Abstract
Epidermal growth factor receptor (EGFR/ErbB1) and HER2 (ErbB2/neu), members of the ErbB receptor tyrosine kinase family, are frequently overexpressed in breast cancer and are known to drive tumor growth and progression, making them promising targets for cancer therapy. Lapatinib is a selective competitive inhibitor of both the HER2 and EGFR tyrosine kinases. Although lapatinib showed significant activity in patients with HER2-positive breast cancer, the role of EGFR in the response of breast cancer to lapatinib has not been defined. Here, we examined the role of EGFR expression levels in the sensitivity of HER2-overexpressing breast cancer cells to lapatinib. Depletion of EGFR by EGFR small-interfering RNA knockdown did not affect lapatinib sensitivity in these cells, whereas treated HER2 siRNA knockdown cells became more resistant to lapatinib. We conclude that the in vitro activity of lapatinib is not dependent on EGFR expression level in HER2-overexpressing breast cancer cells.
Introduction
The ErbB receptor tyrosine kinase family promotes growth and differentiation of both normal breast and malignant human breast cancer cells (1). One member of the family, epidermal growth factor (EGF) receptor (EGFR/ErbB1), is overexpressed in 20% to 80% of breast cancers (2, 3), and another member, HER2 (ErbB2/neu), is amplified and/or overexpressed (i.e., HER2-positive) in 20% to 30% of breast cancers (4, 5). EGFR and HER2 are known to drive tumor growth and progression and have emerged as promising targets for cancer therapy.
A number of small molecules that target individual or dual ErbB receptors have been developed and tested in clinical trials. Lapatinib, a dual inhibitor of EGFR and HER2 tyrosine kinases, has already been approved by the US Food and Drug Administration as a treatment for HER2-positive metastatic breast cancer (6, 7). Lapatinib as a single agent or in combination with trastuzumab or capecitabine exhibited activity against HER2-positive advanced or metastatic breast cancer that has progressed after trastuzumab therapy (8-10). The activity of lapatinib in inflammatory breast cancer was evaluated in an international Phase II trial (11). Although lapatinib showed significant activity in HER2-positive cancers in this clinical study, the authors reported that lapatinib had no significant activity in EGFR-positive/HER2-negative cancers. Furthermore, lapatinib showed only modest activity in EGFR-dominated cancers, such as squamous cell carcinoma of the head and neck and colorectal cancer (12, 13). Although some studies were done to identify the biological and clinical effects of lapatinib on advanced EGFR-overexpressing breast cancers, the limited sample sizes and variant biological effects have left unclear whether lapatinib has meaningful clinical activity against EGFR-overexpressing breast cancer (9, 14).
Whether the antitumor activity of lapatinib is dependent on EGFR expression has not been established. To address the role of EGFR in the response of HER2-overexpressing breast cancer to lapatinib, we examined the involvement of EGFR expression level in lapatinib sensitivity of two HER2-overexpressing breast cancer cell lines, BT-474 and SK-BR-3, which express high levels of HER2 and moderate levels of EGFR. Surprisingly, EGFR small-interfering RNA (siRNA) knockdown did not affect lapatinib sensitivity in these cells. In contrast, cells transfected with HER2 siRNA became significantly more resistant to lapatinib, indicating that lapatinib acts mainly through the HER2 pathway, not the EGFR pathway. Our findings provide the first demonstration that lapatinib sensitivity is independent of EGFR expression level in HER2-overexpressing breast cancer cells.
Materials and Methods
Cell Culture and Reagents
BT-474 and SK-BR-3 breast cancer cells were obtained from the American Type Culture Collection and maintained in DMEM/F12 medium supplemented with 10% fetal bovine serum (Life Technologies/Bethesda Research Laboratories) in a humidified atmosphere containing 5% CO2 at 37°C.
Lapatinib was synthesized and purified according to the published procedure (6). Briefly, we developed a synthesis of lapatinib (free base) with six linear steps. Our first step was to make the mixed ether by the reaction of 2-chloro-4-nitrophenol and 3-fluorobenzylbromide in the presence of K2CO3 in CH3CN, followed by reduction of the nitro group using Fe/EtOAc and HOAc. The aromatic amine was then used in a coupling with 4-chloro-6-iodo-quinazoline [which formed an intermediate between the reaction of 6-iodoquinazolin-4(3H)-one and POCl3] to make the 6-iodo-[4-{(3-fluorobenzyloxy)-3-chlorophenyl}-quinazolin-4-yl]amine. Suzuki coupling of commercially available boronic acid with aryl iodide in the presence of palladium catalyst formed 5-[4-{3-chloro-4-[(3-fluorobenzyl)oxy]anilino}-6-quinazolinyl]-2-furaldehyde in good yield. The title compound was achieved as a purple solid by the reaction of previously made aldehyde with 2-methanesulfonylethylamine followed by reduction with sodium borohydride and purification by flash column chromatography. Stock solutions of lapatinib (10 mmol/L) were prepared in DMSO and stored in aliquots at −20°C.
Western Blot Analysis
Cultured cells were washed twice with ice-cold PBS solution and subjected to lysis in a lysis buffer [50 mmol/L Tris-HCl (pH 7.5), 150 mmol/L NaCl, 1% NP40, 0.5% sodium deoxycholate, 1 mmol/L EDTA, 1 mmol/L sodium fluoride, 1 mmol/L sodium orthovanadate, 1 mmol/L phenylmethylsulfonyl fluoride, 10 μL/ mL phosphatase inhibitor cocktail, and 10 μL/mL protease inhibitor cocktail (Sigma)] for 30 min on ice. Equal amounts of protein extracts (15 μg for HER2 Western blots and 50 μg for other blots) were separated by SDS-PAGE and transferred onto polyvinylidene difluoride membranes (Bio-Rad). The membranes were blocked with 5% nonfat milk in 0.1% Tween 20/TBS solution and then incubated with primary antibodies overnight at 4°C. Primary antibodies were rabbit polyclonal anti-EGFR antibody (Santa Cruz Biotechnology; 1:250), mouse monoclonal anti-HER2/c-neu antibody (Oncogene Research Products; 1:1,000), mouse monoclonal anti–Phospho-EGFR (Y1173) antibody (Santa Cruz Biotechnology; 1:500), mouse monoclonal anti–Phospho-HER2 (Y1248) antibody (Upstate Biotechnology; 1:1,000), mouse monoclonal anti–Phospho-Akt (S473) antibody (Cell Signaling Technology; 1:500), rabbit monoclonal anti–Phospho-extracellular signal-regulated kinase 1/2 (T202/Y204) antibody (Cell Signaling Technology; 1:250), rabbit polyclonal anti–p27 antibody (Santa Cruz Biotechnology; 1:1,000), and mouse monoclonal anti–β-actin antibody (Sigma; 1:5,000). Membranes were washed thrice with 0.1% Tween 20/TBS and then incubated for 1 h with secondary antibodies in blocking buffer. An Odyssey IR imaging system (LI-COR Biosciences) was used to examine the membranes.
WST-1Assay
Cell viability was assayed by application of cell proliferation reagent WST-1 (Roche Applied Science). A cell suspension of 4,000 cells per 90 μL was seeded into each well of a 96-well plate and cultured overnight, after which, 10 AL of lapatinib solution at a final concentration of 0.001, 0.01, 0.1, or 1 μmol/L was added to the individual wells. After 3 d of lapatinib treatment, 10 μL of the ready-to-use WST-1 reagent was added directly into the medium, the plates were incubated at 37°C for 30 min, and absorbance was measured on a plate reader at 450 nm. All experiments were done in triplicate. Cell viability was calculated as the percentage of cells killed by the treatment as measured by differences in absorbance of treated and untreated wells. Median inhibitory concentrations were determined from these calculations.
siRNA Knockdown
ON-TARGET plus siRNA SMART pools against EGFR, HER2, and scrambled control were purchased from Dharmacon Research, Inc., and RNA interference assay was done according to the manufacturer’s protocol. Briefly, cells were seeded in 6-well culture plates at 30% confluence in DMEM/F12 medium supplemented with 10% fetal bovine serum. The next day, cells were transfected with siRNA at a final concentration of 100 nmol/L by using Oligofectamine (Invitrogen).
Statistical Analysis
Statistical analyses were done by commercially available software (Statview, version 5.0; SAS Institute). The data obtained were analyzed by Student’s t test. Values represent the mean ± SE. Statistical significance was defined as having a P value of <0.05.
Results
HER2-overexpressing Breast Cancer Cells Are Sensitive to Lapatinib
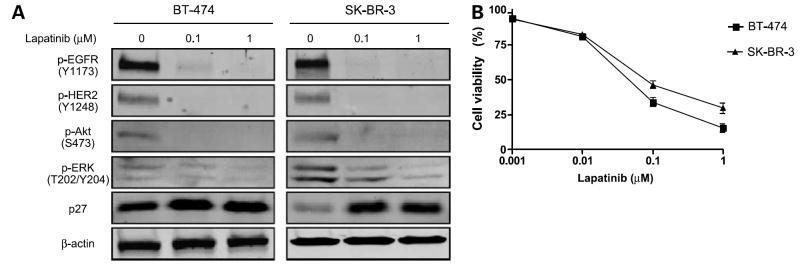
As a first step in our study of the role of EGFR in the response of breast cancer to lapatinib, we examined the sensitivity to lapatinib of two human breast cancer lines, BT-474 and SK-BR-3, which express both HER2 and EGFR. Lapatinib treatment significantly and rapidly inhibited tyrosine phosphorylation of EGFR and HER2 in these cells (Fig. 1A). Activation of Akt and mitogen-activated protein kinase extracellular signal-regulated kinase 1/2, which are downstream of the EGFR and HER2 pathways in cell proliferation and survival mechanisms, is also inhibited by lapatinib treatment. Lapatinib treatment induced phosphorylation of p27, a molecule downstream of EGFR and HER2 involved in cell proliferation (Fig. 1A). We then tested the lapatinib sensitivity of these cells by WST-1 assay and found that the cells were sensitive to lapatinib (IC50 = 0.046 μmol/L for BT-474 and 0.079 μmol/L for SK-BR-3; Fig. 1B).
Figure 1.
BT-474 and SK-BR-3 cells are sensitive to lapatinib. A, BT-474 and SK-BR-3 cells were treated with 0 (vehicle), 0.1, or 1 μmol/L lapatinib for 24 h. Western blot analysis was done to detect phosphorylated EGFR (Y1173), HER2 (Y1248), Akt (S473), extracellular signal-regulated kinase1/2 (p-ERK; T202/Y204), and p27. β-actin was used as a loading control. B, BT-474 and SK-BR-3 cells were plated in 96-well plates and treated with 10-fold serial dilutions of lapatinib ranging from 0.001 to 1 μmol/L for 72 h. The percentage cell viability was evaluated by WST-1 assay. Each experiment was repeated thrice independently.
Lapatinib Sensitivity of HER2-Overexpressing Breast Cancer Cell Lines Is Independent of EGFR Expression Level
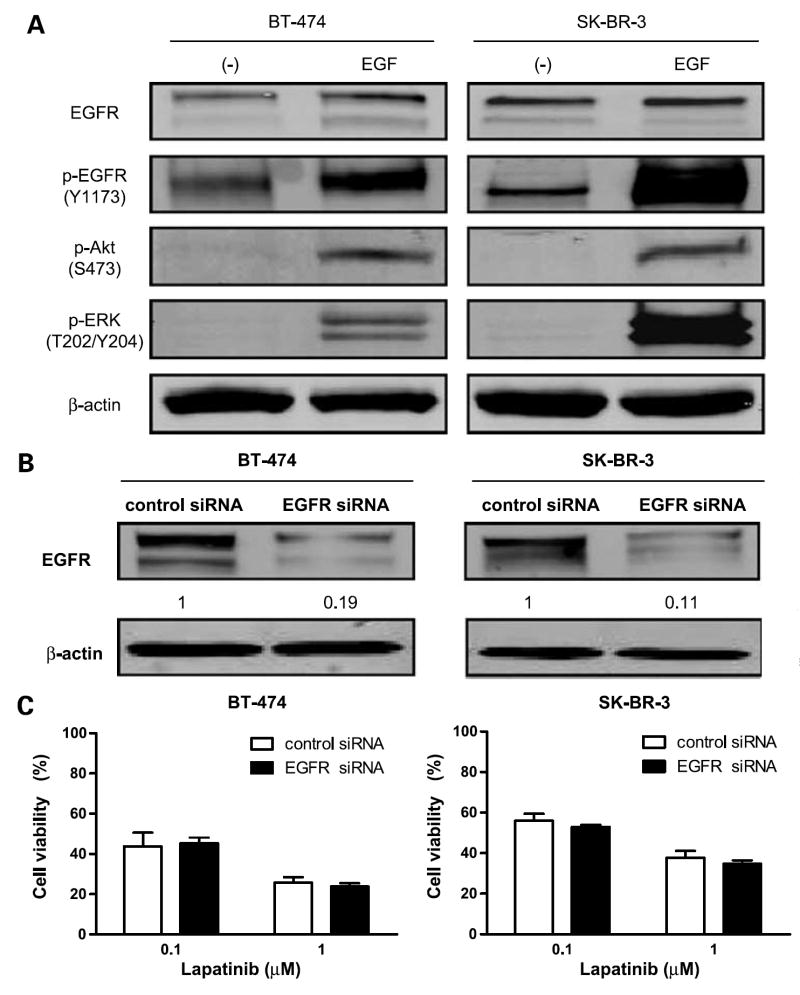
Although lapatinib is a dual EGFR/HER2 tyrosine kinase inhibitor, it is currently indicated only for treatment of HER2-overexpressing breast cancer. Its effectiveness against EGFR-overexpressing breast cancer is unknown. Thus, we studied the role of EGFR expression level in lapatinib activity. First, we tested whether the EGFR pathway is intact in BT-474 and SK-BR-3 cells by treating these cells with EGF stimulation. This stimulation up-regulated EGFR phosphorylation. We then tested the effects of EGF stimulation on expression levels of molecules downstream of the EGFR pathway. EGF stimulation also increased phosphorylation of Akt and extracellular signal-regulated kinase 1/2, suggesting that the EGFR pathway is functional in these cells (Fig. 2A).
Figure 2.
EGFR knockdown does not affect lapatinib sensitivity of HER2-overexpressing cells. A, EGF stimulation leads to EGFR activation. BT-474 and SK-BR-3 cells were cultured in serum-free medium for 24 h before EGF stimulation. Cells were exposed to EGF in cultured medium at a concentration of 100 ng/mL for 5 min. Western blot analysis was done to detect EGFR and phosphorylated EGFR, Akt, and extracellular signal-regulated kinase 1/2. β-actin was used as a loading control. B, BT-474 and SK-BR-3 cells were transfected with control siRNA or EGFR siRNA for 48 h. Western blot analysis was done to determine the expression level of EGFR after siRNA knockdown. β-actin was used as a loading control. The relative intensity of EGFR was quantified with the NIH Image program. Ratios were calculated to compare the densities of EGFR expression in EGFR siRNA –transfected cells versus control siRNA–treated cells. C, at 48 h after siRNA knockdown, the cells were treated with the indicated concentrations of lapatinib for another 72 h. Growth-inhibitory effects of lapatinib on BT-474 and SK-BR-3 cells were studied by WST-1 assay. Viability of EGFR knockdown cells was compared with that of control siRNA knockdown cells (P > 0.05). Each experiment was repeated thrice independently.
We then examined the effect of siRNA-mediated inhibition of EGFR on lapatinib sensitivity. Depletion of EGFR was confirmed by Western blot analysis, which showed significantly decreased EGFR expression at 48 hours after EGFR siRNA knockdown in both BT-474 and SK-BR-3 cells (Fig. 2B). Starting at 48 hours after knockdown, we treated these cells for another 72 hours with 0.1 or 1 μmol/L lapatinib. Knocking down EGFR expression did not affect the antiproliferative activity of lapatinib in these cells compared with control siRNA knockdown cells (P > 0.05; Fig. 2C). These results indicate that response to lapatinib in HER2-overexpressing breast cancer cells is not dependent on EGFR expression level.
Lapatinib Sensitivity of HER2-Overexpressing Breast Cancer Cell Lines Is Dependent on HER2 Expression Level
Because EGFR siRNA knockdown did not affect lapatinib activity in HER2-overexpressing breast cancer cells, we hypothesized that HER2 plays a key role in lapatinib sensitivity. To test this hypothesis, we down-regulated HER2 by HER2 siRNA knockdown in these cells, then tested the antiproliferative activity of lapatinib. Depletion of HER2 was confirmed by Western blot analysis, which showed significantly decreased HER2 expression at 48 hours after HER2 siRNA knockdown in both BT-474 and SK-BR-3 cells (Fig. 3A). Starting at 48 hours after knockdown, we treated these cells for another 72 hours with 0.1 or 1 Amol/L lapatinib. Cells transfected with HER2 siRNA were significantly more resistant to lapatinib treatment (P < 0.05; Fig. 3B).
Figure 3.
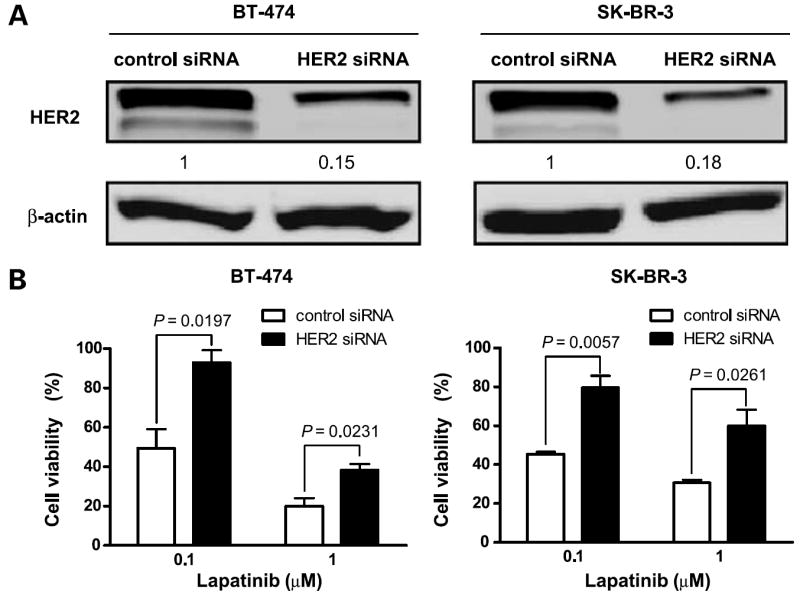
HER2 knockdown leads to lapatinib resistance in HER2-overexpressing cells. A, BT-474 and SK-BR-3 breast cancer cell lines were treated with control siRNA or HER2 siRNA for 48 h. Western blot analysis was done to determine the expression level of HER2 after siRNA knockdown. β-actin was used as a loading control. The relative intensity of HER2 was quantified with the NIH Image program. Ratios were calculated to compare the densities of HER2 expression in HER2 siRNA–transfected cells versus control siRNA–treated cells. B, at 48 h after siRNA knockdown, the cells were treated with the indicated concentrations of lapatinib for another 72 h. Growth-inhibitory effects of lapatinib on BT-474 and SK-BR-3 cells were studied by WST-1 assay. Viability of HER2 knockdown cells was compared with that of control siRNA knockdown cells. P values are indicated. Each experiment was repeated thrice independently.
Discussion
Given the roles of ErbB family tyrosine kinases EGFR and HER2 in breast cancer, treatments that inhibit both kinases are widely considered to be more effective than those that target either kinase alone (15). Lapatinib is a dual inhibitor of EGFR and HER2 tyrosine kinase catalytic activity and induces human breast tumor growth arrest and cell death in vitro and in vivo (7). A recent study by Gottfried et al. (8) presented significant correlation between lapatinib activity and HER2 expression. They also found, by performing a screening comparison of lapatinib growth inhibitory IC50 concentrations with EGFR expression, that response to lapatinib did not correlate with EGFR expression. They did not, however, test whether inhibition of the EGFR pathway affects lapatinib sensitivity. Although some preclinical work suggested that both EGFR and HER-2 were targets of lapatinib, there are no preclinical data showing whether EGFR was an effective target of lapatinib. Therefore, the role of EGFR in lapatinib-targeted breast cancer cells is still unclear.
In this study, we found that lapatinib sensitivity was independent of EGFR expression level in HER2-positive breast cancer cells. This suggests that the antitumor effect of lapatinib is the result of its anti-HER2 activity, not of any combinatorial effect of inhibiting both EGFR and HER2 tyrosine kinase activities. To test whether combination of erlotinib with lapatinib produces additive effect in HER2-overexpressing breast cancer cells, we determined the cell viability in response to erlotinib alone, lapatinib alone, and a combination of erlotinib with lapatinib. We found that erlotinib further enhanced the growth-inhibitory effect of lapatinib in SK-BR-3 cells (data not shown), indicating that the combination of erlotinib and lapatinib may have important implications for the treatment of EGFR/HER2-positive breast cancers, and that development of more powerful dual EGFR/HER2 kinase inhibitors may be warranted.
Both BT-474 and SK-BR-3 cells are moderately sensitive to EGFR tyrosine kinase inhibitor erlotinib (16). To elucidate the relationship between lapatinib sensitivity and acquired resistance against EGFR tyrosine kinase inhibition, we tested the sensitivity to lapatinib of erlotinib-resistant BT-474 and SK-BR-3 cells. These cell lines were established by continuous exposure to increasing concentrations of erlotinib for a total of 6 months. Briefly, BT-474 and SK-BR-3 cells were cultured in 3 μmol/L erlotinib for 1 month, then in 5 μmol/L erlotinib for another month, and finally in 10 μmol/L for 4 more months. We found that lapatinib inhibited proliferation of breast cancer cells with acquired resistance to erlotinib to a similar degree as it inhibited proliferation of parental cells (data not shown), providing additional indirect evidence that the antitumor activity of lapatinib is mainly through the HER2 pathway, not the EGFR pathway.
In this study, we focused on determining the role of EGFR in lapatinib sensitivity in HER2-overexpressing breast cancer cells. Therefore, we do not consider that lapatinib has been ruled out for targeting EGFR in other type of cancers. Additional studies elucidating the mechanism of action of lapatinib in HER2-negative/EGFR-positive cells need be done. Our future work will focus on elucidating this mechanism of action in HER-2 negative/EGFR-positive breast cancer cell lines such as MDA-MB-468.
Overall, our study indicates that inhibiting the EGFR pathway does not play a major role in lapatinib selectivity for HER2-overexpressing breast cancer cells. We believe that additional tests of the clinical efficacy of lapatinib in other EGFR/HER2-overexpressing solid tumors are warranted. For targeting HER2/EGFR-expressing breast cancer, future studies need to compare the biological and clinical effects of lapatinib with specific HER2-targeting therapy.
Acknowledgments
We thank Kathryn Hale (The University of Texas M. D. Anderson Cancer Center) for editorial assistance.
Grant support: NIH grant R01 CA123318-01A1, a donation from Sidney J. Jansma, Jr., and a grant from the In Christ’s Name (I.C.N.) Foundation.
Footnotes
Disclosure of Potential Conflicts of Interest No potential conflicts of interest were disclosed.
References
- 1.Olayioye MA, Neve RM, Lane HA, Hynes NE. The ErbB signaling network: receptor heterodimerization in development and cancer. EMBO J. 2000;19:3159–67. doi: 10.1093/emboj/19.13.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cerra M, Cecco L, Montella M, Tuccillo F, Bonelli P, Botti G. Epidermal growth factor receptor in human breast cancer comparison with steroid receptors and other prognostic factors. Int J Biol Markers. 1995;10:136–42. doi: 10.1177/172460089501000302. [DOI] [PubMed] [Google Scholar]
- 3.Seshadri R, McLeay WR, Horsfall DJ, McCaul K. Prospective study of the prognostic significance of epidermal growth factor receptor in primary breast cancer. Int J Cancer. 1996;69:23–7. doi: 10.1002/(SICI)1097-0215(19960220)69:1<23::AID-IJC5>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 4.Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–82. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 5.Slamon DJ, Godolphin W, Jones LA, et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989;244:707–12. doi: 10.1126/science.2470152. [DOI] [PubMed] [Google Scholar]
- 6.Petrov KG, Zhang YM, Carter M, et al. Optimization and SAR for dual ErbB-1/ErbB-2 tyrosine kinase inhibition in the 6-furanylquinazoline series. Bioorg Med Chem Lett. 2006;16:4686–91. doi: 10.1016/j.bmcl.2006.05.090. [DOI] [PubMed] [Google Scholar]
- 7.Rusnak DW, Lackey K, Affleck K, et al. The effects of the novel, reversible epidermal growth factor receptor/ErbB-2 tyrosine kinase inhibitor, GW2016, on the growth of human normal and tumor-derived cell lines in vitro and in vivo. Mol Cancer Ther. 2001;1:85–94. [PubMed] [Google Scholar]
- 8.Konecny GE, Pegram MD, Venkatesan N, et al. Activity of the dual kinase inhibitor lapatinib ( GW572016) against HER-2-overexpressing and trastuzumab-treated breast cancer cells. Cancer Res. 2006;66:1630–9. doi: 10.1158/0008-5472.CAN-05-1182. [DOI] [PubMed] [Google Scholar]
- 9.Burris HA, III, Hurwitz HI, Dees EC, et al. Phase I safety, pharmacokinetics, and clinical activity study of lapatinib ( GW572016), a reversible dual inhibitor of epidermal growth factor receptor tyrosine kinases, in heavily pretreated patients with metastatic carcinomas. J Clin Oncol. 2005;23:5305–13. doi: 10.1200/JCO.2005.16.584. [DOI] [PubMed] [Google Scholar]
- 10.Geyer CE, Forster J, Lindquist D, et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med. 2006;355:2733–43. doi: 10.1056/NEJMoa064320. [DOI] [PubMed] [Google Scholar]
- 11.Spector NL, Blackwell K, Hurley J. EGF103009, a phase II trial of lapatinib monotherapy in patients with relapsed/refractory inflammatory breast cancer (IBC): clinical activity and biologic predictors of response. Proc Am Soc Clin Oncol. 2006;24(Abstract 502) [Google Scholar]
- 12.Abidoye OO, Cohen EE, Wong SJ. A phase II study of lapatinib ( GW572016) in recurrent/metastatic (R/M) squamous cell carcinoma of the head and neck (SCCHN) Proc Am Soc Clin Oncol. 2006;24(Abstract 5568) [Google Scholar]
- 13.Fields AL, Rinaldi DA, Henderson CA. An open-label multicenter phase II study of oral lapatinib ( GW572016) as single agent, second-line therapy in patients with metastatic colorectal cancer. Proc Am Soc Clin Oncol. 2005;23(Abstract 3583) [Google Scholar]
- 14.Spector NL, Xia W, Burris H, III, et al. Study of the biologic effects of lapatinib, a reversible inhibitor of ErbB1 and ErbB2 tyrosine kinases, on tumor growth and survival pathways in patients with advanced malignancies. J Clin Oncol. 2005;23:2502–12. doi: 10.1200/JCO.2005.12.157. [DOI] [PubMed] [Google Scholar]
- 15.Reid A, Vidal L, Shaw H, de Bono J. Dual inhibition of ErbB1 (EGFR/HER1) and ErbB2 (HER2/neu) Eur J Cancer. 2007;43:481–9. doi: 10.1016/j.ejca.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 16.Yamasaki F, Zhang D, Bartholomeusz C, et al. Sensitivity of breast cancer cells to erlotinib depends on cyclin-dependent kinase 2 activity. Mol Cancer Ther. 2007;6:2168–77. doi: 10.1158/1535-7163.MCT-06-0514. [DOI] [PMC free article] [PubMed] [Google Scholar]