Abstract
Our previous studies have shown that extensive spinal lesions at T12 in the rabbit [ventral hemisection (VHS) or 3/4-section that spares one ventral quadrant (VQ)] severely damaged the postural system. When tested on the platform periodically tilted in the frontal plane, VHS and VQ animals typically were not able to perform postural corrective movements by their hindlimbs, although EMG responses (correctly or incorrectly phased) could be observed. We attempted to restore postural control in VHS and VQ rabbits by applying serotoninergic and noradrenergic drugs to the spinal cord below the lesion through the intrathecal cannula. It was found that serotonin and quipazine (5-HT1,2,3 agonist) did not re-establish postural corrective movements. However, when applied during a 10-day period after lesion, these drugs produced a twofold increase of the proportion of correct EMG responses to tilts. It was also found that methoxamine (α1 noradrenergic agonist), as well as the mixture of methoxamine and quipazine, did not re-establish postural corrective movements and did not increase the proportion of correct EMG responses. Serotonin (at later stages) and methoxamine induced periodical bursting in EMGs, suggesting activation of spinal rhythm-generating networks. Appearance of bursting seems to perturb normal operation of postural mechanisms, as suggested by methoxamine-induced abolishment of postural effects of quipazine. When applied in an intact animal, none of the tested drugs affected the value of postural corrections or evoked periodical bursting. We conclude that activation of the serotoninergic system (but not the noradrenergic one) causes selective enhancement of spinal postural reflexes during the earlier postlesion period.
INTRODUCTION
When standing and walking, animals and humans maintain a specific body orientation and equilibrium because of the activity of the postural system (Deliagina et al. 2006; Horak and Macpherson 1996; Macpherson et al. 1997a; Massion 1998; Massion and Dufosse 1988; Orlovsky et al. 1999). Damage to descending and ascending spinal pathways, caused by a spinal cord injury (SCI), results in an impairment of the postural control. This affects not only equilibrium when standing but also locomotion and voluntary movements that need postural support.
Postural deficits depend on the location and extent of SCI. After a complete lesion of the spinal cord in the thoracic region, chronic spinal cats, when standing, exhibit poor responses to disturbances of posture and are, as a rule, not able to maintain the dorsal-side-up orientation of the caudal part of their body (Barbeau et al. 2002; Macpherson and Fung 1999; Rossignol et al. 1999, 2002). However, some elements of postural control (like weight-bearing standing episodes) may remain (Giuliani and Smith 1985; Grillner 1973; Kellog et al. 1946), and they can be improved by training (De Leon et al. 1998; Edgerton et al. 2001, 2004; Pratt et al. 1994). Balance control during locomotion is also severely damaged, and regular training results in only a minor improvement of this postural function (Grillner 1973).
The effect of different partial SCI on postural control was analyzed during standing on the tilting platform in the rabbit (Lyalka et al. 2005, 2006). These studies show that ventral spinal pathways are of primary importance for the postural control in the hindquarters. After a ventral hemisection (VHS animals), postural corrective movements were absent, although EMG responses (with a correct or incorrect pattern) could be observed. When one ventral quadrant was spared (3/4-section, VQ animals), postural corrective movements were typically absent, suggesting that a single ventral quadrant contains a close-to-critical amount of fibers necessary for the operation of the postural control system. In contrast, when the dorsal pathways were transected leaving the ventral pathways intact [dorsal hemisection (DHS) animals], postural corrections recovered within 1–2 wk after the lesion.
A considerable deterioration of lateral stability of the hindquarters was also observed during locomotion in cats with ventral and ventrolateral spinal lesions (Brustein and Rossignol 1998, 1999), whereas dorsal lesions caused only transient deficits in postural stability (Jiang and Drew 1996).
There are two major ways to account for the poor postural performance in the hindquarters after a complete transection of the spinal cord or after damage to the ventral spinal pathways (Lyalka et al. 2005). The first hypothesis implies a key role of spinal postural mechanisms for the generation of postural corrections. It suggests that spinal lesions result in a reduction or complete abolishment of the supraspinal excitatory tonic drive to the spinal postural mechanisms. Deprived of this drive, the spinal postural networks (responsible for the generation of spinal postural reflexes) are not sufficiently activated and cannot compensate for postural disturbances. The second hypothesis implies a key role of supraspinal motor commands for the generation of postural corrections. It suggests that spinal lesions result in a partial or complete interruption of the loops of spino-brain stem-spinal postural reflexes, which leads to a reduction or abolishment of the supraspinal commands for postural corrections. The relative importance of these two factors at present is not clear (for discussion, see Deliagina et al. 2006, 2008; Horak and Macpherson 1996; Lyalka et al. 2005; Macpherson et al. 1997b).
The aim of this study was to clarify whether the spinal postural networks in subjects with partial SCI (VHS and VQ rabbits) can be reactivated pharmacologically, that is, by application of different drugs to the spinal cord below the lesion by means of the implanted intrathecal cannula (for review of this approach, see Rossignol et al. 1998, 2001). A positive answer to this question would mean that spinal postural reflexes play a significant role in the generation of postural corrections and would suggest a possible way for promoting recovery of this function after SCI. We examined serotoninergic drugs (serotonin and quipazine, 5-HT1,2,3 agonist) and a noradrenergic drug (methoxamine, α1 agonist), as well as a mixture of quipazine and methoxamine, which have been reported to improve weight support and lateral stability during locomotion in the spinal cats (Brustein and Rossignol 1999). We have found that activation of the serotoninergic system (but not the noradrenergic one) causes selective enhancement of spinal postural reflexes during the earlier postlesion period. This result supports the first hypothesis implying a prominent role of spinal reflexes in postural control.
A brief account of part of this study has been published in abstract form (Lyalka et al. 2006).
METHODS
Experiments were carried out on six adult male New Zealand rabbits (weighing, 2.5–3.5 kg). All experiments were conducted with the approval of the local ethical committee (Norra Djurförsöksetiska Nämnden) in Stockholm.
Surgical procedures
Each animal was subjected to two operations under hypnorm-midazolam anesthesia, using aseptic procedures. During the first surgery, bipolar EMG electrodes (0.2-mm flexible stainless steel Teflon-insulated wires) were implanted bilaterally into m. gastrocnemius lateralis (Gast, ankle extensor), m. vastus lateralis (Vast, knee extensor), and m. biceps femoris (Bic, knee flexor). The wires were led subcutaneously toward the head and through a small incision in the skin on the dorsal aspect of the neck. The wound was sutured so that the wires were fastened to the skin. A small connector was soldered to each wire at a distance of 2–3 cm from the skin.
In 3–4 days, when the animal had recovered completely from the first surgery, its postural responses to tilts were tested, and afterward, a second surgery was performed, including implantation of an intrathecal cannula and damage of the spinal cord. An incision was made along the dorsal midline in the lower thoracic region. A laminectomy at the T11 to T12 level was done, and the dura was opened on the dorsum of the cord in T12 segment. A cannula (silicone tube, OD = 0.7 mm, ∼200 mm in length, filled with Ringer solution) was inserted under the dura in the caudal part of the T12 segment and protracted caudally for ∼100 mm to reach the L5–L6 level, as shown schematically in Fig. 1A. The necessary length of the descending arm of the cannula was measured externally by counting spinous processes. The tube was glued to the T11 spinous process by dental cement; its rostral end was led subcutaneously toward the head and through a small incision in the skin on the dorsal aspect of the neck. The wound was sutured so that the tube was fastened to the skin. An opening of the tube was closed with a small plug.
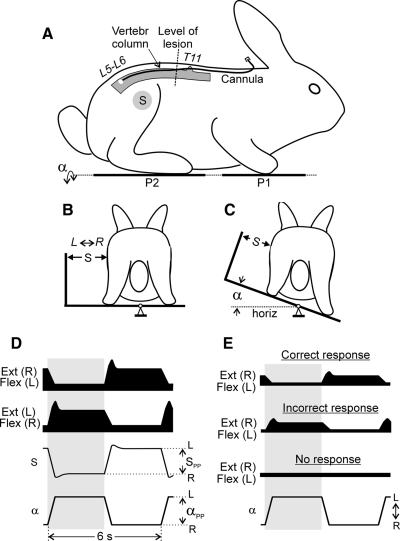
FIG. 1.
Experimental design and postural responses to tilts. Position of the cannula for drug administration and the level of spinal cord damage are shown in A. A–C: testing of postural responses to tilts. The animal was standing on 2 platforms: 1 under the forelimbs (P1) and 1 under the hindlimbs (P2). Platform P2 could be tilted in the transverse plane (α is the platform tilt angle). The sagittal plane of the animal was aligned to the axis of platform rotation. Mechanical sensor S, positioned at the half-height of the body, measured lateral displacements of the caudal part of the trunk in relation to the P2 platform. D: schematic representation of the trajectory of the platform angle (α), corrective movements of the caudal trunk (S), and EMG responses in right (R) and left (L) flexor (Flex) and extensor (Ext) limb muscles. The following values were measured: αPP, the peak-to-peak value of tilt angle of the P2 platform; SPP, the peak-to-peak value of postural corrections in the hindquarters. E: types of EMG responses in the right extensor and left flexor muscles observed in ventral hemisection (VHS) or ventral quadrant (VQ) animals: (i) correct response (Ext EMG is timed to the ipsilateral tilt, and Flex EMG to the contralateral tilt); (ii) incorrect response (opposite phase relations); (iii) no response.
The spinal lesion was performed, which was either 1) ventral hemisection (making VHS animals) or 2) dorsal hemisection combined with transection of one ventral quadrant (making VQ animals). The dura in the rostral part of the T12 segment was opened, and the lesion was done under the dissecting microscope by means of spring scissors, microsurgery forceps, and a small scalpel. Afterward, the incision was closed in anatomical layers. In one animal, the cannula was implanted, but the spinal cord was not damaged.
Animal care
Each rabbit was kept in an individual cage (80 × 70 × 65 cm). The cage was cleaned every day, and its bottom was covered with fresh absorbable tissue. The rabbits were fed dry rabbit food, hay, and carrots and had access to water.
The rabbits were monitored closely after surgery, particularly during the first 24–48 h. An analgesic, buprenorphine hydrochloride (Temgesic, Schering-Plough; 0.01 mg/kg, sc) was given every 12 h for 48 h. To reduce inflammatory reaction caused by surgery, Rimadyl (Orion Pharma, 4 mg/kg, sc) was injected preoperatively and 2 days after surgery. In addition to their own water intake, the first 2 days after the second surgery, the rabbits were administrated, twice daily, 25 ml of Ringer solution. During the 3 days after the first surgery and 5 days after the second surgery, daily antibiotics (Baytril, Bayer HealthCare; 5 mg/kg, im) were administrated prophylactically.
Only two rabbits with spinal cord lesions were kept at any particular time. First, 1–2 days after spinal cord lesion, the bladder was expressed manually twice daily. Later, VQ and VHS rabbits controlled their micturition and did not need manual voiding. The animals were cleaned if necessary.
Experimental design
Postural tests on a tilting platform have been described earlier (Beloozerova et al. 2003; Deliagina et al. 2006; Lyalka et al. 2005). No special training of the rabbits was required before testing. For testing, an animal was positioned on the two platforms (P1 and P2 in Fig. 1A) so that P1 supported the forelimbs and P2 supported the hindlimbs. The sagittal plane of the animal was aligned to the axis of the platform rotation (Fig. 1, B and C). The surface of the platforms was covered with sandpaper to prevent sliding of the animal during tilts.
The platform supporting the hindquarters could be tilted periodically in the frontal (transverse) plane of the animal (angle α; Fig. 1, A and C), while P1 was kept horizontal. A trapezoidal trajectory of tilting was used (Fig. 1D) with transitions between stationary (extreme) positions lasting for 0.5–0.7 s and with each position being maintained for 2–3 s. Tilts were symmetrical in relation to the horizontal position, with the peak-to-peak value of 40°. Smaller values (20 or 30°) were also used in animals with a spinal lesion.
Earlier, it was shown that lateral displacements of the trunk in relation to the tilting platform well characterize the efficacy of stabilization of the dorsal-side-up trunk position (Beloozerova et al. 2003). In this study, we used the same method, and measured lateral displacements of the caudal part of the trunk in relation to the platform P2 (postural corrections in the hindquarters). This was done by means of the mechanical sensor S positioned at the half-height of the body (Fig. 1, B and C). Recording of postural corrections was performed along with recording EMGs from six hindlimb muscles.
Drug injection
The drugs were injected (as a bolus of 100 μl) into the subarachnoid space of the spinal cord through the inlet of the cannula, as described by Giroux et al. (2001). A subsequent bolus injection of saline (100 μl) was made to flush the drug outside the cannula; the dead space of the cannula was 80 μl.
The position of cannula was verified postmortem. In all cases, the tip was positioned in the L5–L6 segments. In two animals, a solution of fast green (100 μl) was injected through the cannula before killing the animal. It was found that practically all parts of the lumbar enlargement of the spinal cord were stained, suggesting that the drugs affected a considerable part of the hindlimbs-related spinal networks.
The following drugs were used in this study: methoxamine (α1 noradrenergic agonist), serotonin (5-HT), quipazine (5-HT1,2,3 agonist), and a mixture of methoxamine and quipazine. The amount of injected drug (concentration 5 mM, and volume of bolus 100 μl) was similar to that used by Brustein and Rossignol (1999) for activation of hindlimb mechanisms of locomotion in chronic spinal cats.
A typical experimental protocol was as follows. Injections began on day 3 after lesion. The four drugs were injected in order, e.g., 1-2-3-4, with the interval between sequential injections of 1–2 days. This sequence was repeated three times with a period of 10 days. Thus our measurements in each animal lasted for three 10-day periods, termed the early, intermediate, and late ones. Postural tests were performed before injection (control) and then each hour for 4–6 h after injection. Between the tests, the animals were kept in the cage. Injection of drugs did not markedly affect their behavior. They were crawling by using the forelimbs and showed normal eating and drinking behavior. After the third 10-day period, the animal was killed.
Recordings and data analysis
The signals from the EMG electrodes and from the position sensors were amplified, digitized with a sampling frequency of 5 (EMGs) and 1 kHz (sensors), and recorded on a computer disk using the data acquisition and analysis software (Power-1401/Spike-2, Cambridge Electronic Design, Cambridge, UK). The EMG signals were rectified and smoothed (time constant, 50 ms).
To evaluate the postural performance in the intact animal, we calculated the gain of postural reflexes defined as: G = SPP/αPP (cm/deg), where αPP is the peak-to-peak value of tilts of the platform, and SPP is the peak-to-peak value of postural corrections in the hindquarters (Fig. 1D).
All quantitative data in this study are presented as mean ± SE. Student's t-test was used to characterize the statistical significance when comparing different means; the significance level was set at P = 0.05.
Histological procedures
At the termination of the experimental series, rabbits were deeply anesthetized with pentobarbital sodium and perfused with isotonic saline followed by a 10% formalin solution. Frozen sections of 30 μm thickness were cut in the region of spinal cord damage. The tissue was stained for Nissl substance with cresyl violet. The position and the extent of lesions were verified by observation of a series of magnified digital images of sections.
RESULTS
Evaluation of the extent of spinal lesions
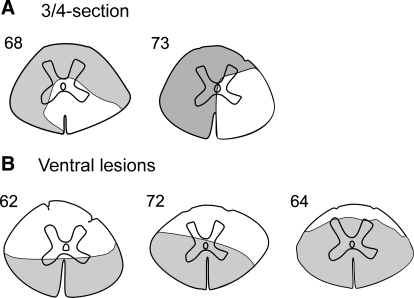
Figure 2A shows the reconstructed lesion sites for two rabbits, in which the aim was to lesion both dorsal quadrants and one ventral quadrant of the spinal cord (3/4-section). In both cases, the right ventral quadrant was not damaged, whereas the three other quadrants were destroyed to a large extent. After these lesions, the right reticulospinal tract (descending in the ventro-medial and ventro-lateral areas; Blessing et al. 1981) was not damaged, as well as the right vestibulospinal tract descending in the ventro-medial area (Akaike and Westerman 1973; Blessing et al. 1981). In rabbit 68, a medial part of the left ventral quadrant remained undamaged, suggesting partial preservation of the left vestibulospinal tract. In rabbit 73, a small part of the right dorsal quadrant was left intact. Rabbits 68 and 73 will be referred to as VQ animals.
FIG. 2.
Extent of the spinal cord damage in rabbits with 3/4-section (A) and with ventral lesions (B). The total extent of the lesion is projected on a spinal cord section taken more rostrally, after inspecting several consecutive sections.
In three rabbits, our aim was to transect both ventral quadrants (ventral hemisection). Figure 2B shows the reconstructed lesion sites for these animals. In two of them (62 and 72), both ventral quadrants were destroyed, whereas the dorsal quadrants remained largely intact. In rabbit 64, the lesion was more extensive than planned, and only a narrow strip in the dorsal part of the spinal cord remained intact. Nevertheless, for simplicity, all these three rabbits will be referred to as VHS animals. In these rabbits, reticulo- and vestibulospinal tracts were destroyed bilaterally.
Postural performance in VQ and VHS rabbits before drug application
As shown in our previous studies, intact rabbits maintain balance when the platform under the hindlimbs (P2 in Fig. 1A) was periodically tilted (Beloozerova et al. 2003; Lyalka et al. 2005). The animals exhibited stereotypic postural responses that included an extension of the hindlimb on the side moving downward and a flexion of the hindlimb on the opposite side, as shown schematically in Fig. 1, B and C. These flexion and extension movements displaced the trunk in the transverse plane, in a direction opposite to the platform tilt (Fig. 1, B and C). The displacements of the trunk (corrective movements, S) were in antiphase to the platform tilts (α), as shown schematically in Fig. 1D. Tilt-related limb movements were caused by a specific pattern of muscle activity (Fig. 1D). When the ipsilateral side of the platform was moving downward, the limb was extending because of activation of extensor muscles. When it was moving upward, the limb was flexing because of a reduction of the extensor activity and an activation of some flexor muscles.
Distortions of postural performance in VHS and VQ rabbits have been described by Lyalka et al. (2005, 2006). In brief, a well-coordinated EMG pattern (observed in control, Fig. 1D) dissociated into an independent activity of individual muscles. They responded to tilts with correct phasing, with incorrect phasing, or did not exhibit any response (Fig. 1E). Each individual muscle could spontaneously switch between these three types of activity. Because of disintegration of the EMG pattern and a decrease of the response value, corrective trunk movements in VHS and VQ animals were absent.
All these postural deficits were observed in this study and were basically similar in the VQ and VHS rabbits. Two examples of responses to tilts before drug injection in VHS rabbit 62 on days 4 and 11 after SCI are shown in Figs. 4A and 6A, respectively. In Fig. 4A, the rabbit exhibited no EMG responses to tilts. In Fig. 6A, the EMG responses were either incorrectly phased in relation to tilts (as in the right Gast) or were absent (as in the left Gast). In both examples, trunk corrective movements were absent, and trunk displacements in relation to the platform (S) were in-phase with platform tilts (α).
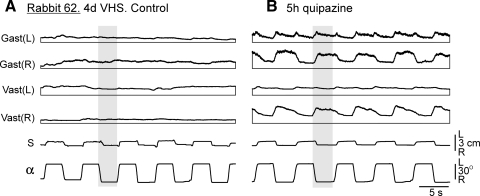
FIG. 4.
Example of the effect of quipazine in a VHS rabbit. A: kinematical and EMG responses to tilts before quipazine application in rabbit 62. B: the same after quipazine application. α, tilt angle of the P2 platform; S, lateral displacement of the caudal part of the trunk in relation to the P2 platform. The EMGs of the following muscles are presented: left (L) and right (R) vastus (Vast) and gastrocnemius (Gast). Shaded column highlights one half of 1 of the tilt cycles to facilitate comparison between curves.
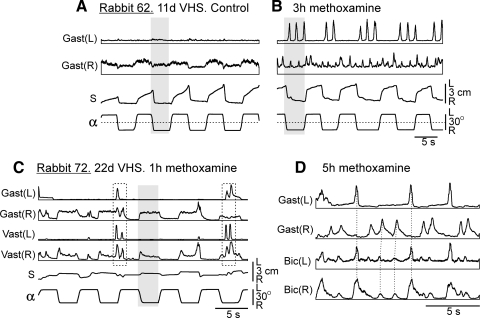
FIG. 6.
Examples of the effects of methoxamine in 2 VHS rabbits. A: kinematical and EMG responses to tilts before methoxamine application in rabbit 62. B: the same after methoxamine application. C: responses to tilts 1 h after methoxamine application in rabbit 72 (2 double-peak bursts are indicated by rectangles). D: periodic EMG bursting, 5 h after methoxamine application in rabbit 72 (synchronous bursts in different EMGs are indicated by interrupted lines). The EMGs of the following muscles are presented: left (L) and right (R) vastus (Vast), gastrocnemius (Gast), and biceps femoris (Bic). Abbreviations as in Fig. 4.
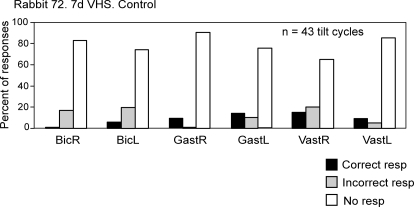
In each trial, we classified all EMG responses in individual muscles into three categories (Fig. 1E): cases with correct responses (extensor EMG is timed to the ipsilateral tilt, and flexor EMG is timed to the contralateral tilt), cases with incorrect responses (opposite phase relations), and cases with no response. We calculated the relative number of tilt cycles with responses in each category for individual muscles. An example of such representation of data (for the rabbit with VHS tested on day 7 after lesion) is shown in Fig. 3. One can see a very low probability (<20%) of postural responses (correct or incorrect) in any of the six muscles. We did not found any marked difference between various muscles in regard to the chance of their activation in the correct or in the incorrect phase of the tilt cycle, and for that reason grouped all muscles together when calculating the proportion of different types of responses.
FIG. 3.
Examples of evaluation of EMG responses to tilts in VHS rabbit. In each trial, all EMG responses in individual muscles were classified into three categories –correct responses (extensor EMG is timed to the ipsilateral tilt, flexor EMG is timed to the contralateral tilt), incorrect responses (opposite phase relations), and no response. Relative number of responses (%) in each category was calculated.
Effects of drug administration in VQ and VHS rabbits
The effects of drug administration in the VQ and VHS rabbits were similar and will be considered together.
EFFECT OF QUIPAZINE.
Injection of 100 μl of 5 mM quipazine strongly affected motor output of the hindlimbs. The effects lasted for a few hours, with a peak in 4–5 h after injection. Figure 4 shows postural responses in VHS rabbit 62 tested on day 4 after lesion, before (Fig. 4A) and 5 h after quipazine injection (Fig. 4B). In control, tilts of the platform caused no corrective motor responses (trunk displacements, S, were in-phase with platform tilts, α). There were also no EMG responses, although some tonic EMG activity could be observed. After quipazine injection, responses to tilts appeared in a part of EMGs. They were correctly phased (i.e., in antiphase to tilts) in the right Gast and Vast, incorrectly phased in the left Gast, and were almost absent in the left Vast. In some cases, correct and incorrect responses could be observed in the muscles of the same limb. Quipazine did not re-establish corrective motor responses to tilts (trunk displacements S were very small and occurred in-phase with tilts). In contrast to serotonin and methoxamine, quipazine did not induce periodical bursting in EMGs during the whole postlesion period.
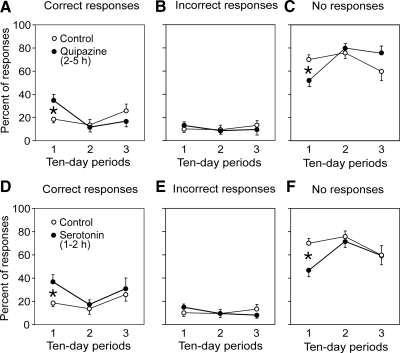
For further analysis, we used the same method as in the tests done before drug application (see above and Fig. 3). For each trial, we calculated (for individual muscles) the number of tilt cycles with correct, incorrect, or no response to tilt. These data were averaged over all muscles. Effects of quipazine are summarized in Fig. 5, A–C, which shows the relative number of different types of EMG responses as a function of postlesion time (1st, 2nd, and 3rd 10-day periods) for the whole population of VQ and VHS animals. One can see that quipazine increased considerably the proportion of correctly phased EMG responses to tilts during the first 10-day period after lesion (Fig. 5A). This increase was statistically significant. An increase of the number of correct responses was accompanied by a decrease of the number of cases with no responses (Fig. 5C), whereas the proportion of incorrect responses did not change (Fig. 5B).
FIG. 5.
Summary of the effects of serotoninergic drugs on EMG responses. A–C: summary of the effects of quipazine. D–F: summary of the effects of serotonin. Proportion of different type of responses to tilts was calculated for control trials (before drug application) and for tests performed after drug application (hours 2, 3, 4, and 5). Data for the 1st, 2nd, and 3rd 10-day period (postlesion) are resented separately (mean ± SE, averaging over all recorded muscles in 3 VHS and 2 VQ rabbits). The difference between the effect of serotoninergic drugs and control was significant only for the 1st 10-day period.
EFFECT OF SEROTONIN.
Effects of serotonin were in general similar to those of quipazine. In 1–2 h after injection of 100 μl of a 5 mM solution of serotonin (performed during the 1st 10-day period after SCI), responses to tilts appeared in a part of EMGs; these responses were correctly phased in some muscles and incorrectly phased in the others. No restoration of postural corrective movements was observed. At later stages (postlesion days 10–30), serotonin evoked periodical bursting in EMGs. This phenomenon will be considered in the section Effect of methoxamine. However, bursting evoked by serotonin was much less pronounced than the bursting evoked by methoxamine.
The effects of serotonin are summarized in Fig. 5, D–F, which shows the relative number of different types of EMG responses to tilts as a function of postlesion time for all VQ and VHS animals taken together. One can see that serotonin increased considerably the proportion of correctly phased EMG responses during the first 10-day period after lesion. This increase was statistically significant. An increase of the number of correct responses was accompanied by a decrease of the number of cases with no responses (Fig. 5F), whereas the proportion of wrong responses did not change (Fig. 5E).
EFFECT OF METHOXAMINE.
Injection of 100 μl of 5 mM solution of methoxamine strongly affected the motor activity of the hindlimbs. The effect lasted for 5–6 h, with a peak at 2–3 h after injection, and was similar in all tested animals. Figure 6 shows postural responses in VHS rabbit 62 tested on day 11 after a lesion, before (Fig. 6A) and at the peak of the effect of methoxamine (3 h after injection; Fig 6B). Figure 6 shows the most characteristic effect of methoxamine—an induction of rhythmical bursting, which was found in both VQ and VHS rabbits. In 3 h after injection, a bursting activity was observed in both left and right Gast, with a period of 1–2 s. The bursting could appear spontaneously or in response to platform tilts. Small stepping-like movements with the corresponding period could be seen in the hindlimbs. If the experimenter lifted the hindquarters of the rabbit, the hindlimbs could perform these movements in the air. When standing, tilts of the platform caused no corrective movements, and trunk displacements (S) occurred in-phase with tilts (Fig. 6B), as before methoxamine injection (Fig. 6A). No static EMG responses to tilts were observed after injection. Instead, the value of periodical EMG bursts was modulated by tilts. In each cycle, tilt to the right caused a decrease of the bursts value in the right Gast as well as a series of bursts in the left Gast. Tilt to the left led to an increase of the burst value in the right Gast and termination of bursting in the left Gastr. A modulation of the bursts value by platform tilts can be considered as interaction of postural responses and rhythmical activity of the limb. Such interaction has been described for the cat walking on the treadmill (Matsuyama and Drew 2000; A. Karayannidou, I. Beloozerova, T. Deliagina, unpublished data). Lateral tilts of the treadmill caused an increase of extensor bursts on the side moving down and a decrease on the opposite side. Reversed relationships were found for flexors. By analogy, in this study, we considered as correct responses the cases when the extensor bursts were larger (and flexor bursts smaller) during ipsilateral tilts compared with contralateral tilts. Reversed relationships (as in Fig. 6B) were considered incorrect responses. The cycles without modulation of the bursts value were considered as the cases with no response.
Figure 6, C and D, shows the effects of methoxamine in another animal (VHS rabbit 72, tested on day 22 after lesion). In 1 h after methoxamine injection, bursting activity was still relatively low (2 double-peak bursts are indicated by rectangles), and this activity did not strongly interfere with static responses to tilts. These responses were correctly phased in the right Gast and Vast but were absent in the left Gast and Vast. When the bursting activity was high (5 h after injection; Fig. 6D), at least two rhythms could be seen in the EMGs (the corresponding bursts in different EMGs are indicated by interrupted lines).
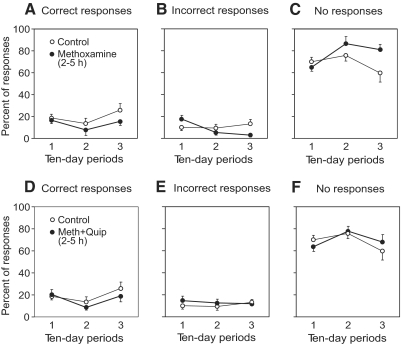
The effects of methoxamine are summarized in Fig. 7, which shows the relative number of different types of EMG responses to tilts as a function of postlesion time (1st, 2nd, and 3rd 10-day periods) for the whole population of VQ and VHS animals. The number of correct EMG responses was small, and no significant changes of this value over time were seen (Fig. 7A). One can conclude that methoxamine did not increase the proportion of correct postural EMG responses; changes in the proportion of incorrect responses were not significant (Fig. 7B). Nor did methoxamine re-establish postural corrective movements.
FIG. 7.
Summary of the effects produced by methoxamine and by combined application of methoxamine and quipazine on EMG responses. A–C: summary of the effects of methoxamine. D–F: summary of the effects of mixture of methoxamine and quipazine. Proportion of different type of responses to tilts was calculated for control trials (before drug application) and for tests performed after drug application (hours 2, 3, 4, and 5). Data for the 1st, 2nd, and 3rd 10-day period (postlesion) are presented separately (mean ± SE, averaging over all recorded muscles in 3 VHS and 2 VQ rabbits). For each point in time, the difference between the effect of drug application and control was not significant.
EFFECT OF MIXTURE OF METHOXAMINE AND QUIPAZINE.
The effects produced by the mixture of methoxamine and quipazine were similar to those produced by methoxamine alone. They included induction of the intense periodical bursting and enhancement of EMG activity. No restoration of postural corrective movements was observed. The proportion of correctly phased EMG responses to tilts was very small and did not differ from control. Effects of injection of these drugs on EMG responses are summarized in Fig. 7, D–F.
Effects of drug administration in intact rabbit
In rabbit 65, the cannula was implanted, but the spinal cord was not damaged. Motor behavior of this rabbit (including the ability to generate postural corrections and to maintain the basic body configuration) did not differ from that observed in intact animals in previous experiments (Beloozerova et al. 2003).
In the intact rabbit 65, we used the dose of drugs (100 μl of 5 mM solution) that was efficient in VQ and VHS rabbits. In the intact rabbit, however, these injections did not seem efficient, as shown in Fig. 8, A and B, for the methoxamine injection. Both before injection (Fig. 8A) and after injection (Fig. 8B), postural corrective motor responses had a value of ∼3 cm peak-to-peak and were generated in antiphase to tilts. The pattern of EMG responses to tilts after injection (activation with ipsilateral tilts) was also similar to that before injection. The periodical bursting activity, which was usually observed in VQ and VHS animals after methoxamine injection (as in Fig. 6B), appeared very rare in the intact rabbit.
FIG. 8.
Drug application produced no effect in intact rabbit. A: responses to tilts in the intact rabbit 65. B: responses in the same rabbit tested in 4 h after methoxamine injection (the dose that was efficient in, e.g., VHS rabbit 72 shown in Fig. 6, C and D). C: the gain of postural reflexes in control and after application of different drugs (mean ± SE, averaging over all tests). Shaded column highlights 1 of the tilt cycles to facilitate comparison between curves.
Figure 8C shows the gain of postural reflexes (see methods) in control and after injection of different drugs (averaging over 5–6 sequential trials in each case; mean ± SE). No significant difference was observed between the gain values in different conditions.
DISCUSSION
The idea to restore impaired motor functions of the spinal networks in SCI subjects by pharmacological stimulation of the spinal cord below the lesion is not a novel one. In particular, in the studies by Rossignol and colleagues (Brustein and Rossignol 1999; Chau et al. 1998, 2002; Giroux et al. 1998, 2001; Rossignol et al. 1998, 2001), the effects of different neurotransmitters and their agonists on the locomotor function were examined in the cats with complete or partial chronic transection of the spinal cord in the lower thoracic region. It was shown that stepping movements of the hindlimbs could be evoked (or significantly improved) by applying specific drugs to the lumbosacral enlargement of the spinal cord.
Another motor function that suffers badly in SCI subjects is the maintenance of body posture and balance (Barbeau et al. 2002; Lyalka et al. 2005). A reason for this is a dramatic impairment of the system of postural reflexes in SCI subjects (Frigon and Rossignol 2006). Despite an essential role of postural function for standing and walking, no attempts to restore postural control in SCI subjects by pharmacological stimulation have been reported. In this study, we tried to reactivate the spinal postural mechanisms of the hindquarters after their damage caused by a partial transection of spinal pathways.
We used two experimental models: the VQ and VHS rabbits. In VHS animals, ventral pathways (including reticulo- and vestibulospinal ones) critically important for the postural control were destroyed bilaterally, whereas in VQ animals, they were destroyed unilaterally. Of these two models, the capacity to recuperate the postural function was completely abolished in VHS animals (Lyalka et al. 2005). In contrast, a small proportion of VQ animals exhibited a limited recovery of postural responses, most likely because of the spared ventral spinal pathways (Lyalka et al. 2006). One characteristic postural deficit in the VQ and VHS rabbits was a considerable reduction of EMG responses to tilts, so that the limb extensors developed insufficient force to compensate for tilts. The other deficit was instability of responses—they could spontaneously change their phase in relation to tilts or disappear altogether. A low probability and small value of correct (properly phased) EMG responses, as well as appearance of incorrect responses, suggests that the spinal reflex chains, necessary for postural control, have not been specifically selected by the supraspinal drive.
For drug application, we used the technique of intrathecal cannula (modified from Chau et al. 1998). The drugs were injected at the L5–L6 level. In special experiments (with injection of fast green), we found that the injected solution spread over the whole enlargement. Thus we stimulated the lower lumbar segments, where most of the motoneuron pools of limb extensors are located (Portal et al. 1991; Romanes 1951; Vanderhorst and Holstege 1997), as well as the corresponding interneurons. One can suggest that this area is involved in the generation of postural reactions, which require a predominant extensor activity. However, we also stimulated the more rostral lumbar segments where the rhythm-generating networks of the locomotor central pattern generator are located (Kjaerulff and Kiehn 1996; Langlet et al. 2005).
We used the drugs affecting the noradrenergic and serotoninergic neurotransmitter systems, namely methoxamine (α1 noradrenergic agonist), serotonin (5-HT), quipazine (5-HT1,2,3 agonist), and a mixture of methoxamine and quipazine. These drugs have been reported to improve weight support and lateral stability during locomotion in cats with spinal lesions (Brustein and Rossignol 1999; Chau et al. 1998). This was our reason to expect that these drugs will reactivate the spinal postural reflex mechanisms, which were severely impaired in VHS and VQ animals. The amount of injected drug was approximately equal to that used for elicitation and modulation of the hindlimb stepping movements in the cats with complete or partial transection of the spinal cord (Brustein and Rossignol 1999; Chau et al. 1998).
It is known that the effects of agonists and antagonists of various neurotransmitters in the damaged spinal cord exhibit temporal changes (for review, see Rossignol et al. 2001). This could be caused by transient up- or down-regulation of various receptors; the up-regulation was shown for α1 noradrenergic and 5-HT1A,2 receptors during the first month after spinalization (Giroux et al. 1999; Lee et al. 2007). Other reasons could be cell degeneration, sprouting, and other secondary processes initiated by partial or complete loss of descending fibers in the lumbar spinal cord after SCI (Holmes et al. 2005; Hultborn and Malmsten 1983; Saruhashi et al. 1996). To characterize these changes, in this study we tested the effect of drugs in each of the three 10-day periods after lesion, termed the early, intermediate, and late periods.
The main result of this study was that, during the early postlesion period, serotonin and quipazine caused a considerable increase of the proportion of correctly phased EMG responses to tilts (Fig. 5). This increase was accompanied by a decrease of the proportion of cases with no responses, whereas the proportion of incorrect EMG responses did not change. Serotonin and quipazine did not re-establish postural corrective movements. Our interpretation of these findings is the following. 1) The serotoninergic system selectively activates the spinal postural networks, but this activation is insufficient to cause full-scale responses to tilts, suggesting that normally the serotoninergic system operates along with other activating systems. 2) Persistence of incorrectly phased EMG responses to tilts, even at the peak of serotonin or quipazine action, suggests that other (not postural) reflex chains have not been sufficiently suppressed. 3) The finding that postural reflexes can be enhanced by serotonin and quipazine only during a 10-day period after lesion suggests considerable postlesion changes in the spinal postural network at later stages after SCI.
There is much evidence for a positive role of serotonin and quipazine in the recovery of motor functions after SCI. In our recent experiments on acute spinal rabbits it was found that the epidural electrical stimulation, if it was combined with application of quipazine, resulted in much better restoration of postural limb reflexes (Musienko et al. 2007). It was also shown that quipazine increased the efficacy of training (Antri et al. 2002; De Leon and Acosta 2006; Fong et al. 2005; Rossignol et al. 2001) and of epidural stimulation (Gerasimenko et al. 2007) for the restoration of locomotor function in SCI animals. One can suggest that, by combining quipazine application with postural training or with epidural stimulation of the spinal cord below the lesion, one can help to restore postural functions, at least during the early post-SCI period.
Positive effects of serotonin are caused by specific characteristics of this neurotransnitter system. First, serotonin has mainly a supraspinal origin (for review, see Schmidt and Jordan 2000), and thoracic SCI induces a substantial loss of serotonin content in lumbar segments (Laporte et al. 1995). Second, serotonin can affect selectively different spinal reflexes, enhancing some of them and suppressing others (Aggelopoulos et al. 1996; Bras et al. 1989, 1990; Jankowska et al. 1993; Miller et al. 1996). There is experimental evidence indicating that serotonin is involved in a selection of specific spinal reflexes, mainly via 5-HT1A receptors (Aggelopoulos et al. 1996). It was also shown that bistable properties of extensor motoneurons, which disappear after acute spinalization, can be restored by serotoninergic drugs (Hounsgaard et al. 1988). These drugs can also restore the extensor tone, which disappears after spinalization (Miller et al. 1996). These modulatory properties of serotonin can contribute to selective enhancement of postural limb reflexes observed in this study. It remains unclear, however, why this effect lasted for only the early post-SCI period and later was replaced by elicitation of rhythmical bursting.
In contrast to serotoninergic drugs, application of methoxamine (α1 noradrenergic agonist) did not increase the proportion of correct EMG responses during the early post-SCI period. Nor did it re-establish postural corrective movements in response to tilts (Fig. 7, A–C). The main effect of methoxamine at all postlesion stages (as well as of serotonin at later stages) was an induction of periodical bursting in EMGs, suggesting activation of spinal rhythm generating networks. At the peak of action of these drugs, alternating stepping-like movements of the hindlimbs could be observed (Fig. 6B), suggesting activation of the spinal locomotor CPG. This result well corresponds to the data that methoxamine promotes initiation of stepping in spinal cats (Chau et al. 1998; for review see Rossignol et al. 2001). However, before and after reaching the peak of methoxamine action, in our experiments, one could simultaneously observe several rhythms in the hindlimb EMGs (as in Fig. 6D): the rhythms could be synchronous in the flexor and extensor muscles in one limb or even in two limbs. These rhythmical patterns are characteristic for clonus, the symptom observed in SCI patients. It seems likely that clonus is caused by central generating mechanism subjected to somatosensory influences (Beres-Jones et al. 2003).
One can suggest that activation of spinal rhythm generating circuits perturbs a normal operation of postural mechanisms. This suggestion is supported by a methoxamine-induced abolishment of postural effects of quipazine, observed in this study (Fig. 7, D–F). Also, methoxamine-evoked bursts were infrequently modulated by tilts (Fig. 6B), similarly to modulation observed in the cat walking on the tilting treadmill (Matsuyama and Drew 2000; A. Karayannidou, I. Beloozerova, T. Deliagina, unpublished data). In this study, however, the modulation equally often was correctly or incorrectly phased in relation to tilt cycles (Fig. 7, A and B).
Our interpretation of the effects of methoxamine is the following. Methoxamine is not able to activate selectively the spinal postural networks. It activates the spinal rhythm generating networks, which perturbs normal operation of the spinal postural networks.
An interesting observation in this study was that all three drugs did not affect postural performance in the intact rabbit (Fig. 8), despite that the same amounts were effective in SCI rabbits. Similar results were obtained in cats walking on the treadmill: the amount of drugs effective in cats with complete or partial spinal lesions was much less effective in intact cats (see Giroux et al. 1998, 2001). One can suggest that intact animals have efficient means of compensating for the neurotransmitter imbalance, which include supraspinal systems.
To conclude, this study showed that activation of the serotoninergic system promotes a partial recovery of postural reflexes after different damages to the spinal cord. In contrast, the noradrenergic system elicits rhythmic activity in the spinal cord and does not promote functioning of postural reflex machinery. It seems probable that effects of serotoninergic drugs can be used beneficially by patients with partial SCI, especially if they still have residual postural capabilities.
GRANTS
This work was supported by National Institute of Neurological Disorders and Stroke Grant R01 NS-049884, the Swedish Research Council (11554), and Gösta Fraenckels Foundation to T. G. Deliagina.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- Aggelopoulos et al. 1996.Aggelopoulos NC, Burton MJ, Cliarke RW, Edgley SA. Characterization of a descending system that enables crossed group II inhibitory reflex pathways in the cat spinal cord. J Neurosci 16: 723–729, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akaike and Westerman 1973.Akaike T, Westerman RA. Spinal segmental levels innervated by different types of vestibulo-spinal tract neurons in rabbit. Exp Brain Res 17: 443–446, 1973. [DOI] [PubMed] [Google Scholar]
- Antri et al. 2002.Antri M, Orsal D, Barthe JY. Locomotor recovery in the chronic spinal rat: effects of long-term treatment with a 5-HT agonist. Eur J Neurosci 16: 467–476, 2002. [DOI] [PubMed] [Google Scholar]
- Barbeau et al. 2002.Barbeau H, Fung J, Leroux A, Ladouceur M. A review of the adaptability and recovery of locomotion after spinal cord injury. Prog Brain Res 137: 9–25, 2002. [DOI] [PubMed] [Google Scholar]
- Beloozerova et al. 2003.Beloozerova IN, Zelenin PV, Popova LB, Orlovsky GN, Grillner S, Deliagina TG. Postural control in the rabbit maintaining balance on the tilting platform. J Neurophysiol 90: 3783–3793, 2003. [DOI] [PubMed] [Google Scholar]
- Beres-Jones et al. 2003.Beres-Jones JA, Johnson TD, Harkema SJ. Clonus after human spinal cord injury cannot be attributed solely to recurrent muscle-tendon stretch. Exp Brain Res 149: 222–236, 2003. [DOI] [PubMed] [Google Scholar]
- Blessing et al. 1981.Blessing WW, Goodchild AK, Dampney RAL, Chalmers JP. Cell groups in the lower brain stem of the rabbit projecting to the spinal cord, with special reference to catecholamine-containing neurons. Brain Res 221: 35–55, 1981. [DOI] [PubMed] [Google Scholar]
- Bras et al. 1989.Bras H, Cavallari P, Jankowska E, McCrea DA. Comparison of effects of monoamines on trasmission in spinal pathways from group I and II muscle afferents in the cat. Exp Brain Res 76: 27–37, 1989. [DOI] [PubMed] [Google Scholar]
- Bras et al. 1990.Bras H, Jankowska E, Noga B, Skoog B. Comparison of effects of various types of NA and 5-HT agonists on transmission from group II muscle afferents in the cat. Eur J Neurosci 2: 1029–1039, 1990. [DOI] [PubMed] [Google Scholar]
- Brustein and Rossignol 1998.Brustein E, Rossignol S. Recovery of locomotion after ventral and ventrolateral spinal lesions in the cat. I. Deficits and adaptive mechanisms. J Neurophysiol 80: 1245–1267, 1998. [DOI] [PubMed] [Google Scholar]
- Brustein and Rossignol 1999.Brustein E, Rossignol S. Recovery of locomotion after ventral and ventrolateral spinal lesions in the cat. II. Effects of noradrenergic and serotoninergic drugs. J Neurophysiol 81: 1513–1530, 1999. [DOI] [PubMed] [Google Scholar]
- Chau et al. 1998.Chau C, Barbeau H, Rossignol S. Effects of intrathecal α1- and α2-noradrenergic agonists and norepinephrine on locomotion in chronic spinal cats. J Neurophysiol 79: 2941–2963, 1998. [DOI] [PubMed] [Google Scholar]
- Chau et al. 2002.Chau C, Giroux N, Barbeau H, Jordal L, Rossignol S. Effects of intrathecal glutamatergic drugs on locomotion. I. NMDA in short-term spinal cats. J Neurophysiol 88: 3032–3045, 2002. [DOI] [PubMed] [Google Scholar]
- De Leon and Acosta 2006.De Leon RD, Acosta CN. Effect of robotic-assisted treadmill training and chronic quipazine treatment on hindlimb stepping in spinally transected rats. J Neurotrauma 23: 1147–1163, 2006. [DOI] [PubMed] [Google Scholar]
- De Leon et al. 1998.De Leon RD, Hodgson JA, Roy RR, Edgerton VR. Full weight-bearing hindlimb standing following stand training in the adult spinal cat. J Neurophysiol 80: 83–91, 1998. [DOI] [PubMed] [Google Scholar]
- Deliagina et al. 2008.Deliagina TG, Beloozerova IN, Zelenin PV, Orlovsky GN. Spinal and supraspinal postural networks. Brain Res Rev 57: 212–221, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deliagina et al. 2006.Deliagina TG, Orlovsky GN, Zelenin PV, Beloozerova IN. Neural bases of postural control. Physiology 21: 216–225, 2006. [DOI] [PubMed] [Google Scholar]
- Edgerton et al. 2001.Edgerton VR, de Leon RD, Tillakaratne NJ, Recktenwald MR, Hodson JA, Roy RR. Retraining the injured spinal cord. J Physiol 533: 15–22, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgerton et al. 2004.Edgerton VR, Tillakaratne NJK, Bigbee AJ, de Leon RD, Roy RR. Plasticity of the spinal neural circuitry after injury. Annu Rev Neurosci 27: 145–167, 2004. [DOI] [PubMed] [Google Scholar]
- Fong et al. 2005.Fong AJ, Cai LL, Reinkensmeyer DJ, Burdick JW, Roy RR, Edgerton VR. Spinal cord-transected mice learn to step in response to quipazine treatment and robotic training. J Neurosci 25: 11738–11747, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frigon and Rossignol 2006.Frigon A, Rossignol S. Functional plasticity following spinal cord lesions. Prog Brain Res 157: 231–260, 2006. [DOI] [PubMed] [Google Scholar]
- Gerasimenko et al. 2007.Gerasimenko YP, Ichiyama RM, Lavrov IA, Courtine G, Cai L, Zhong H, Roy RR, Edgerton VR. Epidural spinal cord stimulation plus quipazine administration enable stepping in complete spinal adult rats. J Neurophysiol 98: 2525–2536, 2007. [DOI] [PubMed] [Google Scholar]
- Giroux et al. 1998.Giroux N, Brustein E, Chau C, Barbeau H, Reader TA, Rossignol S. Differential effects of the noradrenergic agonist clonidine on the locomotion of intact, partially and completely spinalized adult cats. Ann NY Acad Sci 860: 517–520, 1998. [DOI] [PubMed] [Google Scholar]
- Giroux et al. 2001.Giroux N, Reader TA, Rossignol S. Comparison of the effect of intrathecal administration of clonidine and yohimbine on the locomotion of intact and spinal cats. J Neurophysiol 85: 2516–2536, 2001. [DOI] [PubMed] [Google Scholar]
- Giroux et al. 1999.Giroux N, Rossignol S, Reader TA. Autoradiographic study of α1-, α2-noradrenergic and serotonin1 receptors in the spinal cord of normal and chronically transacted cats. J Comp Neurol 406: 402–414, 1999. [PubMed] [Google Scholar]
- Giuliani and Smith 1985.Giuliani CA, Smith JL. Development and characteristics of air-stepping in the chronic spinalized cat. J Neurosci 5: 1276–1282, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillner 1973.Grillner S Locomotion in the spinal cat. In: Control of Posture and Locomotion, edited by Stein RB, Pearson KG, Smith RS, Redford JB. New York: Plenum Press, 1973, p. 515–535.
- Holmes et al. 2005.Holmes GM, Van Meter MJ, Beattie MS, Bresnahan JC. Serotonergic fiber sprouting to external and sphincter motoneurons after spinal cord contusion. Exp Neurol 193: 29–42, 2005. [DOI] [PubMed] [Google Scholar]
- Horak and Macpherson 1996.Horak F, Macpherson J. Postural orientation and equilibrium. In: Handbook of Physiology. Exercise: Regulation and Integration of Multiple Systems, edited by Shepard J, Rowell L. New York: Oxford, 1996, sect. 12, p. 255–292.
- Hounsgaard et al. 1988.Hounsgaard J, Hultborn H, Jespersen J, Kiehn O. Bistability of alpha-motoneurones in the decerebrate cat and in the acute spinal cat after intravenous 5-hydroxytryptophan. J Physiol (Lond) 405: 345–367, 1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultborn and Malmsten 1983.Hultborn H, Malmsten J. Changes in segmental reflexes following chronic spinal cord hemisection in the cat. I. Increased monosynaptic and polysynaptic ventral root discharges. Acta Physiol Scand 119: 405–422, 1983. [DOI] [PubMed] [Google Scholar]
- Jankowska et al. 1993.Jankowska E, Riddell JS, Skoog B, Noga BR. Gating of transmission to motoneurones by stimuli applied in the locus coeruleus and raphe nuclei of the cat. J Physiol 461: 705–722, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang and Drew 1996.Jiang W, Drew T. Effects of bilateral lesions of the dorsolateral funiculi and dorsal columns at the level of the low thoracic spinal cord on the control of locomotion in the adult cat. I. Treadmill walking. J Neurophysiol 76: 849–866, 1996. [DOI] [PubMed] [Google Scholar]
- Kellog et al. 1946.Kellog WN, Deese J, Pronko NH. On the behavior of the lumbo-spinal dog. Exp Psychol 36: 503–511, 1946. [DOI] [PubMed] [Google Scholar]
- Kjaerulff and Kiehn 1996.Kjaerulff O, Kiehn O. Distribution of networks generating and coordinating locomotor activity in the neonatal rat spinal cord in vitro: a lesion study. J Neurosci 16: 5777–5794, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langlet et al. 2005.Langlet C, Leblond H, Rossignol S. Mid-lumbar segments are needed for the expression of locomotion in chronic spinal cats. J Neurophysiol 93: 2474–2488, 2005. [DOI] [PubMed] [Google Scholar]
- Laporte et al. 1995.Laporte AM, Fattaccini CM, Lombard MC, Chauveau J, Hamon M. Effects of dorsal rhizotomy and selective lesion of serotonergic and noradrenergic systems on 5-HT1A, 5-HT1B, and 5-HT3 receptors in the rat spinal cord. J Neural Transm 100: 207–223, 1995. [DOI] [PubMed] [Google Scholar]
- Lee et al. 2007.Lee JK, Johnson CS, Wrathall JR. Up-regulation of 5-HT2 receptors is involved in the increased H-reflex amplitude after contusive spinal cord injury. Exp Neurol 203: 502–511, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyalka et al. 2006.Lyalka VF, Musienko PE, Orlovsky GN, Deliagina TG. Effect of intrathecal application of serotoninergic and noradrenergic drugs on postural performance in rabbits with spinal cord lesions. Soc Neurosci Abstr 32: 88.1, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyalka et al. 2005.Lyalka VF, Zelenin PV, Karayannidou A, Orlovsky GN, Grillner S, Deliagina TG. Impairment and recovery of postural control in rabbits with spinal cord lesions. J Neurophysiol 94: 3677–3690, 2005. [DOI] [PubMed] [Google Scholar]
- Macpherson et al. 1997a.Macpherson JM, Deliagina TG, Orlovsky GN. Control of body orientation and equilibrium in vertebrates. In: Neurons, Networks, and Motor Behaviour, edited by Stein PSG, Grillner S, Selverston AI, and Stuart DG. Cambridge, MA: MIT Press, 1997a, p. 257–267.
- Macpherson and Fung 1999.Macpherson JM, Fung J. Weight support and balance during perturbed stance in the chronic spinal cat. J Neurophysiol 82: 3066–3081, 1999. [DOI] [PubMed] [Google Scholar]
- Macpherson et al. 1997b.Macpherson JM, Fung J, Jacobs R. Postural orientation, equilibrium, and the spinal cord. In: Advances in Neurology: Neuronal Regeneration, Reorganization, and Repair, edited by Seil FJ. Philadelphia, PA: Lippincott-Raven, 1997b, vol. 72, p. 227–232. [PubMed] [Google Scholar]
- Massion 1998.Massion J Postural control systems in developmental perspective. Neurosci Biobehav Rev 22: 465–472, 1998. [DOI] [PubMed] [Google Scholar]
- Massion and Dufosse 1988.Massion L, Dufosse M. Coordination between posture and movement: why and how? News Physiol Sci 3: 88–93, 1988. [Google Scholar]
- Matsuyama and Dew 2000.Matsuyama K, Dew T. Vestibulospinal and reticulospinal neuronal activity during locomotion in the intact cat. II. Walking on a inclined plane. J Neurophysiol 84: 2257–2276, 2000. [DOI] [PubMed] [Google Scholar]
- Miller et al. 1996.Miller JF, Paul KD, Lee RH, Rymer WZ, Heckman CJ. Restoration of extensor excitability in the acute spinal cat by the 5HT2 agonist DOI. J Neurophysiol 75: 620–628, 1996. [DOI] [PubMed] [Google Scholar]
- Musienko et al. 2007.Musienko PE, Orlovsky GN, Deliagina TG. Enhancement of limb reflexes in the spinal rabbit by stimulation of spinal cord. Soc Neurosci Abstr 33: 75.17, 2007. [Google Scholar]
- Orlovsky et al. 1999.Orlovsky GN, Deliagina TG, Grillner S. Neuronal Control of Locomotion. From Mollusc to Man. Oxford, UK: Oxford, 1999.
- Portal et al. 1991.Portal J-J, Corio M, Viala D. Localization of the lumbar pools of motoneurons which provide hindlimb muscles in the rabbit. Neurosci Letters 124: 105–107, 1991. [DOI] [PubMed] [Google Scholar]
- Pratt et al. 1994.Pratt CA, Fung J, Macpherson JM. Stance control in the chronic spinal cat. J Neurophysiol 71: 1981–1985, 1994. [DOI] [PubMed] [Google Scholar]
- Romanes 1951.Romanes G The motor cell columns of the lumbo-sacral spinal cord of the cat. J Comp Neurol 94: 313–336, 1951. [DOI] [PubMed] [Google Scholar]
- Rossignol et al. 2002.Rossignol S, Bouyer L, Barthelemy D, Langlet C, Leblond H. Recovery of locomotion in the cat following spinal cord lesions. Brain Res Rev 40: 257–266, 2002. [DOI] [PubMed] [Google Scholar]
- Rossignol et al. 1998.Rossignol S, Chau C, Brustein E, Giroux N, Bouyer L, Barbeau H, Reader TA. Pharmacological activation and modulation of the central pattern generator for locomotion in the cat. Ann NY Acad Sci 860: 346–359, 1998. [DOI] [PubMed] [Google Scholar]
- Rossignol et al. 1999.Rossignol S, Drew T, Brustein E, Jiang W. Locomotor performance and adaptation after partial or complete spinal cord lesions in the cat. In: Peripheral and Spinal Mechanisms in the Neural Control of Movement, edited by Binder MD. Amsterdam: Elsevier, 1999, p. 349–365. [DOI] [PubMed]
- Rossignol et al. 2001.Rossignol S, Giroux N, Chau C, Marcoux J, Brustein E, Reader TA. Pharmacological aids to locomotor training after spinal injury in the cat. J Physiol 533: 65–74, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saruhashi et al. 1996.Saruhashi Y, Young W, Perkins R. The recovery of 5-HT immunoreactivity in lumbosacral spinal cord and locomotor function after thoracic hemisection. Exp Neurol 139: 203–213, 1996. [DOI] [PubMed] [Google Scholar]
- Schmidt and Jordan 2000.Schmidt BJ, Jordan LM. The role of serotonin in reflex modulation and locomotor rhythm production in the mammalian spinal cord. Brain Res Bull 53: 689–710, 2000. [DOI] [PubMed] [Google Scholar]
- Vanderhorst and Holstege 1997.Vanderhorst VG, Holstege G. Organization of lumbosacral motoneuronal cell groups innervating hindlimb, pelvic floor, and axial muscles in the cat. J Comp Neurol 382: 46–76, 1997. [PubMed] [Google Scholar]