Abstract
This study investigated cellular and synaptic mechanisms of cholinergic neuromodulation in the in vitro lamprey spinal cord. Most spinal neurons tested responded to local application of acetylcholine (ACh) with depolarization and decreased input resistance. The depolarization persisted in the presence of either tetrodotoxin or muscarinic antagonist scopolamine and was abolished with nicotinic antagonist mecamylamine, indicating a direct depolarization through nicotinic ACh receptors. Local application of muscarinic ACh agonists modulated synaptic strength in the spinal cord by decreasing the amplitude of unitary excitatory and inhibitory postsynaptic potentials. The postsynaptic response to direct application of glutamate was unchanged by muscarinic agonists, suggesting a presynaptic mechanism. Cholinergic feedback from motoneurons was assessed using stimulation of a ventral root in the quiescent spinal cord while recording intracellularly from spinal motoneurons or interneurons. Mainly depolarizing potentials were observed, a portion of which was insensitive to removal of extracellular Ca2+, indicating electrotonic coupling. Hyperpolarizing potentials were also observed and were attenuated by the glycinergic antagonist strychnine, whereas depolarizing responses were potentiated by strychnine. Mecamylamine also reduced hyperpolarizing responses. The pharmacology of these responses suggests a Renshaw-like feedback pathway in lamprey. Immunohistochemistry for choline acetyltransferase, performed in combination with retrograde filling of motoneurons, demonstrated a population of nonmotoneuron cholinergic cells in the lamprey spinal cord. Thus endogenous cholinergic modulation of the lamprey spinal locomotor network is likely produced by both motoneurons and cholinergic interneurons acting via combined postsynaptic and presynaptic actions.
INTRODUCTION
Neuromodulation through cholinergic receptors is widespread in the CNS and the effects of acetylcholine (ACh) on the neural network that generates locomotor-like activity in the spinal cord are diverse. Starting with its proper development, the spinal locomotor network depends on cholinergic transmission (Hanson and Landmesser 2003; Myers et al. 2005). During drug-induced locomotor activity in neonatal rat and mouse, exposure of the isolated spinal cord to ACh or cholinergic agents alters the amplitude and frequency of locomotor activity (Miles et al. 2007; Myers et al. 2005) and exposure to ACh alone can induce rhythmic activity in the isolated cord (Cowley and Schmidt 1994). In hatchling Xenopus, cholinergic feedback from spinal motoneurons provides excitation to both motoneurons and interneurons of the locomotor network and cholinergic excitation during locomotion in Xenopus embryos constitutes a significant portion of the depolarizing drive to these neurons (Perrins and Roberts 1995a,b,c). In lamprey, ACh has been shown to be a strong, endogenous neuromodulator involved in fictive swimming, decreasing cycle period and intersegmental phase lag (Quinlan et al. 2004).
At the cellular level, cholinergic neurotransmission from motoneurons and interneurons is involved in modulating neuronal excitability in the spinal cord of many vertebrates. In mammals, collateral axons of motoneurons activate Renshaw cells through nicotinic ACh and ionotropic glutamate receptors (Eccles et al. 1954; Mentis et al. 2005; Nishimaru et al. 2005), which in turn provide inhibition of motoneurons, Ia inhibitory interneurons, and other Renshaw cells in a negative feedback loop during locomotion (Nishimaru et al. 2006; Pratt and Jordan 1987). Evidence for more extensive motoneuron connectivity has been found in the neonatal rat spinal cord, including excitatory feedback to spinal interneurons in the presence of noradrenaline (Machacek and Hochman 2006). Other cholinergic neurons in the mammalian spinal cord include central canal cells (lamina X) and the partition cells, the latter of which extend from the central canal to the lateral edge of the gray matter and have been proposed to participate in locomotor activity on the basis of c-fos staining and electrophysiological recordings (Barber et al. 1984; Borges and Iversen 1986; Carr et al. 1995; Huang et al. 2000; Sherriff and Henderson 1994). Recently, evidence has come forth that medial partition cells give rise to the large cholinergic C-terminals on motoneurons (Miles et al. 2007). Activation of muscarinic cholinergic receptors on motoneurons increases excitability through reduction of the after-spike hyperpolarization (AHP; Chevallier et al. 2006; Miles et al. 2007). In addition to motoneurons, many other spinal neurons of the dorsal and ventral horns and lamina X are responsive to acetylcholine (Bordey et al. 1996a,b; Jiang and Dun 1986; Urban et al. 1989; Zieglgänsberger and Reiter 1974), indicating that both motoneuron and interneuron excitability are modulated by ACh.
The central pattern generator (CPG) for swimming in the lamprey spinal cord has provided a model system in which to study the vertebrate locomotor network. During fictive swimming, sufficient ACh is present in the isolated spinal cord to provide ongoing modulation of the locomotor network through both nicotinic and muscarinic receptors (Quinlan et al. 2004). The goal of the present study was to begin to understand the mechanisms of this cholinergic modulation at the cellular and synaptic levels. To this end we have examined individual neuronal responses to ACh, modulation of synaptic strength through muscarinic receptors, and the possible sources of ongoing cholinergic modulation of fictive swimming, including the identification of a novel class of cholinergic interneurons in the lamprey spinal cord.
METHODS
For these experiments, 86 adult silver lamprey (Ichthyomyzon unicuspis) and 6 sea lamprey (Petromyzon marinus) in the transformer stage were used. No differences were observed between the two species and therefore the data were combined. The experiments were conducted according to the American Physiological Society's Guiding Principles in the Care and Use of Animals and were approved by the Marquette University Institutional Animal Care and Use Committee. The animals were housed in aerated freshwater aquaria at 5°C until their use. Lampreys were immersed in an anesthetizing solution of tricaine (3-aminobenzoic acid ethyl ester, ∼250 mg/l) until responses to tail pinch were lost. The dissection was then performed in cooled Ringer solution of the following composition (in mM): 91 NaCl, 2.6 CaCl2, 2.1 KCl, 1.8 MgCl2, 4.0 glucose, 20 NaHCO3, 8 HEPES (free acid), and 2 HEPES (sodium salt) (pH = 7.4). The dissection began by isolating the body region extending from the caudal end of the gills to the anus with transverse cuts of the body. The viscera, skin, and muscles were then removed, leaving the notochord and spinal cord. The spinal cord was exposed with a midline longitudinal cut of the cartilage overlying the spinal cord. From this midbody region, preparations consisting of 7 to 20 spinal segments were used for experiments in which the notochord was split along the ventral midline, splayed laterally, and pinned to a Sylgard-lined (Dow Corning) chamber, and the pia was removed from the dorsal surface of the spinal cord. The chamber was perfused with oxygenated and cooled (7–10°C) Ringer solution at all times. Tissue was stored in Ringer solution at 4°C until its use, which was always within 3 days of dissection. Unless otherwise noted, all chemicals were obtained from Sigma–Aldrich.
Electrophysiology
Intracellular sharp microelectrodes were pulled from borosilicate filament-containing capillaries (World Precision Instruments [WPI]) and filled with 4 M potassium acetate (resistance between 40 and 100 MΩ). Recordings were made with an Axoclamp 2A amplifier (Axon Instruments), in bridge mode or in discontinuous current-clamp mode, with a sampling rate of 2 kHz. A Cyberamp 320 (Axon Instruments) amplified and filtered the signal (final gain of 50, low-pass filter set at 3 kHz) and a 1401 PC-computer interface (Cambridge Electronic Design) was used for analog-to-digital conversion and storage to disk. The digitizing rate for intracellular recording was ≥6 kHz and recordings were obtained, stored, and analyzed using Spike2 software (Cambridge Electronic Design). Extracellular glass suction electrodes were used to stimulate and record ventral roots by placing the tip onto the root near its exit point from the spinal cord. Glass suction electrodes were also used to stimulate and record the spinal cord activity by splitting the spinal cord down the midline for 1–2 mm at one or both ends of the spinal cord piece and drawing the hemicord into the suction electrode. Stimulation was done through a stimulus isolator (model A360, WPI). A differential AC amplifier (model 1700, A-M Systems) filtered and amplified the extracellular data. The high-pass filter was set at 100 Hz and the low-pass filter at 1,000 Hz. Data were amplified ×10,000 and digitized by the 1401 interface at 2 kHz.
Cell identification
As previously described (Buchanan 1993) spinal neurons were identified on the basis of their axonal projection recorded with extracellular suction electrodes. One-for-one extracellular spikes occurring with constant latency following an intracellular action potential were taken as evidence that the neuron projected to that location. Motoneurons (MNs) projected out the nearby ipsilateral ventral root and CC interneurons (CCINs; locomotor interneurons consisting of both inhibitory and excitatory types) projected to the contralateral and caudal end of the spinal cord piece. In addition, some spinal neurons were first identified by their somatic shape and location. These included the dorsal cells (a class of primary sensory neurons with mechanosensitivity in the skin), lateral interneurons (LINs; large inhibitory interneurons in the rostral half of the spinal cord with an ipsilateral descending axon), and giant interneurons (GINs; large excitatory interneurons in the caudal third of the spinal cord with a contralateral ascending axon). Recorded neurons for which no axonal projection was determined are referred to as unidentified neurons in the text.
Application of drugs
For local application of drugs, a pipette containing ACh (or another drug when specified), diluted in Fast Green and Ringers, was positioned above the bath in close proximity to the target neuron. After the neuron was impaled and a recording was started, the ACh pipette was lowered into the bath and positioned about 100 μm above the dorsal surface of the cord near the recorded neuron. Expulsion of small amounts (∼100 nl) of the pipette solution was controlled by a Picospritzer II (General Valve) and visual confirmation that the solution was applied only to the immediate vicinity of the neuron was possible due to the presence of Fast Green. Drugs applied in this way were ACh (2–10 mM), muscarine (1 mM), oxotremorine (1 mM), nicotine (10 mM), and d-glutamate (1 mM). A second method of drug application was by bath perfusion in Ringers. Bath-applied drugs included tetrodotoxin (TTX, 1–3 μM), the inhibitory blockers strychnine (5 μM) or bicuculline (20 μM), the muscarinic antagonist scopolamine (10 μM), and the nicotinic antagonist mecamylamine (20 μM).
Histology
Three lampreys were used in the following histological procedures. After dissection of the spinal cord/notochord in the midbody region, lengths of 5–11 segments of the spinal cord were exposed in cooled Ringers (4–10°C) and the meninges removed. Depending on the length of the cord, one to four ipsilateral ventral roots were cut and fixable dextran Alexa Fluor 488 (Molecular Probes) was applied to the cut ends to retrogradely label the motoneurons. Care was taken that the ventral roots labeled were at least two segments away from the ends of the spinal cord so the dextran Alexa would not be taken up by axons in the cut ends. The spinal cord/notochord preparation was then allowed to incubate in fresh Ringers for ≥24 h in the dark at 7°C. Tissue was then fixed and mounted in glycerol to verify the labeling of motoneurons. The tissue was fixed by immersion in 4% paraformaldehyde in 0.1 M phosphate buffer (PB) for 4–24 h at 4°C, and cryoprotected by immersion in 15% sucrose PB overnight. Spinal cord pieces were then wrapped in muscle tissue from a rabbit diaphragm for further protection and submerged first in liquid nitrogen-cooled 2-methylbutane (Fischer) then in liquid nitrogen. Frozen tissue was mounted on a block with Tissue-Tek O.C.T. Compound (Miles) and sectioned into 35-μm cross sections in a cryostat and placed on slides. Subsequent steps were carried out in the dark at 7°C. Sections were allowed to air dry for a few hours, washed with phosphate-buffered saline (PBS) three times for 10 min each, preincubated for 1 h in 10% normal donkey serum (NDS) and 0.4% Triton in PBS. After another wash in PBS the tissue was incubated in the primary antibody (goat anti- choline acetyltransferase [ChAT] Ab144P from Chemicon International) diluted 1:200 in 4% NDS, 0.1% Triton, and 0.05% sodium azide in PBS for 40 h. Sections were washed (three times for 10 min each, PBS), incubated 1 h in the secondary antibody (Cy-3 Affinipure donkey anti-goat IgG, Jackson ImmunoResearch), diluted 1:500 in 4% NDS in PBS, washed (three times for 10 min each, PBS), and mounted in glycerol. Sections were visualized using a confocal microscope (Zeiss).
Statistics
To determine statistical significance of the data collected, two-tailed t-tests were used throughout; results described as significant have P values <0.05. For the data on changes in input resistance with ACh, a paired t-test was used to determine significance. In the remaining studies, the data were normalized to 100% and unpaired t-tests were performed. Unpaired t-tests were used rather than paired due to the higher efficiency in detecting small changes in a relatively low n.
RESULTS
Local application of ACh
GENERALIZED SPINAL ACTIVITY.
The aim of the first set of experiments was to determine the response of spinal neurons to ACh in a quiescent preparation. In general, local application of ACh had excitatory nicotinic actions in the lamprey spinal cord. In the two experiments illustrated in Fig. 1, local application of ACh (2 mM) to the dorsal surface of the spinal cord above the impaled neuron resulted in a powerful and prolonged (≤3 min) burst of action potentials as recorded extracellularly from the rostral spinal cord and the nearby ventral root (Fig. 1, Bi and Ci). In 23 of 26 preparations, application of ACh evoked bursts of action potentials in both rostral and caudal ends of the spinal cord. Similarly, in 24 of 25 preparations, ventral root activity was evoked with local application of ACh, although not all of the intracellularly recorded motoneurons reached spike threshold (Fig. 1Ci). In two preparations, rostral and caudal hemicords were recorded using four extracellular recording electrodes while ACh was applied to one side of the cord. Moving the site of application in the rostrocaudal axis made little difference in hemicord responses. However, in both preparations moving the application from left to right sides changed the site of the strongest activity to the hemicord located contralateral to the application site (data not shown).
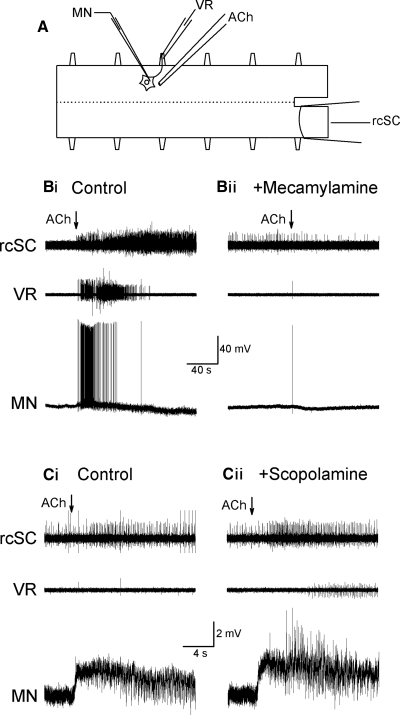
FIG. 1.
Response to local acetylcholine (ACh) application. A: schematic diagram of experimental setup, with extracellular ventral root and spinal cord electrodes recording overall activity and an intracellular microelectrode recording individual cells. Example of responses in Ringers (Bi) and after perfusion of nicotinic antagonist mecamylamine (20 μM; Bii) had attenuated the excitatory responses, revealing a hyperpolarizing response. In another experiment the responses observed in Ringers (Ci) and after perfusion of muscarinic antagonist scopolamine (10 μM; Cii) showing a slightly stronger response.
RESPONSE OF INDIVIDUAL NEURONS TO ACH.
Local application of ACh caused spinal neurons either to spike (Fig. 1Bi) or, in many cases, to depolarize without firing action potentials (Fig. 1Ci). Perfusion of the bath with the nicotinic antagonist mecamylamine (20 μM) for 30 min blocked the response to a second application of ACh in the intracellularly recorded neurons (n = 4, 2 MNs, 1 GIN, and 1 unidentified neuron) (Fig. 1Bii) and in the ventral root and cord recordings (n = 2), thus indicating that the excitatory effect was mediated by nicotinic receptors. Bath perfusion of the muscarinic antagonist scopolamine (10 μM, 30 min) did not attenuate the response in either the cord (n = 4) or the depolarizing response in individual neurons (n = 4, 1 MN, 1 GIN, 2 unidentified neurons; Fig. 1Cii), suggesting that muscarinic receptors are not directly involved in the excitatory response to ACh. Overall, in intracellularly recorded spinal neurons, 57 of 79 cells responded to ACh with depolarization (30/39 MNs; 3/7 GINs, 3/6 LINs, 3/4 CCINs, and 18/23 unidentified neurons). In some cells (7 of 79, including 3 MNs, 1 GIN, 1 CCIN, and 2 unidentified neurons) hyperpolarization alone was observed (Table 1).
TABLE 1.
Responses of individual neurons to application of acetylcholine (ACh) and cholinergic agonists
| Response |
ACh |
Nicotine | Musc or Oxo | |||
|---|---|---|---|---|---|---|
| +Ringer | +TTX | +Scop | +Mec | |||
| Depolarization | 57/79 | 27/33 | 8/8 | 1/11 | 18/19 | 4/68 |
| Mean ± SD, mV | 4.1 ± 2.2* | 5.6 ± 4.3 | 3.0 ± 2.9 | 5.6 ± 2.4 | 4.0 ± 2.6 | |
| Hyperpolarization | 7/79 | 3/33 | 0/8 | 0/11 | 1/19 | 19/68 |
| Mean ± SD, mV | 4.9 ± 2.8 | 5.3 ± 2.6 | 4.9 ± 3.3 | |||
| Depolarization + hyperpolarization | 7/79 | 2/33 | 0/8 | 1/11 | 0/19 | 1/68 |
| No change in membrane potential | 8/79 | 1/33 | 0/8 | 9/11 | 0/19 | 44/68 |
The neuronal responses recorded in normal Ringer solution or in TTX are counted together (except for the first two columns). Scop, muscarinic antagonist scopolamine; mec, nicotinic antagonist mecamylamine; musc and oxo, muscarinic agonists muscarine and oxotremorine, respectively.
Depicts the mean depolarization observed in neurons that were not brought to threshold.
In normal Ringer solution, the local application of ACh not only depolarized neurons but also increased synaptic activity in the intracellularly recorded neuron (Fig. 1Ci). Therefore to confirm that acetylcholine had direct actions independent of spike-mediated synaptic transmission, local application of ACh was repeated in the presence of tetrodotoxin (TTX, 1–3 μM) in the bathing solution. In Fig. 2A, the response of an LIN to local application of ACh (2 mM) is shown in normal Ringer solution (Fig. 2Ai) and in TTX (Fig. 2Aii). After TTX, the LIN still depolarized in response to local application of acetylcholine, indicating a direct response. An interval of ≥20 min separated all applications of Ach because there was desensitization in the subsequent responses for 10–15 min following an application. In TTX, depolarization to ACh was observed in MNs (9/12), LINs (4/5), CCINs (2/2), and unidentified neurons (12/14). To address the pharmacology of the depolarization evoked by ACh, the muscarinic and nicotinic antagonists scopolamine and mecamylamine, respectively, were individually perfused into the bath containing TTX (Fig. 2, B and C). As was the case in normal Ringer solution (Fig. 1C), blocking muscarinic receptors with scopolamine (10 μM) did not attenuate the response to ACh in TTX as shown for a motoneuron in Fig. 2B and as was seen in all 6 neurons (1 MN, 2 LINs, 2 GINs, and 1 CCIN) tested in the presence of both TTX and scopolamine. Perfusion of the nicotinic antagonist mecamylamine (20 μM) with TTX blocked the depolarization in an unidentified neuron in Fig. 2C, and was seen in 6 of 7 neurons tested in TTX and mecamylamine (1 MN, 1 CCIN, and 4 unidentified neurons), similar to the responses in normal Ringers. One unidentified neuron still depolarized with mecamylamine in TTX, although the response was greatly reduced compared with the previous application, also in TTX. Overall, 27 of 33 neurons (82%) depolarized in response to ACh while in TTX, consistent with the results in normal Ringers (57/79 or 72% of neurons depolarized in response to ACh).
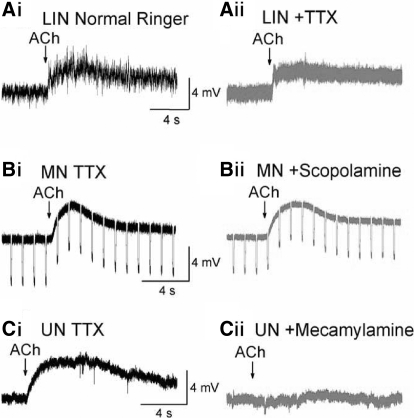
FIG. 2.
Neurons are depolarized directly by ACh application. Ai: a lateral interneuron depolarized and received increased synaptic input after ACh was applied in Ringers. Aii: depolarization persisted in the lateral interneuron after perfusion of 3 μM tetrodotoxin (TTX). Bi: depolarization of a motoneuron in TTX. Bii: depolarization persisted after perfusion of muscarinic antagonist scopolamine (10 μM). Ci: depolarization of an unidentified neuron in TTX. Cii: depolarization was blocked after perfusion of nicotinic antagonist mecamylamine (20 μM).
Local application of nicotine (10 mM) depolarized 15 of 16 neurons tested in normal Ringer and increased synaptic activity in 16/16 cells. In TTX, 3 of 3 neurons tested also depolarized when nicotine was applied locally. Muscarinic receptors were not directly involved in the neuronal response to ACh described earlier, in that the muscarinic agonists oxotremorine or muscarine (both 1 mM) did not produce a change in membrane potential in 44/68 cases (65%). However, in 19 of the 68 neurons (28%), muscarinic agonists elicited a hyperpolarization. Neuronal responses to ACh and other cholinergic agents are summarized in Table 1. Since TTX did not change the nature of neuronal responses to ACh or cholinergic agonists, data were grouped without respect to the presence or absence of TTX in Table 1. The various classes of neurons showed similar responses to ACh, with the possible exception of GINs, which depolarized slightly less often (43%) than overall (72% of neurons in Ringers and 82% of neurons in TTX depolarizing).
Since nicotinic ACh receptors appear to mediate the depolarization, opening of the ionotropic nicotinic ACh receptors should decrease the input resistance of the cell. In 27 of 42 neurons, a decrease in the input resistance of the cell was observed on local application of ACh in normal Ringer, similar to the decrease in input resistance in 12/21 neurons recorded in TTX. The decrease in input resistance followed a time course matching the depolarization evoked by ACh. Overall, mean input resistance after ACh application was significantly reduced (19.4 ± 11.4 to 18.4 ± 10.8 MΩ, n = 42), based on a paired t-test.
Muscarinic modulation of synaptic potentials
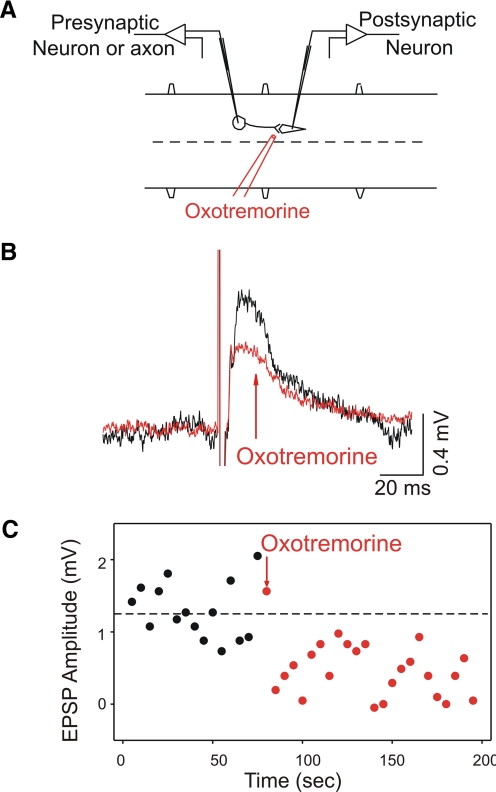
Muscarinic modulation of synaptic strength has been reported in the lamprey brain stem (LeRay et al. 2004), so this possible muscarinic effect was investigated in the spinal cord in the next set of experiments. Neurons were recorded intracellularly in normal Ringer solution, without any antagonists, while the ipsilateral or contralateral rostral spinal cord was stimulated (0.1-Hz, 100-μs pulses, 140–550 μA) to elicit predominantly depolarizing or hyperpolarizing compound postsynaptic potentials (PSPs), respectively (Fig. 3A). Muscarinic agonists (muscarine or oxotremorine, both 1 mM) were then applied locally to the postsynaptic neuron from a micropipette using pressure (n = 12). Averaged compound PSP amplitude was reduced by muscarinic agonists. Mean compound excitatory postsynaptic potentials (EPSPs) evoked in this way were significantly reduced to 73% of control (from 4.6 ± 2.7 to 3.1 ± 2.0 mV) after application of muscarinic agonists (n = 6, all MNs).
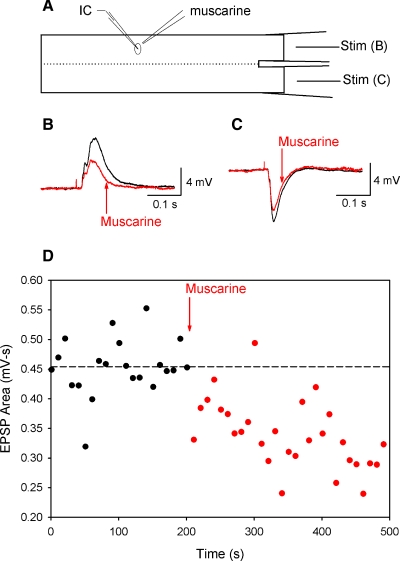
FIG. 3.
Muscarinic agonists decrease compound postsynaptic potentials (PSPs). A: schematic of experimental setup, in which rostral hemicord is stimulated to elicit compound PSPs. Ipsilateral stimulation produces mainly excitatory PSPs (EPSPs), whereas contralateral stimulation produces mainly inhibitory PSPs (IPSPs). B: an example of an EPSP before (black) and after (red) the local application of muscarine (1 mM). C: compound IPSPs were also reduced after muscarine application. D: the reduction in the PSP area was time locked to application of muscarine, as shown for the PSP from B.
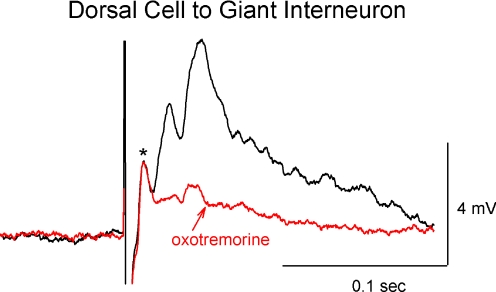
The dorsal cell to giant interneuron synapse was also used to test muscarinic modulation of excitatory synaptic activity. The dorsal cell often produces both electrical and chemical components in the giant interneuron, including a large polysynaptic compound EPSP, as can be seen in the example in Fig. 4. The polysynaptic chemical component of the PSP was significantly reduced to 44% of control after application of oxotremorine (n = 6, 3.3 ± 3.4 mV before and 1.1 ± 0.8 mV after), whereas the presumed electrical component (based on latency; marked with an asterisk) remained unchanged (P = 0.98, mean 2.6 ± 1.3 mV before and 2.5 ± 1.2 mV after). Table 2 summarizes the effects of muscarinic agonists on compound EPSPs from either ipsilateral cord stimulation or from dorsal cell to giant interneuron pairing.
FIG. 4.
The chemical component of the dorsal cell to giant interneuron synapse was reduced by muscarinic agonists. The presumed electrical component of the synapse, marked with an asterisk (*) was unchanged after oxotremorine application, unlike the chemical component, which was greatly reduced. Control is shown in black; after oxotremorine is in red.
TABLE 2.
Muscarinic modulation of postsynaptic potentials
| Category | Compound | Unitary |
|---|---|---|
| All PSPs (n) | 18 | 16 |
| Mean control, mV | 4.0 ± 2.6 | 0.7 ± 0.8 |
| Mean after musc, mV | 2.6 ± 1.8 | 0.4 ± 0.4 |
| Norm mean after musc | 67 ± 25% | 67 ± 31% |
| P value | <0.001 | <0.001 |
| Excitatory PSPs (n) | 12 | 8 |
| Mean control, mV | 3.9 ± 3.0 | 0.7 ± 1.0 |
| Mean after musc, mV | 2.1 ± 1.8 | 0.4 ± 0.4 |
| Norm mean after musc | 57 ± 24% | 70 ± 18% |
| P value | <0.001 | <0.001 |
| Inhibitory PSPs (n) | 6 | 8 |
| Mean control, mV | 4.3 ± 1.7 | 0.6 ± 0.6 |
| Mean after musc, mV | 3.6 ± 1.3 | 0.4 ± 0.5 |
| Norm mean after musc | 87 ± 11% | 63 ± 41% |
| P value | <0.05 | <0.05 |
Values are means ± SD. Compound PSPs were elicited from hemicord stimulation in normal Ringer solution and from dorsal cell to giant interneuron synapses. Unitary PSPs were recorded from neuron pairs or axon–neuron pairs. P values are the result of unpaired t-tests performed on the normalized data.
Mean compound inhibitory postsynaptic potential (IPSP) amplitude was also significantly reduced to 87% of control after application of muscarinic agonists (n = 6, reductions in 3/4 MNs, 2/2 unidentified neurons; see Table 2). Two representative experiments are shown in Fig. 3, B and C. In Fig. 3, B and C, the depolarizing and hyperpolarizing compound PSPs in motoneurons were reduced in amplitude after local application of muscarine. The time course of the reduction in PSP amplitude was locked to the muscarine application, as seen when the area of the PSP in Fig. 3B is plotted in Fig. 3D.
Unitary EPSPs (n = 8) and IPSPs (n = 8) were also tested for sensitivity to muscarinic agonists. Individual neuron pairs or axon–neuron pairs that were synaptically connected were recorded during application of muscarinic agonists. An example in Fig. 5 shows the potentials elicited in a motoneuron from stimulation of an unidentified axon before and after oxotremorine application and the time course of the synaptic depression. Unitary EPSPs significantly decreased in amplitude after application of muscarinic agonists. The postsynaptic cells were motoneurons (4/4 showed reductions in EPSPs) and unidentified neurons (3/4 showed reductions). Unitary IPSPs significantly decreased in the presence of muscarinic agonists. In this set of experiments all six motoneurons recorded showed reductions in IPSPs, whereas neither of two IPSPs recorded in unidentified neurons was reduced with muscarinic agonists. A summary of the results of muscarinic modulation of PSPs is shown in Table 2.
FIG. 5.
Muscarinic agonists reduce unitary EPSPs. A: a schematic of the experimental setup of the paired intracellular recording and local drug application. B: an example of an EPSP before (black) and after (red) local application of muscarinic agonist oxotremorine (1 mM). C: the time course of the decrease in EPSP amplitude from B is shown to be closely associated with drug application.
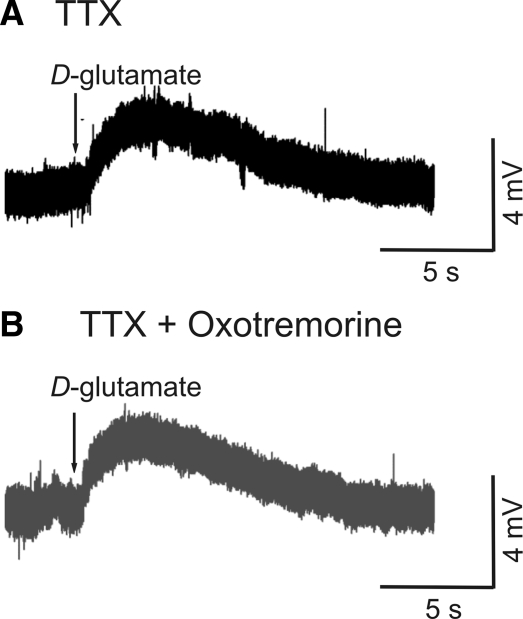
To test whether the muscarinic action on excitatory synaptic potentials could be occurring by a presynaptic mechanism, 4 neurons (1 MN, 3 unidentified neurons) were recorded in TTX while glutamate (1 mM) was applied to the cell by pressure ejection (Fig. 6A), then washed out, and reapplied immediately following the local application of oxotremorine (1 mM; Fig. 6B). The response was unchanged after oxotremorine application (P = 0.4; 5.0 ± 3.3 mV before and 5.0 ± 3.4 mV after), indicating that a presynaptic site of action for muscarinic ACh receptors is likely.
FIG. 6.
Postsynaptic response to glutamate is unchanged by muscarinic agonists. A: the control response to d-glutamate (1 mM) in the presence of TTX. B: response was unchanged after application of oxotremorine, indicating postsynaptic mechanisms are not affected by muscarinic agonists.
Antidromic firing of motoneurons
Previous experiments (e.g., Quinlan et al. 2004) showed that endogenous cholinergic modulation of network activity was taking place during fictive swimming. To investigate the possibility that motoneurons could be contributing to cholinergic neurotransmission in the lamprey spinal cord, antidromic stimulation of motoneurons through an extracellular electrode placed on the ventral root was performed while recording from various types of spinal neurons. For each experiment, the threshold for ventral root stimulation was carefully determined. For experiments in which nonmotoneurons were recorded, 2 to 5 motoneurons were first impaled and a mean threshold for antidromic firing was calculated. The current amplitude was then set at threefold the mean to ensure maximum motoneuron recruitment. When recording intracellularly from motoneurons, the ventral root stimulation was set at a current just below threshold for that motoneuron (but presumably above threshold for some of the motoneurons in the root). Since DC spread from the ventral root electrode to the cord was a concern, in seven preparations the amplitude of stimulation required for direct activation of spinal axons (recorded with a suction electrode on the cut end of the spinal cord) was compared with the stimulation thresholds for antidromic motoneuron activation. Mean stimulation amplitude required for antidromic firing of 23 motoneurons was 100 ± 43 μA, whereas for current spread to the cord 1,200 ± 580 μA was required, 12-fold the mean values for motoneuron recruitment. These values were significantly higher (P < 0.001).
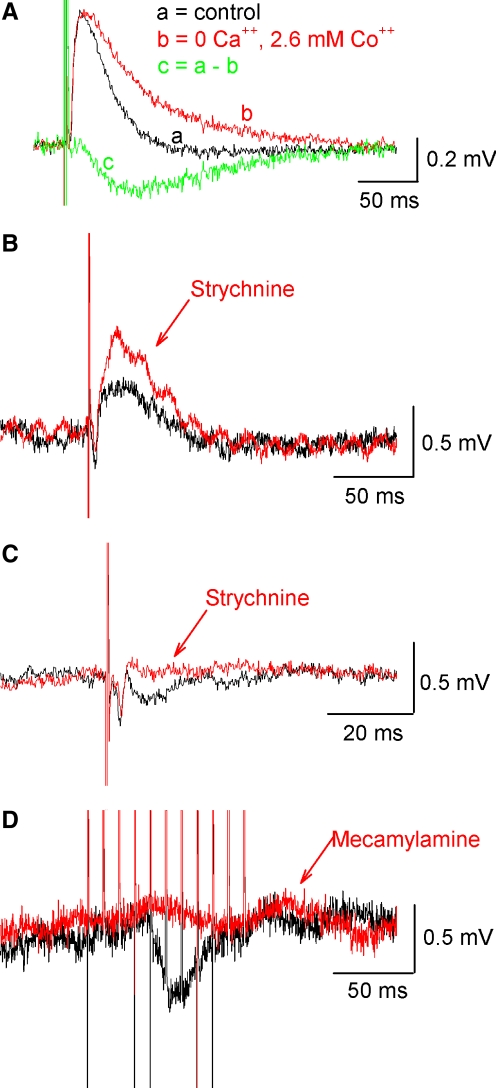
Typically, ventral root stimulation elicited depolarizing responses in motoneurons and interneurons. To test whether these responses required calcium-mediated synaptic transmission, the spinal cord was perfused with a zero-calcium solution in which the calcium was omitted from the Ringer solution (n = 2), replaced by cadmium (1 mM, n = 1) or replaced by cobalt (2.6 mM, n = 6). In all nine cases, the depolarization persisted in 0 Ca2+ with no significant change in the peak amplitude (control = 0.45 ± 0.28 mV; 0 Ca = 0.47 ± 0.15 mV; P = 0.73). However, decay of the response increased in amplitude in 0 Ca2+ as measured 100 ms after the stimulus artifact (control = −0.03 ± 0.03 mV; 0 Ca2+ = 0.1 ± 0.09 mV; P < 0.002), suggesting that a slower hyperpolarizing component was blocked by 0 Ca2+. These results suggest that the antidromic response consists of both an electrotonic component and a Ca2+-dependent chemical synaptic component. An example is shown in Fig. 7A in which subtraction of the averaged responses in cobalt from the averaged control traces revealed a difference curve resembling a hyperpolarizing postsynaptic potential. Three other examples of the neuronal responses to antidromic firing of motoneurons are shown in Fig. 7, B–D. Whereas antidromic stimulation in normal Ringer most often produced a depolarizing response in neurons (24/70; mean 0.4 ± 0.3 mV), in other cases, depolarization was mixed with hyperpolarization (13/70) or hyperpolarization alone was observed (12/70; mean 0.8 ± 0.5 mV). In responses that were mainly depolarizing, as is seen in the LIN in Fig. 7B, the depolarization significantly increased to 160% of control after strychnine (5 μM) was perfused (mean 0.7 ± 0.6 mV before to 0.8 ± 0.7 mV after), indicating the presence of an inhibitory glycinergic component that was masked by a depolarizing response (n = 8, recorded from 4 MNs, 2 GINs, 1 LIN, and 1 unidentified neuron). Figure 7, C and D shows examples of hyperpolarizing responses that were evoked from antidromic motoneuron firing. In Fig. 7C, a CCIN was recorded and a small hyperpolarizing response was visible in the control (black) that was sensitive to blockade by the glycinergic antagonist strychnine (red). Significant reduction of hyperpolarizing responses to 8% of control after strychnine perfusion was observed in 6 neurons tested (3 MNs, 1 CCIN, 1 GIN, and 1 unidentified neuron; mean amplitude of hyperpolarizing response −0.6 ± 0.5 mV before and −0.1 ± 0.3 mV after). In one case, a 1.4-mV IPSP in a motoneuron was reduced to only half that amplitude after 40-min exposure to strychnine, at which point bicuculline was perfused along with strychnine and the hyperpolarizing response was abolished. Responses were also sensitive to the nicotinic receptor blocker mecamylamine (20 μM). In Fig. 7D, a hyperpolarizing response in a giant interneuron evoked by a train of stimuli to the ventral root was attenuated in the presence of mecamylamine; overall, mecamylamine significantly reduced hyperpolarizing potentials to 24% of control (n = 6; from −0.9 ± 1.3 to −0.4 ± 0.8 mV, in 2 MNs, 2 GINs, 1 LIN, and 1 unidentified neuron). Depolarizing potentials were also significantly reduced with mecamylamine to 39% of control (n = 4), indicating motoneurons also have excitatory cholinergic synaptic contacts in the spinal cord (responses observed in 2 MNs, 1 GIN, and 1 unidentified neuron; mean 0.5 ± 0.2 mV before to 0.2 ± 0.2 mV after). Results from all the experiments of antidromic firing of MNs (summarized in Table 3) indicate that in addition to a depolarizing passive current, antidromic firing of MNs can elicit PSPs that are sensitive to blockade by both nicotinic and glycinergic antagonists.
FIG. 7.
Pharmacology of averaged PSPs elicited from antidromic stimulation of motoneurons via the ventral root. A: a depolarizing response was present (black trace) that persisted and was enhanced in 0 Ca2+ Ringer (red trace). Subtraction reveals a hyperpolarizing component (green trace) present in the control. B: in some neurons a depolarizing response was masking a strychnine-sensitive hyperpolarizing potential. Control PSP shown in black and after 5 μM strychnine in red. C: in this example ventral root stimulation evoked a hyperpolarizing potential in a CCIN (locomotor interneuron consisting of both inhibitory and excitatory types), which was abolished with strychnine. D: hyperpolarizing responses were also sensitive to nicotinic receptor blockade, as shown in this example from a giant interneuron. All data are averages of ≥30 traces.
TABLE 3.
Summary of neuronal responses to ventral root (VR) stimulation
| Category | n |
|---|---|
| Response to VR stimulation | |
| Depolarization | 24/70 |
| Hyperpolarization | 12/70 |
| Depolarization + hyperpolarization | 13/70 |
| No response | 21/70 |
| Change in response with strychnine | |
| Increase in depolarization | 6/8 |
| Decrease in depolarization | 1/8 |
| No change in depolarization | 1/8 |
| Decrease in hyperpolarization | 6/6 |
| Change in response with mescamylamine | |
| Decrease in depolarization | 4/7 |
| No change in depolarization | 3/7 |
| Decrease in hyperpolarization | 2/2 |
Summary of neuronal responses to VR stimulation and the changes with glycinergic and cholinergic agents.
ChAT immunohistochemistry
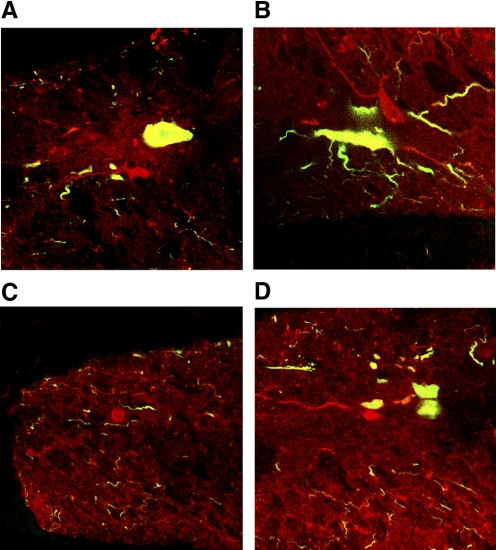
To determine whether the lamprey spinal cord contains cholinergic interneurons, motoneurons were first labeled with a retrograde tracer (fixable dextran Alexa 488) by incubating cut ventral roots in the in vitro preparation with the tracer for 24 h at 7°C. Five such preparations were used to determine the completeness of motoneuron labeling. Individual motoneurons appeared to be completely labeled, including fine dendritic processes. Sections of the labeled spinal cord were then cut and processed for ChAT immunohistochemistry. Examples of the resultant staining are shown in Fig. 8. The combined staining of retrograde green Alexa 488 and red Cy-3 for ChAT resulted in a yellow color in the motoneurons (Fig. 8, A, B, and D). In addition, there were also smaller cells that were stained red, indicating positive staining for ChAT but lacking the green retrograde labeling (Fig. 8, A–D). The mean length of the short axis of motoneuron somata measured here was 22.9 μm (range = 8.9–39.2 μm), whereas the mean short-axis somal length of the neurons labeled for only ChAT was 11.5 μm (range = 8.0–18.5 μm). The difference between the two populations was statistically significant. The nonmotoneuron cholinergic cells were located throughout the gray matter, and one was found outside of the spinal gray matter in the dorsolateral axon tracts where motoneurons were never found (Fig. 8C). To determine whether the ChAT-positive cells did not simply represent motoneurons that failed to be retrogradely labeled, cell counts of both ChAT-positive and retrograde motoneurons were made. From 9 roots in 5 spinal cord preparations, 547 motoneurons were retrogradely labeled, for a mean of 61 motoneurons per ventral root (range of 57–65 per ventral root). Rovainen and Dill (1984) counted axons in ventral roots from electron micrographs and found a mean of 70.6 ± 9.3 axons per ventral root in Ichthyomyzon unicuspis. Assuming no axonal branching, this value indicates 71 motoneurons per hemisegment. Therefore it is possible that about 10 motoneurons per hemisegment failed to retrogradely label. In a group of 28 serial sections of 35 μm in which motoneuron labeling remained constant, 14 nondouble-labeled cholinergic cells were stained. These sections accounted for 1 mm of tissue, 40% of the length of one segment (∼2.5 mm/segment). That would indicate about 35 of these cholinergic neurons would be found per hemisegment, considerably greater than the number of motoneurons that potentially failed to be retrogradely labeled (10/hemisegment). Based on the number, morphology, and location of these cells, they probably constitute one or more populations of cholinergic neurons distinct from the motoneurons.
FIG. 8.
Nonmotoneuron cholinergic cells of the lamprey spinal cord. A–D: red cells are labeled for choline acetyltransferase (ChAT) alone. Motoneurons are visible in A, B, and D as yellow cells, due to the combination of green Alexa tracer and the red Cy-3 marker used in the ChAT immunohistochemistry. Nonmotoneuron ChAT-positive cells were located in the gray matter, with the exception of the cell in C, which was located in the dorsolateral axon tracts.
DISCUSSION
This report describes for the first time the direct depolarizing response of individual neurons of the lamprey spinal cord to nicotinic ACh receptor activation and decreases in both EPSP and IPSP strength with muscarinic receptor activation. The pharmacology of motoneuron and interneuron responses to antidromic firing of motoneurons shows that lamprey, like many other vertebrates, have motoneuron feedback through cholinergic and glycinergic synapses. Furthermore, through histochemical techniques we have found cholinergic spinal interneurons in the lamprey.
Previously, we described endogenous modulation of fictive locomotion in lamprey by ACh, via both nicotinic and muscarinic ACh receptor activation. Activation of both receptor types brought about similar effects on swimming activity—shortening of the cycle period of ventral root bursting and a decrease in intersegmental phase lag. The changes in cellular and synaptic properties with ACh receptor activation and the possible sources of endogenous ACh in the lamprey spinal cord found in this study provide a first step toward understanding the cellular and synaptic mechanisms of this modulation.
Direct nicotinic depolarization
Most of the neurons examined depolarized directly in response to ACh via nicotinic receptors and showed an associated decrease in input resistance. In a previous study in the lamprey spinal cord, giant interneurons were found insensitive to acetylcholine (Martin et al. 1970), although of all the neurons recorded in the present study, giant interneurons depolarized the least often with ACh. However, findings similar to ours have been reported in the lamprey brain stem, where reticulospinal neurons depolarize with nicotinic ACh receptor activation exerted by cholinergic projections from the mesencephalic locomotor region (LeRay et al. 2003). In other vertebrates, nicotinic ACh-mediated depolarization in spinal neurons has also been reported. Rhythmically active neurons in Xenopus embryos (Perrins and Roberts 1994) and neurons from the dorsal horn and central canal of rats (Bordey et al. 1996a,b; Urban et al. 1989) also depolarize through nicotinic ACh receptors. These findings together with the results of this study indicate brain stem neurons involved in initiation of swimming in lamprey as well as neurons located in the area of the spinal locomotor CPG of lamprey and other vertebrates are sensitive to cholinergic excitation. Many neurons were brought to threshold by nicotinic receptor activation, as seen in intracellular recordings and in extracellular recordings from ventral roots and the spinal cord, as well as the increased frequency of postsynaptic potentials recorded in many cells. These results show that not only motoneurons but also interneurons in the lamprey spinal cord could be responding to endogenous ACh present during locomotor activity.
Muscarinic PSP modulation
Although studies performed in other preparations have documented a variety of effects of muscarinic receptor activation such as depolarization through facilitation of a persistent inward Ca2+ current, closing K+ channels, and changes in AHP (Alaburda et al. 2002; Chevallier et al. 2006; Kiehn et al. 1996; Miles et al. 2007; Nowak and Macdonald 1983a,b; Perrier et al. 2000; Rivera-Arconada and Lopez-Garcia 2005; Rivera-Arconada et al. 2004; Smetana et al. 2007), in this study, muscarinic agonists were found to decrease compound and unitary PSPs recorded from spinal neurons. Tonic muscarinic modulation of synaptic strength is also present in the lamprey brain stem, where PSPs evoked in reticulospinal neurons from trigeminal nerve stimulation can be potentiated with muscarinic antagonists (LeRay et al. 2003). In the lamprey spinal cord, presynaptic inhibition of reticulospinal neuron synapses onto spinal neurons by other neurotransmitters such as dopamine (Svensson et al. 2003), metabotropic glutamate receptors (Krieger et al. 1996), neuropeptides (Parker 2000), and 5-HT (Blackmer et al. 2001; Buchanan and Grillner 1991) has previously been reported. Moreover, in other species muscarinic receptors have been implicated in modulation of synaptic strength in the spinal cord. Muscarinic ACh receptors have been localized presynaptically on primary afferents that terminate in the dorsal horn of the rat spinal cord (Gillberg and Askmark 1991). In rat ventral horn neurons muscarinic receptor activation presynaptically suppresses excitatory postsynaptic currents (Jiang and Dun 1986), perhaps the mechanism by which afferent-evoked monosynaptic reflexes are inhibited in the presence of muscarinic agonists (Kurihara et al. 1993). In addition, binding assays have shown that muscarinic ACh receptors (either pre- or postsynaptic) are located in the ventral horn and central canal (lamina X) of rats and cats, some of which must be the muscarinic m2 receptors found postsynaptically on motoneurons (Miles et al. 2007; Seybold 1985; Seybold and Elde 1984). Cholinergic neurotransmission in these areas could be important for locomotion and modulation of sensory information.
Antidromic motoneuron stimulation
Since cholinergic motoneurons could be the source of endogenous cholinergic modulation of fictive swimming in lamprey, an analysis of neuronal responses to antidromic firing of motoneurons through ventral root stimulation was undertaken. Stimulation of ventral roots in the quiescent spinal cord produced postsynaptic potentials that were sensitive to glycinergic and cholinergic agents in many neurons, suggesting the presence of glycinergic Renshaw-like neurons in the lamprey, and nicotinic excitation from motoneurons. It should be noted that some of the depolarization elicited by stimulation of ventral roots was resistant to perfusion of 0 Ca2+ Ringer; therefore it was not mediated through chemical synapses. Rather, this current was likely transmitted through electrical synapses, involving the gap junctions either between motoneurons or between motoneurons and Müller axons and, in turn, Müller axons and several other neuron classes (Buchanan et al. 1992; Rovainen 1974). However, a passive spread of positive current alone would not be changed by the presence of glycinergic or cholinergic agents and could not account for the hyperpolarizing potentials that were present in many cases. Many of the evoked potentials were in fact sensitive to perfusion of strychnine and mecamylamine. Not only were hyperpolarizing responses reduced with the glycinergic antagonist strychnine, many depolarizing responses were increased, indicating the presence of superimposed glycinergic inhibition even in cells that showed depolarization. With the nicotinic blocker mecamylamine both hyperpolarizing and depolarizing responses were reduced, suggesting that synaptically released ACh is involved. It appears that ACh from motoneurons is exciting inhibitory glycinergic cells, which then synapse on other lamprey spinal neurons, similar to the motoneuron–Renshaw cell feedback loop found in other vertebrates. In neonatal rat spinal cord, stimulation of ventral rootlets evoked similar responses in ventral horn neurons, including a glycinergic and cholinergic-sensitive inhibition, a product of the Renshaw cell feedback loop, as well as a zero Ca2+-resistant depolarization in some cases (Jiang et al. 1991), possibly due to electrical coupling between motoneurons in neonates (Mentis et al. 2002; Tresch and Kiehn 2000). In adult frog motoneurons electrical coupling has also been observed (Shapovalov et al. 1978). Motoneuron axon collaterals have been shown in lamprey and there is ample opportunity for en passant synapses between axons as they project out the ventral root (Wallén et al. 1985). It should also be noted that in the ventral roots of lamprey a small number of fibers do not have the morphology of motoneuron axons. Based on antibody staining, these fibers appear to be serotonergic, possibly displaced from the dorsal roots or the cranial nerves in more rostral parts of the cord (Harris-Warrick et al. 1985; Van Dongen et al. 1985). Although with stimulation of ventral roots these fibers could also be activated, the fibers are few in number, so it is unlikely they could account for the number and type of responses found here and, importantly, their activation would not likely bring about postsynaptic potentials with the observed pharmacology.
ChAT immunohistochemistry/retrograde labeling of motoneurons
To assess all possible sources of endogenous ACh in the lamprey spinal cord, we performed a combination of retrograde labeling of motoneurons and choline acetyltransferase immunohistochemistry to identify possible nonmotoneuron cholinergic cells. A previous study in which ChAT immunohistochemistry was performed in lamprey suggested that some cholinergic neurons in the most rostral regions of the spinal cord did not appear to be motoneurons (Pombal et al. 2001). We show here for the first time that these cholinergic neurons in the lamprey are a novel population of spinal interneurons, distinct from motoneurons, and present in the midbody region of the spinal cord. The cells were mainly located in the gray matter, although one was found dorsolateral to the gray (Fig. 8C), and they were significantly smaller than motoneurons. The mean length of the short axis of the soma of nonmotoneuron ChAT-positive cells was less than that of motoneurons and the ChAT-positive cell located in the dorsolateral axon tracts is not consistent with the location of a motoneuron. Furthermore, they are too great in number to merely represent motoneurons that potentially failed to backfill. Even with the most conservative estimates, their numbers were nearly triple that of potentially unlabeled motoneurons. Instead, it is more likely that these cells could be analogous to partition cells and central canal cells found in higher vertebrates. In rats these cholinergic cells project up to six segments and send fibers to the dorsal, intermediate, and ventral gray (Borges and Iversen 1986; Sherriff and Henderson 1994). Recent data from the mouse indicate medial partition cells that at least transiently express the Dbx1 homeodomain protein are the source of the large, cholinergic C-boutons found on motoneurons, and in cat cholinergic, commissurally projecting partition cells were found to be highly active during locomotor activity (Huang et al. 2000; Miles et al. 2007). More investigation needs to be done to determine the specific function of cholinergic interneurons in the lamprey, but these cells could play an important role in cholinergic neurotransmission during locomotor activity in the spinal cord of many vertebrates.
Network implications
In this study and in others, widespread sensitivity of spinal neurons to ACh was found. One study determined 85% of all the neurons recorded in the lumbar cat spinal cord depolarized >5 mV in response to ACh (Zieglgänsberger and Reiter 1974). In other organisms, such as turtles, Xenopus embryos, salamander, and neonatal rats and mice, cholinergic input contributes to excitatory drive during locomotion, inducing or promoting the induction of rhythmic activity (Carlin et al. 2006; Chevallier et al. 2006; Cowley and Schmitt 1994; Guertin and Hounsgaard 1999; Kiehn et al. 1996; Miles et al. 2007; Myers et al. 2005; Perrins and Roberts 1995a,b,c; Zhao and Roberts 1998), although cholinergic effects on network activity are diverse. Like the mammalian spinal cord, cholinergic modulation of locomotor activity in lamprey seems to be taking place through different receptor subtypes and could be originating from more than one neuronal population.
Nicotinic ACh receptor antagonism in both lamprey and mice prolongs the cycle period of locomotor bursts (Myers et al. 2005; Quinlan et al. 2004). One site in which nicotinic receptors are active is the motoneuron–Renshaw cell synapse. Blocking activation of Renshaw cells, or the Renshaw-like cells that we found preliminary evidence for in this study, would decrease recurrent inhibition of motoneurons that could be promoting burst termination. Interestingly, ablation or silencing of the V1 class of interneurons that include Renshaw cells and other ipsilateral inhibitory interneurons in mice also results in a prolonged cycle period of locomotor activity (Gosgnach et al. 2006). However, nicotinic ACh receptors are present in many types of neurons in the lamprey and the effects of nicotinic receptor activation on network activity may also be the result of opening receptors postsynaptic to cholinergic interneurons. Further studies would be required to determine the precise mechanism of the effect on network activity.
Muscarinic receptor activation in the lamprey shortens the cycle period of fictive swimming and brought about a decrease in PSP strength. This modulation of synaptic strength could bring about the observed changes in cycle period by weakening excitation and inhibition during locomotor activity. For example, weakened input from the excitatory interneurons and commissural interneurons could result in premature release of contralateral neurons from inhibition or premature termination of excitation of ipsilateral CPG neurons during the locomotor burst, resulting in an increased left–right oscillation frequency. However, another function of the CINs, left–right coordination, was not altered in the presence of ACh, suggesting altered synaptic excitation may be the primary cause of the reduced cycle period. Reduced excitation from EINs or other excitatory inputs to the spinal cord such as reticulospinal neurons could be contributing to the network effects of ACh as well. To determine the precise mechanism of cholinergic shortening of cycle period would require further investigation.
Possible mechanisms for the cholinergic modulation of intersegmental phase lag reported previously are even less clear, since the generation of this head-to-tail propagation of ventral root bursts in lamprey is, in itself, unclear. Perhaps if the cholinergic interneurons of lamprey have long projection patterns similar to cholinergic interneurons of rat and cat, they could be suited to coordinate this activity or, perhaps, it is a result of weakened ipsilateral excitation.
Taken together these findings present an interesting role for cholinergic neuromodulation in the lamprey, both through the motoneurons and possibly through the newly detected nonmotoneuron cholinergic cells.
GRANTS
This work was supported by National Institute of Neurological Disorders and Stroke Grant NS-35725.
Acknowledgments
We thank R. Dubuc and F. Auclair for help with ChAT immunohistochemistry and S. Kasicki for preliminary electrophysiology.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- Alaburda et al. 2002.Alaburda A, Perrier JF, Hounsgaard J. An M-like outward current regulates the excitability of spinal motoneurons in the adult turtle. J Physiol 540: 875–881, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber et al. 1984.Barber RP, Phelps PE, Houser CR, Crawford GD, Salvaterra PM, Vaughn JE. The morphology and distribution of neurons containing choline acetyltransferase in the adult rat spinal cord: an immunocytochemical study. J Comp Neurol 229: 329–346, 1984. [DOI] [PubMed] [Google Scholar]
- Blackmer et al. 2001.Blackmer T, Larsen EC, Takahashi M, Martin TF, Alford S, Hamm HE. G protein betagamma subunit-mediated presynaptic inhibition: regulation of exocytotic fusion downstream of Ca++ entry. Science 292: 293–297, 2001. [DOI] [PubMed] [Google Scholar]
- Bordey et al. 1996a.Bordey A, Feltz P, Trouslard J. Nicotinic actions on neurones of the central autonomic area in rat spinal cord slices. J Physiol 497: 175–187, 1996a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordey et al. 1996b.Bordey A, Feltz P, Trouslard J. Patch-clamp characterization of nicotinic receptors in a subpopulation of lamina X neurones in rat spinal cord slices. J Physiol 490: 673–678, 1996b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borges and Iversen 1986.Borges LF, Iversen SD. Topography of choline acetyltransferase immunoreactive neurons and fibers in the rat spinal cord. Brain Res 362: 140–148, 1986. [DOI] [PubMed] [Google Scholar]
- Buchanan 1993.Buchanan JT Electrophysiological properties of identified classes of lamprey spinal neurons. J Neurophysiol 70: 2313–2325, 1993. [DOI] [PubMed] [Google Scholar]
- Buchanan and Grillner 1991.Buchanan JT, Grillner S. 5-Hydroxytryptamine depresses reticulospinal excitatory postsynaptic potentials in motoneurons of the lamprey. Neurosci Lett 122: 71–74, 1991. [DOI] [PubMed] [Google Scholar]
- Buchanan et al. 1992.Buchanan JT, Moore LE, Hill R, Wallén P, Grillner S. Synaptic potentials and transfer functions of lamprey spinal neurons. Biol Cybern 67: 123–131, 1992. [DOI] [PubMed] [Google Scholar]
- Carlin et al. 2006.Carlin KP, Dai Y, Jordan LM. Cholinergic and serotonergic excitation of ascending commissural neurons in the thoraco-lumbar spinal cord of the neonatal mouse. J Neurophysiol 95: 1278–1284, 2006. [DOI] [PubMed] [Google Scholar]
- Carr et al. 1995.Carr PA, Huang A, Noga BR, Jordan LM. Cytochemical characteristics of cat spinal neurons activated during fictive locomotion. Brain Res Bull 37: 213–218, 1995. [DOI] [PubMed] [Google Scholar]
- Chevallier et al. 2006.Chevallier S, Nagy F, Cabelguen JM. Cholinergic control of excitability of spinal motoneurones in the salamander. J Physiol 570: 525–540, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowley and Schmidt 1994.Cowley KC, Schmidt BJ. A comparison of motor patterns induced by N-methyl-D-aspartate, acetylcholine and serotonin in the in vitro neonatal rat spinal cord. Neurosci Lett 171: 147–150, 1994. [DOI] [PubMed] [Google Scholar]
- Eccles et al. 1954.Eccles JC, Fatt P, Koketsu K. Cholinergic and inhibitory synapses in a pathway from motor-axon collaterals to motoneurons. J Physiol 126: 524–562, 1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillberg and Askmark 1991.Gillberg PG, Askmark H. Changes in cholinergic and opioid receptors in the rat spinal cord, dorsal root and sciatic nerve after ventral and dorsal root lesion. J Neural Transm 85: 31–39, 1991. [DOI] [PubMed] [Google Scholar]
- Gosgnach et al. 2006.Gosgnach S, Lanuza GM, Butt SJ, Saueressig H, Zhang Y, Velasquez T, Riethmacher D, Callaway EM, Kiehn O, Goulding M. V1 spinal neurons regulate the speed of vertebrate locomotor outputs. Nature 440: 215–219, 2006. [DOI] [PubMed] [Google Scholar]
- Guertin and Hounsgaard 1999.Guertin PA, Hounsgaard J. L-type calcium channels but not N-methyl-D-aspartate receptor channels mediate rhythmic activity induced by cholinergic agonist in motoneurons from turtle spinal cord. Neurosci Lett 261: 81–84, 1999. [DOI] [PubMed] [Google Scholar]
- Hanson and Landmesser 2003.Hanson MG, Landmesser LT. Characterization of the circuits that generate spontaneous episodes of activity in the early embryonic mouse spinal cord. J Neurosci 23: 587–600, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris-Warrick et al. 1985.Harris-Warrick RM, McPhee JC, Filler JA. Distribution of serotonergic neurons and processes in the lamprey spinal cord. Neuroscience 14: 1127–1140, 1985. [DOI] [PubMed] [Google Scholar]
- Huang et al. 2000.Huang A, Noga BR, Carr PA, Fedirchuk B, Jordan LM. Spinal cholinergic neurons activated during locomotion: localization and electrophysiological characterization. J Neurophysiol 83: 3537–3547, 2000. [DOI] [PubMed] [Google Scholar]
- Jiang and Dun 1986.Jiang ZG, Dun NJ. Presynaptic suppression of excitatory postsynaptic potentials in rat ventral horn neurons by muscarinic agonists. Brain Res 381: 182–186, 1986. [DOI] [PubMed] [Google Scholar]
- Jiang et al. 1991.Jiang ZG, Shen E, Wang MY, Dun NJ. Excitatory postsynaptic potentials evoked by ventral root stimulation in neonate rat motoneurons in vitro. J Neurophysiol 65: 57–66, 1991. [DOI] [PubMed] [Google Scholar]
- Kiehn et al. 1996.Kiehn O, Johnson BR, Raastad M. Plateau properties in mammalian spinal interneurons during transmitter-induced locomotor activity. Neuroscience 75: 263–273, 1996. [DOI] [PubMed] [Google Scholar]
- Krieger et al. 1996.Krieger P, El Manira A, Grillner S. Activation of pharmacologically distinct metabotropic glutamate receptors depresses reticulospinal-evoked monosynaptic EPSPs in the lamprey spinal cord. J Neurophysiol 76: 3834–3841, 1996. [DOI] [PubMed] [Google Scholar]
- Kurihara et al. 1993.Kurihara T, Suzuki H, Yanagisawa M, Yoshioka K. Muscarinic excitatory and inhibitory mechanisms involved in afferent fibre-evoked depolarization of motoneurones in the neonatal rat spinal cord. Br J Pharmacol 110: 61–70, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeRay et al. 2003.LeRay D, Brocard F, Bourcier-Lucas C, Auclair F, Lafaille P, Dubuc R. Nicotinic activation of reticulospinal cells involved in the control of swimming in lampreys. Eur J Neurosci 17: 137–148, 2003. [DOI] [PubMed] [Google Scholar]
- LeRay et al. 2004.LeRay D, Brocard F, Dubuc R. Muscarinic modulation of the trigemino-reticular pathway in lampreys. J Neurophysiol 92: 926–938, 2004. [DOI] [PubMed] [Google Scholar]
- Machacek and Hochman 2006.Machacek DW, Hochman S. Noradrenaline unmasks novel, self-reinforcing motor circuits within the mammalian spinal cord. J Neurosci 26: 5920–5928, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin et al. 1970.Martin AR, Wickelgren WO, Beranek R. Effects of iontophoretically applied drugs on spinal interneurones of the lamprey. J Physiol 207: 653–665, 1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mentis et al. 2005.Mentis GZ, Alvarez FJ, Bonnot A, Richards DS, Gonzalez-Forero D, Zerda R, O'Donovan MJ. Noncholinergic excitatory actions of motoneurons in the neonatal mammalian spinal cord. Proc Natl Acad Sci USA 102: 7344–7349, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mentis et al. 2002.Mentis GZ, Diaz E, Moran LB, Navarrete R. Increased incidence of gap junctional coupling between spinal motoneurones following transient blockade of NMDA receptors in neonatal rats. J Physiol 544: 757–764, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles et al. 2007.Miles GB, Hartley R, Todd AJ, Brownstone RM. Spinal cholinergic interneurons regulate the excitability of motoneurons during locomotion. Proc Natl Acad Sci USA 104: 2448–2453, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers et al. 2005.Myers CP, Lewcock JW, Hanson MG, Gosgnach S, Aimone JB, Gage FG, Lee KF, Landmesser LT, Pfaff SL. Cholinergic input is required during embryonic development to mediate proper assembly of spinal locomotor circuits. Neuron 46: 37–49, 2005. [DOI] [PubMed] [Google Scholar]
- Nishimaru et al. 2006.Nishimaru H, Restrepo CE, Kiehn O. Activity of Renshaw cells during locomotor-like rhythmic activity in the isolated spinal cord of neonatal mice. J Neurosci 26: 5320–5328, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimaru et al. 2005.Nishimaru H, Restrepo CE, Ryge J, Yanagawa Y, Kiehn O. Mammalian motor neurons corelease glutamate and acetylcholine at central synapses. Proc Natl Acad Sci USA 102: 5245–5249, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak and Macdonald 1983a.Nowak LM, Macdonald RL. Muscarine-sensitive voltage-dependent potassium current in cultured murine spinal cord neurons. Neurosci Lett 35: 86–91, 1983a. [DOI] [PubMed] [Google Scholar]
- Nowak and Macdonald 1983b.Nowak LM, Macdonald RL. Ionic mechanism of muscarinic cholinergic depolarization of mouse spinal cord neurons in culture. J Neurophysiol 49: 792–803, 1983b. [DOI] [PubMed] [Google Scholar]
- Parker 2000.Parker D Presynaptic and interactive peptidergic modulation of reticulospinal synaptic inputs in the lamprey. J Neurophysiol 83: 2497–2507, 2000. [DOI] [PubMed] [Google Scholar]
- Perrier et al. 2000.Perrier JF, Mejia-Gervacio S, Hounsgaard J. Facilitation of plateau potentials in turtle motoneurones by a pathway dependent on calcium and calmodulin. J Physiol 528: 107–113, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrins and Roberts 1994.Perrins R, Roberts A. Nicotinic and muscarinic ACh receptors in rhythmically active spinal neurones in the Xenopus laevis embryo. J Physiol 8: 221–228, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrins and Roberts 1995a.Perrins R, Roberts A. Cholinergic and electrical synapses between synergistic spinal motoneurones in the Xenopus laevis embryo. J Physiol 485: 135–144, 1995a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrins and Roberts 1995b.Perrins R, Roberts A. Cholinergic and electrical motoneuron-to-motoneuron synapses contribute to on-cycle excitation during swimming in Xenopus embryos. J Neurophysiol 73: 1005–1012, 1995b. [DOI] [PubMed] [Google Scholar]
- Perrins and Roberts 1995c.Perrins R, Roberts A. Cholinergic contribution to excitation in a spinal locomotor central pattern generator in Xenopus embryos. J Neurophysiol 73: 1013–1019, 1995c. [DOI] [PubMed] [Google Scholar]
- Pombal et al. 2001.Pombal MA, Marin O, Gonzalez A. Distribution of choline acetyltransferase-immunoreactive structures in the lamprey brain. J Comp Neurol 431: 105–126, 2001. [DOI] [PubMed] [Google Scholar]
- Pratt and Jordan 1987.Pratt CA, Jordan LM. Ia inhibitory interneurons and Renshaw cells as contributors to the spinal mechanisms of fictive locomotion. J Neurophysiol 57: 56–71, 1987. [DOI] [PubMed] [Google Scholar]
- Quinlan et al. 2004.Quinlan KA, Placas PG, Buchanan JT. Cholinergic modulation of the locomotor network in the lamprey spinal cord. J Neurophysiol 92: 1536–1548, 2004. [DOI] [PubMed] [Google Scholar]
- Rivera-Arconada et al. 2005.Rivera-Arconada I, Lopez-Garcia JA. Effects of M-current modulators on the excitability of immature rat spinal sensory and motor neurones. Eur J Neurosci 22: 3091–3098, 2005. [DOI] [PubMed] [Google Scholar]
- Rivera-Arconada et al. 2004.Rivera-Arconada I, Martinez-Gomez J, Lopez-Garcia JA. M-current modulators alter rat spinal nociceptive transmission: an electrophysiological study in vitro. Neuropharmacology 46: 598–606, 2004. [DOI] [PubMed] [Google Scholar]
- Rovainen 1974.Rovainen CM Synaptic interactions of reticulospinal neurons and nerve cells in the spinal cord of the sea lamprey. J Comp Neurol 154: 207–223, 1974. [DOI] [PubMed] [Google Scholar]
- Rovainen and Dill 1984.Rovainen CM, Dill DA. Counts of axons in electron microscopic sections of ventral roots in lamprey. J Comp Neurol 225: 433–440, 1984. [DOI] [PubMed] [Google Scholar]
- Seybold 1985.Seybold VS Distribution of histaminergic, muscarinic and serotonergic binding sites in cat spinal cord with emphasis on the region surrounding the central canal. Brain Res 342: 291–296, 1985. [DOI] [PubMed] [Google Scholar]
- Seybold and Elde 1984.Seybold VS, Elde RP. Receptor autoradiolgraphy in thoracic spinal cord: correlation of neurotransmitter binding sites with sympathetoadrenal neurons. J Neurosci 4: 2533–2542, 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapovalov et al. 1978.Shapovalov AI, Shiriaev BI, Velumian AA. Mechanisms of post-synaptic excitation in amphibian motoneurons. J Physiol 279: 437–455, 1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherriff and Henderson 1994.Sherriff FE, Henderson Z. A cholinergic propriospinal innervation of the rat spinal cord. Brain Res 634: 150–154, 1994. [DOI] [PubMed] [Google Scholar]
- Smetana et al. 2007.Smetana RW, Alford S, Dubuc R. Muscarinic receptor activation elicits sustained, recurring depolarizations in reticulospinal neurons. J Neurophysiol 97: 3181–3192, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson et al. 2003.Svensson E, Woolley J, Wikström M, Grillner S. Endogenous dopaminergic modulation of the lamprey spinal locomotor network. Brain Res 970: 1–8, 2003. [DOI] [PubMed] [Google Scholar]
- Tresch and Kiehn 2000.Tresch MC, Kiehn O. Motor coordination without action potentials in the mammalian spinal cord. Nat Neursci 6: 593–599, 2000. [DOI] [PubMed] [Google Scholar]
- Urban et al. 1989.Urban L, Willetts J, Murase K, Randić M. Cholinergic effects on spinal dorsal horn neurons in vitro: an intracellular study. Brain Res 500: 12–20, 1989. [DOI] [PubMed] [Google Scholar]
- Von Dongen et al. 1985.Von Dongen PAM, Hökfelt T, Grillner S, Verhofstadt AAJ, Steinbusch HWM, Cuello AC, Terenius L. Immunohistochemical demonstration of some putative neurotransmitters in the lamprey spinal cord and spinal ganglia: 5-hydroxytryptamine-, tachykinin-, and neuropeptide- Y-immunoreactive neurons and fibers. J Comp Neurol 234: 501–522, 1985. [DOI] [PubMed] [Google Scholar]
- Wallén et al. 1985.Wallén P, Grillner S, Feldman JL, Bergelt S. Dorsal and ventral myotome motoneurons and their input during fictive locomotion in lamprey. J Neurosci 5: 654–661, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao and Roberts 1998.Zhao FY, Roberts A. Assessing the roles of glutamatergic and cholinergic synaptic drive in the control of fictive swimming frequency of young Xenopus tadpoles. J Comp Physiol A Sens Neural Behav Physiol 183: 753–758, 1998. [DOI] [PubMed] [Google Scholar]
- Zieglgänsberger and Reiter 1974.Zieglgänsberger W, Reiter CH. A cholinergic mechanism in the spinal cord of cats. Neuropharmacology 13: 519–527, 1974. [DOI] [PubMed] [Google Scholar]