Abstract
Inhibitory interactions shape the activity of output neurons in primary olfactory centers and promote contrast enhancement of odor representations. Patterns of interglomerular connectivity, however, are largely unknown. To test whether the proximity of glomeruli to one another is correlated with interglomerular inhibitory interactions, we used intracellular recording and staining methods to record the responses of projection (output) neurons (PNs) associated with glomeruli of known olfactory tuning in the primary olfactory center of the moth Manduca sexta. We focused on Toroid I, a glomerulus in the male-specific macroglomerular complex (MGC) specialized to one of the two key components of the conspecific females' sex pheromone, and the adjacent, sexually isomorphic glomerulus 35, which is highly sensitive to Z-3-hexenyl acetate (Z3-6:OAc). We used the two odorants to activate these reference glomeruli and tested the effects of olfactory activation in other glomeruli. We found that Toroid-I PNs were not inhibited by input to G35, whereas G35 PNs were inhibited by input to Toroid-I PNs. We also recorded the responses of PNs arborizing in other sexually isomorphic glomeruli to stimulation with the sex pheromone and Z3-6:OAc. We found that inhibitory responses were not related to proximity to the MGC and G35: both distant and adjacent PNs were inhibited by stimulation with the sex pheromone, some others were affected by only one odorant, and yet others by neither. Similar results were obtained in female PNs recorded in proximity to female-specific glomeruli. Our findings indicate that inhibitory interactions among glomeruli are widespread and independent of their spatial proximity.
INTRODUCTION
In the primary olfactory centers of vertebrates (olfactory bulbs, OBs) and insects (antennal lobes, ALs), each glomerulus contains arborizations of, and synaptic connections among, olfactory receptor cells (ORCs), local interneurons (LNs), and projection (output) neurons (PNs) that send axons to higher brain centers (Boeckh and Tolbert 1993; Jefferis et al. 2002; Shepherd 1972; Tolbert and Hildebrand 1981). Because each type of ORC typically expresses one type of odorant receptor (OR), and the axons of ORCs expressing the same OR converge in the same glomerulus (Buck and Axel 1991; Gao et al. 2000; Mombaerts 2004; Mombaerts et al. 1996), a glomerulus is expected to reflect the odor-response profile of the ORCs converging in it (Root et al. 2007; Vosshall et al. 2000). Furthermore, odorants bind to different ORs with different affinities (e.g., Hallem and Carlson 2006; Malnic et al. 1999) and thus evoke characteristic patterns of glomerular activation (Belluscio and Katz 2001; Friedrich and Korsching 1998; Johnson et al. 1998; Sachse et al. 1999; Wang et al. 2003; Xu et al. 2000). Increasing evidence suggests, however, that glomerular output is refined by interglomerular interactions mediated by GABAergic, inhibitory LNs (Aungst et al. 2003; Friedrich and Laurent 2004; Kashiwadani et al. 1999;Nagayama et al. 2004; Olsen and Wilson 2008; Schoppa 2006; Silbering and Galizia 2007; Vucinic et al. 2006; Wilson et al. 2004; Yokoi et al. 1995). Examination of patterns of inhibitory glomerular interaction in various experimental systems has begun only recently: in the OB, responses of mitral cells are modulated by odorants that activate neighboring glomeruli (Nagayama et al. 2004); modeling studies in honey bees show that interactions between glomeruli are proportional to the similarity of their odor-response profiles regardless of their anatomical proximity (Linster et al. 2005), and optical imaging studies suggest the presence of both a glomerulus-specific and a global inhibitory network (Silbering and Galizia 2007); and in the moth Manduca sexta, the male-specific glomeruli that process sensory information about two key components of the female's sex pheromone reciprocally inhibit each other (Christensen and Hildebrand 1997; Lei et al. 2002). These findings suggest that interglomerular inhibitory interactions reflect functional relationships between glomeruli. In this study, we tested whether inhibitory interactions among glomeruli also depend on their spatial relationships. We recorded intracellularly the responses of PNs associated with several neighboring, identified, sexually dimorphic and isomorphic glomeruli with characterized molecular receptive ranges in ALs of male and female M. sexta. We measured odor-driven synaptic events and spiking activity on a millisecond time scale and therefore unambiguously could study odor-driven synaptic inhibition. One of these glomeruli (the Toroid I) belongs to the male-specific macroglomerular complex (MGC) and processes sensory information about one of the two key components of the conspecific females' sex pheromone (Christensen et al. 1989). Each of the two key sex-pheromone components exclusively activates one of two types of male-specific antennal ORCs, and the axons of each of those two types of ORCs project exclusively to one of the two principal MGC glomeruli (Christensen and Hildebrand 1987; Christensen et al. 1995; Kaissling et al. 1989). Therefore by stimulating antennal inputs with these pheromone components, we could activate one or both MGC glomeruli and test the effect of that activation in other glomeruli. Glomerulus 35 (G35) is sexually isomorphic (i.e., its morphological features and physiological properties are equivalent in males and females), responds preferentially and highly sensitively to the plant-derived volatile compound Z-3-hexenyl-acetate (Z3-6:OAc), and is located adjacent to the MGC in males and to a female-specific glomerulus, the lateral large female glomerulus (latLFG), in females (Reisenman et al. 2005). Therefore we could stimulate G35 and test the effect of this odorant activation in glomeruli of the MGC. The latLFG is homologous to the male-specific Toroid I (Rospars and Hildebrand 2000) and responds preferentially to stimulation of the antenna with the plant volatile [±]linalool (Roche King et al. 2000), and especially to the [+] enantiomer (Reisenman et al. 2004). We tested if inhibition is related to glomerular proximity by recording the responses of Toroid-I PNs to the two odorants that activate the adjacent MGC glomerulus (the Cumulus) and G35, the responses of male uniglomerular PNs with arborizations in sexually isomorphic glomeruli (both close and distant to the MGC and G35) to stimulation with the odorants activating these glomeruli, and the responses of female uniglomerular PNs in sexually isomorphic glomeruli to the odorants that preferentially activate the latLFG and G35.
METHODS
Preparation
Male and female M. sexta (L.) (Lepidoptera: Sphingidae), reared in the laboratory on artificial diet, were used 1–3 days after adult emergence. Animals were dissected and prepared for intracellular recording with established procedures (Roche King et al. 2000). After mechanical removal of the perineural sheath covering the AL, the preparation was continuously superfused with physiological saline solution containing (in mM): 150 NaCl, 3 CaCl2, 3 KCl, 10 TES buffer (pH 6.9), and 25 sucrose (Christensen and Hildebrand 1987).
Stimulation
The stimulation procedure has been described elsewhere (Reisenman et al. 2004). Briefly, the cut end of one antenna was inserted into a glass capillary tube filled with physiological saline solution, which served both as a holder to position the antenna and as an electrode for monitoring antennal responses (amplified 50-fold with an amplifier, Model M-707, WPI, Sarasota, FL) to olfactory stimulation. An L-shaped glass tube delivered a constant flow of humidified, charcoal-filtered air to the antenna (1.9 l/min). Plant odorants were injected (2 or 5 ml of odor-bearing air for 200- or 500-ms stimulations, respectively) into the constant air stream by means of a syringe olfactometer (Selchow 1998). Thus odor stimuli injected (at a velocity of 10 ml/s) into the air stream (flowing constantly at 32 ml/s) were diluted ∼1:4. Sex-pheromone components were injected using a solenoid-activated valve (3.8 or 9.6 ml of odor-bearing air for 200- or 500-ms stimulations, respectively). The tip of the syringe containing the odor stimulus was inserted into the air stream through a small hole in the side of the glass tube. In this case, odor stimuli were diluted ∼1:2.5. A funnel connected to a negative-pressure line was positioned near and behind the preparation to remove stimulus volatiles after delivery to the antenna.
The plant-derived odor compounds used in this study were [±]linalool [[±]3,7-dimethyl-1,6-octadien-3-ol, No. L2602, 97% pure from Sigma-Aldrich (St. Louis, MO)] and Z-3-hexenyl acetate [No. H2137, >97%, hereinafter referred to as Z3-6:OAc (Tokyo Chemical Industries, Tokyo, Japan)]. These odor compounds are found among the volatiles emitted by host plants of M. sexta (Fraser et al. 2003; Loughrin et al. 1990; Raguso et al. 2003) and have been shown to evoke responses from antennae (Fraser et al. 2003; Reisenman, unpublished observations) and/or from antennal ORCs in type-A trichoid sensilla (Shields and Hildebrand 2001). Plant-associated volatile compounds were diluted in odorless mineral oil (Sigma-Aldrich) and prepared as described elsewhere (Reisenman et al. 2004). Dilutions ranged from 10-4 to 10-2 (vol/vol). Fifty microliter of solution were applied to a disk of filter paper inserted into a 20-ml stimulus syringe; control syringes contained 50 μl of mineral oil alone.
The two main components of the sex-pheromone (E10,Z12-16:Al [bombykal], and E10,E12,Z14-16:Al or its more chemically stable, biologically active mimic, E11,Z13-15:Al [“C15”] (Kaissling et al. 1989) were obtained from Dr. Jocelyn Millar, (University of California, Riverside, CA). Single components or a blend (1:1) of the two were diluted in cyclohexane and applied (2–500 ng) to a piece of filter paper (0.5 cm2), which was inserted into a 1-ml glass syringe. In each experiment, we first stimulated the antenna with control stimuli, followed by a low stimulus concentration/duration of pheromone components or the blend. If no obvious (mostly inhibitory) responses were observed, the stimulus concentration and/or duration were increased up to a maximum of 500 ng/500 ms. Therefore figures present results from neurons stimulated with different concentrations/durations. Hibiscus and ylang-ylang oils (from Select Oils, Prairie Grove, AR), which contain many odor compounds, were used to evoke measurable antennal responses, widely activate the AL network and test responsiveness of neurons when no obvious responses were observed on stimulation with the sex pheromone or Z3-6:OAc. Male and female PNs, respectively, were stimulated an average of 4.3 ± 0.18 and 3.61 ± 0.16 (means ± SE) times with each concentration of odorant and odor stimuli.
Intracellular recording and staining
Sharp microelectrodes were made from borosilicate glass capillaries with filament (1 mm OD, 0.58 or 0.75 mm ID, Sutter Instruments, Novato, CA) on a laser puller (P-2000, Sutter Instruments). The tip of the micropipette was filled with a solution of Lucifer yellow CH (65 mM, Sigma-Aldrich) in 200 mM LiCl or with a solution of Alexa Fluor 568 hydrazide (10 mM in 200 mM KCl, Molecular Probes, Eugene, OR), and the shaft, with 2 M LiCl; microelectrodes had resistances in the range 100–350 MΩ. Microelectrodes were manipulated into the glomerular region of the AL above the known location of the MGC and G35 so that MGC PNs and G35 PNs, or PNs in neighboring glomeruli or distant glomeruli, could be impaled. The responses of an impaled neuron to stimulation of the ipsilateral antenna were amplified 10- to 50-fold with an amplifier (Axoclamp-2A, Axon Instruments, Molecular Devices, Sunnyvale, CA) coupled to a DC amplifier (LPF 202A, Warner Instruments, Hamden, CT), and digitized at 20 kHz (via a Digidata 1200 series Interface, Axon Instruments, Foster City, CA, or Datapack, Run Technologies, Mission Viejo, CA). Data were analyzed with programs written in Matlab (The Mathworks, Natick, MA).
After physiological characterization, neurons were injected with either Lucifer yellow or Alexa 568 (see preceding text) by passing hyperpolarizing current (0.2–1 nA) for 6–40 min. Different dyes were used in cases in which recordings were obtained from more than one neuron in the same preparation. The duration of intracellular impalements, including both recording and dye injection, was variable (15–20 min, 50-min maximum). On completion of an experiment, the brain was excised and immersed in 2.5% formaldehyde fixative solution (pH 7.2) for ≥3 h, dehydrated through a graded series of aqueous ethanol solutions (from 50 to 100%), and cleared with methyl salicylate (Sigma-Aldrich). Cleared brains were imaged as whole mounts with a laser-scanning confocal microscope (Nikon PCM 2000 or Carl Zeiss 510 Meta, both equipped with a 457-nm Argon laser and a 543-nm Green HeNe laser). When glomerular boundaries could not be visualized in whole mounts, brains were returned to 100% ethanol and embedded in Spurr's resin (Electron Microscopy Sciences, Ft. Washington, PA) for sectioning at 48 μm and then imaged again.
Data analysis
At the end of the experiment we stimulated the antenna with neat hibiscus or ylang-ylang oil, which unambiguously evoked an antennal response. We thus established the earliest time at which any given odor stimulus could reach the antenna in each preparation. This time, so established for each preparation, was used to calculate the physiological response parameters we describe next. The net number of spikes was calculated by subtracting the number of spikes in a 1-s period before antennal stimulation from the number of spikes counted in a 1-s period postantennal stimulation. The membrane potential deflection was calculated as the change in potential occurring in a period of ≤2.2 s after the onset of antennal stimulation. Negative and positive deflections indicate hyperpolarization and depolarization, respectively. Although the selection of this time window was arbitrary, our observations indicate that hyperpolarizations or depolarizations occurring after that time period cannot be attributed to odorant stimulation. Moreover, we found that most odor-evoked changes in membrane potential occurred within 1.5 s after antennal stimulation. In the case of stimulation with control stimuli, we calculated the average membrane potential in a 1-s period after antennal stimulation and subtracted this value from the average resting potential. The net number of spikes and amplitude of the membrane hyperpolarization (or depolarization) were averaged for each combination of PN and stimulus.
To analyze the time course of the spiking response, the spiking activity in a 1-s period after antennal stimulation was divided into 20 50-ms bins. The resting spiking activity was calculated by dividing the 1-s period before odor stimulation into 20 50-ms bins, and the 20 values so obtained were averaged. This single value was subtracted from the activity in each of the 20 50-ms bins of the 1-s period after antennal stimulation. Values were averaged for each combination of PN and stimulus.
Differences between two means were compared with the aid of Wilcoxon-matched pairs tests or Mann Whitney U-tests (Zar 1999). Results were considered statistically significant if P < 0.05.
RESULTS
Results were obtained from a total of 40 PNs in 38 males (neurons from the same animal were stained with different dyes), 9 PNs in 9 females, and 4 LNs in 4 females. Some male PNs could not be identified morphologically with certainty (e.g., owing to incomplete staining, in which cases the text refers to “putative” Toroid-I PNs or G35 PNs) but were recognized on the basis of their physiological response properties. Toroid-I PNs were excited by antennal stimulation with E10,Z12-16:Al and were inhibited (or did not respond) to antennal stimulation with E10,E12,Z14-16:Al (the pheromone component that activates MGC PNs with arborizations in the Cumulus) (Heinbockel et al. 1999; Lei et al. 2002) or its more chemically stable, biologically active mimic, E11,Z13-15:Al [“C15”]. G35 PNs were strongly excited by stimulation with Z3-6:OAc, less excited by butyrate and propionate homologs, and not excited by linalool (Reisenman et al. 2005). PNs can be distinguished from LNs in intracellular recordings on the basis of one or more of the following properties: odor-evoked triphasic response (early, rapid hyperpolarization followed by depolarization with spiking and, thereafter, by long-lasting hyperpolarization), spikes with smaller width at half-amplitude than those of LNs, much higher spiking frequency (≤250 s–1) in response to odor stimulation, and firing irregularity (Christensen et al. 1993; unpublished observations).
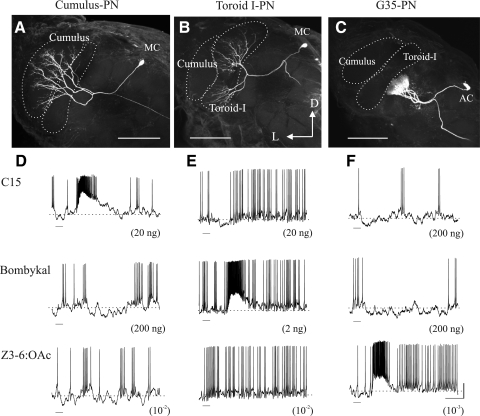
Figure 1 shows the morphological features and physiological responses of PNs with dendritic arborizations restricted to three neighboring glomeruli: the Cumulus (A) and Toroid I (B) of the MGC, and glomerulus 35 (G35, C). Cumulus PNs were excited by antennal stimulation with C15 (Fig. 1D, top) and in most cases, they were hyperpolarized and their spike activity suppressed in response to stimulation with E10,Z12-16:Al (bombykal; Fig. 1D, middle), the pheromone component that activates PNs in the adjacent Toroid I (Fig. 1D, bottom). Likewise, PNs arborizing in Toroid I were excited and inhibited, respectively, by stimulation with bombykal (Fig. 1E, middle) and C15 (Fig. 1E, top). Although these findings have been reported elsewhere (e.g., Heinbockel et al. 1999; Lei et al. 2002), here we replicated some of the experiments with pheromone components as positive controls; i.e., we confirmed that Toroid-I PNs are inhibited by activation of the Cumulus. This ensured that any lack of inhibitory responses in Toroid-I PNs on stimulation of G35 or any glomeruli activated by Z3-6:OAc was not due to uncontrolled factors or low concentration of odorants. PNs with arborizations restricted to G35 were excited by stimulation with the plant odorant Z3-6:OAc (Fig. 1F, bottom) and were hyperpolarized by stimulation with either C15 or bombykal (Fig. 1F, top and middle). In contrast, Toroid-I PNs were not affected by stimulation with Z3-6:OAc (typical examples are shown in Fig. 1, D and E, bottom), even when this odorant was tested at a concentration (10-2 vol/vol) at least two orders of magnitude above the sensitivity threshold of G35 PNs (Reisenman et al. 2005).
FIG. 1.
Morphological features and typical odor responses of projection neurons (PNs) arborizing in 3 neighboring glomeruli in the antennal lobe (AL) of male Manduca sexta. The Cumulus (A) and Toroid I (B) are the 2 main glomeruli of the male-specific macroglomerular complex (MGC); the adjacent glomerulus 35 (G35, C) is a sexually isomorphic glomerulus. MGC PNs and G35 PNs have their cell bodies, respectively, in the medial group of AL neuronal cell bodies (MC in A and B) and in the anterior group (AC in C). Electrophysiological traces obtained from a Cumulus PN (D), a Toroid-I PN (E), and a G35 PN. All PN classes gave excitatory responses to stimulation with their respective key odor inputs, the sex-pheromone components E10,E12,Z14-16:Al (here substituted by the stable chemical mimic, E11,Z13-15:Al or “C15”) and E10,Z12-16:Al (bombykal), and the plant volatile Z-3-hexenyl acetate (Z3-6:OAc). Cumulus PNs and Toroid-I PNs, respectively, were inhibited by stimulation with bombykal and C15 (D and E, deflections below the resting membrane potential indicated by ---), i.e., each by excitatory input to the other MGC glomerulus. These PNs were not inhibited by Z3-6:OAc (D and E, 3rd row), the compound that activates PNs in the adjacent G35. Stimulation with C15 or bombykal inhibited G35 PNs (F, 1st and 2nd rows). The solvents used to dilute the pheromone components and Z3-6:OAc (cyclohexane and mineral oil, respectively—solvent controls) elicited no response (not shown in this figure). —, the activation of the device controlling the stimulus delivery system and the duration (200 ms) of the stimulus. Concentrations or amount of compounds are indicated between parentheses below each record. Calibration: 10 mV, 500 ms. D, dorsal; L, lateral. Scale bars: 150 μm.
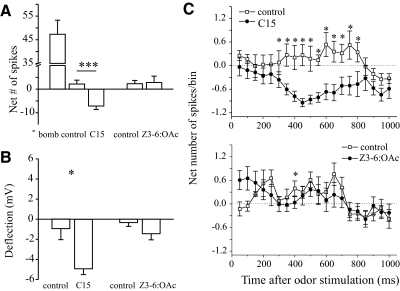
Figure 2A shows a quantitative population analysis of the odor responses of Toroid-I PNs. The response of these PNs to antennal stimulation with C15 (which activates inputs to the Cumulus) was statistically different from the response to the respective control (Wilcoxon matched pair tests, P < 0.05). When the antenna was stimulated with C15, the net number of spikes was lower and the hyperpolarization amplitude was larger than when stimulated with the control cyclohexane stimulus (Fig. 2, A and B, left). In contrast, the response to Z3-6:OAc was not statistically different from the response to the mineral-oil control (P > 0.05 in both cases, Fig. 2, A and B, right). The hyperpolarization evoked by the cyclohexane control stimulus was not different from that evoked by the mineral-oil control (Wilcoxon matched-pairs tests, P > 0.5), and the response to either control was not statistically different from zero (Student's t-test for differences between an observed mean and an hypothesized population mean; P > 0.5 in both cases). Thus although Toroid-I PNs receive inhibitory input from the adjacent Cumulus, they were not inhibited by input to the adjacent G35 or any other putative Z3-6:OAc-activated glomeruli. This finding is not due to weak excitatory input to G35, as lower concentrations of Z3-6:OAc elicited hyperpolarization in other glomeruli (Fig. 3, B–D). We tested a subset of 6 Toroid-I PNs with antennal stimulation using neat hibiscus oil, which is a mixture of many plant volatiles and thus activates sensory inputs to many glomeruli, and observed obvious suppression of spiking activity and hyperpolarization in 50% of PNs. The net number of spikes and hyperpolarization amplitude (control subtracted, means ± SE, n = 6) were, respectively, –6.3 ± 3.7 spikes and –2.2 ± 0.84 mV. This result indicates that Toroid-I PNs receive inhibitory input from at least some sexually isomorphic glomeruli (activated by ≥1 of the constituents of hibiscus oil).
FIG. 2.
Quantification of the responses of Toroid-I PNs (left, means ± SE, n = 11 PNs) to stimulation with bombykal (bomb), C15, Z3-6:OAc, and the 2 respective solvent controls (cyclohexane in the case of pheromone components, mineral oil in the case of Z3-6:OAc). A: the net number of spikes in a 1-s period after the onset of antennal stimulation. B: the amplitude of the odor-evoked deflection (negative values indicate hyperpolarization). PNs were stimulated with 20 ng of bombykal (except 1 PN was stimulated with 2 ng), and with 20, 200, or 500 ng of C15 loaded on filter paper. Stimulus duration was 200 ms (except 500 ms in 3 PNs). Asterisks indicate significant differences between C15 and the respective control (Wilcoxon matched pairs tests; *: P < 0.05, ***: P < 0.005). In neither case was the response to Z3-6:OAc statistically different from the response evoked by mineral oil (Wilcoxon matched pairs tests: P > 0.05). C: time course of the responses of Toroid-I PNs. The net number of spikes (means ± SE, n = 8 PNs) during a 1-s period after the odor had reached the antenna was broken down into 20 50-ms bins. First row: net response to stimulation with the C15 (•) and the cyclohexane control; 2nd row: net response to stimulation with Z3-6:OAc 10−2 vol/vol (•) and the mineral oil control. This panel includes only those PNs that were stimulated with 200-ms pulses. *, statistical differences between the response to the odorant and the respective control for each time bin (Wilcoxon matched pair tests, P < 0.05). Note that the response to C15 was statistically different from that to the control in 11 consecutive time bins. The response to Z3-6:OAc was different from the response to the control in only 1 of the 20 time bins, which is the false positive rate expected under a P = 0.05 criterion. These results show that Toroid-I PNs do not receive inhibitory input from the adjacent G35 glomerulus or any other Z3-6:OAc-activated glomerulus.
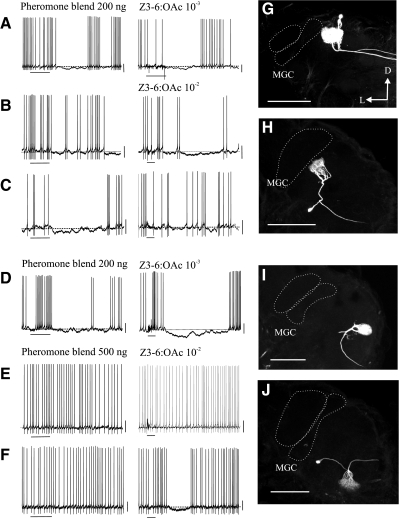
FIG. 3.
Responses and morphological features of PNs in sexually isomorphic glomeruli. A–C, G, and H: PNs in glomeruli adjacent to or 1–2 glomeruli away from the MGC and G35; D–F, I, and J: PNs in glomeruli distant to the MGC. Records shown in A, B, D, and E were obtained, respectively, from the neurons shown in G–J. PNs in both adjacent (A and G) and distant (D and I) glomeruli were inhibited by stimulation with either odorant. PNs in both adjacent (B and H) and distant (F and J) glomeruli were inhibited only by Z3-6:OAc. C shows an example of a PN that was inhibited only by the sex pheromone, and E and J show an example of a PN that was not inhibited by either key component of the sex pheromone. PNs were stimulated with the odor compounds and concentrations indicated. The respective control stimuli (cyclohexane and mineral oil) elicited no response (not shown in this figure). The resting potential is indicated (···). These results show that not all PNs, regardless of their position, receive inhibitory input from the MGC or G35 (or any other glomeruli activated by Z3-6:OAc). Calibration bars: 10 mV in all records. Scale bars = 200 μm. ···, the outline of the MGC glomeruli. The cell body of the PN shown in I (not visible in this section) was in the anterior group of neuronal cell bodies.
We observed that the inhibitory responses of individual Toroid-I PNs were variable in temporal profile, some having more prolonged inhibitory epochs or longer delays to the onset of the inhibition than others, and some not showing obvious suppression of spiking. To address the possibility that we underestimated the degree of inhibition in our population analysis by calculating spiking activity in a relatively long time period (1 s, Fig. 2A), we analyzed the spiking activity of each PN in 20 50-ms bins. The response to stimulation with C15 was statistically different from the control in 11 time bins (Fig. 2C, top, *), started ∼300 ms after the odor reached the antenna, and lasted ∼500–600 ms. By contrast, stimulation with Z3-6:OAc did not cause reduction in spiking activity (the response was statistically different in only 1 of 20 bins, which is the false-positive rate expected under a P = 0.05 criterion; Fig. 2C, bottom). Although Z3-6:OAc might activate other glomeruli besides G35 (Reisenman et al. 2005), this negative result allowed us to conclude unambiguously that Toroid-I PNs do not receive inhibitory input from G35 or from any other glomerulus activated by Z3-6:OAc. We recorded the activity of a small subset of male G35 PNs (n = 5) to Z3-6:OAc and to the two key sex-pheromone components and/or a blend of the two. All G35 PNs, as expected (Reisenman et al. 2005), were excited by antennal stimulation with Z3-6:OAc but were hyperpolarized by stimulation with either of the two key components of the sex-pheromone blend (an example is shown in Fig. 1F; results from responses of three G35 PNs to stimulation with the pheromone blend are included in the analysis presented in Fig. 4, A and B). Because pheromone-responsive ORCs project exclusively to the MGC glomeruli (Christensen and Hildebrand 1987; Christensen et al. 1995; Kaissling et al. 1989), these results indicate that G35 PNs are inhibited by input to either one or both of the main MGC glomeruli (Cumulus and Toroid I). Because we have shown that the opposite is not true (activation of G35 did not inhibit Toroid-I PNs; Fig. 2), these results suggest that inhibitory interactions between the MGC and G35 are not reciprocal.
FIG. 4.
Quantification of the responses of PNs in glomeruli adjacent to or 1–2 glomeruli away from the MGC or distant to the MGC and G35 to stimulation with the sex-pheromone blend (A and B) and Z3-6:OAc (C and D). A and C: the net number of spikes in a 1-s period postantennal stimulation. B–D: the amplitude of the odor-evoked change in membrane potential. ○, the average response (control-subtracted for clarity) of each PN to illustrate the response variability. •, the average response across PNs. The net number of spikes and the change in membrane potential evoked by the pheromone blend were statistically different from those evoked by the cyclohexane solvent-control stimulus in PNs in glomeruli both nearby and distant to the MGC (A and B, *, Wilcoxon matched pairs tests, n = 11 and 14, P < 0.05). The responses evoked by Z3-6:OAc were statistically different from that to the mineral-oil control stimulus only in PNs in glomeruli distant from the MGC and G35 (C and D, *; Wilcoxon matched pairs tests; G35 PNs were not included in this analysis because Z3-6:OAc evokes a strong excitatory response in these PNs). PNs were stimulated with pheromone blend (200 or 500 ng; duration: 200 or 500 ms), the cyclohexane control (200 or 500 ms), Z3-6:OAc (1:100 vol/vol in most cases; G35 PNs were stimulated with 1:1,000 or 1:10,000 vol/vol; duration = 200 ms), and the mineral oil control (200 ms; see methods for an explanation of the different concentration/stimulus duration used). These results indicate that many PNs in sexually isomorphic glomeruli receive inhibitory input from the MGC regardless of their position, and that PNs in distant glomeruli receive inhibitory input from G35 and/or other Z3-6:OAc activated glomeruli.
We next asked if inhibitory interactions among the MGC and sexually isomorphic glomeruli, or between sexually isomorphic glomeruli, are general phenomena. We recorded the activity of 11 and 14 uniglomerular PNs with arborizations in glomeruli adjacent (or 1–2 glomeruli away) and more distant to the MGC and G35, respectively, in response to antennal stimulation with the sex-pheromone blend and Z3-6:OAc. Two examples of each of these PNs are shown in Fig. 3, G–J. All 25 PNs were stained intracellularly so that in each case, the position of the glomerulus containing the arborizations of the recorded PN with respect to the MGC and G35 could be precisely established. We found that some PNs were inhibited by stimulation with both the sex-pheromone blend and Z3-6:OAc regardless of their proximity to the MGC and G35 (e.g., Fig. 3, A and D, shows examples of 2 PNs with arborizations, respectively, in glomeruli adjacent and distant to the MGC). Other PNs were inhibited by stimulation with Z3-6:OAc but not by stimulation with the sex pheromone (e.g., Fig, 3, B and F), and vice versa (e.g., Fig. 3C). Again, this did not depend on the position of the glomerulus from which the recording was obtained. Other PNs were not inhibited by either odor stimulus (e.g., Fig. 3E), even when odorants were tested at high concentrations. In particular, the finding that a lower concentration of Z3-6:OAc (10−3 vol/vol) than that used to stimulate Toroid-I PNs (10−2 vol/vol) was sufficient to hyperpolarize some PNs in sexually isomorphic glomeruli (Fig. 3, A and D), confirms our conclusion about the lack of inhibitory input from G35 (or any Z3-6:OAc) on Toroid-I PNs. At the end of the experiment all PNs were stimulated with hibiscus or ylang-ylang oil to test that they were responsive to odor stimulation even if no response was observed to stimulation with the sex-pheromone blend or Z3-6:OAc.
Figure 4 shows a quantitative population analysis of the responses of PNs with arborizations restricted to sexually isomorphic glomeruli to stimulation with the pheromone blend and Z3-6:OAc, respectively. In this figure, each symbol represents the response (control-subtracted for clarity) of one PN; the • represents the average across PNs. The scattering of data points in this figure illustrates the variability of responses to odor stimulation in PNs with arborizations in glomeruli both adjacent to and distant from the MGC and G35. Despite this variability, the net number of spikes and hyperpolarization amplitude evoked by stimulation with the sex-pheromone blend was statistically different from the respective control in PNs in both neighboring (n = 11 PNs, including 3 G35 PNs, Wilcoxon matched pairs tests, P < 0.05) and distant (n = 14 PNs; P < 0.05) glomeruli. The net number of spikes and hyperpolarization amplitude evoked by pheromone stimulation were not different between neighboring and distant PNs (Mann Whitney U-tests, P < 0.05). These results indicate that pheromonal stimulation inhibits PNs in sexually isomorphic glomeruli regardless of their distance to the MGC (Fig. 4, A and B). The response of PNs to stimulation with Z3-6:OAc was also variable, as indicated by the scattering of data points in Fig. 4, C and D (the responses of G35 PNs are not included in these panels because stimulation with Z3-6:OAc caused a strong excitatory response in these PNs). The net number of spikes and hyperpolarization amplitude of PNs close to the MGC and G35 was not statistically different from the response to the respective control (Wilcoxon matched pairs tests, P > 0.05, Fig. 4, C and D). In contrast, stimulation with Z3-6:OAc caused suppression of spikes and hyperpolarization in many PNs with arborizations in glomeruli distant from the MGC and G35 (Fig. 4, C and D, right). The net number of spikes and hyperpolarization amplitude were statistically different from the respective control (Wilcoxon matched pairs tests, P < 0.05). Because Z3-6:OAc might also activate glomeruli other than G35 (Reisenman et al. 2005), we cannot conclude that the inhibition observed by Z3-6:OAc stimulation in these PNs necessarily reflects connectivity between G35 and the recorded glomerulus. Specific connectivity patterns can be established with certainty only in cases in which we did not observe hyperpolarization and/or suppression of spiking (e.g., Fig. 3, C, E, and F; PNs with values ≈0 in Fig. 4, and Toroid-I PNs, Fig. 2).
We also recorded the activity of nine female PNs with arborizations in glomeruli near the sexually dimorphic latLFG and the sexually isomorphic G35 to stimulation with the odorants that preferentially activate those glomeruli. Figure 5, A and B, shows an example of such a PN that was hyperpolarized by both [±]linalool (which stimulates latLFG PNs) (Roche King et al. 2000) and Z3-6:OAc (which stimulates G35 PNs) (Reisenman et al. 2004). This example illustrates that at least certain PNs in sexually isomorphic glomeruli in the female AL might receive inhibitory input from the sexually dimorphic glomeruli and G35 (or by glomeruli activated by [±]linalool and Z3-6:OAc, respectively). Overall, more PNs were inhibited by stimulation with [±]linalool than with Z3-6:OAc: 66 and 33% of PNs, respectively, were obviously hyperpolarized by these odorants. The hyperpolarization evoked by [±]linalool (but not by Z3-6:OAc) was statistically different from the control (P < 0.05, Fig. 4C *). Thus it appears that activation of the latLFG and/or other glomeruli with linalool (this odorant can activate PNs in other glomeruli besides the latLFG) (Reisenman et al. 2004) has a stronger inhibitory effect in neighboring, sexually isomorphic glomeruli than activation of G35 or any other glomeruli activated by Z3-6:OAc.
FIG. 5.
Responses of PNs in sexually isomorphic glomeruli of female ALs near the female-specific lateral large female glomerulus (latLFG) and G35 to stimulation with linalool and Z3-6:OAc. These odorants respectively strongly stimulated PNs in the latLFG and G35 and to a less extent, PNs in other glomeruli (Reisenman et al. 2004, 2005; Roche King et al. 2000). A: morphology of a PN with arborizations restricted to a glomerulus near the latLFG (···) and G35 (not visible in this section; image obtained after sectioning). This PN had its cell body in the lateral group of neuronal cell bodies (LC). Scale bar: 200 μm. B: electrophysiological responses of this PN to stimulation with the mineral oil control, Z3-6:OAc, and [±]linalool. Note that both Z3-6:OAc and [±]linalool elicited hyperpolarization in this PN (deflections below the resting potential, ···). Calibration bars: 5 mV in all panels. C: deflection evoked by stimulation with [±]linalool and Z3-6:OAc in PNs (n = 9) with dendritic arborizations in the neighborhood of the latLFG and G35. ○ the average response (control-subtracted for clarity) of each PN to illustrate the response variability across neurons (only 6 PNs could be tested with Z3-6:OAc). •, the average response across PNs. Concentration of odorants was 10−3 or 10−2 vol/vol (duration = 200 ms). The response to [±]linalool, but not to Z3-6:OAc, was statistically different from the response to the control (, Wilcoxon matched pairs test, P < 0.05). These results show that the latLFG and/or any other linalool activated glomeruli cause inhibition in PNs in nearby glomeruli.
DISCUSSION
In this study, we tested the whether spatial relationships between olfactory glomeruli determine interglomerular inhibitory interactions. To this end, we used specific odorants (sex-pheromone components and Z3-6:OAc) to activate PNs in morphologically and functionally identified reference glomeruli (the MGC glomeruli and G35, respectively) and tested the effect of this olfactory activation in neighboring and distant glomeruli. We found that interglomerular inhibitory interactions occur throughout the glomerular array in the AL and are not simply a reflection of the spatial relationships among glomeruli. While PNs in Toroid I of the MGC were inhibited by activation of the neighboring MGC Cumulus, these MGC PNs were not inhibited by antennal stimulation with Z3-6:OAc (Figs. 1 and 2), i.e., by activation of input to the neighboring G35 glomerulus. We recorded the responses of PNs arborizing in sexually isomorphic glomeruli (other than G35) to stimulation with the sex pheromone and Z3-6:OAc and found that inhibitory responses were not related to proximity to the MGC and G35: both distant and adjacent PNs were inhibited by stimulation with the sex pheromone, some others were affected by only one odorant and yet others by neither (Figs. 3 and 4). Similar results were obtained in female PNs recorded in the proximity of female-specific glomeruli. These results indicate that inhibitory interglomerular interactions are not determined by proximity of the glomeruli involved in these interactions.
It is thought that a major function of inhibition in the vertebrate OB is sharpening glomerular output through two OB neuronal circuits. One circuit involves reciprocal synapses between the lateral dendrites of output neurons (mitral cells) and GABAergic local interneurons (granule cells), and the other circuit involves inhibitory interactions between glomeruli (Egger et al. 2003; Halabisky and Strowbridge 2003; Schoppa and Urban 2003; Vucinic et al. 2006). Thus in the OB, activation of short-axon cells mediates “center-surround” inhibition of distal mitral cells (Aungst et al. 2003). These in vitro studies, using electrical stimulation in OB brain slices, revealed synaptic circuits underlying inhibition, but they did not use behaviorally relevant stimuli and did not investigate functional interglomerular interactions. Testing patterns of interglomerular connectivity requires knowledge of the natural odor stimuli that activate at least some identifiable glomeruli, a requisite met by the M. sexta olfactory system. In our experiments, we activated a reference glomerulus (e.g., Toroid I) and recorded the activity of PNs in distant and neighboring glomeruli. We could study such interactions unambiguously because the two key sex-pheromone components exclusively activate male-specific ORCs that project exclusively to and activate only the two principal MGC glomeruli (Christensen and Hildebrand 1987; Christensen et al. 1995; Kaissling et al. 1989). The techniques used in this study allowed us to measure synaptic events (hyperpolarization) and spiking activity in single neurons with millisecond resolution and to determine the glomerular associations of the recorded PNs.
As expected, Toroid-I PNs and G35 PNs were strongly excited by antennal stimulation with their respective, preferred odor compounds (bombykal and Z3-6:OAc, respectively). Stimulation with E10,E12,Z14-16:Al or its chemical mimic C15 elicited hyperpolarization and suppression of spiking in Toroid-I PNs (Figs. 1 and 2). Although this finding has been reported previously (Heinbockel et al. 1999, 2004), we repeated some of the earlier experiments to validate the findings discussed in the following text. We found that Toroid-I PNs were not inhibited by stimulation with Z3-6:OAc (Figs. 1E and 2), an odorant that preferentially activates PNs in the adjacent G35 glomerulus. This negative result unambiguously demonstrated that MGC PNs do not receive inhibitory input from G35 or from other glomeruli that might be activated to some extent by Z3-6:OAc. The fact that Toroid-I PNs were inhibited by stimulation with a blend of odorants (hibiscus oil), however, demonstrates that these PNs can receive inhibitory input from glomeruli other than G35 or on stimulation with a high concentration of odorant mixtures. In contrast, G35 PNs were hyperpolarized by antennal stimulation with sex-pheromone components (Fig. 1F). This demonstrates that interactions between glomeruli do not have to be reciprocal in nature. The generality of this finding must be validated by testing the interactions among other glomeruli of characterized odor input.
What might be the functional consequences of such nonreciprocal interaction? In the MGC, reciprocal inhibition between the Cumulus and Toroid-I synchronizes the outputs of the glomeruli processing information about the key components of the sex-pheromone blend (Lei et al. 2002) and thus might “bind” the features of the blend in higher brain centers downstream from the AL. Our findings suggest that the naturally occurring, simultaneous presence of sex pheromone and plant odors such as Z3-6:OAc would not lead to inhibition of the responses of MGC PNs to sex-pheromone stimulation. Because we found that antennal stimulation with sex-pheromone components inhibits PNs in sexually isomorphic glomeruli (Figs. 1F, 3, and 4), this hyperpolarization could enhance the responses of these PNs to their preferred odor input when the two odorants concurrently stimulate the antenna. We have observed that in hyperpolarized PNs, the responses to their preferred odor input is stronger than in PNs at their resting membrane potential (unpublished observations). Another possibility is that activation of the sexually dimorphic glomeruli “shuts down” responses in glomeruli involved in detection and discrimination of plant odors, which in the presence of a conspecific female may become behaviorally less significant to a male moth. These ideas are supported by our finding that stimulation of the sexually dimorphic glomeruli has a strong inhibitory effect in PNs in sexually isomorphic glomeruli (Figs. 3 and 4). Odor input to the brain might be processed in a hierarchical manner such that processing of odorants involved in reproductive behaviors takes priority over processing of food-related odorants (plant volatiles). These speculations remain to be tested both physiologically and behaviorally, e.g., by concurrent stimulation with sex pheromone and plant odorants. Moreover, the finding that MGC PNs were inhibited by sexually isomorphic glomeruli on antennal stimulation with high concentrations of complex odor blends (hibiscus oil) merits further study of the relationship between the sexually dimorphic and isomorphic olfactory subsystems.
Our recordings from PNs with arborizations in glomeruli adjacent to and distant from the MGC and G35 show that inhibitory interactions are not related to glomerular proximity. Overall we found that most PNs, irrespective of their position, were inhibited by antennal stimulation with the sex-pheromone blend (Fig. 4, A and B). Some PNs, mostly those in glomeruli distant from the MGC and G35, also were inhibited by stimulation with Z3-6:OAc (Figs. 3 and 4). Other PNs were inhibited by stimulation with Z3-6:OAc but not by stimulation with the sex pheromone (e.g., Fig. 3, B and F). Again, this did not depend on the positions of the glomeruli containing the arborizations of the recorded PNs. Because pheromone-responsive ORCs project exclusively to the MGC, we could unequivocally establish whether or not PNs in the recorded glomeruli received inhibition from the MGC. In contrast, Z3-6:OAc activates other glomeruli in addition to G35, albeit to a lesser extent (Reisenman et al. 2005). Therefore we could establish conclusive connectivity patterns between G35 and the recorded glomerulus only in those cases in which we did not observe inhibition. For instance, we found that some PNs were inhibited by stimulation with sex pheromone but not by Z3-6:OAc (e.g., Fig. 3C), and other PNs were not inhibited by either odorant (e.g., Fig. 3, E–J) even when odorants were tested at high concentrations. Therefore we can conclude unambiguously that these PNs do not receive inhibitory input from G35 or any other Z3-6:OAc-sensitive glomerulus.
In female M. sexta, we found that antennal stimulation with [±]linalool (which activates PNs in the female-specific latLFG) (Reisenman et al. 2004; Roche King et al. 2000) produced hyperpolarization in most recorded PNs associated with neighboring glomeruli (Fig. 5). Z3-6:OAc stimulation (which activates the adjacent G35) (Reisenman et al. 2005) produced hyperpolarization in some PNs (Fig. 5B), but the response across PNs was not statistically different from the control (Fig. 5C). Furthermore, we have previously shown that G35 PNs are hyperpolarized by antennal stimulation with [±]linalool (Reisenman et al. 2005). Caution should be taken, however, in interpreting these results in terms of glomerular connectivity because these odorants also activate—to a lesser extent—PNs in other glomeruli. Nevertheless these results parallel our findings in males, showing that activation of the sexually dimorphic subsystem appears to have a stronger inhibitory effect in sexually isomorphic glomeruli than does stimulation with Z3-6:OAc.
The finding that activation of Toroid-I PNs (Figs. 3 and 4) inhibited PNs in sexually isomorphic glomeruli shows that interglomerular inhibition extends beyond a cluster of functionally related glomeruli (in this case, the MGC). We observed that Toroid-I PNs received stronger inhibitory input (i.e., larger hyperpolarization) from the Cumulus than did sexually isomorphic PNs from the MGC (compare Figs. 2 and 4). Thus it is possible that sex-pheromonal inhibition has dual roles: one to promote synchronous firing of PNs within a glomerular cluster that processes components of the sex-pheromone blend (Lei et al. 2002) and the other to promote overall inhibition through a mechanism resembling contrast enhancement. Recent studies in Drosophila melanogaster similarly have proposed the parallel existence of glomerulus-specific and global inhibitory networks (Silbering and Galizia 2007).
In both D. melanogaster and M. sexta, odor stimulation can cause suppression of spiking in ORCs (Hallem et al. 2004; Shields and Hildebrand 2001). Because in M. sexta ORCs projecting to sexually isomorphic glomeruli are insensitive to sex-pheromone components (Kalinová et al. 2001; Shields and Hildebrand 2001; B. Kalinová, personal communication), the inhibitory responses we observed in sexually isomorphic glomeruli probably are mediated by inhibitory LNs. In any case, inhibition of ORCs would produce a decrease in the amount of neurotransmitter released by the presynaptic terminal. Because ORCs are excitatory (e.g., Berkowicz et al. 1994; Nickell et al. 1996; Squire et al. 2003), this would at most cause a reduction of spiking activity in postsynaptic AL neurons (but not hyperpolarization). Reduction of spiking activity in PNs also could be caused by other central mechanisms such as presynaptic inhibition of ORC terminals mediated by GABAergic interneurons (Olsen and Wilson 2008; Schoppa and Urban 2003).
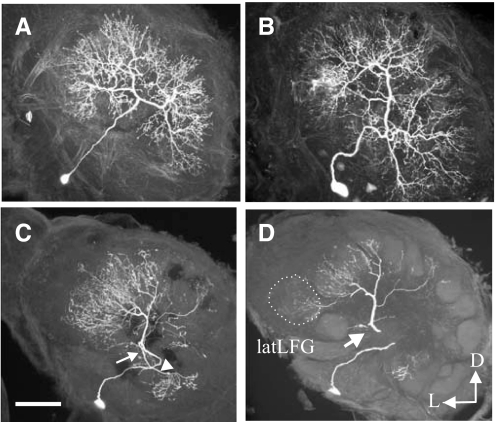
The hypothesis that glomeruli are organized in synaptically interacting clusters is supported by the fact that in M. sexta some LNs, the main inhibitory elements in the AL (Christensen et al. 1993; Hoskins et al. 1986), have primary neurites that arborize in only a few glomeruli (Matsumoto and Hildebrand 1981) (Fig. 6, C and D). These LNs can serve as neural substrates for the selective inhibitory interglomerular interactions described in the preceding text. Although further studies of the synaptic architecture of LNs (e.g., location of input and output synapses) are needed to test this hypothesis, the arborization pattern of such LNs provides at least a neural connection among the glomeruli that process the individual components of an innately significant odor blend. Moreover, each glomerulus receives arborizations from LNs arborizing in different but overlapping sets of glomeruli, and this pattern could provide a combinatorial scheme for coding of odor blends. In M. sexta (Fig. 6, A and B) and other insects, however, there are LNs that arborize in all of the glomeruli (Abel et al. 2001; Matsumoto and Hildebrand 1981; Wilson and Laurent 2005). In D. melanogaster, Wilson and Laurent (2005) found that each LN releases GABA widely throughout the AL, but apparently it does so nonuniformly. In M. sexta, this type of wide-field LN (e.g., Fig, 6, A and B) might have a different function (e.g., overall gain control) than more restricted types such as the one shown in Fig. 6C. These different morphological types of LNs could provide an anatomical substrate for the two parallel inhibitory networks, one that acts globally and one that is glomerulus-specific as proposed for the D. melanogaster AL (Silbering and Galizia 2007). Future studies of the odor-response properties and specificity of such different kinds of LNs, and of the patterns of synaptic connectivity among LNs and PNs, should clarify the issue. Both vertebrate-like GABAA (ionotropic) and GABAB (metabotropic) receptors shape PN odor responses (Christensen et al. 1998; Olsen and Wilson 2008; Root et al. 2007; Silbering and Galizia 2007; Wilson and Laurent 2005). The kinetics of the odor-evoked interglomerular inhibition we observed in M. sexta PNs (slow, long-lasting) suggest that this inhibition is likely mediated by GABAB receptors, but this remains to be investigated. A recent study in D. melanogaster described a population of excitatory (cholinergic) LNs and thus added a new neural element to the AL circuitry in this insect species. The authors suggested that such excitatory LNs could augment PN output through lateral excitation (Shang et al. 2007). In this regard, Olsen et al. (2007) showed that PNs receive indirect excitatory input from other glomeruli and found that the spatial relationships among glomeruli do not predict the strength of these excitatory interactions. These results contrast with those from a recent study in the species that found that ORCs are the main source of excitatory input to PNs and that lateral inhibition is prevalent in the AL (Root et al. 2007).
FIG. 6.
Examples of morphological types of local interneurons (LNs) in the ALs of female M. sexta. LNs are confined to the ALs. A: this type of LN exhibits a symmetrical arborization pattern in which the dendrites branch radially from the major neurite in the central, coarse neuropil and ramify widely in the glomeruli. B: this type of LN is distinguished by the marked asymmetry in the branching pattern of their neurites into the glomeruli and also ramifies widely in the glomeruli. C and D: this type of LN shows an asymmetric pattern, but the arborizations are limited to a smaller number of glomeruli. D: confocal microscopic image obtained from the neuron shown in C after embedding in plastic and sectioning. This neuron had 2 main neurites, one connecting a large number of glomeruli (C and D, →), which includes the latLFG (D, ···), and the other connecting fewer glomeruli (C, ▵). Scale bars: 100 μm. This figure illustrates the different types of LNs that mediate interglomerular interactions. The different morphologies provide a neuronal substrate for proposed global and glomerulus-specific inhibitory networks (Silbering and Galizia 2007).
Overall our results indicate that inhibitory interglomerular interactions are globally distributed in the AL and cannot be explained simply by spatial proximity of the interacting glomeruli. The rules that govern these interactions (e.g., response properties, chemical relatedness, or other functional relationships) merit further study. We expect that patterns of glomerular interaction will be found to reflect a functional and biologically relevant organization of glomeruli because integration of signals among glomeruli is an important mechanism for processing of olfactory information about behaviorally significant, naturally occurring odor blends.
GRANTS
This work was supported by National Institutes of Health Grant R01-DC-02751 to J. G. Hildebrand and by grants from the Whitehall Foundation and the Howard University New Faculty Research Program and National Institutes of Health Grant 2×S06-GM08016×36) to T Heinbockel.
Acknowledgments
We thank four anonymous reviewers, Drs. Andrew Dacks and Hong Lei for valuable comments and corrections on this manuscript, Patricia Jansma for assistance with confocal microscopy, and Suzanne Mackzum for rearing M. sexta. The experiments comply with the “Principles of animal care”, publication No. 86-23, revised 1985 of the National Institute of Health.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- Abel 2001.Abel R, Rybak J, Menzel R. Structure and response patterns of olfactory interneurons in the honeybee, Apis mellifera. J Comp Neurol 437: 363–383, 2001. [DOI] [PubMed] [Google Scholar]
- Aungst 2003.Aungst JL, Heyward PM, Puche AC, Karnup SV, Hayar A, Szabo G, Shipley MT. Centre-surround inhibition among olfactory bulb glomeruli. Nature 426: 623–629, 2003. [DOI] [PubMed] [Google Scholar]
- Belluscio 2001.Belluscio L, Katz LC. Symmetry, stereotypy, and topography of odorant representations in mouse olfactory bulbs. J Neurosci 21: 2113–2122, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkowicz 1994.Berkowicz DA, Trombley PQ, Shepherd GM. Evidence for glutamate as the olfactory receptor cell neurotransmitter. J Neurophysiol 71: 2557–2561, 1994. [DOI] [PubMed] [Google Scholar]
- Boeckh 1993.Boeckh J, Tolbert LP. Synaptic organization and development of the antennal lobe in insects. Microscop Res Techn 24: 260–280, 1993. [DOI] [PubMed] [Google Scholar]
- Buck 1991.Buck LB, Axel R. A novel multigene family may encode odorant receptor: a molecular basis for odor recognition. Cell 65: 175–187, 1991. [DOI] [PubMed] [Google Scholar]
- Christensen 1995.Christensen TA, Harrow ID, Cuzzocrea C, Randolph PW, Hildebrand JG. Distinct projections of two populations of olfactory receptor axons in the antennal lobe of the sphinx moth Manduca sexta. Chem Senses 20: 313–323, 1995. [DOI] [PubMed] [Google Scholar]
- Christensen 1987.Christensen TA, Hildebrand JG. Male-specific, sex pheromone-selective projection neurons in the antennal lobes of the moth Manduca sexta. J Comp Physiol [A] 160: 553–569, 1987. [DOI] [PubMed] [Google Scholar]
- Christensen 1997.Christensen TA, Hildebrand JG. Coincident stimulation with pheromone components improves temporal pattern resolution in central olfactory neurons. J Neurophysiol 77: 775–781, 1997. [DOI] [PubMed] [Google Scholar]
- Christensen 1998.Christensen TA, Waldrop BR, Hildebrand JG. Multitasking in the olfactory system: context-dependent responses to odors reveal dual GABA-regulated coding mechanisms in single olfactory projection neurons. J Neurosci 18: 5999–6008, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen 1989.Christensen TA, Hildebrand JG, Tumlinson JH, Doolittle RE. Sex pheromone blend of Manduca sexta: responses of central olfactory interneurons to antennal stimulation in male moths. Arch Insect Bioch 10: 281–289, 1989. [Google Scholar]
- Christensen 1993.Christensen TA, Waldrop BR, Harrow ID, Hildebrand JG. Local interneurons and information processing in the olfactory glomeruli of the moth Manduca sexta. J Comp Physiol [A] 173: 385–399, 1993. [DOI] [PubMed] [Google Scholar]
- Egger 2003.Egger V, Svodoba K, Mainen ZF. Mechanisms of lateral inhibition in the olfactory bulb: efficiency and modulation of spike-evoked calcium influx into granule cells. J Neurosci 23: 7551–7558, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser 2003.Fraser AM, Mechaber W, Hildebrand JG. Electroantennographic and behavioral responses of the sphinx moth Manduca sexta to host plant headspace volatiles. J Chem Ecol 29: 1813–1833, 2003. [DOI] [PubMed] [Google Scholar]
- Friedrich 1998.Friedrich RW, Korsching S. Chemotopic, combinatorial, and noncombinatorial odorant representations in the olfactory bulb revealed using a voltage-sensitive axon tracer. J Neurosci 18: 9977–9988, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich 2004.Friedrich RW, Laurent G. Dynamics of olfactory bulb input and output activity during odor stimulation in zebrafish. J Neurophysiol 91: 2658–2669, 2004. [DOI] [PubMed] [Google Scholar]
- Gao 2000.Gao Q, Yuan B, Chess A. Convergent projections of Drosophila olfactory neurons to specific glomeruli in the antennal lobe. Nat Neurosci 3: 780–785, 2000. [DOI] [PubMed] [Google Scholar]
- Halabisky 2003.Halabisky B, Strowbridge BW. γ-Frequency excitatory input to granule cells facilitates dendrodendritic inhibition in the rat olfactory bulb. J Neurophysiol 90: 644–654, 2003. [DOI] [PubMed] [Google Scholar]
- Hallem 2004.Hallem EA, Ho MG, Carlson JR. The molecular basis of odor coding in the Drosophila antenna. Cell 117: 965–979, 2004. [DOI] [PubMed] [Google Scholar]
- Hallem 2006.Hallem EA, Carlson JR. Coding of odors by a receptor repertoire. Cell 125: 143–160, 2006. [DOI] [PubMed] [Google Scholar]
- Heinbockel 2004.Heinbockel T, Christensen TA, Hildebrand JG. Representation of binary pheromone blends by glomerulus-specific olfactory projection neurons. J Comp Physiol [A] 190: 1023–1037, 2004. [DOI] [PubMed] [Google Scholar]
- Heinbockel 1999.Heinbockel T, Christensen TA, Hildebrand JG. Temporal tuning of odor responses in pheromone-responsive projection neurons in the brain of the sphinx moth Manduca sexta. J Comp Neurol 409: 1–12, 1999. [PubMed] [Google Scholar]
- Hoskins 1986.Hoskins SG, Homberg U, Kingan TG, Christensen TA, Hildebrand JG. Immunocytochemistry of GABA in the antennal lobes of the sphinx moth Manduca sexta. Cell Tissue Res 244: 243–252, 1986. [DOI] [PubMed] [Google Scholar]
- Jefferis 2002.Jefferis GSXE, Marin EC, Watts RJ, Luo L. Development of neuronal connectivity in Drosophila antennal lobes and mushroom bodies. Curr Opin Neurobiol 12: 80–86, 2002. [DOI] [PubMed] [Google Scholar]
- Johnson 1998.Johnson BA, Woo CC, Leon M. Spatial coding of odorant features in the glomerular layer of the rat olfactory bulb. J Comp Neurol 393: 457–471, 1998. [DOI] [PubMed] [Google Scholar]
- Kaissling 1989.Kaissling KE, Hildebrand JG, Tumlinson JH. Pheromone receptor cells in the male moth Manduca sexta. Arch Insect Bioch 10: 273–279, 1989. [Google Scholar]
- Kalinová 2001.Kalinová B, Hoskovec M, Liblikas I, Unelius CR, Hansson BS. Detection of sex pheromone components in Manduca sexta (L.). Chem Senses 26: 1175–1186, 2001. [DOI] [PubMed] [Google Scholar]
- Kashiwadani 1999.Kashiwadani H, Sasaki YF, Uchida N, Mori K. Synchronized oscillatory discharges of mitral/tufted cells with different molecular receptive ranges in the rabbit olfactory bulb. J Neurophysiol 82: 1786–1792, 1999. [DOI] [PubMed] [Google Scholar]
- Lei 2002.Lei H, Christensen TA, Hildebrand JG. Local inhibition modulates odor-evoked synchronization of glomerulus-specific output neurons. Nat Neurosci 5: 557–565, 2002. [DOI] [PubMed] [Google Scholar]
- Linster 2005.Linster C, Sachse S, Galizia CG. Computational modeling suggests that response properties rather than spatial position determine connectivity between olfactory glomeruli. J Neurophysiol 93: 3410–3417, 2005. [DOI] [PubMed] [Google Scholar]
- Loughrin 1990.Loughrin JH, Hamilton-Kemp TR, Andersen RA, Hildebrand DF. Headspace compounds from flowers of Nicotiana tabacum and related species. J Agri Food Chem 38: 455–460, 1990. [Google Scholar]
- Malnic 1999.Malnic B, Hirono J, Sato T, Buck LB. Combinatorial receptor codes for odors. Cell 96: 713–723, 1999. [DOI] [PubMed] [Google Scholar]
- Matsumoto 1981.Matsumoto SG, Hildebrand JG. Olfactory mechanisms in the moth Manduca sexta: response characteristics and morphology of central neurons in the antennal lobes. P Roy Soc Lond B Biol Sci 213: 249–277, 1981. [Google Scholar]
- Mombaerts 2004.Mombaerts P Genes and ligands for odorant, vomeronasal and taste receptors. Nat Rev Neurosci 5: 263–278, 2004. [DOI] [PubMed] [Google Scholar]
- Mombaerts 1996.Mombaerts P, Wang F, Dulac C, Chao SK, Nemes A, Mendelsohn M, Edmondson J, Axel R. Visualizing an olfactory sensory map. Neuron 87: 675–686, 1996. [DOI] [PubMed] [Google Scholar]
- Nagayama 2004.Nagayama S, Takahashi YK, Yoshihara Y, Mori K. Mitral and tufted cells differ in the decoding manner of odor maps in the rat olfactory bulb. J Neurophysiol 91: 2532–2540, 2004. [DOI] [PubMed] [Google Scholar]
- Nickell 1996.Nickell WT, Shipley MT, Behbehani MM. Orthodromic synaptic activation of rat olfactory bulb mitral cells in isolated slices. Brain Res Bull 39: 57–62, 1996. [DOI] [PubMed] [Google Scholar]
- Olsen 2007.Olsen SR, Bhandawat V, Wilson RI. Excitatory interactions between olfactory processing channels in the drosophila antennal lobe. Neuron 54: 89–103, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen 2008.Olsen SR, Wilson RI. Lateral presynaptic inhibition mediates gain control in an olfactory circuit. Nature 452: 956–960, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raguso 2003.Raguso RA, Levin RA, Foose SE, Holmberg MW, McDade LA. Fragrance chemistry, nocturnal rhythms and pollination “syndromes” in Nicotiana. Phytochemistry 63: 265–284, 2003. [DOI] [PubMed] [Google Scholar]
- Reisenman 2004.Reisenman CE, Christensen TA, Francke W, Hildebrand JG. Enantioselectivity of projection neurons innervating identified olfactory glomeruli. J Neurosci 24: 2602–2611, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisenman 2005.Reisenman CE, Christensen TA, Hildebrand JG. Chemosensory selectivity of output neurons innervating an identified, sexually isomorphic olfactory glomerulus. J Neurosci 25: 8017–8026, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche King 2000.Roche King J, Christensen TA, Hildebrand JG. Response characteristics of an identified, sexually dimorphic olfactory glomerulus. J Neurosci 20: 2391–2399, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Root 2007.Root CM, Semmelhack JL, Wong AM, Flores J, Wang JW. 2007. Propagation of olfactory information in Drosophila. Proc Natl Acad Sci USA 104: 11826–11831, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rospars 2000.Rospars JP, Hildebrand JG. Sexually dimorphic and isomorphic glomeruli in the antennal lobes of the sphinx moth Manduca sexta. Chem Senses 25: 119–129, 2000. [DOI] [PubMed] [Google Scholar]
- Sachse 1999.Sachse S, Rappert A, Galizia CG. The spatial representation of chemical structures in the antennal lobe of honeybees: steps towards the olfactory code. Eur J Neurosci 11: 3970–3982, 1999. [DOI] [PubMed] [Google Scholar]
- Schoppa 2006.Schoppa NE Synchronization of olfactory bulb mitral cells by precisely timed inhibitory inputs. Neuron 49: 271–283, 2006. [DOI] [PubMed] [Google Scholar]
- Schoppa 2003.Schoppa N, Urban NN. Dendritic processing within olfactory bulb circuits. Trends Neurosci 26: 501–506, 2003. [DOI] [PubMed] [Google Scholar]
- Selchow 1998.Selchow K Processing of Plant-Associated Odors by a Subset of projection neurons in the antennal lobe of the Female Moth Manduca sexta (PhD dissertation). Tuscon, AZ: University of Arizona, 1998.
- Shang 2007.Shang Y, Claridge-Chang A, Sjulson L, Pypaert M, Miesenbock G. Excitatory local circuits and their implications for olfactory processing in the fly antennal lobe. Cell 128: 601–612, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd 1972.Shepherd G Synaptic organization of the mammalian olfactory bulb. Physiol Rev 52: 864–917, 1972. [DOI] [PubMed] [Google Scholar]
- Shields 2001.Shields VDC, Hildebrand JG. Responses of a population of antennal olfactory receptor cells in the female moth Manduca sexta to plant-associated volatile organic compounds. J Comp Physiol [A] 186: 1135–1151, 2001. [DOI] [PubMed] [Google Scholar]
- Silbering 2007.Silbering AF, Galizia CG. Processing of odor mixtures in the Drosophila antennal lobe reveals both global inhibition and glomerulus-specific interactions. J Neurosci 7: 11966–11977, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire 2003.Squire L, Roberts J, Spitzer N, Zigmond M, McConnell S, Bloom F. Fundamental Neuroscience (2nd ed.). San Diego, CA: Academic, 2003.
- Tolbert 1981.Tolbert LP, Hildebrand JG. Organization and synaptic ultrastructure of glomeruli in the antennal lobes of the moth Manduca sexta: a study using thin sections and freeze-fracture. Proc Roy Soc Lond B Biol Sci 213: 279–301, 1981. [Google Scholar]
- Vosshall 2000.Vosshall LB, Wong AM, Axel R. An olfactory sensory map in the fly brain. Cell 102: 147–159, 2000. [DOI] [PubMed] [Google Scholar]
- Vucinic 2006.Vucinic D, Cohen LB, Kosmidis EK. Interglomerular center-surround inhibition shapes odorant-evoked input to the mouse olfactory bulb in vivo. J Neurophysiol 95: 1881–1887, 2006. [DOI] [PubMed] [Google Scholar]
- Wang 2003.Wang JW, Wong AM, Flores J, Vosshall LB, Axel R. Two-photon calcium imaging reveals an odor-evoked map of activity in the fly brain. Cell 112: 271–282, 2003. [DOI] [PubMed] [Google Scholar]
- Wilson 2005.Wilson RI, Laurent G. Role of GABAergic inhibition in shaping odor-evoked spatiotemporal patterns in the Drosophila antennal lobe. J Neurosci 25: 9069–9079, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson 2004.Wilson RI, Turner GC, Laurent G. Transformation of olfactory representations in the Drosophila antennal lobe. Science 303: 366–370, 2004. [DOI] [PubMed] [Google Scholar]
- Xu 2000.Xu F, Greer CA, Shepherd G. Odor maps in the olfactory bulb. J Comp Neurol 422: 489–495, 2000. [DOI] [PubMed] [Google Scholar]
- Yokoi 1995.Yokoi M, Mori K, Nakanishi S. Refinement of odor molecule tuning by dendrodendritic synaptic inhibition in the olfactory bulb. Proc Natl Acad Sci USA 92: 3371–3375, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zar 1999.Zar JH Biostatistical Analysis. Upper Saddle River, NJ: Prentice-Hall, 1999.