Abstract
Endocannabinoids released from the postsynaptic neuronal membrane can activate presynaptic CB1 receptors and inhibit neurotransmitter release. In hippocampal slices, depolarization of the CA1 pyramidal neurons elicits an endocannabinoid-mediated inhibition of γ-aminobutyric acid release known as depolarization-induced suppression of inhibition (DSI). Using the highly reduced neuron/synaptic bouton preparation from the CA1 region of hippocampus, we have begun to examine endocannabinoid-dependent short-term depression (STD) of inhibitory synaptic transmission under well-controlled physiological and pharmacological conditions in an environment free of other cells. Application of the CB1 synthetic agonist WIN55212-2 and endogenous cannabinoids 2-AG and anandamide produced a decrease in spontaneous inhibitory postsynaptic current (sIPSC) frequency and amplitude, indicating the presence of CB1 receptors at synapses in this preparation. Endocannabinoid-dependent STD is different from DSI found in hippocampal slices and the neuron/bouton preparation from basolateral amygdala (BLA) since depolarization alone was not sufficient to induce suppression of sIPSCs. However, concurrent application of the metabotropic glutamate receptor (mGluR) agonist (RS)-3,5-dihydroxyphenylglycine (DHPG) and postsynaptic depolarization resulted in a transient (30–50 s) decrease in sIPSC frequency and amplitude. Application of DHPG alone had no effect on sIPSCs. The depolarization/DHPG-induced STD was blocked by the CB1 antagonist SR141716A and the mGluR5 antagonist MPEP and was sensitive to intracellular calcium concentration. Comparing the present findings with earlier work in hippocampal slices and BLA, it appears that endocannabinoid release is less robust in isolated hippocampal neurons.
INTRODUCTION
Cannabinoids are the pharmacologically active substances present in Cannabis sativa (Mackie 2006; Pacher et al. 2006). The effects of cannabinoid administration are various and include alteration of many physiological and psychological processes, such as affective states, sensory perception, antinociception, concentration, and memory (Pacher et al. 2006). The psychological effects of cannabinoids are mediated mainly by activating the CB1 cannabinoid receptor, which is expressed almost ubiquitously in the brain (Herkenham 1991; Matsuda et al. 1990). One of the highest CB1 receptor densities is found in hippocampus, a brain structure associated with memory and learning, and administration of cannabinoids appears to alter memory via CB1 receptor activation (Lichtman and Martin 1996).
The central effects of cannabinoids usually involve modulation of synaptic transmission. Release of endocannabinoids from the postsynaptic neuron and retrograde activation of presynaptic CB1 receptors inhibits the release of γ-aminobutyric acid (GABA). Thus activation of group I metabotropic glutamate receptors (mGluRs), mainly mGluR5, has been shown to suppress inhibitory neurotransmission (Ohno-Shosaku et al. 2002). In addition, depolarization of hippocampal CA1 neurons in slice produces an endocannabinoid-mediated form of short-term plasticity at GABAergic synapses: depolarization-induced suppression of inhibition (DSI) (Ohno-Shosaku et al. 2001; Pitler and Alger 1992, 1994; Wilson and Nicoll 2001). DSI is enhanced by group I mGluR activation in a cooperative manner, indicating that both pathways use common intracellular cascades (Ohno-Shosaku et al. 2002). Although the physiological significance of endocannabinoid-dependent DSI has been questioned (Hampson et al. 2003), recent studies demonstrated that DSI facilitates the induction of long-term potentiation (LTP) in hippocampus (Carlson et al. 2002).
Recently, DSI was found in a highly reduced neuron/synaptic bouton preparation from basolateral amygdala (BLA), supporting the hypothesis of retrograde endocannabinoid signaling (Zhu and Lovinger 2005). In the present study, we used a similar preparation from the CA1 region of hippocampus. Our results demonstrate that in the postsynaptic neuron/synaptic bouton preparation from hippocampal CA1 neurons, DSI could not be induced by depolarization alone. However, concurrent activation of metabotropic glutamate receptor 5 (mGluR5) and postsynaptic depolarization induced an endocannabinoid-dependent form of short-term depression (STD) similar to DSI. Taken together, our results could indicate lower endocannabinoid tone in the hippocampal neuron/synaptic bouton preparation than that found in a similar preparation from BLA.
METHODS
Postsynaptic neuron/presynaptic bouton preparation
Coronal brain slices (400-μm thickness) containing hippocampus were prepared from postnatal day 14 (P14) to P17 Sprague–Dawley rats. Sections were cut with a Vibratome 1000 (Vibratome, St. Louis, MO) in cold buffer oxygenated with 95% O2-5% CO2. The slicing buffer was composed of (in mM): 124 NaCl, 3 KCl, 1.3 Mg2SO4, 2 CaCl2, 1.2 NaH2PO4, 25 NaHCO3, and 10 O glucose. Slices were maintained at room temperature for ≥1 h before dissociation.
Mechanical neuronal dissociation
Single hippocampal neurons were isolated using an enzyme-free mechanical procedure, as described by Vorobjev (1991) and by Akaike and Moorhouse (2003). Briefly, the slices were transferred to a 35-mm culture dish with an external recording buffer containing (in mM): 150 NaCl, 2.5 KCl, 2.5 CaCl2, 1.0 MgCl2, 10 HEPES, 10 glucose, 0.005 2,3-dihydroxy-6-nitro-7-sulfonylbenzo[f]quinoxaline (NBQX), and 0.1 dl-2-amino-5-phosphonopentanoic acid (AP-5), with pH adjusted to 7.4 and osmolarity of about 340 mOsm. A fire-polished glass micropipette was placed on the surface of the CA1 region of the hippocampus. The tip of the pipette was vibrated horizontally along the CA1 region at 30 Hz from about 2 min using a piezoelectric manipulator (LSS-3000, Burleigh/Exfo, Quebec, Canada) triggered by a Grass SD9K stimulator (Grass Technologies, West Warwick, RI). The slice was then removed and the isolated neurons were allowed to settle to the bottom of the dish for 10–15 min. The dish with neurons was placed on the stage of an inverted microscope for visualization and performance of electrophysiological recordings.
Electrophysiology
Whole cell patch-clamp recordings were made at room temperature using the conventional patch-clamp technique and an Axopatch 200B amplifier (Molecular Devices, Union City, CA). Neurons were held at −60 mV unless otherwise indicated. The extracellular solution was the same as that used during neuronal isolation. Patch pipettes had resistances of 3–5 MΩ after filling with a solution containing (in mM): 150 CsCl, 10 HEPES, 2 MgCl2, 0.3 Na-GTP, 3 Mg-ATP, 0.2 BAPTA, with pH adjusted to 7.23 and osmolarity of about 315 mOsm. External solution exchange was achieved by a solenoid valve perfusion system (ValveLink 16, AutoMate Scientific, San Francisco, CA) and rapid lateral movement of three-port square glass pipettes driven by a stepper motor (Fast Step system, Warner Instruments, Hamden, CT).
Data were filtered at 2 kHz and digitized at 5 kHz using pClamp 9 software and a Digidata 1320A interface (Molecular Devices). Detection of spontaneous synaptic currents was done automatically with MiniAnalysis v6.0 software (Synaptosoft, Fort Lee, NJ). The program selected all events that crossed the amplitude threshold (five- to eightfold the root-mean-square baseline noise) and had typical spontaneous inhibitory postsynaptic current (sIPSC) kinetics (Fig. 1B, asterisks). Overlapping events were also considered. Accurate detection was verified by subsequent visual inspection of current traces. All further analyses were done with Igor Pro 5 (WaveMetrics, Lake Oswego, OR) and custom-written macros. All drugs were obtained from Tocris Cookson (Ellisville, MO). When WIN55212-2 {(R)-(+)-[2,3-dihydro-5-methyl-3-(4-morpholinylmethyl)pyrrolo-(1,2,3-de)-1,4-benzoxazin-6-yl]-1-naphthalenylmethanone}, SR141716A [N-(piperidine-1-yl)-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide hydrochloride], AEA (anandamide), or 2-AG (2-arachidonoylglycerol) were used, bovine serum albumin (BSA, 0.05%) was added to the solution as a carrier. Averaged data are presented as means ± SE; the data displayed on inter-event and amplitude histograms were usually binned using 5-s periods unless otherwise noted. Statistical comparisons between conditions were made using paired t-tests. The criterion for significance was P < 0.05.
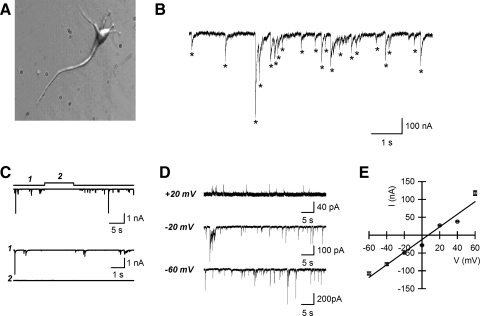
FIG. 1.
GABAergic neurotransmission in the postsynaptic neuron/synaptic bouton preparation from the hippocampal CA1 region. A: a typical mechanically isolated CA1 pyramidal neuron. B: example trace acquired at −60 mV showing spontaneous inward currents. Asterisks indicate the events detected as described in methods. C: spontaneous inward currents acquired at −60 mV were sensitive to the γ-aminobutyric acid type A antagonist bicuculline. 1: currents before application of bicuculline. 2: currents after application of bicuculline (20 μM). D: spontaneous currents acquired at holding potentials of −60, −20, and +20 mV. E: current–voltage (I–V) relationship measured from the cell shown in D. Each data point is an average of 37–695 events.
Immunocytochemistry
Hippocampal neurons were dissociated as described earlier on poly-d-lysine (100 μg/mL in water) coated glass-bottom dishes. Neurons were fixed with 4% formaldehyde in phosphate-buffered saline (PBS, pH 7.4) for 15 min, rinsed with PBS, 0.2% Triton X-100 (PBS-T), and blocked for 1 h with 5% bovine serum albumin (BSA) in PBS-T. Rabbit anti-CB1 (1:200; Dr. K. Mackie) and goat anti-synapsin (1:500, SC #7379; Santa Cruz Biotechnology) primary antibodies were added for 2 h in PBS-T, 1% BSA at room temperature. Neurons were washed three times for 10 min in PBS-T and secondary antibodies were added individually due to species constraints. Alexa 568 donkey anti-goat secondary antibody was added first (1:1,000; Invitrogen) for 1 h and the neurons were washed three times and then incubated with Alexa 488 goat anti-rabbit secondary antibody (1:100; Invitrogen). Negative controls were processed identically except the primary antibodies were omitted. Following three final washes, cells were imaged with a Zeiss Axiovert 200 epifluorescence microscope using 480/35 excitation and 535/35 emission filters for Alexa 488 and 540/25 excitation and 605/55 emission filters for Alexa 568 with a ×100 objective (numerical aperture = 1.3). Individual channels were captured with a monochrome Axiocam (Zeiss) and colors were applied using Axiovision software. Individual channels and the combined images are presented.
RESULTS
Neurons isolated from the CA1 region of rat hippocampus preserved the typical morphology of principal neurons: pyramidal-shaped soma and processes (Fig. 1A). When the neurons were voltage clamped in the whole cell configuration at −60 mV in the presence of NBQX/AP-5 (5 μM/100 μM), spontaneous inward currents were recorded (Fig. 1B). These currents were eliminated by application of 20 μM bicuculline (Fig. 1C) and the current amplitude changed with changes in membrane potential in the manner expected for a Cl− current. The reversal potential for these currents was +9.8 ± 1.6 mV (n = 6; Fig. 1, D and E). These data indicate that the spontaneous currents are sIPSCs mediated by GABA release and activation of postsynaptic GABAA receptors (Fig. 1, C, D, and E).
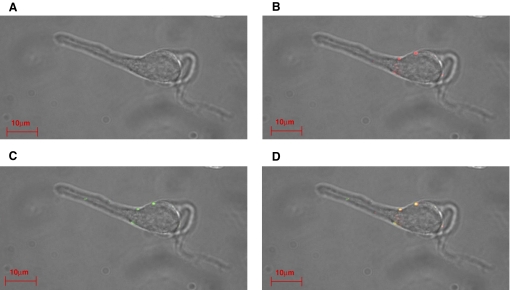
In addition to releasing GABA, the presynaptic terminals possessed functional CB1 receptors. Immunostaining with anti-CB1 receptor and anti-synapsin antibodies revealed the colocalization of CB1 receptor and synapsin at the presynaptic terminals (Fig. 2). Similar staining was observed in 12 cells examined. No fluorescence was detected in control cells, when the primary antibodies were omitted (data not shown).
FIG. 2.
Colocalization of synapsin and CB1 receptors in the isolated neuron/bouton preparation from the hippocampal CA1 region. A: transmitted light image. B: staining with goat anti-synapsin primary antibody and Alexa 568 donkey anti-goat secondary antibody for synapsin. C: staining with rabbit anti-CB1 primary antibody and Alexa 488 anti-rabbit secondary antibody. D: merge.
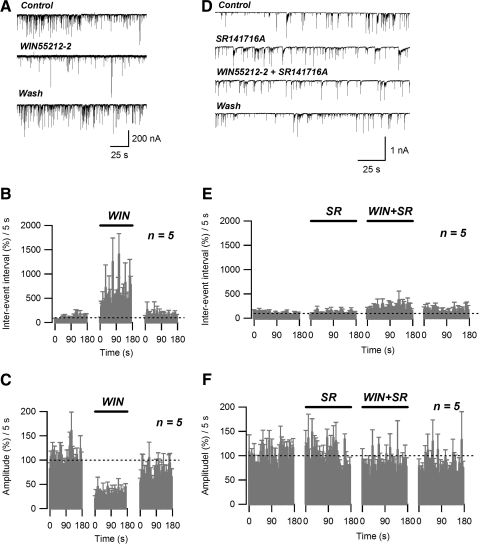
Application of a selective CB1 receptor agonist WIN55212-2 (0.8 μM) reversibly decreased the frequency of sIPSCs, measured as an increase in inter-event interval (Fig. 3, A and B). On average, for a 3-min-long drug application, WIN55212-2 produced a significant (495.51 ± 27.4% of control; n = 5; P < 0.005, paired t-test) increase in mean sIPSC inter-event interval, compared with the control level (Fig. 3B). In addition, WIN55212-2 application resulted in significant (29.6 ± 13.6% of control; n = 5, P < 0.005, paired t-test) reduction of the average sIPSC amplitude (Fig. 3, A and C).
FIG. 3.
Spontaneous inhibitory postsynaptic currents (sIPSCs) are inhibited by the CB1 receptor agonist WIN55212-2. A: sIPSC traces acquired before (Control), during (WIN55212-2), and after (Wash) application of 0.8 μM WIN55212-2. B: running average histogram of sIPSC inter-event interval expressed as a percentage of averaged inter-event interval of control. C: running average histogram of sIPSC event amplitude expressed as a percentage of averaged amplitude of control. Bars (WIN) on B and C indicate the application of WIN55212-2 (0.8 μM). D: sIPSC traces acquired under control conditions (Control), during application of 0.8 μM WIN55212-2 (WIN55212-2) and 0.8 μM WIN55212-2 + 2 μM SR141716A (WIN55212-2 + SR141716A), and after washing out the drugs (Wash). E: running average histogram of sIPSC inter-event interval expressed as a percentage of averaged inter-event interval of control. F: running average histogram of sIPSC event amplitude expressed as a percentage of averaged amplitude of control. Bars in E and F indicate: WIN, application of WIN55212-2 (0.8 μM); SR, application of WIN55212-2 (0.8 μM) + SR141716A (2 μM).
The effect of WIN55212-2 on frequency and amplitude of sIPSCs was completely blocked by 2 μM SR141716A, a competitive CB1 receptor antagonist (Fig. 3, D, E, and F). The average values of sIPSC inter-event interval and amplitude measured in the presence of WIN55212-2/SR141716A were not significantly different from control (186.9 ± 45.0 and 82.3 ± 7.8%; respectively; n = 5; P > 0.05, paired t-test) (Fig. 3, E and F). Interestingly, application of SR141716A alone did not result in significant sIPSC inter-event interval (108.2 ± 8.6% of control; n = 5; P > 0.05, paired t-test) or amplitude changes (101.3 ± 5.0% of control; n = 5; P > 0.05, paired t-test) and could be indicative of relatively low endocannabinoid tone in our preparation.
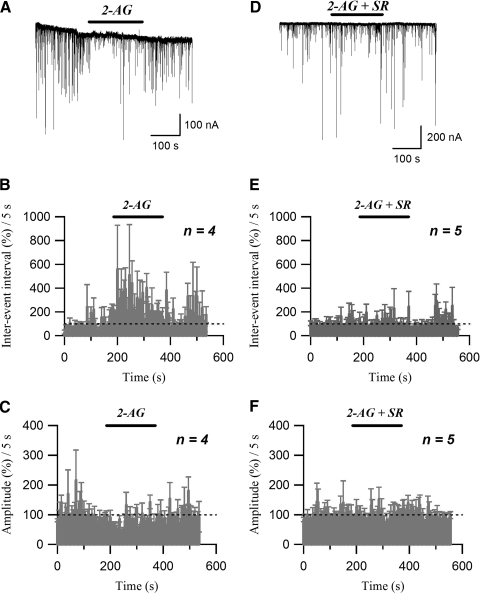
Similarly to WIN55212-2, extracellular application of the endocannabinoid 2-arachidonoylglycerol (2-AG; 0.5 μM) reversibly decreased the frequency of sIPSCs (Fig. 4, A and B). For a 3-min-long drug application, 2-AG produced a significant (247.6 ± 62.3% of control; n = 4; P < 0.05, paired t-test) increase in mean sIPSC inter-event interval, compared with the control level (Fig. 4B). In addition, application of 2-AG significantly decreased the sIPSC amplitude (74.4 ± 2.6% of control; n = 4, P < 0.005, paired t-test; Fig. 4, A and C). The effects of 2-AG on inter-event interval and amplitude of sIPSCs were completely reversed by application of 2 μM SR141716A (inter-event interval: 110.2 ± 17.5% of control; n = 5, P > 0.5, paired t-test; Fig. 4, D and E; amplitude: 95.2 ± 21.6% of control; n = 5, P > 0.5, paired t-test; Fig. 4, D and F).
FIG. 4.
sIPSCs are inhibited by the endocannabinoid 2-AG. A: sIPSC traces acquired before, during (2-AG), and after application of 0.5 μM 2-AG. B: running average histogram of sIPSC inter-event interval expressed as a percentage of averaged inter-event interval of control. C: running average histogram of sIPSC event amplitude expressed as a percentage of averaged amplitude of control. Bars (2-AG) in B and C indicate the application of 2-AG (0.5 μM). D: sIPSC traces acquired under control conditions, during application of 0.5 μM 2-AG (2-AG) and 2 μM SR141716A (2-AG + SR), and after washing out the drugs. E: running average histogram of sIPSC inter-event interval expressed as a percentage of averaged inter-event interval of control. F: running average histogram of sIPSC event amplitude expressed as a percentage of averaged amplitude of control. Bars (2-AG + SR) in E and F indicate the application of 2-AG (0.5 μM) and SR141716A (2 μM).
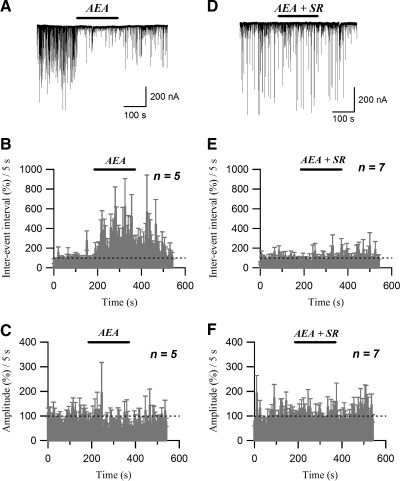
Similarly to 2-AG, extracellular application of another endocannabinoid, arachidonoylethanolamide (AEA, or anandamide; 1 μM) increased the sIPSC inter-event interval (352.8 ± 65.6% of control; n = 6, P < 0.05, paired t-test; Fig. 5, A and B) and decreased the sIPSC amplitude (65.9 ± 9.2% of control; n = 5, P < 0.05, paired t-test; Fig. 5, A and C). One prominent feature of the AEA effect on sIPSC frequency was the slow reversal on washout of the agonist (Fig. 5B). This is similar to the findings of Guo and Ikeda (2004) and Straiker and Mackie (2005) that effects of AEA reverse more slowly than those of 2-AG even in well-washed single-neuron preparations. The effects of AEA on inter-event interval and amplitude were completely blocked by 2 μM SR141716A (inter-event interval: 102.0 ± 13.6% of control; n = 7, P > 0.5, paired t-test, Fig. 5, D and E; amplitude: 103.4 ± 6.3% of control; n = 7, P > 0.5, paired t-test, Fig. 5, D and F).
FIG. 5.
sIPSCs are inhibited by the endocannabinoid anandamide (AEA). A: sIPSC traces acquired before, during (AEA), and after application of 1 μM AEA. B: running average histogram of sIPSC inter-event interval expressed as a percentage of averaged inter-event interval of control. C: running average histogram of sIPSC event amplitude expressed as a percentage of averaged amplitude of control. Bars (AEA) in B and C indicate the application of AEA (1 μM). D: sIPSC traces acquired under control conditions, during application of 1 μM AEA (AEA) and 2 μM SR141716A (AEA + SR), and after washing out the drugs. E: running average histogram of sIPSC inter-event interval expressed as a percentage of averaged inter-event interval of control. F: running average histogram of sIPSC event amplitude expressed as a percentage of averaged amplitude of control. Bars (AEA + SR) in E and F indicate the application of AEA (1 μM) and SR141716A (2 μM).
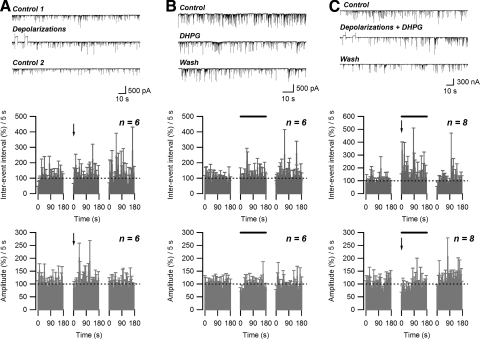
Previously, using a similar neuron/bouton preparation from the BLA, Zhu and Lovinger (2005) have shown that depolarization of the postsynaptic neuron can induce endocannabinoid-mediated DSI. Endocannabinoids are released from postsynaptic neurons by depolarization and then suppress the transmitter release through the activation of presynaptic CB1 receptors. However, in our preparation, no significant changes in either average inter-event interval (108.5 ± 16.7% of control; n = 6; P > 0.05, paired t-test) or average sIPSC amplitude (109.1 ± 10.6% of control; n = 6, P > 0.05, paired t-test) were observed even after two depolarizations (4-s duration each pulse, from −60 to 0 mV; 10-s interval) (Fig. 6A). Since the suppression of inhibitory neurotransmission by retrograde endocannabinoid signaling has also been reported with activation of group I mGluRs (Ohno-Shosaku et al. 2002), we tested the effect of the group I mGluR agonist (RS)-3,5-dihydroxyphenylglycine (DHPG) on sIPSC activity. Again, no significant changes in either inter-event interval (119.6 ± 12.6% of control; n = 6; P > 0.05, paired t-test) or sIPSC amplitude (89.0 ± 17.0% of control; n = 6; P > 0.05, paired t-test) were observed during the application of 50 μM DHPG (Fig. 6B). However, combining two depolarizations with 50 μM DHPG application resulted in an increase in inter-event interval that persisted for about 50 s (average during initial 50-s period: 194.9 ± 27.7% of control; n = 8; P < 0.05, paired t-test). No significant decrease in sIPSC amplitude (average during initial 50-s period: 76.2 ± 10.5% of control; n = 8; P > 0.05, paired t-test) (Fig. 6C) was observed following the two depolarizations in the presence of DHPG.
FIG. 6.
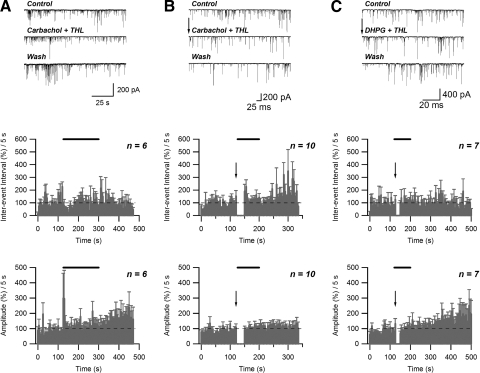
An endocannabinoid-dependent form of short-term depression (STD) is induced by concurrent depolarization and activation of metabotropic glutamate receptor 5 (mGluR5). A: failure to induce depolarization-induced suppression of inhibition (DSI) in postsynaptic neuron/presynaptic bouton preparation: no effect of two 4-s-long depolarizations (−60 to 0 mV) on sIPSC inter-event interval and amplitude. B: no effect of extracellular application of DHPG (50 μM) on sIPSC inter-event interval and amplitude. C: induction of STD by concurrent application of DHPG (50 μM) and 2 depolarizations: increase in sIPSC inter-event interval and no change in amplitude. Top panels: raw data. Middle and bottom panels: effect of treatment (solid bars) on normalized sIPSC inter-event interval and amplitude, respectively. Arrows indicate the end of 2nd depolarization.
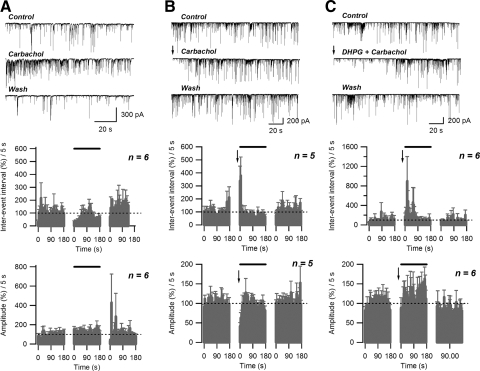
Previous studies indicated that activation of muscarinic acetylcholine receptors (mAChRs) can stimulate endocannabinoid-mediated suppression of inhibition in the hippocampus (Kim et al. 2002). To activate mAChRs we applied 5 μM carbachol. Carbachol treatment alone potentiated GABAergic transmission, as evidenced by a significant decrease of average sIPSC inter-event interval that persisted for about 50 s during continuous carbachol application (average during initial 50-s period: 41.9 ± 5.0 of control; n = 5, P < 0.005, paired t-test) and an increase in sIPSC amplitude (average during initial 50-s period: 127.7 ± 6.0 of control; n = 5, P < 0.05, paired t-test) (Fig. 7A). However, the combination of two depolarizations and 5 μM carbachol application, beginning after the facilitation had subsided, resulted in a 10- to 15-s-long increase of inter-event interval (average during initial 15-s period: 293.1 ± 34.4% of control; n = 5; P < 0.005, paired t-test) and a decrease in amplitude of sIPSC events (average during initial 15-s period: 59.0 ± 10.2% of control; n = 5; P < 0.05, paired t-test) (Fig. 7B). Moreover, combining two depolarizations with application of both 5 μM carbachol and 50 μM DHPG application resulted in a significant increase in inter-event interval that was longer-lasting than that observed with depolarization in the presence of either drug alone (average during initial 60-s period: 289.4 ± 66.5% of control; n = 6; P < 0.05, paired t-test). No significant changes in average sIPSC amplitude were observed over the same time period (initial 60 s: 106.2 ± 7.6% of control; n = 6; P > 0.05, paired t-test) (Fig. 7C).
FIG. 7.
Activation of muscarinic receptors can increase STD induced by DHPG/depolarization. A: application of carbachol (5 μM) produces a transient decrease in sIPSC inter-event interval and increase of amplitude. B: combination of carbachol (5 μM) application and 2 depolarizations (−60 to 0 mV) can induce STD. C: carbachol (5 μM) application increases the magnitude and duration of STD induced by concurrent application of DHPG (50 μM) and 2 depolarizations. Top panels: raw data. Middle and bottom panels: effect of treatment (solid bar) on normalized sIPSC inter-event interval and amplitude, respectively. Arrows indicate the end of 2nd depolarization.
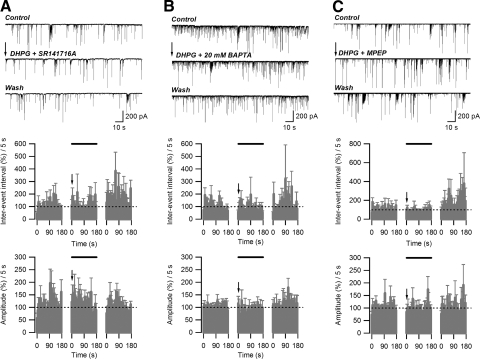
The increase in inter-event interval observed after depolarization/DHPG application was eliminated by application of SR141716A (2 μM), indicating the involvement of CB1 receptor activation (average during initial 50-s period: 104.2 ± 7.1% of control; n = 6; P > 0.5, paired t-test). No changes in average sIPSC amplitude were observed (average during initial 50-s period: 129.9 ± 8.6% of control; n = 5; P > 0.05, paired t-test) (Fig. 8A). In addition, the depolarization/DHPG-induced increase in inter-event interval was not observed in recordings with 20 mM BAPTA-containing postsynaptic internal solution (average during initial 50-s period: 100.6 ± 11.0% of control; n = 6; P > 0.5, paired t-test). The average amplitude of sIPSCs acquired in the presence of 20 mM BAPTA in the internal solution was not different from that of control (average during initial 50-s period: 91.6 ± 6.7% of control; n = 6; P > 0.05, paired t-test) (Fig. 8B). Application of the mGluR5 antagonist 2-methyl-6-(phenylethynyl)pyridine hydrochloride (MPEP, 10 μM) abolished the depolarization/DHPG-induced increase in inter-event interval (average during initial 50-s period: 86.4 ± 5.4% of control; n = 5; P < 0.005, paired t-test), without affecting the sIPSC amplitude (average during initial 50-s period: 96.2 ± 4.9% of control; n = 5; P > 0.05, paired t-test) (Fig. 8C). When applied alone, 10 μM MPEP did not significantly affect the sIPSC inter-event interval (average during initial 50-s period: 80.5 ± 6.4% of control; n = 5; P > 0.05, paired t-test; data not shown) or amplitude (95.8 ± 10.6% of control; n = 5; P > 0.5, paired t-test; data not shown). These observations indicate that STD requires increased postsynaptic calcium, mGluR5 activation, and activation of presynaptic CB1 receptors.
FIG. 8.
Dependence of depolarization/DHPG-induced STD on CB1, mGluR5 receptor activation, and postsynaptic intracellular calcium concentration. A: application of SR141716A (2 μM) abolished the STD induced by depolarization/DHPG. B: increasing BAPTA concentration (20 mM) inside the patch pipette abolished STD induction. C: application of MPEP (5 μM) abolished STD induction. Top panels: raw data (arrow indicates the end of the 2nd depolarization pulse). Middle and bottom panels: effect of treatment (solid bar) on normalized sIPSC inter-event interval and amplitude, respectively. Arrows indicate the end of 2nd depolarization.
To test for the mechanism of endocannabinoid signaling modulation by activation of muscarinic and mGluR5 receptors, we repeated the experiments with carbachol, carbachol + depolarizations, and carbachol + DHPG + depolarization while including 10 μM of the diacylglycerol (DAG) lipase inhibitor tetrahydrolipstatin (THL) inside the patch pipette. The presence of THL inside the patch pipette did not alter the effect of 5 μM carbachol application because this treatment still produced a significant decrease in inter-event interval that persisted for about 50 s (58.1 ± 5.9% of control; n = 6, P < 0.005, paired t-test) (Fig. 9A). In addition, the average amplitude of sIPSC events over the same (50-s) time period was still increased in the presence of THL (146.5 ± 6.0% of control; n = 6, P < 0.05, paired t-test) (Fig. 9A). The intracellular application of THL only slightly reduced the effect of combined 5 μM carbachol application and two depolarizations on inter-event interval (average during initial 15-s period: 186.4 ± 25.8% of control; n = 10; P < 0.005, paired t-test). No changes in sIPSC amplitude were observed (average during initial 15-s period: 93.8 ± 0.7% of control; n = 10; P > 0.5, paired t-test) (Fig. 9B). Similar results were obtained when 10 μM of THL was applied extracellularly during application of 5 μM carbachol and two depolarizations (average inter-event interval during 15-s period: 182.3 ± 22.3% of control; n = 6; P < 0.005, paired t-test; average sIPSC amplitude during 15-s period: 99.7 ± 3.2% of control; n = 6; P > 0.5, paired t-test; data not shown). However, intracellular application of THL abolished the effect of 50 μM DHPG and two depolarizations on inter-event interval (average during initial 50-s afterdepolarization period: 110.1 ± 7.7% of control; n = 7; P > 0.05, paired t-test) (Fig. 9C). As in the case of DHPG application + postsynaptic depolarization (Fig. 6C), no significant changes in sIPSC amplitude were observed in the presence of THL (average during initial 50-s afterdepolarization period: 98.4 ± 5.6% of control; n = 7; P > 0.5, paired t-test) (Fig. 9C).
FIG. 9.
Inhibition of diacylglycerol (DAG) lipase by tetrahydrolipstatin (THL) has differential effects on muscarinic acetylcholine receptor (mAChR)–and mGluR-dependent STD. A: intracellular application of 10 μM THL does not block the carbachol (5 μM)-induced increase in frequency and amplitude of sIPSCs. B: intracellular THL (10 μM) only slightly inhibits the carbachol (5 μM)/depolarization-induced STD. C: intracellular THL (10 μM) completely abolishes the DHPG (50 μM)/depolarization-induced STD. Top panels: raw data (for B and C: arrows indicate the end of the 2nd depolarization pulse). Middle and bottom panels: effect of treatment (solid bar) on normalized sIPSC inter-event interval and amplitude, respectively. Arrows indicate the end of 2nd depolarization.
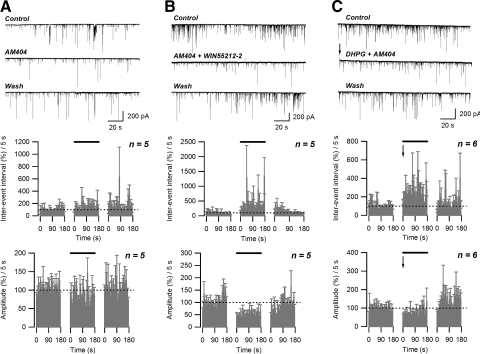
It has been shown that inhibitors of endocannabinoid uptake can mimic DSI by increasing the endocannabinoid concentration in the synaptic cleft (Wilson and Nicoll 2001). In our preparation, extracellular application of 5 μM N-arachidonoylphenol (AM404) resulted in a significant increase in sIPSC inter-event interval (149.2 ± 8.4% of control; n = 5; P < 0.05, paired t-test) and decrease in amplitude (82.5 ± 5.8% of control; n = 5; P < 0.05, paired t-test) (Fig. 10A). Concurrent application of 5 μM AM404 and 0.8 μM WIN55212-2 produced a significant increase in the inter-event interval (381.251 ± 46.7%; P < 0.005, paired t-test) (Fig. 10B, middle) and a decrease in the sIPSC amplitude (48.0 ± 10.0%; n = 5; P < 0.005, paired t-test) (Fig. 9, bottom) similar to that seen with application of WIN55212-2 alone (Fig. 3). This finding indicates that AM404 has little or no direct effect on CB1 receptors in this preparation. Combining AM404 (5 μM) application with the two depolarizations plus 50 μM DHPG resulted in a reversible increase in sIPSC inter-event interval (235.9 ± 43.0% of control; n = 6; P < 0.05, paired t-test) and decrease in amplitude (76.1 ± 6.7% of control; n = 6; P < 0.05, paired t-test) that persisted throughout the 3-min AM404 application (Fig. 10C). Thus application of AM404 prolongs the duration of STD.
FIG. 10.
Inhibition of AEA uptake prolongs depolarization/DHPG-induced STD. A: application of AM404 (5 μM) increased the sIPSC inter-event interval and decreased the sIPSC amplitude. B: application of AM404 does not alter sIPSC inhibition produced by WIN555212-2. Increases in sIPSC inter-event interval and decreases in amplitude were similar to those observed in the presence of WIN555212-2 alone (Fig. 3). C: application of AM404 (5 μM) prolongs depolarization/DHPG-induced STD. Top panels: raw data (for B: arrow indicates the end of the 2nd depolarization pulse); middle and lower panels: effect of treatment (solid bar) on normalized sIPSC inter-event interval and amplitude, respectively. Arrows indicate the end of 2nd depolarization.
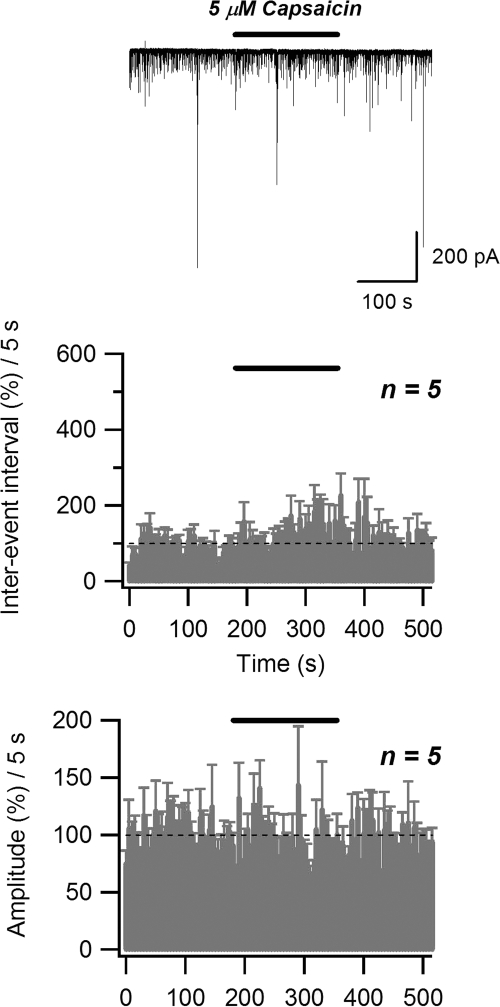
In addition to its inhibitory action on endocannabinoid uptake, AM404 could possibly stimulate transient receptor potential vanilloid 1 (TRPV1) receptors that are highly expressed in the CA1 region of hippocampus at the postsynaptic level (De Petrocellis et al. 2000; Zygmunt et al. 2000). Theoretically, activation of TRPV1 could result in postsynaptic depolarization and, as a consequence, increase of endocannabinoid release. To determine whether TRPV1 activation alters GABAergic transmission in this preparation, we applied the selective TRPV1 agonist capsaicin. A 3-min-long application of 1 μM capsaicin did not result in any significant changes in sIPSC amplitude (100.1 ± 5.1% of control; n = 5; P > 0.05, paired t-test) or inter-event interval (105.2 ± 16.4% of control; n = 5; P > 0.05, paired t-test) (data not shown). Similarly, no changes in sIPSC amplitude (95.1 ± 1.3% of control; n = 5; P > 0.05, paired t-test) or inter-event interval (119.9 ± 5.1% of control; n = 5; P > 0.05, paired t-test) were observed with 5 μM capsaicin application (Fig. 11). Thus TRPV1 activation does not mimic the effect of AM404 in the hippocampal neuron/bouton preparation.
FIG. 11.
Selective vanilloid receptor type 1 (TRPV1) agonist capsaicin fails to affect the sIPSC inter-event interval and amplitude. Top panel: raw data (solid bar indicates the application of 5 μM capsaicin). Middle and bottom panels: effect of treatment (solid bar) on normalized sIPSC inter-event interval and amplitude, respectively.
Application of AM404 could also inhibit the activity of the postsynaptic fatty acid amide hydrolase (FAAH), thereby increasing the effective level of anandamide (Glaser et al. 2003; Vandevoorde and Fowler 2005). Using a selective FAAH inhibitor [3-(3-carbamoylphenyl)phenyl]-N-cyclohexylcarbamate (URB597), we tested for the possible influence of FAAH inhibition on observed STD. A 3-min-long application of 1 μM URB597 did not result in any significant changes in sIPSC amplitude (88.1 ± 3.4% of control; n = 5; P > 0.05, paired t-test) or inter-event interval (106.9 ± 13.3% of control; n = 5; P > 0.05, paired t-test). When applied together with 5 μM carbachol and two depolarizations, 1 μM URB597 did not produce any additional increase in sIPSC inter-event interval (average during 15-s period: 214.9 ± 29.9% of control; n = 9; P < 0.05, paired t-test) beyond that produced by carbachol/depolarization application (Fig. 7B). Similar to the effect of carbachol/depolarization application (Fig. 7B), the sIPSC event amplitude was significantly decreased (average during 15-s period: 76.6 ± 11.5% of control; n = 9; P < 0.05, paired t-test) (data are shown). Application of 1 μM URB597 also did not alter the effect of 50 μM DHPG/depolarization application on either sIPSC inter-event interval (average during 50-s period: 147.4 ± 12.5% of control; n = 5; P < 0.05, paired t-test) or amplitude (average during 50-s period: 118.2 ± 7.1% of control; n = 5; P > 0.05, paired t-test) (data not shown). Taken together, these results indicate that inhibition of FAAH does not influence the STD observed with muscarinic or metabotropic glutamate receptor activation.
DISCUSSION
In the present study, we observed a novel form of STD in the mechanically isolated neuron/bouton preparation from the CA1 region of rat hippocampus. The STD was induced by concurrent postsynaptic depolarization and activation of mGluR5 receptors and mediated by retrograde endocannabinoid signaling.
As a preparation for our study, we have chosen a highly reduced preparation consisting of a postsynaptic neuron and attached presynaptic boutons. This simple preparation has many advantages over brain slices and neuronal cultures. First, synaptic transmission observed in the neuron/bouton preparation is independent of other cells that are found in brain slices or neuronal cultures. Second, since the preparation contains only a few postsynaptic processes, it allows for more precise space clamp than other preparations. Finally, our preparation allows faster exchange of solution around the neuron and thus better control of pharmacological conditions compared with experiments in brain slices.
Presynaptic boutons on neurons from the CA1 region of hippocampus retain spontaneous GABA release (Fig. 1). Moreover, the presynaptic boutons appear to have functional CB1 receptors, as evidenced by both immunostaining and agonist actions. Staining with an anti-CB1 antibody revealed the presence of receptors colocalized with synapsin in putative presynaptic boutons. In addition, activation of CB1 receptors by the synthetic cannabinoid WIN55212-2 and endocannabinoids 2-AG and AEA (Figs. 3, 4, and 5) reversibly decreased the frequency and amplitude of sIPSCs. Comparison of effects of WIN55212-2, 2-AG, and AEA on sIPSC inter-event interval revealed that application of 0.8 μM WIN55212-2 was approximately 1.4-fold as effective as the application of 1 μM AEA and about twofold as effective as the application of 0.5 μM 2-AG. A similar trend was found in efficacy of cannabinoid compounds on sIPSC amplitude: the application of 0.8 μM WIN55212-2 was about 2.2-fold as effective as the application of 1 μM AEA and about 2.5-fold as effective as the application of 0.5 μM 2-AG. We could not accurately measure the maximal efficacies of these agonists because we did not want to use higher concentrations of the drugs that can produce nonspecific effects. However, the trend seen in comparing the efficacies of these compounds at concentrations that should produce near-maximal effects is WIN55212-2 > AEA > 2-AG. The fact that AEA was more effective than 2-AG is somewhat surprising, given that AEA acts as a weak partial agonist in some experimental settings (Guo and Ikeda 2004). However, AEA has been shown to produce efficacious inhibition of transmission in cultured neurons (Straiker and Mackie 2005) and thus there is precedent for this sort of effect. The longer-lasting effect of AEA is consistent with previous findings in isolated cells (Guo and Ikeda 2004; Straiker and Mackie 2005). It is unlikely that this prolonged effect is due to persistent binding of AEA to the CB1 receptors because these preparations are constantly superfused and thus AEA should be removed within milliseconds to seconds. There may be some aspect of the signal transduction activated by AEA that differs from that of the other agonists. The antagonist blockade of the effects of all agonists strongly supports the idea that these effects are mediated through CB1 receptors.
Although the decrease in sIPSC amplitude could be attributed to postsynaptic actions of cannabinoids and endocannabinoids, previous studies demonstrated that this effect of WIN55212-2 is observed only when multiquantal events are examined (Zhu and Lovinger 2005). In addition, previous studies confirmed the exclusive presynaptic localization of CB1 receptors, as our immunolocalization data also indicate, and the presynaptic mechanism of WIN55212-2 action (Hajos et al. 2000; Katona et al. 1999).
The decrease in amplitude of evoked IPSCs after depolarization of the postsynaptic neuron (DSI) was originally reported in hippocampal slices and subsequent studies strongly supported the hypothesis of an endocannabinoid-mediated mechanism of DSI (Pitler and Alger 1992, 1994). During acquisition of spontaneous IPSCs, DSI can be monitored as a decrease in amplitude and frequency of sIPSCs (Alger et al. 1996). Later, a similar DSI was observed in the neuron/bouton preparation from BLA (Zhu and Lovinger 2005). However, we were unable to induce simple DSI in the neuron/bouton preparation from the CA1 region of the hippocampus (Fig. 6). In addition, activation of postsynaptic mGluR5 receptors, known to trigger the release of endocannabinoids in slice (Ohno-Shosaku et al. 2002), did not change the frequency or amplitude of sIPSC events (Fig. 6). These results indicate that there is a relatively low level of endocannabinoid production or release from the postsynaptic elements of our preparation (endocannabinoid “tone”) compared with hippocampal slices or neuron/bouton preparations from the BLA region. The difference in tone is also underscored by the lack of effect of CB1 antagonists/inverse agonists applied in the hippocampal neuron/bouton preparation (Fig. 3), which contrasts with the increased sIPSC frequency produced by these compounds in the same preparation from BLA (Zhu and Lovinger 2005). In both preparations these compounds appear to act as neutral antagonists (see Zhu and Lovinger 2005 for discussion of the data from BLA). Combining activation of mGluR5 receptors with postsynaptic depolarization resulted in a decrease in sIPSC frequency that lasted about 50 s (Fig. 6). This observation supports the idea of cooperativity between depolarization and mGluR5-triggered release of endocannabinoids, similar to that previously observed in the slice preparation (Ohno-Shosaku et al. 2002; Varma et al. 2001). The lack of effect of DHPG application/depolarization on sIPSC amplitude supports a presynaptic mechanism of STD.
Similar to the DSI observed in the neuron/bouton preparation from BLA, the STD in our preparation was dependent on postsynaptic intracellular calcium concentration (Fig. 8). This finding is also consistent with the proposed intracellular calcium-dependent release of endocannabinoids (Di Marzo et al. 1994).
Another similarity of STD observed in isolated hippocampal neurons with DSI is its enhancement by activation of mAChRs. It has been shown that application of carbachol enhances DSI of sIPSCs recorded in hippocampal slices, possibly by increasing the endocannabinoid release from postsynaptic elements (Kim et al. 2002). In our study, application of carbachol produced two effects. The first was a transient enhancement of sIPSC frequency and amplitude (Fig. 7A). This is reminiscent of mAChR-mediated enhancement of GABAergic transmission that has been observed in hippocampal slices, which appears to involve presynaptic mechanisms (Kim et al. 2002). Carbachol application along with depolarization of the postsynaptic neuron decreased the frequency of sIPSCs for a relatively short period of time (Fig. 7B). This effect is similar to the DSI enhancement observed in hippocampal slices, which has been attributed to actions of postsynaptic mAChRs that enhance endocannabinoid production (Kim et al. 2002). Combining DHPG, carbachol application, and postsynaptic depolarization resulted in a decrease in sIPSC frequency that was prolonged relative to effects of either agonist alone when combined with depolarization (Fig. 7C). This finding suggests that the mechanisms downstream of mAChRs and mGluRs are not completely overlapping. In hippocampal slices, mGluR5 receptor stimulation causes production of 2-AG but not anandamide (Jung et al. 2005). Conversely, muscarinic receptor stimulation in DRG neurons was found to preferentially stimulate anandamide formation (van der Stelt et al. 2005). The idea that the mechanisms of carbachol and DHPG action are nonoverlapping is also supported by the finding that DAG lipase inhibition prevents the actions of the mGluR agonist, but not the mAChR agonist (Fig. 9, B and C). DAG lipase is thought to be involved in 2-AG production within the postsynaptic neuron (Hashimotodani et al. 2008; Piomelli 2003). The increase in sIPSC event frequency and amplitude induced by carabachol alone also persisted during the intracellular application of THL (Fig. 9A), indicating the independence of muscarinic modulation pathway on DAG lipase. Our findings differ somewhat from those of Edwards et al. (2006) who observed a role for DAG lipase in mAChR-induced endocannabinoid production. This difference may have to do with the dependence on depolarization that works via a DAG lipase-independent pathway, as suggested by Edwards et al. (2006).
Our findings with the FAAH inhibitor URB597 suggest that anandamide does not play a role in STD produced by depolarization plus either of the receptor antagonists. Thus 2-AG may yet prove to be the endocannabinoid that mediates STD produced by carbachol plus depolarization. The lack of effect of DAG lipase inhibition on this carbachol action may indicate that this form of STD involves “mobilization” of preexisting endocannabinoid, similar to that proposed by Edwards et al. (2006).
Interestingly, although the application of carbachol and depolarization of the postsynaptic neuron significantly decreased the sIPSC amplitude, the combination of DHPG, carbachol application, and postsynaptic depolarization did not affect the sIPSC amplitude. The reduction of sIPSC amplitude in the presence of carbachol and depolarization could indicate the involvement of some postsynaptic mechanisms coupled to muscarinic receptors and affecting the activation of postsynaptic GABAA receptors. In addition, other postsynaptic mechanisms responsible for the reduction of sIPSC amplitude in the presence of carbachol and depolarization could be activated. For example, anandamide production stimulated by the muscarinic receptor activation could possibly increase the intracellular calcium mobilization by activation of the postsynaptic vanilloid receptor type I (TRPV1) (De Petrocellis and Di Marzo 2005; Karai et al. 2004; Olah et al. 2001; Zygmunt et al. 1999). The increase in intracellular calcium concentration could possibly decrease sIPSC amplitude. This indirect effect of muscarinic receptor activation on sIPSC amplitude might be diminished in the presence of DHPG, which increases the postsynaptic production of 2-AG and does not appear to activate the TRPV1 receptor (Zygmunt et al. 1999). However, the TRPV1-related modulation of sIPSC event amplitude is unlikely to occur in our preparation because the selective TRPV1 agonist capsaicin did not decrease sIPSC amplitude. Furthermore, anandamide did not alter sIPSCs when applied in the presence of a CB1 antagonist (Fig. 5). This lack of effect indicates that this known TRPV1 agonist has no CB1-independent (i.e., no TRPV1-mediated) effect on GABAergic transmission in this preparation.
Extracellular application of the endocannabinoid transport inhibitor AM404 transiently decreased sIPSC frequency and amplitude (Fig. 10A), which is consistent with the previously published results of Wilson and Nicoll (2001), in which extracellularly applied AM404 decreased the amplitude of evoked IPSCs. This effect is presumed to occur via the accumulation of endocannabinoids at synapses due to decreased cellular uptake (Wilson and Nicoll 2001), although other mechanisms such as stimulation of TRPV1 receptors or inhibition of FAAH could also be involved (Vandevoorde and Fowler 2005; Zygmunt et al. 2000). As mentioned earlier, application of the selective TRPV1 agonist capsaicin did not affect the amplitude or frequency of sIPSCs in our preparation (Fig. 11), indicating that the actions of AM404 that we have observed are most likely not mediated by actions at TRPV1. In addition, the lack of effect of the FAAH inhibitor URB597 indicates that the AM404 effects are not due to inhibition of this enzyme.
Accumulation of endocannabinoids in the synapse could also account for the prolonged decrease in frequency and amplitude of sIPSCs during depolarization/DHPG-induced STD and application of AM404 (Fig. 10C). The observation that AM404 prolongs endocannabinoid effects in the neuron/bouton preparation indicates that molecules involved in terminating the endocannabinoid signal (e.g., a membrane transporter) are present in neuronal elements at these inhibitory synapses.
The reasons for the difference in endocannabinoid tone and ease of DSI induction between the hippocampal neuron/bouton preparation and the slice preparation are not yet clear. It is possible that cellular elements other than the postsynaptic neuron and the presynaptic terminal regulate endocannabinoid production in the slice preparation. Differences in resting or depolarization-activated postsynaptic intracellular calcium levels could also play a role in setting the endocannabinoid tone and stimulated production. It is surprising in this regard that DSI was easily evoked by depolarization in the neuron/bouton preparation from BLA (Zhu and Lovinger 2005). However, this might reflect differences in glutamatergic action in the two brain regions. We observed evidence of glutamatergic transmission in the BLA preparation (Zhu and Lovinger 2005), but not in the hippocampal neurons examined at present. Determining the factors that regulate endocannabinoid production and release in the different preparations and brain regions may lead us to a better understanding of the mechanisms that are necessary for this retrograde signaling system.
To conclude, similarly to the previously reported DSI in the isolated neuron/presynaptic bouton preparation from BLA, the STD observed in the hippocampal neuron/bouton preparation was dependent on retrograde endocannabinoid signaling and sensitive to the postsynaptic intracellular calcium concentration. In contrast to DSI in a similar preparation from BLA, STD in hippocampal neuron/boutons required much stronger stimulation to elicit endocannabinoid release (i.e., both depolarization of the postsynaptic neuron and activation of mGluR5 receptors). The main finding of the present study is that the level of endocannabinoids available for release (“endocannabinoid tone”) is much lower in the isolated hippocampal neuron/bouton preparation compared with that of a similar preparation from BLA, and this difference could explain the requirement of both postsynaptic mGluR5 and postsynaptic depolarization for STD induction in hippocampal preparation. These findings support the idea that cooperativity between these mechanisms can serve as a regulatory mechanism for cannabinoid signaling under conditions in which a low level of endocannabinoids is available for release. This sort of cooperative regulatory mechanism has the capacity to regulate the activity of the neuronal networks, particularly under conditions of high levels of released glutamate (“glutamate spillover”) (Kullmann and Asztely 1998).
GRANTS
This work was supported by the Division of Intramural Clinical and Basic Research of the National Institute on Alcohol Abuse and Alcoholism. Dr. Ken Mackie was supported by National Institute on Drug Abuse Grant DA-11322.
Acknowledgments
We thank Dr. Ken Mackie (Indiana University, Bloomington) for the generous gift of rabbit anti-CB1 receptor antibody.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- Akaike and Moorhouse 2003.Akaike N, Moorhouse AJ. Techniques: applications of the nerve-bouton preparation in neuropharmacology. Trends Pharmacol Sci 24: 44–47, 2003. [DOI] [PubMed] [Google Scholar]
- Alger et al. 1996.Alger BE, Pitler TA, Wagner JJ, Martin LA, Morishita W, Kirov SA, Lenz RA. Retrograde signalling in depolarization-induced suppression of inhibition in rat hippocampal CA1 cells. J Physiol 496: 197–209, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson et al. 2002.Carlson G, Wang Y, Alger BE. Endocannabinoids facilitate the induction of LTP in the hippocampus. Nat Neurosci 5: 723–724, 2002. [DOI] [PubMed] [Google Scholar]
- De Petrocellis et al. 2000.De Petrocellis L, Bisogno T, Davis JB, Pertwee RG, Di Marzo V. Overlap between the ligand recognition properties of the anandamide transporter and the VR1 vanilloid receptor: inhibitors of anandamide uptake with negligible capsaicin-like activity. FEBS Lett 483: 52–56, 2000. [DOI] [PubMed] [Google Scholar]
- De Petrocellis and Di Marzo 2005.De Petrocellis L, Di Marzo V. Lipids as regulators of the activity of transient receptor potential type V1 (TRPV1) channels. Life Sci 77: 1651–1666, 2005. [DOI] [PubMed] [Google Scholar]
- Di Marzo et al. 1994.Di Marzo V, Fontana A, Cadas H, Schinelli S, Cimino G, Schwartz JC, Piomelli D. Formation and inactivation of endogenous cannabinoid anandamide in central neurons. Nature 372: 686–691, 1994. [DOI] [PubMed] [Google Scholar]
- Edwards et al. 2006.Edwards DA, Kim J, Alger BE. Multiple mechanisms of endocannabinoid response initiation in hippocampus. J Neurophysiol 95: 67–75, 2006. [DOI] [PubMed] [Google Scholar]
- Glaser et al. 2003.Glaser ST, Abumrad NA, Fatade F, Kaczocha M, Studholme KM, Deutsch DG. Evidence against the presence of an anandamide transporter. Proc Natl Acad Sci USA 100: 4269–4274, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo and Ikeda 2004.Guo J, Ikeda SR. Endocannabinoids modulate N-type calcium channels and G-protein-coupled inwardly rectifying potassium channels via CB1 cannabinoid receptors heterologously expressed in mammalian neurons. Mol Pharmacol 65: 665–674, 2004. [DOI] [PubMed] [Google Scholar]
- Hajos et al. 2000.Hajos N, Katona I, Naiem SS, MacKie K, Ledent C, Mody I, Freund TF. Cannabinoids inhibit hippocampal GABAergic transmission and network oscillations. Eur J Neurosci 12: 3239–3249, 2000. [DOI] [PubMed] [Google Scholar]
- Hampson et al. 2003.Hampson RE, Zhuang SY, Weiner JL, Deadwyler SA. Functional significance of cannabinoid-mediated, depolarization-induced suppression of inhibition (DSI) in the hippocampus. J Neurophysiol 90: 55–64, 2003. [DOI] [PubMed] [Google Scholar]
- Hashimotodani et al. 2008.Hashimotodani Y, Ohno-Shosaku T, Maejima T, Fukami K, Kano M. Pharmacological evidence for the involvement of diacylglycerol lipase in depolarization-induced endocannabinoid release. Neuropharmacology 54: 58–67, 2008. [DOI] [PubMed] [Google Scholar]
- Herkenham 1991.Herkenham M Characterization and localization of cannabinoid receptors in brain: an in vitro technique using slide-mounted tissue sections. NIDA Res Monogr 112: 129–145, 1991. [PubMed] [Google Scholar]
- Jung et al. 2005.Jung KM, Mangieri R, Stapleton C, Kim J, Fegley D, Wallace M, Mackie K, Piomelli D. Stimulation of endocannabinoid formation in brain slice cultures through activation of group I metabotropic glutamate receptors. Mol Pharmacol 68: 1196–1202, 2005. [DOI] [PubMed] [Google Scholar]
- Karai et al. 2004.Karai LJ, Russell JT, Iadarola MJ, Olah Z. Vanilloid receptor 1 regulates multiple calcium compartments and contributes to Ca2+-induced Ca2+ release in sensory neurons. J Biol Chem 279: 16377–16387, 2004. [DOI] [PubMed] [Google Scholar]
- Katona et al. 1999.Katona I, Sperlagh B, Sik A, Kafalvi A, Vizi ES, Mackie K, Freund TF. Presynaptically located CB1 cannabinoid receptors regulate GABA release from axon terminals of specific hippocampal interneurons. J Neurosci 19: 4544–4558, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim et al. 2002.Kim J, Isokawa M, Ledent C, Alger BE. Activation of muscarinic acetylcholine receptors enhances the release of endogenous cannabinoids in the hippocampus. J Neurosci 22: 10182–10191, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullmann and Asztely 1998.Kullmann DM, Asztely F. Extrasynaptic glutamate spillover in the hippocampus: evidence and implications. Trends Neurosci 21: 8–14, 1998. [DOI] [PubMed] [Google Scholar]
- Lichtman and Martin 1996.Lichtman AH, Martin BR. Delta 9-tetrahydrocannabinol impairs spatial memory through a cannabinoid receptor mechanism. Psychopharmacology (Berl) 126: 125–131, 1996. [DOI] [PubMed] [Google Scholar]
- Mackie 2006.Mackie K Cannabinoid receptors as therapeutic targets. Annu Rev Pharmacol Toxicol 46: 101–122, 2006. [DOI] [PubMed] [Google Scholar]
- Matsuda et al. 1990.Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature 346: 561–564, 1990. [DOI] [PubMed] [Google Scholar]
- Ohno-Shosaku et al. 2001.Ohno-Shosaku T, Maejima T, Kano M. Endogenous cannabinoids mediate retrograde signals from depolarized postsynaptic neurons to presynaptic terminals. Neuron 29: 729–738, 2001. [DOI] [PubMed] [Google Scholar]
- Ohno-Shosaku et al. 2002.Ohno-Shosaku T, Shosaku J, Tsubokawa H, Kano M. Cooperative endocannabinoid production by neuronal depolarization and group I metabotropic glutamate receptor activation. Eur J Neurosci 15: 953–961, 2002. [DOI] [PubMed] [Google Scholar]
- Olah et al. 2001.Olah Z, Karai L, Iadarola MJ. Anandamide activates vanilloid receptor 1 (VR1) at acidic pH in dorsal root ganglia neurons and cells ectopically expressing VR1. J Biol Chem 276: 31163–31170, 2001. [DOI] [PubMed] [Google Scholar]
- Pacher et al. 2006.Pacher P, Batkai S, Kunos G. The endocannabinoid system as an emerging target of pharmacotherapy. Pharmacol Rev 58: 389–462, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piomelli 2003.Piomelli D The molecular logic of endocannabinoid signalling. Nat Rev Neurosci 4: 873–884, 2003. [DOI] [PubMed] [Google Scholar]
- Pitler and Alger 1992.Pitler TA, Alger BE. Postsynaptic spike firing reduces synaptic GABAA responses in hippocampal pyramidal cells. J Neurosci 12: 4122–4132, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitler and Alger 1994.Pitler TA, Alger BE. Depolarization-induced suppression of GABAergic inhibition in rat hippocampal pyramidal cells: G protein involvement in a presynaptic mechanism. Neuron 13: 1447–1455, 1994. [DOI] [PubMed] [Google Scholar]
- Straiker and Mackie 2005.Straiker A, Mackie K. Depolarization-induced suppression of excitation in murine autaptic hippocampal neurones. J Physiol 569: 501–517, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Stelt et al. 2005.van der Stelt M, Trevisani M, Vellani V, De Petrocellis L, Schiano Moriello A, Campi B, McNaughton P, Geppetti P, Di Marzo V. Anandamide acts as an intracellular messenger amplifying Ca2+ influx via TRPV1 channels. EMBO J 24: 3026–3037, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandevoorde and Fowler 2005.Vandevoorde S, Fowler CJ. Inhibition of fatty acid amide hydrolase and monoacylglycerol lipase by the anandamide uptake inhibitor VDM11: evidence that VDM11 acts as an FAAH substrate. Br J Pharmacol 145: 885–893, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varma et al. 2001.Varma N, Carlson GC, Ledent C, Alger BE. Metabotropic glutamate receptors drive the endocannabinoid system in hippocampus. J Neurosci 21: RC188, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorobjev 1991.Vorobjev VS Vibrodissociation of sliced mammalian nervous tissue. J Neurosci Methods 38: 145–150, 1991. [DOI] [PubMed] [Google Scholar]
- Wilson and Nicoll 2001.Wilson RI, Nicoll RA. Endogenous cannabinoids mediate retrograde signalling at hippocampal synapses. Nature 410: 588–592, 2001. [DOI] [PubMed] [Google Scholar]
- Zhu and Lovinger 2005.Zhu PJ, Lovinger DM. Retrograde endocannabinoid signaling in a postsynaptic neuron/synaptic bouton preparation from basolateral amygdala. J Neurosci 25: 6199–6207, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zygmunt et al. 2000.Zygmunt PM, Chuang H, Movahed P, Julius D, Hogestatt ED. The anandamide transport inhibitor AM404 activates vanilloid receptors. Eur J Pharmacol 396: 39–42, 2000. [DOI] [PubMed] [Google Scholar]
- Zygmunt et al. 1999.Zygmunt PM, Petersson J, Andersson DA, Chuang H, Sorgard M, Di Marzo V, Julius D, Hogestatt ED. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature 400: 452–457, 1999. [DOI] [PubMed] [Google Scholar]