer C, Peter F, Balch WE. Purification of His6-tagged rab1 proteins using bacterial and insect cell expression systems. Methods Enzymol. 1995;257:3–9. doi: 10.1016/s0076-6879(95)57003-9. [DOI] [PubMed] [Google Scholar]
- Ossig R, Dascher C, Trepte HH, Schmitt HD, Gallwitz D. The yeast SLY gene-products, suppressors of defects in the essential GTP-binding YPT1 protein, may act in endoplasmic reticulum-to-Golgi transport. Mol Cell Biol. 1991;11:2980–2993. doi: 10.1128/mcb.11.6.2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez L, Stirling CJ, Woodman PG. Multiple N-ethylmaleimide-sensitive components are required for endosomal vesicle fusion. Mol Biol Cell. 1994;5:773–783. doi: 10.1091/mbc.5.7.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Viciana P, Warne PH, Van Haesebroeck B, Waterfield MD, Downward J. Activation of phosphoinositide 3-kinase by interaction with ras and by point mutation. EMBO J. 1996;15:2442–2451. [PMC free article] [PubMed] [Google Scholar]
- Rybin V, Ullrich O, Rubino M, Alexandrov K, Simon I, Seabra M, Goody R, Zerial M. GTPase activity of rab5 acts as a timer for endocytic membrane fusion. Nature. 1996;383:266–268. doi: 10.1038/383266a0. [DOI] [PubMed] [Google Scholar]
- Schu PV, Takegawa K, Fry MJ, Stack JH, Waterfield MD, Emr SD. Phosphatidylinositol 3-kinase encoded by yeast VPS34 gene essential for protein sorting. Science. 1993;260:88–91. doi: 10.1126/science.8385367. [DOI] [PubMed] [Google Scholar]
- Shibata H, Omata W, Kojima I. Insulin stimulates guanine nucleotide exchange on rab4 via a wortmannin-sensitive signaling pathway in rat adipocytes. J Biol Chem. 1997;272:14542–14546. doi: 10.1074/jbc.272.23.14542. [DOI] [PubMed] [Google Scholar]
- Spiro DJ, Boll W, Kirchhausen T, Wessling-Resnick M. Wortmannin alters the transferrin receptor endocytic pathway in vivo and in vitro. Mol Biol Cell. 1996;7:355–367. doi: 10.1091/mbc.7.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenmark H, Parton RG, Steele-Mortimer O, Lutcke A, Gruenberg J, Zerial M. Inhibition of rab5 GTPase activity stimulates membrane fusion in endocytosis. EMBO J. 1994;13:1287–1296. doi: 10.1002/j.1460-2075.1994.tb06381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich O, Horiuchi H, Bucci C, Zerial M. Membrane association of rab5 mediated by GDP-dissociation inhibitor and accompanied by GDP/GTP exchange. Nature. 1994;368:157–160. doi: 10.1038/368157a0. [DOI] [PubMed] [Google Scholar]
- Ullrich O, Stenmark H, Alexandrov K, Huber LA, Kaibuchi K, Sasaki T, Takai Y, Zerial M. Rab GDP dissociation inhibitor as a general regulator for the membrane association of rab proteins. J Biol Chem. 1993;268:18143–18150. [PubMed] [Google Scholar]
- Vlahos CJ, Matter WF, Hui KY, Brown RF. A specific inhibitor of phosphatidyl 3-kinase, 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002) J Biol Chem. 1994;269:5241–5248. [PubMed] [Google Scholar]
- Wymann MP, Bulgarelli-Leva G, Zvelebil MJ, Pirola L, Vanhaesebroeck B, Waterfield MD, Panayatou G. Wortmannin inactivates phosphoinositide 3-kinase by covalent modification of Lys-802, a residue involved in the phosphate transfer reaction. Mol Cell Biol. 1996;16:1722–1733. doi: 10.1128/mcb.16.4.1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao GH, Shoarinejad F, Jin F, Golemis EA, Yeung RS. The tuberous sclerosis 2 gene product, tuberin, functions as a rab5 GTPase activating protein (GAP) in modulating endocytosis. J Biol Chem. 1997;272:6097–6100. doi: 10.1074/jbc.272.10.6097. [DOI] [PubMed] [Google Scholar]
- Zvelebil MJ, MacDougall LK, Leevers S, Volinia S, Vanhaesebroeck B, Gout I, Panayotou G, Domin J, Stein R, Pages F, Koga H, Salim K, Linacre J, Das P, Panaretou C, Wetzger R, Waterfield M. Structural and functional diversity of phosphoinositide 3-kinases. Philos Trans R Soc Lond B Biol Sci. 1996;351:217–223. doi: 10.1098/rstb.1996.0019. [DOI] [PubMed] [Google Scholar]