Abstract
The sensitivity of αβγ rat epithelial Na+ channel (rENaC) to osmotically or mechanically induced changes of membrane tension was investigated in the Xenopus oocyte expression system, using both dual electrode voltage clamp and cell-attached patch clamp methodologies. ENaC whole-cell currents were insensitive to mechanical cell swelling caused by direct injection of 90 or 180 nl of 100-mM KCl. Similarly, ENaC whole-cell currents were insensitive to osmotic cell swelling caused by a 33% decrease of bathing solution osmolarity. The lack of an effect of cell swelling on ENaC was independent of the status of the actin cytoskeleton, as ENaC remained insensitive to osmotic and mechanical cell swelling in oocytes pretreated with cytochalasin B for 2–5 h. This apparent insensitivity of ENaC to increased cell volume and changes of membrane tension was also observed at the single channel level in membrane patches subjected to negative or positive pressures of 5 or 10 in. of water. However, and contrary to the lack of an effect of cell swelling, ENaC currents were inhibited by cell shrinking. A 45-min incubation in a 260-mosmol solution (a 25% increase of solution osmolarity) caused a decrease of ENaC currents (at −100 mV) from −3.42 ± 0.34 to −2.02 ± 0.23 μA (n = 6). This decrease of current with cell shrinking was completely blocked by pretreatment of oocytes with cytochalasin B, indicating that these changes of current are not likely related to a direct effect of cell shrinking. We conclude that αβγ rENaC is not directly mechanosensitive when expressed in a system that can produce a channel with identical properties to those found in native epithelia.
Keywords: epithelial Na channel, mechanosensitivity, Xenopus oocytes, patch clamp, osmolarity
introduction
Epithelial Na+ channels historically have been classified according to their kinetics, pharmacology, and single channel conductance (see Palmer, 1992; Smith and Benos, 1991). The molecular identity of these channels was unknown until the cloning of the Na(5) channel (classification of Palmer, 1992) independently by Canessa et al. (1993, 1994), by Lingueglia et al. (1993), and Voilley et al. (1994). This channel is also referred to as the epithelial Na+ channel (ENaC)1 and is characterized by a 5-pS single channel conductance, long open and close times, high amiloride sensitivity (K i < 100 nM), and high Na+ to K+ selectivity. ENaC is composed of three subunits, α, β, and γ, that are necessary to reconstitute the in vivo single channel properties and maximal amiloride-sensitive currents in Xenopus oocytes (Canessa et al., 1994).
The epithelial Na+ channel shares little molecular homology with other known ion channels (for review see Garty and Palmer, 1997). However, this channel belongs to a family of proteins that include the degenerins from the nematode Caenorhabditis elegans. As these degenerins are known to participate in touch reception by this nematode, it is thought that they may form mechanosensitive ion channels. This observation has led to speculation that ENaCs may also form mechanosensitive ion channels.
The issue of ENaC and mechanosensitivity is of particular physiological importance given ENaC's presence in many native epithelia that not only undergo moment to moment changes of hydrostatic pressure, but also dynamic changes of luminal osmolarity. To date, there are four reports examining the issue of mechanosensitivity of ENaCs. We have previously reported (Awayda et al., 1995) that the alpha subunit of the bovine homolog (αbENaC) forms a mechanosensitive channel when incorporated into planar lipid bilayers. However, the properties of the channel formed by incorporation of the alpha subunit were not the same as those of the αβγ ENaC channel observed in native epithelia.
Second, and consistent with the bilayer experiments on αbENaC, transfection of αrENaC into mammalian fibroblasts coincided with the appearance of a nonselective stretch-activated channel that was presumably absent before transfection (Kizer et al., 1997). This finding was also consistent with the observation that osteoclasts contain the message for the alpha subunit alone, and also contain the same type of nonselective stretch-activated channel as the αrENaC-transfected fibroblasts.
Third, the channel formed by all three rat subunits (αβγ rENaC) was also found to be mechanosensitive when incorporated into artificial lipid membrane (Ismailov et al., 1996). This conclusion is in disagreement with a recent report by Palmer and Frindt (1996), who found that the open probability (P o) of the Na(5) channel in native epithelia (cortical collecting tubule) was not consistently altered by increasing membrane tension by varying patch pipette hydrostatic pressure (an effect of pressure was observed in only 6 of 22 cell-attached membrane patches). Given the differences between the properties of the cloned channel in both the bilayer and fibroblast systems and those of the native Na(5) channel, it is possible that the discrepancy in the observed effects of membrane tension may be attributed to differences among these expression systems, or in the protein composition of the channel complex in these systems (see discussion).
To address the controversy regarding mechanosensitivity of the ENaC, we used the oocyte expression system and a combination of dual electrode voltage clamp and cell-attached patch clamp methodologies to systematically examine the effects of changes of membrane tension on αβγ ENaC expressed in a biological membrane at the whole-cell and single channel levels. We report that αβγ rENaC, when studied in the biological membrane of Xenopus oocytes, is not mechanosensitive at either the whole-cell or single channel levels. Moreover, this channel remained insensitive to mechanical perturbations even after disruption of the actin cytoskeleton, a condition that may enhance the mechanosensitivity of other ion channels (see Sachs, 1986). The absence of direct mechanosensitivity of ENaC indicates the presence of other indirect mechanisms that allow some Na+-transporting epithelia to respond to changes of luminal osmolarity (Wills et al., 1991).
materials and methods
Oocyte Manipulation
Toads were obtained from Xenopus I (Ann Arbor, MI) and were kept in dechlorinated tap water at 18°C. Oocytes were surgically removed from anesthetized toads and processed as previously described (Awayda et al., 1996, 1997). αβγ rENaC-expressing oocytes were injected with 2.5 ng of each subunit cRNA in 50 nl of nuclease-free water. Injected oocytes were incubated at 18–20°C for 1–3 d until recording. All recordings were performed at 19–21°C.
In experiments that required the direct injection of KCl to increase the oocyte volume, oocytes were first impaled with the injecting electrode, followed by impalement with the two recording microelectrodes as previously described (Awayda et al., 1996). In these experiments, the injecting electrode was filled with 100 mM KCl.
RNA Synthesis
RNA synthesis was performed as previously described (Awayda et al., 1996, 1997). The plasmids containing α, β, or γ rENaC (a gift of B. Rossier, University of Lausanne, Lausanne, Switzerland) were linearized with the appropriate 3′ restriction enzyme. Linearized plasmid DNA was purified using the Geneclean kit (Bio 101, Vista, CA). Sense RNA was synthesized from purified plasmid DNA using T7 RNA polymerase, according to the manufacturer's instructions (Promega Corp., Madison, WI). RNA was in vitro synthesized in the presence of methylguanosine cap analog, m7G(5′)ppp(5′)G (New England Biolabs Inc., Beverly, MA) in threefold excess to GTP. After two rounds of phenol/chloroform extraction and ethanol precipitation, RNA was resuspended in water and quantitated by measuring OD at 260 nm, and stored at −80°C.
Solutions and Chemicals
The pH of all oocyte solutions was adjusted to 7.5. Oocytes were defoliculated in Ca2+-free Ringers of the following composition (mM): 88 NaCl, 1 MgCl2, 2 KCl, 5 HEPES. The oocyte culture medium was half strength (Leibovitz media (L-15; Sigma Chemical Co., St. Louis, MO) supplemented with 15 mM HEPES and 1% of a 10,000-U/ml solution of penicillin/streptomycin (Gibco Laboratories, Grand Island, NY). The recording medium, ND-96, contained (mM): 96 NaCl, 1 MgCl2, 2 KCl, 1.8 CaCl2, 5 HEPES. In experiments that required hyperosmotic cell shrinking, the control solution was ND-96 and the hyperosmotic solution contained an additional 50 mM sucrose. In experiments that required cell swelling, the control solution was reduced ionic strength ND-96 (25, 33, or 50% decrease of ionic strength, and decrease of NaCl to ∼72, 65, or 48 mM) supplemented with the appropriate amount of sucrose to adjust osmolarity to 210 mosmol. The corresponding hypotonic solution eliminated the sucrose. ENaC-expressing oocytes did not tolerate a 50% reduction of osmolarity, as evident by membrane disruption and release of yolk into the bathing medium in <30 min. For cell-attached patch clamp experiments, ND-96 was used in both the bath and as the pipette filling solution. Oocytes were devitellinized in a hyperosmotic solution containing 25 mM sucrose in ND-96. To minimize the contribution of endogenous currents, the pipette filling solution contained the following concentration of blockers: 5 mM Ba++, 100 μM DIDS (4,4′-diisothiocyanate-stilbene-2,2′-disulfonic acid), and 100 μM niflumic acid. DIDS and niflumic acid were obtained from Sigma Chemical Co. and were dissolved in ethanol at a stock concentration of 100 mM. Cytochalasin B (Sigma Chemical Co.) was dissolved in ethanol at a stock concentration of 20 mM and was used at a final concentration of 20 μM. Amiloride was a gift from Merck-Sharp & Dohme (Rahway, NJ), and was used at a final concentration of 10 μM from a stock of 10 mM in water.
Dual Electrode Clamp
Whole-cell currents were recorded and analyzed as described by Awayda et al. (1996). Oocytes were perfused with solution at the rate of 2 ml/min, or approximately two chamber volumes/min. Whole-cell currents were obtained by clamping to voltages between −100 and +60 mV, in 20-mV increments. Currents at −100 and at −80 mV were summarized from the averaged value of the last five current points at the end of the voltage episode. Inward slope conductance was calculated as the conductance between −100 and −80 mV. Amiloride-sensitive inward slope conductances were calculated by subtracting the inward slope conductance of the amiloride-insensitive currents after the application of 10 μM amiloride. By convention, inward flow of cations is designated as inward current (negative current), and all voltages are reported with respect to ground or bath. Except where noted, all data are reported as mean ± SEM.
Patch Clamp
Oocytes were incubated in ND-96 supplemented with 25 mM sucrose for 10 min. This caused shrinking of the oocyte and separation of the plasma membrane from the vitellin membrane, and allowed the mechanical removal of the vitellin membrane. Devitellinized oocytes were transferred to the recording chamber containing ND-96 and allowed to recover for ∼20 min before being patched. Electrodes were pulled in three stages on a P87 horizontal puller (Sutter Instruments, Co., Novato, CA). Electrode resistances were reproducible and in the range of 2 MΩ. Recordings were carried out as described by Hamill et al. (1981) using a BioLogic RK-400 (Molecular Kinetics, Pullman, WA) patch clamp amplifier. In oocytes that had no obvious leakage of yolk into the chamber, gigaohm seals (>20 GΩ) were observed in >80% of cases with <2 in. applied negative water pressure. Patches that required greater than 2 in. H2O sealing pressure were discarded so as to reduce the chances of altering the existing membrane tension.
Single channel data were stored on digital audio tape (DAT 200; Vetter Instruments, Rebersburg, PA). Upon playback for data acquisition (TL-1 interface; Axon Instruments, Foster City, CA), data were filtered at 100 Hz and acquired at 200 Hz. Data were analyzed using pCLAMP 6.0.3 (Axon Instruments). All voltages are reported intracellular with respect to patch pipettes, and all positive currents represent the forward flow of cations from the pipette to the inside of the cell.
Single channel analysis was performed on records obtained from experiments with seal resistances >20 GΩ that remained stable for >30 min. An event list was generated by the Fetchan subroutine of pCLAMP and was used to analyze the single channel parameters, including the channel's open and close times, open probability, and amplitude using the pSTAT subroutine of pCLAMP. Single channel open probability was calculated according to the following equation:
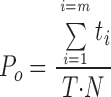 |
where t i is an event dwell time, N is the number of channels, and m and T are the number of events and the duration of the analyzed record, respectively. Single channel current was determined from the Gaussian fit to the event amplitude histograms.
Statistical analyses for both the whole cell and single channel data were carried out using paired Student's t test where appropriate. Significance was determined at the 95% confidence levels (P < 0.05).
results
Experiments reported here used oocytes expressing all three subunits of the rat epithelial Na+ channel (αβγ rENaC). For the sake of simplicity, these will be referred to as ENaC-expressing oocytes. Amiloride-sensitive whole-cell currents in ENaC-expressing oocytes averaged −2.82 ± 0.15 μA (n = 41) at −100 mV, and were 30–40-fold larger than those reported in oocytes expressing the alpha subunit alone (Canessa et al., 1994; Voilley et al., 1994; McDonald et al., 1995; Awayda et al., 1996, 1997). Moreover, the currents in ENaC-expressing oocytes treated with 10 μM amiloride were −69 ± 7 nA (n = 16) at −100 mV, and were similar to the currents in control oocytes. The large difference of these currents made it relatively easy to determine changes of the amiloride-sensitive or -insensitive currents subsequent to any experimental manipulation. Moreover, in experiments where the amiloride-insensitive currents were constant, the changes of whole-cell currents could be used as an index of changes of ENaC currents.
Effects of Osmotic Swelling
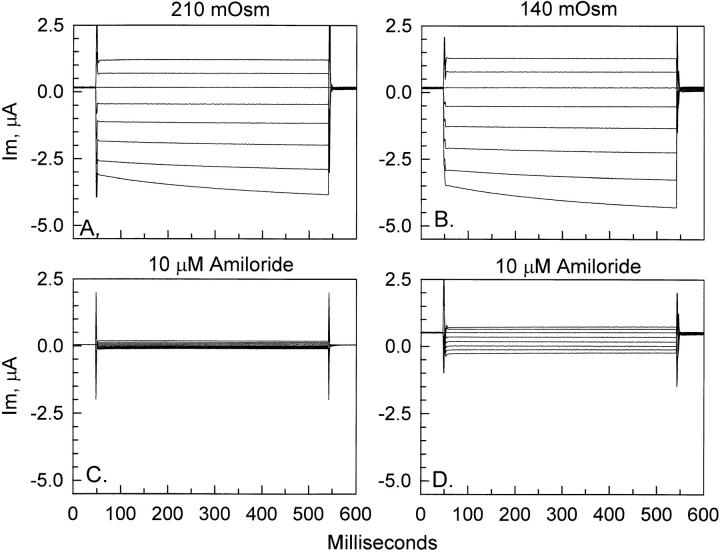
As a first measure of assessing the mechanosensitive properties of ENaC, we tested the effects of osmotic cell swelling on the whole-cell currents. Oocytes were equilibrated in the control 210 mosmol solution for a period of 30 min, followed by cell swelling in 140 mosmol solution for 30 min. As shown in Fig. 1, a 33% reduction of solution osmolarity caused a small increase of the whole cell currents observed at 30 min after the osmolarity change. These small changes were accompanied by a similar increase of the amiloride-insensitive currents (Fig. 1, C and D). Examination of the summary data in Fig. 2 indicated that the −439-nA increase of control current subsequent to cell swelling was relatively small (as compared with a whole-cell current of −3,376 nA) and was similar in magnitude to the −318-nA increase of the amiloride-insensitive currents. Thus, these small changes of current were not likely related to an increase of ENaC activity.
Figure 1.
Effect of osmotic cell swelling on ENaC whole-cell currents. Representative example of the effect of a 33% decrease of solution osmolarity. Whole-cell currents were measured as described in materials and methods. As seen in A and B, there were no appreciable changes in the currents between the control 210 mosmol and the hypo-osmotic 140 mosmol solutions. The small increase of current with hypo-osmolarity was accompanied by a similar increase of the amiloride-insensitive current before (C ) and after (D) hypo-osmolarity.
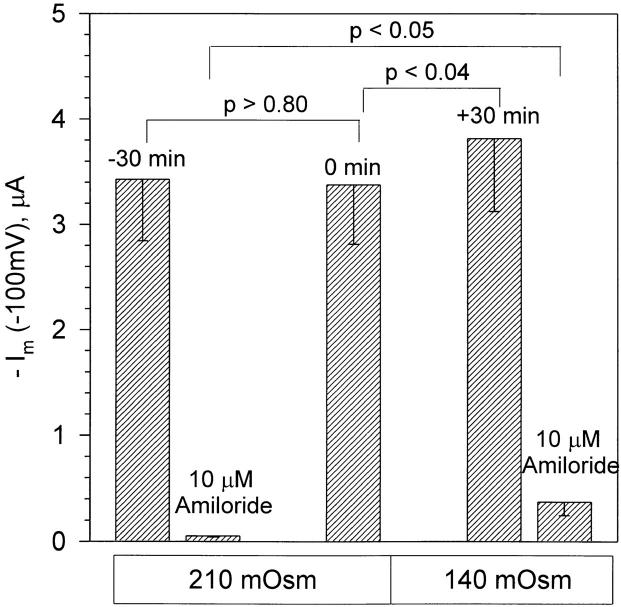
Figure 2.
ENaC currents are insensitive to osmotic cell swelling. Currents are summarized at a holding voltage of −100 mV. In this group of experiments, the baseline current was stable during the 30 min control period (compare the values of −30 min and 0 min in the 210 mOsm solution). The currents at 30 min after cell swelling (+30 min, 140 mOsm) were elevated above the iso-osmotic control values (0 min). However, this increase of current with cell swelling was likely due to the accompanying increase of the amiloride-insensitive current (compare 10 μM amiloride in 210 mOsm and 140 mOsm). n = 6.
Incubation of oocytes in a 50% hypo-osmotic or 105 mosmol solution resulted in membrane disruption and yolk leakage within 30 min (“oozing”). Thus, in our experiments, the 33% decrease of osmolarity for 30 min represented the maximal hypo-osmotic challenge that could be tolerated by ENaC-expressing oocytes without compromising their membrane integrity. At this near maximal level of hypo-osmolarity, it was clear that ENaC-expressing oocytes were insensitive to osmotically induced increases in membrane tension.
Effects of Mechanical Swelling
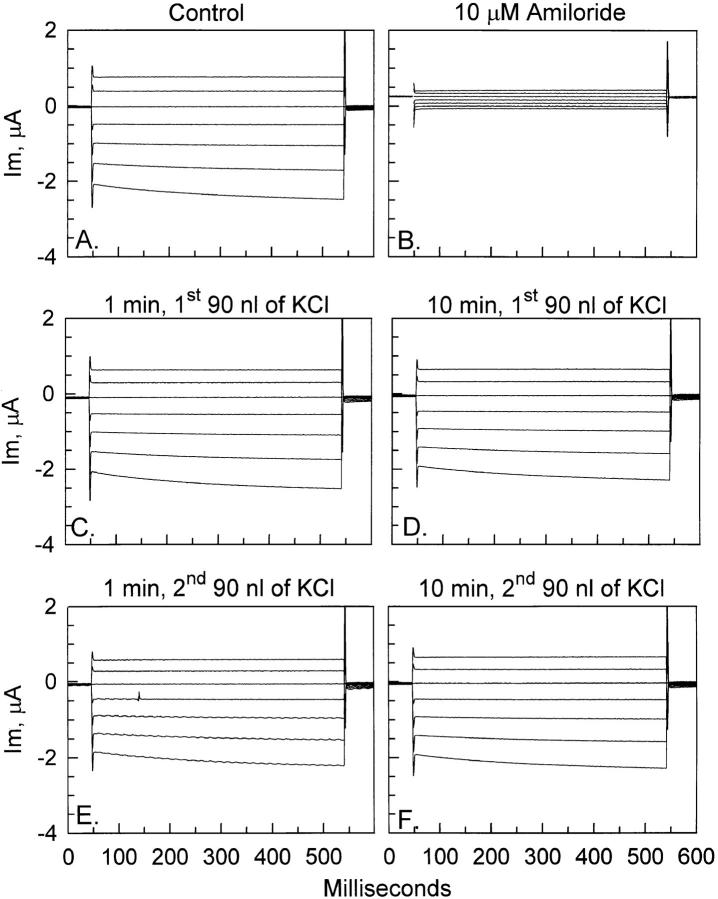
To rule out the presence of any potential volume-regulatory changes that may occur over a period of 30 min and may compensate for any osmotic swelling–mediated activation of ENaC, we carried out experiments in oocytes impaled with a third electrode that allowed the immediate increase of cell volume by intracellular injection of isotonic KCl. As shown in Fig. 3, injection of 90 nl of 100-mM KCl did not result in an increase of ENaC currents. In this representative example, there were no appreciable immediate (within 1 min; Fig. 3 C ) or long term (within 10 min; Fig. 3 D) changes of whole cell currents. Some oocytes were injected with an additional 90 nl of KCl. This was done 10–15 min after the first injection and was used to test whether additional volume changes can produce a large enough increase of membrane tension to cause ENaC stimulation. As seen in Fig. 3, E and F, these larger volume changes were still not able to elicit appreciable ENaC stimulation despite the larger increase of cell volume. This is the maximum increase an oocyte membrane can tolerate before oozing, and therefore represents a similar increase of membrane tension to that caused by osmotic swelling in response to a 33% decrease of solution osmolarity, except that this effect is more immediate.
Figure 3.
Effect of mechanical cell swelling on ENaC whole-cell currents. Representative example testing the effects of 90- and 180-nl increases of cell volume. The baseline ENaC currents are shown in A, and the amiloride insensitive currents are shown in B. A 90-nl increase of cell volume (see text) did not result in appreciable immediate (1 min, C) or long term (10 min, D) changes of current. A total increase of cell volume by 180 nl, by a second 90-nl injection 10 min after the first one, was still without immediate (1 min, E ) or long term (10 min, F ) effects on currents.
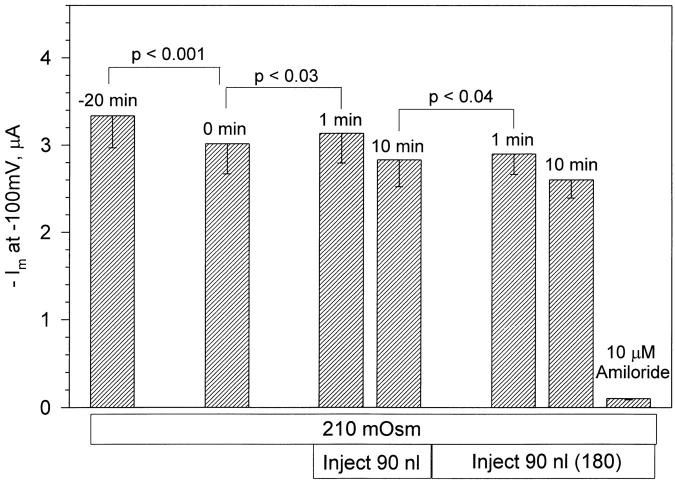
Summarized in Fig. 4 are the effects of volume injection on ENaC currents. A small (<10%) but statistically significant increase of whole-cell current (at −100 mV) was observed 1 min after the first 90-nl injection. Likewise, there was also a small but significant increase of current after the second 90-nl injection. This small initial increase of current within the first minute of volume injection was transient and currents returned to baseline within 10 min (currents actually returned to values below baseline; however, this is accounted for by the small drift during the control period). This initial current increase is likely unrelated to activation of ENaC, as a similar but larger response was observed in control oocytes pretreated with cytochalasin B (see below).
Figure 4.
ENaC currents are insensitive to mechanical cell swelling. Currents are summarized at a holding voltage of −100 mV. There was a small (<10%) increase of current 1 min after the first injection. This increase was transient and currents decayed to values expected of control. Similar results were observed in oocytes injected twice for a total cell-volume increase of 180 nl. This transient stimulation was similar to, but smaller than, that observed in control oocytes pretreated with cytochalasin B (Figs. 7 and 8). Therefore, it is likely unrelated to ENaC stimulation. Thus, ENaC currents were insensitive to both osmotic and mechanical cell swelling. n = 6 for the first 90-nl injection and n = 4 for the second 90-nl injection.
Effects of the Actin Cytoskeleton on Cell Swelling
The possibility that the differences in mechanosensitivity between the channel expressed in oocytes and that incorporated into planar lipid bilayers were attributed to the presence of an actin cytoskeleton that may stabilize the native channel was tested. Similar cell-swelling experiments were carried out in oocytes pretreated for 2–5 h with 20 μM cytochalasin B. Oocytes pretreated with cytochalasin B were more sensitive to changes of cell volume, presumably owing to the loss of some cytoskeletal integrity. As shown in the example in Fig. 5, oocytes pretreated with cytochalasin B were still insensitive to osmotically induced cell swelling and membrane stretch. In cytochalasin-pretreated oocytes, a decrease of solution osmolarity of >25% for 30 min was not well tolerated by the oocyte membrane, and thus experiments are limited to this osmolarity change.
Figure 5.
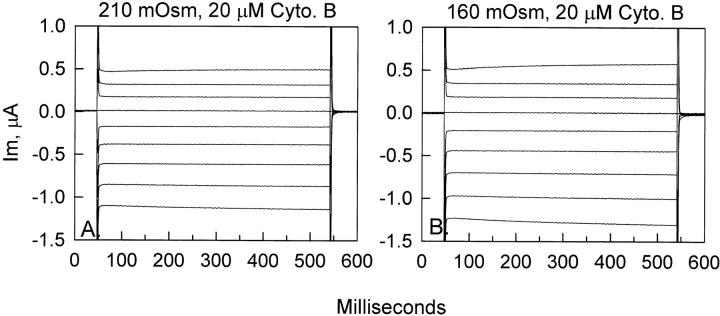
Effect of hypo-osmolarity on ENaC whole-cell currents in oocytes pretreated with cytochalasin B. Representative ENaC currents in oocytes pretreated with cytochalasin B (A) were not altered after 30 min of hypo-osmotic cell swelling (B). In this group of oocytes, a 25% decrease of osmolarity was the maximum decrease that could be tolerated before oozing.
As summarized in Fig. 6, there was no significant difference between the control currents and those observed after 30 min in the 160-mosmol solution. In this group of experiments there was a tendency for a small decrease of current during the control period. During a 30-min control period, the inward current decreased by −137 nA from −1,589 ± 222 (−30 min) to −1,452 ± 232 (0 min) nA. The values of current before and after cell swelling were not statistically different and averaged −1,452 ± 232 (0 min) and −1,439 ± 194 (+30 min) nA, respectively. Moreover, even with a −137-nA drift in 30 min, these two values would not be significantly different from each other. Thus, consistent with the lack of effect of cell swelling in untreated oocytes (see Figs. 1 and 2, above), ENaC currents in cytochalasin B-pretreated oocytes were also insensitive to osmotic cell swelling.
Figure 6.
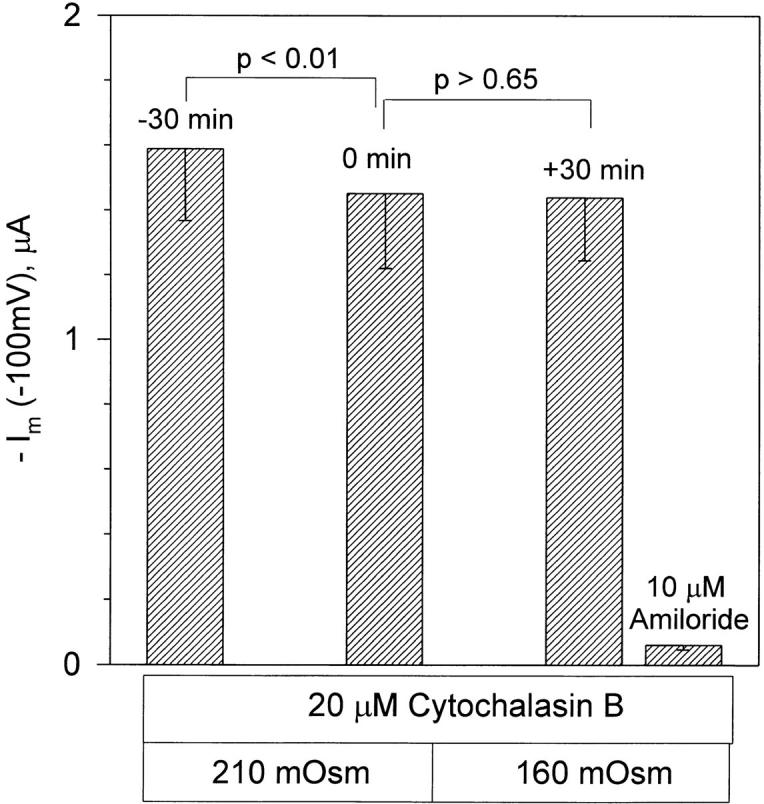
ENaC currents are also insensitive to osmotic cell swelling in the presence of cytochalasin B. Oocytes were pretreated with cytochalasin B for 2–5 h. Currents are summarized at a holding voltage of −100 mV. In this group of experiments there were no differences between the 0 min control currents recorded in the 210-mosmol solution and +30 min currents recorded 30 min after incubation in the 160-mosmol solution. There were also no differences between the amiloride-insensitive currents in oocytes treated with cytochalasin B and those untreated (see 10 μM amiloride in Fig. 4). n = 6.
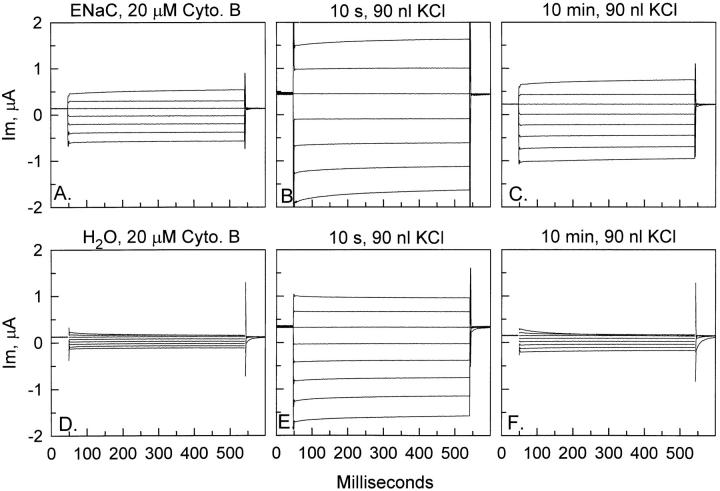
To test the immediate effects of cell swelling, cytochalasin B-pretreated ENaC-expressing oocytes were injected with 90 nl of isotonic KCl. As observed with osmotic swelling, cytochalasin treatment resulted in increased membrane fragility and these oocytes could not tolerate injections >90 nl. As shown in Fig. 7, ENaC-expressing oocytes pretreated with cytochalasin B were found to be transiently stimulated by cell swelling. Within 10 s of injection of 90 nl of isotonic KCl and subsequent increase of membrane tension, a large (∼−1 μA) stimulation of current at −100 mV was observed (Fig. 7 B). This current gradually returned to values slightly higher than baseline within 10 min (Fig. 7 C ). However, this same pattern of transient stimulation was also observed in water-injected oocytes (Fig. 7, E and F ).
Figure 7.
Effect of mechanical swelling on whole-cell currents in ENaC and water-injected oocytes pretreated with cytochalasin B. Currents in ENaC-expressing oocytes (A) responded to injection of 90 nl of 100-mM KCl with a transient stimulation (10 s, B) that gradually declined toward baseline by 10 min (C ). However, the same pattern of stimulation was observed in water-injected oocytes (D) that exhibited an initial increase (10 s, E ) that declined toward baseline within 10 min (F ). The two examples shown here were chosen to reflect the potential for very large initial stimulation in response to volume injection in both control oocytes and ENaC-expressing oocytes.
Summarized in Fig. 8 are the effects of mechanical cell swelling on control and ENaC-expressing oocytes. Currents in ENaC-expressing oocytes increased by −652 nA within 20 s and returned toward control within 10 min (Fig. 8 A). This increase was unrelated to activation of ENaC as a similar increase of −482 nA was observed in ENaC-expressing oocytes treated with 10 μM amiloride (Fig. 8 B). Moreover, this increase was also observed in control oocytes and averaged −538 nA. Thus, the increase summarized in Fig. 8 is likely due to activation of an endogenous channel, and may be mediated via the channel activated after the application of a positive pipette pressure of 10 in. of water (see the cell-attached patch experiments below).
Figure 8.
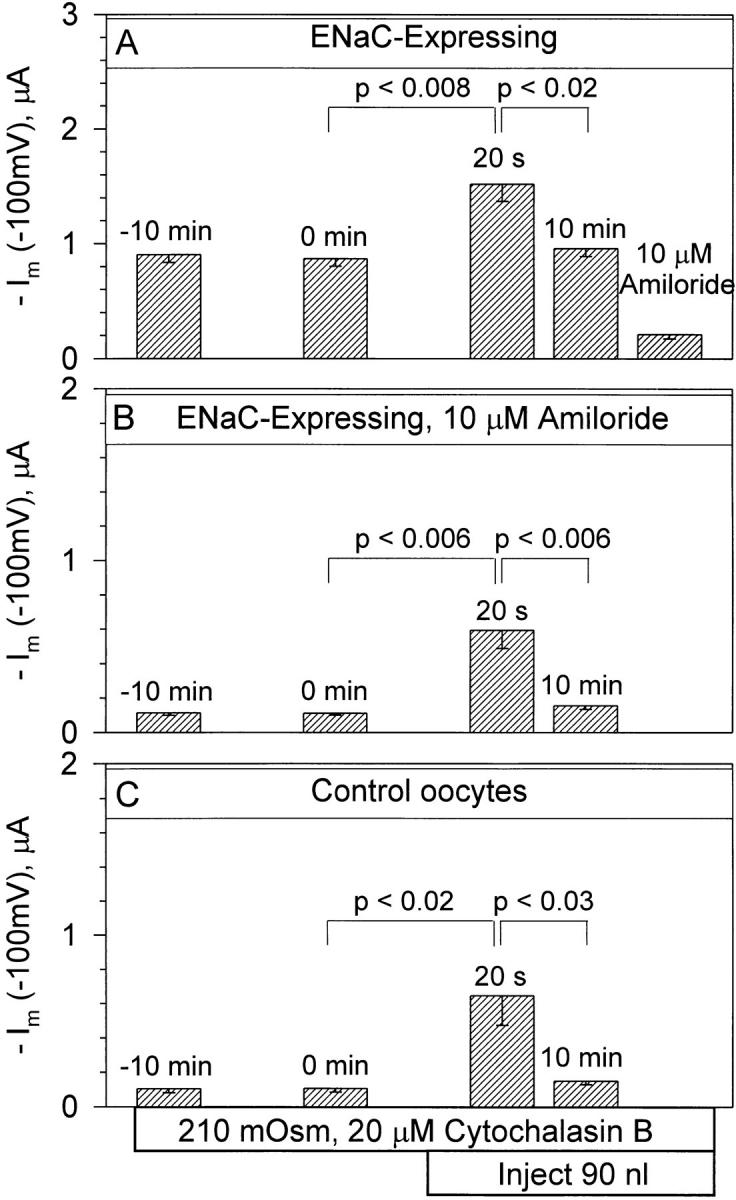
Mechanical cell swelling activates an endogenous current in cytochalasin B-pretreated oocytes. ENaC-expressing oocytes responded by a transient increase of current at −100 mV 20 s after the 90-nl volume increase (A). However a similar response was observed in ENaC-expressing oocytes treated with 10 μM amiloride (B). This response was also observed in control oocytes (C ) and is, therefore, unrelated to the presence of ENaC. The initial stimulation is summarized at 20 s rather than 10 s, as this value was not measured in all groups of oocytes. However, the stimulation at 20 s was only slightly lower than that observed at 10 s. n = 7 for each group.
Effect of Cell Shrinking
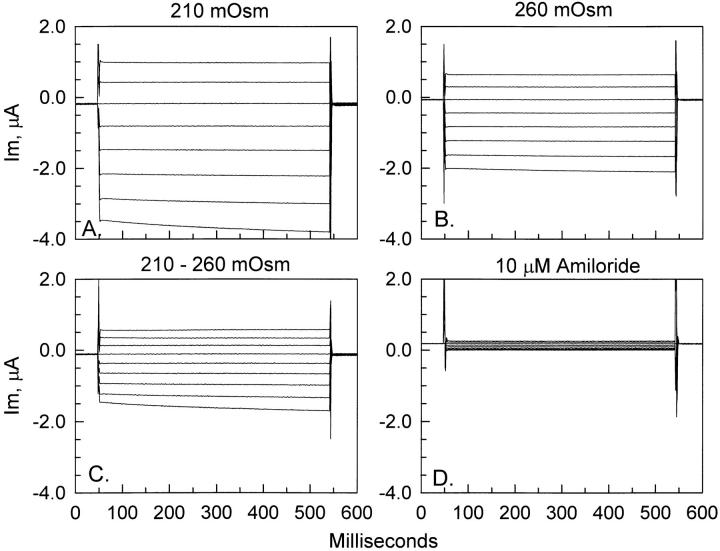
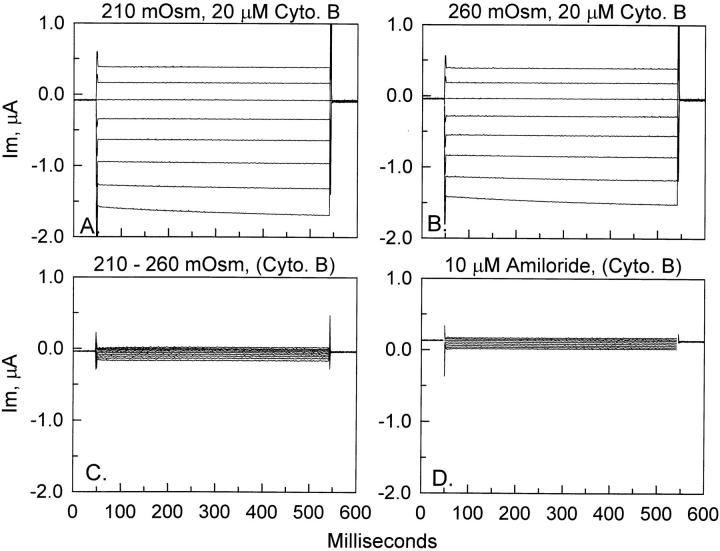
Without a priori knowledge of the existing membrane tension in oocyte membranes, we cannot rule out the possibility that cell shrinking may also alter ENaC currents. To test the effects of a decrease of cell volume, oocytes were incubated in hyperosmotic solution containing sucrose. In response to a 25% increase of bathing solution osmolarity, oocytes exhibited a gradual decrease of current. Fig. 9 shows the effect of a 45-min incubation of ENaC-expressing oocytes in hyperosmotic solution of 260 mosmol. It is clear from Fig. 9, A and B, that cell-shrinking causes an inhibition of ENaC currents. The currents inhibited by cell shrinking are shown in Fig. 9 C and are indistinguishable in characteristics from the ENaC whole-cell currents. Moreover, the currents in Fig. 9 C are also amiloride sensitive, as evident from the fact that they are larger in magnitude than the amiloride-insensitive currents observed at the beginning of any experiment (see Fig. 1 C ) or at the end of this experiment (Fig. 9 D).
Figure 9.
Effect of hyperosmolarity on ENaC currents. ENaC-expressing oocytes (A) responded to a 45-min incubation in 260 mosmol hyperosmotic solution by a decrease of whole-cell currents (B). The magnitude of the shrinking-inhibitable currents is shown in C and is indistinguishable from ENaC currents, and is, moreover, amiloride sensitive, as indicated by the relatively small values of amiloride-insensitive currents observed at the end of the experiment (D).
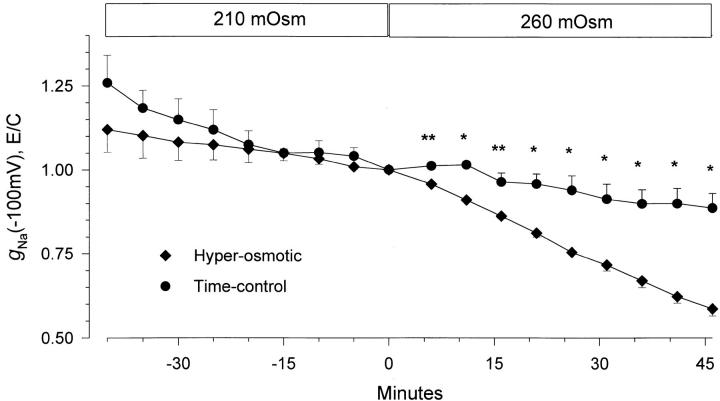
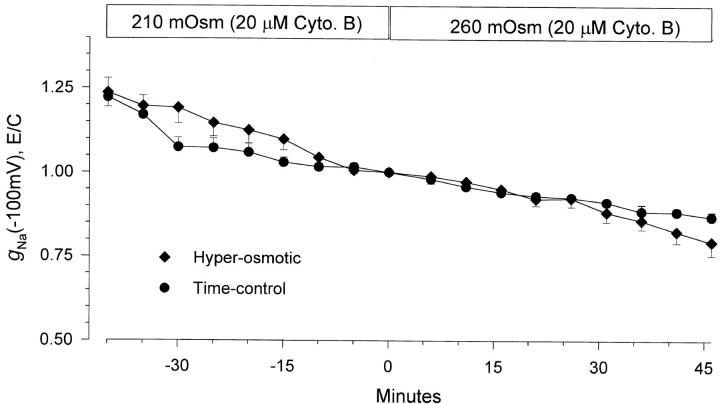
Summarized in Fig. 10 is the time course of the changes from control of the amiloride-sensitive ENaC slope conductance at −100 mV. This data is summarized as changes of conductance rather than changes of currents at −100 mV because the value of the slope conductance is independent of the oocyte reversal potential. Moreover, as ENaC exhibits inward rectification, and the overall conductance of oocyte-endogenous channels exhibit outward rectification, the contribution of other, albeit small, conductances is further minimized in the hyperpolarizing range. Cell shrinking caused a gradual inhibition of the inward slope conductance. At 45 min, conductance decreased by 41.4 ± 2.1%, from 38.2 ± 3.9 to 22.4 ± 2.8 μS (n = 6). Conductance in ENaC-expressing oocytes untreated with the hyperosmotic solution (time-control oocytes) decreased by 11.4 ± 4.4% (n = 7) during the same time period. Thus, it is clear that cell shrinking caused a gradual inhibition of ENaC's conductance. However, the slow time course of this change provided justifiable concerns as to the nature of this inhibition, especially as oocytes placed in the hyperosmotic solution were verified by visual inspection to shrink within 5–10 min. Thus, the inhibitory effect of cell shrinking may be indirectly mediated via other regulatory mechanisms, rather than a direct effect of changes of membrane tension.
Figure 10.
Time-course of inhibition of amiloride-sensitive slope conductance after hyperosmotic cell shrinking. Data were corrected for the value of the amiloride-insensitive slope conductance as described in materials and methods and are plotted normalized to the conductance immediately before the hyperosmotic solution change. The time-control data were obtained from oocytes treated in a similar manner except that they were not incubated in the hyperosmotic solution. It is clear that a 25% increase of solution osmolarity caused a gradual decrease of ENaC conductance. This decrease of conductance was not observed in the time-control group. n = 6 and 7 for the hyperosmotic and time-control groups, respectively. *P < 0.001 and **P < 0.004 using a nonpaired Student's t test. All other data points were not significantly different from each other when compared between the two groups of oocytes at the same time interval.
Attempts at decreasing cell volume by using a third electrode were not successful, as electrode tips were always clogged by intracellular yolk that prevented emptying of some of the intracellular contents of the oocytes. Therefore, this approach was abandoned. Since actin depolymerization was found to heighten the sensitivity of the oocyte-endogenous channel to cell-swelling mediated membrane stretch, we repeated the cell-shrinking experiments in cytochalasin B pretreated oocytes.
Effects of the Actin Cytoskeleton on Cell Shrinking
As shown in Fig. 11, pretreatment of ENaC-expressing oocytes with cytochalasin B nearly completely reversed the inhibitory effect of cell shrinking. In this example, the shrinking inhibitable currents (Fig. 11 C ) were much smaller than those observed in oocytes placed in the hyperosmotic solution in the absence of cytochalasin B pretreatment (see Fig. 9 C ).
Figure 11.
Effect of hyperosmolarity on ENaC currents in cytochalasin B-pretreated oocytes. ENaC expressing oocytes pretreated with cytochalasin B for 2–5 h (A) were not responsive to a 45-min incubation in 260 mosmol hyperosmotic solution (B). Indeed, the shrinking-inhibitable currents (C ) were much smaller than those observed in the absence of cytochalasin pretreatment (Fig. 9 C ) and may be explained by the small baseline drift observed during the control period (see Fig. 12). The amiloride-insensitive currents recorded at the end of the experiment were not affected by cytochalasin pretreatment (D).
Examination of the time course of changes of amiloride-sensitive whole-cell slope conductance at −100 mV indicated that cytochalasin B pretreatment eliminated the inhibitory effect of cell shrinking. As seen in Fig. 12, the time-dependent decrease of conductance in the presence of cytochalasin B was only slightly different between oocytes incubated in the hyperosmotic solution and those left in the iso-osmotic solution. Indeed, at 45 min there was a small tendency for a larger decrease of conductance in oocytes incubated in the hyperosmotic solution versus those left in the iso-osmotic solution, a 20.6 ± 4.0% (n = 6) vs. a 13.2 ± 1.5% (n = 7) decrease. However, these two changes were not significantly different from each other (P = 0.11). Thus, cytochalasin B pretreatment eliminated the inhibitory effect of cell shrinking. The finding that inhibition by cell shrinking depends on the presence of an intact cytoskeleton, in combination with the slow time course of this inhibition in the absence of cytochalasin B, indicated that ENaC's inhibition with cell shrinking is not likely related to a direct effect of changes of membrane tension.
Figure 12.
Time course of the changes of the amiloride-sensitive inward slope conductance in response to cell shrinking in cytochalasin B-pretreated oocytes. See Fig. 10, legend, for details. In this group of oocytes, cell-shrinking did not cause a large decrease of ENaC conductance as observed in the absence of cytochalasin B (Fig. 10). Moreover, there were no significant differences between the experimental and time-control groups of data. n = 6 and 7 for the hyperosmotic and time-control groups, respectively.
Single Channel Correlates
The observation that ENaC whole-cell currents were insensitive to mechanical perturbations indicated that, on average, ENaC activity could not be appreciably altered by changes of membrane tension. However, it was not clear whether ENaC was also mechano-insensitive at the single channel level. Single channel recordings of the effect of membrane tension are important for two reasons. First, this data may reconcile the discrepancy with the previously reported bilayer data as these experiments are more comparable with the single channel data. Second, given the changes of membrane geometry and tension that can be introduced by the formation of a cell-attached patch, single channel experiments would test the effects of changes of tension under conditions of altered membrane geometry.
Experiments with cell-attached membrane patches were carried out in devitellinized oocytes bathed in ND-96. Patch electrodes were constructed as described in materials and methods and conformed to resistances of ∼2 MΩ and tip diameters of ∼3 μm. To reduce the chance of encountering endogenous channels, the pipette filling solution contained 5 mM Ba++, 100 μM DIDS, and 100 μM niflumic acid. To minimize membrane disruption, only patches that formed a GΩ seal with a gentle suction of no more than 2 in. of water were used. In >50% of cases, this resulted in the formation of an ∼50 GΩ seal that if left unperturbed was stable for hours.
Cell-attached membrane patches were clamped to −80 mV, intracellular with respect to patch pipette. In ENaC-expressing oocytes, a channel with 3–5 pS conductance was observed with open and closed time constants on the order of seconds. This channel was never observed in water-injected or noninjected oocytes. A representative recording is shown in Fig. 13. As shown in this example, channel activity was unaffected by the application of negative (Fig. 13 B) or positive (Fig. 13 C ) pipette pressure of 5 in. of water, indicating the lack of a direct effect of membrane tension. However, given the relatively long recording periods that were necessary to properly describe a channel with long open and closed times, it was not uncommon to observe spontaneous changes of the open probability. The presence of these spontaneous changes required the examination of the time course of P o in each individual experiment.
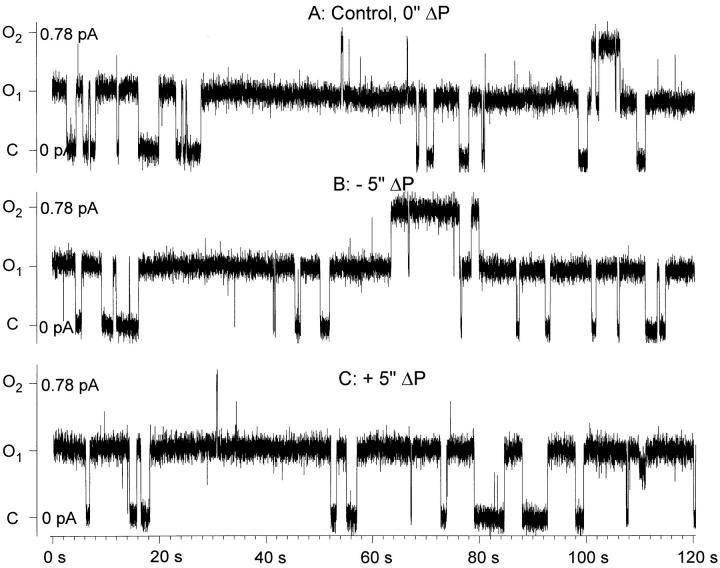
Figure 13.
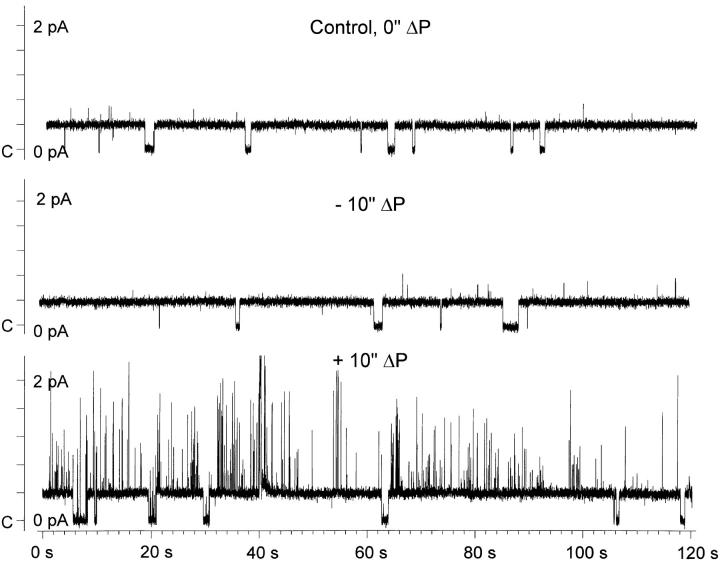
Effect of a 5-in. H2O pipette pressure difference on ENaC single channel activity. Representative single channel records from a cell-attached patch in an ENaC-expressing oocyte demonstrating the lack of effect of applied pipette pressure. Patch voltage was −80 mV (inside the cell with respect to ground or bath). Upward deflections indicate channel openings. The open probability under control conditions (A) was 0.35. Application of a negative (B) or positive (C ) pipette pressure of 5 in. H2O did not significantly alter the open probability. Conditions were as described in materials and methods.
A typical effect of changing pipette patch pressure on the P o time course is shown in Fig. 14. The values of open probability are binned into 10-s intervals and are plotted for the duration of the experiment. It is clear that even during the control period, in the absence of any additional manipulations, the channel spontaneously switched from high to low P o modes. These gating modes are similar to those described by Palmer and Frindt (1996) on the Na(5) channel in native epithelia and may represent a higher order stochastic process that regulates long term channel activity over many minutes to hours. This phenomenon is not to be confused with rundown, where channel activity decreases irreversibly with time, as this P o switch was sometimes observed as a switch from low to high P o modes. Indeed, it can be seen from the example in Fig. 14 that during the last experimental period (after ∼28 min of recording) the channel switched from a P o of ∼0.35 to a P o of ∼0.65. These spontaneously observed changes of P o lasted many minutes, but could be easily observed in experiments that were 30–120 min in duration. These changes of P o were unrelated to changes of pipette patch pressure as observed from the absence of immediate changes of P o after the pipette pressure was changed from 0 to −5 in. H2O, and again from −5 to +5 in. H2O.
Figure 14.
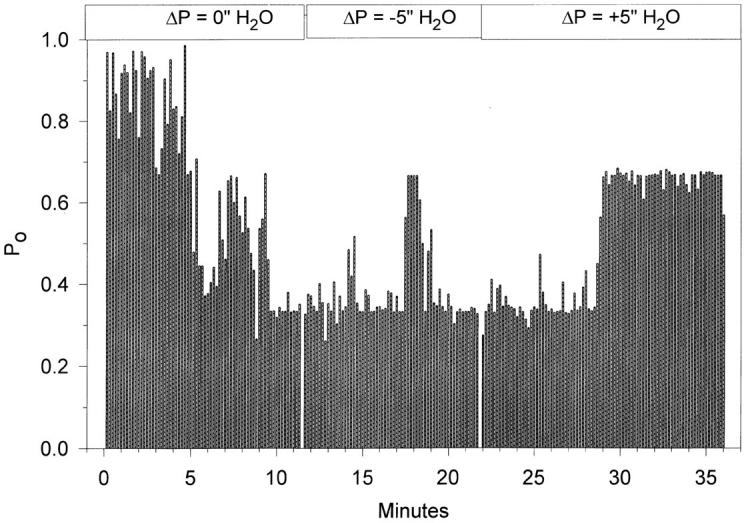
Time course of the changes of the single channel open probability in response to changes of pipette pressure. Open probability data were binned into 10-s intervals and were plotted as a continuous function of time. Despite the noticeable spontaneous changes of P o that were observed in the control period (0 in. ΔP) and toward the end of the experiment (+5 in. ΔP), there were no changes between the control period and each of the experimental periods, indicating lack of sensitivity of ENaC to either positive or negative pipette pressure difference.
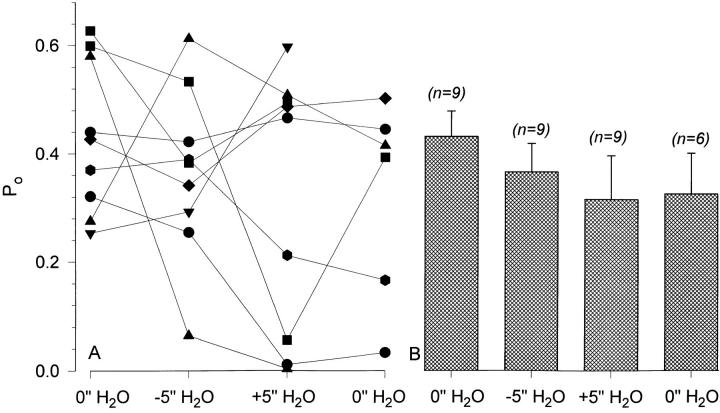
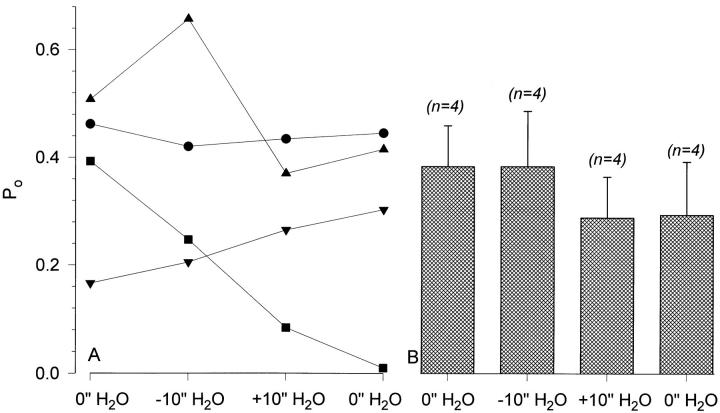
The effect of −5 and +5 in. H2O pipette pressure difference was studied in nine experiments. The data from these individual experiments are consistent with the data shown in Figs. 13 and 14 in that there were no immediate effects of pipette pressure difference on P o. The results of these experiments are summarized in Fig. 15. There were no changes of P o in individual experiments (Fig. 15 A). Moreover, mean P o exhibited a small decrease rather than an increase with both negative and positive pipette pressures (Fig. 15 B). Mean P o was also unchanged at the end of the experiment when the pipette pressure was returned to 0 from +5 in. H2O (P o = 0.315 ± 0.081 and 0.326 ± 0.075 in +5 and 0 in. H2O, respectively). As seen in Fig. 15 A, this decrease of P o was not attributed to any particular pattern of change of the individual experiment P o and was also not due to rundown of the control P o. Thus, the single channel data is consistent with that observed at the whole-cell level, indicating that ENaC present in a biological membrane is not directly mechanosensitive.
Figure 15.
Summary data indicating the lack of effect of a 5 in. H2O pipette pressure difference on ENaC's open probability. As observed from the individual experiment data (A), there were no reproducible effects of negative or positive pipette pressure on P o. Examination of the P o in individual experiments as illustrated in Fig. 14 indicated that none of these experiments exhibited any measurable changes of activity immediately after applying either the positive or negative pipette pressures. The data from these nine experiments are summarized in B and also indicate the lack of significant effect of pipette pressure difference on the channel's mean open probability (P > 0.3 between all four groups).
Pipette pressures of negative or positive 10 in. of water were also used in five experiments. A representative example is shown in Fig. 16 and demonstrates that there were no effects of the pressure change on ENaC activity in either the positive or negative directions. There were, however, effects on a non-ENaC, presumably endogenous, channel that were observed with +10 in. H2O pressure. As the kinetics of this channel were remarkably different from those of ENaC, the activity of these two channels could be easily differentiated.
Figure 16.
Effect of a 10-in. H2O pipette pressure difference on ENaC single channel activity. This larger pressure difference was also without effect on ENaC activity. However, an endogenous channel was stimulated by the application of +10 in. H2O despite the presence of blockers of endogenous channels in the pipette (see materials and methods). This channel could be easily differentiated from ENaC given the large difference in their kinetics. Conditions were the same as described in Fig. 13.
The data with 10 in. H2O pressure is summarized in Fig 17. One patch exhibited a very low P o throughout the experiment (<0.05) and was not included in the summary data. However, it is clear from the remaining four experiments that there were no effects of the larger pipette pressure change on ENaC's P o. Larger changes of pressure, such as 15 in. of water, caused patch membrane disruption and were abandoned. It is important to note that the changes of pipette pressure in the current experiments are comparable to much larger pressures if pipettes with smaller tip diameters were used, as membrane tension is related to patch diameter and pressure by the Laplace equation (see Sackin, 1994, for an excellent review). For example, the 10 in. H2O pressure in our pipettes is equivalent to a pressure of 18.4 mmHg, and is similar to a pressure of 165 mmHg if applied through a pipette with a tip diameter of 0.5 μM (assuming that pipette tip area is the same as the true planar area of the membrane).
Figure 17.
Summary data indicating the lack of effect of a 10-in. H2O pipette pressure difference on ENaC's open probability. See text and Fig. 15, legend, for details.
discussion
We have used the Xenopus oocyte expression system for its ability to faithfully reproduce the electrophysiological properties of the epithelial Na+ channel to study the effects of cell volume and membrane tension changes on the gating of this channel. We found that the channel formed by αβγ rENaC was mechanoinsensitive in a biological membrane, at both the whole cell and single channel levels and in response to large changes of membrane tension close to those that could cause membrane breakdown. Moreover, disruption of the actin cytoskeleton with cytochalasin B, a condition that was found to uncover the presence of an endogenous mechanosensitive channel, did not alter the lack of mechanosensitivity of ENaC.
Comparison with the Bilayer Data
Ismailov et al. (1996) have reported that the channel formed by all three ENaC subunits is mechanosensitive when incorporated into planar lipid bilayers. This channel was obtained from either oocyte membrane vesicles or from an in vitro translation reaction that used nuclease-treated rabbit reticulocyte cell lysate in the presence of canine microsomes. The gating kinetics and cationic selectivity of the channel incorporated into lipid bilayers differed dramatically from those of the Na(5) channel observed in native epithelia, and from those observed from ENaC in oocytes. Moreover, the channel incorporated into bilayers displayed cooperative gating with three subconductance states, a property that is not observed when recording ENaC in oocytes (Puoti et al., 1995; Schild et al., 1997; and see Figs. 13 and 16), or in native epithelia (Palmer and Frindt, 1996). However, the channel incorporated into bilayers retained some of the properties of ENaC observed in native membranes or in oocytes, namely, amiloride sensitivity and kinase regulation (Awayda et al., 1996).
The identity of the complex incorporated into bilayers from either the in vitro translation or oocyte vesicle preparations is unknown. The amount of protein that is actually incorporated into artificial lipid bilayers is very small, and therefore it is a formidable task to characterize the incorporated proteins. The possibility of carryover of other non-ENaC-related proteins from the translation reaction cannot be ruled out. Moreover, it is also not clear whether separately translated subunits will combine in the proper ratios when mixed together to reconstitute an oligomeric channel with identical composition and stoichiometry to that of the native channel. Additionally, artificial lipid bilayers by virtue of their reductionist approach may alter the native properties of the incorporated channels, especially if these channels are regulated by cytoskeletal interactions and/or membrane lipid composition and charge (Busath and Szabo, 1988).
The utility of any reconstitution or expression system such as artificial lipid bilayers or Xenopus oocytes is dependent on its ability to faithfully replicate the properties of the channel found in its native environment. In this regard, it is important to note that the lipid bilayer system did not reconstitute many of the properties of the Na(5) channel, while the oocyte system has proven to produce a channel with properties indistinguishable from those of the native channel. The question of mechanosensitivity is one of these issues where the oocyte data and the bilayer data are at odds. For these issues to have physiological implications, and given the limitations of the bilayer experiments, the findings from oocytes are more physiologically relevant than those obtained from artificial lipid bilayers. Nevertheless, the finding of ENaC mechanosensitivity in bilayers is interesting from a biophysical standpoint, but its lack of direct physiological pertinence cautions against over interpreting data without clear extrapolation to biological systems. While it is possible that conditions may exist that allow ENaC to indirectly respond to membrane tension, these findings would ultimately have to be validated on a case by case basis in native epithelia.
Discrepancy with Data from the Channel Formed by Alpha Alone
In mammalian fibroblasts, transfection of αrENaC causes the appearance of a high-conductance low-cationic selectivity channel that is gated by membrane stretch. The channel's P o was close to zero (not detectable) in the absence of applied membrane stretch, and was reversibly stimulated by applied pipette suction (Kizer et al., 1997). The properties of this channel were similar to those observed from a mechanosensitive channel that is endogenous to osteoclasts. Moreover, osteoclasts were also found to contain detectable levels of αENaC message and not β- or γENaC. The conclusion from both the fibroblast and osteoclast data is that this channel is caused by alpha ENaC alone. This conclusion is tentative in that it is not known what other endogenous proteins, if any, may associate with this subunit to form this nonselective channel. Moreover, it is also not known if there are any conditions other than the transfection of αrENaC that can cause the induction of this channel in fibroblasts. Nonetheless, this data is consistent with the idea that the channel potentially formed by the alpha subunit alone is mechanosensitive in a biological membrane.
Whole-Cell versus Single-Channel Data
The mere act of forming a gigaohm seal causes additional constraints on the membrane trapped in the pipette. This membrane is forced to conform to a new geometry that is not only dictated by the existing cell shape but also by the pipette shape, taper, and opening (Sokabe et al., 1991). Thus, it is expected that single channel recordings of mechanosensitive channels are difficult to interpret given the existing forces that may alter the channel's behavior. Indeed, as described by Morris (1992), whole-cell correlates of single channel mechanosensitivity are usually not sought out, but are necessary for the demonstration that a channel is mechanosensitive.
In the oocyte system, the differences between the two configurations (whole-cell and cell-attached) are even more apparent owing to the enormously large surface area of an oocyte compared with that of the membrane trapped in the pipette during single channel recordings. Thus, it is expected that there would be large differences in the existing tensions in the two configurations and, moreover, different levels of stimuli are necessary to cause the activation of any potential mechanosensitive channels (for review see Sackin, 1994). This issue of differences in tension with applied forces between the two different configurations was circumvented in the present study by examining the effects of membrane tension under conditions that cause extreme changes of this parameter. Thus, under these conditions the data from the whole-cell and cell-attached configuration can be easily compared.
There are also enormous differences between the number of channels studied in whole-cell and cell-attached configurations. In the cell-attached configuration, the activity of a few channels is recorded, while in the whole-cell mode the activity of a few million channels is being recorded (using an amiloride-sensitive current of 1 μA at −80 mV, a single channel current of 0.33 pA, and a P o of 0.5, this results in a density of six million channels per oocyte). Therefore, it is expected that when dealing with mechanosensitivity, data from the whole-cell currents is usually more statistically reliable than data that could be obtained from single channel recordings. Despite the differences in the number of channels examined, there are no differences in the conclusion that ENaC is not directly sensitive to changes in membrane tension.
Conclusions
We conclude that the classical Na+ channel found in tight epithelia is not mechanosensitive. It is unclear whether other proteins that may associate with the αβγ channel in a biological membrane render this complex mechanically stable. Moreover, it is also unclear whether other combinations of the channel, such as αβ or αγ, or any potential covalent or noncovalent modifications of the αβγ channel, can induce mechanosensitivity. However, it appears that, under conditions that are known to produce a channel with biophysical properties identical to those found in native membranes, there is no observed mechanosensitivity.
Acknowledgments
We thank Dr. Bernard Rossier for the gift of rat ENaC subunits and Ms. Roxanne Reger for reading the manuscript.
This work was supported by a Grant-In-Aid from the Louisiana American Heart Association and by a Louisiana Educational Quality Support Fund grant from the Louisiana Board of Regents to M.S. Awayda.
Abbreviation used in this paper
- ENaC
epithelial Na+ channel
references
- Awayda MS, Ismailov II, Berdiev BK, Benos DJ. A cloned renal epithelial Na+channel protein displays stretch activation in planar lipid bilayers. Am J Physiol. 1995;268:C1450–C1459. doi: 10.1152/ajpcell.1995.268.6.C1450. [DOI] [PubMed] [Google Scholar]
- Awayda MS, Ismailov II, Berdiev BK, Fuller CM, Benos DJ. Protein kinase regulation of a cloned epithelial Na+-channel. J Gen Physiol. 1996;108:49–65. doi: 10.1085/jgp.108.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awayda MS, Tousson A, Benos DJ. Regulation of a cloned epithelial Na+-channel by its beta and gamma subunits. Am J Physiol. 1997;273:1889–1899. doi: 10.1152/ajpcell.1997.273.6.C1889. [DOI] [PubMed] [Google Scholar]
- Busath D, Szabo G. Low conductance gramicidin A channels are head-to-head dimers of β6.3-helices. Biophys J. 1988;53:689–695. doi: 10.1016/S0006-3495(88)83150-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canessa CM, Horisberger J-D, Rossier BC. Epithelial sodium channel related to proteins involved in neurodegeneration. Nature. 1993;361:467–470. doi: 10.1038/361467a0. [DOI] [PubMed] [Google Scholar]
- Canessa CM, Schild L, Buell G, Thoreus B, Gautschi I, Horisberger J-D, Rossier BC. Amiloride sensitive Na+channel is made of three homologous subunits. Nature. 1994;367:463–467. doi: 10.1038/367463a0. [DOI] [PubMed] [Google Scholar]
- Garty H, Palmer LG. Epithelial sodium channels: functions, structure, and regulation. Physiol Rev. 1997;77:359–396. doi: 10.1152/physrev.1997.77.2.359. [DOI] [PubMed] [Google Scholar]
- Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflügers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Ismailov II, Awayda MS, Berdiev BK, Bubien JK, Lucas JE, Fuller CM, Benos DJ. Triple-barrel organization of ENaC, a cloned epithelial Na+channel. J Biol Chem. 1996;271:807–816. doi: 10.1074/jbc.271.2.807. [DOI] [PubMed] [Google Scholar]
- Kizer NL, Guo X-L, Hruska K. Reconstitution of stretch activated cation channels by expression of the α subunit of the epithelial sodium channel cloned from osteoblasts. Proc Natl Acad Sci USA. 1997;94:1013–1018. doi: 10.1073/pnas.94.3.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lingueglia E, Voilley N, Waldmann R, Lazdunski M, Barbry P. Expression cloning of an epithelial amiloride-sensitive Na+ channel. A new channel type with homologies to Caenorhabditis elegansdegenerins. FEBS Lett. 1993;318:95–99. doi: 10.1016/0014-5793(93)81336-x. [DOI] [PubMed] [Google Scholar]
- McDonald FJ, Price MP, Snyder PM, Welsh MJ. Cloning and expression of the β- and γ-subunits of the human epithelial sodium channel. Am J Physiol. 1995;268:C1157–C1163. doi: 10.1152/ajpcell.1995.268.5.C1157. [DOI] [PubMed] [Google Scholar]
- Morris CE. Are stretch-sensitive channels in molluscan cells and elsewhere physiological mechanotransducers? . Experientia (Basel) 1992;48:852–858. doi: 10.1007/BF02118418. [DOI] [PubMed] [Google Scholar]
- Palmer LG. Epithelial Na channels: function and diversity. Annu Rev Physiol. 1992;54:51–66. doi: 10.1146/annurev.ph.54.030192.000411. [DOI] [PubMed] [Google Scholar]
- Palmer LG, Frindt G. Gating of Na channels in the rat cortical collecting tubule: effects of voltage and membrane stretch. J Gen Physiol. 1996;107:35–45. doi: 10.1085/jgp.107.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puoti A, May A, Canessa CM, Horisberger J-D, Schild L, Rossier BC. The highly selective low-conductance epithelial Na channel of Xenopus laevisA6 kidney cells. Am J Physiol. 1995;269:C188–C197. doi: 10.1152/ajpcell.1995.269.1.C188. [DOI] [PubMed] [Google Scholar]
- Sachs, F. 1986. Mechanotransducing ion channels. In Ionic Channels in Cells and Model Systems. R. Latorre, editor. Plenum Publishing Corp., New York. 181–193.
- Sackin, H. 1994. Stretch activated ion channels. In Cellular and Molecular Physiology of Cell Volume Regulation. K.L. Strange, editor. CRC Press, Boca Raton, FL. 215–240.
- Schild L, Schneeberger E, Gautschi I, Firsov D. Identification of amino acid residues in the alpha, beta, and gamma subunits of the epithelial sodium channel (ENaC) involved in amiloride block and ion permeation. J Gen Physiol. 1997;109:15–26. doi: 10.1085/jgp.109.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PR, Benos DJ. Epithelial Na+channels. Annu Rev Physiol. 1991;53:509–530. doi: 10.1146/annurev.ph.53.030191.002453. [DOI] [PubMed] [Google Scholar]
- Sokabe M, Sachs F, Jing Z. Quantitative video microscopy of patch clamped membranes: stress, strain, capacitance, and stretch activation. Biophys J. 1991;59:722–728. doi: 10.1016/S0006-3495(91)82285-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voilley N, Lingueglia E, Champigny G, Mattei M-G, Waldmann R, Lazdunski M, Barbry P. The lung amiloride sensitive Na+channel; biophysical properties, pharmacology, ontogenesis, and molecular cloning. Proc Natl Acad Sci USA. 1994;91:247–251. doi: 10.1073/pnas.91.1.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wills NK, Millinoff LP, Crowe WE. Na+channel activity in cultured renal (A6) epithelium: regulation by solution osmolarity. J Membr Biol. 1991;121:79–90. doi: 10.1007/BF01870653. [DOI] [PubMed] [Google Scholar]