Abstract
Serotonin can produce multiple, contradictory modulatory effects on strength of synaptic transmission in both vertebrate and invertebrate nerve circuits. In crayfish, serotonin (5-HT) can both facilitate and depress transmission to lateral giant escape command neurons; however, which effect is manifest during application, as well as the sign and duration of effects that may continue long after 5-HT washout, may depend on history of application as well as on concentration. We report that protein kinase A (PKA) signaling is essential to the production of facilitation but depression is mediated by non-cAMP/PKA signaling pathways. However, we unexpectedly found that PKA activity is essential for the decay of depression when serotonin is washed out. This, and evidence from the effects of a variety of serotonin application regimens, suggest that facilitatory and depressive states coexist and compete and that the decay of each is dependent on stimulation by the other. A computational model that incorporates these assumptions can account for and rationalize the varied effects of a wide range of serotonin application regimens.
INTRODUCTION
Serotonergic modulation can cause both facilitation and depression of synaptic transmission at the same synapse (Cai et al. 2002; Hori et al. 1996; Huang and Kandel 2007; Li and Zhuo 1998; Nishimura and Akasu 1989; Rygh et al. 2006; Shay et al. 2005). Such contradictory effects have, in particular, been noted in circuits concerned with defensive responses, such as those mediating pain, fear, and protective reflexes, in both vertebrates and invertebrates. Understanding the factors that determine whether facilitation or depression will be manifest should help us comprehend the functions of serotonin in these circuits, and the cellular signaling systems underlying the modulation are interesting as examples of how contradictory modulatory effects are integrated. Understanding the mechanisms underlying bidirectional modulation may also be of practical interest because manipulation of brain serotonin levels in humans is a dominant treatment for a number of psychiatric disorders.
In the circuitry mediating tail-flip escape behavior of the crayfish, serotonin can have either depressive or facilitatory effects on transmission to the lateral giant fibers (LGs), which are command neurons for one type of reflex tail-flip escape reaction. Whether facilitation or depression will be manifest depends on dose as well as on time course of concentration (Krasne and Edwards 2002; Teshiba et al. 2001). Schedule of application can also affect whether depression or facilitation of transmission continues, even hours after serotonin is washed out. Although not investigated here, serotonergic modulation of the LG reflex is of additional interest because reactions to serotonin, remarkably, depend on the social history of the individual crayfish; application regimens that cause facilitation in isolates or dominants cause depression in subordinates (Yeh et al. 1996, 1997).
The present work was undertaken with the aim of understanding the basis for the complex and seemingly arbitrary way that effects on synaptic transmission vary as a function of serotonin application regimen. Additional application regimens were examined in the hope of uncovering regularities not previously apparent, and characterization of intracellular signaling mechanisms mediating the facilitatory and depressive effects, which had been begun by others, was continued. Yeh and Edwards (Yeh et al. 1996, 1997) and Araki et al. (2005) provided evidence for the involvement of cAMP in the production of facilitation, but mediation of depressive effects had not been examined. We found that protein kinase A (PKA) was a crucial mediator of facilitation as might have been expected from the previous work; however, unexpectedly, it was also essential for the termination of depression when serotonin was washed out. This observation, and evidence from the effects of various doses and schedules of application, suggest that serotonergic facilitation and depression are mediated by different signaling pathways—they are not merely two ends of a single continuum; activity or activation within each pathway is inherently persistent after serotonin withdrawal; and activity of each pathway promotes the loss of the active state of the other pathway. We refer to the second and third observations together as reciprocal stimulation of decay. This principle is able to explain all of the complex dependencies on application regimen that have been observed, as was verified with a simple computational version of the hypothesis.
METHODS
Animals
Experiments were on Procambarus clarkii obtained from Atchalafaya Biological Supply and kept isolated in 14 × 20-cm polyethylene dishes for ≥2 wk before experimentation. Juveniles 2–3 cm rostrum-to-telson or adults 8–9 cm long were used as specified in the following text.
Experimental procedures
Physiological saline was 4 mM HEPES-buffered crayfish Ringer, pH 7.2. Animals were cooled gradually to 5°C, the cephalothorax including the rostral part of the nervous system cut away and discarded, the abdomen pinned on silicone elastomer (Sylgard) in crayfish saline, and the abdominal nerve cord exposed dorsally (as in Krasne 1969). Thereafter experiments were at ca 20°C with well-aerated superfusate continually flowed over the cord at 30 ml/ min (also done in the arterially perfused preparations described in the following text).
Recording electrodes (R, Fig. 1A ) filled with 3 M KCl with resistance of about 10 MΩ were placed in the initial segment of LGs, in sixth abdominal ganglion for superfused and third or fourth ganglion for perfused-artery animals. Pairs of Pt wire stimulating electrodes were placed on roots 2–4 of sixth ganglion or root 2 of third or fourth ganglia (S, Fig. 1A). A dorsal cord electrode on the 2–3 connective was used to monitor action potentials of relatively large axons. Test excitatory postsynaptic potentials (EPSPs) were evoked every 2 min by a 0.2-ms voltage pulse at stimulating electrodes, proximal electrode negative. Exposure to 5-HT was begun when the amplitude of evoked EPSPs, which often drifted up or down following dissection and penetration, stabilized (usually 30–60 min). Electrical activity was stored digitally for later analysis.
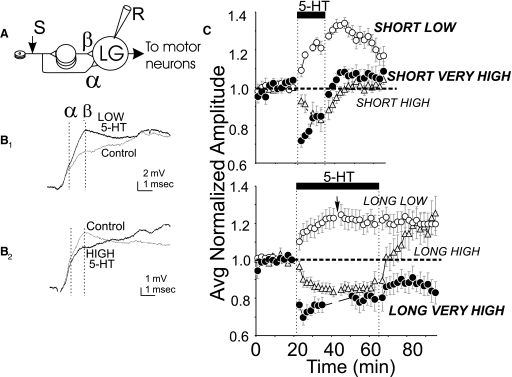
FIG. 1.
Experimental arrangement and effects of serotonin (5-HT) application regimen. A: experimental arrangement and lateral giant fiber (LG) circuit. LGs receive input over both monosynaptic (α) and disynaptic (β) pathways. B: compound excitatory postsynaptic potentials (EPSPs) recorded at base of LG dendrites in last abdominal ganglion in response to shocks to roots 2–4; the α and β components of the EPSP are marked. B1: facilitation produced by low 5-HT. 5-HT trace after ∼10 min of 5-HT (end of a short exposure). B2: depression produced by high 5-HT. 5-HT trace after ∼40 min of 5-HT (end of a long exposure). C: average time courses of normalized EPSP amplitude during 5-HT exposure and wash for various application regimens; normalized to pre-5-HT amplitude. Application regimen properties in caps defined in text. Bold caps indicate data reported here; short high, long high, and long low data are from Teshiba et al. (2001). The arrow indicates omission of a few time points (see methods).
Drug preparation
Pharmacological agents used were 5-HT (creatinine sulfate, Sigma), 8-Bromo cAMP (Sigma), H-89 (LC Labs), SQ22536 (Calbiochem), and chelerythrine (LC Labs).
Drugs were generally stored as specified by suppliers. However, in our hands 5-HT, once opened, often seems to become ineffective after much less than its supposed shelf life. Therefore once opened, we stored it with desiccant at about −5°C and always used it within 1 mo of opening.
All drugs were dissolved just prior to use. 5-HT, the membrane permeant, phosphodiesterase-resistant cAMP analogue, 8-Bromo cAMP, and the adenylate cyclase inhibitor, SQ22539, were all dissolved directly in HEPES Ringer. SQ22539 was used at 10−4 M. H-89 was dissolved in DMSO rather than alcohol because the concentrations of alcohol required had effects of their own. Working H-89 solutions were 10 μM with a residual DMSO content of 0.0125%; at this concentration, H-89 is said by the supplier to be relatively specific for PKA. Solutions were mixed by initially injecting water very slowly into 8 × 10−2 M H-89 in DMSO (very slow injection was essential to avoid precipitation of the H-89) and adding necessary salt solutions only after water had been introduced. Controls had the same concentration of DMSO in perfusate as did H-89 preparations.
The preferential PKC inhibitor, Chelerythrine (LC Labs), used at 8 × 10−6 M, was dissolved in the same way as H-89.
Drug application
Where possible, we applied drugs via superfusion in juveniles. 5-HT appears to pass through the cord sheath easily in such small animals although not in large. However, we found that H-89 and chelerytrine seemed to be without effect when superfused, so in experiments using them, all pharmacological agents were applied by arterial perfusion. This necessitated the use of adult animals to make cannulation of the ventral artery feasible.
As discussed throughout this paper, the qualitative effects of 5-HT were dependent on concentration. Teshiba et al. (2001) found that lower concentrations of 5-HT generally facilitated transmission to the LGs, whereas higher ones depressed it although absolute levels differed between perfused and superfused preparations. Comparable facilitatory effects were produced by 10−8 M 5-HT perfused and 5 × 10−6 M superfused, both of which were denoted “low” 5-HT. Comparable inhibitory effects were produced by 5 × 10−5 M perfused and 10−4 M superfused; these were denoted “high.” We adopt the same conventions here. In addition, we here did some perfusion experiments using a concentration of 10-3 M, which we refer to as “very high.”
Teshiba et al. (2001) also used several standard application schedules that are adopted here. 5-HT applications lasting 10–12 min, referred to as “short,” were usually just long enough to get a near maximal change (up or down) in EPSP amplitude. Applications lasting 30–45 min were referred to as “long.” Drug applications that achieved final concentration within a few minutes (or even faster in the case of perfusion) were described as “fast” by Teshiba et al. (2001). Here fast application is assumed unless otherwise specified. In some previous experiments on superfused animals (Teshiba et al. 2001; Yeh et al. 1996, 1997), 5-HT was dripped at a rate such that that full concentration was reached in ∼20–30 min; Teshiba et al. (2001) referred to such application as “slow.” Effects of slow and fast application of a comparable concentration can be qualitatively different from each other.
In some of the present experiments using H-89, we perfused a gradually increasing concentration of serotonin as follows: starting from zero we increased concentration of 5-HT at 1.25 × 10−7 M per min for 8 min, at 2.5 × 10−7 M for 10 min, and at 2.5 × 10−6 M for 8 min with a final concentration of 5 × 10−5 M (= high).
Superfusion of inexpensive drugs was at 30 ml/min from a large reservoir into a preparation chamber ∼10 ml in volume; superfusate was recirculated back to the reservoir. When we washed drugs out, the preparation chamber was rapidly half-emptied and refilled twice at the start of wash to lower bath concentration of drug rapidly; 700 ml was then superfused without recirculation. For expensive drugs, animals were pinned in a small chamber in which 10 ml of superfusate were continually re-circulated by using an air jet to push fluid through a channel that connected wells at each end of the preparation chamber-proper (design after Robert Pitman). Drugs were introduced by adding to one well the desired amount of drug in 1/10 the volume of the system and removing the same volume from the other well. Washing out drug in this small system sometimes caused artifactual changes of EPSP amplitude; so washout data are not reported from this sort of experiment.
Perfusions were via a polyethylene cannula (PE 10 tubing drawn to ∼0.4 mm diam at its end) inserted into the descending artery, which was maintained intact during the dissection; the cannula was threaded into the abdominal portion of the ventral artery where it was tied in place with a fine thread. Perfusate was flowed continually at a rate of ∼0.5–1 ml/min. In such experiments, we recorded from middle abdominal ganglia because perfused drugs were less likely to be lost by leakage from small cut arterial branches and bubbles less likely to form between the cannula tip and site of recording. At the end of each experiment, we perfused methylene blue; if it didn't reach the recorded ganglion readily, we discarded the experiment.
Data analysis
The EPSPs evoked by stimulation of ganglionic roots generally begin with an early depolarizing peak (α) caused by arrival of monosynaptic input and a slightly later peak (β) produced by disynaptic input (Fig. 1, A and B). Subsequent portions of the response reflect a mixture of polysynaptic excitatory and inhibitory input that follows the stimulus (Vu et al. 1997). In analyzing our data, we measured membrane potential at the times where alpha and beta peaks were located at the beginning of the experiment even when these peaks subsequently shifted somewhat. The α and β peaks generally changed in the same direction in response to 5-HT, but β changes, which were more robust and reliable, were used for all analyses.
For graphing, EPSP amplitudes were normalized to their average value over the last 5–10 trials prior to drug application. Although we attempted to set initial EPSP levels so that LG spikes, which interfered with measurement of EPSP amplitudes, would be unlikely, sometimes serotonin increased EPSPs enough to cause spikes. If spikes were only occasional, we entered into analyses an EPSP amplitude equal to the largest EPSP amplitude of the experiment that was not contaminated by a spike. If spikes were frequent, we moved our beta measurement time for the entire experiment to the latest time uncontaminated by LG spikes. Mean EPSP amplitudes were taken over three trials centered at the time of interest. Statistical tests were always two-tailed. Wilcoxon rather than t-test were used in a few cases where distributions appeared very nonnormal. Error markers on graphs are SE.
The duration of short and long varied somewhat across experiments from 10 to 12 min and 30 to 45 min, respectively. To superimpose the results of experiments for graphing averages, we lined up the initial and final test trials of each period and removed excess trials from the middle of the interval producing breaks in graph (see e.g., the arrow in Fig. 1C, long low).
Model parameters
Parameters (defined in the appendix) used to show that the computational model discussed could account for the major qualitative features of the data were: αF = 0.017, αF,H-89 = 0, αD = 0.05, βF = 0.04, βD = 0.115 σF = 0.4, σD = 0.75, θF = 0.2, θD = 0.5, τF = 0.05, τD = 0.05, SLOW = 0.2, SHIGH = 0.61, SVERY HIGH = 1.0, Emax = 1.3, Emin = 0.7 (which corresponded to serotonergic drives, SF and SD, respectively, of: low: 0.49 and 0.002. high: 0.99 and 0.90. very high: 0.99 and 0.99). For computation, differential equations were approximated by difference equations. Five iterations were taken to correspond to 1 min.
RESULTS
Dependence on application regimen
In social isolates EPSPs are generally increased in amplitude (“facilitated”) by low levels of 5-HT starting at ∼10−8 M (Fig. 1B1), but they are attenuated (“depressed”) by concentrations above ∼10−4 M (Fig. 1B2). Teshiba et al. (2001) studied standard concentrations (defined in methods) referred to as low and high. These were applied either just long enough to produce approximately maximal effects (∼10 min), which are referred to as short exposure, or for periods of ∼40 min, which are referred to as long exposure. With the exception of one application regimen to be discussed later, the sign of serotonin's effect simply depended on concentration. However, washout behavior seemed more complex. Long low exposures caused facilitation that persisted throughout the wash. The depression caused by short high exposures recovered to control levels within a 5- to 10-min wash. Long high exposures, which caused depression during the 5-HT application, led to the development of persisting facilitation when the 5-HT was washed out. These effects are illustrated in Fig. 1C.
In the hope of trying to understand the basis for these seemingly arbitrary variations in washout behavior, we here added to the list of application regimens short low exposures as well as both short and long applications of a higher concentration of 5-HT than used by Teshiba et al. (2001) (very high 5-HT, defined in methods). Thus three levels of 5-HT (low, high, and very high) at each of two durations (short and long) have now been examined.
Average curves for the newly examined regimens are included in Fig. 1C, and their effects are further detailed in Fig. 2.
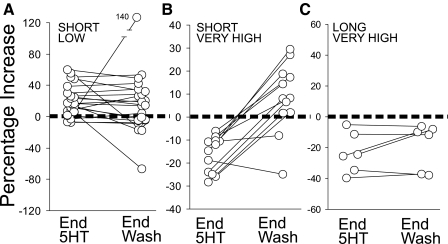
FIG. 2.
Effects of new application regimens: short low and short and long, very high 5-HT. A–C: percentage increase of EPSP amplitude relative to pre-5-HT amplitude at the end of 5-HT exposure and at the end of 30 min wash for individual preparations. One outlying point in A extends beyond the top of the y axis.
The facilitation produced by short low 5-HT exposure (P < 0.001, t13 = 21.2 for difference between pre-5-HT and end 5-HT) persisted during 30 min of wash virtually unabated relative to its level at the end of 5-HT exposure (Figs. 1C and 2A) as had the facilitation due to long low 5-HT studied previously (Fig. 1C). After 30 min of wash, EPSP amplitude was on average 97% of what it had been at the end of 5-HT exposure, and it was still significantly above its pre-5-HT value (P < 0.001, Wilcoxin test, n = 21).
The effects of short very high 5-HT were roughly similar to those produced by the short high 5-HT exposures utilized by Teshiba et al. (2001). Depression, of faster onset in the case of very high than of high 5-HT, developed during the 5-HT (P < 0.001, t10 = 6.7 for difference between pre-5-HT and end 5-HT), and recovery from depression occurred within ∼10 min of wash (Figs. 1C and 2B; P < 0.001, t11 = 6.06 for end 5-HT to end wash). There was a tendency for some facilitation to develop in the later stages of wash, but this was not statistically significant (t11 = 1.9; P > 0.08).
The depression caused by long very high 5-HT exposures (P < 0.02, t6 = 3.67 for difference between pre-5-HT and end 5-HT), in contrast to that caused by short high or by long high exposures, failed to recover during wash (Figs. 1C and 2C). There was no systematic increase of EPSP amplitudes during wash (P > 0.2, t5 = 1.4 for end 5-HT to end wash), and at the end of wash, EPSPs of all preparations were still decreased relative to their preserotonin amplitudes (P < 0.05, t5 = 3.05).
The salient qualitative features of these findings are summarized in Table 1. The new findings do not by themselves make apparent any simple rule that accounts for the various kinds of washout behavior shown in Fig. 1C and Table 1. However, when they are taken together with findings described next on intracellular mediation of these modulatory effects, a plausible hypothesis emerges. Moreover, this hypothesis is of interest in its own right.
TABLE 1.
Effects of 5-HTexposure on EPSP amplitude
| 5-HT schedule | End 5-HT | End Wash |
|---|---|---|
| short low† | Facilitated | Facilitated |
| long low‡ | Facilitated | Facilitated |
| short high† | Depressed | Recovered |
| long high‡ | Depressed | Facilitated |
| short v high† | Depressed | Recovered |
| long v high† | Depressed | Depressed |
| slow high‡ | Facilitated | Facilitated |
| H-89 slow high† | Depressed* | — |
| H-89 long high† | Depressed | Depressed |
| H-89 short v high† | Depressed | Depressed |
Table gives excitatory postsynaptic potential (EPSP) amplitudes relative to pre-5-HT level.
A non-significant trend;
present data;
Intracellular signaling
Previous work has established that cAMP production is necessary and sufficient for the production of facilitation in the present system (Araki et al. 2005; Yeh et al. 1996, 1997). However, mediation of depression has not been studied.
DOES cAMP ALSO MEDIATE DEPRESSION?
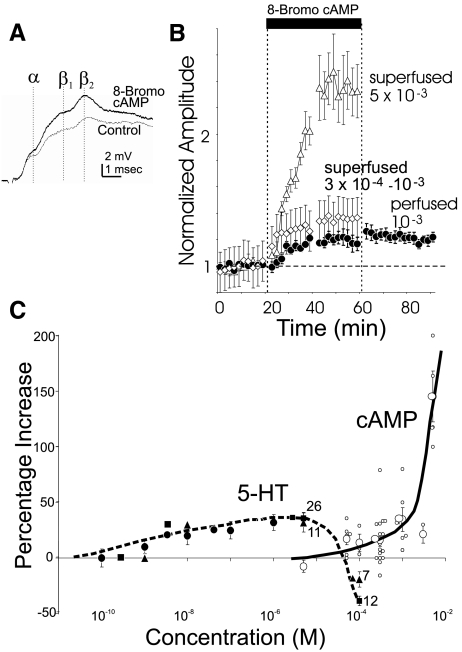
Since low levels of 5-HT cause facilitation, whereas high ones cause depression, it seemed possible that this same relationship might hold for the cAMP signaling that mediates facilitation. We thus explored the effect of a range of concentrations of the cell-permeant, phosphodiesterase-resistant cAMP analogue 8-Bromo cAMP. We tested concentrations ≤5 × 10−3 M (superfused) to see whether sufficiently high concentrations might cause depression. Only facilitation was seen (Fig. 3). If cAMP were common to the facilitation and depression pathways, then it would be expected that concentrations of cAMP greater than those that cause the maximum amount of facilitation producible by 5-HT should recruit depressive mechanisms. The maximal average facilitation producible by 5-HT was about a 25% increase in EPSP amplitude (P < 0.001, t38 = 8.02 for the difference between pre-5-HT end 5 × 10−6 M 5-HT exposure), which occurred at 5 × 10−6 M 5-HT; at higher concentrations of 5-HT, less facilitation or depression occurred. A concentration of 10−3 8-Bromo cAMP was needed to produce the amount of facilitation (∼25%) that was maximal for 5-HT; however, a five times higher concentration of 8-Bromo cAMP, rather than producing less facilitation or depression, produced a 150% increase in EPSP amplitude. This was much higher than ever seen with 5-HT (P < 0.001, t42 = 5.36 for the difference between average percent increase at the end of exposure to 5 × 10−6 M 5-HT and 5 × 10−3 8-Bromo cAMP). Thus high levels of 5-HT do not cause depression by generating high levels of cAMP.
FIG. 3.
cAMP causes facilitation but not depression. A: facilitation after ∼40 min of superfused 10−3 M 8-Bromo cAMP. β1 and β2 are probably due to roughly synchronized 1st and 2nd firings of the interneurons recruited by the ganglionic root stimulus pulse. B: facilitation is produced by 8-Bromo cAMP at several concentrations. Note that facilitation does not abate during washout (measured in perfusion experiments). C: dose-response curve for various concentrations of superfused 8-Bromo cAMP (individual experiments small circles; averages large open circles with SEs indicated) and fast 5-HT (filled squares). Also shown are points from Teshiba et al. (2001) (filled triangles) and from Yeh et al. (1977) (filled circles), who used gradual applications of 5-HT in a concentration range where slow and fast application would be expected to have similar effects. N′s for higher-dose 5-HT experiments are indicated. Whereas 5-HT produces a maximum facilitation of ∼40% at 5 × 10−6 M (low) and becomes inhibitory at higher concentrations, cAMP can produce much greater facilitation presumably because it does not recruit competing depression.
It is additionally of interest that, as seen in Fig. 3B, the facilitation produced by 8-Bromo cAMP was at about the same level at the end of 30-min wash as at the end of cAMP exposure in arterial perfusion experiments (see methods) where the cAMP was likely to have been effectively removed. This suggests that the persistence of facilitation in wash is due to the persistence of an agent downstream of cAMP production.
DOES PKA MEDIATE SEROTONERGIC FACILITATION?
Serotonergic facilitation in other systems has commonly been found to be mediated via cAMP/PKA signaling or pathways that activate PKC, and in some cases, cAMP-mediated serotonergic facilitation appears to be caused by direct (not PKA-mediated) effects of cAMP (e.g., Beaumont and Zucker 2000). Although it is known (as discussed above) that in the present system cAMP mediates facilitation, it is not known whether PKA is involved. We thus tested the effects of the preferential PKA blocker, H-89.
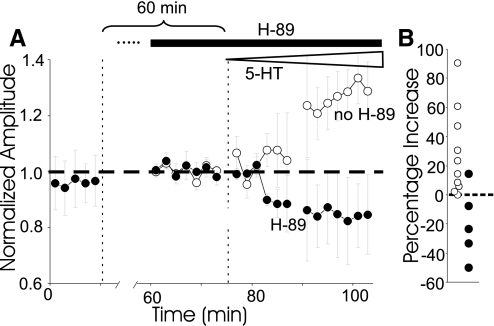
H-89 perfusion was begun about an hour before 5-HT was introduced. The H-89 produced no effect of its own (Fig. 4 A). The effect of H-89 on serotonergic facilitation was tested with a gradually increasing concentration of serotonin, used in both H-89 and control experiments, that would necessarily cross the facilitatory 5-HT threshold before the depressive one. As shown by Teshiba et al. (2001) and discussed in the final section of this paper, gradual application of 5-HT usually produces facilitation even when concentrations are reached that would produce depression if applied rapidly. Facilitation developed in control (P < 0.02, t9 = 3.03 and Wilcoxin P < .005 for pre-5-HT vs. end 5-HT) but not H-89 animals (Fig. 4, A and B; P < 0.01, t12 = 3.33 for the difference between H-89 and controls). In fact in the H-89 animals, 5-HT appeared to cause some depression, perhaps due to the loss of competing facilitation, although this effect was not quite statistically significant. In any case, PKA seems to be essential for production of serotonergic facilitation.
FIG. 4.
Protein kinase A (PKA) inhibitor prevents facilitation. A: effect of H-89 on facilitation produced by slowly increasing concentrations of 5-HT. Note that H-89 alone has no apparent effect. B: individual measurements at the end of the 5-HT increase without and with (preexposed) H-89.
DO cAMP OR PKA SIGNALING PLAY ANY ROLE IN DEPRESSION?
In several systems where serotonergic depression has been studied (referenced in discussion), the depression appears to be mediated by 5-HT receptors that are negatively coupled to adenylate cyclase. This suggests that cAMP/PKA-dependent facilitation operates at rest and is partly reversed by depression-causing concentrations of 5-HT. However, it is unlikely that this is the case here because inhibition of PKA with H-89 did not cause any decrease of EPSP amplitude (Fig. 4A). We also found that the adenylate cyclase inhibitor SQ22536 at a concentration that reduced development of serotonergic facilitation (as in Araki et al. 2005) did not by itself reduce EPSP amplitudes (no decrease in any of 12 experiments; not illustrated).
Although cAMP did not seem to be able to cause depression, it remained possible that PKA nevertheless participates in producing depression in combination with other signaling agents. We thus examined the effect of H-89 on the depression caused by long high 5-HT exposures. Perfusion of H-89 was begun about an hour before introducing 5-HT. Depression still occurred (P < 0.01, t11 = 4.02 for difference between pre-5-HT and end 5-HT) and was, if anything, increased rather than decreased (Fig. 5A1; t43 = 2.57, P < 0.02 for difference between H-89 and control at the end of 5-HT exposure) presumably because H-89 prevented facilitatory effects that normally interfere with the mediation or expression of depression. Thus PKA action is apparently neither necessary nor sufficient for the production of depression.
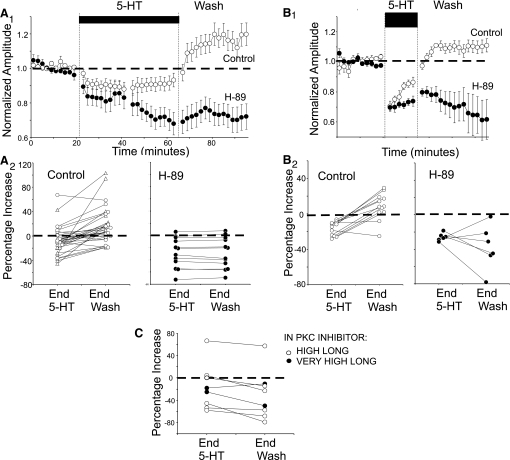
FIG. 5.
PKA and protein kinase C (PKC) inhibitors prevent decay of depression in wash. A: effect of H-89 on high long 5-HT exposures. B: effect of H-89 on very high short 5-HT exposures. A1 and B1 show time courses of development and wash. A2 and B2 compare recovery during washout of 5-HT for individual preparations under control conditions and preparations continually exposed to H-89. C: effect of long exposures to 5-HT in chelerythrine for individual preparations at end of 5-HT exposure and end of wash.
PKA-INVOLVEMENT IN RECOVERY FROM DEPRESSION.
Although PKA appears not to be involved in the production of depression, it does promote recovery from depression. Depression caused by high 5-HT, normally reverses during wash (Teshiba et al. 2001). However, it fails to do so when H-89 sufficient to block production of facilitation is present. This is apparent in the time-course plot of Fig. 5A1, which shows the time course of washout of 5-HT in H-89. Figure 5A2 compares washout in individual experiments with and without (“control”) H-89 present. The control graph also includes results from Teshiba et al. (2001), plotted as open triangles. In control experiments, EPSPs rose on average 24% (P < 0.001, t31 = 5.2) during wash, and over three quarters of the 32 preparations rose by >10%. When H-89 was present, there was virtually no average increase, none of the 12 preparation rose by even 5%, and there was no significant overall change (P > 0.9, t11 = 0.007).
To further evaluate the generality of the persistence of depression during wash when PKA is blocked, we repeated these experiments using short 5-HT exposures, but we used a very high concentration to try to ensure robust depression despite the short exposure. As shown in Fig. 5B, recovery occurred in control experiments but not when H-89 was present throughout. In the H-89 experiments, EPSPs at the end of wash remained depressed relative to both their pre-5-HT levels (P < 0.02, t5 = 3.5, although recovery did occur in one of 6 experiments) and the postwash levels of controls (P < 0.001, t16 = 4.79).
Thus PKA is not only necessary for the production of serotonergic facilitation but also for recovery from depression. We will return below to this finding, which we believe provides the key to understanding the complex effects of application regimen on sign and duration of serotonin's effect on transmission to the LGs.
EFFECTS OF PKC AND PKA INHIBITION ARE SIMILAR.
Given that PKC mediates production of long-term depression of synapses on cerebellar Purkinje cells (Linden and Connor 1991, 1995), we tested the effect of the PKC inhibitor, chelerythrine, in eight experiments using either high or very high 5-HT. Excluding one outlier in which 5-HT produced strong facilitation instead of depression, 5-HT caused depression despite the chelerythrine (Fig. 5C; P < 0.01, t6 = 4.19 for difference between pre-5-HT and end 5-HT, outlier excluded); the decrease of ∼28% (16% with outlier) should be compared with ∼20% for controls. There was no sign of recovery from depression in any of the chelerythrine experiments (there was actually a small but statistically significant increase in depression during wash; P < 0.05, t6 = 2.84). Also, whereas in the absence of protein kinase inhibition, facilitation develops during wash after long high 5-HT (Fig. 5A), there was no sign of this effect in any of the six long high experiments in which chelerythrine was present (Fig. 5C). We, therefore think it unlikely that PKC is part of the signaling pathway mediating serotonergic depression in this system. In fact, inhibition of PKC seems to affect both facilitation and recovery from depression in the same way as does inhibition of PKA though this requires confirmation.
Reciprocal stimulation of decay
The preceding finding that blocking the catalytic activity of PKA, in addition to preventing development of serotonin-induced facilitation, prevents termination of serotonin-induced depression during 5-HT washout, suggests the idea that intracellular signals that cause facilitation might engender decay of depression. One might wonder whether, conversely, signals causing depression would cause termination of facilitation. We refer to these possible paired relationships as reciprocal stimulation of decay. The findings reported in the preceding text support the view that facilitation and depression are in fact mediated by independent signaling pathways whose activity can co-exist. Manifest facilitation and depression are presumably expressions of the relative balance of underlying facilitatory and depressive states. If reciprocal stimulation of decay held, then during washout of serotonin, facilitation and depression would each decay under stimulation by the other until one was fully extinguished; whichever remained at that point would then persist without further decay; this dynamic can account for all of the forms of washout behavior summarized in Fig. 1C and Table 1. Figure 6 shows how different patterns can arise and recapitulates those data displaying each pattern.
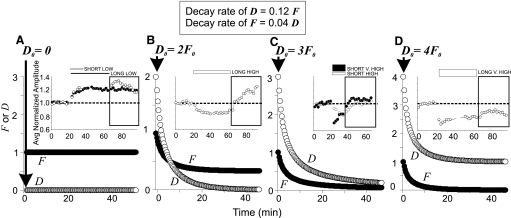
FIG. 6.
Washout behavior is consistent with Reciprocal Stimulation of Decay rule. Explanation in text. Boxed formulas (top center) were used to calculate moment-to-moment exponential decay rates of depression (D) and facilitation (F). A–D: decay of facilitation and depression for a range of starting conditions, indicated at the top of each ordinate. Insets: data that conform to the depression and facilitation decay patterns of its panel; the washout period of each experiment is enclosed in a bold-lined box.
In Fig. 6, we have calculated levels of facilitation (F) and depression (D) during wash for various starting conditions under the assumption that variables F and D each undergo negative exponential decay at a rate that is proportional, from moment to moment, to the level of the other variable according to the boxed rule at the top of the figure. The different patterns of washout we have seen can then be interpreted as being due to different amounts of depression relative to facilitation at the start of wash.
Figure 6A shows the prediction for low 5-HT. This concentration of serotonin seems to cause no depression (D); therefore according to the preceding rule, facilitation (F) would not be at all stimulated to decay, and manifest facilitation produced by low 5-HT should always persist in wash. As we saw above (Fig. 1, Table 1; recapitulated in the inset), facilitation produced by both short and long exposures to low 5-HT do in fact persist throughout our 30-min washout period.
High 5-HT produces manifest depression (Figs. 1 and; Table 1). Hence, at the start of wash, depression is presumably greater than facilitation. If the level of depression at the start of wash is not too much higher than that of facilitation and if the intrinsic rate of decay of depression is greater than that of facilitation (as reflected in the constants of the boxed formulas), then it would be expected that during wash the level of depression would fall below that of facilitation and would fully extinguish while facilitation was still elevated; once depression has extinguished, there is nothing to stimulate further decay of facilitation. This could explain why facilitation becomes manifest and persists during wash following long high 5-HT exposures (recapitulated in inset to Fig. 6B).
If the level of depression at the start of wash is somewhat higher relative to facilitation than is that in Fig. 6B, depression and facilitation will extinguish approximately in tandem as illustrated in Fig. 6C. In that case, depression would abate during wash, as occurs after short exposures to both high and very high 5-HT (recapitulated in inset to Fig. 6C). It should be noted that Fig. 6, B and C, taken together imply that depression is less following a long exposure to high 5-HT than following a short one because facilitation develops in wash after the long exposure but not the short; an explanation for this nonintuitive conclusion is provided in the next section.
Finally, if the level of depression relative to facilitation at the start of wash were sufficiently great, facilitation would decay to zero before depression were does, even if facilitation's intrinsic rate of decay is lower than that of depression. This is illustrated in Fig. 6D. In this case, depression would persist throughout wash, as happens after long very high 5-HT exposures (recapitulated in inset to Fig. 6D).
Further evaluation of reciprocal stimulation of decay
In addition to producing a range of washout behaviors, different application regimens also have some counterintuitive differential effects during 5-HT exposure. One of these is a contrast between the effect of high 5-HT levels reached gradually versus suddenly: Whereas sudden onset of high 5-HT causes manifest depression, the same concentration reached gradually (or in the stepwise fashion used in the experiment of Fig. 3) produces manifest facilitation (Teshiba et al. 2001; Fig. 7 D, left; Table 1). Also difficult to understand, at least in relation to the reciprocal stimulation of decay hypothesis, is the inference from Fig. 6, B and C, discussed in the preceding text, that depression (D) appears to be greater after short exposures to high 5-HT than after long ones.
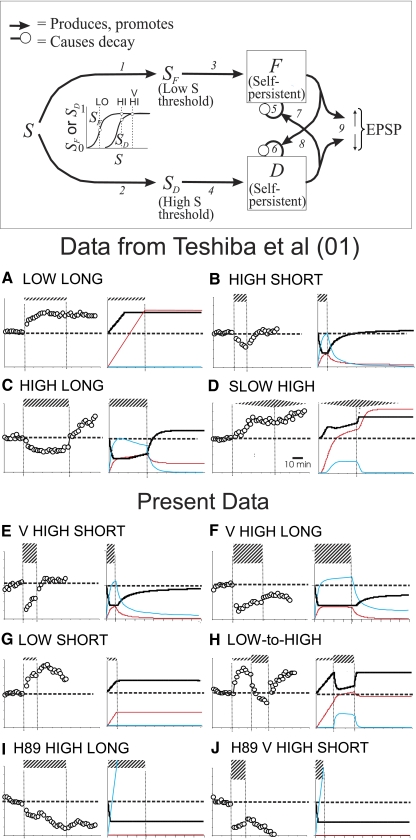
FIG. 7.
Comparison of model predictions with experimental findings. Inset: proposed conceptual model incorporating Reciprocal Stimulation of Decay. Explanation in text. Numbers refer to equations of computational implementation of model given in appendix. In each lettered frame, average experimental results are shown on the left and predictions of model on right. Hatched bars indicate 5-HT exposure; width of bar portrays concentration. In computational graphs, bold solid lines are average normalized EPSP amplitude, and red lines graph F and while blue lines graph D. A–D are as reported by Teshiba et al. (2001). E–J: for conditions studied here. A single set of parameter values, given in methods, was used for all simulations. Five iterations of difference equations given in appendix were taken to correspond to 1 min. For simulations, short and long were 10 and 40 min, respectively. For low, high, and very high S was taken to be 0.2, 0.61, and 1.0, respectively. Slow was modeled by negative exponential rises and falls of serotonin level S with a time constant of 0.024 (time steps of 0.2 min) (see appendix).
One can fairly readily see how the first of these counterintuitive features of the data might follow from the reciprocal stimulation of decay hypothesis. When serotonin is present, facilitation and depression presumably each grow at rates determined by 5-HT concentration, but they might be expected to concurrently decay according to the rules of the reciprocal stimulation of decay hypothesis. If so, net increases in facilitation or depression would then occur whenever the growth rate of either exceeded its rate of decay. During gradual increase of 5-HT concentration, facilitation, which because of its lower threshold would develop first, might elevate the rate of decay of depression sufficiently that depression could never become greater than facilitation, with the result that only facilitation would be expressed.
To move beyond such verbal arguments and evaluate the overall explanatory power of the reciprocal stimulation of decay hypothesis, we added to the assumption of reciprocal stimulation of decay several additional assumptions (detailed in the appendix) that would allow us to make predictions about development of facilitation and depression during 5-HT exposure as well as their decline during washout. 1) We assumed that growth of depression and facilitation are caused by serotonin-derived facilitatory and depressive “drives” SF and SD, each of which is a sigmoid function of log serotonin concentration S (see graph in the box at the top of Fig. 7). These functions were assumed to be such that in low 5-HT SF is ∼50% maximal and SD nil; in high 5-HT, SF is maximal, and SD ∼90% maximal, and in very high 5-HT, SF and SD are both maximal. 2) We assumed that facilitation increases EPSP amplitude while depression decreases it; however, we assumed that the maximum amounts of elevation and depression are limited so that even very high levels of facilitation or depression can only produce limited increases or decreases of EPSP amplitude.
We then asked whether we could find a single set of growth and decay rates that would give correct predictions for all of the kinds of experiments performed here or reported previously, including the counterintuitive features of the data pointed out in the preceding text. The goal was not to fit the data exactly but to see if the model could account for the main qualitative features summarized in Table 1. We found that if, as in Fig. 6, the decay rate for depression is about three times that for facilitation and if this is also true for growth rates in serotonin (exact parameter values are given in methods), all of the main features of existing data can be accounted for including the problematical ones.
In particular, the model accounts for augmentation of EPSPs in low 5-HT (Fig. 7, A and G) and diminution for rapid application of higher concentrations (Fig. 7, B, C, E, F, I, and J). It accounts for the persistence of manifest facilitation in wash after both short and long applications of low 5-HT (Fig. 7, A and G). It accounts for persistent depression of EPSPs in wash after long very high (Fig. 7F) but not after short very high (Fig. 7E) exposures. It also accounts for the effects of a shift to high serotonin after a period in low serotonin (Fig. 7H; not detailed in the preceding text), which was done to critically test and rule out Teshiba et al.'s (2001) hypothesis that facilitation and depression inhibit each other's formation. Had this assumption been correct, a shift from low to high should have produced little or no reduction of EPSP amplitude; in fact it produced a substantial drop. It accounts for the effects of H-89 on direction and longevity of 5-HT effects under the assumption that H-89's sole effect is to lower the growth rate for facilitation to zero (Fig. 7, I and J).
It also accounts for continuing facilitation during gradual application of high 5-HT (Fig. 7D) even when concentrations are reached that would cause depression if applied suddenly (B and C), and it does so for exactly the reasons suggested in the preceding text.
Finally, it accounts for the nonintuitive conclusion that depression is less after long than short exposures to high 5-HT (with the consequence that manifest facilitation develops during washout of high levels of 5-HT following long but not short exposures) (Fig. 7, B and C, respectively). Examination of the right theoretical side of Fig. 7B shows that at the end of short high exposures depression is enough greater than facilitation so that depression and facilitation decay roughly in tandem, which leads to recovery from manifest depression during a short period of 5-HT washout (as in Fig. 6C). However, Fig. 7C shows that during the latter parts of long exposures to high 5-HT the level of depression (blue curve) falls while that of facilitation (red curve) rises. This happens because during long exposures facilitation reaches a sufficiently high level to cause the decay rate of depression to exceed its growth rate. As the level of the depressive state thus declines, decay of facilitation slows leading to the progressive loss of depression and increase of facilitation. Thus by the end of long exposures, the level of depression relative to facilitation is low enough to allow the sort of crossover during wash that was illustrated in the preceding text in Fig. 6B and that results in development of manifest facilitation.
DISCUSSION
Reciprocal stimulation of decay hypothesis
Low levels of serotonin increase (facilitate), whereas high levels decrease (depress) efficacy of synaptic transmission to crayfish LGs. The findings reported here support the view that serotonergic facilitation and depression are mediated by separate signaling pathways that interact in a novel way. The activity or each appears to be inherently self-sustaining after 5-HT is washed out, but each stimulates the decay of the other. We refer to this as reciprocal stimulation of decay.
EVIDENCE IN CRAYFISH.
The evidence for this hypothesis is partially pharmacological and partly inferred from the diverse outcomes of a variety of serotonin application regimens, which the hypothesis rationalizes. Particularly suggestive was the finding that inhibition of PKA, which prevented the development of facilitation, also prevented the decay of depression.
Crucial to the hypothesis is the belief that facilitation and depression are independent states that can coexist and that their relative balance determines whether EPSPs will be increased or decreased. Their independence was first suggested by the observation that whereas low 5-HT causes facilitation that persists even after 5-HT is washed out, prolonged exposures to high 5-HT, although causing manifest depression, nevertheless gives way to facilitation during wash, suggesting that facilitation was present all along but was overshadowed by depression while the high 5-HT was present. Our evidence that production of facilitation and depression seem to depend on different intracellular signaling agents supports this conclusion.
The independence of facilitation and depression is also supported by work on the nature of the functional changes that underlie these states. Transmission to the LGs from primary afferents and interneurons is via rectifying (voltage-dependent) synapses (Edwards et al. 1991). Facilitation produced by low and by slow high applications of 5-HT appears to be in part due to increased junctional conductance of the synapses providing ininput to the LGs and in part to decreased conductance of distal and, at least in the case of slow high, also proximal dendrites (Antonsen and Edwards 2007). Coupling resistance during depression caused by fast high 5-HT has not been studied, but membrane conductance appears to increase distally though not proximally and to cause shunting of EPSPs (Vu and Krasne 1993). Both the membrane conductance decreases seen during facilitation and the increases seen during depression are associated with membrane depolarization (Antonsen and Edwards 2007; Teshiba et al. 2001; Vu and Krasne 1993; Yeh et al. 1997). Consequently, it seems likely that the decreases are due to closure of channels passing K+, which has an equilibrium potential that is hyperpolarized relative to the resting level, whereas the increases are due to opening of channels that pass Cl–, which in these cells has an equilibrium potential above rest. Thus facilitation and depression are at least in part due to cellular alterations that can coexist, which is consonant with our conclusions about signaling pathways.
GENERALITY OF HYPOTHESIS.
It seems likely that the hypothesis of reciprocal stimulation of decay applies well beyond the crayfish circuitry studied here. In a number of kinds of neurons, synaptic transmission or membrane properties that affect excitability can be altered in opposite directions as the result of synaptic or neuromodulatory input, and in many cases these alterations are persistent over at least hours (e.g., Daoudal and Debanne 2003; Daoudal et al. 2002; Fino et al. 2005; Fioravante et al. 2006; Guan et al. 2002; Hansel et al. 2001; Hori et al. 1996; Huang and Kandel 2007; Li and Zhuo 1998; Lisman et al. 2002; Nishimura and Akasu 1989; Shay et al. 2005;Spencer and Murphy 2000). Persistence has been attributed to a variety of inherently stable or self-renewing signaling events including altered activity of PKA, CaMKII, PKC, and metabotropic glutamate receptors (e.g., Casadio et al. 1999; Ghirardi et al. 1995; Hrabetova and Sacktor 2001; Huang and Hsu 2006; Lisman et al. 2002; Sutton et al. 2004; Zhao et al. 2006). Moreover it is not infrequently the case that, as with 5-HT in crayfish, a given external or internal signaling agent, operating at different levels, can cause oppositely directed modulation of transmission or excitability (e.g., Jorntell and Hansel 2006; Lisman 1994). It seems plausible that wherever both persistent up- and downregulations are possible, it may be adaptive for onset of modulation in one direction to terminate the persistence of any previously established modulation of the opposite sign, as proposed here. This has long been believed to be true of hippocampal LTP, where signaling events involved in the production of LTD are thought to dephosphorylate CaMKII and thereby terminate early LTP. We believe that such active termination of intrinsically persistent signaling activities by opposing modulatory pathways will turn out to be a common motif.
Facilitatory and depressive effects of 5-HT at single synapses are not uncommon
To put the present work in perspective, it should be appreciated that both facilitation and depression of synaptic transmission by serotonin appear to be common and, as in the present case, may often occur at the same synapses.
Serotonergic facilitation has been extensively studied at Aplysia sensory-motor synapses, where it is involved in both sensitization and classical conditioning of protective responses (Byrne and Kandel 1996; Hawkins et al. 1983; Kandel 2001). The same synapses are also subject to depression, of unknown function, by neuromodulators, though, so far as is known, not by serotonin itself (Fioravante et al. 2006; Guan et al. 2002). Serotonin also first depresses and then enduringly facilitates transmission from pre- to postganglionic parasympathetic neurons (Nishimura and Akasu 1989); and it can either enhance or reduce GABAA receptor chloride currents of prefrontal pyramidal cells, depending on their concurrent resting potential (Cai et al. 2002).
Perhaps more directly relevant in the present context, excitatory synapses on dorsal horn neurons are subject to both serotonergic facilitation and depression (Hori et al. 1996; Li and Zhuo 1998; Rygh et al. 2006; Shay et al. 2005) that may contribute to hyperalgesia and hypoalgesia, respectively. High concentrations cause a transient depression, whereas low concentrations cause facilitation that persists long after washout, precisely as happens here. Serotonin can also promote activity-dependent long-term potentiation of dorsal horn synapses; LG synapses are subject to LTP (Tsai et al. 2005), but its relationship to serotonergic modulation is unresolved. Transmission from the lateral area of the amygdala to basolateral neurons, which is a segment of the pathway thought to mediate fear and anxiety, is also bidirectionally modulated by serotonin (Huang and Kandel 2007). As in the dorsal horn and in the LG escape circuit, as studied here, depression occurs during exposure to 5-HT, but during wash, long-lasting facilitation develops. This should limit fear and anxiety during naturally occurring exposure to 5-HT but facilitate it later.
In crayfish, serotonin may be released during agonistic encounters (Edwards and Kravitz 1997; Kravitz 2000). It has been proposed that high-serotonin depression might serve to suppress reflex escape during fights in which dominance relationships are being established, whereas low-serotonin facilitation might promote an enhancement of escape that could be useful in the case of unexpected attacks occurring after a dominance relationship has been established but before it is fully entrenched (Krasne and Edwards 2002).
Serotonergic modulation of dorsal horn responses and amygdala transmission, as well as of the LG reflex, may all be seen as affecting defensive and protective behavior, broadly defined. Known facts suggest that that serotonin is released during episodes of acute danger with two effects: 1) development of facilitation of protective responses (including fear or anxiety) that will outlast the period of acute danger and 2) suppression of protective responses acutely, perhaps to allow the animal to deal more effectively with the situation at hand. It may be seen as consonant with this conjecture that serotonergic systems can enhance the acquisition of learned freezing responses to contextual cues while acutely decreasing the freezing responses evoked by direct stimulation to brain regions that may organize freezing and other defensive responses (Martinez et al. 2007).
Signaling pathways for serotonergic facilitation and depression
In so far as it has been determined, facilitation at the mammalian and Aplysia synapses discussed in the preceding text appears to be mediated via receptors that recruit cAMP/PKA or PKC signaling, similar to 5-HT4 and 5-HT2 receptors, respectively, in mammals (Cai et al. 2002; Hori et al. 1996; Huang and Kandel 2007; Li and Zhuo 1998; Nishimura and Akasu 1989; Rygh et al. 2006; Shay et al. 2005). Depression appears to be commonly mediated by receptors such as 5-HT1As that are negatively coupled to cAMP production (Hori et al. 1996; Huang and Kandel 2007; Li and Zhuo 1998; Nishimura and Akasu 1989; Rygh et al. 2006; Shay et al. 2005).
At the LG synapses, cAMP analogues cause facilitation (Araki et al. 2005; Edwards et al. 2002), and the adenylate cyclase inhibitor, SQ22536, prevents serotonergic facilitation (Araki et al. 2005) (see also preceding text). We found here that the PKA inhibitor H-89 also prevents facilitation. This H-89 finding supports a role for cAMP and suggests that it exerts its effect via PKA rather than directly (Beaumont and Zucker 2000).
Experiments by Antonsen and Edwards (2005) suggest that there is additionally an obligatory Ca2+ signaling step in the production of facilitation, which is also true of dorsal horn serotonergic facilitation (Li and Zhuo 1998). There are many possible reasons for this. 1) Our evidence of PKC involvement might account for a Ca2+ dependence. 2) The adenylate cyclase that catalyzes cAMP production might be Ca2+-dependent. And 3) PKA's role might be to promote an essential Ca2+-dependent step as is seen in the PKA-dependent suppression of CaMKII dephosphorylation that promotes production of CA1 LTP in hippocampus (Lisman 1994).
There are as yet only negative findings on serotonergic depression. The possibility that high 5-HT might recruit depression by producing high levels of PKA activation or of cAMP, itself, is rendered unlikely by the inability to induce depression with 8-Bromo cAMP or to prevent it with H89. The possibility that, as in several of the mammalian cases discussed in the preceding text, depression of transmission is mediated by 5-HT1A-receptor-mediated reductions of cAMP and is merely a reduction of a baseline state of facilitation, is also contrary to several of our H-89 findings as well as being contrary to evidence that depression and facilitation are separate states that can coexist. Another possibility is that activation of 5-HT1A receptors might cause inhibition by lowering levels of cAMP and/or of PKA activation below those operating at baseline but not to zero. Low levels of cAMP might cause depression just as low levels of Ca2+ cause hippocampal LTD. If this were so, modest SQ or H-89 action should cause depression, whereas strong action should block both facilitation and depression. However, SQ concentrations that were probably submaximally effective because they did not totally abolish serotonin-induced facilitation, caused some facilitation of their own, not depression; and H-89 concentrations that strongly blocked serotonergic facilitation did not prevent serotonergic depression. So whereas we have ruled out various plausible possibilities, the signaling agents that mediate depression remain to be identified.
Possible therapeutic implications
If bidirectional effects of serotonin that were subject to the reciprocal stimulation of decay hypothesis were to operate at crucial targets of 5-HT therapies, this relationship could have significant implications for designing drug regimens. In particular, effects of serotonin titers would be highly nonmonotonic and dependent on rate of titer increase, and appropriately controlled transient serotonin elevations could produce relatively long-lasting effects that might reduce the need for continual drug exposure.
Conclusion
Until such time as the intracellular signals that underlie both facilitation and depression are fully identified and their levels can actually be measured during and following 5-HT exposure, evidence for or against the reciprocal stimulation of decay hypothesis will necessarily be inferential. When, for example, manifest depression of transmission recovers after a short exposure to high levels of 5-HT, one can only infer the degree to which this apparent recovery is due to decay of the underlying depressive state or growth of the facilitatory one. However, we believe that the case for the hypothesis becomes strong when one takes together 1) that PKA inhibition, which prevents manifest facilitation, also causes all signs of recovery from manifest depression to be lost (and PKC inhibition may do the same), 2) that the hypothesis accounts for the otherwise confusing and arbitrary-seeming effects of a variety of 5-HT application regimens, and 3) that the proposal is inherently plausible.
GRANTS
This work was supported by National Institute of Neurological Disorders and Stroke Grant NS-8108.
Acknowledgments
We thank B. Antonsen, D. Edwards, T. O'Dell, and S. Krasne for helpful discussions and feedback on versions of the manuscript.
APPENDIX
To explore the consequences of the reciprocal stimulation of decay hypothesis, we created a simple computational model. Our purpose was to help us understand the implications of the model's assumptions, not to try to account quantitatively for our data. In this model (Fig. 7, inset) two intermediaries, F and D, are posited. Facilitation is manifest when F is sufficiently greater than D, and conversely.
Italicized equation numbers below refer to the numbered steps in the inset of Fig. 7. Greek letters are parameters.
S, representing an unspecified increasing function of serotonin concentration, produces facilitation and depression-specific “drives” SF and SD
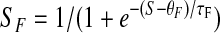 |
(A1) |
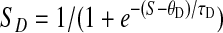 |
(A2) |
both translated and rescaled so that they equal 0 at S = 0 and 1 asymptotically.
F and D are produced at rates proportional to SF and SD, respectively. Thus
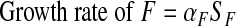 |
(A3) |
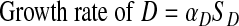 |
(A4) |
F and D also continually decay.
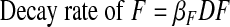 |
(A5) |
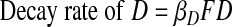 |
(A6) |
Thus
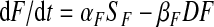 |
(A7) |
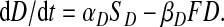 |
(A8) |
Normalized EPSP amplitude is given by
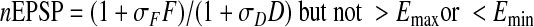 |
(A9) |
Understanding the behavior of Model 2 is greatly aided by considering how the variables D and F, both of which are functions of time, covary. In what follows, it is convenient to define rF = αFSF /βF and rD = αDSD/βD. We refer to these as production/breakdown ratios. Note that these are not fixed parameters but depend on serotonin concentration. Given these definitions
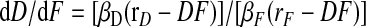 |
(A10) |
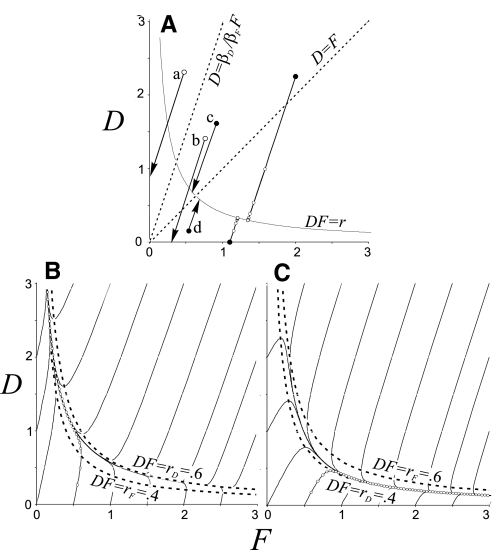
The behavior of the model is extremely simple if 5-HT is applied at a concentration for which rF = rD = r, which also includes the wash condition in which rF = rD = 0: then D and F always move along a straight line with slope βD/βF. They move from their starting value toward the hyperbola defined by DF = r or during wash (r = 0) toward the axes. When they reach the hyperbola or axes, no further change occurs. This is shown in Fig. A1A in which trajectories for illustrative starting situations (indicated by filled and the larger open circles) are shown. On the two right-most trajectories there are marks (small open circles) every 5 processing steps (∼1 min) to show the negatively accelerating rate of approach to the asymptotic levels. In the special case that serotonin concentration starts at zero, D and F will increase along the line D = βD/βF F until they reach the hyperbola at D = √(rβD/βF) and F = √(rβF/βD). During wash, both variables decrease along the a line of slope βD/βF until one of them becomes 0. If the starting point is above the line, D = βD/βF F, depression will persist after washout, and if the starting point is below that line, facilitation will persist (e.g., lines a and b, respectively).
FIG. A1.
Plots of D as a function of F. Explanation and interpretation in appendix. βD = 0.12; βF = 0.04 throughout. Various reference lines, defined on the graphs, are dashed. A: rD = rF = r. Trajectories starting at filled points show behavior of model 2 in the presence of serotonin when r = 0.4. Trajectories starting at open circles show washout behavior (r = 0). Tiny open circles along trajectories are 1-min time marks. B and C: behavior in the presence of serotonin when rD = 0.6 and rF = 0.4 (B) or conversely (C) (discussed in text).
If serotonin is present at an effective dose such that rF and rD are not equal, then the changes in D and F over time are less straightforward. Several features are apparent from Eq. A5 Fig. A1B for rD > rF. If the product DF is small, F and D initially follow a trajectory with initial slope approximately (βD/βF)(rD/rF) toward the hyperbola DF = rF; if DF starts large, then the trajectory, with initial slope βD/βF, is toward the hyperbola DF = r. In either case, the trajectories cross the hyperbola they reach first and then remain sandwiched in-between them, with D eventually increasing without bound along the lower hyperbola. If initially rF > rD, the situation is reversed as illustrated in Fig. A1C.
Using this characterization, a number of predictions pertinent to our experiments follow. We assume that βD > βF, and for the sake of discussion we also assume that facilitation is manifest when F > D, and conversely (this will be so when σF = σD).
Asymptotic behavior. No matter what the starting values of D and F, if rD > rF, ultimately F will go to zero and D will increase without bound; and conversely. However, if rD = rF, D and F [i.e., the point (D,F)] will approach the hyperbola DF = r at a location that depends on the starting values of D and F. Asymptotically D will then be greater than F if D and F start above the line of slope βD/βF that runs through the point D = F = √r (e.g., trajectory c of A1A), and conversely (trajectory d).
If D and F are initially zero. Depression will be manifest initially if rD/rF > βF/βD, and conversely; however, eventually the preceding relationship A1 will obtain. If rD = rF = r, then asymptotically D = √(rβD/βF) and F = √(rβF/βD). If the production/breakdown ratios are approximately equal, then for a considerable period D and F will behave as though there were a common r, but eventually the asymptotic behavior described in the previous paragraph will prevail.
Behavior during wash. Facilitation tends to persist in wash if it is present at the start of wash, as is the case after exposures to low 5-HT. This happens because if facilitation is expressed, the initial point (D,F) is to the right of the line D = F, and, following a trajectory along a straight line of slope βD/βF, it will stay to the right of that line so long as βD > βF, which it is for in the parameter set that is consistent with all of our data. If at the start of wash, D > F and D/F > βD/βF (e.g., a in Fig. A1A), depression will persist in wash, as happens after long exposure to very high 5-HT, whereas if D/F < βD/βF (e.g., b in Fig. A1A), facilitation will develop in wash, as happens after long exposures to high 5-HT.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- Antonsen and Edwards 2005.Antonsen BL, Edwards DH. Mechanisms of serotonergic modulation of the crayfish lateral giant escape circuit. Soc Neurosci Abstr 754.713, 2005.
- Antonsen and Edwards 2007.Antonsen BL, Edwards DH. Mechanisms of serotonergic facilitation at a command neuron. J Neurophysiol 98: 3494–3504, 2007. [DOI] [PubMed] [Google Scholar]
- Araki et al. 2005.Araki M, Nagayama T, Sprayberry J. Cyclic AMP mediates serotonin-induced synaptic enhancement of lateral giant interneuron of the crayfish. J Neurophysiol 94: 2644–2652, 2005. [DOI] [PubMed] [Google Scholar]
- Beaumont and Zucker 2000.Beaumont V, Zucker RS. Enhancement of synaptic transmission by cyclic AMP modulation of presynaptic Ih channels. Nat Neurosci 3: 133–141, 2000. [DOI] [PubMed] [Google Scholar]
- Byrne and Kandel 1996.Byrne JH, Kandel ER. Presynaptic facilitation revisited: state and time dependence. J Neurosci 16: 425–435, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai et al. 2002.Cai X, Flores-Hernandez J, Feng J, Yan Z. Activity-dependent bidirectional regulation of GABA(A) receptor channels by the 5-HT(4) receptor-mediated signalling in rat prefrontal cortical pyramidal neurons. J Physiol 540: 743–759, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casadio et al. 1999.Casadio A, Martin KC, Giustetto M, Zhu H, Chen M, Bartsch D, Bailey CH, Kandel ER. A transient, neuron-wide form of CREB-mediated long-term facilitation can be stabilized at specific synapses by local protein synthesis. Cell 99: 221–237, 1999. [DOI] [PubMed] [Google Scholar]
- Daoudal and Debanne 2003.Daoudal G, Debanne D. Long-term plasticity of intrinsic excitability: learning rules and mechanisms. Learn Mem 10: 456–465, 2003. [DOI] [PubMed] [Google Scholar]
- Daoudal et al. 2002.Daoudal G, Hanada Y, Debanne D. Bidirectional plasticity of excitatory postsynaptic potential (EPSP)-spike coupling in CA1 hippocampal pyramidal neurons. Proc Natl Acad Sci USA 99: 14512–14517, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards et al. 1991.Edwards DH, Heitler WJ, Leise EM, Fricke RA. Postsynaptic modulation of rectifying electrical synaptic inputs to the LG escape command neuron in crayfish. J Neurosci 11: 2117–2129, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards and Kravitz 1997.Edwards DH, Kravitz EA. Serotonin, social status and aggression. Curr Opin Neurobiol 7: 812–819, 1997. [DOI] [PubMed] [Google Scholar]
- Edwards et al. 2002.Edwards DH, Yeh SR, Musolf BE, Antonsen BL, Krasne FB. Metamodulation of the crayfish escape circuit. Brain Behav Evol 60: 360–369, 2002. [DOI] [PubMed] [Google Scholar]
- Fino et al. 2005.Fino E, Glowinski J, Venance L. Bidirectional activity-dependent plasticity at corticostriatal synapses. J Neurosci 25: 11279–11287, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fioravante et al. 2006.Fioravante D, Smolen PD, Byrne JH. The 5-HT- and FMRFa-activated signaling pathways interact at the level of the Erk MAPK cascade: potential inhibitory constraints on memory formation. Neurosci Lett 396: 235–240, 2006. [DOI] [PubMed] [Google Scholar]
- Ghirardi et al. 1995.Ghirardi M, Montarolo PG, Kandel ER. A novel intermediate stage in the transition between short- and long-term facilitation in the sensory to motor neuron synapse of aplysia. Neuron 14: 413–420, 1995. [DOI] [PubMed] [Google Scholar]
- Guan et al. 2002.Guan Z, Giustetto M, Lomvardas S, Kim JH, Miniaci MC, Schwartz JH, Thanos D, Kandel ER. Integration of long-term-memory-related synaptic plasticity involves bidirectional regulation of gene expression and chromatin structure. Cell 111: 483–493, 2002. [DOI] [PubMed] [Google Scholar]
- Hansel et al. 2001.Hansel C, Linden DJ, D'Angelo E. Beyond parallel fiber LTD: the diversity of synaptic and non-synaptic plasticity in the cerebellum. Nat Neurosci 4: 467–475, 2001. [DOI] [PubMed] [Google Scholar]
- Hawkins et al. 1983.Hawkins RD, Abrams TW, Carew TJ, Kandel ER. A cellular mechanism of classical conditioning in Aplysia: activity-dependent amplification of presynaptic facilitation. Science 219: 400–405, 1983. [DOI] [PubMed] [Google Scholar]
- Hori et al. 1996.Hori Y, Endo K, Takahashi T. Long-lasting synaptic facilitation induced by serotonin in superficial dorsal horn neurones of the rat spinal cord. J Physiol 492: 867–876, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrabetova and Sacktor 2001.Hrabetova S, Sacktor TC. Transient translocation of conventional protein kinase C isoforms and persistent downregulation of atypical protein kinase Mzeta in long-term depression. Brain Res Mol Brain Res 95: 146–152, 2001. [DOI] [PubMed] [Google Scholar]
- Huang and Kandel 2007.Huang YY, Kandel ER. 5-Hydroxytryptamine induces a protein kinase A/mitogen-activated protein kinase-mediated and macromolecular synthesis-dependent late phase of long-term potentiation in the amygdala. J Neurosci 27: 3111–3119, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorntell and Hansel 2006.Jorntell H, Hansel C. Synaptic memories upside down: bidirectional plasticity at cerebellar parallel fiber-Purkinje cell synapses. Neuron 52: 227–238, 2006. [DOI] [PubMed] [Google Scholar]
- Kandel 2001.Kandel ER The molecular biology of memory storage: a dialogue between genes and synapses. Science 294: 1030–1038, 2001. [DOI] [PubMed] [Google Scholar]
- Krasne 1969.Krasne FB Excitation and habituation of the crayfish escape reflex: the depolarizing response in lateral giant fibres of the isolated abdomen. J Exp Biol 50: 29–46, 1969. [DOI] [PubMed] [Google Scholar]
- Krasne and Edwards 2002.Krasne FB, Edwards DH. Modulation of the Crayfish Escape Reflex. Integrative Comp Biol 42: 705–715, 2002. [DOI] [PubMed] [Google Scholar]
- Kravitz 2000.Kravitz EA Serotonin and aggression: insights gained from a lobster model system and speculations on the role of amine neurons in a complex behavior. J Comp Physiol [A] 186: 221–238, 2000. [DOI] [PubMed] [Google Scholar]
- Li and Zhuo 1998.Li P, Zhuo M. Silent glutamatergic synapses and nociception in mammalian spinal cord. Nature 393: 695–698, 1998. [DOI] [PubMed] [Google Scholar]
- Linden and Connor 1991.Linden DJ, Connor JA. Participation of postsynaptic PKC in cerebellar long-term depression in culture. Science 254: 1656–1659, 1991. [DOI] [PubMed] [Google Scholar]
- Linden and Connor 1995.Linden DJ, Connor JA. Long-term synaptic depression. Annu Rev Neurosci 18: 319–357, 1995. [DOI] [PubMed] [Google Scholar]
- Lisman 1994.Lisman J The CaM kinase II hypothesis for the storage of synaptic memory. Trends Neurosci 17: 406–412, 1994. [DOI] [PubMed] [Google Scholar]
- Lisman et al. 2002.Lisman J, Schulman H, Cline H. The molecular basis of CaMKII function in synaptic and behavioural memory. Nat Rev Neurosci 3: 175–190, 2002. [DOI] [PubMed] [Google Scholar]
- Martinez et al. 2007.Martinez RC, Ribeiro de Oliveira A, Brandao ML. Serotonergic mechanisms in the basolateral amygdala differentially regulate the conditioned and unconditioned fear organized in the periaqueductal gray. Eur Neuropsychopharmacol 17: 717–724, 2007. [DOI] [PubMed] [Google Scholar]
- Nishimura and Akasu 1989.Nishimura T, Akasu T. 5-Hydroxytryptamine produces presynaptic facilitation of cholinergic transmission in rabbit parasympathetic ganglia. J Auton Nerv Syst 26: 251–260, 1989. [DOI] [PubMed] [Google Scholar]
- Rygh et al. 2006.Rygh LJ, Suzuki R, Rahman W, Wong Y, Vonsy JL, Sandhu H, Webber M, Hunt S, Dickenson AH. Local and descending circuits regulate long-term potentiation and zif268 expression in spinal neurons. Eur J Neurosci 24: 761–772, 2006. [DOI] [PubMed] [Google Scholar]
- Shay et al. 2005.Shay BL, Sawchuk M, Machacek DW, Hochman S. Serotonin 5-HT2 receptors induce a long-lasting facilitation of spinal reflexes independent of ionotropic receptor activity. J Neurophysiol 94: 2867–2877, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer and Murphy 2000.Spencer JP, Murphy KP. Bi-directional changes in synaptic plasticity induced at corticostriatal synapses in vitro. Exp Brain Res 135: 497–503, 2000. [DOI] [PubMed] [Google Scholar]
- Sutton et al. 2004.Sutton MA, Bagnall MW, Sharma SK, Shobe J, Carew TJ. Intermediate-term memory for site-specific sensitization in aplysia is maintained by persistent activation of protein kinase C. J Neurosci 24: 3600–3609, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teshiba et al. 2001.Teshiba T, Shamsian A, Yashar B, Yeh SR, Edwards DH, Krasne FB. Dual and opposing modulatory effects of serotonin on crayfish lateral giant escape command neurons. J Neurosci 21: 4523–4529, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai et al. 2005.Tsai LY, Tseng SH, Yeh SR. Long-lasting potentiation of excitatory synaptic signaling to the crayfish lateral giant neuron. J Comp Physiol [A] Neuroethol Sens Neural Behav Physiol 191: 347–354, 2005. [DOI] [PubMed] [Google Scholar]
- Vu et al. 1997.Vu ET, Berkowitz A, Krasne FB. Postexcitatory inhibition of the crayfish lateral giant neuron: a mechanism for sensory temporal filtering. J Neurosci 17: 8867–8879, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vu and Krasne 1993.Vu ET, Krasne FB. Crayfish tonic inhibition: prolonged modulation of behavioral excitability by classical GABAergic inhibition. J Neurosci 13: 4394–4402, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh et al. 1996.Yeh SR, Fricke RA, Edwards DH. The effect of social experience on serotonergic modulation of the escape circuit of crayfish [see comments]. Science 271: 366–369, 1996. [DOI] [PubMed] [Google Scholar]
- Yeh et al. 1997.Yeh SR, Musolf BE, Edwards DH. Neuronal adaptations to changes in the social dominance status of crayfish. J Neurosci 17: 697–708, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao et al. 2006.Zhao Y, Leal K, Abi-Farah C, Martin KC, Sossin WS, Klein M. Isoform specificity of PKC translocation in living Aplysia sensory neurons and a role for Ca2+-dependent PKC APL I in the induction of intermediate-term facilitation. J Neurosci 26: 8847–8856, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]