Abstract
Under physiological conditions, potassium channels are extraordinarily selective for potassium over other ions. However, in the absence of potassium, certain potassium channels can conduct sodium. Sodium flux is blocked by the addition of low concentrations of potassium. Potassium affinity, and therefore the ability to block sodium current, varies among potassium channel subtypes (Korn, S.J., and S.R. Ikeda. 1995. Science. 269:410–412; Starkus, J.G., L. Kuschel, M.D. Rayner, and S.H. Heinemann. 1997. J. Gen. Physiol. 110:539–550). The Shaker potassium channel conducts sodium poorly in the presence of very low (micromolar) potassium due to its high potassium affinity (Starkus, J.G., L. Kuschel, M.D. Rayner, and S.H. Heinemann. 1997. J. Gen. Physiol. 110:539–550; Ogielska, E.M., and R.W. Aldrich. 1997. Biophys. J. 72:A233 [Abstr.]). We show that changing a single residue in S6, A463C, decreases the apparent internal potassium affinity of the Shaker channel pore from the micromolar to the millimolar range, as determined from the ability of potassium to block the sodium currents. Independent evidence that A463C decreases the apparent affinity of a binding site in the pore comes from a study of barium block of potassium currents. The A463C mutation decreases the internal barium affinity of the channel, as expected if barium blocks current by binding to a potassium site in the pore. The decrease in the apparent potassium affinity in A463C channels allows further study of possible ion interactions in the pore. Our results indicate that sodium and potassium can occupy the pore simultaneously and that multiple occupancy results in interactions between ions in the channel pore.
Keywords: Shaker, Na+ permeation, ion selectivity, S6, K+ affinity
introduction
Potassium channels exert a stabilizing influence on the membrane potential of excitable cells. Their ability to select for potassium over sodium is critical to their function of conducting outward current across the membrane. Sodium exclusion is thought to take place in the narrowest region of the potassium channel pore, appropriately named the selectivity filter. In physiological solutions, these channels conduct sodium at a very low rate compared with potassium, although the relative sodium permeability can be increased by extreme depolarizations (Bezanilla and Armstrong, 1972; French and Wells, 1977). Potassium channels also discriminate between other monovalent ions, only allowing four to pass efficiently. The relative permeability is Tl+ > K+ > Rb+ > NH4 + (Hille, 1973); although Cs+ is weakly permeant in some cases (Cukierman et al., 1985; Heginbotham and MacKinnon, 1993).
Voltage-gated potassium channels are tetramers (MacKinnon, 1991; Liman et al., 1992; Kavanaugh et al., 1992; Doyle et al., 1998) with six membrane spanning regions (S1 through S6) in each of the four subunits. The loop between the fifth and sixth membrane spanning domains has been termed the P region because mutations in this loop affect several pore properties of the channel, including ion selectivity (Armstrong and Hille, 1998). The crystal structure of a Streptomyces lividans potassium channel (KscA), a proton-gated channel with two membrane spanning domains and a P region, has recently been determined (Doyle et al., 1998). Although this is a two-transmembrane channel, it is highly homologous to voltage-gated potassium channels within the regions that form the ion conducting pore. The molecular fingerprint of a potassium channel is the highly conserved TXXTXGYG sequence found in the P region and it is revealed as the finely tuned selectivity filter. The narrow ion-selective region of KscA is lined by the backbone carbonyls from the signature sequence amino acids, while S6 helices form the wide internal vestibule of the channel (Doyle et al., 1998). The carbonyls form three ion binding sites within the narrow region, one external site and two internal sites that cannot be simultaneously occupied due to their close proximity. The structure revealed two dehydrated permeant ions residing in the narrow region, while a third hydrated ion is found in the water-filled cavity in the internal vestibule of the channel.
It appears that potassium channels, like Ca channels, select for potassium by an affinity mechanism (Hille and Schwarz, 1978; Yellen, 1984; Neyton and Miller, 1988a , 1988b ; Korn and Ikeda, 1995; Baukrovitz and Yellen, 1996). This idea proposes that a channel selects for potassium by binding potassium more tightly than all other ions and suggests that the channel pore contains high affinity potassium binding sites. This prediction has been verified experimentally for several potassium channels (Yellen, 1984; Neyton and Miller, 1988a , 1988b ; Ikeda and Korn, 1995; Baukrovitz and Yellen, 1996; Starkus et al., 1997) and is also supported by the crystal structure, where permeant ions are presumably bound tightly to the pore lining to compensate for the loss of hydration (Doyle et al., 1998).
An affinity mechanism of selectivity must also incorporate a means of achieving high flux rates through the channel. One idea is that the pore contains several high affinity potassium binding sites. Multiple pore occupancy decreases potassium affinity through ion–ion interactions, and results in high flux rates through the channel (Hille and Schwarz, 1978). Experimentally, it has been shown that the potassium channel pore can be multiply occupied and ion–ion interactions in the pore can occur (Hodgkin and Keynes, 1955; Hagiwara et al., 1977; Adelman and French, 1978; Newland et al., 1992; Heginbotham and MacKinnon, 1993; Perez-Cornejo and Begenesich, 1994; Stampe and Begenesich, 1996; Doyle et al., 1998). An alternative affinity model proposes a single high affinity site flanked by two lower affinity sites (Dang and McCleskey, 1998). In this view, high rates of conduction are dependent on multiple occupancy, but not on ion–ion interactions within the pore. Several observed permeation properties of potassium channels are approximated well by either of these models (Kiss et al., 1998; Hille and Schwarz, 1978; Neyton and Miller, 1988b ). In both models, potassium channel selectivity is contingent on the existence of at least one high affinity potassium binding site.
Since it is precisely the presence of potassium that renders the channels potassium selective, one prediction of the affinity mechanism is that in the absence of the high affinity K ion other, lower affinity ions should readily conduct through the channel. Sodium conduction in the absence of potassium has been reported (Zhu and Ikeda, 1993; Callahan and Korn, 1994; Korn and Ikeda, 1995; Ogielska and Aldrich, 1997; Block and Jones, 1996, 1997; Starkus et al., 1997), and the addition of low concentrations of potassium readily blocks the observed sodium currents (Ikeda and Korn, 1995; Ogielska and Aldrich, 1997; Starkus et al., 1997). Differences in sodium conduction among channel subtypes appears to be governed by the differences in their potassium affinity.
The mammalian potassium channels Kv2.1, Kv1.5, and Kv1.3 behave differently in the absence of potassium (Korn and Ikeda, 1995; Kiss et al., 1998). Kv2.1 readily conducts sodium in the virtual absence of potassium, and millimolar concentrations of potassium are required to block the observed sodium fluxes. Kv1.3 and Kv1.5, however, do not conduct sodium under these ionic conditions. Either the Kv1 channels have a much higher affinity for potassium than Kv2.1, and the residual potassium ions are sufficient to block any sodium conduction, or these two channel subtypes select among monovalent cations by different mechanisms (Korn and Ikeda, 1995; Kiss et al., 1998). Shaker, the Drosophila homolog of the mammalian Kv1 channels, conducts sodium in the virtual absence of potassium, but does so poorly due to its high, micromolar internal potassium affinity (Ogielska and Aldrich, 1996; Starkus et al., 1997). The affinity differences between potassium channel subtypes motivated us to identify the residues that determine the difference in potassium affinity between Shaker and Kv2.1.
It was shown recently that a chimera transplanting the P region of Kv1.3 into Kv2.1 cannot fully account for the differences between the two channels (Kiss et al., 1998). Therefore, we chose to focus on the differences in sequence between Shaker and Kv2.1 in areas outside the P region and their effect on potassium affinity and sodium conduction. Because of previous studies suggesting a role for S6 in various pore functions (Choi et al., 1993; Aiyar et al., 1994; Tagliatela et al., 1994; Lopez et al., 1994), we changed residues in the S6 region of Shaker to their counterparts in Kv2.1. Sodium conduction in the virtual absence of potassium and the ability of potassium to block the sodium current was taken as a measure of changes in potassium affinity. We found that a single point mutation in S6 (A463C) decreases the internal potassium affinity of the channel by a factor of ∼1,000, into the millimolar range. Our results also suggest that in the absence of potassium an external binding site can be occupied by sodium in the A463C mutant and that the presence of a sodium ion at that site affects the internal potassium affinity through ion–ion interactions in the pore. Preliminary reports of this data have been presented in abstract form (Ogielska and Aldrich, 1996, 1997, 1998).
materials and methods
Molecular Biology
The S460G:A463C:T469V and the A463C mutations were made by generating PCR primers using noninactivating ShBΔ6-44 as a template. The PCR product was gel purified and spliced into the channel using NsiI and SpeI restriction sites. The S460G and the T469V mutations were generated using the Transformer CloneTech Site-directed Mutagenesis Kit (Clontech, Palo Alto, CA). Briefly, this method anneals two oligonucleotide primers to one strand of DNA. One primer introduces the mutation and the other a unique restriction site for the purpose of selection. The mutants were sequenced through the mutated region to check that no secondary mutations occurred. Finally, the mutants were linearized with EcoR1 or Ava1 and RNA was synthesized using the T7 polymerase (Ambion Inc., Austin, TX). The transcribed RNA was injected into Xenopus laevis oocytes as previously described (Zagotta et al., 1989).
Electrophysiology
Macroscopic recordings were done in cell free patches (either inside-out or outside-out) 3–9 d after injection. Patch pipettes were made of borosilicate glass (VWR Micropipettes, West Chester, PA). Their tips were coated with wax (Sticky Wax, Emeryville, CA) and fire polished before use. Pipettes had initial resistances <2 MΩ and all recordings were done at 21°C. Leak subtraction was done using the P/4 protocol and no corrections were made for series resistance.
Data were acquired using either an Axopatch 200-A (Axon Instruments, Foster City, CA) or an EPC 7 (Adams and List Associates Ltd., Westbury, NY) patch clamp amplifier. The data were digitized using a Macintosh-based computer system using Pulse Acquisition software (HEKA Electronik, Lambrecht, Germany) and the ITC-16 hardware interface (Instrutech Corp., Great Neck, NY). Data were analyzed using Igor Pro graphing and curve fitting software (Wavemetrics Inc., Lake Oswego, OR).
Solutions
Unless otherwise indicated, the internal solution contained (mM): 10 EGTA, 10 HEPES, and either 140 NaCl or 140 KCl. The external solution was composed of (mM): 2 CaCl2, 5 HEPES, and either 2 KCl, 140 NaCl, or 0 KCl, 140 XCl (where X denotes either K, N-methyl-d-glucamine [NMG],1 Na, or tetraethylammonium [TEA]). Solutions used in the potassium blocking experiments also contained symmetrical 30 mM NMGCl. To conserve osmolarity and keep the sodium concentration constant, NMG was replaced by equimolar amounts of potassium. The pH was 7.1 for all internal solutions and 7.2 for the external solutions.
In solutions without added potassium, the free potassium concentration was measured by flame photometry and was determined to be <40 μM (Scientific Environmental Laboratories Inc., Palo Alto, CA). Internal barium solutions were made taking the EGTA barium buffering capacity into account (EGTA Ba++ K d = 2.8 × 10−5 M). Patches were extremely unstable in barium solution of >500 μM barium. Solutions were exchanged using a sewer pipe flow system (DAD 12) purchased from Adams and List Associates Ltd. The system consisted of 12 syringe reservoirs, each fitted with a solenoid valve and a thin tube leading to a single polyacrylamide-coated quartz pipe (i.d. 100 μM) that formed the output. The patch was situated in front of the pipe and immersed in the laminar flow. Flow was aided by air pressure applied to each reservoir (200 psi), solution exchange was computer controlled and occurred in <1 s. Sometimes the solution changes were incomplete due to air bubble formation in the tubing and/or vesicle formation. When these technical problems were encountered, the experiments were terminated and discounted.
results
Sodium Conduction through the Wild-Type Shaker Channel
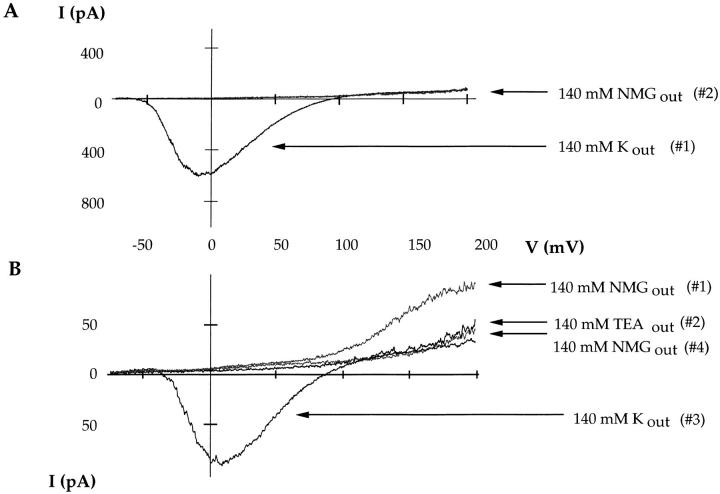
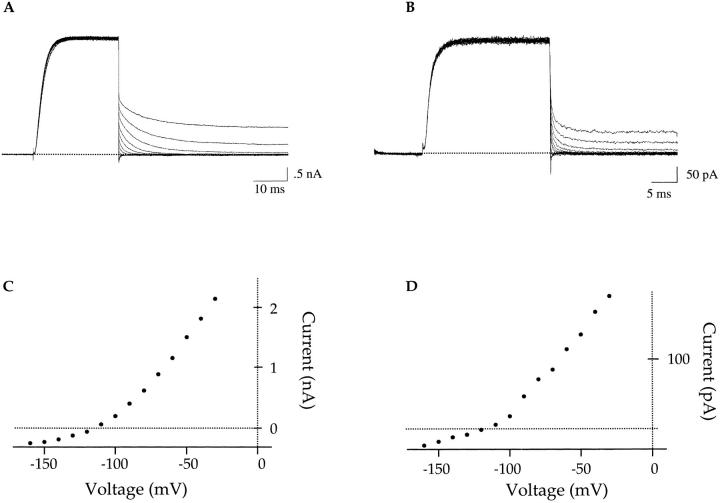
The Shaker channel is the Drosophila homolog of the mammalian Kv1.3 channel, which does not readily conduct sodium in the nominal absence of potassium (Korn and Ikeda, 1995). In our first set of experiments, we examined the ability of the Shaker channel to conduct sodium upon the removal of potassium. Fig. 1 A displays current sweeps acquired from outside-out patches with 76-ms voltage ramps from −100 to +200 mV. The first sweep (Fig. 1 A, #1) represents the behavior of wild-type Shaker channels with 140 mM KCl outside and 140 mM NaCl inside the pipette. Only a prominent inward potassium current is observed, indicating the presence of thousands of potassium-selective channels. In the second trace (Fig. 1 A, #2), the external potassium solution has been replaced with 140 mM NMG+. We use NMG+ as an ion substitute that preserves the osmolarity of the solutions but does not conduct through the channel or interfere with channel function. An identical voltage protocol does not elicit any additional outward or inward current.
Figure 1.
The wild-type Shaker channel conducts sodium poorly in the absence of added potassium. Experiments were done on outside-out patches. Current traces were elicited by 76-ms voltage ramps from −100 to +200 mV. The pipette solution contains 140 mM NaCl, 0 added KCl. (A) In the presence of 140 mM external KCl, a prominent inward current is observed (#1). No additional outward current is observed with 140 mM external NMG (#2). (B) The patch was extensively washed with nominally K-free solution (140 mM NMGCl) and the order of solution changes was reversed. Under these conditions, a ramp protocol elicits an outward current (#1). The current is blocked by 140 mM external TEA (#2). Upon washout, 140 mM KCl is washed back onto the patch to demonstrate the viability of the channels (#3). A subsequent attempt to observe sodium currents with 140 mM NMGCl fails (#4).
Three interpretations of these data are possible: (a) channels are rendered nonfunctional in the absence of potassium, (b) residual potassium ions in the solution are blocking sodium permeation, or (c) wild-type Shaker channels do not conduct sodium in the absence of potassium. Fig. 1 B illustrates an experiment attempting to distinguish between these three possibilities. The data are collected in the same manner as those in Fig. 1 A. The difference between the two sets of experiments (Fig. 1, A and B) is in the order of solution changes and current amplitude. We chose a patch with relatively small potassium currents to minimize the residual potassium contamination. The outside-out patch in Fig. 1 B was formed in symmetrical 140 mM NaCl solutions, excepting the potassium present in the oocyte upon seal formation. Initially, the patch was extensively washed with external 140 mM NMG+ to eliminate residual potassium. In the first trace (Fig. 1 B, #1), an identical voltage protocol as in Fig. 1 A elicits a discernible outward current. Given the makeup of the solutions, that current is probably due to sodium ions conducting through the wild-type Shaker channel. It is unlikely that the current is carried by potassium because outward current is not observed until the patch is extensively washed with potassium-free solutions. To discount the possibility that the observed current in Fig. 1, #1, is due to endogenous oocyte currents, 140 mM external TEA was washed onto the patch and the protocol was repeated. Fig. 1, #2, shows block of the outward current by external TEA, further identifying it as sodium conducting through the wild-type Shaker channels. Subsequently, TEA was washed out and replaced with 140 mM external KCl. The experiment was repeated and the potassium conductance was restored (Fig. 1, #3). It should be noted that several sweeps with 140 mM KCl were necessary to wash out the bound TEA. If the potassium is subsequently replaced with NMG+ but the patch is not thoroughly perfused with potassium-free solution, the outward sodium current is no longer present. We propose that the residual potassium ions, present in our solutions due to accumulation of potassium in the pipette, prevent sodium permeation by binding with a very high affinity to sites in the channel.
The wild-type Shaker channel conducts sodium in the absence of added potassium, but it does so poorly. Our solutions do contain trace amounts of potassium contamination and the residual accumulation of potassium ions must be sufficient to prevent the detection of large sodium currents. Similarly, Starkus et al. (1997) have shown that Shaker channels conduct sodium in the absence of added potassium and that submillimolar concentrations of internal potassium block the sodium current. The wild-type channel inactivated rapidly in the absence of added potassium (Starkus et al., 1997). Since channels in both the open and C-type inactivated conformation can conduct sodium in the absence of added potassium (Starkus et al., 1997), the observed sodium currents in our experiments may be the result of sodium conducting through both the open and inactivated states of the channel. Although these results do not differentiate between these two possibilities, it is clear that the wild-type Shaker channel does not conduct sodium well in the absence of added potassium and that the channel has a high potassium affinity (Fig. 1 B and Starkus et al., 1997). This is unlike the mammalian Kv2.1 channel studied by Korn and Ikeda (1995), where millimolar concentrations of potassium are required to block the observed sodium current.
Mutations in S6 Allow for Large Sodium Fluxes in the Absence of Potassium
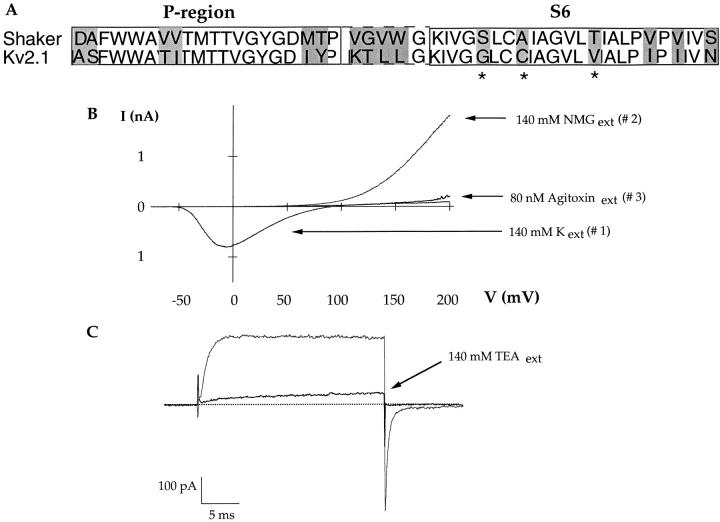
A chimeric channel containing the P region and external linkers of Kv1.3 in the Kv2.1 background conducts sodium in the absence of potassium and the observed currents are blocked by micromolar concentrations of externally applied potassium (Kiss et al., 1998). These results indicated that, although the P region is clearly an important determinant of potassium affinity, the chimeric channel behaves more like an intermediate between Kv1.3 and Kv2.1. Clearly, other portions of the channel protein must play a role in determining the affinities of ion binding sites in the pore. A sequence comparison between Shaker and Kv2.1 is shown in Fig. 2 (A). The amino acid differences between the two channels in the P region and in S6 are highlighted. To determine if residues in S6 are important in determining the channels' affinity for potassium, a partial chimera between Shaker and Kv2.1 was constructed in the Shaker background. In the alpha helical region of S6 (before the presumed helix-breaking proline residues), there are three amino acid differences between Shaker and Kv2.1 (see Fig. 2 A). We mutated those three residues in Shaker (S, A, T) to their counterparts in Kv2.1 (G, C, V) and asked whether this partial chimera was better able to conduct sodium in the absence of potassium.
Figure 2.
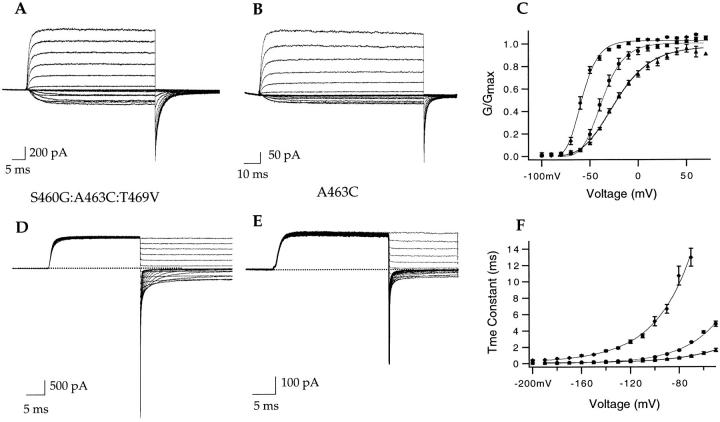
The S460G:A463C:T469V mutant conducts large sodium currents in the absence of potassium. (A) Sequence comparison between the pore and the S6 region of the Kv2.1 and the Shaker channel. The amino acid differences between the two channels are highlighted. In this study, the starred residues in the S6 of Shaker were mutated to their counterparts in Kv2.1. (B) Current sweeps are from outside-out patches and were elicited by 76-ms voltage ramps from −100 to +200 mV. The pipette solution contained 140 mM NaCl and 0 KCl. With external 140 mM KCl (#1), a large inward potassium current is observed. Replacement of external K with 140 mM NMGCl (#2) allows for large outward sodium currents. The outward current is entirely blocked by 80 nM Agitoxin (#3). (C) Current traces are from an outside-out patch in symmetrical 140 mM NaCl. The currents were elicited by a step from −100 to +100 mV and a repolarization back to −100 mV. Both outward and inward currents are readily blocked by 140 mM external TEA.
The experiment described in Fig. 1 A for the wild-type Shaker channel was repeated with the S460G: A463C:T469V chimera. Fig. 2 B, #1, was elicited in the presence of 140 mM external KCl and 140 mM internal NaCl. As observed with the wild-type channel, only an inward potassium current is elicited. Subsequently, 140 mM external NMGCl was washed onto the patch and the voltage protocol was repeated. Unlike what was observed for the wild-type channel, a large outward sodium current is evident in the chimera. The sodium current is blocked by 80 nM Agitoxin (Fig. 2, #3), a specific Shaker channel blocker, indicating that the large current is indeed due to sodium conducting through the S460G:A463C:T469V chimera. Fig. 2 (C) shows, with voltage steps applied to a patch in symmetrical 140 mM NaCl, that the chimera is able to pass sodium in both the inward and outward directions and that external TEA can block the observed sodium current.
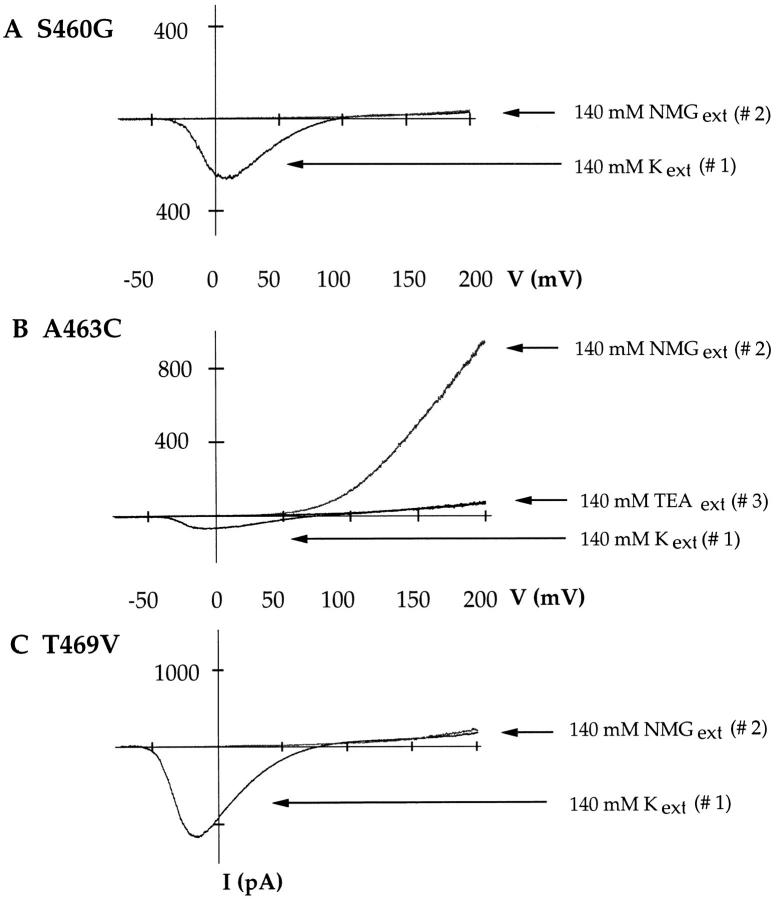
It is clear that mutations in S6 increase the channels' ability to conduct sodium in the absence of potassium. We next set out to determine whether all three substitutions were necessary to confer the observed effect or whether a single point mutation was sufficient. We mutated each of the three residues separately and tested the channels' ability to conduct sodium. The results are shown in Fig. 3, neither S460G (A) nor T469V (C) alone conduct appreciable sodium once potassium is removed. However, the A463C mutation is sufficient to reproduce the effect observed with S460G:A463C:T469V chimera (Fig. 3 B) and the Kv2.1 channel (Korn and Ikeda, 1995). As in the case of the triple mutant, the outward sodium current can be blocked with a potassium channel blocker, in this case with 140 mM external TEA (Fig. 3, #3).
Figure 3.
A463C is sufficient for large sodium currents to be observed in the absence of potassium. In all panels, the current sweeps are from outside-out patches and were elicited by voltage ramps from −100 to +200 mV. The pipette solution contained 140 mM NaCl, 0 KCl in all experiments. #1 in all three panels is the current elicited in the presence of 140 mM external KCl. #2 is the current elicited in the presence of 140 mM external NMGCl. S460G (A) and T469V (C) do not readily conduct sodium in the absence of potassium. A463C (B), however, readily conducts sodium in the absence of potassium. The observed sodium current is blocked by 140 mM external TEA (#3).
In all cases (Figs. 1 B, 2 B, and 3 B), the sodium currents elicited by ramps appear to activate at more depolarized voltages then in potassium solutions. This apparent shift is not due to a change in channel gating (see Fig. 6), but rather is the result of block by residual potassium ions that is relieved as the membrane potential is increased.
Figure 6.
In Na+ solutions, the S460G:A463C:T469V mutant has both a shallower steady state voltage dependence and slower deactivation kinetics as compared with K+ solutions. (A) The currents were elicited by 50-ms steps from −100 to +60 mV followed by a 25-ms step to −150 mV. (C) Tail current families were generated by stepping to +150 mV for 20 ms, followed by 20-ms steps from +140 to −200 mV in 10-mV decrements. The families in both panels were recorded in symmetrical 140 mM NaCl. (B) Conductance–voltage relationships in K+ (♦) and Na+ (▴). The K+ data points (n = 12) were best fit with a fourth power Boltzmann with V1/2 = −54.5 mV and z = 2.0 e. The Na+ data points (n = 3) were best fit with V1/2 = −67.4 mV and z = 1.2 e. (D) The time constant of deactivation changes e-fold per 24.1 mV when the permeating ions are K+ (♦; n = 16). Deactivation is slower and the time constant of deactivation changes e-fold per 21.4 mV when the permeating ions are Na+ (▴, n = 9).
A single point mutation in S6, A463C, is sufficient for appreciable sodium conduction to be observed in the absence of potassium. Qualitatively, it appears that A463C channels conduct larger sodium currents than do the S460G:A463C:T469V chimera, although this was not studied further. In symmetrical sodium conditions, both S460G:A463C:T469V and A463C reverse at the predicted sodium reversal potential of 0 mV (data not shown).
Selectivity Is Not Affected in Physiological Ionic Conditions
It is important to establish that both S460G:A463C: T469V and A463C are selective for potassium under physiological conditions and that we have not simply constructed nonselective channels. The experiments described in this section were performed with the following monovalent ion concentrations: 140 mM Na with 2 mM K+ in the external solution and 140 mM K+ in the internal solution. Under these ionic conditions, the Nernst equation predicts a reversal potential of −110 mV if the channels are potassium selective. Wild-type Shaker currents elicited in these solutions indeed reverse at the predicted Nernst potential (data not shown). Fig. 4, A and B, depict the reversal potential experiments for S460G:A463C:T469V and A463C, respectively. The voltage was stepped to +50 mV to open all the channels, and subsequently the voltage was changed from −20 to −160 mV in 10-mV decrements. The current reversed direction at −112 ± 4 mV for S460G:A463C:T469V and at −106 ± 9 mV for A463C. It is clear from the data that in physiological conditions both mutants are strongly potassium selective like the wild-type Shaker channel. The observed sodium conductance in both S460G:A463C:T469V and A463C is therefore contingent upon the absence of potassium and is not the result of a disturbance in the overall selectivity of the channel.
Figure 4.
S460G:A463C:T469V and A463C are both selective for K+ in physiological conditions. Representative current traces from S460G:A463C:T469V (A) and A463C (B) elicited by a depolarizing step from a holding potential of −100 to +50 mV and followed by hyperpolarizing steps ranging from −30 to −160 mV in 10-mV decrements. The external solution (A and B) contained (mM): 140 NaCl, 2 KCl, 4 MgCl2, 2 CaCl2, 5 HEPES, pH 7.1. The internal solution contained (mM): 140 KCl, 2 MgCl2, 10 EGTA, 10 HEPES, pH 7.2. (C) Instantaneous current–voltage relationship as measured isochronally from the tail current family shown in A. The predicted Nernst reversal potential for K+ under these ionic conditions is −110 mV. The measured reversal potential is −112 ± 4 mV (n = 4). (D) Instantaneous current–voltage relationship as measured from the current family shown in B. The predicted Nernst reversal potential for K+ under these ionic conditions is −110 mV. The measured reversal potential is −106 ± 9 mV (n = 4).
Activation and Deactivation in the Mutant Channels
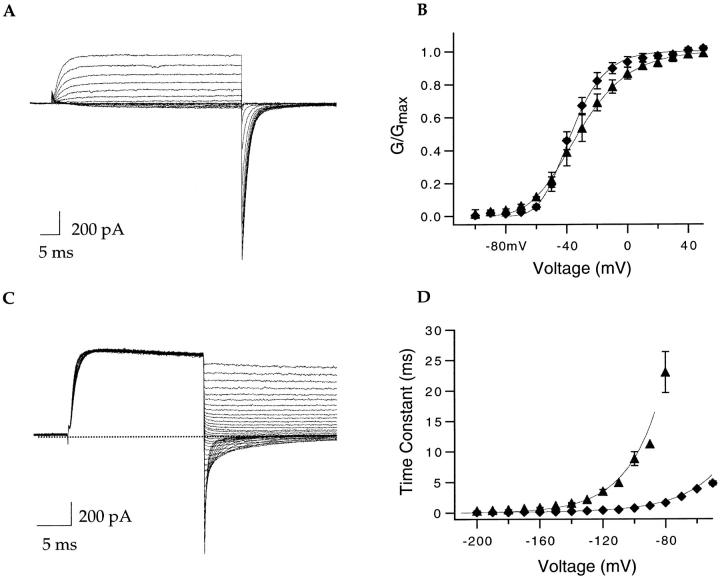
Mutations that alter pore properties of potassium channels often drastically affect the activation gating (Heginbotham et al., 1994). Activation and deactivation of ionic currents in our mutant channels were examined in symmetrical 140 mM potassium. The activation families for S460G:A463C:T469V and A463C are shown in Fig. 5, A and B, respectively. The resultant tail current conductance–voltage relationships (GVs) were fitted with fourth power Boltzmann functions and are shown in Fig. 5 C along with the wild-type data (♦). The GVs of both mutants are shifted slightly rightward and the slopes are shallower (see Fig. 5, legend). The rate of channel closing was also affected by the S6 mutations. Fig. 5 shows tail current families for S460G:A463C:T469V and A463C. The tail currents were well fitted with single exponentials and the resultant time constants are plotted in Fig. 5 F with wild-type Shaker data shown as a reference (♦). Both mutants S460G:A463C:T469V (•) and A463C (▴) display faster deactivation kinetics compared with the wild-type channel. The mutations clearly affect the gating of the channel, although not nearly as drastically as many other mutations that affect the pore properties of the channel (Heginbotham et al., 1994).
Figure 5.
The gating of S460G:A463C:T469V and A463C in symmetrical potassium solutions. All experiments were performed in symmetrical 140 mM KCl. Representative activation current families for S460G:A463C:T469V (A) and A463C (B). The currents were elicited by 50-ms steps from −100 to +60 mV, followed by a 25-ms step to −65 (A) and −75 (B) mV. (C) Conductance–voltage relationships for ShBΔ6-46 (♦), S460G:A463C:T469V (•), and A463C (▴) were calculated from tail currents such as those in A and B. Error bars represent SEM. The smooth curves through the data represent fourth power Boltzmann fits. The ShBΔ6-46 data points (n = 10) were best fit with V1/2 = −75.7 mV and z = 2.4 e. The triple mutant data (n = 5) were best fit with V1/2 = −60.2 mV and z = 1.8 e. The A463C data (n = 7) were best fit with V1/2 = −61.7 mV and z = 1.1 e. The steady state GV relationships for S460G and T469V were best fitted with V1/2 = −71.5 mV (n = 8) and −76.5 mV (n = 3), respectively (data not shown). Deactivation was studied by tail current families generated by stepping to +50 mV for 25 ms, followed by 25-ms steps from +50 to −200 mV in 10-mV decrements for S460G:A463C:T469V (D) and 15-ms repolarizing steps for A463C (E). Deactivation time constants were obtained from single exponential fits to tail currents such as those shown in D and E. The wild-type (ShBΔ6-46) (♦; n = 7) time constant of deactivation changes e-fold per 30.0 mV, the triple mutant (•, n = 7) changes e-fold per 27.9 mV, and A463C (▴; n = 9) time constant of deactivation changes e-fold per 32 mV. The time constants of deactivation changes for S460G and T469V were e-fold per 31.7 (n = 9) and 28.9 (n = 5) mV (data not shown).
One interpretation of a change in the slope of a GV is that the mutation has altered the amount of charge translocated during activation. It seems unlikely that uncharged substitutions in S6 are affecting the movement of gating charge, although it is possible that these mutations do so indirectly. We favor an alternative hypothesis. Several activation models (Zagotta et al., 1994 a; Schoppa and Sigworth, 1998; Smith-Maxwell et al., 1998) incorporate an energetic stabilization of the open state so that the first closing transition is slower than expected for an independent model. The added stability may be due to hydration, cooperativity among subunits, or the interactions between conducting ions and the channel protein. Alteration of this stability factor can change the slope of the GV and also the deactivation rate of the channel (Zagotta et al., 1994 a; Smith-Maxwell et al., 1998). Specifically, the alterations in gating could be explained if the S6 mutations (S460G: A463C:T469V and A463C) disturb the stability of the open state, perhaps due to the lowering of the potassium affinity of the channel. Supporting this hypothesis, the two substitutions that do not conduct appreciable sodium in the absence of potassium, S460G and T469V, deactivate more slowly and retain steep conductance voltage relationships (see Fig. 5, legend).
It has previously been shown that permeant ions can affect the rate of potassium channel closing (Swenson and Armstrong, 1981; Matteson and Swenson, 1986; Zagotta et al., 1994 b). Therefore, we next examined whether gating of the mutant channel S460G:A463C:T469V is affected by the nature of the conducting ion. Representative current families obtained in symmetrical sodium are shown in Fig. 6, A and C. Fig. 6 B compares the steady state properties of S460G:A463C:T469V in symmetrical sodium (▴) or symmetrical potassium (♦). The slope of the steady state GV curve is more shallow with sodium as the conducting ion. The deactivation kinetics in symmetrical potassium (Fig. 6 D, ♦) are much faster than in symmetrical sodium (Fig. 6 D, ▴).
According to the occupancy hypothesis, the slow deactivation can be explained if sodium remains bound at a site in the pore for a longer time than potassium and in this manner prevents the closing of the channel (Swenson and Armstrong, 1981). The potassium channel pore contains several potassium ion binding sites and can be multiply occupied. We will show that the A463C mutation decreases the potassium affinity of an internal binding site in the pore. However, our data also indicates that in the A463C mutant a more external binding site can be occupied by sodium. We are presently investigating whether the increased sodium occupancy at the external site results in slower closing kinetics. Alternatively, the slow deactivation kinetics may be due to altered gating resulting from the absence of potassium. Channels can enter into either an inactivated state or a debased state in the absence of potassium and the slow channel closing could be the result of such a conformational change (Almers and Armstrong, 1980; Khodakhan et al., 1997; Gomez-Lagunas, 1997; Starkus et al., 1997). However, for reasons detailed in the discussion, we believe that the currents shown in this paper are due to sodium conducting through the open state of the channel.
A463C Specifically Decreases the Potassium Affinity of the Channel
Given that the A463C mutation neither altered the selectivity of the channel as measured in physiological conditions nor grossly affected the gating of the channel, we next examined the possibility that we have in fact specifically affected the potassium affinity of the channel. To test this hypothesis, we asked how much internal potassium was necessary to block the observed sodium current.
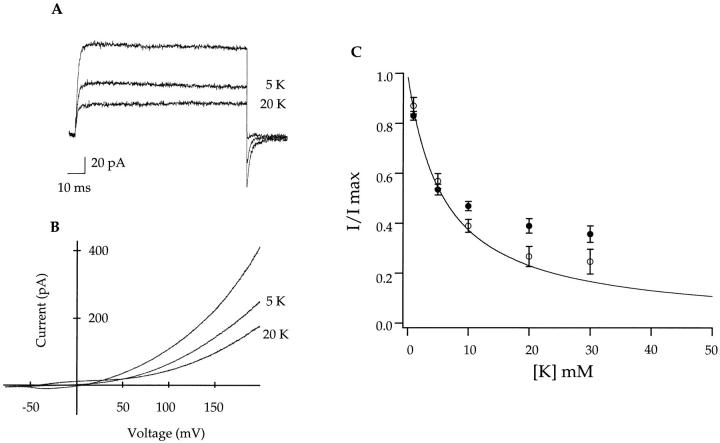
The blocking experiments were performed with inside out patches expressing A463C in symmetrical 140 mM Na+. Percent block of outward currents was measured from both steps to +100 mV (Fig. 7 A) and at +100 mV in ramps from −100 to +200 mV (Fig. 7 B). The results were identical regardless of which voltage protocol was used, and the data were pooled. Similarly, we measured percent block of inward tail currents at −100 mV (Fig. 7 A). The results are plotted as I/Imax vs. internal K concentration and fitted with a single site binding isotherm (Fig. 7 C). Block at +100 mV is shown as • and block at −100 mV is shown as ○. It is evident that the A463C mutation drastically decreases the internal potassium affinity well into the millimolar range (K d ∼ 6 mM). The measured affinity was not voltage dependent, comparing the measurement at +100 vs. −100 mV, suggesting that the ion binding site is quite superficial.
Figure 7.
A463C decreases the apparent potassium affinity of the channel. Data were obtained from inside-out patches in symmetrical 140 mM NaCl. Currents were elicited by voltage steps to +100 mV followed by a step to −100 mV (A) or by voltage ramps from −100 to +200 mV (B). Internally applied potassium blocked the observed currents in a concentration-dependent manner. The data were quantified by measuring percent current block at +100 mV in either steps or ramps versus internal K+ concentration (•) or percent current block at −100 mV in step data (○). (C) The error bars represent SEM. A fit to the data with a single binding isotherm yields an approximate K d of 6 mM.
One possible worry is that the measured potassium affinity appears decreased due to the permeant nature of the blocking ion. As is evident from the graph (Fig. 7), at higher potassium concentrations, the current amplitude deviates from the fit presumably due to blocker (K) conduction through the channel. Perhaps the error introduced by potassium conduction contributes to the observed decrease in the measured potassium affinity. One argument against this possibility is that the measured affinity is in the millimolar range regardless of the applied electric field or the overall direction of current.
Potassium is not as efficient at out-competing sodium in the A463C mutant as it is in the wild-type channel. The decreased potassium affinity allows for large sodium fluxes in the nominal absence of potassium in the A463C mutant. Another possible caveat of this measurement is that the affinity may be influenced by ions residing at neighboring sites in the pore. Ions present at adjacent sites may be electrostatically or otherwise affecting the stability of the bound potassium ion and as a result the effective ∼6-mM K d may be an overestimate of the affinity decrease in the A463C mutant. The previously described potassium blocking experiments that yielded a 6-mM potassium affinity were performed in the presence of 140 mM external sodium. If the external sodium is able to enter and bind in the pore of the channel, then it could possibly interact with the potassium at its internal binding site. This ion–ion interaction could destabilize the potassium ion and lead to a decreased apparent internal potassium affinity.
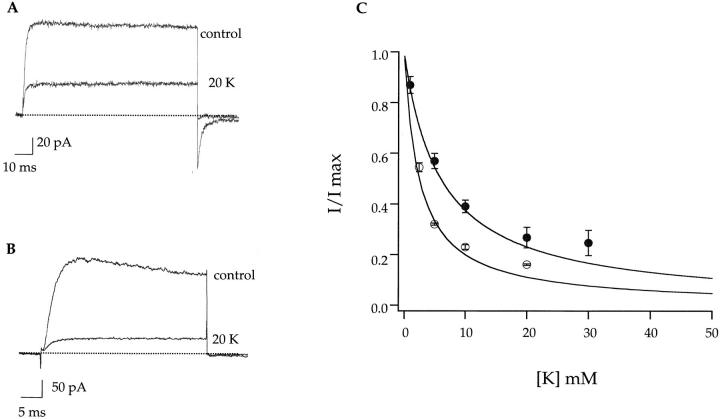
This hypothesis was tested by measuring internal K+ block of sodium currents with NMG+ as the major external cation. According to the hypothesis, an external binding site is significantly occupied in the presence of external sodium and the presence of the bound sodium ion is felt by the potassium bound at the internal site. If that were the case, then in the presence of external NMG+ the external site should be occupied less frequently and the apparent potassium affinity should increase. Indeed, lower concentrations of potassium were necessary to produce the equivalent amount of block in the presence of external NMG+ (Fig. 8, A and B). Under these ionic conditions, the apparent internal potassium affinity is increased to 2.5 mM (Fig. 8 C) as measured at +100 mV. Evidently, both sodium and potassium can simultaneously occupy the pore and mutually destabilize each other. Ion–ion interactions, however, cannot fully account for the observed decrease in potassium affinity in the A463C mutant. The internal potassium affinity is decreased into the millimolar range regardless of the presence or absence of external sodium.
Figure 8.
The potassium affinity is influenced by ions residing at neighboring sites. (A) Representative internal K block experiments in the presence of 140 mM symmetrical NaCl are taken from Fig. 7 A and are shown for comparison. (B) Currents elicited by an identical experimental protocol except in the presence of 140 mM external NMGCl and 140 mM internal NaCl. The current was elicited by a voltage step to +100 mV, followed by a step to −100 mV in both cases. (C) The data are quantified as described in Fig. 7 C except that in the external NMGCl experiments only data elicited by voltage steps were used. (•) Blocking data from 140 mM symmetrical NaCl, as shown in Fig. 7 C. (○) Blocking data from experiments with 140 mM external NMGCl and 140 mM internal NaCl. The fits are single binding isotherms yielding values of 2.5 mM for the external NMGCl data and 6.0 mM for the external 140 mM NaCl data.
A463C Decreases the Internal Barium Affinity
An independent measure of changes in potassium affinity can be obtained by studying barium block. Barium has the same crystal radius as potassium and therefore binds to the same ion binding sites in the channel pore. Unlike potassium, however, barium is doubly charged and therefore binds with an extremely high affinity, blocking current rather than conducting through the channel (Eaton and Brodwick, 1980; Armstrong et al., 1982; Neyton and Miller, 1988a , 1988b ; Hurst et al., 1995). We reasoned that, if A463C specifically decreases the potassium affinity of the channel, then barium affinity should also be decreased if both ions bind to the same site in the channel pore.
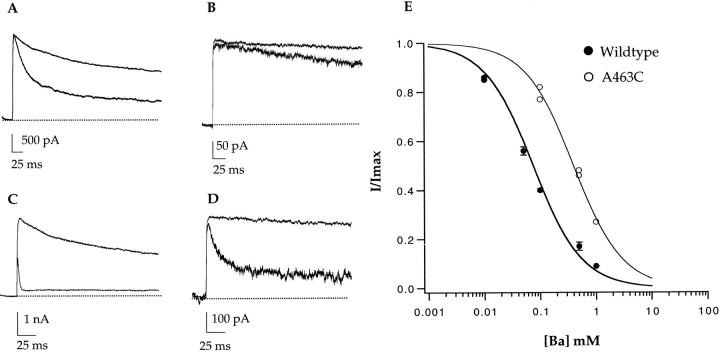
Internally applied barium blocks the Shaker channel with a high affinity and residues in S6 were shown previously to affect internal Ba++ block (Lopez et al., 1994). We found that the barium affinity of the wild-type Shaker channel is 75 μM when measured in 140 mM symmetrical potassium during a step to +100 mV (Fig. 9, A and C). The extent of block is severely decreased in the A463C mutant (Fig. 9, B and D), and this decrease in blocking ability translates to a roughly fivefold decrease in apparent affinity (350 μM). Although the voltage dependence of barium block was not quantitatively assessed in this study, it should be noted that the block is voltage dependent for both mutant and wild type. Whether there is a difference in the voltage dependence between the wild-type channel and A463C was not investigated. It appears from the representative traces that the rate of barium block was also affected by the A463C mutation.
Figure 9.
A463C decreases the internal Ba++ affinity of the channel. Internal Ba++ affinity was measured in both the wild-type channel and A463C with steps to +100 mV in the presence of symmetrical 140 mM KCl. Wild-type channel block in the presence of 100 (A) and 1,000 (C) μM internal Ba++. A463C channel block in the presence of 100 (B) and 1,000 (D) μM internal Ba++. The data are quantified as percent current versus Ba++ concentration (E). (•) Wild-type data, (○) A463C data. The single binding isotherm fits yield K ds of 75 μM for the wild-type channel and 350 μM for A463C.
We measured the ability of barium to block potassium currents to get an independent estimate of changes in affinity at a potassium ion binding site. This result also allows us to eliminate the possibility that the decrease in K+ affinity as measured by K+ block of sodium currents was solely dependent on the absence of potassium. Similarly, we can rule out the possibility that the decrease in potassium affinity is somehow dependent on sodium occupancy of the pore.
discussion
We have found that a single point mutation in S6 greatly decreases the potassium affinity of the Shaker channel. The wild-type Shaker channel has a micromolar potassium affinity as measured by internal K+ block of Na+ currents (Fig 1 B and Starkus et al., 1997) and by an elegant indirect measurement of the dwell time of the last K+ ion in the pore (Baukrowitz and Yellen, 1996). Mutating the alanine at position 463 into its counterpart in Kv2.1, a cysteine, is sufficient to decrease the apparent internal potassium affinity of Shaker well into the millimolar range. The block is not voltage dependent, nor does it depend on the direction of current. The measured affinity, however, is affected by the external permeant ion concentration, which indicates that the binding site is most likely in the channel pore. However, because the affinity is not dependent on voltage, we predict that the potassium ion binding site is located near the narrowing of the internal mouth of the channel. Our measurements further indicate that in A463C sodium and potassium can simultaneously occupy the pore. Externally present sodium enters the pore and interacts with a blocking potassium ion, decreasing its apparent affinity. In accordance with this hypothesis, replacement of external sodium with impermeant NMG+ results in an increase in the internal potassium affinity. We take this as evidence that ion– ion interactions do occur in the Shaker potassium channel and are a part of the normal permeation process. This finding argues against a strict permeation model without any electrostatic ion–ion interactions in the pore (Dang and McCleskey, 1998; Kiss et al., 1998). However, our data does not rule out the possibility of a pore containing ion binding sites with different potassium affinities.
The observed decrease in potassium affinity is further substantiated by a concomitant decrease in internal barium affinity. Barium has historically been a favorite probe of ion binding sites in the potassium channel pore as it is thought to bind to the same sites, although it blocks current rather than conducting through the channel. The barium affinity was measured in the presence of 140 mM symmetrical potassium in both wild type and A463C. Barium affinity decreased roughly fivefold in A463C as compared with the wild-type channel. The change in barium affinity between wild type and A463C is smaller then the measured change in potassium affinity (micro- to several millimolar). Since we know that blocker affinity can be affected by ion occupancy, one possible explanation may be that pore occupancy differs depending on the ionic conditions. Pore occupancy may also differ between wild type and A463C and the level of occupancy may differentially affect the binding of the monovalent potassium ion vs. the divalent barium ion. Also, in the A463C mutant the affinity is decreased for both the blocker and the competing potassium ion. If ionic competition is decreased in the A463C mutant, then a smaller change in barium affinity versus the potassium affinity may be expected. Differences in pore occupancy in this multi-ion pore may also explain the observation that potassium block of sodium currents is voltage independent while barium block of potassium currents is sensitive to voltage. Alternatively, the voltage dependance of barium block may be more apparent than for potassium block because it is doubly charged.
Since the barium affinity was measured in symmetrical potassium, we can rule out the possibility that the observed decrease in potassium affinity in A463C is contingent on the absence of potassium. This observation argues against the idea that the absence of potassium results in a general collapse of the pore, which then leads to a decrease in potassium affinity (Korn and Ikeda, 1995). Similarly, external TEA or Agitoxin block equally well regardless of whether the conducting ion is sodium or potassium. Since Agitoxin especially is quite sensitive to the architecture of the external vestibule (Miller, 1995), we would predict that a conformational change of the external mouth of the pore would have resulted in an altered blocker affinity.
It has recently been shown that the C-type inactivated state of the Shaker channel also conducts sodium in the absence of potassium, albeit less efficiently than the open state (Starkus et al., 1997). With sodium as the conducting ion, the wild-type Shaker channel inactivates extremely rapidly (Starkus et al., 1997), indicating that when conducting sodium the channels progress from open to (C-type) inactivated states. Furthermore, these results indicate that either sodium is not as effective as potassium at slowing C-type inactivation or sodium does not spend much time at that site in the wild-type channel. Unlike for the wild-type channel, the presence of external sodium greatly decreases the C-type inactivation rate of the A463C mutant as a direct consequence of the decreased affinity at the internal ion binding site (Ogielska, E.M., and R.W. Aldrich, manuscript in preparation). Based on our observations concerning the interaction of sodium with the C-type inactivation gate, we believe that the sodium currents presented in this study are due to sodium conducting through the open state of the channel. In the presence of external NMG+, however, we do see currents conducting through both the open and inactivated states. The blocking experiments performed in the presence of external NMG+ were done with short depolarizing pulses to separate these two conducting states and specifically assess the potassium block of open A463C channels.
Some potassium channels have been shown to lose function in the total absence of potassium (Chandler and Meves, 1970; Almers and Armstrong, 1980). In the absence of potassium and with prolonged depolarizations, they enter either a long-lived nonconducting state or an inactivated state (Khodakhan et al., 1997; Gomez-Lagunas, 1997; Starkus et al., 1997). The presence of a potassium ion at an external site in Shaker appears necessary to prevent the entry into a nonconducting or inactivated state (Gomez-Lagunas, 1997; Baukrowitz and Yellen, 1995; Starkus et al., 1997). Based on our experiments, we propose that in the A463C mutant the presence of external sodium prevents the extensive loss of channels previously observed in wild-type channels. Our currents in sodium are quite stable with repeated pulses and we do recover a stable potassium conductance upon returning to potassium solutions (data not shown). However, this has not been studied quantitatively and we cannot rule out the possibility that in the absence of potassium some channels become nonfunctional as a result of dekalification.
Our results indicate that the residue at position 463 in S6 participates in forming a potassium ion binding site in the pore. Mutations at position 463 in Shaker are known to alter single channel conductance, internal blocker efficacy, and the interaction of external permeant ions with channel closing (Hoshi et al., 1991; Avdonin et al., 1997; Ogielska, E.M., P.C. Zei, and R.W. Aldrich, unpublished observations). In accordance with the crystal structure of KscA, recent cysteine accessibility studies indicate that residues in S6 show state-dependent binding, being more accessible in the open state, and indicate that these residues line the internal vestibule of the channel (Liu et al., 1997). The activation gate influences the access of blockers into this vestibule and the activation gate itself involves residues in the carboxy terminal of S6 (Liu et al., 1997). The residue at the position equivalent to A463 in the KscA channel is a methionine. Although the methionine side chain does not itself project into the lumen of the pore, it is in direct contact with a conserved valine in signature sequence (R. MacKinnon, personal communication). This valine (V443 in Shaker) contributes its backbone carbonyl to stabilize a permeant ion at the internal binding site in the narrow region of the pore. We propose that in the Shaker channel the A463C mutant decreases potassium affinity of the internal ion binding site in the pore by altering the interaction between the 463 side chain and the side chain of the binding site valine at position 443.
Acknowledgments
We thank T. Middendorf, M. Kanevsky, D. Cox, and G. Talukder for helpful comments on the manuscript and D. Cox, R.W. Tsien, and R. MacKinnon for helpful discussions, R. MacKinnon for the kind gift of Agitoxin and the structural insights, and C. Toman for help with the molecular biology.
This work was supported by a grant from the National Institutes of Health (NS-23294) and a National Institutes of Health Training Grant (GM08327). R.W. Aldrich is an investigator with the Howard Hughes Medical Institute.
Abbreviations used in this paper
- GV
conductance–voltage
- NMG
N-methyl-d-glucamine
- TEA
tetraethylammonium
references
- Adelman WJ, French RJ. Blocking of the squid axon potassium channel by external caesium ions. J Physiol (Camb) 1978;276:13–25. doi: 10.1113/jphysiol.1978.sp012217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aiyar J, Nguyen AN, Chandy KG, Grissmer S. The P-region and S6 of Kv3.1 contribute to the formation of the ion conduction pathway. Biophys J. 1994;67:2261–2264. doi: 10.1016/S0006-3495(94)80710-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almers W, Armstrong C. Survival of K+ permeability and gating currents in squid axons perfused in K+free media. J Gen Physiol. 1980;75:61–78. doi: 10.1085/jgp.75.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong CM, Hille B. Voltage-gated ion channels and electrical excitability. Neuron. 1998;20:371–380. doi: 10.1016/s0896-6273(00)80981-2. [DOI] [PubMed] [Google Scholar]
- Armstrong CM, Swenson RP, Taylor SR. Block of squid axon K+channels by internally and externally applied barium ions. J Gen Physiol. 1982;80:663–682. doi: 10.1085/jgp.80.5.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avdonin V, Shibata E, Hoshi T. Dihydropyridine action on voltage-dependent potassium channels expressed in Xenopusoocytes. J Gen Physiol. 1997;109:169–180. doi: 10.1085/jgp.109.2.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baukrowitz T, Yellen G. Modulation of K+ current by frequency and external K+: a tale of two inactivation mechanisms. Neuron. 1995;15:951–960. doi: 10.1016/0896-6273(95)90185-x. [DOI] [PubMed] [Google Scholar]
- Baukrowitz T, Yellen G. Use-dependent blockers and exit rate of the last ion from the multi-ion. Science. 1996;271:653–656. doi: 10.1126/science.271.5249.653. [DOI] [PubMed] [Google Scholar]
- Bezanilla F, Armstrong C. Negative conductance caused by entry of sodium and cesium ions into the potassium channels of squid axons. J Gen Physiol. 1972;60:588–608. doi: 10.1085/jgp.60.5.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block BM, Jones SW. Ion permeation and block of M-type and delayed rectifier potassium. J Gen Physiol. 1996;107:473–488. doi: 10.1085/jgp.107.4.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block BM, Jones SW. Delayed rectifier current of bullfrog sympathetic neurons: ion-ion. J Physiol (Camb) 1997;499:403–416. doi: 10.1113/jphysiol.1997.sp021937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callahan MJ, Korn SJ. Permeation of Na+ through a delayed rectifier K+channel in chick dorsal root ganglion neurons. J Gen Physiol. 1994;104:747–771. doi: 10.1085/jgp.104.4.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler WK, Meves H. Sodium and potassium currents in squid axons perfused with fluoride solutions. J Physiol (Camb) 1970;211:623–652. doi: 10.1113/jphysiol.1970.sp009297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi KL, Mossman C, Aube J, Yellen G. The internal quaternary ammonium receptor site of Shakerpotassium channels. Neuron. 1993;10:533–541. doi: 10.1016/0896-6273(93)90340-w. [DOI] [PubMed] [Google Scholar]
- Cukierman, S., G. Yellen, and C. Miller. The K+ channel of sarcoplasmic reticulum. A new look at Cs+ block. Biophys. J. 48:477–484. [DOI] [PMC free article] [PubMed]
- Dang T, McCleskey E. Ion channel selectivity through stepwise changes in binding affinity. J Gen Physiol. 1998;111:185–193. doi: 10.1085/jgp.111.2.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle DA, Cabral JM, Pfuetzner RA, Kuo A, Gulbis JM, Cohen SL, Chait BT, MacKinnon R. The structure of the potassium channel: molecular basis of K+conduction and selectivity. Science. 1998;280:69–77. doi: 10.1126/science.280.5360.69. [DOI] [PubMed] [Google Scholar]
- Eaton DC, Brodwick MS. Effects of barium on the potassium conductance of the squid axon. J Gen Physiol. 1980;75:727–750. doi: 10.1085/jgp.75.6.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- French R, Wells J. Sodium ions as blocking agents and charge carriers in the potassium channels of the squid giant axon. J Gen Physiol. 1977;70:707–724. doi: 10.1085/jgp.70.6.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Lagunas F. Shaker B K+ conductance in Na+ solutions lacking K+ions: a remarkably stable non-conducting state produced by membrane depolarizations. J Physiol (Camb) 1997;499:3–15. doi: 10.1113/jphysiol.1997.sp021907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S, Miyazaki S, Krasne S, Ciani S. Anomalous permeabilities of the egg cell membrane of a starfish in K+-Tl+mixtures. J Gen Physiol. 1977;70:269–281. doi: 10.1085/jgp.70.3.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heginbotham L, MacKinnon R. Conduction properties of the cloned Shaker K+channel. Biophys J. 1993;65:2089–2096. doi: 10.1016/S0006-3495(93)81244-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heginbotham L, Lu Z, Abramson T, MacKinnon R. Mutations in the K+channel signature sequence. Biophys J. 1994;66:1061–1067. doi: 10.1016/S0006-3495(94)80887-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. Potassium channels in myelinated nerve; selective permeability to small cations. J Gen Physiol. 1973;61:669–686. doi: 10.1085/jgp.61.6.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B, Schwarz W. Potassium channels as multi-ion single file pores. J Gen Physiol. 1978;72:409–442. doi: 10.1085/jgp.72.4.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin AL, Keynes R. The potassium permeability of a giant nerve fibre. J Physiol (Camb) 1955;128:61–88. doi: 10.1113/jphysiol.1955.sp005291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshi T, Zagotta W, Aldrich R. Biophysical and molecular mechanisms of Shakerpotassium channel inactivation. Science. 1990;250:533–538. doi: 10.1126/science.2122519. [DOI] [PubMed] [Google Scholar]
- Hoshi T, Zagotta W, Aldrich R. Two types of inactivation in Shaker K+channels: effects of alterations in the carboxy terminal region. Neuron. 1991;7:547–556. doi: 10.1016/0896-6273(91)90367-9. [DOI] [PubMed] [Google Scholar]
- Hurst RS, Latorre R, Toro L, Stefani E. External barium block of ShakerPotassium channels: evidence for two binding sites. J Gen Physiol. 1995;106:1069–1087. doi: 10.1085/jgp.106.6.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda SR, Korn SJ. Influence of permeating ions on potassium channel block by external TEA. J Physiol (Camb) 1995;486:267–272. doi: 10.1113/jphysiol.1995.sp020809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavanaugh MP, Hurst RS, Yakel J, Varnum MD, Adelman JP, North RA. Multiple subunits of a voltage-dependent potassium channel contribute to the binding site for tetraethylammonium. Neuron. 1992;8:493–497. doi: 10.1016/0896-6273(92)90277-k. [DOI] [PubMed] [Google Scholar]
- Khodakhah K, Melishchuk A, Armstrong C. Killing channels with TEA+ . Proc Natl Acad Sci USA. 1997;94:13335–13338. doi: 10.1073/pnas.94.24.13335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss L, Immke D, LoTurco J, Korn S. The interaction of Na+ and K+in voltage-gated potassium channels: evidence for cation binding sites of different affinity. J Gen Physiol. 1998;111:195–206. doi: 10.1085/jgp.111.2.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn SJ, Ikeda SR. Permeation selectivity by competition in a delayed rectifier potassium. Science. 1995;269:410–412. doi: 10.1126/science.7618108. [DOI] [PubMed] [Google Scholar]
- Liman ER, Tytgat J, Hess P. Subunit stoichiometry of a mammalian K+channel determined by construction of multimeric cDNAs. Neuron. 1992;9:861–871. doi: 10.1016/0896-6273(92)90239-a. [DOI] [PubMed] [Google Scholar]
- Liu Y, Holmgren M, Jurman M, Yellen G. Gated access to the pore of a voltage dependent K+channel. Neuron. 1997;19:175–184. doi: 10.1016/s0896-6273(00)80357-8. [DOI] [PubMed] [Google Scholar]
- Lopez G, Jan Y, Jan L. Evidence that the S6 segment of the Shaker voltage gated K+channel comprises part of the pore. Nature. 1994;367:179–182. doi: 10.1038/367179a0. [DOI] [PubMed] [Google Scholar]
- MacKinnon R. Determination of the subunit stoichiometry of a voltage-activated potassium channel. Nature. 1991;350:232–235. doi: 10.1038/350232a0. [DOI] [PubMed] [Google Scholar]
- MacKinnon R. Pore loops: an emerging theme in ion channel structure. Neuron. 1995;14:889–892. doi: 10.1016/0896-6273(95)90327-5. [DOI] [PubMed] [Google Scholar]
- Mattenson D, Swenson R. External monovalent cations that impede the closing of K+channels. J Gen Physiol. 1986;87:795–816. doi: 10.1085/jgp.87.5.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C. The charybdotoxin family of K+channel-blocking peptides. Neuron. 1995;15:5–10. doi: 10.1016/0896-6273(95)90057-8. [DOI] [PubMed] [Google Scholar]
- Newland C, Adelman J, Tempel B, Almers W. Repulsion between tetramethylammonium ions in cloned voltage-gated potassium channels. Neuron. 1992;8:975–982. doi: 10.1016/0896-6273(92)90212-v. [DOI] [PubMed] [Google Scholar]
- Neyton J, Miller C. Potassium blocks barium permeation through a calcium activated potassium channel. J Gen Physiol. 1988a;92:549–567. doi: 10.1085/jgp.92.5.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neyton J, Miller C. Discrete Ba++ block as a probe of ion occupancy and pore structure of the high conductance Ca++ activated K+channel. J Gen Physiol. 1988b;92:569–586. doi: 10.1085/jgp.92.5.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogielska EM, Aldrich RW. Mutations in the S6 segment of Shakerchannels allow sodium permeation in the absence of potassium. Biophys J. 1996;70:A191. . (Abstr.) [Google Scholar]
- Ogielska EM, Aldrich RW. A single amino acid substitution in the S6 of Shakerdecreases potassium affinity and allows for sodium permeation in the absence of potassium. Biophys J. 1997;72:A233. . (Abstr.) [Google Scholar]
- Ogielska EM, Aldrich RW. A Shaker S6 mutation that affects K+ binding also alters Ba++affinity and ion interactions with the C-type inactivation gate. Biophys J. 1998;74:A19. . (Abstr.) [Google Scholar]
- Perez-Cornejo P, Begenesich T. The multi-ion nature of the pore in Shaker K+channels. Biophys J. 1994;66:1929–1938. doi: 10.1016/S0006-3495(94)80986-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoppa N, Sigworth FJ. Activation of Shakerpotassium channels III. An activation gating model for wild-type and V2 mutant channels. J Gen Physiol. 1998;111:313–342. doi: 10.1085/jgp.111.2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith-Maxwell C, Ledwell J, Aldrich RW. Uncharged S4 residues and cooperativity in voltage-dependent potassium channel activation. J Gen Physiol. 1998;111:421–439. doi: 10.1085/jgp.111.3.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stampe P, Begenesich T. Unidirectional K+ fluxes through recombinant Shaker potassium channels expressed in single Xenopusoocytes. J Gen Physiol. 1996;107:449–457. doi: 10.1085/jgp.107.4.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starkus JG, Kuschel L, Rayner MD, Heinemann SH. Ion conduction through C-type inactivated Shakerchannels. J Gen Physiol. 1997;110:539–550. doi: 10.1085/jgp.110.5.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swenson R, Armstrong C. K+ channels close more slowly in the presence of external K+ and Rb+ . Nature. 1981;291:427–429. doi: 10.1038/291427a0. [DOI] [PubMed] [Google Scholar]
- Tagliatela, M., M. Champagne, J. Drewe, and A.M. Brown. 1994. Comparison of H5, S6 and H5-S6 exchanges on pore properties of voltage dependent K+ channels. J.B.C. 269:13867–13873. [PubMed]
- Yellen G. Relief of Na+ block of Ca++-activated K+channels by external cations. J Gen Physiol. 1984;84:187–199. doi: 10.1085/jgp.84.2.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagotta WN, Hoshi T, Aldrich RW. Gating of single Shaker potassium channels in Drosophila muscle and in Xenopus oocytes injected with ShakermRNA. Proc Natl Acad Sci USA. 1989;86:7243–7247. doi: 10.1073/pnas.86.18.7243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagotta W, Hoshi T, Aldrich R. a. Shakerpotassium channel gating III: evaluation of kinetic models for activation. J Gen Physiol. 1994;103:279–319. doi: 10.1085/jgp.103.2.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagotta W, Hoshi T, Dittman J, Aldrich R. b. Shakerpotassium channel gating II: transitions in the activation pathway. J Gen Physiol. 1994;103:279–319. doi: 10.1085/jgp.103.2.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Ikeda SR. Anomalous permeation of Na+ through a putative K+channel in rat. J Physiol (Camb) 1993;468:441–461. doi: 10.1113/jphysiol.1993.sp019781. [DOI] [PMC free article] [PubMed] [Google Scholar]