Abstract
This study was designed to differentiate between two models of motor lateralization: “feedback corrections” and dynamic dominance. Whereas the feedback correction hypothesis suggests that handedness reflects a dominant hemisphere advantage for visual-mediated correction processes, dynamic dominance proposes that each hemisphere has become specialized for distinct aspects of control. This model suggests that the dominant hemisphere is specialized for controlling task dynamics, as required for coordinating efficient trajectories, and the nondominant hemisphere is specialized for controlling limb impedance, as required for maintaining stable postures. To differentiate between these two models, we examined whether visuomotor corrections are mediated differently for the nondominant and dominant arms. Participants performed targeted reaches in a virtual reality environment in which visuomotor rotations occurred in two directions that elicited corrections with different coordination requirements. The feedback correction model predicts a dominant arm advantage for the timing and accuracy of corrections in both directions. Dynamic dominance predicts that correction timing and accuracy will be similar for both arms, but that interlimb differences in the quality of corrections will depend on the coordination requirements, and thus, direction of corrections. Our results indicated that correction time and accuracy did not depend on arm. However, correction quality, as reflected by trajectory curvature, depended on both arm and rotation direction. Nondominant trajectories were systematically more curvilinear than dominant trajectories for corrections with the highest coordination requirement. These results support the dynamic dominance hypothesis.
INTRODUCTION
Lateralization is a well-documented feature of neural organization in many animals (Halpern et al. 2005; Rogers et al. 2004; Vallortigara and Rogers 2005). Handedness is one behavioral manifestation of neural lateralization that is prominent in both humans and nonhuman primates (Hopkins and Pearson 2000; Hopkins et al. 2003) and is characterized by the consistent preference for one arm over the other for performance of certain tasks. More than a century ago, Liepmann (1905) suggested that handedness emerges because the “dominant hemisphere,” reflecting the hemisphere that is contralateral to the dominant arm, is used to plan movements of both arms. According to this idea, performance disadvantages of the nondominant arm result from interhemispheric transmission delays associated with dominant hemisphere commands for nondominant arm movements (Derakhshan 2006). This model of handedness predicts performance advantages of the dominant arm for all aspects of motor control and coordination. We have recently proposed the dynamic dominance model of handedness, in which each hemisphere contributes unique features of control to each arm (Sainburg 2005). This has been supported by data indicating advantages of the nondominant arm for specific aspects of control (Bagesteiro and Sainburg 2003, 2005; Duff and Sainburg 2007; Wang and Sainburg 2007). However, it has been difficult to differentiate between these two models of handedness, because the neurobehavioral processes subserved by motor lateralization remain incompletely understood.
The most longstanding neurobehavioral correlate to Leipmann's model of motor lateralization has been the hypothesis that dominant arm advantages in performance reflect specialization of the dominant hemisphere for visual-mediated correction processes (for review, see Carson 1989). This model has been supported by studies that have indicated that dominant arm movements tend to be shorter in duration (Elliott et al. 1994; Mieschke et al. 2001; Todor and Cisneros 1985) and more accurate (Roy 1983; Todor and Cisneros 1985) than nondominant arm movements. In addition, interlimb differences in movement accuracy tend to be reduced when movements are performed very rapidly, diminishing the role of visual-based error corrections (Flowers 1975). These findings have led to the speculation that dominant hemisphere advantages for the speed and efficacy of visual-mediated corrections confer accuracy advantages to the dominant arm. Some previous studies, however, have questioned this hypothesis after failing to show an effect of visual conditions on performance asymmetries (Buekers and Helsen 2000; Carnahan 1998; Carson et al. 1990, 1992, 1993; Elliott et al. 1993; Roy and Elliott 1986, 1989; Roy et al. 1994). Nevertheless, the feedback correction model of lateralization predicts that the dominant arm should show distinct advantages in the timing and efficacy of corrections to visual errors.
The dynamic dominance hypothesis of motor lateralization (Sainburg 2002, 2005) provides an alternative explanation for dominant arm advantages during reaching. This model is based on the idea that a movement is initially planned by specifying trajectory parameters in extrapersonal space (Sarlegna and Sainburg 2007) and that this plan is transformed into commands that specify the dynamic properties that reflect the forces required to initiate the movement (Sainburg 2002). Our hypothesis suggests that dominant arm control has become specialized for the latter process of dynamic transformations. In support of this model, our laboratory has shown clear dominant arm advantages in intersegmental coordination during multijoint movements and for adaptation to novel dynamic conditions (Bagesteiro and Sainburg 2002; Duff and Sainburg 2007; Sainburg and Kalakanis 2000; Wang and Sainburg 2004, 2007). Recent findings have also showed nondominant arm advantages for load compensation responses (Bagesteiro and Sainburg 2003, 2005) and for impedance control during adaptation to novel dynamic conditions (Duff and Sainburg 2007; Schabowsky et al. 2007). We thus hypothesized that the nondominant arm controller has become specialized for regulating limb impedance, as required to achieve steady-state limb positions. This hypothesis does not predict advantages in either the speed or efficacy of visual-mediated correction mechanisms. However, our model does predict dominant arm advantages for intersegmental coordination in conditions where intersegmental dynamic interactions are substantial (Sainburg and Kalakanis 2000).
In this study, we differentiate between the feedback correction and dynamic dominance hypotheses by determining whether the dominant and nondominant arms perform differently in response to visual errors that elicit corrections with different coordination requirements. We use a virtual reality environment to impose unexpected visuomotor rotations in two directions during targeted reaching. Our predictions are based on three main dependent variables: final position accuracy, correction time, and trajectory curvature. The feedback correction model of lateralization predicts a dominant arm advantage in the timing and accuracy of error corrections that does not vary with rotation direction. This is because the “advantage” of the dominant arm should arise from more effective visuomotor correction mechanisms, regardless of coordination requirements. Alternatively, the dynamic dominance hypothesis predicts that correction timing and accuracy will be similar between the arms, but that dominant advantages in coordination, reflected by trajectory curvature, will depend on rotation direction. We have designed the task such that the intersegmental coordination requirement of corrections to lateral rotations (clockwise for the dominant arm and counterclockwise for the nondominant arm) is larger than that of corrections to medial rotations, and expect dominant advantages in coordination to be more pronounced for corrections to lateral rotations.
METHODS
Subjects
Fourteen neurologically intact right-handed adults (5 males and 9 females), aged from 18 to 28 yr old, were recruited to participate in this study. Handedness was determined using a 35-item version of the Edinburgh inventory (Oldfield 1971), and only those classified as right-handers were used for the experiment. Each participant performed a session consisting of 140 trials with one arm; seven participants performed the task with their nondominant arm and seven participants performed the task with their dominant arm. All participants gave informed consent before the start of the experiment, which was approved by the Biomedical Institutional Review Board of the Pennsylvania State University (IRB 15084).
Experimental setup
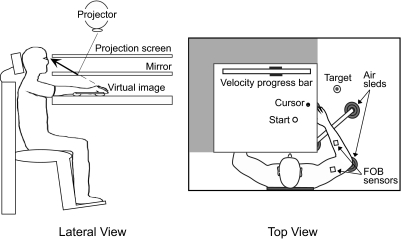
Figure 1 depicts the experimental setup. Participants sat facing a projection screen with their arm supported over a horizontal table top, positioned just below shoulder height. The arm was supported by an air jet system that reduced the effects of gravity and friction. Participants were fitted with an adjustable arm brace to eliminate movements of the wrist and a butterfly-shaped chest restraint to restrict movements of the trunk and scapula. A start circle, target, and cross-hair cursor, representing index fingertip position, were projected on a horizontal back-projection screen just above the arm. A mirror below this screen reflected the visual display such that it was perceived to be in the same horizontal plane as the arm and fingertip. Calibration of the display assured that this projection was veridical. Positions and orientations of the measured segments were sampled using a Flock-of-Birds (FoB; Ascension-Technology) magnetic 6 degree-of-freedom movement-recording system. A single sensor was attached to the upper arm segment via an adjustable plastic cuff, and another sensor was fixed to the air sled where the forearm was fitted. These sensors were positioned approximately at the center of each arm segment. The positions of three bony landmarks were digitized using a stylus that was rigidly fixed to a FoB sensor: index finger tip, the lateral epicondyle of the humerus, and the acromion, directly posterior to the acromio-clavicular joint. These positions, relative to the sensors attached to each arm segment, remained constant throughout the experimental session. The cross-hair cursor (1.4 cm diam) was projected on the screen at a rate of 85 Hz, which was fast enough to maintain the cursor on the fingertip throughout the sampled movements. During the experiment, the light was turned off, and the arm was covered such that participants were unable to view their movements. Data were digitized at 100 Hz using a Macintosh computer, which controlled the sensors through separated serial ports, and stored on disk for further analysis. Custom computer algorithms for experiment control and data analysis were written in REAL BASIC (REAL Software), C (CodeWarrior), and IGOR Pro (WaveMetrics).
FIG. 1.
Lateral and top view of the experimental apparatus.
Experimental task
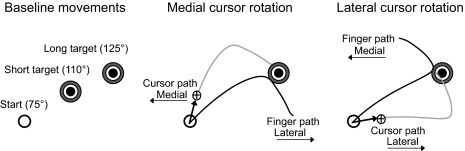
Before all trials, the index finger position was displayed in real time as a screen cross-hair cursor. Targets, defined using shoulder and elbow angles, were customized to each participant. The start circle was located at a 75° elbow angle and 45° shoulder angle, whereas the short distance target was located at a 110° elbow angle and 45° shoulder angle and the long distance target at a 125° elbow angle and 45° shoulder angle (Fig. 2, left). Thus the task was designed such that shoulder excursion was not required for baseline performance, since subjects could complete baseline movements by extending their elbow 35° for the short target and 50° for the long target. Before the start of a trial, the target was presented on the screen. Participants were instructed to bring their index finger into the start circle and to remain there for a period of 300 ms. At that time, an audiovisual signal prompted the beginning of the trial and participants were instructed to make a fast movement directly to the target. Feedback regarding peak velocity was provided as a progress-bar display. Participants were trained to produce peak velocities ranging from 0.8 to 1.2 m/s during the first 25 trials. Visual feedback of the cursor was provided during the entire session, and points were awarded for final position accuracy when the movement also satisfied the peak velocity requirement. The target was 2 cm in diameter, and final position errors of <1 cm were awarded 10 points, those between 1 and 2 cm were awarded 3 points, and final position errors between 2 and 3 cm were awarded 1 point. Following the first 25 trials, participants were randomly exposed to a 30° visuomotor cursor rotation every 6–8 trials for the remaining 115 trials. During these trials, the position of the cursor, corresponding to the index fingertip, was rotated relative to the start circle by the specified angle. Rotations occurred in either the clockwise (CW) or counterclockwise (CCW) direction. However, because rotations were mirror-imaged between the arms, CCW rotations in the dominant arm and CW rotations in the nondominant arm will hereinafter be referred to as medial cursor rotations. Conversely, CW rotations in the dominant arm and CCW rotations in the nondominant arm will hereinafter be referred to as lateral cursor rotations. To successfully bring the cursor into the target, participants were required to initiate corrective responses that were opposite in direction to the rotation. Thus medial cursor rotations elicited lateral directed corrections (Fig. 2, middle), mediated by shoulder extension. Conversely, lateral cursor rotations elicited medial directed corrections (Fig. 2, right), mediated by shoulder flexion. Thus our task was designed such that baseline movements required primarily elbow motion, whereas error corrections required shoulder motion. This facilitated the dissociation of corrective responses through the analysis of joint kinematics. In total, there were six experimental conditions: 1) baseline, short target; 2) baseline, long target; 3) lateral rotation, short target; 4) lateral rotation, long target; 5) medial rotation, short target; 6) medial rotation, long target. Trials were removed when subjects failed to initiate a clearly identifiable response to the rotation. This accounted for only 4.8% of the relevant data.
FIG. 2.
Experimental task required elbow extension during baseline conditions (left), shoulder extension during lateral directed responses to medial cursor rotations (middle), and shoulder flexion during medial directed responses to lateral cursor rotations (right). Handpaths are depicted in dominant-arm coordinates.
Kinematic data
The three-dimensional (3D) position of the index finger, elbow, and shoulder were calculated from the sensor position and orientation, and joint angles were calculated using these measures. All kinematic data were low-pass filtered at 8 Hz (3rd-order, dual-pass Butterworth) and differentiated to yield velocity and acceleration values. The onset of movement was defined by the last minimum (<3% peak tangential finger velocity) before the maximum in tangential finger velocity. Movement termination was similarly defined by the first minimum (<3% peak tangential finger velocity) following the maximum in tangential finger velocity. Measures including initial direction, absolute final position error, and peak tangential acceleration were determined to compare baseline and rotation conditions within each arm. Initial direction was measured in a dominant-arm coordinate system relative to the line connecting the finger at movement start and the target location. Initial direction was calculated as the deviation between this target line and the line originating at the starting location of the hand and ending at the position of the hand at peak acceleration. Positive values indicate hand paths that were directed lateral to the target line, whereas negative values indicate hand paths that were directed medial to the target line. Movement accuracy was measured as absolute final position error and was calculated as the shortest distance between the index finger location at movement termination and the center of the target. Finally, peak acceleration was calculated as the maximum in tangential acceleration.
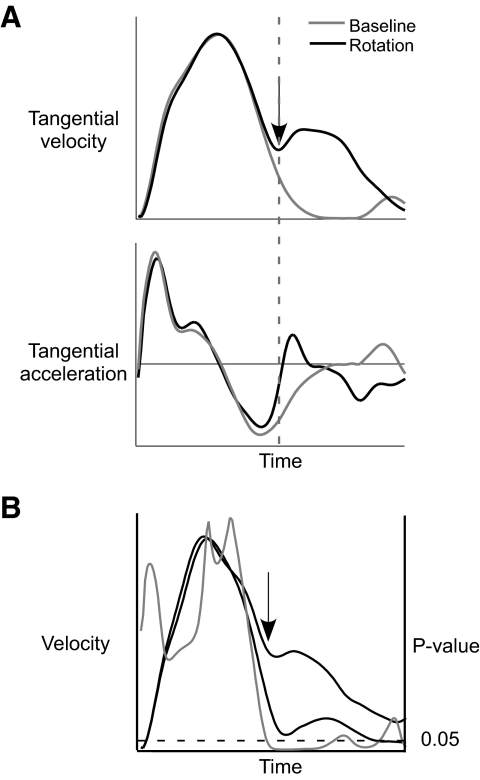
Corrective segments of the rotated movements were isolated and analyzed separately to quantify the efficiency of the corrections. As depicted in the schematic shown in Fig. 3A, the tangential velocity profile of a rotated movement (black) showed a divergence from that of a baseline movement (gray), corresponding to the correction (indicated by arrow). However, because corrections did not always result in a pronounced inflection in velocity, corrective segments were isolated using tangential acceleration. Thus the onset of the corrective segment was defined as the point following the negative peak in tangential finger acceleration that was 5% of that peak (dotted line). Although this method was used to isolate the corrective segment of each perturbed trial for subsequent kinematic and kinetic analysis, a more rigorous statistical-based routine was used to determine correction time (see Statistical analysis). Following the isolation of the corrective segment of the movement, trajectory curvature was calculated as the difference in the cumulative distance and shortest distance from the finger position at the onset of the corrective segment to movement end. Trajectory curvature is an indication of the spatial efficiency of the corrective movement segments, where larger curvatures indicate less spatially efficient responses. Additionally, elbow angle was calculated at the end of movement to assess coordination requirements for the two rotation directions.
FIG. 3.
A: schematic of the method used to define the onset of corrective segments. B: schematic of the method used to identify correction time.
Kinetic data
Shoulder and elbow torques were calculated using equations that model the upper arm and forearm as rigid interconnected units with frictionless joints at the shoulder and elbow. The shoulder was allowed to move freely, and the torques resulting from linear accelerations of the shoulder were included in the equations of motion for each joint. To separately analyze the effects of intersegmental forces and muscle forces on arm motion, we partitioned the terms of the equations of motion at the joint into three main components: interaction torque, muscle torque, and net torque (Sainburg et al. 1995, 1999). At each joint, interaction torque represents the rotational effect of the forces caused by the rotation and linear motion of the other segment. The muscle torque predominantly represents the rotational effect of muscle forces acting on the segment. However, muscle joint torque also includes the passive effects of soft tissue deformation and does not distinguish muscle forces that counter one another during co-contraction. Finally, the net torque is equal to the combined muscle and interaction torques, which is directly proportional to the joint acceleration.
Torques were computed and analyzed for the shoulder and elbow joint as detailed in the equations below. The mass of the forearm support is 0.58 kg, whereas the inertia is 0.0247 kg/m2. Arm segment inertia, center of mass, and mass were computed from regression equations using subjects' body mass and measured arm segment lengths (Winter 1990) Elbow joint torques
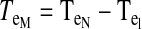 |
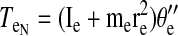 |
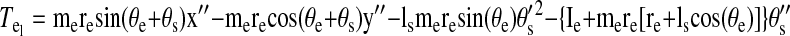 |
Shoulder joint torques
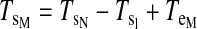 |
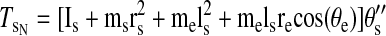 |
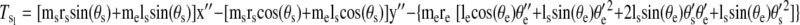 |
where m is segment mass, r is distance from proximal joint to center of mass, l is segment length, I is segment moment of inertia about the point of rotation, θs is shoulder angle, θe is elbow angle, x is shoulder position along the x-direction, y is shoulder position along the y-direction, TeI is elbow interaction torque, TeM is elbow muscle torque, TeN is elbow net torque, TsI is shoulder interaction torque, TsM is shoulder muscle torque, and TsN is shoulder net torque. Subscript e denotes lower arm segment, and s denotes upper arm segment. Derivations of joint torques have been described previously (Bagesteiro and Sainburg 2002).
Shoulder and elbow torque impulse was calculated by integrating the absolute values of the torque profiles at each joint over the interval corresponding to the corrective segment (onset of corrective segment to movement end). Total torque impulse for the corrective segment was calculated as the sum of the torque impulses.
Statistical analysis
The independent measures of interest in this experiment were arm, target distance, and rotation condition. When rotation trials were compared with baseline trials, a 2 × 3 × 2 repeated-measures ANOVA was used with target distance (short, long) and rotation condition (baseline condition, lateral cursor rotation, medial cursor rotation) as within-subject factors and arm (nondominant, dominant) as a between-subject factor. Following the isolation of the corrective segment of the rotation trials, a different ANOVA was used because baseline movements were not included in this analysis. Thus a 2 × 2 × 2 repeated-measures ANOVA was used with target distance and rotation condition (lateral cursor rotation, medial cursor rotation) as within-subject factors and arm as a between-subject factor. When only lateral cursor rotations were examined, a 2 × 2 repeated-measures ANOVA with target distance as the within-subject factor and arm as the between-subject factor was used. In all cases, post hoc analysis was done using the Tukey-Kramer honestly significantly different (HSD) test. The Student's t-test was used to assess differences in coordination requirements for the medial and lateral rotation directions. Finally, we conducted a within-subject linear regression analysis to assess the dependence of trajectory curvature on elbow interaction torque for the corrective segments of lateral rotation trials. Correlation coefficients were normalized using Fisher's z' transformation, and mean slope and z' values for the nondominant and dominant arms were compared using the Student's t-test.
To determine the earliest measurable response to the rotations, finger and shoulder velocities were subjected to an iterative t-test routine. This analysis is based on a timing routine previously used by Shapiro et al. (2002, 2004) and modified by our laboratory (Bagesteiro and Sainburg 2003) to identify changes from baseline performance for kinematic data. All rotation trials for a given target distance and response direction were compared with an equal number of baseline trials to the same distance target. Baseline trials were chosen as the median trial between two rotation trials. All profiles were synchronized to the onset of the movement and were subsequently subjected to the iterative t-test, resulting in a P value at each instant in time. Figure 3B shows a schematic of ensemble averaged velocity plots (black) with the P value time series overlaid (gray). The first measurable response was defined as the point in time in which the iterative t-test yielded a P value below an α value of 0.05 (indicated by arrow) and remained significant for a minimum of three consecutive points, which was equivalent to ∼30 ms. Correction time was calculated as the time from the onset of movement to the first statistically measurable response.
RESULTS
Validation of task design
This study was designed to assess responses to unexpected visuomotor rotations, occurring during the course of movement. To guarantee that rotations were not anticipated before movement onset, we first compared early trajectory features between baseline and rotated trials within each arm. If subjects anticipated cursor rotations before movement initiation, the early trajectory features of baseline and rotated movements should differ. Statistical analysis showed that both initial directions and peak tangential accelerations were comparable across rotation conditions for each arm. This was indicated by a lack of an effect of rotation condition (initial direction error, F2,13 = 1.4320; P = 0.25; peak tangential acceleration, F2,13 = 0.5792; P = 0.56) and a lack of an interaction of hand with rotation condition (initial direction error, F2,13 = 0.2730; P = 0.76; peak tangential acceleration, F2,13 = 0.8304; P = 0.44). This tendency to initiate baseline and rotated trials with the same initial trajectories supports the idea that subjects did not anticipate the rotations before movement onset.
General performance during baseline and rotated trials
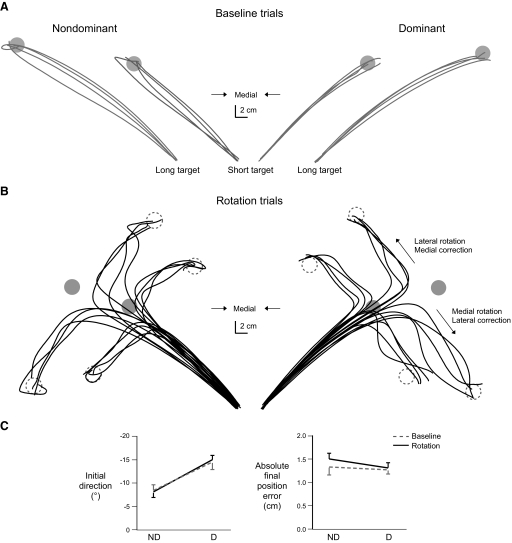
We now describe performance under veridical conditions, to establish a baseline for assessing rotated trials. Figure 4A shows typical nondominant (left) and dominant (right) hand paths from representative subjects during baseline conditions. Dominant hand paths tended to be directed medial to the target line at movement onset and gently curved back to the target during the course of motion, whereas nondominant hand paths tended to be straighter and directed toward the target at movement onset.
FIG. 4.
A: hand paths of baseline movements from representative subjects for the nondominant and dominant arms. B: hand paths of rotated movements from representative subjects for the nondominant and dominant arms. C: means and SE of initial direction (left) and absolute final position error (right) for baseline and rotated movements of the nondominant (ND) and dominant (D) arm.
These findings were consistent across subjects and were also characteristic of the initial trajectories of rotated trials (Fig. 4B). Figure 4C (left) shows the initial directions of baseline and rotated trials for each arm. As described above, dominant arm movements were more curved and therefore showed substantially more negative (medial) initial directions than nondominant arm movements (F1,13 = 8.3720; P < 0.05). This finding is consistent with our previous work, which showed that the dominant arm more effectively coordinates elbow and shoulder action, to better account for inertial interactions. For movements characterized primarily by elbow extension, more curved trajectories reflect synergistic action at the elbow and shoulder, whereas straighter movements result from counteraction at the joints (Sainburg et al. 1999).
Despite initial differences in nondominant and dominant arm trajectories, both arms acquired final positions with similar accuracies, as is exemplified by the examples in Fig. 4, A and B. Additionally, final position accuracies during movements with rotations were comparable to the final position accuracies of baseline movements. Figure 4C (right) shows the means and SE of final position error for the baseline and perturbed movements of the dominant and nondominant arms. This pattern was shown across all subjects. Absolute final position error was similar for the nondominant and dominant arms (F1,13 = 0.6530; P = 0.43). Interestingly, the rotation had no effect on final position accuracy, showing that both arms effectively corrected for the altered cursor position. This was shown by a lack of a main effect of rotation condition (F2,13 = 2.3956; P = 0.10) and a lack of an interaction of arm with rotation condition (F2,13 = 2.1916; P = 0.12). In summary, the final position accuracy of rotated trials was comparable between the arms and did not differ from the final position accuracy of baseline trials.
Timing of corrections to rotations
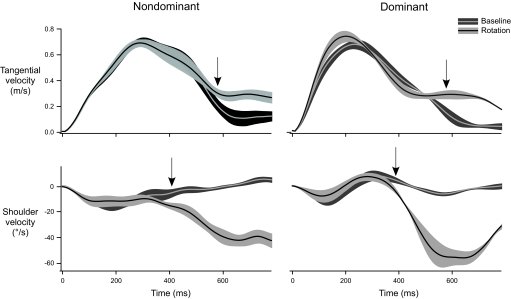
Our task was designed to require elbow excursions during baseline trials and shoulder excursions to correct for visual rotations. To determine the earliest measurable response to the rotation, we thus subjected tangential finger velocity and shoulder joint velocity profiles to an iterative t-test routine, using an α value of 0.05 (see Fig. 3B). Figure 5 shows sample ensemble averages (4 trials each) of baseline (gray) and rotation (black) tangential finger velocities (top) and shoulder velocities (bottom). SE is depicted as gray bands surrounding each average. The earliest responses, measured in this way, are indicated with an arrow, and profiles are shown until 200 ms following the response at the finger. Across subjects, the first measurable response at the finger occurred at 543.6 ± 16.4 ms and was not significantly different between the arms (F1,13 = 1.2879; P = 0.28). Responses at the shoulder were measured some 150 ms earlier than the finger, on average 394.6 ± 7.9 ms following movement onset. There was an interaction of arm with rotation condition (medial, lateral; F1,13 = 5.6063; P < 0.05), such that nondominant corrections occurred earlier for the medial rotation than the lateral rotation. However, post hoc analysis conducted between arms and within rotation conditions was not significant (Tukey-Kramer; in all cases, P > 0.05). Thus both finger and shoulder correction times were comparable between arms within each rotation condition.
FIG. 5.
Ensemble averages and SE (gray bands) of tangential velocity (top) and shoulder velocity (bottom) from representative subjects for the nondominant and dominant arms, with the 1st measurable response to the rotation indicated by an arrow.
It should be stressed that the 395-ms time to correction, measured in this study, cannot be directly compared with studies that were specifically designed to assess minimum visuomotor response latencies. Such studies have reported latencies ranging from 110 to 450 ms (Carlton 1981; Day and Lyon 2000; Johnson et al. 2002; Khan et al. 2003; Prablanc and Martin 1992; Saunders and Knill 2003, 2004; Soechting and Lacquaniti 1983). However, although the angle of the visuomotor rotation used in this study was constant, the displacement error between the finger location and the cursor position evolved during the course of the movement. The time at which this error exceeded a threshold to initiate a response could not be determined, and thus absolute latencies could not be analyzed. Therefore we were only concerned with the relative difference in response time between the arms for this paradigm.
Coordination of corrections to rotations
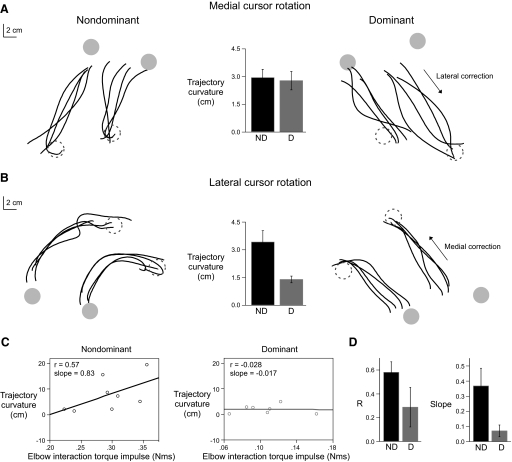
As described above, neither the timing of responses nor the accuracy of corrections differed between the arms. However, consistent with our previous work, the dominant arm showed more efficiently coordinated initial trajectories during baseline and rotation conditions. To further assess coordination during responses to rotations, corrective trajectories were isolated and compared between the arms. Figure 6A shows sample trajectories of corrections to medial rotations (lateral directed responses), whereas Fig. 6B shows sample trajectories of corrections to lateral rotations (medial directed responses). The short and long targets are provided for reference. The most striking difference between the arms occurred for lateral but not medial rotations. For these medial directed corrections, nondominant hand trajectories were highly curved compared with dominant hand trajectories. In contrast, lateral directed corrections to medial cursor rotations showed very little differences in trajectory curvature between the arms. This is indicated by the insets in Fig. 6, A and B, and as reflected by our ANOVA (interaction of arm with rotation direction; F1,13 = 7.7870; P < 0.05). Dominant arm corrections were systematically less curved than nondominant arm corrections for lateral rotations (Tukey-Kramer; P < 0.05), but not medial rotations (Tukey-Kramer; P > 0.05). This striking difference in coordination between the medial and lateral directed corrections is likely related to previously reported direction dependent inertial anisotropies for planar arm movements (Gordon et al. 1994; Hogan 1985; Sainburg et al. 1999). These studies have shown that medially directed movements have larger inertial resistances and larger acceleration-dependent intersegmental dynamic interactions than laterally directed movements. This is because effective limb inertia and acceleration dependent interaction torques vary with the cosine of the elbow angle, becoming greater as the angle deviates from 90°. In fact, the elbow angle at the end of each movement was systematically >90° (122.8 ± 2.1°) for medial directed responses to lateral rotations and was systematically <90° (83.6 ± 1.7°) for lateral directed responses to medial rotations (t-test; P < 0.05). As a result, the total interaction torque was significantly larger for responses to lateral rotations (0.45 ± 0.04 Nms) than medial rotations (0.36 ± 0.03 Nms; t-test, P < 0.05). These findings suggest that medial directed corrections required greater dynamic coordination than laterally directed corrections. Figure 6C shows sample scatterplots and linear regressions showing the dependence of trajectory curvature on elbow interaction torque impulse for medial directed corrections. The correlation coefficients and slope values were larger for the nondominant arm (left) than the dominant (middle) arm. Figure 6D shows the means and SE for the correlation coefficients (left) and slopes (right) of the regression analysis across subjects. Although the correlation coefficients (following Fisher's z' transformation) were not significantly different between the nondominant and dominant arms (t-test, P > 0.05), mean slopes were significantly greater for the nondominant arm than the dominant arm (t-test, P < 0.05). These results indicate that the dominant arm more effectively accounted for intersegmental dynamics than did the nondominant arm, resulting in smaller increases in trajectory curvature given an increase in interaction torque impulse. These findings are consistent with previous work from our laboratory that has shown that the nondominant and dominant systems use different coordination strategies during reaching (Sainburg and Kalakanis 2000).
FIG. 6.
A: sample corrective segments for responses to medial cursor rotations from the nondominant and dominant arms. Inset: means and SE of trajectory curvature for responses to medial cursor rotations, separated by arm. B: sample corrective segments for responses to lateral cursor rotations from the nondominant and dominant arms. Inset: means and SE of trajectory curvature for responses to lateral cursor rotations, separated by arm. C: sample scatterplots and linear regressions showing the dependence of trajectory curvature on elbow interaction torque impulse for medial directed corrections to lateral rotations, from representative subjects for the nondominant (left) and dominant (right) arms. D: means and SE of the correlation coefficients (left) and slope values (right) from the regression analysis, separated by arm.
In summary, interlimb differences in the timing and accuracy of corrections to visual rotations were not significant, whereas differences in the coordination of those responses varied substantially with the dynamic requirements of the correction. When coordination requirements were high, as was the case for medial directed corrections, the curvatures of nondominant but not dominant arm corrections depended on elbow interaction torque impulse. These results support the dynamic dominance hypothesis.
DISCUSSION
This study was designed to differentiate between the dynamic dominance and feedback correction models of motor lateralization. Each hypothesis predicts a different pattern of results with respect to visual-based error corrections. The dynamic dominance hypothesis predicts that dominant arm advantages in coordination will be independent of visual-based correction processes, whereas the feedback correction model predicts a dominant arm advantage in the timing and accuracy of error corrections. To distinguish between hypotheses, we designed a task that required corrections in two directions that elicited different coordination requirements. Thus interlimb differences in coordination should depend on both arm and rotation direction, whereas interlimb differences in visuomotor processing should depend on arm but not rotation direction. Our findings indicated no main effect of arm for correction timing or accuracy. Our findings also showed the predicted interaction between arm and rotation direction for coordination, indicated by our measure of trajectory curvature. In addition, the quality of this interaction showed no differences for lateral directed corrections (medial rotations) and large differences for medial directed corrections (lateral rotations). This pattern is consistent with the dynamic dominance hypothesis, because medial directed movements present greater intersegmental coordination requirements than do lateral directed movements (Gordon et al. 1994; Sainburg and Kalakanis 2000). Accordingly, trajectories of the dominant arm during medial, but not lateral directed corrections were shown to reflect more efficiently coordinated dynamics. The fact that lateral directed responses to medial rotations did not show interlimb differences in coordination emphasizes that visuomotor corrections in themselves do not produce interlimb differences in coordination. Taken together, the results of this study do not support the feedback correction model of motor lateralization but do provide support for the dynamic dominance hypothesis.
Visuomotor response latencies
Responses to visuomotor rotations in this study occurred some 395 ms following the onset of the movement. Previous studies have reported a wide variety of latencies for correcting visual-detected errors, ranging from 110 to 450 ms (Brenner and Smeets 1997, 2003; Carlton 1981; Johnson et al. 2002; Keele and Posner 1968; Prablanc and Martin 1992; Saunders and Knill 2003, 2004; Soechting and Lacquaniti 1983; Woodworth 1899; Zelaznik et al. 1983). However, these reported response latencies include qualitatively different types of responses: short latency responses, on the order of 100–150 ms, and long latency responses, on the order of 200–400 ms. Short latency responses have been attributed to two main mechanisms: errors computed from efference copy information (Desmurget and Grafton 2000; Miall et al. 1993; Wolpert and Miall 1996) and triggered reactions, or motor commands that are released by sensory stimuli (Carlsen et al. 2003, 2004; Valls-Sole et al. 1999). The efference copy from the ongoing hand movement can mediate short latency corrections by using the current target location to predict sensory errors (Prablanc et al. 2003), an idea supported by studies that have shown short latency corrections to target displacements when visual feedback about hand position is prevented (Goodale et al. 1986; Komilis et al. 1993). However, in this study, the hand position, with respect to the target location, is not affected by our visual rotation. Thus the hypothesized error information required for this short latency mechanism was not available in our paradigm. Triggered reactions, on the other hand, depend on the ability to predict the rotation. This is because triggered reactions are thought to reflect stored responses that are startled or released by the onset of a rotation or by some other sensory stimulus. However, in this study, there was a low probability of receiving any one rotation. Following a short training phase, rotations occurred on only 7% of the remaining trials. Thus given that there were two target distances and two rotation directions (see methods), there was less than a 2% chance of any condition on any given trial. It therefore seems reasonable that short latency responses were not elicited in this study.
Nondominant and dominant arms show similar response timing and efficacy
The hypothesis that neural processes underlying movement control might be lateralized is consistent with the performance asymmetries of the nondominant and dominant arms. Early work by Flowers (1975) attributed interlimb differences during reciprocal aiming tasks to a dominant system advantage in the speed of visuomotor processing. This proposal was supported by findings indicating that dominant arm movements could be completed at a faster rate, but with similar accuracies to that of the nondominant arm, and that such asymmetries were reduced during very rapid ballistic movements, mediated predominantly through feedforward processes (Elliott et al. 1994; Hodges et al. 1997; Mieschke et al. 2001; Roy 1983; Todor and Cisneros 1985). Dominant specialization for sensory feedback processing would be consistent with dominant arm advantages in both the accuracy and the timing of corrections to visual rotations. However, these findings contradict this prediction. Corrective responses in the nondominant and dominant arms occurred at similar times and resulted in similar final position accuracies. Moreover, the presence of the rotation had no effect on our measures of final position accuracy, indicating that both arms effectively corrected for the rotations. Differences in coordination of corrections occurred for directions with substantial coordination requirements but did not occur for the direction in which intersegmental dynamic effects were small. Thus asymmetries in coordination could not be attributed to differences in visuomotor processes but instead reflected the same coordination patterns evident during unperturbed movements. These findings support and extend previous studies, which have shown that motor lateralization cannot be accounted for by differences in visually mediated processes to correct movement errors (Buekers and Helsen 2000; Carnahan 1998; Carson et al. 1990, 1992, 1993; Elliott et al. 1993, 1994; Mieschke et al. 2001; Roy and Elliott 1986, 1989; Roy et al. 1994).
Dynamic dominance hypothesis
The dynamic dominance hypothesis predicts that each hemisphere has become specialized for specific aspects of control. Thus in contrast to earlier models, which suggested that one hemisphere controls the planning of movements in both arms, our model suggests that ipsilateral and contralateral hemisphere activations during unilateral reaching movements are associated with planning different features of movement. Recently, this idea has been examined by assessing the effects of unilateral brain damage on the ipsilesional or “unaffected” arm in stroke patients. Significant damage to the sensorimotor cortex typically results in varying degrees of hemiparesis in the contralesional arm of stroke patients, suggesting a large role for the contralateral hemisphere in movement execution. However, deficits in the ipsilesional arm would suggest that the ipsilateral hemisphere, although to a lesser extent, also contributes to movement. In fact, these studies have shown coordination deficits in the ipsilesional arm of patients (Fisk and Goodale 1988; Haaland and Harrington 1989a,b; Schaefer et al. 2007; Winstein and Pohl 1995; Yarosh et al. 2004) that were significant enough to diminish functional performance of activities of daily living (Desrosiers et al. 1996; Sainburg and Duff 2006; Sunderland 2000; Wetter et al. 2005). Interestingly, these deficits seem to vary with the hemisphere that is lesioned, suggesting that contributions from each hemisphere are lateralized. Schaefer et al. (2007) recently found deficits in the initial trajectory features of ipsilesional arm movements following dominant but not nondominant hemisphere damage. Conversely, patients with damage to the nondominant hemisphere showed deficits in final position accuracy when reaching with their ipsilesional arm, whereas patients with dominant hemisphere damage did not. These results reflect the predictions of the dynamic dominance hypothesis of motor lateralization, which suggests that the dominant hemisphere has become specialized for controlling task dynamics, as required for coordinating efficient trajectories, whereas the nondominant hemisphere has become specialized for controlling limb impedance, as required for achieving stable postures. This study further supports this hypothesis by showing that trajectory efficiency of visual-mediated corrections varied according to the coordination requirements of the task. Dominant arm corrections were significantly more efficient than nondominant arm corrections when inertial interactions of the limb segments were substantial.
In summary, this experiment both supports and extends our dynamic dominance model of lateralization by showing that dominant arm advantages in coordination are not dependent on visuomotor correction processes. Thus the results of this experiment do not support the hypothesis that asymmetries in reaching behaviors result from specialization of the dominant hemisphere for visual-based correction processes, as previously proposed.
GRANTS
This research was supported by National Institute of Child Health and Human Development Grant R01 HD-39311.
Acknowledgments
We thank F. Sarlegna and S. Schaefer for scholarly discussions regarding this manuscript.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- Bagesteiro 2002.Bagesteiro LB, Sainburg RL. Handedness: dominant arm advantages in control of limb dynamics. J Neurophysiol 88: 2408–2421, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagesteiro 2003.Bagesteiro LB, Sainburg RL. Nondominant arm advantages in load compensation during rapid elbow joint movements. J Neurophysiol 90: 1503–1513, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagesteiro 2005.Bagesteiro LB, Sainburg RL. Interlimb transfer of load compensation during rapid elbow joint movements. Exp Brain Res 161: 155–165, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner 1997.Brenner E, Smeets JB. Fast responses of the human hand to changes in target position. J Motor Behav 29: 297–310, 1997. [DOI] [PubMed] [Google Scholar]
- Brenner 2003.Brenner E, Smeets JB. Fast corrections of movements with a computer mouse. Spat Vis 16: 365–376, 2003. [DOI] [PubMed] [Google Scholar]
- Buekers 2000.Buekers MJ, Helsen WF. Vision and laterality: does occlusion disclose a feedback processing advantage for the right hand system? Cortex 36: 507–519, 2000. [DOI] [PubMed] [Google Scholar]
- Carlsen 2004.Carlsen AN, Chua R, Inglis JT, Sanderson DJ, Franks IM. Prepared movements are elicited early by startle. J Motor Behav 36: 253–264, 2004. [DOI] [PubMed] [Google Scholar]
- Carlsen 2003.Carlsen AN, Hunt MA, Inglis JT, Sanderson DJ, Chua R. Altered triggering of a prepared movement by a startling stimulus. J Neurophysiol 89: 1857–1863, 2003. [DOI] [PubMed] [Google Scholar]
- Carlton 1981.Carlton LG Processing visual feedback information for movement control. J Exp Psychol 7: 1019–1030, 1981. [DOI] [PubMed] [Google Scholar]
- Carnahan 1998.Carnahan H Manual asymmetries in response to rapid target movement. Brain Cogn 37: 237–253, 1998. [DOI] [PubMed] [Google Scholar]
- Carson 1989.Carson RG Manual asymmetries: Feedback processing, output variability, and spatial complexity-resolving some inconsistencies. J Motor Behav 21: 38–47, 1989. [DOI] [PubMed] [Google Scholar]
- Carson 1990.Carson RG, Chua R, Elliott D, Goodman D. The contribution of vision to asymmetries in manual aiming. Neuropsychologia 28: 1215–1220, 1990. [DOI] [PubMed] [Google Scholar]
- Carson 1993.Carson RG, Goodman D, Chua R, Elliott D. Asymmetries in the regulation of visually guided aiming. J Motor Behav 25: 21–32, 1993. [DOI] [PubMed] [Google Scholar]
- Carson 1992.Carson RG, Goodman D, Elliott D. Asymmetries in the discrete and pseudocontinuous regulation of visually guided reaching. Brain Cogn 18: 169–191, 1992. [DOI] [PubMed] [Google Scholar]
- Day 2000.Day BL, Lyon IN. Voluntary modification of automatic arm movements evoked by motion of a visual target. Exp Brain Res 130: 159–168, 2000. [DOI] [PubMed] [Google Scholar]
- Derakhshan 2006.Derakhshan I Laterality of the command center in relation to handedness and simple reaction time: a clinical perspective. J Neurophysiol 96: 3556, 2006. [DOI] [PubMed] [Google Scholar]
- Desmurget 2000.Desmurget M, Grafton S. Forward modeling allows feedback control for fast reaching movements. Trends Cogn Sci 4: 423–431, 2000. [DOI] [PubMed] [Google Scholar]
- Desrosiers 1996.Desrosiers J, Bourbonnais D, Bravo G, Roy PM, Guay M. Performance of the ‘unaffected’ upper extremity of elderly stroke patients. Stroke 27: 1564–1570, 1996. [DOI] [PubMed] [Google Scholar]
- Duff 2007.Duff SV, Sainburg RL. Lateralization of motor adaptation reveals independence in control of trajectory and steady-state position. Exp Brain Res 179: 551–561, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott 1994.Elliott D, Chua R, Pollock BJ. The influence of intermittent vision on manual aiming. Acta Psychol 85: 1–13, 1994. [DOI] [PubMed] [Google Scholar]
- Elliott 1993.Elliott D, Roy EA, Goodman D, Chua R, Maraj BKV. Asymmetries in the preparation and control of manual aiming movements. Can J Physiol Pharmacol 47: 570–589, 1993. [Google Scholar]
- Fisk 1988.Fisk JD, Goodale MA. The effects of unilateral brain damage on visually guided reaching: hemispheric differences in the nature of the deficit. Exp Brain Res 72: 425–435, 1988. [DOI] [PubMed] [Google Scholar]
- Flowers 1975.Flowers K Handedness and controlled movement. Br J Psychol 66: 39–52, 1975. [DOI] [PubMed] [Google Scholar]
- Goodale 1986.Goodale MA, Pelisson D, Prablanc C. Large adjustments in visually guided reaching do not depend on vision of the hand or perception of target displacement. Nature 320: 748–750, 1986. [DOI] [PubMed] [Google Scholar]
- Gordon 1994.Gordon J, Ghilardi MF, Cooper SE, Ghez C. Accuracy of planar reaching movements. II. Systematic extent errors resulting from inertial anisotropy. Exp Brain Res 99: 112–130, 1994. [DOI] [PubMed] [Google Scholar]
- Haaland 1989a.Haaland KY, Harrington D. The role of the hemispheres in closed loop movements. Brain Cogn 9: 158–180, 1989a. [DOI] [PubMed] [Google Scholar]
- Haaland 1989b.Haaland KY, Harrington DL. Hemispheric control of the initial and corrective components of aiming movements. Neuropsychologia 27: 961–969, 1989b. [DOI] [PubMed] [Google Scholar]
- Halpern 2005.Halpern ME, Gunturkun O, Hopkins WD, Rogers LJ. Lateralization of the vertebrate brain: taking the side of model systems. J Neurosci 25: 10351–10357, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges 1997.Hodges NJ, Lyons J, Cockell D, Reed A, Elliott D. Hand, space and attentional asymmetries in goal-directed manual aiming. Cortex 33: 251–269, 1997. [DOI] [PubMed] [Google Scholar]
- Hogan 1985.Hogan N The mechanics of multi-joint posture and movement control. Biol Cybern 52: 315–331, 1985. [DOI] [PubMed] [Google Scholar]
- Hopkins 2003.Hopkins WD, Hook M, Braccini S, Schapiro SJ. Population-level right handedness for a coordinated bimanual task in chimpanzees: replication and extension in a second colony of apes. Int J Primatol 24: 677–689, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins 2000.Hopkins WD, Pearson K. Chimpanzee (Pan troglodytes) handedness: variability across multiple measures of hand use. J Comp Psychol 114: 126–135, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson 2002.Johnson H, Van Beers RJ, Haggard P. Action and awareness in pointing tasks. Exp Brain Res 146: 451–459, 2002. [DOI] [PubMed] [Google Scholar]
- Keele 1968.Keele SW, Posner MI. Processing of visual feedback in rapid movements. J Exp Psychol 77: 155–158, 1968. [DOI] [PubMed] [Google Scholar]
- Khan 2003.Khan MA, Lawrence G, Fourkas A, Franks IM, Elliott D, Pembroke S. Online versus offline processing of visual feedback in the control of movement amplitude. Acta Psychol 113: 83–97, 2003. [DOI] [PubMed] [Google Scholar]
- Komilis 1993.Komilis E, Pelisson D, Prablanc C. Error processing in pointing at randomly feedback-induced double-step stimuli. J Motor Behav 25: 299–308, 1993. [DOI] [PubMed] [Google Scholar]
- Liepmann 1905.Liepmann H Die linke hemisphäre und das handeln. Münchener Medizinische Wochenschrift 49: 2375–2378, 1905. [Google Scholar]
- Miall 1993.Miall RC, Weir DJ, Wolpert DM, Stein JF. Is the cerebellum a Smith predictor? J Motor Behav 25: 203–216, 1993. [DOI] [PubMed] [Google Scholar]
- Mieschke 2001.Mieschke PE, Elliott D, Helsen WF, Carson RG, Coull JA. Manual asymmetries in the preparation and control of goal-directed movements. Brain Cogn 45: 129–140, 2001. [DOI] [PubMed] [Google Scholar]
- Oldfield 1971.Oldfield RC The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9: 97–113, 1971. [DOI] [PubMed] [Google Scholar]
- Prablanc 2003.Prablanc C, Desmurget M, Grea H. Neural control of on-line guidance of hand reaching movements. Prog Brain Res 142: 155–170, 2003. [DOI] [PubMed] [Google Scholar]
- Prablanc 1992.Prablanc C, Martin O. Automatic control during hand reaching at undetected two-dimensional target displacements. J Neurophysiol 67: 455–469, 1992. [DOI] [PubMed] [Google Scholar]
- Rogers 2004.Rogers LJ, Zucca P, Vallortigara G. Advantages of having a lateralized brain. Proceedings Biological Sciences/The Royal Society 271 (Suppl 6): S420–S422, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy 1983.Roy EA Manual performance asymmetries and motor control processes: subject-generated changes in response parameters. Hum Mov Sci 2: 271–277, 1983. [Google Scholar]
- Roy 1986.Roy EA, Elliott D. Manual asymmetries in visually directed aiming. Can J Psychol 40: 109–121, 1986. [DOI] [PubMed] [Google Scholar]
- Roy 1989.Roy EA, Elliott D. Manual asymmetries in aimed movements. Q J Exp Psychol 41A: 501–516, 1989. [Google Scholar]
- Roy 1994.Roy EA, Kalbfleisch L, Elliott D. Kinematic analyses of manual asymmetries in visual aiming movements. Brain Cogn 24: 289–295, 1994. [DOI] [PubMed] [Google Scholar]
- Sainburg 2002.Sainburg RL Evidence for a dynamic-dominance hypothesis of handedness. Exp Brain Res 142: 241–258, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainburg 2005.Sainburg RL Handedness: differential specializations for control of trajectory and position. Exerc Sport Sci Rev 33: 206–213, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainburg 2006.Sainburg RL, Duff SV. Does motor lateralization have implications for stroke rehabilitation? J Rehab Res Dev 43: 311–322, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainburg 1999.Sainburg RL, Ghez C, Kalakanis D. Intersegmental dynamics are controlled by sequential anticipatory, error correction, and postural mechanisms. J Neurophysiol 81: 1045–1056, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainburg 1995.Sainburg RL, Ghilardi MF, Poizner H, Ghez C. Control of limb dynamics in normal subjects and patients without proprioception. J Neurophysiol 73: 820–835, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainburg 2000.Sainburg RL, Kalakanis D. Differences in control of limb dynamics during dominant and nondominant arm reaching. J Neurophysiol 83: 2661–2675, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarlegna 2007.Sarlegna FR, Sainburg RL. The effect of target modality on visual and proprioceptive contributions to the control of movement distance. Exp Brain Res 176: 267–280, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders 2003.Saunders JA, Knill DC. Humans use continuous visual feedback from the hand to control fast reaching movements. Exp Brain Res 152: 341–352, 2003. [DOI] [PubMed] [Google Scholar]
- Saunders 2004.Saunders JA, Knill DC. Visual feedback control of hand movements. J Neurosci 24: 3223–3234, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schabowsky 2007.Schabowsky CN, Hidler JM, Lum PS. Greater reliance on impedance control in the nondominant arm compared with the dominant arm when adapting to a novel dynamic environment. Exp Brain Res 182: 567–577, 2007. [DOI] [PubMed] [Google Scholar]
- Schaefer 2007.Schaefer SY, Haaland KY, Sainburg RL. Ipsilesional motor deficits following stroke reflect hemispheric specializations for movement control. Brain 130: 2146–2158, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro 2004.Shapiro MB, Gottlieb GL, Corcos DM. EMG responses to an unexpected load in fast movements are delayed with an increase in the expected movement time. J Neurophysiol 91: 2135–2147, 2004. [DOI] [PubMed] [Google Scholar]
- Shapiro 2002.Shapiro MB, Gottlieb GL, Moore CG, Corcos DM. Electromyographic responses to an unexpected load in fast voluntary movements: descending regulation of segmental reflexes. J Neurophysiol 88: 1059–1063, 2002. [DOI] [PubMed] [Google Scholar]
- Soechting 1983.Soechting JF, Lacquaniti F. Modification of trajectory of a pointing movement in response to a change in target location. J Neurophysiol 49: 548–564, 1983. [DOI] [PubMed] [Google Scholar]
- Sunderland 2000.Sunderland A Recovery of ipsilateral dexterity after stroke. Stroke 31: 430–433, 2000. [DOI] [PubMed] [Google Scholar]
- Todor 1985.Todor JI, Cisneros J. Accommodation to increased accuracy demands by the right and left hands. J Motor Behav 17: 355–372, 1985. [DOI] [PubMed] [Google Scholar]
- Vallortigara 2005.Vallortigara G, Rogers LJ. Survival with an asymmetrical brain: advantages and disadvantages of cerebral lateralization. Behav Brain Sci 28: 575–589, 2005. [DOI] [PubMed] [Google Scholar]
- Valls-Sole 1999.Valls-Sole J, Rothwell JC, Goulart F, Cossu G, Munoz E. Patterned ballistic movements triggered by a startle in healthy humans. J Physiol 516: 931–938, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang 2004.Wang J, Sainburg RL. Interlimb transfer of novel inertial dynamics is asymmetrical. J Neurophysiol 92: 349–360, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang 2007.Wang J, Sainburg RL. The dominant and nondominant arms are specialized for stabilizing different features of task performance. Exp Brain Res 178: 565–570, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetter 2005.Wetter S, Poole JL, Haaland KY. Functional implications of ipsilesional motor deficits after unilateral stroke. Arch Phys Med Rehabil 86: 776–781, 2005. [DOI] [PubMed] [Google Scholar]
- Winstein 1995.Winstein CJ, Pohl PS. Effects of unilateral brain damage on the control of goal-directed hand movements. Exp Brain Res 105: 163–174, 1995. [DOI] [PubMed] [Google Scholar]
- Winter 1990.Winter D Biomechanics and Motor Control of Human Movement. New York: Wiley, 1990.
- Wolpert 1996.Wolpert DM, Miall RC. Forward models for physiological motor control. Neural Netw 9: 1265–1279, 1996. [DOI] [PubMed] [Google Scholar]
- Woodworth 1899.Woodworth RS The accuracy of voluntary movement. Psychol Rev: 1–119, 1899.
- Yarosh 2004.Yarosh CA, Hoffman DS, Strick PL. Deficits in movements of the wrist ipsilateral to a stroke in hemiparetic subjects. J Neurophysiol 92: 3276–3285, 2004. [DOI] [PubMed] [Google Scholar]
- Zelaznik 1983.Zelaznik HZ, Hawkins B, Kisselburgh L. Rapid visual feedback processing in single-aiming movements. J Motor Behav 15: 217–236, 1983. [DOI] [PubMed] [Google Scholar]