Abstract
The interaural time difference (ITD) is the primary auditory cue used by the barn owl for localization in the horizontal direction. ITD is initially computed by circuits consisting of axonal delay lines from one of the cochlear nuclei and coincidence detector neurons in the nucleus laminaris (NL). NL projects directly to the anterior part of the dorsal lateral lemniscal nucleus (LLDa), and this area projects to the core of the central nucleus of the inferior colliculus (ICcc) in the midbrain. To show the selectivity of an NL neuron for ITD requires averaging of responses over several stimulus presentations for each ITD. In contrast, ICcc neurons detect their preferred ITD in a single burst of stimulus. We recorded extracellularly the responses of LLDa neurons to ITD in anesthetized barn owls and show that this ability is already present in LLDa, raising the possibility that ICcc inherits its noise reduction property from LLDa.
INTRODUCTION
The interaural time difference (ITD) is the primary auditory spatial cue used by the barn owl for localization in the horizontal direction (Moiseff 1989; Moiseff and Konishi 1981; Poganiatz et al. 2001). ITD is initially computed by neural circuits that consist of axonal delay lines provided by cochlear nucleus magnocellularis and coincidence detectors provided by nucleus laminaris (NL) and is then processed in multiple stages of the hind- and midbrain auditory localization pathway leading to the optic tectum (Carr and Konishi 1990; Moiseff and Konishi 1983; Fujita and Konishi 1991; Olsen et al. 1989). Near the apex of this pathway, in the auditory space map in the external nucleus of the inferior colliculus (ICx), single neurons show sensitivity to ITD in spike count responses that meets or exceeds the behavioral discrimination performance of the owl (Bala et al. 2003). Moreover, barn owls localize a sound source after hearing a single noise burst <100 ms (Knudsen et al. 1979). In contrast, it takes coincidence detector neurons in NL several noise bursts of 100 ms in duration before their ITD preference can be seen by averaging their responses (Christianson and Peña 2006). These behavioral and physiological observations suggest that one component of the transformation of spike count responses to ITD within the localization pathway is a noise reduction in the representation of the auditory spatial localization cue ITD.
Noise reduction in spike count ITD tuning curves, relative to coincidence detector responses, is observed in the core of the central nucleus of the inferior colliculus (ICcc), suggesting that ICcc neurons pool over the responses of multiple coincidence detector neurons (Christianson and Peña 2006). ICcc receives input from NL (Takahashi and Konishi 1988a) and the anterior part of the dorsal lateral lemniscal nucleus [LLDa, previously known as nucleus ventralis lemnisci lateralis, pars anterior, or VLVa (Wild et al. 2001)] (Adolphs 1993), which also receives input from the contralateral NL (Takahashi and Konishi 1988b). It is unclear what role LLDa plays in transforming spike count responses to ITD within the owl's localization pathway because the variability of single neuron responses to ITD has not been described. It is therefore an open question whether noise reduction of coincidence detector responses to ITD occurs in LLDa.
To determine whether noise reduction of ITD tuning occurs in LLDa, and to compare the spike count responses to ITD of single neurons in NL, LLDa, and ICcc, we recorded extracellularly the responses of LLDa neurons to ITD and compared the responses to those measured for NL and ICcc neurons by Christianson and Peña (2006).
METHODS
Extracellular recordings of isolated neurons were made in four anesthetized adult female barn owls (Tyto alba). Experimental procedures followed the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of the California Institute of Technology. Methods for surgery, stimulus delivery, and aspects of data collection followed previously published descriptions (Christianson and Peña 2006; Fischer et al. 2007).
Surgery
Owls were anesthetized with intramuscular injections of ketamine (20 mg/kg; Ketaject; Phoenix Pharmaceuticals, St. Joseph, MO) and xylazine (2 mg/kg; Xyla-Ject; Phoenix Pharmaceuticals). Owls were also given an intramuscular injection of antibiotics (oxytetracycline, 20 mg; maxim-200; Phoenix Pharmaceuticals) and a subcutaneous injection of lactated Ringer solution (B. Braun Medical, Irvine, CA) at the beginning of each session. Subsequent injections of ketamine and xylazine were given as needed to maintain an adequate level of anesthesia (approximately every 2 h). The owl was restrained with a soft leather jacket and covered with a water-based heating pad (k-20-f; American Medical Systems, Cincinnati, OH) to maintain the body temperature. In the initial experiment, a head plate and zero post were fixed to the owl's skull to facilitate stereotaxic localization of the recording area. The head plate holds the owl's head with the beak rotated 30° below horizontal in the sagittal plane (Arthur 2004). A craniotomy was made to expose the area between 1 and 3 mm lateral to the zero post. At each recording session, the dura was incised and reflected with a hypodermic needle, and an electrode was lowered to the desired depth. At the end of the recording session, the opening in the skull was filled with dental cement and the wound was sutured. The owl remained isolated in a heated cage overnight.
Acoustic stimuli
Custom software was used to generate sound stimuli (Arthur 2004). Stimuli consisted of broadband noise and tones 100 ms in duration with 5-ms linear rise and fall ramps. Broadband noise stimuli had a passband of 0.5–12 kHz. Stimulus ITD was varied in steps of 30 μs and frequency in steps of 500 Hz.
All recordings took place in a double-walled sound-attenuating chamber (Industrial Acoustics, Bronx, NY). Acoustic stimuli were delivered by a stereo analog interface (DD1; Tucker Davis Technologies, Alachua, FL) through a calibrated earphone assembly. Each earphone consisted of a transducer (Knowles 1914; Knowles, Itasca, IL) and a microphone (Knowles 1319) encased in a custom-made metal delivery piece that fits in the owl's ear canal. Gaps between the earphone assembly and the ear canal were filled with silicon impression material (gold Velvet II; All American Mold Laboratories, Oklahoma City, OK). The microphone was used to measure the transducer's frequency response. The inverse of the transducer's frequency response was then applied to outgoing stimuli (Arthur 2004).
Data collection
Extracellular recordings of single LLDa neurons were made with tungsten electrodes (1 MΩ, 0.005-in; A-M Systems, Carlsborg, WA). Neurons were considered isolated based on the presence of a refractory period in the interspike interval histogram and the similarity of spike shape.
The data for this study consist of spike counts in response to sound stimuli with fixed ITD and, for tones, frequency. The following protocol was employed once a neuron was isolated. The spike count response to ITD values between ±250 μs (positive corresponds to right ear leading and negative corresponds to left ear leading) of a broadband noise stimulus was collected at a stimulus level that was 15–20 dB above threshold. The average spike count response over trials to ITD values between ±250 μs is called the ITD tuning curve. Also the response to the frequency of a tonal stimulus at a fixed stimulus intensity was collected at the best ITD at a stimulus level that was 15–20 dB above threshold. The average spike count response over trials to stimulus frequency at a fixed stimulus intensity is called the isointensity frequency tuning curve. If possible, the response of the neuron to a larger range of ITD values, ±2,100 μs, was collected using broadband noise stimuli. The average spike count response over trials to the larger range of ITD is called the long-range ITD tuning curve. ITD tuning curves and isointensity frequency tuning curves were obtained with randomized sequences of ITD and frequency, respectively. Spike counts were averaged over 5–10 repetitions for each parameter value.
Data analysis
FREQUENCY TUNING.
The best frequency is the frequency that elicits the maximum spike count in the isointensity frequency tuning curve.
TRIAL-TO-TRIAL VARIABILITY OF ITD TUNING CURVES.
We examined the convergence of ITD tuning curves obtained using 1–9 trials to the 10-trial average for neurons where 10 trials were collected for each ITD value. The similarity between the tuning curve obtained with 10 trials, denoted r10, and the tuning curve obtained using a randomly selected set of n trials, denoted rn, is given by the correlation coefficient of the responses (Christianson and Peña 2006)
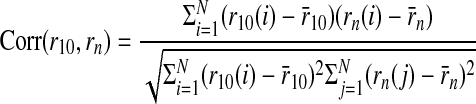 |
(1) |
where rn(i) is the response of the n-trial tuning curve at the ith ITD, r̄n is the average response of the n-trial tuning curve over all ITDs, and N is the number of ITD values used to compute the ITD tuning curve. We computed the average value of Corr(r10,rn) over multiple instances of n randomly selected trials.
We quantified the regularity of spike count responses to ITD using the Fano factor. The Fano factor for each ITD was computed as the ratio of the sample variance of the spike count to the sample mean of the spike count.
ITD TUNING CURVE SHAPE.
The trough firing rate is the minimum value of the sinusoid-shaped ITD tuning curve expressed in spikes per second.
ITD tuning curves of neurons in the barn owl's interaural time difference pathway show varying degrees of rectification (Christianson and Peña 2006). Rectification of long-range ITD tuning curves was quantified by the rectification index (RI), which measures the symmetry of the tuning curve about the mean value of the curve at large ITDs (Christianson and Peña 2006). The rectification index is the average of the responses to the 15 most negative ITDs (less than –1,000 μs) and the 15 most positive ITDs (>1,000 μs) of the long-range ITD curve after normalization to have values between 0 and 1, denoted R10, yielding
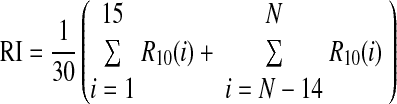 |
(2) |
where N is the number of ITD values used to compute the long-range ITD tuning curve. The RI takes a value of 0.5 for an ITD curve that is symmetrical about the mean value of the curve at large ITDs. The RI is <0.5 if the curve is rectified and is >0.5 if the mean value of the curve at large ITDs is closer to the peak than to the trough.
The peak-trough difference is the difference between the maximum and minimum values of the ITD tuning curve expressed in spikes per second.
The half-height width of the ITD tuning curve is the width of the peak in the tuning curve at half the distance between the minimum and maximum responses. If one flank of the tuning curve remains above the half-maximum height over the range of ITD values used, then the tuning curve half-height width is undefined. The half-height width is expressed as a percent of period by multiplying the ITD range by the neuron's best frequency.
FISHER INFORMATION.
We investigated the accuracy of ITD estimation from spike count responses of single neurons using the Fisher information. The Fisher information (FI) for a single neuron is defined as a function of stimulus ITD as
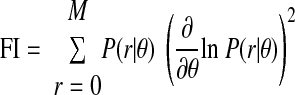 |
(3) |
where r denotes the spike count, M is the maximum spike count, and θ denotes ITD. The probability distribution over spike counts given the stimulus ITD, P(r|θ), was estimated by convolving the histogram of spike count responses with a Gaussian-shaped filter having SDs of 0.5 spikes and 15 μs and then normalizing each column to sum to one (Dean et al. 2005).
ESTIMATING THE NUMBER OF CONVERGING NL NEURONS IN LLDA.
We estimated the number of NL neurons converging on an LLDa neuron by assuming that LLDa responses are a linear combination of n NL inputs. The linearity assumption is supported by the similarity of ITD tuning curve shapes in NL and LLDa (see results). Specifically, we assume that the spike count response of an LLDa neuron, denoted rLLDa, can be written in terms of NL responses as 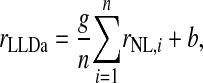 where the constants g and b reflect the differences in peak and trough firing rates of NL and LLDa neurons. We also assume, for computational convenience, that all NL pairs have the same spike count correlation coefficient ρ. While there are no data available to constrain the spike count correlation coefficient, we assume that LLDa neurons pool NL neurons with the same best frequency and best ITD, and therefore it is reasonable to assume that the spike count correlation coefficient does not vary greatly among pairs in this small population. Under this model, the variance of the LLDa spike count response, denoted σLLDa2, can be written in terms of the average NL spike count variance, denoted 〈σNL,i2〉, and the average NL spike count covariance, denoted ρ〈σNL,i, σNL,j〉, as
where the constants g and b reflect the differences in peak and trough firing rates of NL and LLDa neurons. We also assume, for computational convenience, that all NL pairs have the same spike count correlation coefficient ρ. While there are no data available to constrain the spike count correlation coefficient, we assume that LLDa neurons pool NL neurons with the same best frequency and best ITD, and therefore it is reasonable to assume that the spike count correlation coefficient does not vary greatly among pairs in this small population. Under this model, the variance of the LLDa spike count response, denoted σLLDa2, can be written in terms of the average NL spike count variance, denoted 〈σNL,i2〉, and the average NL spike count covariance, denoted ρ〈σNL,i, σNL,j〉, as
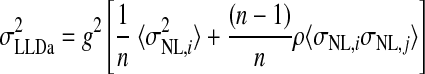 |
(4) |
where 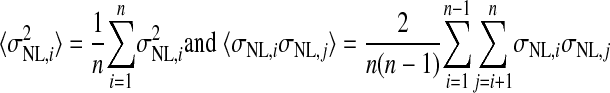 (see APPENDIX). Therefore the number of converging inputs is found by solving this equation for n, yielding
(see APPENDIX). Therefore the number of converging inputs is found by solving this equation for n, yielding
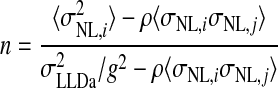 |
(5) |
which is greater than zero for ρ < σLLDa2/(g2〈σNL,i σNL,j〉). If the NL inputs are matched in best frequency and best ITD, then the constant g that produces the desired peak-trough difference in LLDa is given by the ratio of the peak-trough difference of the LLDa neuron to the average of the NL peak-trough differences, yielding 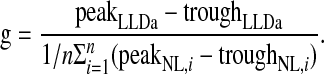 The value of b does not factor into the calculation of the number of converging NL neurons and is left as a free parameter that determines the LLDa neuron's trough firing rate.
The value of b does not factor into the calculation of the number of converging NL neurons and is left as a free parameter that determines the LLDa neuron's trough firing rate.
Histology
To verify recording sites, iontophoretic injections of 10,000 molecular-weight (MW) biotinylated dextran amine (BDA) (Molecular Probes, Eugene, OR) [10% in 25 mM phosphate buffer (PB), pH 7.5] were made in LLDa in two owls and iontophoretic injection of Alexa Fluor 488-dextran amine (Molecular Probes) (10,000 MW in a 10% solution of PB) was made in LLDa in one owl (Akutagawa and Konishi 2005). The tracers were iontophoresed with positive-current 5-μA pulses (7 s on/7 s off) for 10 min through a glass pipette (15 μm diam). Survival times varied from 6 to 9 days. Animals were given a lethal dose of pentobarbital (Abbott, Abbott Park, IL) and perfused through the left ventricle of the heart with 0.9% NaCl until clearing, followed by 1 h of 2% paraformaldehyde (PF) in 25 mM PB. After 1 day of postfixing at 4°C, the brains were cryoprotected by immersing them in a solution of 30% sucrose in 2% PF in PB for 2 days (4°C). The brains were cut on a freezing microtome (30 μm), and the sections were serially collected in cold PB. Fluorescent-labeled sections were mounted as soon as possible from PB onto precleaned slides, dried for 30 min in the dark, and coverslipped with Vectashield (Vector Laboratories, Burlingame, CA). Brain sections with BDA microinjections were similarly cut and collected in PB. After a 15-min wash in PB, the sections were incubated in the ABC Elite kit (Vector Laboratories) for 1 h at room temperature, followed by three washes in PB with 0.1% Triton X-100 (TX; Sigma, St. Louis, MO) for 15 min each. The reaction product was visualized by incubating the sections in 0.02% 3–3′-diaminobenzidine tetrahydrochloride (Sigma) and 0.009% hydrogen peroxide in PB with or without cobalt chloride intensification. The sections were washed twice for 15 min in PB, mounted onto subbed slides, and dried. The slides were coverslipped unstained in Permount (Sigma).
RESULTS
We measured ITD tuning curves and isointensity frequency tuning curves for 31 isolated LLDa neurons. Injections of anatomical tracers verified LLDa as the recording site (Fig. 1). We compared the ITD tuning curves measured for LLDa neurons with those obtained for 83 NL neurons and 28 ICcc neurons by Christianson and Peña (2006). ITD tuning curves for LLDa, NL, and ICcc neurons were obtained using broadband noise stimuli (Christianson and Peña 2006). ITD tuning curves were compared over the physiological range of ITDs, ±250 μs (Keller et al. 1998; Poganiatz et al. 2001) with the exception of the analysis of ITD tuning curve rectification. Sample sizes are indicated when subsets of the data are used.
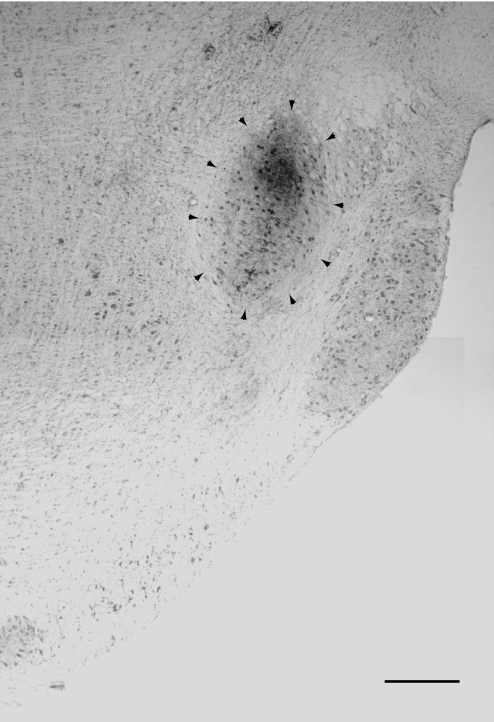
FIG. 1.
Identification of the recording site as the anterior part of the dorsal lateral lemniscal nucleus (LLDa) by injection of biotinylated dextran amine. The figure is a montage of 2 photographs. The scale bar represents 0.5 mm. Dorsal is up and medial is left.
Trial-to-trial variability of ITD tuning
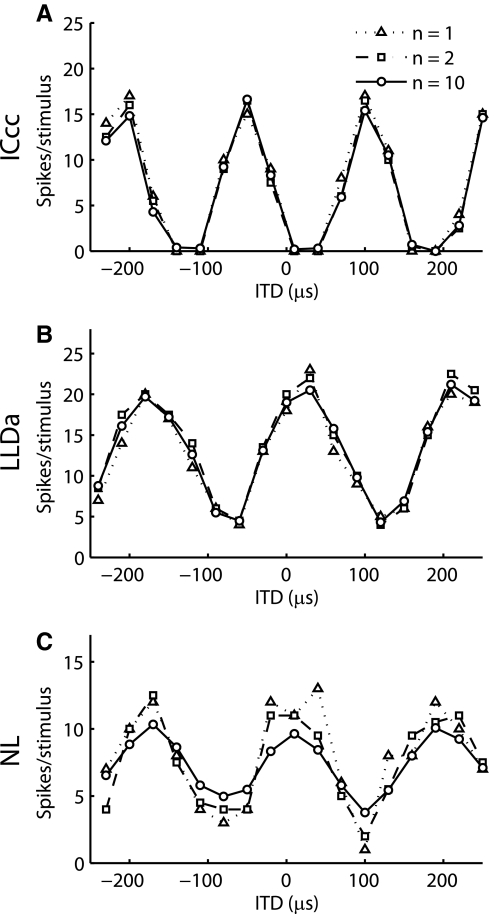
Spike count responses to ITD in LLDa showed little trial-to-trial variability. For most LLDa neurons, the ITD tuning curves obtained in single trials resemble the 10-trial average tuning curve (Figs. 2B and 3). We quantified the similarity between the ITD tuning curve obtained using n trials, denoted rn, and the ITD tuning curve obtained using ten trials, denoted r10, using the correlation coefficient of rn and r10 (see methods). Consistent with the similarity between r1 and r10 seen in Fig. 2B, there is a very high median correlation coefficient of r1 and r10 in LLDa [median: 0.94; interquartile range (IQR): 0.86–0.97; Fig. 3A]. A similarly high correlation coefficient of r1 and r10 was observed in ICcc [ICcc, 23 neurons; median: 0.96; IQR: 0.93–0.98; LLDa and ICcc medians not significantly different by Kruskal-Wallis (KW), P > 0.15] (Christianson and Peña 2006). The median correlation coefficient of r1 and r10 is significantly larger in both LLDa and ICcc than in NL (NL, 24 neurons; median: 0.76; IQR: 0.68–0.81; NL and LLDa medians significantly different by KW, P < 10−7; NL and ICcc medians significantly different by KW, P < 10−7). The value of the correlation coefficient of r1 and r10 was robust to selection of the trial used to compute r1 as quantified by the ratio of the mean value of the correlation coefficient over trials to the SD of the correlation coefficient over trials (LLDa, median: 32.81; NL, 24 neurons; median: 7.25; ICcc, 23 neurons; median: 38.35) (Christianson and Peña 2006).
FIG. 2.
Interaural time difference (ITD) tuning curves in nucleus laminaris (NL), LLDa, and the core of the central nucleus of the inferior colliculus (ICcc) obtained using broadband noise stimuli. Example n-trial ITD curves for ICcc (A), LLDa (B), and NL (C) neurons. The trials selected for the 1- and 2-trial tuning curves are those that gave the best correlation with the 10-trial tuning curve.
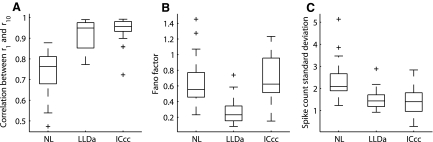
FIG. 3.
Trial-to-trial variability of ITD tuning. A: correlation between the ITD tuning curve obtained with a single trial and the 10-trial ITD tuning curve for NL, LLDa, and ICcc neurons. The box extends from the lower quartile to the upper quartile of the sample. Outliers (+) are data points that lie >1.5-fold the interquartile range of the sample beyond the box. B: the ITD-averaged Fano factor for NL, LLDa, and ICcc neurons. C: the ITD-averaged spike count SD for NL, LLDa, and ICcc neurons.
Trial-to-trial variability of spike count responses to ITD is smaller in LLDa than in both NL and ICcc when quantified with the Fano factor. The Fano factor is a noise-to-signal ratio that takes a value of one for a Poisson spike train (Dayan and Abbott 2001; Zador 1998). For each neuron, we computed the Fano factor at all ITD values and report the average value over ITD. Over populations, the medians of the ITD-averaged Fano factors for NL, LLDa, and ICcc are less than one, indicating a greater regularity in spiking than a Poisson spike train (Fig. 3B). The median ITD-averaged Fano factor is smaller in LLDa than in both NL and ICcc (LLDa, median: 0.23; IQR: 0.15–0.34; NL, 29 neurons; median: 0.55; IQR: 0.46–0.77; LLDa and NL medians significantly different by KW, P < 10−6; ICcc, 23 neurons; median: 0.62; IQR: 0.51–0.96; LLDa and ICcc medians significantly different by KW, P < 10−6). The median ITD-averaged Fano factor is not significantly different between ICcc and NL (KW, P > 0.27). Similarly, the Fano factor computed at the best ITD is smaller in LLDa than in both NL and ICcc and is not significantly different between ICcc and NL (LLDa, median: 0.23; IQR: 0.11–0.39; NL, 29 neurons; median: 0.42; IQR: 0.27–0.93; LLDa and NL medians significantly different by KW, P < 10−3; ICcc, 23 neurons; median: 0.36; IQR: 0.29 –0.56; LLDa and ICcc medians significantly different by KW, P < 10−2; NL and ICcc medians not significantly different by KW, P > 0.57).
We also computed the spike count SD to determine its contribution to differences in the Fano factor among NL, LLDa, and ICcc. The ITD-averaged spike count SD is similar in LLDa and ICcc (LLDa; median: 1.4 spikes/stimulus; IQR: 1.2–1.7 spikes/stimulus; ICcc, 23 neurons; median: 1.4 spikes/stimulus; IQR: 1.0–1.8 spikes/stimulus; LLDa and ICcc medians not significantly different by KW, P > 0.55; Fig. 3C). The ITD-averaged spike count SD is larger in NL than in both LLDa and ICcc (NL, 29 neurons; median: 2.1 spikes/stimulus; IQR: 1.9–2.7 spikes/stimulus; NL and LLDa medians significantly different by KW, P < 10−5; NL and ICcc medians significantly different by KW, P < 10−4).
The degree of trial-to-trial variability in ITD tuning displayed by NL, LLDa, and ICcc neurons was weakly correlated with both the neuron's best frequency and the neuron's best ITD. Best frequencies ranged from 1.9 to 6.1 kHz [3.63 ± 1.17 (SD) kHz] in LLDa, from 0.7 to 6.5 kHz (4.09 ± 1.36 kHz) in NL, and from 1.1 to 6.6 kHz (4.03 ± 1.65 kHz) in ICcc. Weak correlation was observed between the best frequency and 1) the correlation coefficient of r1 and r10 (LLDa, r = −0.53; NL, r = 0.03; ICcc, r = −0.41), 2) the ITD-averaged Fano factor (LLDa, r = 0.02; NL, r = 0.01; ICcc, r = −0.06), and 3) the ITD-averaged spike count SD (LLDa, r = 0.36; NL, r = −0.49; ICcc, r = −0.24). Best ITDs for NL, LLDa, and ICcc neurons were largely within the physiological range of ITDs, ±250 μs (Keller et al. 1998; Poganiatz et al. 2001) (LLDa, −210–270 μs; NL, −240–240 μs; ICcc, −210–200 μs). Weak correlation was observed between the absolute value of the best ITD and 1) the correlation coefficient of r1 and r10 (LLDa, r = 0.14; NL, r = 0.05; ICcc, r = 0.02), 2) the ITD-averaged Fano factor (LLDa, r = 0.12; NL, r = −0.23; ICcc, r = 0.36), and 3) the ITD-averaged spike count SD (LLDa, r = −0.13; NL, r = 0.21; ICcc, r = −0.28).
ITD tuning curve shape
The accuracy of ITD estimation from spike count responses of single neurons depends on the probability distribution of responses for each ITD, which encompasses not only the variability in the response to ITD but also the shape of the ITD curve (Dayan and Abbott 2001). Therefore we characterized ITD tuning curve shapes by quantifying tuning curve rectification, peak-trough differences, and tuning curve widths.
Rectification of ITD tuning curves
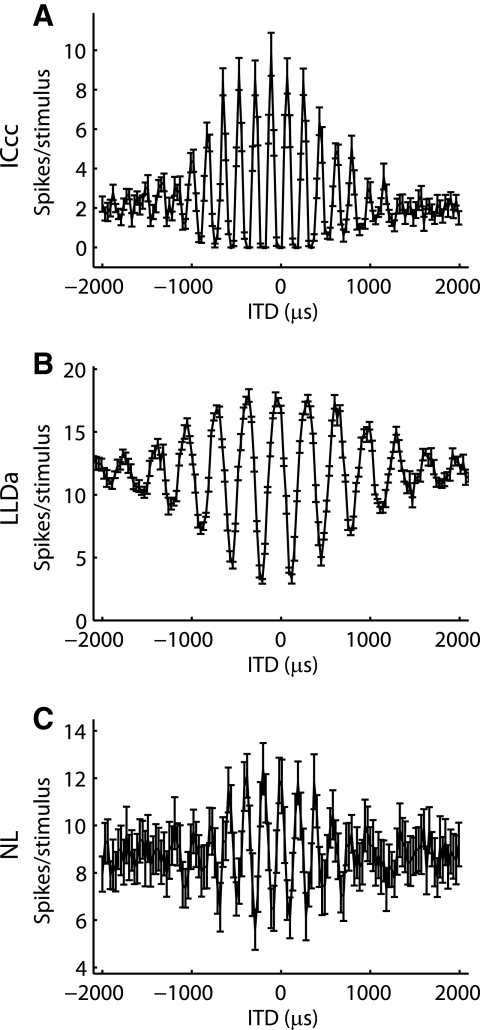
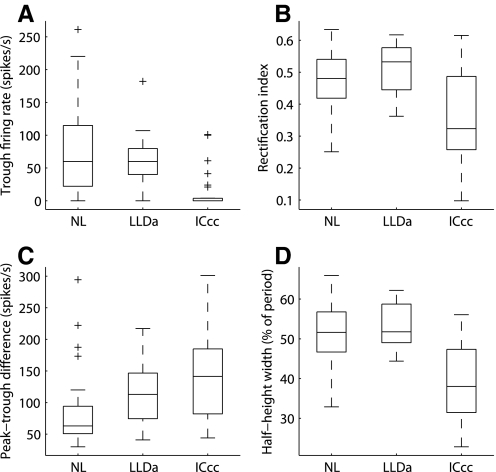
Consistent with previous descriptions, most NL and LLDa neurons have ITD tuning curves with troughs lying above 0 spikes/s, whereas most ICcc neurons have ITD tuning curves with large troughs where zero spikes are produced (Christianson and Peña 2006; Fujita and Konishi 1991; Moiseff and Konishi 1983) (Figs. 2, 4, and 5A). Consequently, ITD tuning curves in NL and LLDa appear symmetrical about their mean value, but ITD tuning curves in ICcc are asymmetrical, resembling a rectification of the curves seen in NL and LLDa. For quantitative comparison of tuning curve rectification in NL, LLDa, and ICcc neurons, we computed the trough firing rate and the rectification index (Fig. 5, A and B, see methods). The firing rate at the trough of the ITD tuning curve is above 0 spikes/s for most NL and LLDa neurons but is equal to 0 spikes/s for most ICcc neurons (LLDa, median: 60 spikes/s; IQR: 40–79.8 spikes/s; NL, median: 60 spikes/s; IQR: 22.3–114.9 spikes/s; LLDa and NL medians not significantly different by KW, P > 0.6; ICcc, median: 0 spikes/s; IQR: 0–3.8 spikes/s; ICcc and LLDa medians significantly different by KW, P < 10−6; ICcc and NL medians significantly different by KW, P < 10−8; Fig. 5A). The rectification index measures symmetry of the ITD tuning curve and takes a value of 0.5 for a symmetrical curve and a value <0.5 for curves that are rectified. The rectification index shows that ITD tuning curves in NL and LLDa do not display rectification, but ITD tuning curves in ICcc are rectified (LLDa, 15 neurons; median: 0.53; IQR 0.44–0.58; NL, 82 neurons; median: 0.48; IQR: 0.42–0.54; LLDa and NL medians not significantly different by KW, P > 0.17; ICcc, median: 0.32; IQR: 0.26–0.49; ICcc and LLDa medians significantly different by KW, P < 10−3; ICcc and NL medians significantly different by KW, P < 10−4; Fig. 5B).
FIG. 4.
Rectification of ITD tuning curves. Example long-range ITD curves for ICcc (A), LLDa (B), and NL (C) neurons. The error bars represent the SE over 10 trials. The ITD tuning curves of NL and LLDa neurons are approximately symmetrical about the mean, but the ITD tuning curve of the ICcc neuron shows rectification.
FIG. 5.
ITD tuning curve shape. ITD tuning curve shapes of NL, LLDa, and ICcc neurons quantified by the trough firing rate (A), the rectification index (B), the peak-trough difference (C), and the half-height width (D) expressed as percent of period. Box plots are as in Fig. 3.
Peak-trough difference
The peak-trough difference of ITD tuning curves is larger in ICcc than in NL and LLDa (Christianson and Peña 2006; Fujita and Konishi 1991). The median peak-trough difference in LLDa of 113 spikes/s is significantly larger than the median value of 63 spikes/s observed in NL and smaller, but not significantly, than the median value of 141.5 spikes/s observed in ICcc (LLDa, IQR: 74.5–146.8; NL, 31 neurons; IQR: 50.9–94.1; LLDa and NL medians significantly different by KW, P < 10−2; ICcc, 28 neurons; IQR: 82–184.8; LLDa and ICcc medians not significantly different by KW, P > 0.1; ICcc and NL medians significantly different by KW, P < 10−3; Fig. 5C).
Tuning widths
The half-height width of ITD tuning curves in NL and LLDa is near the half-period predicted by sinusoidal tuning, but is less than one half-period in ICcc (Fig. 5D) (Fujita and Konishi 1991). The median half-height width of ITD tuning curves in LLDa expressed as a percent of period is 51.8%, which is consistent with sinusoidal tuning (17 neurons, IQR: 49.0–58.8%). Similar to responses in LLDa, the median half-height width of ITD tuning curves in NL is 51.6% (61 neurons; IQR: 46.7–56.8%; NL and LLDa medians not significantly different by KW, P > 0.4). The median half-height width of ITD tuning curves in ICcc is 38.0%, indicating sharper tuning than sinusoidal (22 neurons; IQR: 31.4–47.4%; ICcc and LLDa medians significantly different by KW, P < 10−4; ICcc and NL medians significantly different by KW, P < 10−6).
Fisher information
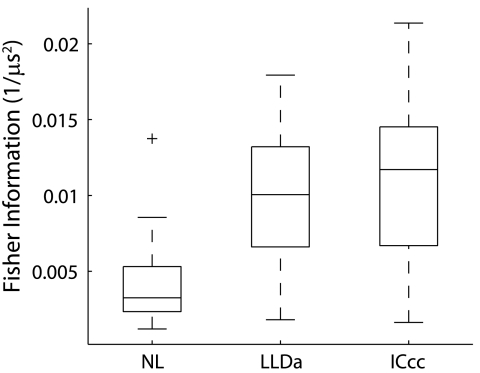
The accuracy of ITD estimation from spike count responses of single neurons is greater in both LLDa and ICcc than in NL as quantified by the Fisher information. The inverse of the Fisher information describes how well ITD can be estimated from a neuron's response by providing a lower bound on the mean square error of an unbiased estimate derived from the neural response (Dayan and Abbott 2001). Fisher information is maximal at points of steepest slope of the tuning curve where neurons are most sensitive to changes in ITD (Dayan and Abbott 2001). For each neuron, we computed the maximum value of the Fisher information over stimulus ITD. The population median of the maximum Fisher information over ITD is larger in both LLDa and ICcc than in NL (Fig. 6; LLDa and NL medians significantly different by KW, P < 10−5; ICcc and NL medians significantly different by KW, P < 10−5) (Christianson and Peña 2006). The maximum Fisher information over ITD is not significantly different in LLDa and ICcc (KW, P > 0.3). We note that specific values of the Fisher information depend on the parameters of the smoothing filer used to estimate the response probability (see methods). Nevertheless, the increase in the Fisher information in both LLDa and ICcc relative to NL was robust to changes in the parameters.
FIG. 6.
Fisher information. The maximum over ITD of the Fisher information for NL, LLDa, and ICcc neurons. Box plots are as in Fig. 3.
Estimating neural convergence from NL to LLDa
The lower variability of spike count responses to ITD in LLDa than in NL suggests that LLDa neurons pool the responses of multiple coincidence detector neurons. We estimated the degree of neural convergence required to produce the observed noise reduction under the assumption that LLDa responses are a linear combination of multiple NL responses. This assumption is supported by the similarity of ITD tuning curve shapes between NL and LLDa neurons. In particular, the results here show that both NL and LLDa neurons have near-sinusoidal ITD tuning curves with little rectification. Given the linear model, the number of converging NL neurons can be expressed in terms of the spike count variances of the NL neurons and the target LLDa neuron, the LLDa and NL peak-trough differences, and the spike count correlation coefficient of NL neurons (Eq. 5). Assuming the NL responses are uncorrelated, the median number of pooled NL neurons required to produce the LLDa spike count variance is five (IQR: 2–9.5 neurons). For each LLDa neuron, there is a maximum spike count correlation coefficient of NL responses that will allow the LLDa neuron's spike count variance to be produced from a linear combination of the NL responses (see methods). The median, computed over the LLDa population, of the maximum correlation coefficient between NL neurons allowable to produce the LLDa neuron's spike count variance is 0.20 (IQR: 0.11–0.61). For the 22 of 31 LLDa neurons with a maximum allowable spike count correlation coefficient between NL neurons of >0.1, the median number of pooled NL neurons, assuming an NL correlation coefficient of 0.1, required to produce the LLDa spike count variance is 3.5 (IQR: 2–11 neurons).
DISCUSSION
Our results show that LLDa is a site of noise reduction of coincidence detector responses to ITD in the owl's auditory localization pathway. Given that NL is the only site of ITD-sensitive input to LLDa (Takahashi and Konishi 1988a,b), the noise reduction observed in LLDa must result from a pooling of coincidence detector responses by LLDa neurons. Based on a linear model of NL convergence in LLDa, we estimate that the number of NL neurons converging on an LLDa neuron is relatively small (<25) under the assumption of moderate correlation between NL neurons. The owl's auditory localization pathway is one of the few experimentally identified examples of a noise reduction process (see Christianson and Peña 2006 and references therein), and the results presented here identify LLDa as a site where the noise reduction occurs.
The presence of noise reduction of NL coincidence detector responses in LLDa implies that noise reduction may be inherited in ICcc. As stated in Christianson and Peña (2006, 2007), noise reduction of NL coincidence detector responses in ICcc may result from a pooling of NL inputs by ICcc neurons. However, the results here suggest that pooling of NL neurons by ICcc neurons is not necessary to produce noise reduction in ICcc. We demonstrate that LLDa neurons show similar trial-to-trial variability in ITD tuning to that displayed by ICcc neurons. Given the projection of LLDa to ICcc (Adolphs 1993), noise reduction in ICcc may be an inherited property of ITD tuning derived from LLDa. In particular, selection of the LLDa neurons with the smallest trial-to-trial variability in ITD tuning or convergence of multiple LLDa neurons in ICcc would be sufficient to produce the trial-to-trial variability in ITD tuning observed in ICcc.
It remains an open question why the owl's auditory system contains both a direct pathway from NL to ICcc (Takahashi and Konishi 1988a) and an indirect pathway from NL to ICcc via LLDa (Adolphs 1993; Takahashi and Konishi 1988b). A possible explanation for the presence of both direct and indirect pathways from NL to ICcc can be drawn from the observation that NL projects contralaterally to both LLDa and ICcc (Takahashi and Konishi 1988a,b) and LLDa projects contralaterally to ICcc (Adolphs 1993). This circuit configuration allows for signals derived from NL neurons on both sides of the brain to converge on single neurons in ICcc. Bilateral convergence of signals originating in NL would allow for greater noise reduction in ICcc through averaging of signals originally derived from different NL neurons. This may explain why trial-to-trial variability of ITD tuning is smaller, although not significantly, in ICcc than in LLDa (Fig. 3A). Another reason for the existence of both a direct and an indirect pathway to ICcc is to create the temporal delay required to produce motion-direction sensitivity in ICcc neurons (Wagner and Takahashi 1992). It is unknown, however, if LLDa and NL neurons synapse on the same neurons in ICcc. It is therefore possible that there are separate pathways on a fine scale such that NL and LLDa inputs to ICcc are segregated, and thus pooling of NL neurons to reduce variability would occur twice in the owl's time pathway.
The cumulative effect of processing ITD at successive stages in the owl's localization pathway produces a population code where units accurately represent ITD and ITD-independent spikes are eliminated. Previous work on the owl's time pathway, coupled with the current results, shows that neurons in post-NL nuclei display smaller overall rates of spiking, more narrow ITD curves, and more accurate rate coding of ITD than displayed by coincidence detectors (Bala et al. 2003; Christianson and Peña 2006; Fujita and Konishi 1991). We found that in NL, LLDa, and ICcc, the Fano factor was less than one, indicating greater regularity in the spike count response than that produced by a Poisson process. The Fano factor provides a complementary measure of response regularity to those assessing phase locking by looking at the regularity of the spike count without addressing the precise timing of spikes. An interesting aspect of the transformation of ITD tuning from LLDa to ICcc is that spike count responses are more regular in LLDa than in ICcc, as quantified by the Fano factor. This raises the question, what is the benefit of introducing a transformation that decreases spike count response regularity when such a decrease may lead to a decrease in the accuracy of the population code representation of ITD? One possibility is that the system is acting to increase energy efficiency while maintaining accuracy in representing ITD. Examining the spike count variance (Fig. 3C) and the trough firing rate (Fig. 5A) shows that the decrease in the Fano factor from LLDa to ICcc results from a decrease in spike number, not from a change in spike count variance. Inhibition is known to cause ITD tuning troughs to fall to zero in ICcc and, consequently, reduce the number of spikes produced at each ITD by removing the ITD-independent part of the signal that is present in LLDa responses (Fujita and Konishi 1991). The presence of inhibition in ICcc also leads to sharper ITD tuning curves in ICcc than in LLDa (Fujita and Konishi 1991). The sharpening of ITD tuning curves in ICcc produces a small, but not significant, increase in the Fisher information in ICcc relative to LLDa. While the elimination of the ITD-independent component of the signal in ICcc through inhibition produces a smaller Fano factor in ICcc than in LLDa, the net effect of inhibition acting on ICcc neurons is to keep approximately the same single-neuron Fisher information in ICcc and LLDa, and therefore equal estimation and detection accuracy but with fewer spikes in ICcc. The presence of inhibition that produces narrowly tuned units that fire few spikes per stimulus presentation is furthered in ICx (Fujita and Konishi 1991; Peña and Konishi 2002). The system may therefore be organized to provide accurate localization performance with as few spikes as possible.
In conclusion, we find that LLDa is a site of noise reduction of coincidence detector responses in the owl's localization pathway. This raises the possibility that ICcc inherits its noise reduction property from LLDa. A comparison of ITD tuning at successive stages in the owl's localization pathway suggests that processing is geared toward producing a population code where single neurons accurately represent ITD and ITD-independent spikes are eliminated.
GRANTS
This work was funded by National Institute of Deafness and Other Communication Disorders Grants DC-00134 to M. Konishi and DC-007690 to J. L. Peña.
Acknowledgments
We thank G. B. Christianson and J. L. Peña for providing the nucleus laminaris and inferior colliculus data, E. Akutagawa for histology, and J. L. Peña for comments on the manuscript.
APPENDIX
Here we derive a formula for the number of NL neurons converging on an LLDa neuron. We assume that the spike count response of an LLDa neuron, denoted rLLDa, can be written in terms of NL responses as 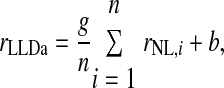 where g and b are constants. Under this model, the spike count variance of the LLDa neuron is given by
where g and b are constants. Under this model, the spike count variance of the LLDa neuron is given by
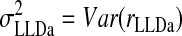 |
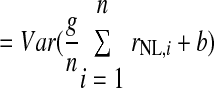 |
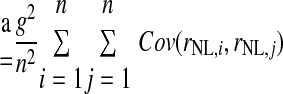 |
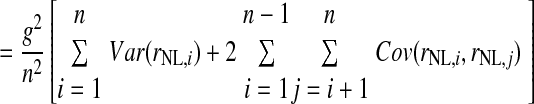 |
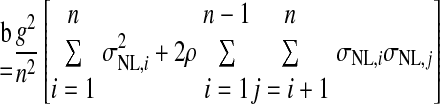 |
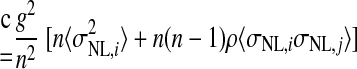 |
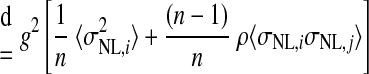 |
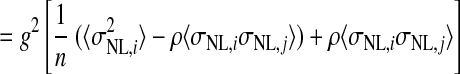 |
where Var(x) denotes the variance of x and Cov(x,y) denotes the covariance of x and y. In the preceding, equality (a) follows from the definition of the variance of an affine combination of random variables, equality (b) uses the notation Var(rNL,i) = σNL,i2 and employs the assumption that all NL pairs have the same spike count correlation coefficient ρ, equality (c) includes the averages of the spike count variance and the product of spike count SDs defined as
 |
and
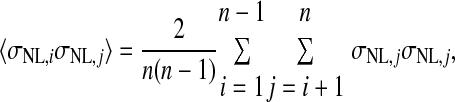 |
and equality (d) yields Eq. 4. This equation can be solved for n to yield Eq. 5.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- Adolphs 1993.Adolphs R Bilateral inhibition generates neuronal responses tuned to interaural level differences in the auditory brain stem of the barn owl. J Neurosci 13: 3647–3668, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akutagawa and Konishi 2005.Akutagawa E, Konishi M. Connections of thalamic modulatory centers to the vocal control system of the zebra finch. Proc Natl Acad Sci USA 102: 14086–14091, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthur 2004.Arthur BJ Sensitivity to spectral interaural intensity difference cues in space-specific neurons of the barn owl. J Comp Physiol [A] 190: 91–104, 2004. [DOI] [PubMed] [Google Scholar]
- Bala et al. 2003.Bala ADS, Spitzer MW, Takahashi TT. Prediction of auditory spatial acuity from neural images on the owl's auditory space map. Nature 424: 771–774, 2003. [DOI] [PubMed] [Google Scholar]
- Carr and Konishi 1990.Carr CE, Konishi M. A circuit for detection of interaural time differences in the brain stem of the barn owl. J Neurosci 10: 3227–3246, 1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christianson and Peña 2006.Christianson GB, Peña JL. Noise reduction of coincidence detector outputs by the inferior colliculus of the barn owl. J Neurosci 26: 5948–5954, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christianson and Peña 2007.Christianson GB, Peña JL. Preservation of spectrotemporal tuning between the nucleus laminaris and the inferior colliculus of the barn owl. J Neurophysiol 97: 3544–3553, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayan and Abbott 2001.Dayan P, Abbott LF. Theoretical Neuroscience: Computational and Mathematical Modeling of Neural Systems. Cambridge, MA: MIT Press, 2001.
- Dean et al. 2005.Dean I, Harper NS, McAlpine D. Neural population coding of sound level adapts to stimulus statistics. Nat Neurosci 8: 1684–1689, 2005. [DOI] [PubMed] [Google Scholar]
- Fischer et al. 2007.Fischer BJ, Peña JL, Konishi M. Emergence of multiplicative auditory responses in the midbrain of the barn owl. J Neurophysiol 98: 1181–1193, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita and Konishi 1991.Fujita I, Konishi M. The role of GABAergic inhibition in processing of interaural time difference in the owl's auditory system. J Neurosci 11: 722–739, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller et al. 1998.Keller CH, Hartung K, Takahashi TT. Head-related transfer functions of the barn owl: measurement and neural responses. Hear Res 118: 13–34, 1998. [DOI] [PubMed] [Google Scholar]
- Knudsen 1983.Knudsen EI Subdivisions of the inferior colliculus in the barn owl (Tyto alba). J Comp Neurol 218: 174–186, 1983. [DOI] [PubMed] [Google Scholar]
- Knudsen et al. 1979.Knudsen EI, Blasdel GG, Konishi M. Sound localization by the barn owl (Tyto alba) measured with the search coil technique. J Comp Physiol [A] 133: 1–11, 1979. [Google Scholar]
- Knudsen and Knudsen 1983.Knudsen EI, Knudsen PF. Space-mapped auditory projections from the inferior colliculus to the optic tectum in the barn owl (Tyto alba). J Comp Neurol 218: 187–196, 1983. [DOI] [PubMed] [Google Scholar]
- Moiseff 1989.Moiseff A Bi-coordinate sound localization by the barn owl. J Comp Physiol [A] 164: 637–644, 1989. [DOI] [PubMed] [Google Scholar]
- Moiseff and Konishi 1981.Moiseff A, Konishi M. Neuronal and behavioral sensitivity to binaural time differences in the owl. J Neurosci 1: 40–48, 1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moiseff and Konishi 1983.Moiseff A, Konishi M. Binaural characteristics of units in the owl's brain stem auditory pathway: precursors of restricted spatial receptive fields. J Neurosci 3: 2553–2562, 1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen et al. 1989.Olsen JF, Knudsen EI, Esterly SD. Neural maps of interaural time and intensity differences in the optic tectum of the barn owl. J Neurosci 9: 2591–2605, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peña and Konishi 2002.Peña JL, Konishi M. From postsynaptic potentials to spikes in the genesis of auditory spatial receptive fields. J Neurosci 22: 5652–5658, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poganiatz et al. 2001.Poganiatz I, Nelken I, Wagner H. Sound-localization experiments with barn owls in virtual space: influence of interaural time difference on head-turning behavior. J Assoc Res Otolaryngol 2: 1–21, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi and Konishi 1988a.Takahashi TT, Konishi M. Projections of the cochlear nuclei and nucleus laminaris to the inferior colliculus of the barn owl. J Comp Neurol 274: 190–211, 1988a. [DOI] [PubMed] [Google Scholar]
- Takahashi and Konishi 1988b.Takahashi TT, Konishi M. Projections of nucleus angularis and nucleus laminaris to the lateral lemniscal nuclear complex of the barn owl. J Comp Neurol 274: 212–238, 1988b. [DOI] [PubMed] [Google Scholar]
- Takahashi et al. 1989.Takahashi TT, Wagner H, Konishi M. Role of commissural projections in the representation of bilateral auditory space in the barn owl's inferior colliculus. J Comp Neurol 281: 545–554, 1989. [DOI] [PubMed] [Google Scholar]
- Wagner and Takahashi 1992.Wagner H, Takahashi TT. Influence of temporal cues on acoustic motion-direction sensitivity of auditory neurons in the owl. J Neurophysiol 68: 2063–2076, 1992. [DOI] [PubMed] [Google Scholar]
- Wild et al. 2001.Wild JM, Kubke MF, Carr CE. Tonotopic and somatotopic representation in the nucleus basalis of the barn owl Tyto alba. Brain Behav Evol 57: 39–62, 2001. [DOI] [PubMed] [Google Scholar]
- Zador 1998.Zador A Impact of synaptic unreliability on the information transmitted by spiking neurons. J Neurophysiol 79: 1219–1229, 1998. [DOI] [PubMed] [Google Scholar]