Abstract
In vertebrate auditory systems, specialized combination-sensitive neurons analyze complex vocal signals by integrating information across multiple frequency bands. We studied combination-sensitive interactions in neurons of the inferior colliculus (IC) of awake mustached bats, using intracellular somatic recording with sharp electrodes. Facilitated combinatorial neurons are coincidence detectors, showing maximum facilitation when excitation from low- and high-frequency stimuli coincide. Previous work showed that facilitatory interactions originate in the IC, require both low and high frequency–tuned glycinergic inputs, and are independent of glutamatergic inputs. These results suggest that glycinergic inputs evoke facilitation through either postinhibitory rebound or direct depolarizing mechanisms. However, in 35 of 36 facilitated neurons, we observed no evidence of low frequency–evoked transient hyperpolarization or depolarization that was closely related to response facilitation. Furthermore, we observed no evidence of shunting inhibition that might conceal inhibitory inputs. Since these facilitatory interactions originate in IC neurons, the results suggest that inputs underlying facilitation are electrically segregated from the soma. We also recorded inhibitory combinatorial interactions, in which low frequency sounds suppress responses to higher frequency signals. In 43% of 118 neurons, we observed low frequency–evoked hyperpolarizations associated with combinatorial inhibition. For these neurons, we conclude that low frequency–tuned inhibitory inputs terminate on neurons primarily excited by high-frequency signals; these inhibitory inputs may create or enhance inhibitory combinatorial interactions. In the remainder of inhibited combinatorial neurons (57%), we observed no evidence of low frequency–evoked hyperpolarizations, consistent with observations that inhibitory combinatorial responses may originate in lateral lemniscal nuclei.
INTRODUCTION
How do sensory neurons create an output from the assortment of inputs that they receive? In the auditory midbrain, or inferior colliculus (IC), many neurons display response selectivity that seems to originate in the IC (Casseday et al. 1994; Faingold et al. 1991; Fuzessery and Hall 1996; Mittmann and Wenstrup 1995; Park and Pollak 1994). These responses result from the inputs of multiple brain stem, cortical, and nonauditory sources (Cant 2005; Saldaña and Merchán 2005; Schofield 2005; Winer 2005). To understand how neurons in the IC or elsewhere create a selective output, an evaluation is required of the response properties and selectivities of their inputs, the types of synaptic inputs that play a role in the selectivity, and the manner in which the neuron integrates these inputs to generate the spike-based output. Although extracellular recording methods contribute to an understanding of the response selectivities of inputs and the types of synaptic inputs, intracellular methods are required for a fuller understanding of the integration that is performed.
This report uses intracellular recording methods to examine integration by a class of selective IC neurons called combination-sensitive, because their responses depend on combinations of acoustic elements in vocal signals. Like other IC neurons, they integrate a range of auditory brain stem and descending inputs (Marsh et al. 2002; Wenstrup et al. 1999). Their response also integrates information from distinct, often widely separated frequency bands (Leroy and Wenstrup 2000; Portfors and Wenstrup 1999, 2002). The mechanisms that create combination-sensitive interactions are not fully understood but are of broad significance in understanding strategies used to create selective responses to complex sensory stimuli. Integration across the spectral and temporal features of frequency-modulated sounds may involve similar mechanisms (Fuzessery et al. 2006; Voytenko and Galazyuk 2007; Xie et al. 2007).
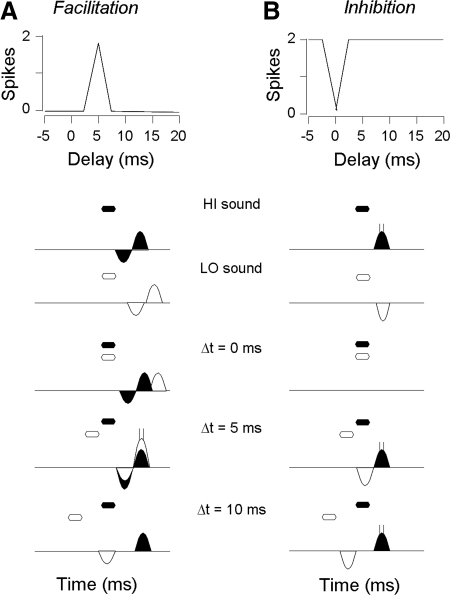
Two types of combination-sensitive responses occur in the IC of the mustached bat. Facilitatory responses are characterized by an enhanced response when two spectrally distinct signals are combined in an appropriate temporal relationship. Such facilitation does not occur in auditory brain stem structures (Marsh et al. 2006; Portfors and Wenstrup 2001) but instead seems to originate in high-frequency regions of the IC (Nataraj and Wenstrup 2005; Wenstrup and Leroy 2001; Wenstrup et al. 1999). Recent work has shown that response facilitation in IC neurons is independent of glutamatergic inputs but requires glycinergic inputs (Sanchez et al. 2008). Glycine-dependant facilitation may result from a postinhibitory rebound mechanism (Nataraj and Wenstrup 2005), in which both low- and high-frequency glycinergic inputs generate inhibitory postsynaptic potentials (IPSPs) followed by rebound excitation (Sanchez et al. 2008). The coincidence of these rebounds is hypothesized to be necessary for response facilitation (Fig. 1A). Using intracellular recording with sharp electrodes, we tested whether facilitating sounds evoke hyperpolarization and rebound excitation in neurons of the mustached bat IC.
FIG. 1.
Facilitatory and inhibitory combination-sensitive neurons may arise from different combinations of excitatory and inhibitory inputs. Top: delay curves show the spike discharge as a function of the delay of the high-frequency sounds (HI) after the low-frequency sound (LO). Bottom: representation of spiking responses and the sum of excitatory and inhibitory postsynaptic potentials (EPSPs and IPSPs, respectively) in response to high- and low-frequency sweeps alone and in response to combination stimuli at 3 different delays. A: our operating hypothesis is that facilitation arises in inferior colliculus (IC) through a postinhibitory rebound mechanism. Thus IPSPs should be observed in response to both HI and LO signals, and facilitation should occur when the 2 rebounds are aligned (5-ms delay). B: inhibitory combination sensitivity seems to originate mostly within the NLL but may include low frequency inhibitory inputs to IC. Thus EPSPs should be observed in response to the HI sound, whereas IPSPs may (shown in B) or may not (not shown) be observed in response to the LO sound. Filled hexagons and response curves indicate HI stimulus and HI PSPs, respectively, whereas unfilled hexagons and response curves indicate LO stimulus and PSPs, respectively. Depolarizing and hyperpolarizing responses are shown as areas above and below the horizontal lines, respectively.
A second class of combination-sensitive neurons (Mittmann and Wenstrup 1995; O'Neill 1985), showing inhibitory interactions between spectral elements (Fig. 1B), may originate below the IC. Such neurons occur in nuclei of the lateral lemniscus (NLL) (Portfors and Wenstrup 2001), which provide a major input to IC neurons (Wenstrup et al. 1999). Nataraj and Wenstrup (2006), proposed that glutamatergic inputs from NLL to IC impose combination-sensitive inhibitory response properties onto IC neurons, but they also suggested that some neurons might receive additional, low frequency–tuned inhibitory input that enhances the response inherited from NLL inputs. This study tests whether intracellular recordings of combination-sensitive inhibitory neurons show hyperpolarizations associated with low-frequency suppression of responses to high-frequency sounds.
METHODS
Intracellular recordings in response to acoustic stimuli were obtained from neurons in the IC of five awake mustached bats (Pteronotus parnellii). The animals were wild-caught in Trinidad and Tobago, West Indies. All procedures were approved by the Northeastern Ohio Universities College of Medicine Animal Care and Use Committee and followed the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals.
Surgery
Before surgery, each bat received an intraperitoneal injection of a sedative (butorphanol, 5 mg/kg, Fort Dodge Animal Health, Fort Dodge, IA) and was anesthetized by isoflurane inhalation (1.5–2.0% in oxygen; Abbott Laboratories, North Chicago, IL). It was placed in a stereotaxic holder, a depilatory lotion was used to remove hair over the skull, and the skin was disinfected with betadine (Henry Schein, Melville, NY). A midline incision was made in the skin, and the underlying muscles were reflected laterally to expose the dorsal surface of the skull. A metal pin was cemented (OptiBond, Henry Schein, Melville, NY) onto the rostral portion of the skull to secure the head during physiological experiments. Using surface and stereotaxic coordinates, a small hole (<0.5 mm diam) was opened to expose the IC, which lies on the dorsal surface of the brain. Lidocaine (4%, Ferndale Laboratories, Ferndale, MI) was applied to the surgical areas, and the bat was returned to the holding cage. The bat recovered for 2–3 days before physiological experiments were initiated.
Acoustic stimulation
Acoustic stimuli were generated using SigGen software and System III hardware (Tucker-Davis Technologies, Alachua, FL). Digital signals were converted to analog waveforms at a rate of 200 kHz (model RP2, Tucker-Davis Technologies) and fed to an attenuator (model PA5, Tucker-Davis Technologies) and to a driver and loudspeaker (model US-LS, UltraSound Advice) that was placed 10 cm from the bat and 25° into the sound field contralateral to the IC recording site. The performance of the entire acoustic system was monitored using a calibrated 1/4-in microphone (Brüel and Kjaer model 4135) and Quest sound level meter (model 1800).
All stimuli were frequency modulated (FM) downsweeps (0.5-ms rise/fall, 4-ms total duration) or combinations of sweeps. The low-frequency stimulus swept from 30 to 22 kHz at 75 ± 1 dB SPL. This sweep included all frequencies within the fundamental component (both constant frequency and FM components) of the mustached bat's sonar signal. These frequencies are required for response facilitation in most combination-sensitive neurons in the mustached bat (Nataraj and Wenstrup 2005; Portfors and Wenstrup 1999). The high-frequency stimulus contained two harmonically related sweeps, corresponding to the second and third harmonics of the sonar signal (61–47 kHz at 65 ± 6 dB SPL; 91.5–67.5 kHz at 67 ± 3 dB SPL). Unlike our previous studies that used tone burst stimuli (Nataraj and Wenstrup 2005; Portfors and Wenstrup 1999), we used sweeps to stimulate combination-sensitive neurons because there was insufficient time with intracellular recordings to characterize each unit's best frequency. The frequency ranges of the sweeps in the high-frequency stimulus, while similar to those in natural echolocation signals, were designed to activate combination-sensitive neurons with best frequencies in the second (∼59–48 kHz) or third (∼88–72) harmonics of these echolocation sounds. The decibel values represent sound pressure levels of tonal signals with equivalent peak pressure, whereas the variation in SPL represents the range in SPL across the frequencies of the sweep caused by the frequency response of the acoustic system. The sound levels were chosen to be ∼20 dB above the threshold for combination-sensitive interactions in most units (Nataraj and Wenstrup 2005; Portfors and Wenstrup 1999).
Intracellular recording
Bats were placed in a stereotaxic apparatus within a heated, single-walled acoustic chamber. To minimize distress, bats were lightly sedated with butorphanol (5 mg/kg, ip). Recording sessions never exceeded 6 h in a single day.
Intracellular recordings were obtained with 1.2-mm-diam quartz microelectrodes (Sutter Instruments, Novato, CA) filled with 3 M potassium acetate. Electrodes were pulled on a Flaming-Brown micropipette puller (Sutter model P2000) and had impedances between 80 and 150 MΩ. After placement of the electrode on the surface of the IC, the exposure was filled with 4% agar. Using a precision microdrive (Kopf model 660), the electrode was advanced in 2- to 3-μm steps from dorsal to ventral through IC regions that represent frequencies >55 kHz (O'Neill et al. 1989; Zook et al. 1985).
Intracellular responses of IC neurons were amplified (Cygnus Technology model IR183A) and monitored on a digital oscilloscope (Yokogawa model DL1640). Waveforms were digitized using a data acquisition system (Heka model EPC-10) at a sampling rate of 100 kHz and transferred to a computer's disk.
Neurons were impaled via vertical advancement of the micropipette in 3-μm steps. Stable intracellular impalements were signaled by a sustained drop in the DC potential. (see results for inclusion criteria.) After a unit was impaled, we initiated a fixed protocol of acoustic stimulation that tested for combination-sensitive responses. In this protocol, there were three repetitions for each of the following stimuli: the low-frequency stimulus (see Acoustic stimulation), the high-frequency stimulus, and combinations of the low- and high-frequency stimuli at delays ranging from –9 (high-frequency stimulus leads) to +15 ms (high-frequency stimulus lags). Delay increments were either 2 or 3 ms. Each single or combination stimulus was repeated at a rate of 4/s, and the entire protocol lasted for ∼24 s. The protocol was repeated for as long as the resting membrane potential remained stable. Total repetitions of each stimulus ranged between 3 and 144, with a median of 6.
In some experiments, long (75–100 ms) depolarizing and/or hyperpolarizing current pulses were injected into neurons through the recording electrode to identify potentials that may be hidden by shunting inhibition. Current injection was synchronized with sound onset. We only included neurons for which the current injection maintained membrane potential at a depolarized or hyperpolarized state (±6–30 mV) for at least three successive stimulus presentations. These changes in membrane potential were sufficient to observe amplitude changes in sound-evoked depolarization or hyperpolarization.
Analysis
COMBINATION SENSITIVITY.
This study examined potentials from IC neurons that show combination-sensitive facilitation or inhibition. Based on our extracellular studies (Nataraj and Wenstrup 2005, 2006; Portfors and Wenstrup 1999), neurons were considered to be combination-sensitive if their spike discharge to combination stimuli was ≥20% higher (for facilitation) or ≥20% lower (for inhibition) than the sum of their spikes in response to separate high- and low-frequency stimuli. Both facilitatory and inhibitory interactions were sensitive to the relative timing of the low- and high-frequency signals. We characterized these interactions by the delay at which the interaction was at its maximum (best delay), the range of delays over which the interaction occurred (delay width), and the maximum strength of the interaction, computed as the percent facilitation or inhibition (see above). In some units, high background discharge affected spike counts and distorted the calculation of the interaction index. In these neurons, we analyzed spikes within a restricted time window 10–20 ms wide to minimize this distortion.
INTRACELLULAR RECORDINGS.
For combination-sensitive neurons, we examined the amplitude and latency of potentials evoked by acoustic stimuli. In this analysis, we averaged all waveforms from a neuron obtained in response to the repetitions of a particular stimulus. The resting membrane potential was calculated from the entire duration of these averaged waveforms: we first obtained the mean value of the averaged waveform, then eliminated all values that exceeded the standard deviation (SD) from the mean, and finally recalculated a new mean. This calculation of resting potential eliminated the influence of spikes or large postsynaptic potentials. Sound-evoked potentials were defined as transient depolarizing or hyperpolarizing fluctuations from the resting membrane potential that exceeded 2 SD (95% confidence limits (CLs)) and began 2–20 ms after stimulus onset. Events such as afterhyperpolarizations were excluded. The latencies of these depolarizing and hyperpolarizing responses were calculated as the interval between the sound onset and the time when the potential change exceeded a 95% CL. The duration of these responses was calculated as the duration of time in which the membrane potential exceeded the 95% CLs.
RESULTS
This report is based on intracellular recordings from 198 neurons in the IC of awake mustached bats. Neurons were included in the study only if their recordings met several criteria: 1) the resting membrane potential remained more negative than −40 mV throughout the recording period, 2) the resting membrane potential varied by <5 mV throughout the recording period (mean, 1.7 mV), and 3) spike height exceeded 30 mV (typically >40 mV). Of the 198 neurons, 71% responded with spikes to at least one of the types of acoustic stimuli presented. We expect that this percentage would have been larger if we had more time to present a broader range of signals. For each of these neurons, we analyzed spike discharge rate to characterize combination-sensitive response properties and sound-evoked depolarizing and hyperpolarizing potentials to examine the synaptic mechanisms underlying these properties.
Within the recorded sample, 136 neurons displayed combination-sensitive response properties. Neurons were considered to be combination-sensitive if their spike discharge to combination stimuli was ≥20% higher (for facilitation) or ≥20% lower (for inhibition) than the sum of their spikes in response to separate high and low frequency stimuli. Of combination-sensitive neurons, 36 (26%) displayed facilitatory interactions in response to spectrally distinct signals. Facilitated neurons differed in the delay of the high-frequency signal at which facilitation was strongest (best delay) as well as in the range of delays over which facilitation was observed (delay width). Inhibitory combination-sensitive responses were recorded in 118 (87%) of combination-sensitive neurons, including 18 of the facilitated neurons. Inhibitory interactions typically were strongest when the low and high-frequency signals were presented simultaneously. Across both facilitated and inhibited combination-sensitive responses, separate high- and low-frequency signals could evoke action potentials. First-spike latency averaged ∼8 ms for responses to both high- and low-frequency sweeps. All of these response properties, summarized in Table 1, are within ranges previously observed in extracellular recordings of combination-sensitive IC neurons using tonal stimuli (Nataraj and Wenstrup 2005, 2006; Portfors and Wenstrup 1999). Such results suggest that the use of FM sweeps rather than tonal signals did not fundamentally alter combination-sensitive interactions.
TABLE 1.
Properties of combination-sensitive neurons
| Number of Neurons | Best Delay, ms [mean (range)] | Delay Width, ms [mean (range)] | Latency HI, ms [mean (SD)] | Latency LO, ms [mean (SD)] | |
|---|---|---|---|---|---|
| Facilitation* | 36 | 4.8 (−2–14) | 4.5 (0.5–18.3) | 8.2 (3.8) | 6.9 (3.7) |
| Inhibition* | 118 | 0.8 (−4–14) | 11 (1–18) | 7.9 (3.9) | 8.1 (3.6) |
Eighteen neurons showed both combination-sensitive facilitation and inhibition.
Sound-evoked transient potentials in facilitated combination-sensitive neurons
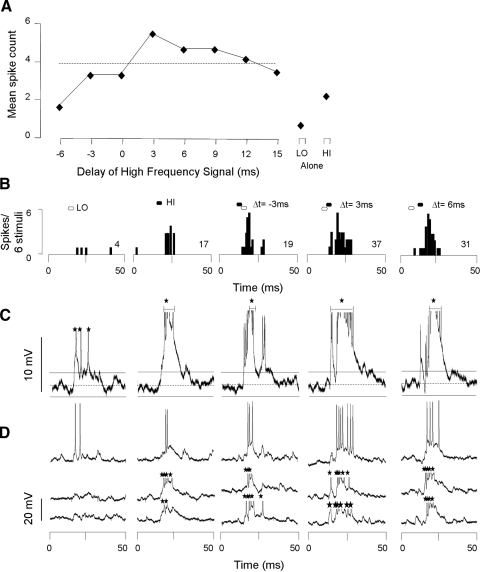
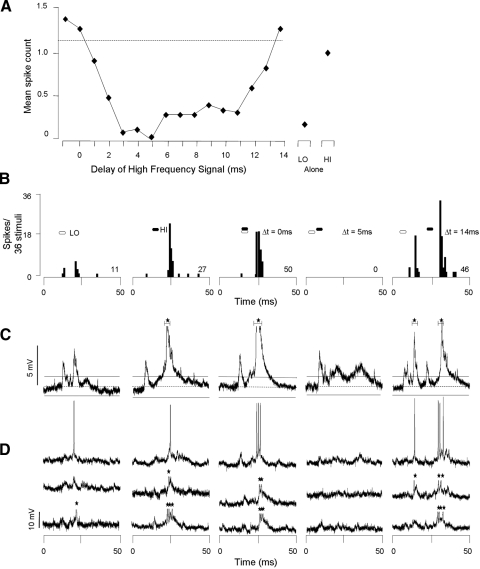
Since glycinergic inputs are essential for the expression of combination-sensitive facilitation in IC neurons (Nataraj and Wenstrup 2005; Sanchez et al. 2008; Wenstrup and Leroy 2001), we examined intracellular responses to both low-frequency and high-frequency facilitating signals for sound-evoked hyperpolarizing potentials. Our major observations were that most facilitated neurons showed no evidence of low frequency–evoked hyperpolarizations but occasionally showed evidence of high frequency–evoked hyperpolarizations. An example is the facilitated neuron shown in Fig. 2. Spike discharge by this neuron showed a poor response to low-frequency sweeps (4 spikes/6 stimuli) and a moderate response to high-frequency sweeps (17 spikes/6 stimuli; Fig. 2, A and B). When the high-frequency sweep followed the low-frequency sweep by 3–12 ms, spike discharge was facilitated. The strongest response was obtained at a high-frequency delay of 3 ms (best delay: 37 spikes/6 stimuli). This response was 76% greater than the sum of the responses to the separate low- and high-frequency signals, exceeding our criterion for response facilitation.
FIG. 2.
Facilitatory combination-sensitive neuron does not show hyperpolarization in response to low- or high-frequency stimuli. A: spike-based response of neuron as function of delay between low- and high-frequency stimuli. Response to separate low (LO) and high (HI) frequency sweeps shown at right. Dashed line, criterion for facilitation; values above the line exceed our criterion for facilitation. B: poststimulus time histograms (PSTHs) display spike response to particular stimulus combinations. Filled and unfilled hexagons above PSTHs represent the timing of HI and LO signals, respectively, and apply to B–D. Numbers at right in PSTHs indicate total spike count for the indicated number of stimulus repetitions. C: averaged voltage traces (n = 6) for all intracellular responses to the stimulus indicated above B. On the averaged trace, the dashed horizontal line indicates the resting membrane potential (−72 mV) and the horizontal solid lines indicate the 95% CLs of the resting membrane potential. Only values exceeding the 95% CLs are judged to be significant responses to sound. Neither the LO nor HI signals generated IPSPs. Note that spike height is altered by the averaging process. D: 3 of 6 individual voltage traces that contribute to the average in C. Only averaged voltage tracers were used to assess the presence or absence of transient membrane potential changes. Black stars indicate truncated spikes. Spike height = 45 mV.
Intracellular recordings from this neuron showed no evidence of low or high frequency–evoked hyperpolarization. Averaged voltage responses (Fig. 2C) and individual samples (Fig. 2D) showed that the low-frequency sweep evoked a brief depolarization with a latency of 5.6 ms. The high-frequency sweep evoked a more sustained depolarization with a latency of 6.5 ms, but similarly showed no evidence of hyperpolarization. In this neuron, the facilitated response to the combination stimulus was marked by a long-duration depolarization that lasted >18 ms, supporting an average of six spikes per stimulus. For the facilitated response, we also observed no evidence of hyperpolarization.
Across the sample of 36 facilitated neurons, 35 neurons displayed no hyperpolarization in association with the low frequency stimulus and 21 neurons displayed no hyperpolarization in association with the high-frequency stimulus. In 14 facilitated neurons, we recorded hyperpolarization in response to the high-frequency sweep alone but not to the low-frequency sweep (Fig. 3). When the two signals were presented together at significant delays (Fig. 3; Δt = 10 ms), it can be seen in the same recording trace that the high-frequency signal, but not the low-frequency signal, evoked a transient hyperpolarization. Such results suggest that the lack of observable low frequency-evoked hyperpolarizations may not result from technical issues. A later section describes many inhibitory combination-sensitive neurons in which low frequency–evoked hyperpolarizations were observed.
FIG. 3.
Facilitatory combination-sensitive neuron displayed transient hyperpolarization in response to high- but not low-frequency stimuli. A: delay-tuned spike response. This neuron was facilitated when the low-frequency sweep preceded the high-frequency sweep by 2–8 ms. B: PSTHs display spike response to different stimuli. C: averaged trace (n = 6) in response to stimuli indicated above B. Average resting membrane potential = −75 mV. No evidence of low frequency–evoked hyperpolarization was observed, but the HI stimulus clearly evoked hyperpolarizing responses. D: 3 consecutive voltage traces that contributed to the averaged trace. Spike height = 40 mV. See Fig. 2 for protocol.
In one neuron we recorded hyperpolarizations in response to both the low- and high-frequency stimuli (Fig. 4). This neuron did not discharge any spikes in response to the low- or high-frequency sweeps presented separately but showed a relatively weak spike discharge to the combination stimulus at delays of 0 and 2 ms (Fig. 4, A and B). Both low- and high-frequency sweeps evoked significant hyperpolarizations of ∼2 mV, each with a latency of 4 ms (Fig. 4, C and D). When the hyperpolarizations coincided at a delay of 0 ms (simultaneous presentation), the resulting hyperpolarization was sometimes followed by a spike. This neuron's response is consistent with the hypothesis that postinhibitory rebound excitation underlies combination-sensitive facilitation, but it was observed in only one facilitated neuron.
FIG. 4.
Facilitated unit displayed transient hyperpolarization in response to both low- and high-frequency sweeps. A: delay function showed spikes only when the LO sweep preceded the HI sweep by 0 or 2 ms. B: PSTHs show no response to single stimuli or to combination stimuli at nonpreferred delays; a weak response occurred at the best delays. C and D: averaged (n = 18) and individual voltage traces show reliable hyperpolarizing responses to both LO and HI stimuli. Facilitation occurred at delays in which the transient hyperpolarizations coincided. Resting membrane potential = −62 mV, spike height = 55 mV. See Fig. 2 for protocol.
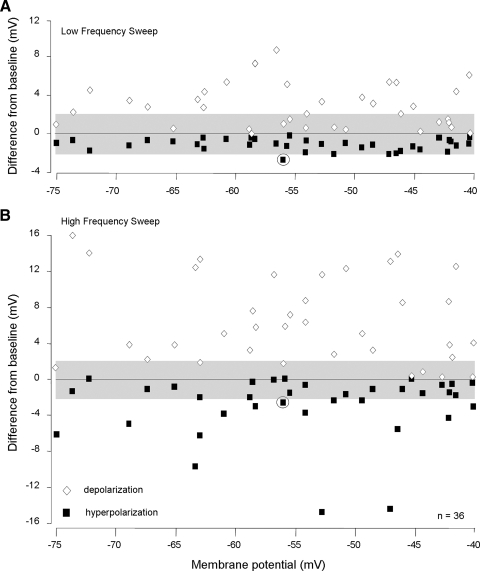
We next examined whether the resting membrane potential of neurons in some way biased our observation of facilitation or of sound-evoked hyperpolarization. Figure 5 plots each neuron's maximum transient depolarizing and hyperpolarizing response to low-frequency (Fig. 5A) and high-frequency (Fig. 5B) stimuli. Only data points above or below the shaded regions represent statistically reliable deviations from the resting membrane potential. Across the broad range of resting membrane potentials (−40 to −75 mV), there was no evidence of a relationship with the amplitudes of either depolarizations or hyperpolarizations. Only one neuron (shown in Fig. 4) displayed a reliable hyperpolarizing response to low-frequency sweeps. These results suggest that the resting potential of a neuron within the range −40 to −75 mV does not influence the recording of either combination-sensitive facilitation or of hyperpolarization for these neurons. This point is strengthened in a subsequent section: for inhibitory combination-sensitive neurons, there likewise was no relationship between resting membrane potential and the size of sound-evoked changes in membrane potential.
FIG. 5.
Neurons with facilitatory combination-sensitive responses had a wide range of resting membrane potentials. A: for each facilitated neuron, the graph shows maximal averaged poststimulus depolarization and hyperpolarization in response to low-frequency sweeps. The grayed area indicates ±2 mV that approximates the 95% CLs for each neuron. Only values exceeding the 95% CLs were considered to be significant depolarizing or hyperpolarizing responses. The circled symbols designate the only neuron with a significant low frequency–evoked hyperpolarization (see Fig. 4). B: graph shows maximal averaged poststimulus depolarization and hyperpolarization in response to high-frequency sweeps. Data in A and B show that the size of hyperpolarization was not related to resting membrane potential. The greater magnitude of high frequency–evoked potentials likely occurs because these neurons were recorded in high-frequency representations of the IC. In this figure, only symbols located completely outside the grayed area represent values that exceed the 95% CL.
Since we found no relationship between facilitatory interactions and hyperpolarization, we examined whether low and high frequency–evoked depolarizations could be related to response facilitation. We examined whether coincidence of transient depolarizations occurs at delays that evoke facilitation. The analysis in Fig. 6 shows no observable relationship. It represents the timing of depolarizing responses evoked by low- and high-frequency sweeps for the neuron in Fig. 2. Facilitatory interactions occur at high-frequency delays where there is no overlap of low and high frequency–evoked depolarizations (e.g., 6–12 ms). Moreover, there was no facilitation at delays where coincidence of depolarizations was observed (e.g., 0 ms). This was the observation in 35 of 36 facilitated neurons. These results suggest that somatically recorded depolarizations do not interact to create response facilitation.
FIG. 6.
Observed depolarizing responses cannot explain the duration or timing of facilitation. Graph schematically represents the timing of HI and LO frequency sweeps (black and white hexagons, respectively) and of depolarizing responses to the HI and LO frequency sweeps (black and white bars on delay line, respectively). Hatched rectangles indicate an overlap of HI and LO frequency-evoked depolarizations. Facilitated delays are indicated by an asterisk at left. There is little overlap of observable depolarizations at facilitated delays.
Sound-evoked transient potentials in inhibitory combination-sensitive neurons
We studied whether inhibitory combination-sensitive neurons showed membrane hyperpolarizations associated with low-frequency suppression of high-frequency excitatory responses. Our main observation was that the majority of inhibited combination-sensitive neurons (57% of 118 neurons) displayed no low frequency–evoked hyperpolarizations. Figure 7 shows an example of such a neuron. This neuron discharged moderately in response to high-frequency sweeps (27 spikes/36 stimuli) and weakly to low-frequency sweeps (11 spikes/36 stimuli; Fig. 7, A and B). For combination stimuli, the high frequency–evoked spike discharge was very strongly suppressed, by as much as 100%, when the low-frequency signal preceded the high-frequency signal by 2–10 ms. Despite this very strong low frequency–evoked suppression, we observed no evidence of low frequency–evoked hyperpolarization in the intracellular recordings (Fig. 7, C and D). In about one half (52%) of the 67 inhibitory neurons that showed no low frequency–evoked hyperpolarization, we observed that high-frequency stimuli evoked hyperpolarizing responses. This indicates, for these neurons, that the absence of low frequency–evoked hyperpolarization was not explained by shunting inhibition.
FIG. 7.
Many inhibitory combination-sensitive interactions are not associated with low frequency–evoked hyperpolarization. A: delay curve shows strong spike suppression when the low-frequency sweep preceded the high-frequency sweep by 2–12 ms. Dashed line indicates criterion for combination-sensitive inhibition; values below the line exceed that criterion. B: PSTHs suggest that both LO and HI sweeps suppressed the response to the other sweep. C and D: averaged (n = 36) and individual voltage traces show no evidence of hyperpolarization to explain spike suppression. Resting membrane potential = −66 mV, spike height = 38 mV. See Fig. 2 for protocol.
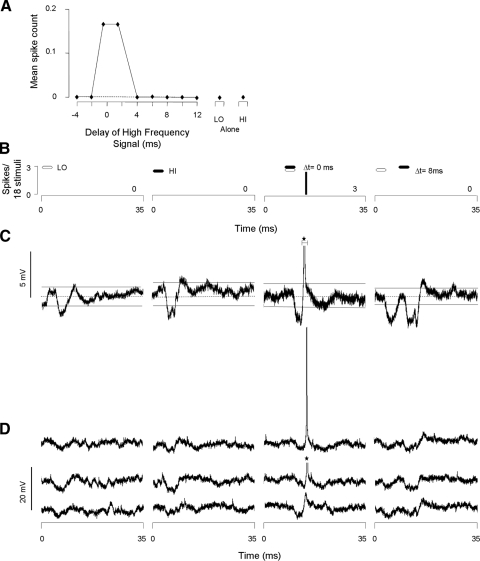
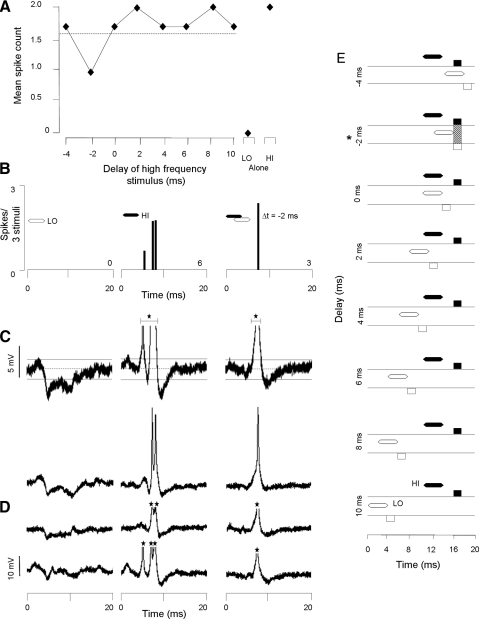
In somewhat less than one half of inhibited neurons (43%), we observed low frequency–evoked hyperpolarization. Figure 8 shows an example of this population. This neuron displayed depolarization with action potentials (6 spikes/3 stimuli) 6 ms after the high-frequency sweeps and a transient hyperpolarization 4 ms after the low-frequency sweep (Fig. 8, A–C). Because the relative timing of the sweeps was varied, the high frequency–evoked spike discharge was suppressed (3 spikes/3 stimuli) at a delay of −2 ms. The timing of this suppression coincided with the overlap of the low frequency–evoked hyperpolarization and the high frequency–evoked depolarization (see shaded region Fig. 8E). In 53% of the 51 inhibited neurons that displayed low frequency–evoked hyperpolarizing responses, high-frequency stimuli did not evoke hyperpolarizing responses.
FIG. 8.
Some inhibitory combination-sensitive interactions are associated with low frequency–evoked transient hyperpolarization. A: delay curve shows spike suppression at a delay of −2 ms. Dashed line indicates criterion for combination-sensitive inhibition. B: PSTHs show spiking responses to the LO and HI sweeps alone as well as at the best delay (−2 ms). C and D: averaged (n = 3) and individual voltage traces show low frequency–evoked hyperpolarization that suppresses high frequency–evoked spike discharge. Resting membrane potential = −51 mV, spike height = 35 mV. See Fig. 2 for protocol. E: graph schematically represents the timing of hyperpolarizations (boxes below the horizontal lines) and depolarizations (boxes above the horizontal lines) in response to the HI and LO frequency sweeps (black and white, respectively). The timing of sound presentations is shown by black (HI) and white (LO) hexagons above the horizontal lines. Hatched rectangles indicate an overlap of HI and LO frequency-evoked membrane potential changes. Inhibited delays are indicated by an asterisk at left. Note that the spike discharge of the neuron is suppressed when the LO-evoked hyperpolarization is aligned with the HI-evoked depolarization.
In a subset of the inhibitory neurons that showed low frequency–evoked hyperpolarization (n = 7), responses to both the high- and low-frequency sweeps consisted of a hyperpolarization followed by depolarization. Among these neurons, suppression of the high frequency–evoked spikes occurred when the low frequency–evoked depolarization overlapped with the hyperpolarizing response to the high-frequency sweep. The neuron in Fig. 9 displayed combined hyperpolarization/depolarization responses to both the low- and high-frequency sweeps (Fig. 9, C and D). The low-frequency sweep evoked a hyperpolarization 5 ms after the sweep that lasted for 5 ms and a depolarizing response beginning 10 ms after the sweep that lasted for 5 ms. The high-frequency sweep evoked a hyperpolarization 5 ms after the sweep that lasted for 3.7 ms and a depolarization beginning 9 ms after the sweep that lasted for 3 ms. When these stimuli were presented together, the spiking response to the high-frequency sweep was suppressed when the low-frequency preceded the high-frequency sweep by 6 ms. At that delay, the low-frequency depolarizing response completely overlapped with the high-frequency hyperpolarizing response (see shaded region in Fig. 9E). Thus the depolarizing response seems to reduce the hyperpolarization that may be necessary for spike discharge. This result, obtained in all seven neurons showing this pattern, suggests that the spiking response to the high-frequency stimulus results from a rebound from inhibition. We hypothesize that the low-frequency response is also formed by rebound from inhibition, since each of these neurons had hyperpolarizations preceding the depolarizing response.
FIG. 9.
Some inhibitory combination-sensitive interactions may be created by disinhibition of rebound excitation. Delay curve shows suppression of high-frequency spike discharge when the high-frequency sweep is delayed by 6 ms. Dashed line indicates criterion for combination-sensitive inhibition. B: PSTHs show spiking responses to different stimuli. Gray bars indicate the timing of a windowed response. C and D: averaged (n = 3) and individual voltage traces show LO- and HI-evoked transient hyperpolarizations followed by depolarizations. Resting membrane potential = −77 mV. Spike height = 55 mV. See Fig. 2 for protocol. E: graph schematically represents the timing of hyperpolarizations (boxes below the horizontal lines) and depolarizations (boxes above the horizontal lines) responses to the HI and LO frequency sweeps (black and white, respectively). The timing of sound presentations is shown by black (HI) and white (LO) hexagons above the horizontal lines. Hatched rectangles indicate an overlap of HI- and LO-evoked potentials. Inhibited delays are indicated by an asterisk at left. Note that spike suppression occurred when the LO-evoked depolarization was aligned with the HI-evoked hyperpolarization.
As with facilitated neurons, we examined whether the resting membrane potential of inhibited combination-sensitive neurons in some way biased our observation of either spike suppression or hyperpolarizing potentials. Figure 10 plots each neuron's maximum depolarizing and hyperpolarizing response to low (Fig. 10A) and high (Fig. 10B) frequency stimuli. Across the broad range of resting membrane potentials (−40 to −85 mV), there was no evidence of a relationship with either hyperpolarization or depolarization. It is noteworthy that transient hyperpolarizations of 10–15 mV could be observed, in different neurons, at resting potentials of 43–74 mV. These results suggest that the resting potential of a neuron within this range does not in itself influence the recording of either combination-sensitive inhibition or of sound-evoked hyperpolarization.
FIG. 10.
Neurons with inhibitory combination-sensitive responses had a wide range of resting membrane potentials and many showed strong hyperpolarization. A: maximal averaged poststimulus depolarization and hyperpolarization for each inhibited neuron, in response to low-frequency sweeps. B: maximal averaged poststimulus depolarization and hyperpolarization for each inhibited neuron, in response to high-frequency sweep. The grayed area indicates ±2 mV that approximates the 95% CLs for each neuron. Only values exceeding the 95% CLs were considered to be significant depolarizations and hyperpolarizations. In A, 8 of 12 diamonds (depolarizations) and 4 of 24 filled squares (hyperpolarizations) that touched the ±2-mV line exceeded the 95% CL. In B, 5 of 9 diamonds and 5 or 12 filled squares that touched the ±2-mV line exceeded the 95% CLs. Data in A and B show that amplitude of transient membrane potential changes was not related to resting membrane potential.
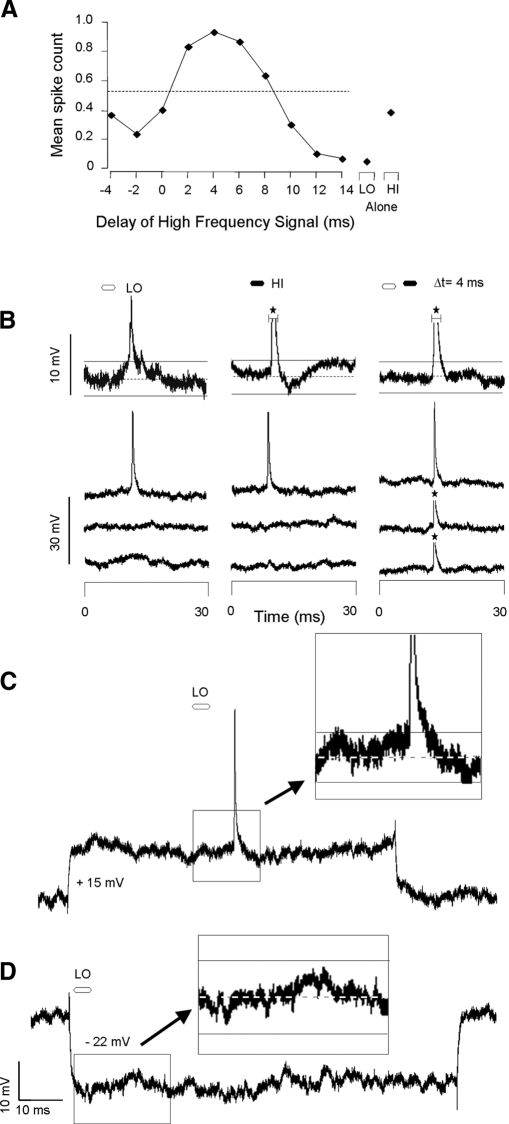
In some facilitatory and inhibitory neurons, the absence of hyperpolarization evoked by low-frequency stimuli could result from inhibitory conductances that shunted current rather than hyperpolarized the neuron. Although this cannot be an explanation for any neuron that displayed transient hyperpolarizing responses to high-frequency signals, including 14 facilitated neurons and 35 inhibited combination-sensitive neurons, it remained a possibility for neurons in which no hyperpolarization was observed. We therefore tested for the presence of shunting conductances in 6 facilitated and 20 inhibited neurons by examining sound-evoked responses while applying a hyperpolarizing and/or depolarizing current. An example is shown in Fig. 11. The facilitated neuron showed a strongly facilitated response at a best delay of 4 ms (Fig. 11A). No hyperpolarizations were observed in response to either the low- or high-frequency stimuli (Fig. 11B). When we injected depolarizing and hyperpolarizing currents sufficient to change membrane potential by +15 and −22 mV, respectively, no transient potential change was shown (Fig. 11, C and D). To show that current injection was capable of altering the amplitude of sound-evoked hyperpolarization, Fig. 12 shows, in another neuron, that the amplitude of a sound-evoked hyperpolarization changed with application of depolarizing and hyperpolarizing current. In all neurons tested, application of depolarizing or hyperpolarizing current showed no evidence of shunting inhibition evoked by low-frequency stimuli.
FIG. 11.
Lack of observable transient hyperpolarizations is not explained by shunting inhibition. A: delay curve shows facilitation when the high-frequency sweep preceded the low-frequency sweep by 2–8 ms. Dashed line indicates criterion for facilitation; values above the line exceed our criterion for facilitation. B: averaged (top trace; n = 9) and individual voltage traces show no IPSPs. Resting membrane potential = −58 mV; spike height = 35 mV. C: intracellular trace with a depolarizing current injection. For this trace, resting membrane potential = −59 mV. D: intracellular trace with hyperpolarizing current injection. For this trace, resting membrane potential = −57 mV. Current injection did not show potentially inhibitory conductances. B–D: dashed horizontal line indicates the resting membrane potential and the horizontal solid lines indicate the 95% CLs of the resting membrane potential. See Fig. 2 for protocol.
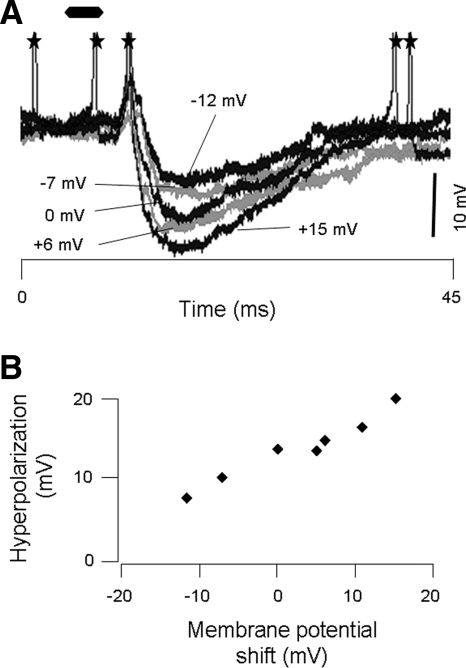
FIG. 12.
Current injection affects magnitude of high frequency–evoked hyperpolarization. Top: overlay of 5 traces with different levels of current injection. The membrane potential in each trace has been vertically aligned so that the amplitude of the hyperpolarizations can be compared. Numbers pointing to each trace indicate the shift in membrane potential from rest that results from current injection. Bottom: summary of relationship between induced change in membrane potential and the amplitude of sound-evoked hyperpolarizations. The linear relationship indicates that current injection successfully altered membrane potential. Resting membrane potential = −62 mV.
DISCUSSION
In vertebrate auditory systems, the analysis of complex vocal signals involves the integration of information across spectrally and temporally distinct elements of these vocalizations. The mustached bat, like many other species, uses a specific form of spectrotemporal integration called combination sensitivity, whereby a neuron's response to a complex sound is based on facilitative and/or inhibitory interactions resulting from the combination of spectrally discrete inputs. This study used intracellular recording to examine sound-evoked membrane potential changes that may be associated with combination-sensitive interactions in IC neurons. We were particularly interested in low frequency–evoked responses, since these may be based on a nontonotopic input (i.e., not matched to the BF of the neuron) to high-frequency neurons in the IC (Wenstrup et al. 1999) and represent one of the novel features associated with combination-sensitive responses in these neurons. Moreover, since both combination-sensitive inhibition and facilitation have been associated with inhibitory inputs to IC neurons, we were particularly interested in sound-evoked hyperpolarizing responses.
For neurons that displayed combination-sensitive inhibitory interactions, we found that low frequency–evoked hyperpolarization was present in 43% of neurons. This suggests that low frequency–tuned inhibitory inputs project onto some high-frequency IC neurons. However, for the larger number of neurons displaying combination-sensitive inhibition, the lack of observable low frequency–evoked transient hyperpolarization suggests that the interactions that produce this response property may originate at lower levels of the ascending pathway. For combination-sensitive facilitation, synaptic inputs related to the facilitation were rarely observed. We interpret the lack of observable postsynaptic potentials differently in these neurons (compared with inhibitory neurons) because studies have shown that facilitatory interactions originate in the IC. Thus we propose that synaptic inputs underlying facilitation are placed at sites that are electrically segregated from the soma, probably on dendrites.
Issues in the interpretation of intracellular recordings
Some conclusions outlined above and detailed in the following sections are based on the absence of sound-evoked membrane potential changes in response to stimuli that contribute to combination sensitivity. Such conclusions require caution, because there are several circumstances that limit the observation of transient membrane potential changes during intracellular recording. We consider these below.
MEMBRANE SHUNT.
When the chloride equilibrium potential is very near the resting potential of a neuron, opening of chloride channels associated with GABAA or glycine receptors will cause little or no potential change or current. When we fail to see hyperpolarizing potentials in response to any sound, this is a potential explanation. However, this cannot be an explanation if, in the same neuron, one sound evokes transient hyperpolarization but another fails to do so. That situation occurred in 39% of facilitated neurons and 53% of inhibited neurons. (Because of the time course of the hyperpolarizing responses and combination-sensitive interactions, it is unlikely that receptor-activated potassium currents mediate these responses.)
Shunting conductances also affect whether a membrane can display postinhibitory rebound. This occurs because the currents associated with rebound excitation (h-current, low threshold calcium current, low threshold potassium current) require a hyperpolarization to become active under most conditions (Dodla et al. 2006; Koch and Grothe 2003; Llinás and Yarom 1981; Sivaramakrishnan and Oliver 2001). Thus an absence of low frequency–evoked hyperpolarization in somatic recording suggests that a low frequency–activated rebound is likewise absent from the soma. When this condition occurred in neurons that displayed combination-sensitive facilitation, we concluded that the facilitation either does not depend on rebound or that both the hyperpolarization and rebound occur in an electrically segregated part of the neuron.
A variant of the shunting hypothesis is the chloride depolarization hypothesis: a chloride equilibrium potential more positive than the resting potential would result in depolarization during activation of GABAA or glycine receptors. This phenomenon is well known in developing mammals and both developing and mature birds (Kandler and Friauf 1995; Lu and Trussell 2001; Monsivais and Rubel 2001). It seems to be common in adult mammals as well (Gulledge and Stuart 2003; Martina et al. 2001). Its occurrence is sometimes restricted to compartments of neurons, e.g., the spike initiation zone (Szabadics et al. 2006), axon terminal (Turecek and Trussell 2001), or dendrites (Avoli 1992; Blaxter and Carlen 1988; Gavrikov et al. 2006). Aware of this possibility, we examined whether sound-evoked depolarization could underlie combination-sensitive interactions. When fast hyperpolarizing responses are recorded in response to some sounds, it is unlikely that other sounds could activate a chloride-based depolarization in the vicinity of the soma.
MEMBRANE LEAK.
Sharp electrodes introduce some additional leak in the membrane that permits ion flow and lowers the input resistance of the neuron. The amplitude of postsynaptic potentials is thus reduced. We did not test the input resistance of neurons, so we cannot exclude its influence on our data. However, the membrane leak hypothesis is weak in cases where one type of sound evokes a strong membrane depolarization but another sound, one that clearly alters the neuron's spike discharge to combination stimuli, evokes no observable response on its own. As with a shunting conductance, it is not clear how a membrane leak that resists membrane potential changes would permit sufficient hyperpolarization to activate rebound mechanisms.
MIXED INPUTS.
When a sound activates both depolarizing and hyperpolarizing conductances simultaneously, postsynaptic potentials that each conductance might generate separately may not be observed. Can such conductances contribute to facilitatory or inhibitory interactions with other inputs when no potential change occurs? This seems unlikely for facilitatory interactions, since membrane potential changes are necessary both for a direct depolarization to spike threshold or for a postinhibitory rebound generation of action potentials. The presence of a mixed response to a sound that activates both depolarizing and hyperpolarizing conductances would always reduce the purely excitatory response to a second sound and would be unlikely to activate facilitation. For inhibitory interactions, it is possible that mixed inputs in response to one sound could obscure the overall inhibitory influence of that sound on responses to a different sound. Thus a mixed input, in which no transient potential change is observed, could mask a postsynaptic response contributing to combination-sensitive inhibition but is unlikely to mask a postsynaptic response contributing to combination-sensitive facilitation.
ELECTRICALLY ISOLATED POTENTIALS.
The passive properties of neurons dictate that synaptic inputs placed throughout the dendritic tree do not uniformly affect somatic potentials. Voltage-gated channels in dendrites may either enhance these differential influences or counteract them. For example, voltage-gated sodium channels in cortical pyramidal neurons may amplify distal EPSPs or generate dendritic spikes (Benardo et al. 1982; Oviedo and Reyes 2002; Schwindt and Crill 1995; Stuart et al. 1997). In contrast, neurons of the medial superior olive use low-threshold potassium currents to restrict current flow between the soma, the site of integration of binaural inputs, and the spike initiation zone on the axon (Scott et al. 2005). In this study, the absence of synaptic potentials associated with inputs that clearly influence a neuron's spike discharge raises the possibility that these inputs occur in electrically isolated portions of a neuron and that only the result of the interactions among these inputs is of sufficient strength to influence somatic potentials or spike generation. The electrical compartmentalization of dendrites is well established (Gulledge et al. 2005; Häusser et al. 2000; Magee 2000).
The constraints of intracellular recording in awake animals preclude data collection that comprehensively tests these issues. We will base our interpretations in part on the considerations we have outlined here and in part on related data and conclusions from extracellular recordings, micro-iontophoretic studies, and anatomical tract-tracing.
Combination-sensitive inhibitory interactions arise below and within the IC
Combination-sensitive inhibition is one of several terms for spectrotemporal interactions in which the response to sounds within a neuron's excitatory tuning curve is suppressed by sounds in a distinct, often remote, frequency band. Such neurons are common throughout the mustached bat IC (Leroy and Wenstrup 2000; Mittmann and Wenstrup 1995; Nataraj and Wenstrup 2006; O'Neill 1985; Portfors and Wenstrup 1999) and occur in other species and auditory centers (Imig et al. 1997; Kadia and Wang 2003; Portfors and Felix 2005; Rauschecker et al. 1995; Sutter et al. 1999).
In this study, we found that the majority of such responses in IC neurons are not accompanied by transient hyperpolarization in response to low-frequency stimuli. In 35 of the 67 inhibitory neurons (52%) that show no low frequency–evoked hyperpolarization, shunting and leak explanations are unlikely because we observed hyperpolarizing responses to high-frequency stimuli. From a broader perspective, the absence in low frequency–evoked hyperpolarization in these neurons is consistent with our previous work. Blockade of GABAA and/or glycine receptors on IC neurons that display this interaction only rarely eliminated inhibitory combination sensitivity (Nataraj and Wenstrup 2005, 2006). Together, these results suggest that combination-sensitive inhibition arises below the IC and that sub-collicular neurons impose this response property onto IC neurons via excitatory inputs. The most likely sources are the NLL, which contain significant numbers of neurons showing this response property (Coomes et al. 2006; Portfors and Wenstrup 2001) and which project strongly to regions of the IC that exhibit combination-sensitive inhibition (Wenstrup et al. 1999).
In a significant number of combination-sensitive inhibitory neurons recorded (43%), low frequency–evoked transient hyperpolarizations were observed. Consistent with these results, Nataraj and Wenstrup (2006) found that blockade of inhibitory neurotransmitters often reduced the strength of inhibitory interactions. Together, these results indicate that inhibitory inputs tuned to low frequencies terminate on high-frequency neurons in the IC. These low frequency–evoked hyperpolarizations are likely to interact with high frequency–evoked excitatory inputs, some of which show combination-sensitive inhibitory properties, to further shape combination-sensitive inhibition. Overall, these results suggest that there are multiple sites within the ascending auditory pathway where inhibitory input tuned to frequencies distant from a neuron's BF modifies its response to sounds within its excitatory tuning curve.
Combination-sensitive facilitatory interactions may occur within electrically isolated parts of IC neurons
Combination-sensitive facilitation occurs in about one quarter of mustached bat IC neurons (Nataraj and Wenstrup 2005) but not in the cochlear nucleus (Marsh et al. 2006) or in NLL (Portfors and Wenstrup 2001). Facilitatory interactions in IC are always eliminated or nearly eliminated through blockade of glycine receptors in IC (Nataraj and Wenstrup 2005; Sanchez et al. 2008; Wenstrup and Leroy 2001). These results strongly suggest that every facilitated combination-sensitive response in the IC is the result of interactions of glycinergic inputs onto the facilitated IC neurons. Furthermore, Sanchez et al. (2008) showed that facilitation in IC neurons depends only on glycinergic inputs and that the facilitatory interaction is unaffected during blockade of ionotropic glutamatergic and GABAergic receptors that eliminates all spikes in response to single tonal stimuli. These data strongly suggest that combination-sensitive facilitation arises in IC neurons and depends on a set of synaptic inputs that operate independent of the inputs that generate an excitatory response at the neuron's best frequency. Sanchez and colleagues hypothesized that each facilitating glycinergic input creates an inhibitory postsynaptic potential followed by rebound excitation and that the coincidence of these excitations results in the facilitatory response. An alternate hypothesis was that these glycinergic inputs are purely depolarizing. This study sought to test these hypotheses.
Surprisingly, we were generally unable to identify either transient hyperpolarizations or depolarizations that could be associated with the facilitating low-frequency input. Since all previous work indicates that facilitatory interactions arise in IC neurons and since the recorded neurons displayed combination-sensitive facilitation in the absence of transient potentials associated with facilitating input, we conclude that the synaptic inputs mediating facilitation must be located sufficiently far from the soma so that their individual postsynaptic potentials were not observable.
The principal alternative to this conclusion is that our methods were insufficient to detect the synaptic potentials associated with facilitation, because of shunting or leakage currents. This is unlikely for the 14 neurons that displayed high frequency–evoked hyperpolarization but not low frequency–evoked hyperpolarization, but it is possible for the 21 facilitated neurons that showed no low or high frequency–evoked hyperpolarization. However, as indicated in a previous section, the presence of shunting or leakage currents that render inhibitory conductances unobservable would also make hyperpolarization-triggered rebound unlikely. The fact that we were able to observe facilitation in these 21 neurons in the absence of low frequency–evoked hyperpolarization suggests either that rebound does not occur near the soma or that the influences are purely depolarizing. However, other analyses indicate no close association between sound-evoked depolarizing potentials and facilitation.
From a population perspective, the argument that we were selectively unable to observe hyperpolarization in facilitating neurons caused by technical issues (shunting, leakage currents, mixed inputs) is suspect. Comparing combination-sensitive facilitated and inhibited neurons, there is a large difference in the proportion of neurons for which we observed low frequency–evoked hyperpolarization (facilitation, 3%; inhibition, 43%). This result is the opposite of what we predicted based on previous iontophoretic studies. These studies showed that combination-sensitive facilitation, but not combination-sensitive inhibition, is eliminated by glycine or GABAA receptor blockade at IC neurons (Nataraj and Wenstrup 2005, 2006; Sanchez et al. 2008; Wenstrup and Leroy 2001). There is no reason to suspect that the above technical difficulties would be more prevalent in the facilitated neurons.
Our conclusion that facilitating inputs are segregated from other inputs to IC neurons is consistent with several observations obtained from extracellular recording in combination with microiontophoresis (Sanchez et al. 2008). First, blockade of glutamatergic and GABAergic inputs had no effect on the number of facilitation spikes discharged by these neurons. This striking result suggests that inputs underlying combination-sensitive facilitation interact in a manner independent of the glutamatergic inputs that relay excitatory influences from auditory brain stem nuclei or the GABAergic and glycinergic inhibitory inputs that shape the excitatory response near the best frequency. In particular, blockade of glycinergic and GABAergic receptors often increased the response to BF tones, whereas, in the same neuron, glycine blockade eliminated the combination-sensitive facilitation (Nataraj and Wenstrup 2005; Sanchez et al. 2008). Thus whereas some chloride-based synaptic inputs suppressed the response to the BF sound, others contributed to facilitation. It is unclear how this can occur unless the inhibitory inputs are segregated from the facilitating glycinergic inputs.
Finally, the conclusion regarding segregated processing of facilitating inputs is consistent with the temporal features of the facilitation. For many facilitated neurons, facilitation occurs when the high-frequency stimulus is delayed—by ≤30 ms in some neurons (Nataraj and Wenstrup 2005; Portfors and Wenstrup 1999). This requires that responses to the low-frequency stimulus have longer latencies than the responses to the high-frequency stimulus. However, low-frequency inputs to the IC do not have sufficiently long latencies, ≤40 ms, to explain this delayed response (Haplea et al. 1994; Klug et al. 2000; Marsh et al. 2006; Portfors and Wenstrup 2002). Longer delays could be accomplished by low-frequency inputs onto more peripheral parts of the dendrites, perhaps in conjunction with mechanisms that lengthen inhibitory duration or delay the rebound. It is difficult to imagine how the delay tuning of facilitated combination-sensitive neurons can arise if the synaptic inputs from low- and high-frequency tuned neurons terminate near the soma. When these considerations are combined with the present observations that facilitation is not accompanied by low frequency–evoked hyperpolarization, we believe that there is a strong basis for concluding that the facilitating inputs act at an electrically isolated site away from the soma and spike initiation zone.
Although our results suggest where these interactions occur, they do not identify the mechanism by which these glycinergic inputs have a net excitatory effect. We propose that facilitating inputs cause hyperpolarization followed by rebound but recognize that these inputs may be purely depolarizing. In fact, the electrical isolation of these inputs would permit local alterations in the chloride concentration ratio, thus changing the direction of postsynaptic potential changes of the glycinergic input.
It is even more unclear how the facilitation signal is amplified relative to the contributing postsynaptic responses. We hypothesize that a particular site in the dendritic tree contains a voltage-sensitive conductance that boosts the depolarization resulting from the separate glycinergic inputs. These may depend on voltage-gated sodium, low-threshold calcium, low-threshold potassium, or h currents. The overall effect, however, is that the depolarization that signals the time-dependent facilitatory interaction reaches the soma and spike generation site, whereas the individual postsynaptic responses do not.
Processing domains for individual IC neurons
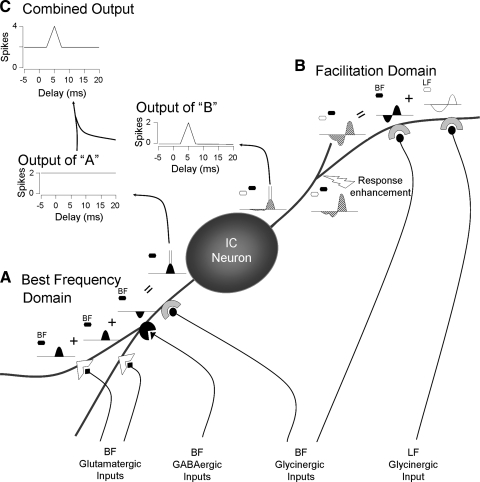
It has long been recognized that ascending inputs to the central nucleus of IC are distributed unequally, suggesting that different parts of the central nucleus process different sets of information (Brunso-Bechtold et al. 1981; Roth et al. 1978). Further work extended this concept to individual frequency representations (Ross and Pollak 1989) and led to the concept of “synaptic domains,” in which different brain stem inputs terminate within subregions of individual laminae (Loftus et al. 2004; Oliver and Huerta 1992; Oliver et al. 1997). The results of this and previous work (Sanchez et al. 2008) extend this concept to individual neurons. We hypothesize that inputs onto single IC neurons segregate into “processing domains.” Some of the inputs interact within the same processing domain, whereas others are segregated into different domains.
Figure 13 schematically depicts how such processing domains may be implemented in a delay-tuned, facilitated combination-sensitive neuron. In the example neuron, a disc-shaped cell of the central nucleus of IC, the response to a BF signal is facilitated when a low-frequency signal (LF) leads by 6 ms. Within one domain (Fig. 13A), the excitatory response to the BF tone is relayed via glutamatergic inputs from multiple auditory brain stem centers. These glutamatergic synapses, from multiple brain stem sources, interact with BF-tuned GABAergic and glycinergic inputs that have potential effects on several response properties: rate-level functions, temporal response patterns, sharpness of frequency tuning, and responses to dynamic signals like frequency sweeps and amplitude modulated sounds. Each of these functions could be segregated into separate domains but are grouped in this example. This domain mediates a broad range of responses to signals near the neuron's BF.
FIG. 13.
Schematic representation of processing domains for an IC neuron. We propose that ascending inputs onto IC neurons are functionally and perhaps anatomically localized. In this figure, sounds (hexagons) and postsynaptic potentials (curved shapes) in response to best frequency (BF) sounds are indicated by filled hexagons and curves, respectively; unfilled shapes refer to lower frequency (LF) sounds and potentials. Hatched potentials result from combinations of BF and LF sounds. A: glutamatergic, GABAergic, and glycinergic inputs tuned near the BF interact among themselves to generate and shape responses near the neuron's BF (bottom left). These inputs generate action potentials that are part of the neuron's output. Since inputs within this domain are affected only by sounds near the BF, spikes generated by interactions within this domain are independent of the delay between a LF sound and a sound near BF. B: the activity within the BF domain does not affect the facilitatory interaction within the facilitation domain (top right). These results and previous work (Sanchez et al. 2008) strongly suggest that 1) the BF and LF glycinergic inputs contributing to facilitation are segregated from other inputs, 2) that they are electrically isolated from the soma, and 3) that an active mechanism (response enhancement) is required to trigger facilitation spikes. Because of selective enhancement of the depolarization and overall decrement caused by cable properties, the voltage changes arriving at the soma and spike generator are sufficient to trigger spikes but separate postsynaptic potentials are too small to be observed. C: the combined output reflects the activity in both domains. Blockade of glycine receptors typically results in a delay function similar to that of the BF domain (Nataraj and Wenstrup 2005; Wenstrup and Leroy 2001). In contrast, blockade of ionotropic glutamate receptors results in a delay function like the output of the facilitation domain (Sanchez et al. 2008). Figure modified from Sanchez et al. (2008).
Current and previous data indicate that facilitating inputs are isolated from the BF-related inputs described above. This is accomplished in the model by a separate domain (Fig. 13B) that receives LF- and BF-tuned glycinergic inputs contributing to combination-sensitive facilitation. In this example, these glycinergic inputs produce hyperpolarization followed by rebound excitation. Their interaction occurs at a site remote from the soma and from the influences of the other domain. At some nearby site, a mechanism based on voltage-gated channels provides an enhancement (response enhancement) of the resultant depolarization. Both the postsynaptic potentials and the resultant, enhanced depolarization are attenuated as they spread toward the soma. The enhanced depolarization is sufficient to generate spikes, but the postsynaptic potentials generated by each signal separately do not influence the membrane potential at the soma or the spike initiation site. Furthermore, their interactions are removed from the influence of many of the ionotropic glutamate receptors and their associated GABA and glycine receptors, since these seem to have little influence on the expression of combination-sensitive facilitation (Sanchez et al. 2008). This same principle may segregate other types of inputs that converge on single IC neurons, such as those carrying monaural and binaural information. The output of a neuron presumably reflects activity across these different processing domains (Fig. 13C).
While speculative, this identifies a working hypothesis that can be tested with other approaches. Key to further studies is an ability to relate sound processing features, such as combination sensitivity, to cell morphology. Once that occurs, physiological responses to sound can be related to the physiology of different cellular compartments and the local distribution of transmitter-specific terminals and membrane-bound proteins (glycine receptors and voltage-gated channels in this case).
GRANTS
This work was supported by National Institute on Deafness and Other Communication Disorders Grants R01 DC-005377 to A. Galazyuk, R01 DC-00937 to J. J. Wenstrup, and F32 DC-007786 to D. C. Peterson.
Acknowledgments
We thank Y. Lu, B. Schofield, and S. Sivaramakrishnan for discussion and comments on the manuscript and the Wildlife Section of the Ministry of Agriculture, Land and Marine Resources of Trinidad and Tobago for permission to exports bats.
The costs of publication of this article were defrayed in part by the payment of page charges. The article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
REFERENCES
- Avoli 1992.Avoli M Synaptic activation of GABAA receptors causes a depolarizing potential under physiological conditions in rat hippocampal pyramidal cells. Eur J Neurosci 4: 16–26, 1992. [DOI] [PubMed] [Google Scholar]
- Benardo 1982.Benardo LS, Masukawa LM, Prince DA. Electrophysiology of isolated hippocampal pyramidal dendrites. J Neurosci 2: 1614–1622, 1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaxter 1988.Blaxter TJ, Carlen PL. GABA responses in rat dentate granule neurons are mediated by chloride. Can J Physiol Pharmacol 66: 637–642, 1988. [DOI] [PubMed] [Google Scholar]
- Brunso-Bechtold 1981.Brunso-Bechtold JK, Thompson GC, Masterton RB. HRP study of the organization of auditory afferents ascending to central nucleus of inferior colliculus in cat. J Comp Neurol 197: 705–722, 1981. [DOI] [PubMed] [Google Scholar]
- Cant 2005.Cant NB Projections from the cochlear nuclear complex to the inferior colliculus. In: The Inferior Colliculus, edited by Winer JA, Schreiner CE. New York: Springer Science, 2005.
- Casseday 1994.Casseday JH, Ehrlich D, Covey E. Neural tuning for sound duration: role of inhibitory mechanisms in the inferior colliculus. Science 246: 847–850, 1994. [DOI] [PubMed] [Google Scholar]
- Coomes 2006.Coomes DL, Nataraj K, Wenstrup J. Glycine but not GABA contributes to spectral integration in nuclei of the lateral lemniscus. Soc Neurosci 544.8, 2006. [Google Scholar]
- Dodla 2006.Dodla R, Svirskis G, Rinzel J. Well-timed, brief inhibition can promote spiking: postinhibitory facilitation. J Neurophysiol 95: 2664–2677, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faingold 1991.Faingold CL, Boersma Anderson CA, Caspary DM. Involvement of GABA in acoustically-evoked inhibition in inferior colliculus neurons. Hear Res 52: 201–216, 1991. [DOI] [PubMed] [Google Scholar]
- Fuzessery 1996.Fuzessery ZM, Hall JC. Role of GABA in shaping frequency tuning and creating FM sweep selectivity in the inferior colliculus. J Neurophysiol 76: 1059–1073, 1996. [DOI] [PubMed] [Google Scholar]
- Fuzessery 2006.Fuzessery ZM, Richardson MD, Coburn MS. Neural mechanisms underlying selectivity for the rate and direction of frequency modulated sweeps in the auditory cortex of the pallid bat. J Neurophysiol 96: 1320–1336, 2006. [DOI] [PubMed] [Google Scholar]
- Gavrikov 2006.Gavrikov KE, Nilson JE, Dmitriev AV, Zucker CL, Mangel SC. Dendritic compartmentalization of chloride cotransporters underlies directional responses of starburst amacrine cells in retina. Proc Natl Acad Sci USA 103: 18793–18798, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulledge 2003.Gulledge AT, Stuart GJ. Excitatory actions of GABA in the cortex. Neuron 37: 299–309, 2003. [DOI] [PubMed] [Google Scholar]
- Gulledge 2005.Gulledge AT, Kampa BM, Stuart GJ. Synaptic integration in dendritic trees. J Neurobiol 64: 75–90, 2005. [DOI] [PubMed] [Google Scholar]
- Haplea 1994.Haplea S, Covey E, Casseday JH. Frequency tuning and response latencies at three levels in the brainstem of the echolocating bat, Eptescus fuscus. J Comp Physiol 174: 671–683, 1994. [DOI] [PubMed] [Google Scholar]
- Häusser 2000.Häusser M, Spruston N, Stuart GJ. Diversity and dynamics of dendritic signaling. Science 290: 739–744, 2000. [DOI] [PubMed] [Google Scholar]
- Imig 1997.Imig TJ, Poirier P, Irons WA, Samson FK. Monaural spectral contrast mechanism for neural sensitivity to sound direction in the medial geniculate body of the cat. J Neurophysiol 78: 2754–2771, 1997. [DOI] [PubMed] [Google Scholar]
- Kadia 2003.Kadia SC, Wang X. Spectral integration in A1 of awake primates: neurons with single- and multipeaked tuning characteristics. J Neurophysiol 89: 1603–1622, 2003. [DOI] [PubMed] [Google Scholar]
- Kandler 1995.Kandler K, Friauf E. Development of glycinergic and glutamatergic synaptic transmission in the auditory brainstem of perinatal rats. J Neurosci 15: 6890–6904, 1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klug 2000.Klug A, Khan A, Burger RM, Bauer EE, Hurley LM, Yang L, Grothe B, Halvorsen MG, Park TJ. Latency as a function of intensity in auditory neurons: influences of central processing. Hear Res 148: 107–123, 2000. [DOI] [PubMed] [Google Scholar]
- Koch 2003.Koch U, Grothe B. Hyperpolarization-activated current (Ih) in the inferior colliculus: distribution and contribution to temporal processing. J Neurophysiol 90: 3679–3687, 2003. [DOI] [PubMed] [Google Scholar]
- Leroy 2000.Leroy SA, Wenstrup JJ. Spectral integration in the inferior colliculus of the mustached bat. J Neurosci 20: 8533–8541, 2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llinás 1981.Llinás R, Yarom Y. Electrophysiology of mammalian inferior olivary neurones in vitro. Different types of voltage-dependent ionic conductances. J Physiol 315: 549–567, 1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loftus 2004.Loftus WC, Bishop DC, Saint Marie RL, Oliver DL. Organization of binaural excitatory and inhibitory inputs to the inferior colliculus from the superior olive. J Comp Neurol 472: 330–344, 2004. [DOI] [PubMed] [Google Scholar]
- Lu 2001.Lu T, Trussell LO. Mixed excitatory and inhibitory GABA-mediated transmission in chick cochlear nucleus. J Physiol 535: 125–131, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee 2000.Magee JC Dendritic integration of excitatory synaptic input. Nat Rev Neurosci 1: 181–190, 2000. [DOI] [PubMed] [Google Scholar]
- Marsh 2002.Marsh RA, Fuzessery ZM, Grose CD, Wenstrup JJ. Projection to the inferior colliculus from the basal nucleus of the amygdala. J Neurosci 22: 10449–10460, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh 2006.Marsh RA, Nataraj K, Gans D, Portfors CV, Wenstrup JJ. Auditory responses in the cochlear nucleus of awake mustached bats: precursors to spectral integration in the auditory midbrain. J Neurophysiol 95: 88–105, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martina 2001.Martina M, Royer S, Pare′ D. Cell-type-specific GABA responses and chloride homeostasis in the cortex and amygdala. J Neurophysiol 86: 2887–2895, 2001. [DOI] [PubMed] [Google Scholar]
- Mittmann 1995.Mittmann DH, Wenstrup JJ. Combination-sensitive neurons in the inferior colliculus. Hear Res 90: 185–191, 1995. [DOI] [PubMed] [Google Scholar]
- Monsivais 2001.Monsivais P, Rubel EW. Accomodation enhances depolarizing inhibition in central neurons. J Neurosci 21: 7823–7830, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nataraj 2005.Nataraj K, Wenstrup JJ. Roles of inhibition in creating complex auditory responses in the inferior colliculus: facilitated combination-sensitive neurons. J Neurophysiol 93: 3294–3312, 2005. [DOI] [PubMed] [Google Scholar]
- Nataraj 2006.Nataraj K, Wenstrup JJ. Roles of inhibition in complex auditory responses in the inferior colliculus: inhibited combination-sensitive neurons. J Neurophysiol 95: 2179–2192, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver 1997.Oliver DL, Beckius GE, Bishop DC, Kuwada S. Simultaneous anterograde labeling of axonal layers from lateral superior olive and dorsal cochlear nucleus in the inferior colliculus of cat. J Comp Neurol 382: 215–229, 1997. [DOI] [PubMed] [Google Scholar]
- Oliver 1992.Oliver DL, Huerta MF. Anatomy of the colliculi. In: The Mammalian Auditory Pathway: Neuroanatomy, edited by Webster DB, Popper AN, Fay RR. New York: Springer-Verlag, 1992.
- O'Neill 1985.O'Neill WE Responses to pure tones and linear FM components of the CF-FM biosonar signal by single units in the inferior colliculus of the mustached bat. J Comp Physiol 157: 797–815, 1985. [DOI] [PubMed] [Google Scholar]
- O'Neill 1989.O'Neill WE, Frisina RD, Gooler DM. Functional organization of mustached bat inferior colliculus. I. Representation of FM frequency bands important for target ranging revealed by 14C-2-deoxyglucose autoradiography and single unit mapping. J Comp Neurol 284: 60–84, 1989. [DOI] [PubMed] [Google Scholar]
- Oviedo 2002.Oviedo H, Reyes AD. Boosting of neuronal firing evoked with asynchronous and synchronous inputs to the dendrite. Nat Neurosci 5: 261–266, 2002. [DOI] [PubMed] [Google Scholar]
- Park 1994.Park TJ, Pollak GD. Azimuthal receptive fields are shaped by GABAergic inhibition in the inferior colliculus of the mustache bat. J Neurophysiol 72: 1080–1102, 1994. [DOI] [PubMed] [Google Scholar]
- Portfors 2005.Portfors CV, Felix RA II. Spectral integration in the inferior colliculus of the CBA/CaJ mouse. Neuroscience 136: 1159–1170, 2005. [DOI] [PubMed] [Google Scholar]
- Portfors 1999.Portfors CV, Wenstrup JJ. Delay-tuned neurons in the inferior colliculus of the mustached bat: implications for analyses of target distance. J Neurophysiol 82: 1326–1338, 1999. [DOI] [PubMed] [Google Scholar]
- Portfors 2001.Portfors CV, Wenstrup JJ. Responses to combinations of tones in the nuclei of the lateral lemniscus. J Assoc Res Otolaryngol 2: 104–117, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portfors 2002.Portfors CV, Wenstrup JJ. Excitatory and facilitatory frequency response areas in the inferior colliculus of the mustached bat. Hear Res 168: 131–138, 2002. [DOI] [PubMed] [Google Scholar]
- Rauschecker 1995.Rauschecker JP, Tian B, Hauser M. Processing of complex sounds in the macaque nonprimary auditory cortex. Science 268: 111–114, 1995. [DOI] [PubMed] [Google Scholar]
- Ross 1989.Ross LS, Pollak GD. Differential ascending projections to aural regions in the 60 kHz contour of the mustache bat's inferior colliculus. J Neurosci 9: 2819–2834, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth 1978.Roth GL, Aitkin LM, Andersen RA, Merzenich MM. Some features of the spatial organization of the central nucleus of the inferior colliculus of the cat. J Comp Neurol 182: 661–680, 1978. [DOI] [PubMed] [Google Scholar]
- Saldaña 2005.Saldaña E, Merchán MA. Intrinsic and commissural connections of the inferior colliculus. In: The Inferior Colliculus, edited by Winer JA, Schreiner CE. New York: Springer Science, 2005.
- Sanchez 2008.Sanchez JT, Gans D, Wenstrup JJ. Glycinergic “inhibition” mediates selective excitatory response to combinations of sounds. J Neurosci 28: 80–90, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schofield 2005.Schofield BR Superior olivary complex and lateral lemniscal connections of the auditory midbrain. In: The Inferior Colliculus, edited by Winer JA, Schreiner CE. New York: Springer Science, 2005.
- Schwindt 1995.Schwindt PC, Crill WE. Amplification of synaptic current by persistent sodium conductance in apical dendrite of neocortical neurons. J Neurophysiol 74: 2220–2224, 1995. [DOI] [PubMed] [Google Scholar]
- Scott 2005.Scott LL, Mathews PJ, Golding NL. Posthearing developmental refinement of temporal processing in principal neurons of the medial superior olive. J Neurosci 25: 7887–7895, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivaramakrishnan 2001.Sivaramakrishnan S, Oliver DL. Distinct K currents result in physiologically distinct cell types in the inferior colliculus. J Neurosci 21: 2861–2877, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart 1997.Stuart G, Schiller J, Sakmann B. Action potential initiation and propagation in rat neocortical pyramidal neurons. J Physiol 505: 617–632, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutter 1999.Sutter ML, Schreiner CE, McLean M, O'conner KN, Loftus WC. Organization of inhibitory frequency receptive fields in cat primary auditory cortex. J Neurophysiol 82: 2358–2371, 1999. [DOI] [PubMed] [Google Scholar]
- Szabadics 2006.Szabadics J, Varga C, Molnar G, Olah S, Barzo P, Tamas G. Excitatory effect of GABAergic axo-axonic cells in cortical microcircuits. Science 311: 233–235, 2006. [DOI] [PubMed] [Google Scholar]
- Turecek 2001.Turecek R, Trussell LO. Presynaptic glycine receptors enhance transmitter release at a mammalian central synapse. Nature 411: 587–590, 2001. [DOI] [PubMed] [Google Scholar]
- Voytenko 2007.Voytenko SV, Galazyuk AV. Intracellular recording reveals temporal integration in inferior colliculus neurons of awake bats. J Neurophysiol 97: 1368–1378, 2007. [DOI] [PubMed] [Google Scholar]
- Wenstrup 2001.Wenstrup J, Leroy SA. Spectral integration in the inferior colliculus: role of glycinergic inhibition in response facilitation. J Neurosci 21: RC124, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenstrup 1999.Wenstrup JJ, Mittmann DH, Grose CD. Inputs to combination-sensitive neurons of the inferior colliculus. J Comp Neurol 409: 509–528, 1999. [DOI] [PubMed] [Google Scholar]
- Winer 2005.Winer JA Decoding the auditory corticofugal systems. Hear Res 207: 1–9, 2005. [DOI] [PubMed] [Google Scholar]
- Xie 2007.Xie R, Gittelman JX, Pollak GD. Rethinking tuning: in vivo whole-cell recordings of the inferior colliculus in awake bats. J Neurosci 27: 9469–9481, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zook 1985.Zook JM, Winer JA, Pollak GD, Bodenhamer RD. Topology of the central nucleus of the mustache bat's inferior colliculus: correlation of single unit properties and neuronal architecture. J Comp Neurol 231: 530–546, 1985. [DOI] [PubMed] [Google Scholar]