Abstract
Unlike adult spinalized rats, approximately 20% of rats spinalized as postnatal day 1 or 2 (P1/P2) neonates achieve autonomous hindlimb weight support. Cortical representations of mid/low trunk occur only in such rats with high weight support. However, the importance of hindlimb/trunk motor cortex in function of spinalized rats remains unclear. We tested the importance of trunk sensorimotor cortex in their locomotion using lesions guided by cortical microstimulation in P1/P2 weight-supporting neonatal spinalized rats and controls. In four intact control rats, lesions of hindlimb/trunk cortex caused no treadmill deficits. All spinalized rats lesioned in trunk cortex (n = 16: 4 transplant, 6 transect, 6 transect + fibrin glue) lost an average of about 40% of their weight support. Intact trunk cortex was essential to their level of function. Lesion of trunk cortex substantially increased roll of the hindquarters, which correlated to diminished weight support, but other kinematic stepping parameters showed little change. Embryonic day 14 (E14) transplants support development of the trunk motor representations in their normal location. We tested the role of novel relay circuits arising from the grafts in such cortical representations in E14 transplants using the rats that received (noncellular) fibrin glue grafting at P1/P2 (8 allografts and 32 xenografts). Fibrin-repaired rats with autonomous weight support also had trunk cortical representations similar to those of E14 transplant rats. Thus acellular repair and intrinsic plasticity were sufficient to support the observed features. Our data show that effective cortical mechanisms for trunk control are essential for autonomous weight support in P1/P2 spinalized rats and these can be achieved by intrinsic plasticity.
INTRODUCTION
Presently, the only rats that develop independent hindlimb weight support after a complete thoracic spinal transection are those injured at postnatal day 1 or 2 (P1/P2). This is despite many therapeutic efforts in other rats (e.g., Orsal et al. 2002). About 20% of P1/P2 spinalized rats develop such autonomous hindlimb support (Stelzner et al. 1975). Transplants of embryonic day 14 (E14) spinal cord (immediately after lesion) double the likelihood (Miya et al. 1997). In such rats intracranial microstimulation (ICMS) reveals mid- to low-trunk motor representations that correlate 1:1 with the development of weight support (Giszter et al. 1998). However, the role of these cortical areas and representations in function has been unclear. The role of the cortex in both intact and injured rats' locomotion has often been considered very minor.
The cortical motor representations of mid-to-low trunk in neonatal spinalized rats could be causal in the developed weight support of these animals or they could simply correlate with its achievement and be of little other significance. Research on locomotion in mammals and lower tetrapods suggests that modular spinal locomotor pattern generators and pattern shaper systems are responsible for many aspects of the organization of locomotion (Barbeau and Rossignol 1987; Deleon et al. 2002; Edgerton et al. 1992). Nonetheless, cortex clearly can play a significant role in locomotion in cats (Bretzner and Drew 2005a,b). In man, a stroke in leg or trunk cortex often produces serious locomotor deficits (as reviewed in Nudo 2006). However, in rodent models deficits in locomotion after similar lesions are minor, requiring subtle motor tests (e.g., Hicks and D'Amato 1975; Muir and Whishaw 1999).
Although the role of hindlimb/trunk motor cortex in intact rats may be modest in normal locomotor control of hindlimbs, it might nonetheless become very significant after P1/P2 neonatal spinal cord injuries. The normal motor representation of hindlimb and lumbar axial musculature in intact rats is contained in an area that is caudal to bregma and within 2.5 mm of the midline. The same area also contains a sensory representation of the trunk and hindlimbs and is a kind of sensorimotor amalgam (e.g., Hall and Lindholm 1974; Hummelsheim and Weisendanger 1986). Both the motor and sensory representations in this area are vulnerable to spinal cord injury (Giszter et al. 1998; Jain et al. 2003). The P1/P2 injuries used here occur before various critical periods in cortical organization. Sensory representations are developed in this region in all such P1/P2 rats but are reported to be lost after the critical periods (Jain et al. 2003). Cortex might be engaged differently in locomotion developed by the P1/P2 rats that were injured preceding critical periods compared with the intact or later injured rats. To test the possible role of this cortical region and its representations, we used ICMS to guide focal lesions placed in the normal location of the trunk area of cortex.
One of the most significant qualitative differences between P1/P2 neonatal transplant (TP) and transect (TX) rats that achieve weight support is the presence of trunk motor representations located in the caudal (axial/hindlimb) cortex. These representations in TP rats (Giszter et al. 1998), revealed by ICMS, were never observed in TX rats. E14 transplants thus caused a qualitative change in cortical development after spinalization, but the mechanism has been unclear. The more normal cortical representations observed after such transplants could depend on formation of novel E14 tissue circuits and cellular relays in spinal cord that are dependent on the transplanted cells (e.g., see Itoh et al. 1998). Alternatively, other effects could represent the major changes allowing the cortical representations. In contrast to E14 transplants, fibrin glue accomplishes hemostasis, cord stabilization, and potentially could bridge host fibers across a lesion site (e.g., Iwazawa et al. 1999), but it contains no cellular components. Fibrin glue thus clearly excludes novel neural relays as a mechanism. We tested the potential importance of novel relays by using repairs of neonatal spinal transections with noncellular fibrin glues. We tested both a rat-derived glue (likely to maximize tissue compatibility of the glue) and a human-derived surgical fibrin glue that was very pure, commercially available, and standardized (although likely less compatible). We compared the cortical organization and function of such rats with similar spinalized rats with E14 transplants. Our data showed the rats' cortices were very similar and thus the recovery of normally located trunk motor representations does not depend directly on the cellular components of the E14 transplants, but rather can be achieved by the actions of fibrin glues and host neurons and their plasticity alone.
Our experiments reported here show that, after P1/P2 spinalization, the role of the trunk cortex in the rats' locomotion is significantly increased, likely by developmental plasticity. Trunk cortex becomes an essential participant in the weight-supporting locomotion of these rats.
METHODS
We examined the representations in motor cortex and lesion effects in adult Sprague–Dawley rats spinalized at segment T8–T10 as neonates and tested the effects of two varieties of repair with fibrin glue and implantation of E14 fetal spinal transplants. In all, 99 rats were assessed in the course of this study. All procedures were carried out in accordance with US Department of Agriculture and Institutional Animal Care and Use Committee (IACUC) guidelines and with IACUC approval.
Neonatal surgery
Animals were prepared by neonatal surgery at postnatal days 1 and 2 (P1/P2). Surgery is described in detail in Miya et al. (1997). Neonates were placed under anesthesia by hypothermia, with a total surgical duration of 20 min. At least one complete segment of spinal cord was removed in the transection using aspiration. The lesion cavity created was either filled with gelfoam (spinal transect or TX rat), filled with fibrin glue (fibrin or FG rat; see following text) or filled with an E14 fetal spinal cord transplant (transplant or TP rat), as described in Miya et al. (1997) and Giszter et al. (1998).
Fibrin glue (see Redl and Schlag 1986) was derived in one of two ways: 1) rat-derived fibrin glue (FGR rats) was manufactured in house according to a recipe developed by Dr. N Kuwahara; 2) Tisseel fibrin glue (FGH rats) was prepared as recommended by the manufacturer (Baxter Healthcare, Vienna, Austria). In this way, a noncellular allograft (FGR rats) or noncellular xenograft matrix (FGH rats) was used and compared with the fetal spinal cord cellular allografts.
We prepared five groups of animals: 1) T8–T10 spinal cord transections (TX rats, n = 32), 2) T8–T10 spinal cord transection plus Tisseel fibrin glue repair of the spinal cord (FGH rats, n = 32), 3) T8–T10 spinal cord transection plus rat-derived fibrin glue repair of the spinal cord (FGR rats, n = 8), 4) fetal transplant rats (TP rats, n = 19), and 5) unoperated littermates (n = 8) serving as controls. Rat pups were returned to the dam and weaned at 3 wk or when body weight exceeded 45 g. We also prepared more FGH and TX rats because the likelihood of such rats achieving weight support was low (Giszter et al. 1998; Miya et al. 1997; Stelzner et al. 1975), and we here found it to be similarly low in the FGH rats. Rat numbers were also further reduced by some surgical losses. For this reason there could be differences in the numbers of rats in each group assessed and completed in different aspects of our study.
Tisseel fibrin glue preparation
After transection, hemostasis and stabilization of the injury cavity were achieved with injection of Tisseel vapor-heated sealant (75–115 mg fibrinogen/ml; Baxter Healthcare) prepared as specified by the manufacturer.
Fibrin glue preparation from rat
A rat-derived fibrin glue was prepared in house (recipe developed by Dr. N. Kuwahara) as follows.
On Day 1, 20 ml of whole blood was collected from a rat under anesthesia using a heparin-primed syringe. The plasma was separated by centrifugation at 3,000 rpm for 10 min at a temperature of 4°C. The plasma was then frozen at −70°C for 24 h.
On Day 2 the plasma was thawed to 1–6°C (∼15 h) and centrifuged at 1,000 rpm for 15 min. The plasma was decanted, leaving the cryoprecipitated fibrinogen and factor pellet. This concentrate was stored at −30°C.
At neonate surgery the thawed concentrate material was rapidly combined with a solution of 500 units of bovine thrombin and 400 mg calcium chloride in 1 ml of distilled water to form the activated fibrin glue.
Training and testing
Rats were trained on several locomotor tasks beginning at weaning, around 3 wk postpartum, as described previously (Giszter et al. 1998). Briefly, rats were exercised on a motorized treadmill set at speeds from 4 to 8 cm/s and trained to cross a narrow (2.5-in.) runway. Animals were water restricted and rewarded on the treadmill or narrow runway with a dilute sucrose solution. Test animals were trained at least three times weekly and videotaped weekly for evaluation. Animals were videotaped during training as they locomoted completely unassisted by the experimenter. Hindlimb steps on the camera side of a rat were classified and counted as weight-supporting or nonweight-supporting over a 3-min interval at a treadmill speed of 5 cm/s. Weight-supporting steps were recognized based on the criteria of no contact of the trunk, belly, or proximal joints (hip or knee) with the substrate in any limb during the swing and stance phases of the stepping limb. Plantar foot placement was not required; “knuckle walking” was permitted because our focus was on proximal balanced weight support. All other types of steps were considered nonweight-supporting. Using this method Miya et al. (1997) showed that percentage weight-supported steps during locomotion of adult animals that had been operated on as neonates formed a bimodal distribution with peaks centered on about 20 and 75% weight-supported steps. Accordingly, here we classified animals' locomotion into two categories: 1) weight supporting (WS) and 2) nonweight supporting (NWS). The classification we used here was based on observations of achievement of consistent (>50%) weight-supported steps in the WS class during the 2 mo postweaning, as compared with never achieving this level of function and routine sweeping/scissoring of limbs in the NWS class (<40% weight-supported steps). Our NWS group corresponds approximately to the Basso–Beattie–Bresnehan (BBB) scale ratings of ≤8 and our WS group corresponds to BBB ratings of 12–14 (see Basso et al. 1995).
Kinematics
Before and after cortical lesions rats kinematics were digitized from 60-Hz field-rate shuttered video with a 1-ms shutter time, captured to computer. Stick figures of several step cycles were constructed using a custom digitizing system that preserved tibial and femur length from frame to frame after initial calibration. Hip, knee, and ankle angles were calculated from the captured stick figures. Roll of the pelvis was assessed qualitatively from video. Steps with roll judged to be >45° about the long body axis were counted in a 3-min interval and expressed as a fraction of total steps. This measure thus included both incidents of loss of weight support through pelvic roll and large roll events that were corrected by the rat and did not cause stumbling. A probability of roll per step was estimated directly from this fraction of steps. The number and probability of steps with incidents of high pelvic roll were also related to the percentage weight-supported stepping measure obtained over the same interval using regression analysis.
ICMS cortical mapping
Using microstimulation, we mapped the motor cortex of operated rats and compared these maps to those presented previously by our laboratory (Giszter et al. 1998). Stimulation recruited muscles polysynaptically via activation of the corticospinal tract (CST).
At about 3 mo of age rats were anesthetized using an injection of 0.1–0.3 ml. The anesthetic cocktail consisted of ketamine hydrochloride (dose 50 mg/kg), xylazine (dose 5 mg/kg), and acepromazine (dose 0.75 mg/kg) in saline. Rats were also injected with dexamethasone (dose 5 mg/kg, administered intramuscularly) to control blood pressure and brain swelling. Subsequent anesthesia maintenance injections consisted of ketamine and acepromazine only. The rat was placed in a stereotaxic apparatus and bone pins for a headpiece/cap were placed in the skull, which was prepared at that time for future dental cement. Bregma was located and noted, a window in the skull (∼8 mm rostral × 10 mm lateral, to allow complete ICMS motor mapping) surface was removed, and the dura was reflected to expose the cortical surface. The animal was electrically isolated from the stereotaxic instrument with rubber caps on ear and mouth bars and the preparation checked carefully before and after mapping for shortcircuits or capacitative loads that might compromise current pulse delivery. The cortical surface was kept moist with a shallow saline bath and cotton reservoir. The motor responses that could be elicited from motor areas of both hemispheres of cortex were mapped by ICMS using fine stainless steel electrodes (∼10 MΩ, initial impedance at 1 kHz; shank diameter, 125 microns; and tip <1-micron diameter, exposed tip about 5 square microns; FHC). Mapping penetrations were arrayed across motor areas in a continuous 0.5-mm grid and were vertical with respect to the stereotaxic instrument. The electrodes were driven through pia, retreated to the pial surface, and advanced in 200-micron increments down to a 1,400-micron depth. Stimulation of 50 μA was tested at each depth and, at the region of strongest response, the threshold was checked. Over the area of interest, distortions arising from cortical surface curvature or from slight movements off precise grid placements due to blood vessels were small. Stimuli were applied as 0.2-ms total duration constant-current bipolar pulses with anodal current leading, at 333 Hz in trains of 300-ms duration. The longer trains allowed us to observe effects in which temporal facilitation or ventilatory synergistic or gating effects on stimuli were present (see Giszter et al. 1998). These ventilatory interactions with stimulus efficacy could be frequent in spinalized rats (Giszter et al. 1998). The threshold response (defined here as detectable motion or electromyographic [EMG] changes in recorded muscles) and responses at 50 μA were routinely examined. In “silent” areas the maximal currents used were 100 μA to confirm the absence of any response. Thus data points in the maps shown herein were all collected at 50 μA, but silent areas were stimulated at ≤100 μA. To estimate area we considered each penetration at 50-μA current to represent the response in a 250- to 700-micron radius circle (based on Yeomans 1990). Areas of recruited tissue were thus expected to range between just touching square packed circles and overlapping circles with full coverage of the tested cortical surface. For analysis, areas were quantified as numbers of such contiguous and adjacent penetrations or, in the case of divided or patchy representations (such as the trunk in the normal rat), were assessed using the total numbers of penetrations. Sites with two response types (e.g., leg and low trunk in an intact rat) were counted as a contributing area to two representations. Pulse amplitudes and rise times were monitored using a Tektronix oscilloscope to examine voltage changes across a 10-kΩ resistor interposed in series between the preparation ground and the stimulator. Electrodes were replaced if pulse shapes altered radically or desired currents were not achieved.
Stimulation length parameters were chosen that were longer than conventionally used (300-ms as opposed to the standard 30-ms-long train). As in previous work (Giszter et al. 1998), we used longer trains with the goal of allowing the greatest opportunity for temporal facilitation of activity elicited by microstimulation at each of the synapses along the several possible cascades of connections between cortex and motoneurons in the spinal-lesioned rats. [Our train lengths were similar to those used in some of the earliest maps of rat cortex (Settlage et al. 1949). The use of long trains minimized the possibility of false negatives in our procedures and provided the greatest chance of detecting a functionally relevant physiological connection. Hall and Lindholm (1974) compared 50- and 250-ms stimulation regimes and reported little difference between these.] In Giszter et al. (1998) we also established that the longer train parameters did not alter the map features of interest or the basic map structure in our intact control rats.
ICMS-induced movements and responding muscles were identified. To identify the caudal extent of response we used palpation or EMGs recorded from chosen muscles using bipolar stainless steel EMG pairs (fish-hook, patch, or ball electrodes). Electrodes were positioned acutely during the experiment in surgically exposed and identified muscles. Investigators were not blind to animal's spinalizations during mapping because the haunches of spinalized rats are significantly smaller and there is scoliosis in some rats. Histology was also used to confirm that cortical stimulation sites were appropriately placed in nonlesioned rats as in Giszter et al. (1998). Statistics of standard maps were analyzed and compared using the Matlab, Minitab, R or S-plus statistical packages. Fifty rats were mapped in all.
Cortical lesions
In normal rats, following mapping, the area of low trunk/hindlimb was identified and lesioned using a heat cautery penetrating to a depth of 0.8–1 mm. We also lesioned all WS rats studied except FGR. FGR rats were expensive to prepare and in other ways did not differ from FGH rats. In neonatal injured rats in which a low-trunk representation was identifiable in the caudal motor cortex (bregma and behind) we lesioned this representation. In neonatal injured rats in which this region was silent to ICMS we relied on stereotaxic coordinates to identify this area (see Giszter et al. 1998). Lesion sizes were similar among these three groups. The dural flap was replaced as far as possible and the skull resealed with Durelon dental acrylic (Schein) or Reprosil dental compound. The rat was removed from the stereotaxic frame and returned to the cage when sternally recumbent. Buprenex analgesia was administered every 6 h for 2 days. Rats were then again treadmill trained and retested over the next 2 mo before sacrifice. All rats survived lesion surgery. We collected data from 28 rats that had lesions in trunk cortex (including 4 normals, 16 spinalized rats with weight support and cortical lesions, 4 intact rats with cortical lesions, and 4 nonweight-supporting rats with cortical lesion) that fulfilled all criteria for inclusion in analysis. In two additional TP rats lesions were placed in forelimb areas 1 mm rostral to bregma. These lesions induced weight-support stepping reductions in these rats of 20 and 30%; these reductions were less than the average with lesions behind bregma. Lesions out of motor regions were not assessed.
Histology
Following the postlesion testing/training the rats were overdosed with an anesthetic cocktail and perfused transcardially with buffered Ringer solution followed by Zamboni's fixative (4% paraformaldehyde, 0.3% picric acid, and 0.1 M phosphate buffer) or 4% buffered paraformaldehyde. The following day the spinal cord and cortex were removed. The spinal cord was placed in 30% sucrose solution made with 0.1 M sodium phosphate buffer. Histological procedures to examine the spinal cord repair are described in Miya et al. (1997) and Kim et al. (1999). Briefly, consecutive 20-μm sections of the tissue were stained with either Nissl-myelin stain (Cyanine R followed by cresyl violet; Clark 1981) or antibodies to serotonin (5-hydroxytryptamine [5HT]). The tissue was examined by evaluators blind to surgical procedures or behavioral level achieved. The distribution of 5HT rostral and caudal to the lesion was evaluated by three independent examiners who were blind to the intervention procedures used and the level of function. We used tissue histology to assess repair effects, completeness of transection, and to detect the qualitative possibility of serotonergic bridging.
Cortical lesions were identified in 50-μm Nissl-myelin–stained coronal frozen or wax-embedded sections of the cortex and in several rats using a cryopolycut block-face imaging technique. In all cases included herein the lesions were well localized, extended to layer V, and were located appropriately rostrocaudally.
Histological status of surgery and transplants
After sacrifice, the completeness of the spinal cord lesion and the FG rat's neural bridging effects were examined in Nissl-stained parasagittal sections. All transections and data reported here were histologically confirmed as complete. Axon growth into lumbar cord due to transplant-mediated bridging and regeneration or development was examined with antibodies to 5HT. All significant 5HT axons found in lumbar cord derive from descending brain-stem pathways. Histology confirmed that some bridging function was provided for some descending (5HT) pathways by both TP and FG repairs as previously observed in TP rats (Giszter et al. 1998; Miya et al. 1997). As in all earlier work in such transplants (Giszter et al. 1998; Miya et al. 1997) it was impossible to directly correlate serotonin sprouting to functional level. Some NWS FG and TP rats could also show serotonergic fibers below the lesion. For this reason we remark here only that we observed qualitatively that some bridging was possible with both FG and TP interventions and do not pursue this further.
Statistics and group comparisons
Groups were compared using standard parametric statistics where feasible. However, because the achievement of weight support seems to have a bimodal distribution, based on percentage weight-supported stepping (Miya et al. 1997), we also compared distributions of weight support using nonparametric statistics. We classified the number of rats in each group into weight-supporting or nonweight-supporting and then compared their frequencies using Z-scores. Similarly, the numbers of rats with particular axial extent of representation present were compared with Z-scores. Kinematics were compared using t-tests of upper and lower ranges of motion, mean angles, and mean ranges. Numbers of roll events were quantified and compared statistically with pre- and postlesion t-tests. Linear regressions were used to relate roll event number and probability of roll per step and number to the percentages of weight-supported and nonweight-supported steps.
RESULTS
Our results fall into two main categories. First they relate to the importance of the trunk area of sensorimotor cortex in weight-bearing locomotion after neonatal spinalization. Second, they examine the possible need for cellular relays for rats with neonatal spinalizations to achieve trunk representation in intact rats' location in the caudal motor cortex.
We compared rats with cellular and noncellular grafts and neonatal spinalization with gelfoam alone. Comparisons were made in three ways: 1) The number of rats that achieved our behavioral criteria of autonomous weight support were compared between fibrin glue interventions [FGR (allograft), FGH (xenograft)], transect (TX), and transplant (TP) rats. This comparison helps define the neural mechanisms most important for improving the likelihood of the development of function. 2) The motor cortical organization of the rats in ICMS were compared among TX, TP, and FG interventions to see whether these affected cortical organization differently. 3) The levels of weight support of rats with autonomous hindlimb weight support as adults were assessed before and after lesions of the caudal (trunk) area of motor cortex. All rats were confirmed in histology to have received complete transections, with a few serotonergic fibers bridging the lesion site in some of the TP, FGR, and FGH repaired rats.
Behavioral recovery after differing interventions in the neonatal injuries
We first examine how locomotion of injured rats developed after neonatal spinal cord injury (SCI) in the different interventions. Comparing the cellular and acellular interventions allowed us to assess the importance of circuits engaging or using E14 grafted neurons and our earlier results. For assessments, we examined weekly bouts of 3 min of continuous treadmill locomotion in which animals were motivated by a water reward. In 3 min of treadmill stepping, on average our animals made between 60 and 150 steps in the test interval. We found that total steps executed in a 3-min period varied among groups: TX rats with low weight support (n = 8) made 57 ± 25.2 steps. Weight-supporting TX, WS transplant rats, and WS fibrin glue rats made similar numbers of mean steps: e.g., transplant (TP) rats (n = 19) made 92.7 ± 14.7 steps. Normal intact rats (n = 8) made about 104.7 ± 7.8 steps. The TX rats with low weight support both stepped significantly less and lost weight support more often when they stepped. However, we found the stride length of TX rats was no less than that of TP or normal rats (t-test, P > 0.1). The stepping of all rats was characterized by calculating the percentage of executed steps that were weight-supporting steps.
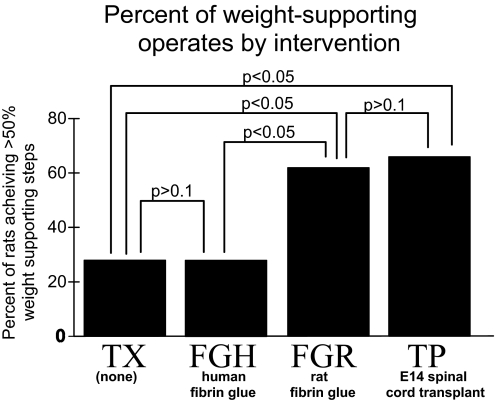
Rats were placed into one of two classes based on consistency of the percentage weight-support measure. Animals having <50% weight-supported hindlimb steps were placed in the nonweight-supporting (NWS) class and those with >50% weight-supported steps, in the weight-supporting (WS) class (see methods). Figure 1 shows the percentage of WS rats in each group.
FIG. 1.
Percentage of neonatal spinal transected rats achieving weight support (>50% of steps weight supported on the treadmill) after various interventions. Spinal transect (TX): no intervention (n = 8); TP: embryonic day 14 (E14) spinal transplants (n = 19); FGR: rat–derived fibrin glue (n = 8); FGH: Tisseel (human) fibrin glue (n = 32). Z-scores show TX and FGH are not different, TP and FGR are not different (P > 0.1, t-test), but TX and FGH each differ significantly from either TP or FGR in their weight support (P < 0.05, t-test).
We found that there were no statistical differences in the distributions of percentage weight-supported steps achieved between transplant rats reported previously (Giszter et al. 1998), those TP rats tested here, and the FGR rats in this study (P > 0.1, t-test; totals: FGR, n = 8; TP, n = 19). Although 68% of transplants had 50% weight-supported steps or better (13/19 WS in TP rats), 62% of FGR rats functioned at that level (5/8 WS in FGR rats). In contrast, in FGH rats (n = 32) that received Tisseel (human-derived) fibrin glue the frequency of WS rats resembled the frequency of WS rats among transect (TX) rats. The distributions of weight-supported steps showed no difference between FGH and TX (TX, n = 8; FGH, n = 32, P > 0.1). Both Miya et al. (1997) and Giszter et al. (1998) reported previously that the distributions of motor performance in the TX and TP groups differed significantly. This was also true here for both FGR rats and TX rats compared with TP rats using a Kruskal–Wallis or one-tailed Mann–Whitney U test (P < 0.05, for both comparisons). Thus the rat-derived fibrin allografts in FGR rats, which were acellular, were similar to fetal transplants in their degree of enabling of weight support. In contrast, acellular Tisseel fibrin glue (xenograft) in FGH rats was no more effective than gelfoam in promoting weight support. However, we found that despite this lack of difference, the lesser number of FGH rats that achieved weight support nonetheless resembled FGR and TP rats in their cortical organization and in this way differed from TX rats (see following text).
Cortical organization of FG-repaired rats without cellular transplants
We compared motor cortex organization in TX rats, rats with E14 fetal spinal cord transplants, and rats repaired with fibrin glue (FGR and FGH rats).
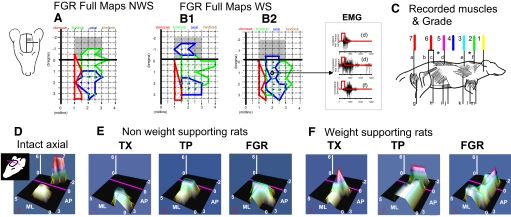
At 2–3 mo of age all our rats were anesthetized and the motor cortex was mapped using intracranial microstimulation. We used longer trains [300 ms, 50–100 μA, 0.1-ms biphasic pulses at 100 Hz, following Giszter et al. (1998)]. Our rationale was to allow the greatest opportunity for temporal facilitation of activity elicited by microstimulation at synapses along the several possible cascades of connections between cortex and motoneurons in lesioned rats. Caudal to the normal hindlimb/trunk area the cortex was always motorically silent, i.e., there were no motor responses to our microstimulation across the range of parameters used. Figure 2 shows detailed FGR rat maps.
FIG. 2.
Trunk representation in intracranial microstimulation (ICMS) maps of cortex is compared across function and intervention. Note the orientation of the maps indicated by the cartoon on the left. A, B1, and B2: cortical microstimulation examples from rats transected as neonates with FGR repairs, along with sample electromyograms (EMGs) from their mapping. A: in rats without weight support the shaded area was unresponsive to microstimulation (see Giszter et al. 1998). B1 and B2: rats with weight support all showed mid- to low-trunk motor representations (in blue regions) when mapped using 50-μA current pulses in 300-ms trains. In intact rats these representations would in general occur caudal to bregma (in the gray-shaded regions in A and B). Cortical representations similar to normal trunk representation developed without cellular elements of an E14 transplant. In fibrin glue repaired rats the mid- to low-trunk motor representations were similarly located (B1) or sometimes overlapping (B2) the low-trunk motor areas used in the normal intact rat. The midthoracic ipsilateral and contralateral latissimus dorsi and contralateral supraspinatus were activated at the site circled (see C for diagram of locations of these). C: to create microstimulation maps and assess trunk control at different segmental levels the following muscles were recorded: a, semitendinosus; b, iliopsoas; c, multifidus; d, longissimus; e, trapezius; f, supraspinatus; g, biceps femoris; h, external oblique; i, internal oblique; j, rectus abdominis; k, latissimus; l, triceps brachii; m, biceps brachii. Leg muscles (a, g) were never recruited in spinalized rats. Mid- to low-trunk muscles (c, d, and i, j, k; very rarely b) could cause observable pelvic motion either directly or through reflex and mechanical couplings. Trunk or hindleg segmental level found in ICMS maps was scored from 1 to 7 as shown: 1, upper cervical; 2, upper back; 3, upper shoulder/thorax; 4, midback; 5, mid to low back; 6, low back/lumbar; 7, legs. The scored values for trunk alone were represented in D, E, and F in the figure in 2 ways: they were used as the height parameter for the surface and a false color mesh was applied to the surface with color related to height. The values were interpolated across the ICMS map to construct a continuous surface in which height represents the segmental level score and thus the caudal extent of motor recruitment of trunk from each site in the map. In the false color mesh red represents low trunk (color assignments as shown). D: for the normal rat, hindlimb recruitment (level 7) is achieved in the caudal region of the map behind bregma (anteroposterior [AP] coordinate 0 and purple line in each map, gray-shaded region in A, B1, and B2). E: nonweight-supported rats are unresponsive to microstimulation in the area behind bregma and the purple line regardless of intervention. F: in spinalized rats the maximum height was always ≤6. Weight-supporting spinalized rats show peaks at level 5–6 but in TX rats these peaks are rostral to bregma, whereas in TP and FGR rats these are behind bregma in the normal intact rat's location.
The presence of a cortical hindlimb representation was not necessary for good weight-supported locomotion in TX, FGR, FGH, or TP operated rats. Despite good weight support, there was no indication of any hindlimb muscle representation in motor cortex in any TX, TP, FGR or FGH rats. This replicates earlier work in TX and TP rats (Giszter et al. 1998). We found that both FGR and FGH weight-supporting (WS) rats developed their low-trunk motor functions in the normal hindlimb/trunk area (see Fig. 2 for examples of detailed map). In this way they were similar to the WS TP rats (see Fig. 2, B1 and F). In all WS TP, FGR, and FGH rats this same area represented mid to low trunk. However, the frequency of representation of such caudal motor representation of the trunk differed in the groups of rats. The caudal motor representation developed in 6 of 8 FGR rats, but in only 6 of 32 FGH rats. These differing numbers paralleled the development of autonomous hindlimb weight support in these rats. Caudal trunk motor representations were matched with the achievement of autonomous weight support: i.e., those rats with weight support all possessed the caudal trunk motor representations. (A single exception was one FGR rat that had weight support marginally <50%, but possessed the caudal trunk representation.) In this way the FGH xenografted rats were similar to TX rats in the likelihood of their achieving weight support, but they differed significantly from the same TX rats because they developed motor representations of trunk in this caudal area of cortex, if they had autonomous weight support. In no TX rat did we ever observe this motor response or representation, regardless of the quality of its weight support, in keeping with Giszter et al. (1998).
We examined the segmental levels of trunk representation in ICMS maps of motor cortex in TX, TP, and FGR rats. We assigned hindlimb and trunk muscle representations grades (Grades: 1, neck; 2, rostral thoracic; 3, midthoracic; 4, low-thoracic; 5, thoracic-abdominal; 6, low abdominal-lumbar; 7, leg/tail; see Fig. 2C). The level of locomotor function in the awake behaving animal was strongly related to the maximum grade (i.e., the maximum caudal segmental extent in the trunk of the axial muscles represented). The maximum axial grade that was achieved in a map correlated closely with percentage weight support of the mapped rat (linear regression r2 = 0.75 for relation of axial score and weight-support score, P < 0.05).
No animal that lacked 40% weight support was able to recruit midthoracic musculature in the axial representations (Fig. 2E). FGR and FGH rats that developed caudal trunk motor representations had these representations in the same location as TP and normal rats and always exhibited good weight-supported locomotion in motor tests (Fig. 2, D and F). Similarly, WS FGH and FGR rats, without exception, had axial muscle representations from which midthoracic or more caudal muscles could be recruited (Fig. 3 C). As previously reported in TP rats (Giszter et al. 1998), we also observed that such low to midthoracic muscle activity routinely produced forces and motion at the pelvis either via mechanical coupling or via reflex coupling. However, the extent of the trunk representation extending caudal to bregma could vary in FG rats (e.g., see Fig. 2, B1 and B2, FGR WS rats).
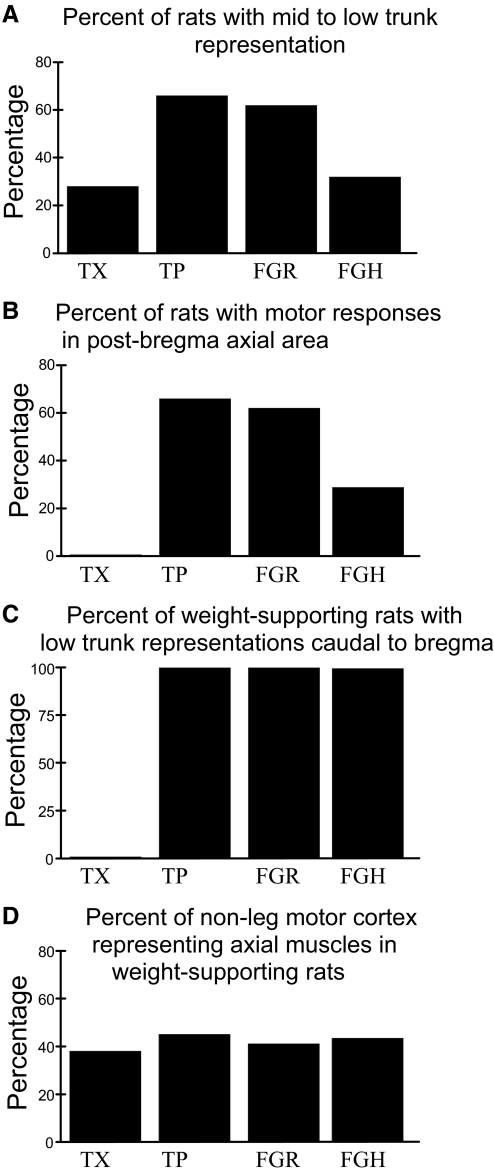
FIG. 3.
Organization of rats spinalized but achieving hindlimb weight support. A: percentage of rats with each treatment possessing specific mid- to low-trunk motor representation in cortex. The percentages match exactly those in Fig. 1. B: percentage of all rats with each treatment representing mid-to-low trunk behind bregma. C: percentage of weight-supporting rats representing mid-to-low trunk behind bregma. No TX rats possess motor representations in the normal hindlimb trunk area. All weight-supporting rats in TP, FGR, and FGH groups do. D: percentage of ICMS responsive cortical area devoted to trunk control was statistically indistinguishable in the treatment groups. This percentage of cortex also resembles the intact rat percentage of cortex after pure hindlimb areas of representation are removed from consideration (not shown).
We made a more detailed analysis of FGR and FGH cortical maps in relation to TP cortical maps to see whether we could find any significant differences. We assessed 1) the caudal extent in the trunk of the axial muscles recruited in FGR and FGH rats (Figs. 2 and 3A); 2) the percentage of all FGR and other rats possessing representations of axial muscles in the normal hindlimb/trunk area (Fig. 3B); 3) the percentage of the weight-supporting rats occurring in each group with representation of axial muscles in the normal hindlimb/trunk area (Fig. 3C); and 4) the area of motor cortex representing axial muscles was measured and expressed as a percentage of the total area of nonfacial motor responses (Fig. 3D). FGR, FGH, and TP rats with weight support did not differ significantly on any of these bases (t-test, P > 0.1, all comparisons). We found that the trunk representation was a significantly greater fraction of all nonfacial motor areas in all the spinalized rats compared with normal (each t-test, P < 0.05). However, this was primarily due to loss of the hindlimb representations in the operate rats and its subsumption for trunk representation. After we removed those sites that elicited exclusively hindlimb responses from consideration in the normal rats' data we found there were no statistically significant differences between percentages of sites dedicated to representation of trunk and forelimb between any of the normal and TX, TP, FGR, or FGH weight-supporting rats (each t-test, P > 0.1). The prominent qualitative and quantitative difference was that in TX rats the low trunk representation was “squeezed” in the more rostral motor areas, as previously reported in Giszter et al. (1998). There were no major representational differences between FG and TP rats.
Cortex role: lesion of the caudal hindlimb/trunk area abolishes or diminishes weight support in injured rats
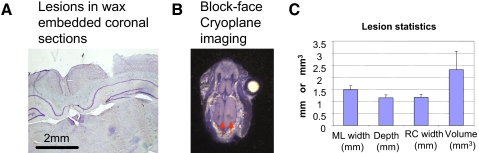
The importance of trunk cortex in locomotion functions developed after P1/P2 neonatal spinalization has thus far been unclear. Representation of mid to low trunk could simply be a correlated outcome of achievement of autonomous weight support. Alternatively, they could play a crucial role in maintaining autonomous weight support. To distinguish these possibilities we performed cortical lesions. Lesions were completed and confirmed in 16 WS class rats (i.e., with >50% weight-supported steps in the month after weaning). These rats comprised 6 TX rats, 6 FGH rats, and 4 TP rats. We also examined cortical lesions in 8 NWS rats and 4 intact control rats. In all rats we lesioned the normal hindlimb/trunk area (i.e., the area representing low trunk and hindlimb in normal rats). Injured rats could vary in their prelesion weight-support level, depending on their body weight. However, all 16 WS rats that were lesioned walked well in the first several months postweaning. In lesioned WS FGH and WS TP rats we first elicited mid- to low-trunk motor responses with ICMS during the lesion surgery. In the TX rats, as predicted, this area of cortex was unresponsive to ICMS during surgery for lesion. Lesions in TX rats were instead based on remaining map structure and stereotaxic locations. Lesions were bilateral and average tissue loss and cortical damage of slightly >2 mm3 on each side were seen in histology (Fig. 4). Lesions were confined to a region from 1 to 3.4 mm caudal to bregma for the data reported. The depth of cell loss extended to lamina V, usually lamina VI, and in one instance deeper into axonal regions (Fig. 4). Following a week of recovery, the lesioned rats were again trained and tested for ≥2 mo. NWS and intact controls were unaffected by the lesions in our testing.
FIG. 4.
Cortical lesion histology. Nissl-myelin–stained sections were used for reconstruction of cortical lesions in one of two ways. A: lesions were reconstructed by coronal sections after wax embedding. B: lesions were also reconstructed by horizontal frozen sections using block-face imaging in situ (right, arrows in a bilaterally lesioned rat). C: statistics of lesions sizes from a sampling of 8 of the lesioned rats. Vertical bars: SEs.
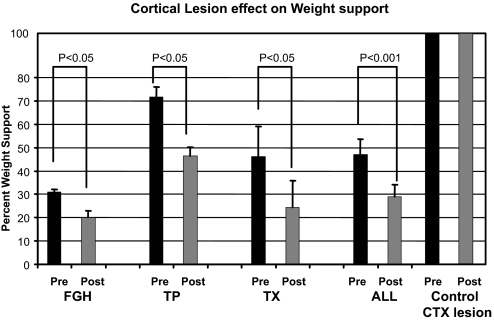
After the focal lesions, all WS spinalized rats except one showed large deficits in percentage weight support compared with their prelesion weight-support values. Each group (FGH, TP, TX) of rats showed significant decrements in percentage weight support (P < 0.05, paired t-test, Fig. 5) as did the combined group of all WS spinalized rats (P < 0.001, paired t-test, Fig. 5). WS rats on average diminished in their independent weight-support score after the lesion by about 40%. TP, TX, and FGH rats did not differ significantly among one another in lesion effect (P > 0.1, t-test, and ANOVA). It was initially surprising to us that the TX WS rats were as affected by the lesion as the other WS rats (P < 0.05, paired t-test). However, we believe the lesioned “trunk” cortex in TX WS rats is likely to have sensory representations and integrative functions since it is a sensorimotor overlap area (see discussion). Normal rats (n = 4) with similar lesions in the trunk area showed absolutely no treadmill deficits, all remaining at their prelesion levels of 100% WS. The universality of the importance of this area of cortex in recovered function in all our spinalized WS rats was a clear outcome.
FIG. 5.
Significant loss of weight support occurred in all groups of weight-supporting rats as a result of localized lesions regardless of explicit motor representation in ICMS. Lesions were made in the cortical area in which the low-trunk hindlimb motor representation resides in normal rats. Losses were significant in each group (P < 0.05, t-test) and in the pooled data (P < 0.001, t-test). Control rats with similar lesions showed no deficits. Mean fractional decrements from the spinal cord injury (SCI) data after lesion of caudal trunk cortex varied from 0.32 to 0.52 and were not significantly different among the treatments. The ensemble average for SCI rats was a 42% decrease. SEs are shown for each bar graph. All SCI rats required intact trunk cortex to generate autonomous weight-supporting locomotion. Lesioned control rats all showed 0% change.
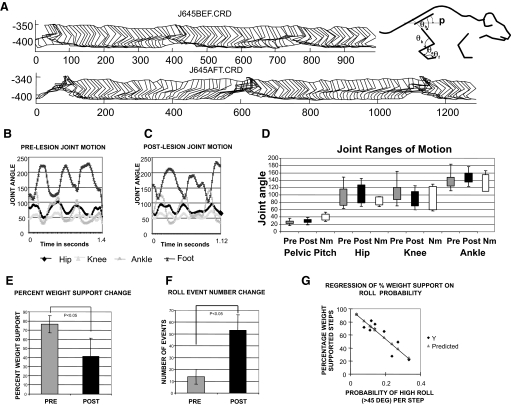
We next examined detailed kinematics pre- and postlesion in eight of the spinalized rats (four TP and four TX). We tested hindlimb stepping, pelvic roll events where roll exceeded 45° off the sagittal plane, and joint angle kinematics in the parasagittal plane in 3-min periods. Measures were compared before and after the lesions to check for alteration of stepping kinematics that were likely mostly lumbar pattern generator based (Fig. 6). Rats showed no obvious quantitative changes in their hindlimb step kinematics. We examined pelvis, hip, knee, ankle, and paw parasagittal kinematics and hip, knee, and ankle angles. Neonatal spinalized rats during autonomous weight support show less systematic kinematics than that observed during air stepping or during spinal rats' and cats' bipedal stepping (e.g., see Murray et al. 2004) so our analysis did not focus on joint phase plots. However, none of the joint angle or kinematic parameters we compared was significantly different in mean, range, or variance pre- and postlesion (P > 0.2, n = 8; and see Fig. 6, B–D). Ranges were also roughly comparable to those of our normal intact rats on the treadmill and to published data for fast walking in intact rats (Thota et al. 2005). Ankle heights during swing were slightly higher in several rats (P < 0.2), perhaps due to increased pelvic roll, and swing was often shorter and more abbreviated, again likely due to increased roll. However, these were not statistically significant effects (P > 0.1).
FIG. 6.
Kinematics of hindlimbs and pelvis in rats before and after cortical lesions. A: parasagittal stick figure motion was digitized for multiple step cycles before and after lesion. An example of data from one rat is shown. The measured internal angles are displayed to the right. Prelesion (B) and postlesion joint angles (C). Pelvic pitch orientation and joint angles of hip, knee, ankle, and foot were measured from the captured stick figures pre- and postlesion. D: range of motion for each angle's time series were compared pre/post for 8 rats and for normal rats. Ranges for data from B/C are shown, together with normal intact rat ranges measured similarly. The maximum and minimum joint angles and their SDs in the group of 8 rats tested in detail for pre- and postlesion data were compared. In the 8 rats tested (4 TP and 4 TX), statistical comparisons of the kinematic features measured in the parasagittal plane were not significantly different. Neither the ranges of motion, the basic pattern of coordination among joints, nor the period of the hindlimb stepping was significantly altered by the cortical lesions (n = 8, P > 0.1). E: after lesions, the group percentage weight support decreased significantly (paired t-test, P < 0.05). F: after lesion there were increased numbers of pelvic roll events where roll clearly exceeded 45° (paired t-test, P < 0.05). The probability of 45° roll for each step was calculated from these data and more than doubled postlesion (paired t-test, P < 0.05). The number of nonweight-supporting steps in rats was also linearly related to the number of roll events (r2 = 0.83; slope coefficient ∼2; and significance P < 0.0005). G: the percentage of weight-supported steps in rats was negatively correlated to the probability of roll per step (r2 = 0.81, slope coefficient significance P < 0.0001). Thus hindlimb kinematics were not altered significantly, but pelvic roll was increased and related to quality of weight support.
Despite the very similar within-limb step joint angle kinematics and ranges of motion after the lesion, our measures showed that the frequency of high-roll (i.e., >45 degree) events in the haunches increased substantially. The prelesion group of eight rats showed an average of 14 high-roll events per 3-min analysis epoch. Postlesion this number increased to 35 events per epoch (Fig. 6F). These changes in roll were statistically significant (t-test, P < 0.005). The likelihood of high roll in a step cycle increased. A prelesion probability per step of high roll of 0.1 increased to a postlesion probability per step of 0.25 (statistically significant, t-test, P < 0.005). The roll event probability in a recording epoch correlated negatively with the percentage weight-support measure in that epoch (regression r2 = 0.81, regression intercept 98.8% at 0 probability of events, slope coefficient of −221, both coefficients highly significant, P < 0.0001, Fig. 6G), and probability of roll correlated positively with the number of nonweight-supporting step cycles (regression r2 = 0.83, slope 2, coefficient P < 0.0005). On average it took rats two or more step cycles to recover from a roll-induced stumble.
The increased frequency of high roll of the pelvis coupled with similar step cycle kinematic organization in the hindlimbs suggest strongly that the cortical lesions did not directly disrupt the control of limbs or directly alter the developed pattern generator function. Rather, the hindlimb/trunk cortex lesions disrupted aspects of control of roll, pelvic balance, and the integration of forelimb and hindlimb mechanics. Presumably, lesions acted by degrading voluntary and precise control of the trunk musculature.
In summary, the caudal-most segmental level of muscles recruited in the trunk representation correlated well with weight support in all rats; lesion of the caudal region of cortex (which in intact rats represents trunk and hindlimbs) seriously compromised all spinal transected rats' weight support. The data support an important use of trunk cortex in locomotion after complete neonatal spinalization (compared with a very small locomotor contribution of trunk cortex in intact rats) and show intercalated novel neural relays derived from transplant cells are not essential for the cortical or functional improvements observed with such interventions.
DISCUSSION
Many aspects of quadrupedal locomotion are automatic and largely use mechanisms embedded in the spinal cord (Barbeau and Rossignol 1987; Belanger et al. 1996; Edgerton et al. 1992). The hindlimbs in rats are often considered to receive only limited cortical control (Hicks and Damato 1977; Muir and Whishaw 1999). After selective CST lesions in normal adult rats, locomotion on treadmills and most overground locomotion are unaffected. Deficits are observed only in pedestal- or ladder-stepping tasks that require high precision (Hicks and Damato 1977), consistent with a role in fractionated responses and motor skill acquisition. After spinalization, many of the alterations that support hindlimb stepping and load bearing in the hindlimbs are localized to the pattern generators and hindlimb musculature that are isolated from the brain after the lesion (e.g., Petruska et al. 2007). However, recovery from injury and compensation can be thought of as a problem in novel skill development. Consistent with this, motor cortex plays a clear role in recovery from trauma and reorganizes after injuries of various kinds, including immobilization, limb amputation, stroke, deafferentation, and SCI (e.g., see Dancause et al. 2006; Donoghue and Sanes 1987, 1988; Emerick et al. 2003; Kim et al. 2006; Nudo 2006). Cortex plays roles both in voluntary skill development and in spinal reflex plasticity (see Chen et al. 2006; Wolpaw 2006). The balance and cooperation of these voluntary and automatic mechanisms in recovery of function constitute an important issue in SCI and may become significant in developing therapies in the future. Our data here test the importance of trunk cortex in functional locomotion developed after P1/P2 spinalization of rats.
The role of trunk/hindlimb sensorimotor cortex in development of autonomous weight support after neonatal spinal transection
Our results show that the hindlimb/trunk region of sensorimotor cortex plays a crucial role in weight support in all rats transected as neonates. Lesions of this area reduced by almost half the independent weight support achieved in most rats tested. Intact rats showed no treadmill deficits with similar lesions, replicating published data.
This result is at first surprising: our rats were thoracic transected and cortical control was absent in hindlimbs and limited in the trunk muscles (e.g., see Giszter et al. 1998). However, some trunk muscles physically span the lesioned segments and they may have distributed motor pools spanning the lesion. It is documented that trunk muscles may be coordinated across a lesion by reflex chaining. For example, emetic and other trunk responses remain coordinated and effective in thoracic spinalized cats (e.g., see Iscoe 1998). Cortical systems probably do not begin to contribute to locomotion until about P14–P21 when their representations and roles in movement mature (Gramsbergen 1998; Vinay et al. 2002; Westerga and Gramsbergen 1990, 1993). Cortical motor control of trunk may thus in several ways provide a means of interacting with autonomous lumbar stepping. Cortical integration of trunk-related information and development of highly skilled trunk use may partly compensate for the loss of the normal communication pathways following the neonatal transection. Through trunk controls the cortex might potentially help coordinate forelimb–hindlimb mechanical transmissions and shape the mechanical environment provided by trunk in which lumbar stepping occurs. Such mechanical shaping is known to play a role in pattern generator function after SCI (Barbeau and Rossignol 1987; Deleon et al. 2002; Edgerton et al. 1992).
In adults, cortex involvement in adapting locomotion is also likely to be large. Cortex can play an essential role in down-conditioning in adult rats and thus setting the balance and strength of reflex gains in rats (Chen et al. 2006b). Functions involving the operations of trunk cortex appeared crucial for the full expression of weight support in our spinalized rats in adulthood. Trunk cortex contributed to function regardless of the presence of explicit motor representations: some spinalized rats with no intervention showed good weight support without an explicit motor representation in this sensorimotor area, but they were equally affected by its lesion. The lack of any explicit motor response in ICMS in this area of the cortex in the TX rats had led us at the outset to discount the role of the area in function in these rats. However, the lesioned area is an area of sensorimotor overlap cortex containing both motor and sensory representations. Presumably, important sensory and sensorimotor integration mechanisms in this area play roles in operate weight support. All neonatal injured WS rats appeared to have a strong reliance on the functions of this area of cortex to achieve independent weight support. Normal rats (n = 4) with similar lesions in the area showed absolutely no treadmill deficits, all remaining at their prelesion levels of 100% WS. The universality of the importance of cortex in recovered function in all our spinalized WS rats could be significant for understanding therapies tested in adult spinalized rats. The lesioned region of cortex may provide important integrative sensorimotor functions in the P1/P2 transected rats.
Our kinematic data are consistent with the idea that the cortical lesions disrupted trunk mechanical integration without directly affecting lumbar pattern generation or hindlimb kinematics. There were no major hindlimb kinematic changes or deficits after lesions. However, the rats' pelvises showed increased roll after the lesions. There were higher frequencies of balance problems with the haunches that could be associated with roll. Taken together, these data suggest that the lesions did not significantly alter lumbar limb pattern generators per se, but rather disrupted trunk integration of the lumbar stepping and pattern generation into whole body locomotion (see Giszter et al. 2008).
Conceivably, the cortex-dependent skills developed in neonates might also be achievable after rats' adult spinalization, using intrinsic plasticity (e.g., see Bareyre et al. 2004). Such skill development could be a fundamental component needed for adult recovery as in the neonatal spinalized rats. However, caution is needed. Both cortical and spinal differences are expected following spinal transections as neonates compared with adults. Differing patterns of cortical cell loss may occur (e.g., Hains et al. 2003). Cortical representations and the organization developed in the context of P1/P2 SCI might differ strongly from adult injured cortical organization (Chakrabarty and Martin 2005; Friel and Martin 2005; Friel et al. 2007). The pattern of corticospinal system projections in spinal cord probably differs after neonatal injury (Martin 2005; Martin et al. 2004). Further, the state and capabilities of lumbar pattern generators following adult injury might differ in their suitability for the strategies used by neonatal spinalized rats. For example, hindlimb stepping in rats and cats spinalized as adults requires tail-pinch or epidural stimulation (e.g., Gerasimenko et al. 2006) and motoneuron properties may differ from those of neonates (Petruska et al. 2007). Various compensations and alterations in descending systems can cause alterations in the spinal cord even in adults (Rossignol et al. 1999; Wolpaw 2006) and spinal pattern generators play an important role in recovery from partial lesions (Barrière et al. 2008). Nonetheless, the possibility of training cortex in adults to help replicate the weight-supporting functions achieved by P1/P2 neonates is intriguing, using strategies such as rehabilitation robotics and BMI training (see Chapin et al. 1999; also see Giszter et al. 2005; Udoekwere et al. 2006).
Cortical representations and functionally important physiological mechanisms enabled by transplant interventions
Our data offer clues to effects of neonatal transplants. E14 spinal tissue provides a spatially structured and partly differentiated graft. Neural and glial precursors, progenitors, and perhaps stem cells may be available. Limited synaptic connections of E14 spinal grafts with host central neurons and afferents are possible (Houle et al. 1996; Itoh et al. 1998), potentially forming relays, along with bridging of host axons. However, the specific mechanisms important in supporting the improved recovery following transplants are largely unknown.
Cellular transplants can promote function in various ways after complete or incomplete SCI (Bregman 1987; Bregman and Reier 1986; Lepore et al. 2006; Liu et al. 2002; Murray et al. 2002). Five classes of mechanism have roles in recovery after transplantation (e.g., see Bregman 1987): bridge, relay, rescue, increasing intrinsic plasticity, and supply of neuromodulators and trophins (Orsal et al. 2002). In bridging, host axons cross the lesion (Bernstein-Goral and Bregman 1993; Bregman 1987). In relay mechanisms, transplant neurons are intercalated in host circuits spanning the lesion (e.g., Itoh et al. 1996). In rescue mechanisms, neuronal circuits are more likely to survive in the presence of a transplant (Bregman and Reier 1986; Mori et al. 1997). In plasticity mechanisms, transplants promote formation of plasticity, novel terminals, and dendritic sprouting. Finally, transplant neurons can supply neuromodulator or trophins, substituting for lesioned descending sources (Orsal et al. 2002; Ribotta et al. 2000).
E14 transplants can be shown to alter cortical motor representation in lesioned animals with independent weight support (Giszter et al. 1998). Our data support a significant role of these cortical areas and of cortical trunk motor representations. We tested whether transplant-derived relays played important roles in the developed cortical organization. Fibrin glues, lacking graft cells, help identify the most crucial contributions organizing neonatal rat cortex and weight-support recovery. Fibrin glue can in some instances allow bridging (Iwazawa et al. 1999).
We found fibrin glue–repaired rats exhibited both good weight support and the cortical trunk motor representations characteristic of E14 recipient rats. Using long stimulation trains of 300 ms gave us the best possible opportunity to observe any differential effects that could be due to cellular relays, including any synergy with ventilation, although none was detectable. In all regards tested, fibrin glue rescued motor representations of axial musculature in the caudal areas of normal hindlimb/trunk cortex in a fashion similar to E14 spinal cord transplants, although this happened with lower probability in the xenograft FGH rats. The caudal trunk motor areas give rise to earlier developing fibers of the CST, which could be at or close to the transection site at the time of the surgery (Schreyer and Jones 1982, 1988). No neural or other cells were introduced in the FGR and FGH rats. Thus these rats had no possibility of novel neural relays using graft neurons. It thus appears that novel relays involving transplant cells are not necessary for the cortical organizations and weight-support recovery observed in FG and TP rats. Given the similar high likelihood of function and similar cortical organizations in both FGR and TP rats, it seems likely that any novel relay mechanisms must primarily play other roles in the TP rats.
The presence of relays using novel neural elements are thus not likely strictly required for the cortical representations in E14 transplant rats. Of course, both relay and modulation supported by the cells of the E14 transplant may have other roles in the recovery, not tested here. Rat-derived fibrin glue was about as effective as E14 spinal cord and both were significantly better than human-derived fibrin glue, presumably because of better compatibility with the tissue and perhaps reduced immunological responses over time. The data here are consistent with forms of plasticity following more limited lesions in P1/P2 rats shown in Z'Graggen et al. (2000). Our data suggest that possible bridging of host fibers, rescue of host tissues around the lesion, and plasticity in cortex and spinal cord may be the most significant processes in the P1/P2 rats' development of locomotor function. These mechanisms are consistent both with support of the cortical roles we found and with the patterns of recovery in both cellular and acellular grafting described here. The results suggest various bridging interventions using host tissues (e.g., Campos et al. 2004) might be very functionally effective in thoracic transected rats.
Representation and roles of trunk/hindlimb motor cortex in recovery
Specific motor cortex representations (of mid- to low-trunk muscles) correlated 1:1 with autonomous weight support after neonatal spinalization. Low-trunk cortical motor representations in caudal motor cortex were observed only in rats that achieved independent weight support following E14 or fibrin glue repairs. However, our lesions also demonstrated this same region was important in spinalized rats receiving gelfoam. These rats did not develop an overt motor response in this area, but instead in more frontal regions of cortex. Roles in recovery of both the explicit motor responses revealed by ICMS and/or a more covert cortical motor integration were indicated by the substantial lesion effects we found in weight-supporting spinalized rats. Sensory representations are also found in this area of cortex and these have early critical periods (Jain et al. 2003). These representations may have important roles in the locomotion developed and lesion effects observed. It is also likely that specific combinations of trunk muscles in the representations developed in cortical sensorimotor representations after neonatal injury are significant in allowing weight support, but these are not well understood at this time. It would be of interest to lesion the trunk hindlimb sensorimotor amalgam at P1/P2 at the same time as the spinalization to discover whether weight support is achieved in such rats and if other regions of cortex could substitute representations.
Conclusions
In conclusion, the trunk region of cortex in rats plays a crucial role in weight support in neonatal spinalized rats that achieve good locomotion as adults. Cortical mechanisms may be substituting for lost controls at lower levels of CNS. The development of overt motor function in the caudal trunk cortex found in some neonatal spinalized rats appears to rely primarily on bridging or plasticity of host circuits. Novel intercalated circuits from fetal spinal grafts are not crucial. A natural prediction of our study for future work is that in adult animals that are spinal transected at thoracic levels it is likely that engagement of cortical mechanisms and appropriate trunk rehabilitation will play a significant role in advancing recovery of function.
GRANTS
This work was supported by National Institute of Neurological Disorders and Stroke Grants NS-24707 and NS-44564 and the Allegheny Singer Research Institute.
TABLE 1.
Training and testing regime
| Postnatal Days 1 and 2 | Postnatal Days 21–28 | 60 Days of Training and Testing on Treadmill | Postnatal Days 90–105 | 7 Days of Surgical Recovery | 60–120 Days of Training and Testing on Treadmill |
|---|---|---|---|---|---|
| Thoracic spinal transection + treatment | Weaning based on a pup achieving weight of 40 g | 15 min per day; 3 days per wk on treadmill | Cortical map and/or cortical lesion | Rest | 15 min per day; 3 days per wk on treadmill |
Acknowledgments
We thank G. Hockensmith, B. Kilby, C. Agnew, J. Young, J. Scabich, T. Connors; Drs. Tim Himes, Motohide Shibayama, and Nao Kuwahara for assistance in different technical aspects of this study; Dr. Jonathan Nissanov and laboratory staff for assistance with cryopolycut assessment of cortical lesions; M. Lemay and M. Murray for readings of manuscript drafts; and M. Murray, I. Fischer, and the Spinal Cord Group for invaluable and unflagging support.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- Barbeau and Rossignol 1987.Barbeau H, Rossignol S. Recovery of locomotion after chronic spinalization in the adult cat. Brain Res 412: 84–95, 1987. [DOI] [PubMed] [Google Scholar]
- Bareyre et al. 2004.Bareyre FM, Kerschensteiner M, Raineteau O, Mettenleiter TC, Weinmann O, Schwab ME. The injured spinal cord spontaneously forms a new intraspinal circuit in adult rats. Nat Neurosci 7: 269–277, 2004. [DOI] [PubMed] [Google Scholar]
- Barrière et al. 2008.Barrière G, Leblond H, Provencher J, Rossignol S. Prominent role of the spinal central pattern generator in the recovery of locomotion after partial spinal cord injuries. J Neurosci 28: 3976–3987, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basso et al. 1995.Basso DM, Beattie MS, Bresnehan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma 12: 1–21, 1995. [DOI] [PubMed] [Google Scholar]
- Bates and Stelzner 1993.Bates CA, Stelzner DJ. Extension and regeneration of corticospinal axons after early spinal injury and the maintenance of corticospinal topography. Exp Neurol 123: 106–117, 1993. [DOI] [PubMed] [Google Scholar]
- Belanger et al. 1996.Belanger M, Drew T, Provencher J, Rossignol S. A comparison of treadmill locomotion in adult cats before and after spinal transection. J Neurophysiol 76: 471–491, 1996. [DOI] [PubMed] [Google Scholar]
- Bernstein-Goral and Bregman 1993.Bernstein-Goral H, Bregman BS. Spinal cord transplants support the regeneration of axotomized neurons after spinal cord lesions at birth: a quantitative double-labeling study. Exp Neurol 123: 118–132, 1993. [DOI] [PubMed] [Google Scholar]
- Bregman 1987.Bregman BS Spinal cord transplants permit the growth of serotonergic axons across the site of neonatal spinal cord transection. Brain Res 431: 265–279, 1987. [DOI] [PubMed] [Google Scholar]
- Bregman and Reier 1986.Bregman BS, Reier PJ. Neural tissue fetal transplants rescue axotomized rubrospinal cells from retrograde death. J Comp Neurol 244: 86–95, 1986. [DOI] [PubMed] [Google Scholar]
- Bretzner and Drew 2005a.Bretzner F, Drew T. Contribution of the motor cortex to the structure and the timing of hindlimb locomotion in the cat: a microstimulation study. J Neurophysiol 94: 657–672, 2005a. [DOI] [PubMed] [Google Scholar]
- Bretzner and Drew 2005b.Bretzner F, Drew T. Changes in corticospinal efficacy contribute to the locomotor plasticity observed after unilateral cutaneous denervation of the hindpaw in the cat. J Neurophysiol 94: 2911–2927, 2005b. [DOI] [PubMed] [Google Scholar]
- Campos et al. 2004.Campos L, Meng Z, Hu G, Chiu DT, Ambron RT, Martin JH. Engineering novel spinal circuits to promote recovery after spinal injury. J Neurosci 24: 2090–2101, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarty and Martin 2005.Chakrabarty S, Martin JH. Motor but not sensory representation in motor cortex depends on postsynaptic activity during development and in maturity. J Neurophysiol 94: 3192–3198, 2005. [DOI] [PubMed] [Google Scholar]
- Chapin et al. 1999.Chapin JK, Moxon KA, Marlowitz RS, Nicolelis MAL. Realtime control of a robot arm using simultaneously recorded neurons in the motor cortex. Nat Neurosci 2: 664–670, 1999. [DOI] [PubMed] [Google Scholar]
- Chen et al. 2006.Chen XY, Chen Y, Chen L, Tennissen AM, Wolpaw JR. Corticospinal tract transection permanently abolishes H-reflex down-conditioning in rats. J Neurotrauma 23: 1705–1712, 2006. [DOI] [PubMed] [Google Scholar]
- Chen et al. 2006.Chen Y, Chen XY, Jakeman LB, Chen L, Stokes BT, Wolpaw JR. Operant conditioning of H-reflex can correct a locomotor abnormality after spinal cord injury in rats. J Neurosci 26: 12537–12543, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dancause et al. 2006.Dancause N, Barbay S, Frost SB, Zoubina EV, Plautz EJ, Mahnken JD, Nudo RJ. Effects of small ischemic lesions in the primary motor cortex on neurophysiological organization in ventral premotor cortex. J Neurophysiol 96: 3506–3511, 2006. [DOI] [PubMed] [Google Scholar]
- DeLeon et al. 2002.DeLeon RD, Kubasak MD, Phelps PE, Timoszyk WK, Reinkensmeyer DJ, Roy RR, Edgerton VR. Using robotics to teach the spinal cord to walk. Brain Res Rev 40: 267–273, 2002. [DOI] [PubMed] [Google Scholar]
- Donoghue and Sanes 1987.Donoghue JP, Sanes JN. Peripheral nerve injury in developing rats reorganizes representation pattern in motor cortex. Proc Natl Acad Sci USA 84: 1123–1126, 1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donoghue and Sanes 1988.Donoghue JP, Sanes JN. Organization of adult motor cortex representation patterns following neonatal forelimb nerve injury in rats. J Neurosci 8: 3221–3232, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgerton et al. 1992.Edgerton VR, Roy RR, Hodgson JA, Prober RJ, De Guzman CP, de Leon R. Potential of adult mammalian lumbosacral spinal cord to execute and acquire improved locomotion in the absence of supraspinal input. J Neurotrauma 9: 110–127, 1992. [PubMed] [Google Scholar]
- Emerick et al. 2003.Emerick AJ, Neafsey EJ, Schwab ME, Kartje GL. Functional reorganization of the motor cortex in adult rats after cortical lesion and treatment with monoclonal antibody IN-1. J Neurosci 23: 4826–4830, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friel et al. 2007.Friel KM, Drew T, Martin JH. Differential activity-dependent development of corticospinal control of movement and final limb position during visually guided locomotion. J Neurophysiol 97: 3396–3406, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friel and Martin 2005.Friel KM, Martin JH. Role of sensory-motor cortex activity in postnatal development of corticospinal axon terminals in the cat. J Comp Neurol 485: 43–56, 2005. [DOI] [PubMed] [Google Scholar]
- Gerasimenko et al. 2006.Gerasimenko YP, Lavrov IA, Courtine G, Ichiyama RM, Dy CJ, Zhong H, Roy RR, Edgerton VR. Spinal cord reflexes induced by epidural spinal cord stimulation in normal awake rats. J Neurosci Methods 157: 253–263, 2006. [DOI] [PubMed] [Google Scholar]
- Giszter et al. 2007.Giszter SF, Davies MR, Graziani V. Motor strategies used by rats spinalized at birth to maintain stance in response to imposed perturbations. J Neurophysiol 97: 2663–2675, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giszter et al. 2005.Giszter SF, Davies MR, Graziani V. Coordination strategies for limb forces during weight-bearing locomotion in normal rats, and in rats spinalized as neonates. Exp Brain Res In press. [DOI] [PMC free article] [PubMed]
- Giszter et al. 2005.Giszter SF, Hart CB, Udoekwere UI, Markin S, Barbe C. A real-time system for small animal neurorobotics at spinal or cortical levels. Proc 2nd Int Conf IEEE/EMBS on Neuroengineering, Washington, DC, 2005.
- Giszter et al. 1998.Giszter SF, Kargo WJ, Davies MR, Shibayama M. Fetal transplants rescue axial muscle representations in M1 cortex of neonatally transected rats that develop weight support. J Neurophysiol 80: 3021–3030, 1998. [DOI] [PubMed] [Google Scholar]
- Gramsbergen 1998.Gramsbergen A Posture and locomotion in the rat: independent or interdependent development? Neurosci Biobehav Rev 22: 547–553, 1998. [PubMed] [Google Scholar]
- Hains et al. 2003.Hains BC, Black JA, Waxman SG. Primary cortical motor neurons undergo apoptosis after axotomizing spinal cord injury. J Comp Neurol 462: 328–341, 2003. [DOI] [PubMed] [Google Scholar]
- Hall and Lindholm 1974.Hall RD, Lindholm EP. Organization of motor and sensory neocortex in the albino rat. Brain Res 66: 23–28, 1974. [Google Scholar]
- Hicks and D'Amato 1977.Hicks SP, D'Amato CJ. Motor-sensory cortex-corticospinal system and developing locomotion and placing in rats. Am J Anat 143: 1–42, 1977. [DOI] [PubMed] [Google Scholar]
- Houle et al. 1996.Houle JD, Skinner RD, Garcia-Rill E, Turner KL. Synaptic evoked potentials from regenerating dorsal root axons within fetal spinal cord tissue transplants. Exp Neurol 139: 278–290, 1996. [DOI] [PubMed] [Google Scholar]
- Hummelsheim and Wiesendanger 1986.Hummelsheim H, Wiesendanger M. Is the hind-limb representation of the rat's cortex a sensorimotor amalgam? Brain Res 346: 75–81, 1986. [DOI] [PubMed] [Google Scholar]
- Iscoe 1998.Iscoe S Control of abdominal muscles. Prog Neurobiol 56: 433–506, 1998. [DOI] [PubMed] [Google Scholar]
- Itoh et al. 1998.Itoh Y, Waldeck RF, Tessler A, Pinter MJ. Regenerated dorsal root fibers form functional synapses in embryonic spinal cord fetal transplants. J Neurophysiol 76: 1236–1245, 1998. [DOI] [PubMed] [Google Scholar]
- Iwaya et al. 1999.Iwaya K, Mizoi K, Tessler A, Itoh Y. Neurotrophic agents in fibrin glue mediate adult dorsal root regeneration into spinal cord. Neurosurgery 44: 589–595, 1999. [DOI] [PubMed] [Google Scholar]
- Jain et al. 2003.Jain N, Diener PS, Coq JO, Kaas JH. Patterned activity via spinal dorsal quadrant inputs is necessary for the formation of organized somatosensory maps. J Neurosci 23: 10321–10330, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim et al. 2006.Kim BG, Dai HN, McAtee M, Vicini S, Bregman BS. Remodeling of synaptic structures in the motor cortex following spinal cord injury. Exp Neurol 198: 401–415, 2006. [DOI] [PubMed] [Google Scholar]
- Kim et al. 1999.Kim D, Adipudi VJ, Giszter SF, Shibayama M, Tessler A, Murray M, Simansky KJ. Direct agonists for serotonin (5HT2) receptors enhance locomotor function in rats that received neural fetal transplants after neonatal spinal transection. J Neurosci 19: 6213–6224, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavrov et al. 2006.Lavrov I, Gerasimenko YP, Ichiyama RM, Courtine G, Zhong H, Roy RR, Edgerton VR. Plasticity of spinal cord reflexes after a complete transection in adult rats: relationship to stepping ability. J Neurophysiol 96: 1699–1710, 2006. [DOI] [PubMed] [Google Scholar]
- Lepore et al. 2006.Lepore AC, Neuhuber B, Connors TM, Han SS, Liu Y, Daniels MP, Rao MS, Fischer I. Long-term fate of neural precursor cells following transplantation into developing and adult CNS. Neuroscience 142: 287–304, 2006. [DOI] [PubMed] [Google Scholar]
- Liu et al. 2002.Liu Y, Himes BT, Murray M, Tessler A, Fischer I. Grafts of BDNF-producing fibroblasts rescue axotomized rubrospinal neurons and prevent their atrophy. Exp Neurol 178: 150–164, 2002. [DOI] [PubMed] [Google Scholar]
- Martin 2005.Martin JH The corticospinal system: from development to motor control. Neuroscientist 11: 161–173, 2005. [DOI] [PubMed] [Google Scholar]
- Martin et al. 2004.Martin JH, Choy M, Pullman S, Meng Z. Corticospinal system development depends on motor experience. J Neurosci 24: 2122–2132, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miya et al. 1997.Miya D, Giszter S, Mori F, Adipudi V, Tessler A, Murray M. Fetal transplants alter the development of function after spinal cord transection in newborn rats. J Neurosci 17: 4856–4872, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori et al. 1997.Mori F, Himes BT, Kuwada M, Murray M, Tessler A. Fetal transplants rescue some axotomized rubrospinal neurons from retrograde cell death in adult rats. Exp Neurol 143: 45–60, 1997. [DOI] [PubMed] [Google Scholar]
- Muir and Webb 2000.Muir GD, Webb AA. Assessment of behavioral recovery following spinal cord injury in rats. Eur J Neurosci 12: 3079–3086, 2000. [DOI] [PubMed] [Google Scholar]
- Muir and Whishaw 1999.Muir GD, Whishaw IQ. Complete locomotor recovery following corticospinal tract lesions: measurement of ground reaction forces during overground locomotion in rats. Behav Brain Res 103: 45–53, 1999. [DOI] [PubMed] [Google Scholar]
- Murray et al. 2004.Murray M, Fischer I, Smeraski C, Tessler A, Giszter S. Towards a definition of recovery of function. J Neurotrauma 21: 405–413, 2004. [DOI] [PubMed] [Google Scholar]
- Murray et al. 2002.Murray M, Kim D, Liu Y, Tobias C, Tessler A, Fischer I. Transplantation of genetically modified cells contributes to repair and recovery from spinal injury. Brain Res Brain Res Rev 40: 292–300, 2002. [DOI] [PubMed] [Google Scholar]
- Nudo 2006.Nudo RJ Mechanisms for recovery of motor function following cortical damage. Curr Opin Neurobiol 16: 638–644, 2006. [DOI] [PubMed] [Google Scholar]
- Orsal et al. 2002.Orsal D, Barthe JY, Antri M, Feraboli-Lohnherr D, Yakovleff A, Gimenez y Ribotta M, Privat A, Provencher J, Rossignol S. Locomotor recovery in chronic spinal rat: long-term pharmacological treatment or transplantation of embryonic neurons? Prog Brain Res 137: 213–230, 2002. [DOI] [PubMed] [Google Scholar]
- Petruska et al. 2007.Petruska JC, Ichiyama RM, Jindrich DL, Crown ED, Tansey KE, Roy RR, Edgerton VR, Mendell LM. Changes in motoneuron properties and synaptic inputs related to step training after spinal cord transection in rats. J Neurosci 27: 4460–4471, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redl and Schlag 1986.Redl H, Schlag G. Fibrin sealant and its modes of application. In: Fibrin Sealant in Operative Medicine, General Surgery and Abdominal Surgery, edited by Schlag G, Redl H. Berlin: Springer-Verlag, 1986, vol. 6, p. 13–26.
- Reisman et al. 2005.Reisman DS, Block HJ, Bastian AJ. Interlimb coordination during locomotion: what can be adapted and stored? J Neurophysiol 94: 2403–2415, 2005. [DOI] [PubMed] [Google Scholar]
- Remple et al. 2002.Remple MS, Bruneau RM, Vandenberg PM, Goertzen C, Kleim JA. Sensitivity of cortical movement representations to motor experience: evidence that skill learning but not strength training induces cortical reorganization. Behav Brain Res 123: 133–141, 2002. [DOI] [PubMed] [Google Scholar]
- Ribotta et al. 2000.Ribotta MG, Provencher J, Feraboli-Lohnherr D, Rossignol S, Privat A, Orsal D. Activation of locomotion in adult chronic spinal rats is achieved by transplantation of embryonic raphe cells reinnervating a precise lumbar level. J Neurosci 20: 5144–5152, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossignol et al. 1999.Rossignol S, Drew T, Brustein E, Jiang W. Locomotor performance and adaptation after partial or complete spinal cord lesions in the cat. Prog Brain Res 123: 349–365, 1999. [DOI] [PubMed] [Google Scholar]
- Schreyer and Jones 1983.Schreyer DJ, Jones EG. Growing corticospinal axons by-pass lesions of neonatal rat spinal cord. Neuroscience 9: 31–40, 1983. [DOI] [PubMed] [Google Scholar]
- Schreyer and Jones 1988.Schreyer DJ, Jones EG. Topographic sequence of outgrowth of corticospinal axons in the rat: a study using retrograde axonal labeling with Fast blue. Dev Brain Res 38: 89–101, 1988. [DOI] [PubMed] [Google Scholar]
- Stelzner et al. 1975.Stelzner DJ, Ershler WB, Weber ED. Effects of spinal transection in neonatal and weanling rats: survival of function. Exp Neurol 46: 156–177, 1975. [DOI] [PubMed] [Google Scholar]
- Thota et al. 2005.Thota AK, Watson SC, Knapp E, Thompson B, Jung R. Neuromechanical control of locomotion in the rat. J Neurotrauma 22: 442–465, 2005. [DOI] [PubMed] [Google Scholar]
- Udoekwere et al. 2006.Udoekwere UI, Mbi LT, Ramakrishnan A, Giszter SF. Robot applied elastic fields at the pelvis of the spinal transected rat: a tool for detailed assessment and rehabilitation. Proc IEEE/EMBC Conf, New York, 2006. [DOI] [PMC free article] [PubMed]
- Vinay et al. 2002.Vinay L, Brocard F, Clarac F, Norreel JC, Pearlstein E, Pflieger JF. Development of posture and locomotion: an interplay of endogenously generated activities and neurotrophic actions by descending pathways. Brain Res Brain Res Rev 40: 118–129, 2002. [DOI] [PubMed] [Google Scholar]
- Westerga, and Gramsbergen 1990.Westerga J, Gramsbergen A. Development of locomotion in the rat. Dev Brain Res 57: 163–174, 1990. [DOI] [PubMed] [Google Scholar]
- Westerga, and Gramsbergen 1993.Westerga J, Gramsbergen A. Development of locomotion in the rat: the significance of early movements. Early Hum Dev 34: 89–100, 1993. [DOI] [PubMed] [Google Scholar]
- Wise and Donoghue 1986.Wise SP, Donoghue JP. The motor cortex of rodents. In: The Cerebral Cortex: Sensory-Motor Areas and Aspects of Cortical Connectivity, edited by Jones EG, Peters A. New York: Plenum Press, 1986, vol. 5, p. 243–270.
- Wolpaw 2006.Wolpaw JR The education and re-education of the spinal cord. Prog Brain Res 157: 261–280, 2006. [DOI] [PubMed] [Google Scholar]
- Yeomans 1990.Yeomans JS Principles of Brain Stimulation. New York: Oxford Univ. Press, 1990.
- Z'Graggen et al. 2000.Z'Graggen WJ, Fouad K, Raineteau O, Metz GAS, Schwab ME, Kartje GL. Compensatory sprouting and impulse rerouting after unilateral pyramidal tract lesion in neonatal rats. J Neurosci 20: 6561–6569, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]