Abstract
Locusts have two large collision-detecting neurons, the descending contralateral movement detectors (DCMDs) that signal object approach and trigger evasive glides during flight. We sought to investigate whether vision for action, when the locust is in an aroused state rather than a passive viewer, significantly alters visual processing in this collision-detecting pathway. To do this we used two different approaches to determine how the arousal state of a locust affects the prolonged periods of high-frequency spikes typical of the DCMD response to approaching objects that trigger evasive glides. First, we manipulated arousal state in the locust by applying a brief mechanical stimulation to the hind leg; this type of change of state occurs when gregarious locusts accumulate in high-density swarms. Second, we examined DCMD responses during flight because flight produces a heightened physiological state of arousal in locusts. When arousal was induced by either method we found that the DCMD response recovered from a previously habituated state; that it followed object motion throughout approach; and—most important—that it was significantly more likely to generate the maintained spike frequencies capable of evoking gliding dives even with extremely short intervals (1.8 s) between approaches. Overall, tethered flying locusts responded to 41% of simulated approaching objects (sets of 6 with 1.8 s ISI). When we injected epinastine, the neuronal octopamine receptor antagonist, into the hemolymph responsiveness declined to 12%, suggesting that octopamine plays a significant role in maintaining responsiveness of the DCMD and the locust to visual stimuli during flight.
INTRODUCTION
How is vision for action different from vision during passive observation? Attention and arousal are well known to influence processing of visual information either by sharpening response tuning of neurons in visual areas processing motion or by increasing sensitivity to a specific stimulus (Aston-Jones et al. 1992; Devauges and Sara 1990; Harley 1987; Womelsdorf et al. 2006). Without the change in state accompanying arousal, the responses of the visual system to a repeated stimulus often decline. This process of decline can be reversed either by the sudden presentation of a new stimulus or by a change in state caused by the release of neuromodulatory substances from the nervous system (Bacon et al. 1995; Berridge et al. 1993; Bicker and Menzel 1989; Cole and Robbins 1992; Evans 1985; Gray 2005; Gu 2002; Lapiz and Morilak 2006; Orchard et al. 1993; Ramirez and Pearson 1991; Rowell 1971b; Stern et al. 1995, 1999; Stevenson et al. 2005; Vankov et al. 1995; Weisel-Eichler and Liebersat 1996).
In insects, the neuromodulator octopamine is thought to perform functions similar to those of norepinephrine in mammals (Evans 1985). Rapid rises in octopamine level produce an increase in the general level of arousal, changing the insect from a resting state into an active state, ready to process incoming sensory stimuli and prepared for sustained activity or aggression (Bacon et al. 1995; Bicker and Menzel 1989; Evans 1985; Orchard et al. 1993; Ramirez and Pearson 1991; Rowell 1971b; Stevenson et al. 2005; Weisel-Eichler and Liebersat 1996). Octopamine is released both locally within the CNS, to modulate activity of target visual neurons (Bacon et al. 1995; Stern et al. 1995, 1999), and in a more widespread way into the blood, increasing the insect's preparedness either to escape a threatening stimulus by flight (Bicker and Menzel 1989; Orchard et al. 1993; Ramirez and Pearson 1991; Simpson et al. 2001; Weisel-Eichler and Liebersat 1996) or to maintain a fight (Stevenson et al. 2005). When locusts or crickets are forced to fly, or the mechanosensory pathways in their hind legs are stimulated, octopamine levels in the hemolymph and optic lobes increase (Bacon et al. 1995; Simpson et al. 2001). Octopamine elevation is particularly marked in the first 10 min of flight and at the time of flight initiation (David et al. 1985; Goosey and Candy 1980). Epinastine is a drug that specifically binds to neuronally located octopamine receptors in locusts and it can be used to block octopamine-induced behavior (Roeder et al. 1998; Stevenson et al. 2005).
Locusts are good subjects for investigating the role of arousal on identified neurons and behavior because they have some large identified visual neurons (O'Shea and Williams 1974; Rowell 1971a) and have evolved a high level of sensitivity to looming objects, probably as an adaptation to predation (Gabbiani et al. 1999; Hatsopoulos et al. 1995; Matheson et al. 2004; Paron et al. 2007; Rind and Simmons 1992, 1997; Schlotterer 1977). This sensitivity is mediated by two large identified neurons: the lobula giant movement detector (LGMD; O'Shea and Williams 1974) and its postsynaptic target, the descending contralateral movement detector (DCMD; Rowell 1971a). These neurons respond most strongly to rapidly approaching objects (Gabbiani et al. 1999, 2002, 2005; Hatsopoulos et al. 1995; Peron et al. 2007; Rind and Simmons 1992, 1997; Schlotterer 1977), with the DCMD following 1–1 spikes in the LGMD (Rind 1984). The DCMD excites some flight motoneurons (Simmons 1980) and can trigger predator avoidance during flight (Santer et al. 2005, 2006). In response to a looming stimulus that is indicative of a predator's approach, a tethered, flying locust will perform a “gliding dive,” in which it ceases to beat its wings for one or more wingbeat cycles; the wings are held elevated in stereotyped gliding posture (Santer et al. 2005). These gliding dives are evasive maneuvers and occur in response to approaching objects when a DCMD produces consecutive spikes at a frequency of ≥150 Hz during the elevation phase of a wingbeat cycle (Santer et al. 2006).
Previously, several studies have shown that the responses of the LGMD and DCMD neurons to both small moving objects and looming ones are subject to habituation (Bacon et al. 1995; Gray 2005; Matheson et al. 2004; Rogers et al. 2007; Rowell 1971b). The extent of the decline in the overall spike numbers, peak spike frequency, and variability in DCMD responses to repeated looming stimuli depends on the phase of the locust (Gray 2005; Matheson et al. 2004; Rogers et al. 2007). When Matheson et al. (2004) and Rogers et al. (2007) delivered 30 stimuli at 60-s intervals, to both solitary and gregarious locusts, they found a decline in spike numbers and peak spike frequency. The decline was more pronounced in solitary locusts where peak DCMD responses rapidly fell to <100 Hz (by the 15th and 7th stimulus in the respective studies).
A habituated DCMD still responds well to the presentation of a looming object of a novel size or approaching along a novel trajectory (Gray 2005). However, evidence for an arousal-based modulation of habituation comes from other accounts, which report that LGMD and DCMD neurons' responses can be restored by mechanical stimulation of the body, head, or ipsilateral antenna, or by application of octopamine (Bacon et al. 1995; Gauglitz and Stevenson 1993; Ramirez and Pearson 1991; Roeder et al. 1998; Rowell 1971b; Stern 1999). For example, a previously habituated DCMD response to a moving object can be partially restored by the intracellular depolarization of the identified octopaminergic PM4 neurons (Bacon et al. 1995). These cells are also excited by the same mechanical stimuli that have been shown to mediate dishabituation of the DCMD response (Rowell 1971b). Although such studies have correlated physiological parameters, such as constitutive octopamine levels, with the spiking activity of the LGMD–DCMD in response to various visual stimuli, none has measured DCMD responses to looming stimuli following changes in the state of arousal induced by natural behavior itself.
In this study we used two different behavioral manipulations to modify the level of arousal in locusts. First, we raised the level of arousal by stimulating the hind legs of locusts. This stimulation has already been shown to induce changes in the phase of a locust, eventually causing a solitary individual to become gregarious, with an altered response to visual stimuli and an accompanying change in behavior (Simpson et al. 2001). Second, we flew individual locusts in a wind tunnel to simulate the first 10 min of flight. Not only did these manipulations greatly reduce habituation of the spiking activity in an identified neuron in the visual system—the DCMD—but the change in arousal state induced in each case also increased the probability that this neuron produced the code that provoked evasive gliding behavior (Santer et al. 2006).
METHODS
Visual stimuli
The computer program to calculate the geometry of object approach was written in Borland Delphi programming language. Graphic commands were sent to a Cambridge Research Systems VSG2/3 image synthesizer and RG2 raster generator system. The resulting signals were displayed on a Kikusui COS1611 X-Y monitor using a green P31 phosphor. The RG2 raster generator produced a display with 438 dots × 437 lines, resulting in a pixel that subtended 0.09° at the eye. This is smaller than the acceptance angle of locust photoreceptors under either light- or dark-adapted conditions. The screen had a width of 110 mm (71.1° at the eye) and a height of 80 mm (59.5° at the eye). The components were chosen for the high speeds at which they could animate and display simple graphics.
The animation rate of approaching and receding objects was kept at 200 Hz (the maximum refresh rate of the RG2 unit). The front of the monitor was placed parallel to the long axis of the locust and stimuli were viewed monocularly, by the lateral surface of the compound eye. The object, a dark disc of 80-mm diameter, approached from 207 to 7 cm from the locust, at one of a range of constant velocities from 0.25 to 5 ms−1. Unless stated otherwise, each simulated object approach stopped when the disc subtended an angle of 59° at the eye. Consequently, collision would have occurred 280 ms after the end of object approach at 0.25 ms−1; 140 ms after the end of object approach at 0.5 ms−1; 70 ms after the end of object approach at 1 ms−1; 35 ms after at 2 ms−1; 23.3 ms after at 3 ms−1; and 14 ms after at 5 ms−1.
EXPERIMENTAL PROCEDURES
Experiments were performed on 152 adult male and female Locusta migratoria, obtained from a crowded colony kept at the University of Newcastle, or from an outside supplier (Blades Biological). Experiments were conducted at temperatures of 22–30°C. Locusts were either minimally dissected and restrained or tethered.
1) Minimally dissected, restrained locusts. The locust was mounted ventral side uppermost on a plasticine block and a fine minuten pin mounted on a piece of circuit board was manipulated into a 0.5-mm hole in the cervical sternite and lowered into contact with the nerve cord. An earth electrode was inserted into the thorax of the locust. DCMD spike trains were analyzed using Spike2 software (Cambridge Electronic Design). To manipulate levels of arousal, 10 s before each looming stimulus we delivered brush strokes to the hind leg, lasting 1 s. Stroking the leg is known to excite the octopaminergic brain neuron PM4, which increases DCMD responsiveness to brief translating visual stimuli (Bacon et al. 1995; Stern 1999).
2) Tethered, wind-stimulated, or flying locusts. A square window was cut in the ventral cuticle of the rostral thorax, just forward of the mesothoracic ganglion. Hook electrodes were implanted as in Santer et al. (2005). The square of removed cuticle was then waxed over the window as a lid and the locust mounted, dorsal side up by its pronotum to a brass rod. Locusts were tethered via the dorsal pronotum throughout the experiment. The locust grasped a paper ball and was relatively free to move. In trials where the locust was wind stimulated but not flying it continued to grasp the paper ball but was constantly stimulated with a 1.5 ms−1 laminar air flow from a simple wind tunnel powered by a DC ball-bearing fan (Papst TYP 4212 NH; RS Components, Northants, UK). Air flow was made laminar by passing it through an array of aligned straws (Santer et al. 2005). Flying was induced by removing the paper ball and increasing the air to 3.0 ms−1. DCMD records were taken from four locusts during wind stimulation and in the first 5 min of flight. Stimuli, consisting of 80-mm-diameter objects approaching at 3 ms−1, were delivered in six blocks of six with a 2 min rest period between each block of six stimuli. The six stimuli were delivered at interstimulus intervals (ISIs) of 40, 20, 10, and 1.8 s. Each locust received 432 looming stimuli: 144 without wind stimulation or flight, 144 while wind stimulated, and 144 in flight.
Movement artifacts were removed from the extracellular DCMD records by digital filtering. Power spectra of nerve cord recordings with and without DCMD spikes were compared in Spike2. On the basis of this information, data were high-pass filtered in Spike2. In most recordings, the DCMD was clearly the largest spike from the nerve cord.
When looms were presented during flight, locusts regularly performed gliding behaviors. Following these behaviors, flight normally resumed after one or two wingbeat cycles but occasionally locusts stopped flying briefly after they performed the glide. When this happened the locust was given a paper ball so tarsal contact was regained. Flight was then resumed after tarsal contact with the ball was lost and the experiment continued. These periods were usually brief compared with the stimulus delivery regime, with a mean time of 2.18 ± 0.37 s (SE) as measured with an infrared sensor and occurred infrequently. For example, for one locust, during a delivery regime of 42 stimuli, at an ISI of 1.8 s, there were nine occasions when the locust stopped flying following object approach. Trials in which the locust was not flying were excluded from the analysis.
To investigate the role of the neuromodulator octopamine in the flight-induced arousal increase and predator avoidance we injected epinastine, an octopamine antagonist (Roeder et al. 1998) that prevents octopamine from binding to its neuronally located receptor, into the thorax of six locusts. Epinastine was applied by injecting 25 μl of a 20 mM solution in saline into the thorax, through the arthrodial membrane at the base of the prothoracic leg. This site was used because it does not disrupt vision or wing musculature and has recently been shown to be effective in raising octopamine levels in other insect brains: octopamine injected into the thorax of the bee was recovered from the brain after 15 min and elevation persisted for ≥60 min (Barron et al. 2007). After allowing 45 min for the antagonist to enter the optic lobes (Stevenson et al. 2005), we flew each locust in a laminar 3-m/s air flow and we used the infrared beam to monitor wingtip position during object approach. Stimuli were delivered in groups of six each separated by a 1.8-s interval. As a control, six locusts were injected with saline and treated as described earlier. In total, 540 stimuli were delivered to control animals and 462 to animals injected with epinastine.
Statistical analysis of arousal effects
To statistically analyze the effect of arousal changes on the DCMD responses we used the statistical analysis software (SAS Institute) generalized linear modeling (Genmod) procedure, in which the data were plotted as a Poisson distribution on a logarithmic scale. A Type III analysis of the sum of means squared was made to assess the significance of each treatment either on DCMD activity or on behavior. This technique is good for analyzing experiments where repeated measures are made on individual subjects following various treatments so that interactions between the treatments can be analyzed simultaneously (Der and Everitt 2002). Additional analyses were used when simple comparisons were sufficient. Nonparametric tests were used if the data were found to have a nonnormal distribution.
RESULTS
Without arousal, DCMD spiking responses habituate
Previous studies using static, translating, and looming visual stimuli have shown that the DCMD response habituates to repeated stimulus presentations and that it can be dishabituated by various visual and mechanical stimuli (Bacon et al. 1995; Gray 2005; Rowell 1971a,b). In the first stage of our study, we wanted to build on these experiments to gather new information of direct relevance to a locust's performance of escape behavior during flight. For this reason we use looming visual stimuli that might represent the attacks of aerial predators and analyze aspects of the DCMD response previously shown to underlie the performance of an emergency gliding dive (Santer et al. 2006).
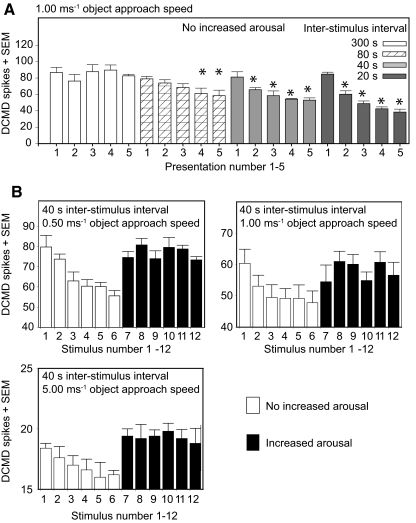
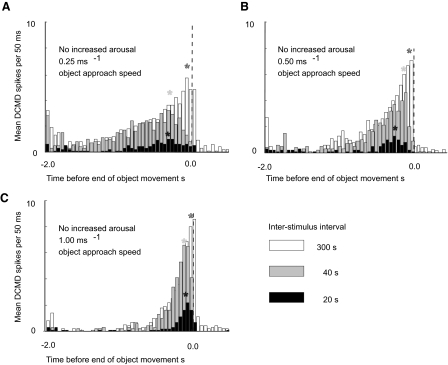
As predicted by previous work on static, translating, or looming stimuli (Bacon et al. 1995; Gray 2005; Rogers et al. 2007; Rowell 1971a,b), we observed that when a locust passively viewed the stimulus without being aroused the DCMD response to a looming stimulus habituated when the stimulus was repeated (Fig. 1). Furthermore, the rate of DCMD habituation was greatest when the ISIs were shortest (Fig. 1A). In these experiments habituation showed as a significant effect of the stimulus number, from first to sixth, on the size of the DCMD response. For unaroused locusts a logistic regression analysis (see methods) showed a significant effect of stimulus number and ISI on DCMD spike number per stimulus (χ12 = 70.41, P < 0.001). The analysis also confirmed that the rate of DCMD response decline depended on the interval between the consecutive stimuli (ISIs), with significant decline in spike numbers between first and subsequent responses at ISIs of 20, 40, and 80 s but not at 300 s (statistical analysis for ISI = 20 s: stimulus 1 vs. stimulus 2 χ12 = 16.64, P < 0.0001; 1 vs. 3 χ12 = 39.32, P < 0.0001; 1 vs. 4 χ12 = 57.19, P < 0.0001; 1 vs. 5 χ12 = 71.15, P < 0.0001; for ISI = 40 s: stimulus 1 vs. stimulus 2 χ12 = 6.55, P = 0.0105; 1 vs. 3 χ12 = 14.88, P = 0.0001; 1 vs. 4 χ12 = 21.67, P < 0.0001; 1 vs. 5 χ12 = 23.96, P < 0.0001; for ISI = 80: stimulus 1 vs. stimulus 2 χ12 = 0.65, P = 0.4188; 1 vs. 3 χ12 = 2.99, P = 0.0837; 1 vs. 4 χ12 = 9.01, P = 0.0027; 1 vs. 5 χ12 = 12.27, P = 0.0005; for ISI = 300 there was no significant decline: stimulus 1 vs. stimulus 2 χ12 = 0.92, P = 0.3382; 1 vs. 3 χ12 = 3.25, P = 0.0716; 1 vs. 4 χ12 = 0.07, P = 0.7876; 1 vs. 5 χ12 = 0.84, P = 0.3584). DCMD habituation included a decline both in the number of spikes per stimulus (Fig. 1) and in the maximum spike rate during a stimulus (Fig. 2). In locusts where the DCMD was habituated, the DCMD response declined or ceased early during an approach (Fig. 2). The earliest decline in spike numbers occurred for locusts stimulated with the shortest intervals between looming stimuli, which was 20 s in these experiments (Fig. 2, A–C).
FIG. 1.
Descending contralateral movement detector (DCMD) responses depended on the state of arousal. A: without an increase in arousal the DCMD response declined following repeated stimulation. Over the range tested, the rate of decline in the number of DCMD spikes generated increased as the interstimulus interval (ISI) was decreased. B: with approach speeds from 0.50 to 5.00 ms−1, this decline in total DCMD spike numbers could be reversed by increasing arousal by mechanical stimulation of the hind-leg (filled bars).
FIG. 2.
Without arousal, the shape of the DCMD response envelope to looming stimuli, as well as DCMD spike numbers, depended on the interval between stimuli. Each graph shows DCMD responses to object approach at a particular speed but with different ISIs (20, 40, or 300 s). Approach speeds were 0.25, 0.50, and 1.00 ms−1 in A, B, and C, respectively. Bars show the mean DCMD spike frequency in 50-ms bins, for 5 stimulus repetitions with a single locust. Ten spikes per bin would generate a maintained spike frequency of ≥200 Hz. With shorter ISIs, the number of spikes in the DCMD decreased and the maximum spike frequency occurred earlier in the approach (marked by *). The shift in the timing of the maximum response was greater for the slower stimulus approach speeds. Data from this experiment were also used in Fig. 5B.
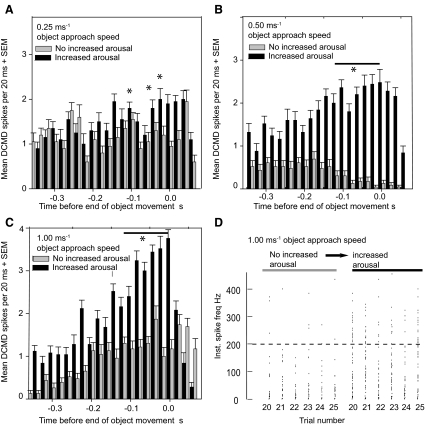
Previous reports show that a mechanical stimulus to a locust's head, body, or antennae can restore a habituated DCMD response to a static or translating visual stimulus (Bacon et al. 1995; Rowell 1971a,b). In the current study, we sought to determine whether mechanical stimulation of the hind leg could do the same to the DCMD response to a behaviorally important looming stimulus. When a locust was aroused between stimulus presentations with a 1-s-long mechanosensory stimulus to the hind-leg tibia, habituation was suppressed (Fig. 1B). The general effects of hind-leg stimulation on habituation were robust, repeatable, and reversible (Fig. 1B). Specifically, in habituated subjects, increasing arousal by hind-leg stimulation restored the overall DCMD spike numbers to a prehabituated level, as observed in experiments with nonlooming visual stimuli (Rowell 1971b). A logistic regression analysis confirmed that the negative effect of stimulus number on the DCMD response (habituation), which was significant in nonaroused locusts, was no longer significant in aroused ones (effect of stimulus number: with an approach speed of 0.50 ms−1 without arousal χ12 = 32.25, P < 0.0001, with arousal χ12 = 3.38, P = 0.6411; with an approach speed of 1.0 ms−1 without arousal χ52 = 20.65, P = 0.0009, with arousal χ12 = 6.66, P = 0.2470 and with an approach speed of 5.0 ms−1 without arousal χ12 = 1.20, P = 0.9446, with arousal χ12 = 0.14, P = 0.9996). DCMD responses were elicited throughout the entire duration of the loom (Figs. 2 and 3) and the high-frequency DCMD spikes during the final stages of object approach were also restored. This effect of arousal on peak spike frequency is illustrated in Fig. 3, where the bars represent the DCMD mean spike frequency in 20 ms bins over 50 stimulus repetitions with 20-s intervals between each one. Object approach speeds were 0.25, 0.50, or 1.00 ms−1. The first 25 stimuli (gray bars) were delivered without any increase in arousal and the second 25 stimuli (dark bars) were each preceded by a stroke to the hind-leg tibia to increase the state of arousal. In the 60 ms before projected collision, the increase in arousal significantly increased the frequency of the DCMD response with approach speeds of 0.25, 0.50, and 1.00 ms−1 (*P < 0.01, Mann–Whitney rank-sum test). The highest instantaneous spike rate was observed in aroused locusts immediately before object approach ceased (Fig. 3, A–D).
FIG. 3.
Arousal restored the peak firing frequency and the ability of the DCMD to follow the increase in image size throughout object approach. With arousal, responses reach mean spike rates per 20-ms bin of almost 200 Hz. A–C: bars represent the DCMD mean spike frequency, over 50 stimulus repetitions at 20-s intervals, with approach speeds of 0.25, 0.50, and 1.00 ms−1. The first 25 stimuli (gray bars) were delivered without any increase in arousal; then a further 25 stimuli (colored bars) were each preceded by a stroke to the hind leg tibia, raising the state of arousal. At 60 ms before projected collision, arousal significantly increases the DCMD response with approach speeds of 0.50 and 1.00 ms−1 (P < 0.01; Mann–Whitney rank-sum test). D: instantaneous DCMD spike frequencies during the last 6 approaches are shown for the aroused and nonaroused trials.
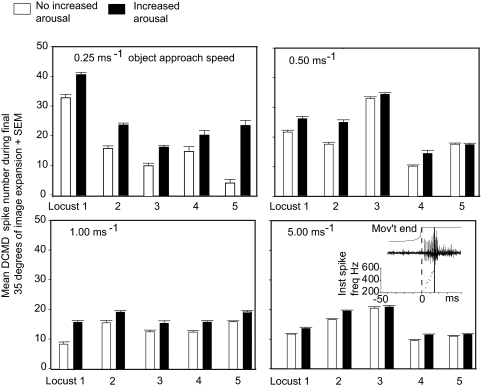
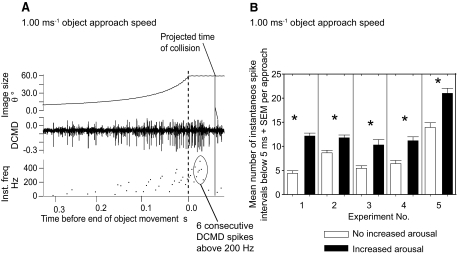
High-frequency spikes during the final period of a DCMD's response to a looming stimulus have been implicated in triggering emergency predator-avoidance glides (Santer et al. 2006). These high-frequency spikes are therefore a behaviorally important parameter of the DCMD response. We concentrated on these last-minute DCMD spikes in a further five locusts (locusts 1–5, Fig. 4), where a comparison of the DCMD spike numbers in aroused and nonaroused locusts in the final milliseconds before collision confirmed our earlier observations that DCMD spike numbers were always significantly greater for aroused subjects, irrespective of loom speed over the range 0.25–5.00 ms−1 (χ12 = 39.2, P < 0.001 for loom speeds in the range 0.25–1.00 ms−1 and χ12 = 12.8, P < 0.001 for loom speeds of 5 ms−1). Spike numbers obtained from aroused locusts were 26–50% (95% confidence limits) greater than the equivalent values recorded from nonaroused subjects with looming speeds in the range 0.25–1.00 ms−1. For looming speeds of 5 ms−1, the spike numbers were 7–26% (95% confidence limits) greater for aroused locusts than in the nonaroused (Fig. 4). In these experiments, maximum instantaneous frequencies of 500–600 Hz were generated in aroused locusts (Fig. 4, inset, bottom right graph). To permit a direct comparison of the DCMD responses at various looming speeds, spike numbers were counted over time intervals that coincided with a growth of 35° in the angular subtense of the stimulus, at each speed (see Fig. 4 legend for exact intervals). When we looked at the DCMD responses in these experiments, particularly at spike frequencies around 200 Hz as collision was imminent (Fig. 5A), we found that, in nonaroused locusts, the numbers of DCMD spikes occurring at frequencies >200 Hz was low and that when the arousal of the locust was increased, the number of spikes at frequencies >200 Hz increased significantly (Fig. 5B; paired Student's t-test, *P < 0.002). The DCMD responses where arousal had been increased contained six or more consecutive DCMD spikes at frequencies >200 Hz (example circled in Fig. 5A) in 56 of 148 occasions (38%) in aroused locusts, but in only 2 of 122 occasions (0.016%) in nonaroused subjects.
FIG. 4.
Arousal increased the number of spikes in the DCMD in response to approaching objects during the final stages of object approach. When the arousal of the locust was increased by stroking the hind leg tibia (dark bars), the number of spikes increased significantly. The inset in the bottom right graph shows the increasing DCMD spike frequency throughout the response to a 5 ms−1 object approach. Projected collision (solid line) would have occurred 14 ms after motion ceased (dotted line). The duration of the DCMD response and section of the response analyzed for each object approach velocity depended on the velocity and was the final 400 ms for approach speeds of 0.25 ms−1 (−400 to 0 ms), the final 200 ms for approach speeds of 0.50 ms−1 (−220 to 0 ms), and the final 100 ms for approach speeds of 1 ms−1 (−100 to 0 ms). The final 25 ms were used for approaches at 5 ms−1. These intervals allowed a comparison of the DCMD responses to the same change in angular subtense (from 25 to 59°) with different loom speeds.
FIG. 5.
Arousal increased the number of high-frequency DCMD spikes. A: during object approach in an aroused locust, instantaneous spike frequency in the DCMD reached >200 Hz, particularly when collision was imminent. In this example, spike frequencies exceeded 200 Hz and 6 consecutive spikes occurred at ≥200 Hz (circled). B: approaches were presented at intervals of 40 s to either nonaroused (open bars) or aroused (filled bars) locusts. For nonaroused locusts, the numbers of DCMD spikes occurring at frequencies >200 Hz was low. When the arousal state of the locust was increased, the number of spikes at frequencies >200 Hz increased significantly (*P < 0.002, paired Student's t-test). In particular, increased levels of arousal led to increases in the number of approaches generating >6 consecutive spikes at 200 Hz: 56 of 148 approaches with increased levels of arousal and only 2 of 122 without arousal. Trials were in blocks of 6, with a total of 18 to 42 approaches per block. The analysis shown in Fig. 6B excluded the first 3 responses of the experiment in nonaroused locusts.
Flying boosts arousal and stops significant DCMD habituation
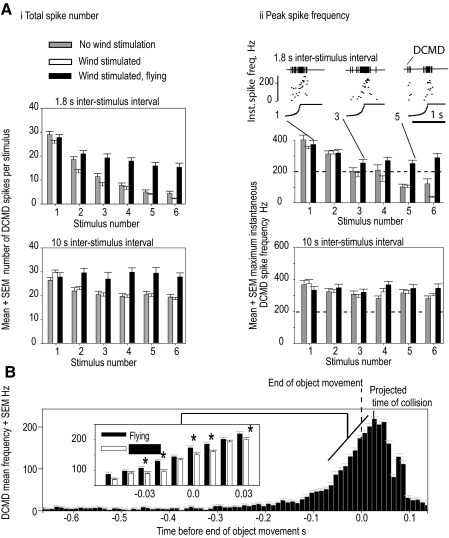
Our experiments on quiescent locusts showed that the rate of DCMD habituation was greatest when the intervals between stimuli were shortest and included both a reduction in the numbers of spikes per stimulus and a decline in the peak spike rate (Gray 2005) with the response ceasing early, before the loom had stopped. We wanted to see the effect of a specific behavioral action on these aspects of DCMD habituation and we chose flight as the behavioral manipulation. This behavior is also a relevant one since evasive glides can occur only when a locust is in flight. Since evasive glides occur in flight in response to imminent collision and are triggered by high-frequency spikes in the final stage of the DCMD response, the observed effects of flight and arousal on these spikes are crucial in understanding the triggering of antipredator behavior. Just as we observed in subjects aroused by hind-leg tibial stimulation, we expected that the act of flying would produce an aroused state in locusts that would concomitantly reduce our ability to habituate the DCMD to the repeated looming stimuli. To examine this, we compared the DCMD responses of flying locusts with those of wind-stimulated nonflying locusts and nonflying locusts without wind stimulation (Fig. 6, A and B). Wind stimulation was used as a control in this experiment because wind stimulates hairs in the same manner a flying locust would experience during air movement, although the subjects do not undergo the change in physiological state that accompanies the activation of flight muscles and sustained activity. For these experiments we chose ISIs equal to or less than those that had caused strong DCMD habituation in our previous experiments. We examined the three measures of DCMD response strength that we had shown earlier to be affected by habituation: the number of spikes during a stimulus, the peak instantaneous spike frequency, and the persistence of the response throughout the loom. As we predicted, all three measures of response strength showed the effects of habituation in wind-stimulated nonflying locusts (Fig. 6A). We could also see that flight had not increased the overall DCMD response compared with wind stimulation because the responses to the first stimulus were either the same in both cases or higher during wind stimulation (stimulus 1 in Fig. 6A, i and ii. Least-squares multiple comparisons of spike number: for ISI = 1.8 s χ12 = 2.56, P = 0.1094; for ISI = 10 s χ12 = 1.43, P = 0.2314; for ISI = 20 s χ12 = 3.21, P = 0.0732; least-squares multiple comparisons of peak instantaneous spike frequency: for ISI = 1.8 s χ12 = 397.99, P < 0.0001; for ISI = 10 s χ12 = 7.53, P < 0.0061; for ISI = 20 s χ12 = 17.45, P < 0.0001). When we statistically compared DCMD responses to approaching objects in flying and nonflying locusts, across three ISIs (1.8, 10, and 20 s), we found that both spike number and peak instantaneous frequency were significantly greater in aroused flying locusts compared with wind-stimulated ones (logistic regression analysis gave χ12 = 6.56, P < 0.0104 for spike number and χ12 = 277.75, P < 0.0001 for peak instantaneous frequency). Looking at the DCMD responses to each ISI separately revealed the greatest boost induced by flying occurred at the shortest interval. With 1.8 s between approaches, the number of DCMD spikes was 33% higher (95% confidence level; range = 13–51%) and peak instantaneous spike frequency was 56% higher (95% confidence level; range = 51–61%) in flying subjects.
FIG. 6.
Flying protects the DCMD from habituation, enabling the locust to react to impending collisions even with very short intervals between successive stimuli. DCMD responses to looming objects in tethered flying locusts were very robust. Stimuli consisted of a dark disc of diameter 80 mm approaching the eye at 3 ms−1. The end of disc movement is shown by a dashed vertical line. Experiments were performed on 4 locusts with 144 stimuli delivered when the locust was not flying, 144 stimuli during wind stimulation, and 144 during flight, each separated by an ISI of either 1.8, 10, 20, or 40 s (data from 20 and 40 s separation between stimuli are not shown). A: comparison of habituation in the DCMD neuron during repeated presentation of a stimulus looming at a velocity of 3 ms−1 in 2 different behavioral states: wind stimulated and flying. i: total spike number per stimulus response. ii: peak instantaneous spike frequency per response. In flying locusts the peak instantaneous spike frequency remained >200 Hz at all ISIs. Top right: DCMD activity is shown for 3 looming stimuli (numbers 1, 3, and 5 of a sequence of 6), separated by an ISI of 1.8 s. B: the time course of the mean DCMD response to looming stimuli in the flying locusts is shown, divided into 10 ms bins. The responses persisted beyond the end of object approach (dashed line), with the maximum response occurring at the projected time of collision (solid line). The inset then compares DCMD responses in flying and nonflying wind-stimulated locusts in the 90 ms immediately prior to collision. Intervals during which flight had a statistically significant effect are marked with an asterisk (P < 0.02, Mann–Whitney rank-sum test).
The rate of decline in both spike number and peak instantaneous frequency with successive approaches was significantly reduced by flight compared with wind stimulation over all ISIs and at each ISI (logistic regression analysis gave: χ12 = 572.78, P < 0.0001 for spike number and χ12 = 4,774.71, P < 0.0001 for maximum spike frequency taking all ISIs together; for ISI = 1.8 s χ12 = 295.22, P < 0.0001 for spike number and χ12 = 4,068.68, P < 0.0001 for peak instantaneous frequency; for ISI = 10 s χ12 = 38.4, P < 0.0001 for spike number and χ12 = 134.83, P < 0.0001 for peak instantaneous frequency; finally, for ISI = 20 s χ12 = 17.12, P < 0.0001 for spike number and χ12 = 31.68, P < 0.0001 for peak instantaneous frequency). The wind stimulation provided during flight was twice the velocity of that for nonflying locusts, although this factor was unlikely to underlie the effect of flight on habituation because rather than preventing habituation, wind stimulation of a quiescent locust was either not significant or was associated with an increase in habituation (Fig. 6, gray bars compared with unfilled bars).
In flying locusts, we consistently found that the DCMD response to a looming stimulus persisted beyond the end of object motion and that high rates of spiking were produced at the end of object approach (Fig. 6B). The DCMD spike rates in an aroused flying locust in these experiments were >150 Hz for 40 ms (Fig. 6B), which is close to the duration of a whole wingbeat cycle (Santer et al. 2005; Wilson and Weis-Fogh 1962), and would be sufficient to evoke gliding dives (Santer et al. 2006). Short evasive glides were regularly observed in response to looming stimuli delivered during flight but not without a looming stimulus.
Boost to predator-avoidance responses during flight can be prevented by epinastine
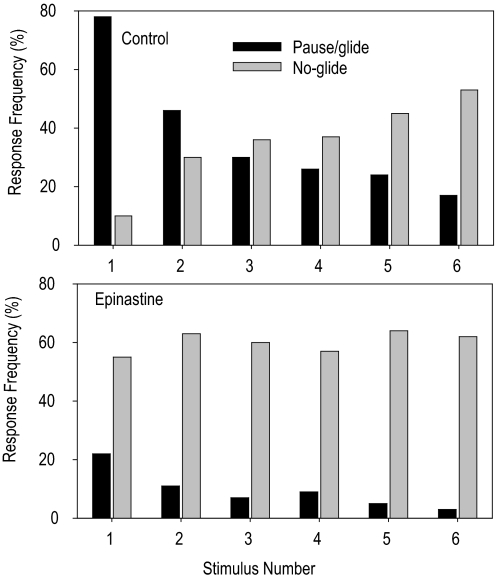
We hypothesize that the boost in the LGMD response during flight is mediated by octopamine released in the optic lobe. To test this hypothesis, we measured the occurrence of evasive gliding in the presence of epinastine, a blocker of neuronally located octopamine receptors. In the presence of epinastine we would expect strong habituation in the evasive gliding behavior because the response of the DCMD in producing high spike frequencies (which is necessary for the gliding behavior) would no longer be protected from habituation during flight. Also if there were any additional preflight role for octopamine on arousal we may find that the response to the first stimulus may be reduced. For these experiments, we chose ISIs equal to or less than those that had caused strong DCMD habituation in our previous experiments (shown in Fig. 6). Six stimuli were delivered at 1.8-s intervals, a total of 540 to control locusts and 462 to locusts injected with epinastine. We categorized responses as a “pause” or “glide” if there was an interruption in flight >25% of the mean duration of the preceding 10 wingbeats (Santer et al. 2005); otherwise, the response was classified as “no glide.” The number of responses when flight was not resumed during the 1.8 s between stimuli was also recorded. The results of these experiments are shown in Fig. 7.
FIG. 7.
Injection of epinastine, a specific blocker of neuronal octopamine receptors, significantly reduces the protective effect of flight on the evasive gliding behavior. Stimuli consisted of a dark disc of diameter 80 mm approaching the eye at 3 ms−1 and were given in groups of 6, each stimulus separated by an ISI of 1.8 s. Experiments were performed on 12 locusts; 45 min prior to flight 6 had been injected with epinastine and 6 with saline as controls. An infrared beam was used to monitor wing-tip position and responses by flying locusts to imminent collision are plotted as the percentage of stimuli resulting in either 1) a pause or glide during flight lasting <1.8 s (dark bars); or 2) no-glide (gray bars). Stimuli delivered to nonflying locusts have not been plotted.
In total, out of these 1,002 stimulus presentations, control locusts showed 221/540 pauses or glides compared with 57/462 in epinastine-treated ones; and control locusts showed 53/540 no-glides compared with 361/462 in treated ones. In the remaining 320 trials the locust was not flying. The probability that a locust would glide in response to a looming stimulus on any repetition was significantly reduced in animals in which octopamine receptor activity had been blocked (108 compared with 44; χ12 = 27.2, P < 0.001). However, we also found that the probability a locust would glide decreased as a function of the number of repetitions in the same manner for both the control and epinastine-treated locusts (χ12 = 0.04, P = 0.849). Conversely, epinastine injection significantly increased the probability that a locust would show a no-glide regardless of stimulus repetition number (1–6) (χ12 = 97.7, P < 0.001). Whereas epinastine-treated locusts exhibited a high probability of no-glides in response to all stimuli regardless of the repetition number, the control animals exhibited a repetition-dependent increase in no-glides (χ12 = 31.1, P < 0.001).
DISCUSSION
In this study we have investigated the link between arousal, sensory responses, and behavioral action in a locust's preparedness for the performance of a DCMD-triggered gliding dive. The evasive glide of a flying locust is probably a last-chance maneuver to avoid an attacking bird and a critical event in the decision to perform a glide is a sustained spike rate of ≥150 Hz in the DCMD neuron during wing elevation (Santer et al. 2005, 2006). Strong arousal is required for the DCMD neuron to reach this level of response, particularly with multiple approaches separated by only a few seconds. This strong arousal can be mediated either by flight itself or by mechanosensory stimulation of the locust's hind leg.
When we increased arousal, using either a tactile stimulus to the locust's hind leg or inducing a locust to fly (David et al. 1985; Rowell 1971b; Stern et al. 1995), we found three effects on the way the DCMD responded to approaching stimuli. First, the maximum frequency of spikes was high, often reaching a rate of ≥200 Hz for 40 ms, the level found to trigger an evasive glide if occurring at the correct phase of the wingbeat cycle (Santer et al. 2006). Second, the DCMD's response increased throughout image growth until the end of stimulus movement. An increase in arousal could restore high rates of discharge and the relationship of peak firing to the end of approach in a previously habituated DCMD neuron. Third, the DCMD responded to approaches separated by as little as 1.8 s with a spike rate that could trigger a collision-avoidance glide.
In crickets, flying has already been shown to alter behavioral thresholds and to promote aggression in previously defeated individuals (Hofmann and Stevenson 2000; Stevenson et al. 2005). In crickets the increase in aggression due to flight is thought to be mediated by a neuronal octopamine receptor because the effects of a period of flight on aggression are abolished by octopamine depletion and by injection of epinastine, a specific and selective neuronal octopamine receptor antagonist (Hofmann and Stevenson 2000; Stevenson et al. 2005). Flight had specific effects on aggressive behavior without enhancing the cricket's general level of excitability (Stevenson et al. 2005). In the locust, flight increased the likelihood of high-frequency discharge in the DCMD. The effect was stronger than that of wind stimulation applied to a nonflying locust, which suggests that flight specifically increases DCMD responsiveness to a level that would trigger collision-avoidance behaviors. A role for neuronal octopamine receptors in preventing DCMD habituation has been suggested by Roeder et al. (1998) who injected epinastine into the locust prothorax and found that DCMD activity in response to repeated moving objects was significantly reduced in a dose-dependent way.
When we injected epinastine into the locust, the effect of flight in protecting collision-avoidance behavior from habituation was significantly reduced. Even the response to the first stimulus was reduced compared with controls following epinastine injection, confirming a role for octopamine in the flight-induced increase in the response of DCMD and in collision-avoidance behavior. Antibodies against the neuronal octopamine receptors have recently been developed (Degen et al. 2000; Farooqui et al. 2004) and may allow these neuronal octopamine receptors to be localized to specific neurons on the LGMD–DCMD pathway.
At what stage in the LGMD–DCMD pathway could protection from habituation occur? One likely site is at the synapses made by the afferents onto the main LGMD dendritic tree in the distal lobula, in that these synapses are thought to be prone to decrement (O'Shea and Rowell 1976). In the crayfish, transmission at sensory afferent synapses onto the lateral giant (LG) interneuron, which mediates escape tail flips, also decrements with repeated stimulation (Wine and Krasne 1972; Yeh et al. 1996, 1997). There is clear evidence that this synaptic decrement is subject to modulation so that the synapses are protected from habituation in dominant individuals by neuromodulators such as octopamine and 5-hydroxytryptamine (5-HT) (Araki and Nagayama 2003; Yeh et al. 1996, 1997). 5-HT has recently been found to facilitate synaptic input onto the LG by interacting directly with a transmembrane receptor on the LG itself (Araki et al. 2005). In the locust, where the DCMD and the LGMD responses to approaching objects are protected from habituation, it may be that the excitatory postsynaptic potentials evoked in the LGMD by visual afferents from the medulla are maintained in strength during repeated stimulation by arousal and by flight. The octopaminergic PM4 neurons are candidates for this protective role because they have arborizations in the same area of the lobula as the dendritic fan of the LGMD (Bacon et al. 1995). Their excitation can partially restore a habituated DCMD response to a small moving object, although it is not known whether they make direct or indirect contact with the afferents or the LGMD itself (Bacon et al. 1995).
Adult locusts in a swarm occur at high densities, averaging ≤50 million individuals per km2 (Rainey 1954). In early 1954, air reconnaissance observations registered 50 swarms that invaded Kenya, covering an area of about 1,000 km2 and rising to 1,000 to 1,500 m above the ground (Rainey 1954). The largest of these 50 swarms covered 200 km2 and consisted of about 10 billion locusts. Typically, in a locust swarm, daily flight activity begins with short rolling flights with frequent landings, followed by more sustained flights by midmorning (Symmons and Cressman 2001). During these take-offs, adult migratory locusts need persistent collision-avoidance responses. Not only could locusts collide with other locusts, but also predatory birds gather around swarms, and many of these bird species have been seen attacking flying locusts (Smith and Popov 1953). Prior to take-off, locusts descend from vegetation to bask on the ground (Symmons and Cressman 2001). As the densities of adult locusts on the ground increase, frequent mechanical stimulation occurs between individuals. These same mechanical stimuli to the hind legs by neighboring locusts are implicated in triggering swarm formation, causing locusts to become gregarious and aggregate together (Buhl et al. 2006; Rainey 1954; Simpson et al. 2001). In this overcrowded state, as mimicked by our mechanical stimulus to the hind leg, the DCMD in a swarming locust would be primed for its action in triggering collision avoidance during flight. After take-off, arousal mediated by increased octopamine levels would then increase again for the first 10 min of flight (David et al. 1985; Goosey and Candy 1980). The result would be that DCMD-mediated collision-avoidance glides are triggered reliably even with so many potential predator attacks or collisions occurring in a short space of time.
In summary, the DCMD response to approaching objects is protected by flight and mechanical stimulation of the hind leg, both of which are known to increase octopamine levels and are now shown to increase arousal. This effect is not seen in response to other stimuli such as wind stimulation. Arousal increases the response of an identified collision-detecting visual neuron to enhance collision avoidance during normal behavior of the locust; the increase in arousal is mediated via neuronal receptors for octopamine. The precise way in which this is done is as yet unknown but the pathways and the mechanism involved may shed light on attention and vigilance in a wide range of animals, less amenable to detailed scrutiny.
GRANTS
This work was supported by the Biotechnology and Biological Sciences Research Council and by the European Union Information Society Technologies Programme Grant IST-2001-38097 (LOCUST Project).
Acknowledgments
We thank P. Simmons and B. Simmons for helpful discussions and for critically reading the manuscript. All authors contributed to writing of the paper, F. C. Rind and R. D. Santer carried out the experiments; and G. A. Wright concentrated on the statistical analysis.
Present address of R. D. Santer: School of Biological Sciences, University of Nebraska–Lincoln, Lincoln, NE 68588.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- Araki and Nagayama 2003.Araki M, Nagayama T. Direct chemically mediated synaptic transmission from mechanosensory afferents contributes to habituation of crayfish lateral giant escape reaction. J Comp Physiol A Sens Neural Behav Physiol 189: 731–739, 2003. [DOI] [PubMed] [Google Scholar]
- Araki et al. 2005.Araki M, Nagayama T, Sprayberry J. Cyclic AMP mediates serotonin-induced synaptic enhancement of lateral giant interneuron of the crayfish. J Neurophysiol 94: 2644–2652, 2005. [DOI] [PubMed] [Google Scholar]
- Aston-Jones et al. 1992.Aston-Jones G, Chiang C, Alexinsky T. Discharge of noradrenergic locus coeruleus neurons in behaving rats and monkeys suggests a role in vigilance. Prog Brain Res 88: 501–520, 1992. [DOI] [PubMed] [Google Scholar]
- Bacon et al. 1995.Bacon JP, Thompson KSJ, Stern M. Identified octopaminergic neurons provide an arousal mechanism in the locust brain. J Neurophysiol 74: 2739–2743, 1995. [DOI] [PubMed] [Google Scholar]
- Barron et al. 2007.Barron AB, Maleszka J,. Van der Meer RK, Robinson GE, Maleszka R. Comparing injection, feeding and topical application methods for treatment of honey bees with octopamine. J Insect Physiol 53: 187–194, 2007. [DOI] [PubMed] [Google Scholar]
- Berridge et al. 1993.Berridge CW, Page ME, Valentino RJ, Foote SL. Effects of locus coeruleus inactivation on electroencephalographic activity in neocortex and hippocampus.Neuroscience 55: 381–393, 1993. [DOI] [PubMed] [Google Scholar]
- Bicker and Menzel 1989.Bicker G, Menzel R. Chemical codes for the control of behavior in arthropods. Nature 337: 33–39, 1989. [DOI] [PubMed] [Google Scholar]
- Buhl et al. 2006.Buhl J, Sumpter DJT, Couzin ID, Hale JJ, Despland E, Miller ER, Simpson SJ. From disorder to order in marching locusts. Science 312: 1402–1406, 2006. [DOI] [PubMed] [Google Scholar]
- Cole and Robbins 1992.Cole BJ, Robbins TW. Forebrain norepinephrine role in controlled information processing in the rat. Neuropsychopharmacology 7: 129–142, 1992. [PubMed] [Google Scholar]
- David et al. 1985.David JC, Coulon JF, Lafon-Cazal M. Octopamine changes in nervous and non-nervous tissues of the locust, Locusta-migratoria L after different flight conditions. Comp Biochem Physiol C Pharmacol Toxicol Endocrinol 82: 427–432, 1985. [Google Scholar]
- Degen et al. 2000.Degen J, Gewecke M, Roeder T. Octopamine receptors in the honey bee and locust nervous system: pharmacological similarities between homologous receptors of distantly related species. Br J Pharmacol 130: 587–594, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Der and Everitt 2002.Der G, Everitt BS. A Handbook of Statistical Analyses Using SAS. Boca Raton, FL: Chapman & Hall/CRC Press, 2002.
- Devauges and Sara 1990.Devauges V, Sara SJ. Activation of the noradrenergic system facilitates an attentional shift in the rat. Behav Brain Res 39: 19–28, 1990. [DOI] [PubMed] [Google Scholar]
- Evans 1985.Evans PD Octopamine. In: Comprehensive Insect Physiology, Biochemistry, and Pharmacology, edited by Kerkut GA, Gilbert LI. Oxford, UK: Pergamon, 1985, vol. 11, p. 499–530.
- Farooqui et al. 2004.Farooqui T, Vaessin H, Smith BH. Octopamine receptors in the honeybee (Apis mellifera) brain and their disruption by RNA-mediated interference. J Insect Physiol 50: 701–713, 2004. [DOI] [PubMed] [Google Scholar]
- Gabbiani et al. 2005.Gabbiani F, Cohen I, Laurent G. Time-dependent activation of feed-forward inhibition in a looming-sensitive neuron J Neurophysiol 94: 2150–2161, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbiani et al. 2002.Gabbiani F, Krapp HG, Koch C, Laurent G. Multiplicative computation in a visual neuron sensitive to looming. Nature 420: 320–324, 2002. [DOI] [PubMed] [Google Scholar]
- Gabbiani et al. 1999.Gabbiani F, Krapp HG, Laurent G. Computation of object approach by a wide-field, motion-sensitive neuron. J Neurosci 19: 1122–1141, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauglitz and Stevenson 1993.Gauglitz S, Stevenson P. Octopamine enhances responsiveness of a locust movement detector interneurone (DCMD). In: Gene, Brain, Behaviour, edited by Elsner N, Heisenberg M. New York: Thieme, 1993, p. 608.
- Goosey and Candy 1980.Goosey MW, Candy DJ. The D-octopamine content of the haemolymph of the locust, Schistocerca americana gregaria and its elevation during flight. Insect Biochem 10: 393–397, 1980. [Google Scholar]
- Gray 2005.Gray JR Habituated visual neurons in locusts remain sensitive to novel looming objects. J Exp Biol 208: 2515–2532, 2005. [DOI] [PubMed] [Google Scholar]
- Gu 2002.Gu Q Neuromodulatory transmitter systems in the cortex and their role in cortical plasticity. Neuroscience 111: 815–835, 2002. [DOI] [PubMed] [Google Scholar]
- Harley 1987.Harley CW A role of norepinephrine in arousal, emotion and learning? Limbic modulation by norepinephrine and the Kety hypothesis. Prog Neuropsychopharmacol Biol Psychiatry 11: 419–458, 1987. [DOI] [PubMed] [Google Scholar]
- Hatsopoulos et al. 1995.Hatsopoulos JK, Gabbiani F, Laurent G. Elementary computation of object approach by a wide-field visual neuron. Science 270: 1000–1003, 1995. [DOI] [PubMed] [Google Scholar]
- Hofmann and Stevenson 2000.Hofmann HA, Stevenson PA. Flight restores fight in crickets (Abstract). Nature 403: 613, 2000. [DOI] [PubMed] [Google Scholar]
- Lapiz and Morilak 2006.Lapiz MDS, Morilak DA. Noradrenergic modulation of cognitive function in rat medial prefrontal cortex as measured by attentional set shifting capability. Neuroscience 137: 1039–1049, 2006. [DOI] [PubMed] [Google Scholar]
- Matheson et al. 2004.Matheson T, Rogers SM, Krapp HG. Plasticity in the visual system is correlated with a change in lifestyle of solitarious and gregarious locusts. J Neurophysiol 91: 1–12, 2004. [DOI] [PubMed] [Google Scholar]
- Orchard et al. 1993.Orchard I, Ramirez J-M, Lange AB. A multifunctional role for octopamine in locust flight. Annu Rev Entomol 38: 227–249, 1993. [Google Scholar]
- O'Shea and Williams 1974.O'Shea M, Williams JLD. The anatomy and output connection of a locust visual interneurone; the lobular giant movement detector (LGMD) neurone. J Comp Physiol A Sens Neural Behav Physiol 91: 257–266, 1974. [Google Scholar]
- Peron et al. 2007.Peron SP, Krapp HG, Gabbiani F. Influence of electrotonic structure and synaptic mapping on the receptive field properties of a collision-detecting neuron. J Neurophysiol 97: 159–177, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainey 1954.Rainey RC Recent developments in the use of insecticides from aircraft against locusts. Rep 6th Commonw Entomol Conf London 6: 48–51, 1954. [Google Scholar]
- Ramirez and Pearson 1991.Ramirez JM, Pearson K. Octopaminergic modulation of interneurons in the flight system of the locust. J Neurophysiol 166: 1522–1537, 1991. [DOI] [PubMed] [Google Scholar]
- Rind 1984.Rind FC A chemical synapse between two motion detecting neurons in the locust brain. J Exp Biol 110: 143–167, 1984. [DOI] [PubMed] [Google Scholar]
- Rind and Simmons 1992.Rind FC, Simmons PJ. Orthopteran DCMD neurone: a re-evaluation of responses to moving objects. I. Selective responses to approaching objects. J Neurophysiol 68: 1654–1666, 1992. [DOI] [PubMed] [Google Scholar]
- Rind and Simmons 1997.Rind FC, Simmons PJ. The signaling of object approach by the DCMD neuron of the locust. J Neurophysiol 77: 1029–1033, 1997. [DOI] [PubMed] [Google Scholar]
- Rind and Simmons 1999.Rind FC, Simmons PJ. Seeing what is coming: building collision sensitive neurones. Trends Neurosci 22: 215–220, 1999. [DOI] [PubMed] [Google Scholar]
- Roeder et al. 1998.Roeder T, Degen J, Gewecke M. Epinastine, a highly specific antagonist of insect neuronal octopamine receptors. Eur J Pharmacol 349: 171–177, 1998. [DOI] [PubMed] [Google Scholar]
- Rogers et al. 2007.Rogers SM, Krapp HG, Burrows M, Matheson T. Compensatory plasticity at an identified synapse tunes a visuomotor pathway. J Neurosci 27: 4621–4633, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowell 1971a.Rowell CHF The Orthopteran descending movement detector (DMD) neurons: a characterization and review. Z Vergl Physiol 73: 167–194, 1971a. [Google Scholar]
- Rowell 1971b.Rowell CHF Variable responsiveness of a visual interneurone in the freely-moving locust, and its relation to behaviour and arousal. J Exp Biol 55: 727–747, 1971b. [Google Scholar]
- Santer et al. 2006.Santer RD, Rind FC, Stafford R, Simmons PJ. The role of an identified looming-sensitive neuron in triggering a flying locust's escape. J Neurophysiol 95: 3391–3400, 2006. [DOI] [PubMed] [Google Scholar]
- Santer et al. 2005.Santer RD, Simmons PJ, Rind FC. Gliding behaviour elicited by lateral looming stimuli in flying locusts. J Comp Physiol A Sens Neural Behav Physiol 191: 61–73, 2005. [DOI] [PubMed] [Google Scholar]
- Schlotterer 1977.Schlotterer GR Response of the locust descending movement detector neuron to rapidly approaching and withdrawing visual stimuli. Can J Zool 55: 1372–1376, 1977. [Google Scholar]
- Simmons 1980.Simmons PJ Connexions between a movement-detecting visual interneurone and flight motoneurones of a locust. J Exp Biol 86: 87–97, 1980. [Google Scholar]
- Simpson et al. 2001.Simpson SJ, Despland E, Hagele BF, Dodgson T. Gregarious behavior in desert locusts is evoked by touching their back legs. Proc Natl Acad Sci USA 98: 3895–3897, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith and Popov 1953.Smith KD, Popov GB. On birds attacking desert locust swarms in Eritrea. Entomologist 86: 3–7, 1953. [Google Scholar]
- Stern 1999.Stern M Octopamine in the locust brain: cellular distribution and functional significance in an arousal mechanism. Microsc Res Tech 45: 135–141, 1999. [DOI] [PubMed] [Google Scholar]
- Stern et al. 1995.Stern M, Thompson KSJ, Zhou P, Watson DG, Midgley JM, Gewecke M, Bacon JP. Morphological, biochemical and electrophysiological characterization of potential modulators of the visual-system. J Comp Physiol A Sens Neural Behav Physiol 177: 611–625, 1995. [Google Scholar]
- Stevenson et al. 2005.Stevenson PA, Dyakonova V, Rillich J, Schildberger K. Octopamine and experience-dependent modulation of aggression in crickets. J Neurosci 25: 1431–1441, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symmons and Cressman 2001.Symmons PM, Cressman K. Desert Locust Guidelines. 1. Biology and Behaviour (2nd ed.). Rome: Food and Agriculture Organization of the United Nations, 2001.
- Vankov et al. 1995.Vankov A, Hervé-Minvielle A, Sara SJ. Response to novelty and its rapid habituation in locus coeruleus neurons of the freely exploring rat. Eur J Neurosci 7: 1180–1187, 1995. [DOI] [PubMed] [Google Scholar]
- Weisel-Eichler and Libersat 1996.Weisel-Eichler A, Libersat F. Neuromodulation of flight initiation by octopamine in the cockroach Periplaneta americana. J Comp Physiol A Sens Neural Behav Physiol 179: 103–112, 1996. [Google Scholar]
- Wilson and Weis-Fogh 1962.Wilson DM, Weis-Fogh T. Patterned activity of co-ordinated motor units, studied in flying locusts. J Exp Biol 39: 643–667, 1962. [Google Scholar]
- Wine and Krasne 1972.Wine JJ, Krasne FB. The organization of escape behaviour in the crayfish. J Exp Biol 56: 1–18, 1972. [DOI] [PubMed] [Google Scholar]
- Womelsdorf et al. 2006.Womelsdorf T, Anton-Erxleben K, Pieper F, Treue S. Dynamic shifts of visual receptive fields in cortical area MT by spatial attention. Nat Neurosci 9: 1156–1160, 2006. [DOI] [PubMed] [Google Scholar]
- Yeh et al. 1996.Yeh S-R, Fricke RA, Edwards DH. The effect of social experience on serotonergic modulation of the escape circuit of crayfish.Science 271: 366–369, 1996. [DOI] [PubMed] [Google Scholar]
- Yeh et al. 1997.Yeh S-R, Musolf BE, Edwards DH. Neuronal adaptations to changes in the social dominance status of crayfish. J Neurosci 17: 697–708, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]