Abstract
Escherichia coli Nissle 1917 (EcN) is a well-characterized probiotic bacterium. Although genomic comparisons of EcN with the uropathogenic E. coli strain CFT073 revealed high degrees of similarity, EcN is generally considered a non-pathogenic organism. However, as recent evidence suggests that EcN is capable of inducing inflammatory responses in host intestinal epithelial cells, we aimed to investigate potential pathogenic properties of EcN in an in vivo model using various germ-free (GF) mouse strains. With the exception of C3H/HeJZtm mice, which carry a defective toll-like receptor (TLR)4-allele, no lesions were obvious in mice of different strains orally inoculated with EcN for 1 week, although organ cultures (blood, lung, mesenteric lymph node, pancreas, spleen, liver and kidney) tested positive to various degrees. C3H/HeJZtm mice inoculated with EcN became clinically ill and the majority died or had to be euthanized. Organs of all gnotobiotic C3H/HeJZtm mice were positive for EcN by culture; major histological findings were moderate to severe pyogranulomatous serositis, typhlitis and pancreatitis. Histological findings were corroborated by highly elevated tumour necrosis factor (TNF) serum levels. Lesions were not detected in specified pathogen free maintained C3H/HeJZtm mice, GF C3H/HeJ mice lacking the interleukin-10 gene, or GF C3H/HeJZtm mice that were inoculated with E. coli K12 strain MG1655 as a control. In addition, mild histological lesions were detected in Ztm:NMRI mice 3 months after oral inoculation with EcN. This study shows that EcN is capable of displaying a virulent phenotype in GF C3H/HeJZtm mice. Whether this phenotype is linked to the bacterium’s probiotic nature should be the focus of further studies.
Keywords: E. coli Nissle 1917, EcN, germ-free mice, probiotics
Escherichia coli strain Nissle 1917 (EcN) of the serotype 06:K5:H1 is a well-characterized probiotic bacterium originally isolated by Dr Alfred Nissle in 1917 from the faeces of a soldier who stayed unaffected during a severe outbreak of shigellosis (Nissle 1918). Probiotics are live microorganisms considered to confer health benefits to the host (Guarner & Schaafsma 1998), and their use for therapeutic manipulation of the enteric microflora is promising for treating intestinal conditions such as inflammatory bowel disease (IBD) (Shanahan 2000, 2001; Colombel et al. 2001; Sartor 2004; Bleich & Mähler 2005). For example, administration of EcN was recently shown to be as effective as standard medication in maintaining remission of ulcerative colitis in human patients (Rembacken et al. 1999; Kruis et al. 2004).
The mechanisms underlying the probiotic nature of EcN have yet to be elucidated. Attempts to identify strain-specific characteristics and analysis of gene expression patterns resulting from interaction of this bacterium with the host have been used to determine the beneficial effect of EcN (Grozdanov et al. 2004; Sun et al. 2005; Ukena et al. 2005). In a recently published study, we have shown that most genes specifically upregulated by EcN interaction with host cells in vitro were coding for proinflammatory cytokines or molecules that are part of inflammatory pathways (Ukena et al. 2005). Furthermore, genomic comparisons of probiotic EcN with the uropathogenic E. coli strain CFT073 (Welch et al. 2002) revealed a high degree of similarity at genome level between these two strains, including factors considered to be virulence associated in pathogenic bacteria. These factors might contribute to the fitness and adaptability of EcN (Grozdanov et al. 2004; Sun et al. 2005).
Regardless of these similarities, to the best of our knowledge there have been no reports of the induction of pathogenic effects following EcN treatment. The aim of this study was to determine if this is also the case when mice with a naïve gut physiology are exposed to EcN. We therefore inoculated germ-free (GF) mice of different genetic backgrounds with EcN or with the non-pathogenic K12 E. coli strain MG1655, whose genome sequence has been fully determined (Blattner et al. 1997).
Materials and methods
Mice
Mice (all 56 days of age) of the following strains and stocks were obtained from colonies maintained GF in flexible film isolators (Metall + Plastik, Radolfzell-Stahringen, Germany) at the Central Animal Facility, Hannover, Germany: Ztm:NMRI (six females, seven males), BALB/cJZtm (four females, six males), C3H/HeJZtm (eight females, five males), C3H/HeJBir.129P2-Il10tm1Cgn (C3.129P2-Il10−/−, four females, four males), C57BL/6JZtm (three females), ZtmTac:SW (five females). Pelleted irradiated (50 kGy) diet (ssniff® M-Z, ssniff Spezialdiäten, Soest, Germany) containing 22.0% protein, 4.5% fat and 3.9% fibre, and autoclaved R/O water were provided ad libitum. The light:dark cycle was set at 12:12 h.
C3H/HeJZtm and C3.129P2-Il10−/− mice were tested for the Tlr4Lps-d allele (Poltorak et al. 1998) by restriction fragment length polymorphism (RFLP) analysis of a 325 bp PCR amplicon spanning the region of the Tlr4 gene containing a point mutation leading to a Pro→His substitution at amino acid 712 of the mature protein (protocol kindly provided by Dr S. Goyert). The following oligonucleotide primers were used to amplify the target region: 5′-AGA-ATG-AGG-ACT-GGG-TGA-GA-3′ and 5′-CTG-CTA-AGA-AGG-CGA-TAC-AA-3′. PCR was performed with the REDExtract-N-AMP-PCR ReadyMix (Sigma-Aldrich, Munich, Germany). Products yielded after 40 amplification cycles with an annealing temperature of 60 °C were digested with BstNI (New England Biolabs, Frankfurt, Germany) according to the manufacturer’s protocol and separated on a 3% NuSieve agarose gel (Biozym Scientific, Hessisch-Oldendorf, Germany) containing SYBR Green (Gel Star, 4 μl/100 ml; Biozym Scientific).
A total of eight C3H/HeJZtm mice (four females, four males) were produced and maintained in a room with controlled environment (21 ± 2 °C, 55 ± 5% relative humidity, 12 h light:dark cycle, 12-14 changes of air per hour). Personnel entering the room were required to wear a gown, cap, surgical mask, overshoes and gloves. Mice were housed separately by sex in individually ventilated cages (440 cm2 floor area) with a maximum of five animals per cage on bedding of non-sterilized, dust-free softwood fibres. Pelleted diet (ALTROMIN® 1314; Altromin Spezialfutter, Lage, Germany) containing 22.5% protein, 5.0% fat and 4.5% fibre, and tap water treated with UV light were provided ad libitum. Routine microbiological monitoring according to FELASA recommendations (Nicklas et al. 2002) did not reveal any evidence of infection with common mouse pathogens except for Pasteurella pneumotropica. These mice are henceforth designated as specified pathogen-free (SPF).
This study was conducted in accordance with the German Animal Welfare Law and with the European Communities Council Directive 86/609/EEC for the protection of animals used for experimental purposes. All experiments were approved by the Local Institutional Animal Care and Research Advisory committee and authorized by the local government.
Preparation of bacteria and colonization of mice
Escherichia coli Nissle 1917 and E. coli K12 laboratory strain MG1655 were prepared as described previously (Ukena et al. 2005). Both strains were grown overnight in Luria Bertani (LB) medium (Invitrogen, Karlsruhe, Germany) at 37 °C on a shaker. The cultures were then diluted 1:500 in LB media, grown at 37 °C, harvested in the late logarithmic phase after reaching an OD600 = 1 [containing approximately 109 colony-forming units (CFU) per millilitre], and centrifuged. Each animal received an inoculum (109 CFU) via oral gavage redissolved in 100 μl sterile PBS. This application was repeated after 3 days. For chronic treatment, bacteria were redissolved in sterile drinking water given to the mice for 1 week ad libitum. Individual group sizes of mice colonized with EcN or E. coli MG1655 are given in the results section (Tables 1–3).
Table 1.
Escherichia coli Nissle 1917 (EcN) detected in organs by culture (in %)
| Strain | n | Blood | Lung | MLN | Pancreas | Spleen | Liver | Kidney |
|---|---|---|---|---|---|---|---|---|
| Ztm:NMRI (7 days) | 5 | 0 | 40 | 100 | 20 | 40 | 20 | 0 |
| Ztm:NMRI (3 months) | 8 | 0 | 0 | 38 | 0 | 0 | 0 | 0 |
| C57BL/6JZtm | 3 | 0 | 67 | 67 | 33 | 33 | 0 | 33 |
| BALB/cJZtm | 5 | 0 | 60 | 60 | 80 | 60 | 40 | 60 |
| ZtmTac:SW | 5 | 20 | 20 | 100 | 60 | 60 | 60 | 80 |
| C3H/HeJZtm | 9 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| C3H/HeJZtm-SPF | 8 | 0 | 25 | 0 | 0 | 0 | 0 | 0 |
| Mean | 17 | 45 | 66 | 42 | 42 | 31 | 39 |
SPF, specified pathogen free; MLN, mesenteric lymph node.
Histological and bacteriological examination
Mice were euthanized by CO2 asphyxiation 1 week or 3 months after inoculation. Organs were removed aseptically and one part cultured as described below. The remaining samples from lung, lymph nodes, kidney, liver, spleen, pancreas, small intestine, cecum and large intestine were fixed in neutral buffered 4% formalin, processed routinely, embedded in paraffin, sectioned at 5–6 μm, and stained with haematoxylin and eosin (H&E).
For bacterial examination, the organs listed above, blood and faeces were cultured in thioglycollate broth (Oxoid, Basingstoke, UK) at 37 °C for up to 1 week. Positive cultures were plated on blood and Gassner agar, and bacteria were identified by API20E system (bioMérieux, Marcy l’Etoile, France) and EcN-specific PCR using primers (Muta 5/6) specific for the pMUT1 plasmid (Blum-Oehler et al. 2003). Primer sequences were: 5′-AAC-TGT-GAA-GCG-ATG-AAC-CC-3′ and 5′-GGA-CTG-TTC-AGA-GAG-CTA-TC-3′; the annealing temperature was set to 60 °C.
For quantitative bacterial cultures, livers, spleens, kidneys and mesenteric lymph nodes of gnotobiotic C3H/HeJZtm, Ztm:NMRI and BALB/cZtm mice were collected 3 days after inoculation with EcN, weighed, and homogenized in 1 ml PBS with a Xenox motorized hand tool (Proxxon, Niersbach, Germany). The homogenates were serially diluted in PBS, and each dilution was cultured on blood agar plates. The number of colonies per plate was counted and expressed as CFU per organ.
Assessment of cytokine serum levels
Serum samples were stored at −80 °C until they were processed for cytokine analysis. Levels of tumour necrosis factor (TNF) were quantified using fluorescent-labelled microspheres (Fluorokine MAP System; R&D Systems, Wiesbaden-Nordenstadt, Germany) and the Luminex 100 instrument (Luminex BV, Oosterhout, the Netherlands). All procedures closely followed the manufacturer’s instructions. The data were analyzed with luminex 2.3 software using five-parametric curve fitting. A standard curve was generated from a threefold dilution series made with the standard cocktail provided in the Fluorokine MAP Base Kit (R&D Systems) with the lowest standard concentration being 6 pg/ml. Sera were diluted fourfold and measured in duplicates.
Results
Germ-free mice tolerated inoculation with EcN well with the exception of C3H/HeJZtm mice, all of which developed clinical disease within 3–7 days after inoculation. In total, three out of nine mice died, and three moribund mice were euthanized on day four or five. Organs of these mice were taken for bacterial culture and histological examination as described above. The remaining three mice survived until day seven, showing moderate clinical signs (ruff fur and dehydration as evidenced by tenting of the skin). No GF mice of the other strains inoculated with EcN exhibited clinical signs. As only GF C3H/HeJZtm mice developed disease, we investigated the effect of EcN given to SPF mice of this strain. However, no clinical signs could be observed in these animals. We then questioned whether pathogenic effects might also be observed in GF mice of resistant strains when infected with EcN for a period of 3 months. Ztm:NMRI mice were chosen for this because they showed less positive organ cultures than the other strains (see below). In contrast to the previous experiments, EcN was given via the drinking water. Again, these chronically infected mice did not exhibit any clinical signs for up to 3 months.
Gross necropsy and histology
Necropsies revealed mild to moderate enlargement of the mesenteric lymph nodes and spleens of gnotobiotic mice of all strains except for BALB/cJZtm mice and C3H/HeJZtm mice inoculated with E. coli MG1655. Germ-free C3H/HeJZtm mice inoculated with EcN showed signs of dehydration as well as moderate to severe enlargement of the mesenteric lymph nodes and spleens. A markedly oedematous pancreas was seen in one mouse that underwent necropsy on day five.
Histological lesions were limited to the C3H/HeJZtm inbred strain inoculated with EcN and maintained as gnotobiotic animals and to the gnotobiotic Ztm:NMRI mice exposed to EcN for 3 months. No lesions were observed in C3H/HeJZtm mice maintained under SPF conditions, in C3H/HeJZtm and BALB/cJZtm mice inoculated with E. coli MG1655, nor in GF mice of the other strains inoculated with EcN. These findings indicate that C3H/HeJZtm mice, which are a substrain of C3H/HeJ mice that carry the Tlr4Lps-d allele (Poltorak et al. 1998), are susceptible under GF conditions to morbidity and mortality when associated with EcN.
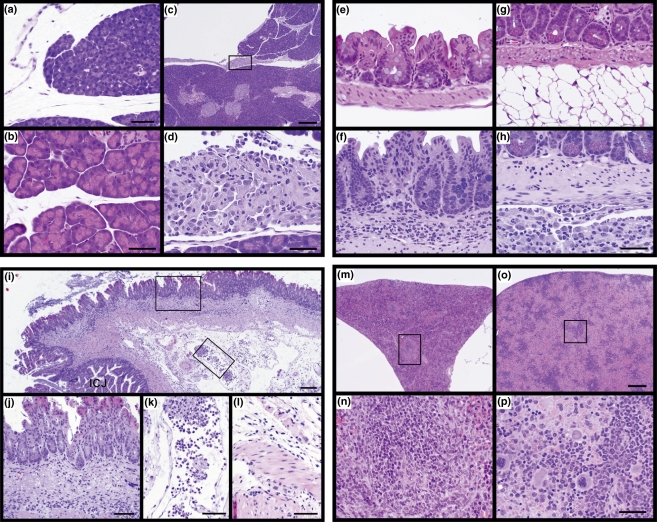
In C3H/HeJZtm mice inoculated with EcN, the pancreatic acini were compressed and there was a clear space separating the connective tissue surrounding the pancreatic lobules. In addition, the spaces between lobules were enlarged compared with controls (Figure 1a,b). There was also an accumulation of macrophages and granulocytes, both neutrophils and eosinophils, in the interstitium between lobules (Figure 1c,d). In the intestines, lesions were limited to moderate, diffuse granulocytic infiltrations within the submucosa of the cecum (Figure 1e,f,i,j). However, moderate to severe pyogranulomatous inflammatory cell infiltrates were present on the serosal surface (Figure 1g,h). Veins in the mesenteric attachments were dilated with large numbers of inflammatory cells, fibrin, and bacteria (Figure 1k). Bacilli were present in the submucosa, muscle layer and serosa (Figure 1j–l). Spleens of affected mice were responsive to the EcN infection as evidenced by their marked enlargement. Microscopically, normal spleens of unaffected C3H/HeJZtm mice inoculated with E. coli MG1655 were small and triangular in shape, with areas of small white and red pulp that were not very active (Figure 1m,n). By contrast, the spleens of affected mice were markedly enlarged and filled with blood. While the white pulp was not very active at low magnification, it was actively producing large numbers of immature granulocytes (Figure 1o,p). The livers and kidneys were essentially normal, although mild accumulations of macrophages were present on their serosal surfaces. Lungs and mediastinal tissues had various degrees of pyogranulomatous inflammation on their surfaces similar to that found in the abdomen. One liver had a small focus of acute coagulative hepatic necrosis with paracentric inflammation. These lesions are relatively common in many inbred strains of mice (Sundberg et al. 1997).
Figure 1.
(a–d) C3H/HeJZtm germ-free (GF) mice given Escherichia coli Nissle 1917 (EcN) had oedema fluid separating the connective tissue surrounding the pancreatic lobules and between the lobules themselves (a) unlike the C3H/HeJZtm controls inoculated with E. coli MG1655 (b). Accumulations of macrophages and lesser numbers of granulocytes, both neutrophils and eosinophils, also separated the lobules of the pancreas in C3H/HeJZtm mice (c) given EcN. This can be seen in the boxed enlargement in c (d). Bar = 150 μm (a), 100 μm (b,d), 700 μm (c). (e–h) The cecum of a GF C3H/HeJZtm mouse inoculated with E. coli MG1655 had a very thin submucosa with essentially no cells (e). In contrast, those inoculated with EcN showed dilation of the submucosa filled with a mixed, mostly granulocytic, inflammatory cell infiltrate (f). The mesenteric surface of the intestines of C3H/HeJZtm mice inoculated with E. coli MG1655 had no abnormalities indicated by the normal white fat in this region of the small intestine (g). By contrast, GF C3H/HeJZtm mice inoculated with EcN had a prominent pyogranulomatous infiltration in the mesentery (h). Bar = 100 μm. (i–l) Ileocecal junction of an EcN infected C3H/HeJZtm mouse. While the mucosa is only slightly thickened compared to that of control mice, the submucosa is markedly thickened with a mixed inflammatory cell infiltrate predominated by neutrophils (i). Numerous bacilli are diffusely growing in this area and many are being phagocytosed by the inflammatory cells (j). Veins in the mesenteric attachments are dilated with large numbers of neutrophils, small numbers of macrophages phagocytosing bacilli, and fibrin (k). Throughout the muscular layers there is moderate oedema fluid accumulation and diffuse growth of bacilli (l). Bar = 400 μm (i), 150 μm (j–l). (m–p) The normal spleen from a C3H/HeJZtm mouse inoculated with E. coli MG1655 in cross section is shrunken and triangular in shape (m). At higher magnification (boxed area in m), the white pulp is seen to produce lymphocytes (n). In contrast, the GF C3H/HeJZtm mice given EcN had markedly enlarged spleens filled with mature red blood cells (o). The enlarged area in o illustrates the abundant mature red blood cells and production of large numbers of immature granulocytes in response to the infection (p). Bar = 700 μm (o), 100 μm (p).
Of the eight Ztm:NMRI mice inoculated with EcN for 3 months, three showed small granulomas and lymphoid aggregates widely scattered throughout the mesenteric fat around the cecum and/or colon. In one mouse, a locally extensive area of mild neutrophilic infiltration was evident in the submucosa of the small intestine; in addition, there were pyogranulomatous foci associated with and surrounding medium-sized arteries in this area within the mesenteric fat. In one pancreas, a mild to moderate lymphocytic infiltrate was evident around a solitary islet. Mild to moderate chronic interstitial nephritis was seen in three Ztm:NMRI mice.
Bacteriological examinations
Escherichia coli Nissle 1917 was isolated to various degrees from organs of all GF mice inoculated for 1 week (Table 1). Testing showed monocultures in organs and faeces, indicating that no contamination occurred. Specificity of EcN was confirmed by PCR. By contrast to the other strains and stocks, all organs of all C3H/HeJZtm mice tested positive for EcN. When C3H/HeJZtm mice were inoculated with E. coli MG1655 bacteria could only be grown from livers, kidneys, lungs and mesenteric lymph nodes as indicated in Table 2, and only two organs were positive in the C3H/HeJZtm-SPF mice inoculated with EcN (Table 1). These results suggest that GF C3H/HeJZtm mice are susceptible to systemic infection with the probiotic EcN.
Table 2.
Escherichia coli MG1655 detected in organs by culture (in %)
| Strain | n | Blood | Lung | MLN | Pancreas | Spleen | Liver | Kidney |
|---|---|---|---|---|---|---|---|---|
| C3H/HeJZtm | 4 | 0 | 75 | 100 | 0 | 0 | 25 | 50 |
| BALB/cJZtm | 5 | 0 | 0 | 20 | 0 | 0 | 20 | 60 |
| Mean | 0 | 38 | 60 | 0 | 0 | 23 | 55 |
MLN, mesenteric lymph node.
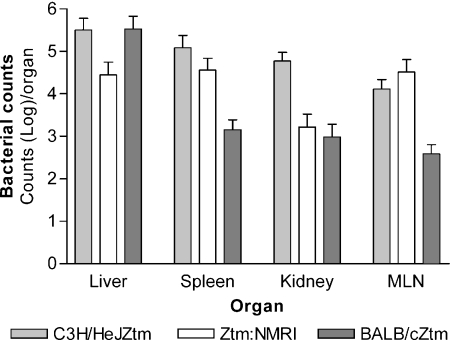
As with the C3H/HeJZtm mice, there were fewer positive cultures from BALB/cJZtm mice inoculated with E. coli MG1655 than in BALB/cJZtm mice inoculated with EcN (Table 2), indicating that EcN is more invasive than E. coli MG1655. However, invasiveness of EcN in gnotobiotic mice seems not to be the sole reason for lesions seen in C3H/HeJZtm mice because a great proportion of organs was also positive in ZtmTac:SW and BALB/cJZtm mice inoculated with EcN, and those mice developed neither clinical disease nor pathological lesions. Furthermore, quantitative culture of organs of C3H/HeJZtm, Ztm:NMRI, and BALB/cJZtm revealed higher bacterial counts only in spleens and kidneys of C3H/HeJZtm mice than in both other strains; however, these results were not statistically significant (Figure 2). Thus, quantitative cultures did not indicate that occurrence of pathological lesions in C3H/HeJZtm is solely due to a high extra-intestinal bacterial count in these mice.
Figure 2.
Quantitative culture of organs from Escherichia coli Nissle 1917 (EcN)-infected germ-free (GF) mice 3 days after inoculation. C3H/HeJZtm mice show higher colonization levels in the spleens and kidneys than Ztm:NMRI and BALB/cZtm mice, but differences are not statistically significant. MLN, mesenteric lymph node.
Interestingly, almost all organs of some of the Ztm:NMRI mice inoculated for 1 week were positive to a certain degree, while only mesenteric lymph nodes were positive in the group exposed to EcN for 3 months. Almost all GF mice inoculated with EcN for 1 week yielded positive culture results for their lymph nodes, except for one C57BL6/J and two BALB/cJZtm mice.
Serum TNF levels
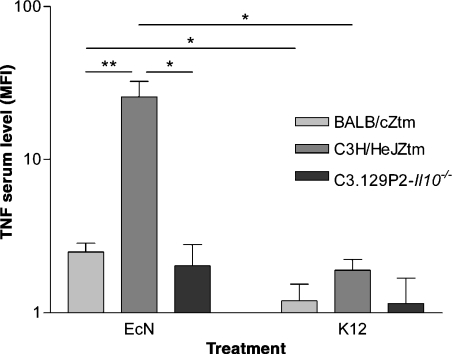
Tumour necrosis factor was measured by a fluorescent microbead assay in sera of GF BALB/cZtm, C3H/HeJZtm and C3.129P2-Il10−/− (see below) mice after inoculation with EcN or E. coli MG1655 (Figure 3). Serum levels above 6 pg/ml were detected only in GF C3H/HeJZtm mice inoculated with EcN. These considerably high amounts of TNF probably mirror the pathological lesions seen in these mice. Comparison of the mean fluorescence index (MFI), i.e. the amount of fluorescence particles bound to serum TNF, resulted in significantly higher TNF serum levels in EcN treated BALB/c and C3H/HeJZtm mice than in mice inoculated with E. coli MG1655.
Figure 3.
Detection of tumor necrosis factor (TNF) by fluorescent microbead array in serum samples of Escherichia coli Nissle 1917 (EcN) and E. coli MG1655 treated mice. Amount of TNF is expressed as mean fluorescence index (MFI) because only C3H/HeJZtm mice displayed serum levels above 6 pg/ml (the lowest standard concentration recommended by the manufacturer). Two-way anova revealed significant differences between strains (P = 0.0002), treatment (EcN or E. coli MG1655, P = 0.0006), and strain/treatment interaction (P = 0.0006). Significance levels of subsequent t-tests were set to P < 0.05 (*) and P < 0.01 (**). EcN treated BALB/cZtm and C3H/HeJZtm mice displayed higher TNF serum levels than E. coli MG1655 treated mice. Furthermore, inflammatory lesions seen in C3H/HeJZtm mice after EcN inoculation went along with considerably high TNF levels (up to 100 pg/ml). In addition to abrogation of pathological lesions after EcN treatment, deletion of the Il10-gene in susceptible C3H/HeJ mice led to reduction of TNF comparable to a level detected in non-susceptible BALB/cZtm mice.
Infection of C3.129P2-Il10−/− mice
It is known that C3H/HeJ substrains are susceptible to gram-negative infections (Wang et al. 1999, 2002; Bernheiden et al. 2001; Vallance et al. 2003; Branger et al. 2004) and that IL10 plays an important role in the pathogenesis of disease induced by gram-negative pathogens in mice (Greenberger et al. 1995; Wang et al. 1999). Neutralization of IL10 enhanced the resistance of C3H/HeJ (Wang et al. 1999) and CD-1 (Greenberger et al. 1995) mice to Klebsiella pneumoniae infection. Therefore we questioned if C3H/HeJ mice are still susceptible to EcN infection when they lack the Il10 gene. None of the infected C3.129P2-Il10−/− mice inoculated with EcN or E. coli MG1655 developed any sign of disease or histopathological lesion. In contrast to C3H/HeJZtm mice (that were positive to EcN in all organs investigated), EcN was detected only in mesenteric lymph nodes and two of four lungs of EcN inoculated mice. All other organs were negative by culture (Table 3). Serum levels of TNF were significantly lowered in EcN infected C3.129P2-Il10−/− mice compared with C3H/HeJZtm mice (P = 0.0141, t-test) and comparable with EcN inoculated BALB/c mice.
Table 3.
EcN and Escherichia coli (E. coli) MG1655 detected in organs of C3.129P2-Il10−/− mice by culture (in %)
| Inoculation | n | Blood | Lung | MLN | Pancreas | Spleen | Liver | Kidney |
|---|---|---|---|---|---|---|---|---|
| EcN | 4 | 0 | 50 | 100 | 0 | 0 | 0 | 0 |
| MG1655 | 4 | 0 | 0 | 75 | 0 | 0 | 0 | 0 |
EcN, E. coli Nissle 1917; MLN, mesenteric lymph node.
Verification of the Tlr4Lps-d allele in germ-free C3H/HeJZtm and C3.129P2-Il10−/− mice
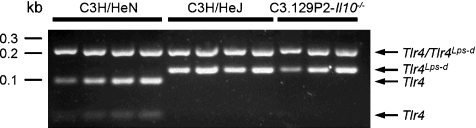
As the defective TLR4 of C3H/HeJ substrains is likely to mediate susceptibility to EcN induced lesions and IL10-deficiency seems to reverse this effect, we determined whether the point mutation in the Tlr4 gene leading to the Pro→ His substitution at amino acid 712 of the protein is present in the GF C3H/HeJZtm and C3.129P2-Il10−/− mouse colonies. As shown in Figure 4, restriction digest of a 325 bp PCR amplicon with BstNI resulted in the expected two fragments with a size of 202 and 123 bp in C3H/HeJZtm and C3.129P2-Il10−/− mice, and in the predicted three fragments of 202, 94 and 29 bp in C3H/HeN mice carrying a wild-type Tlr4 allele. Thus, we could verify the presence of the Tlr4Lps-d allele in C3H/HeJZtm and C3.129P2-Il10−/− mice, leading to a defective TLR4 in these animals.
Figure 4.
Germ-free (GF) C3H/HeJZtm and C3.129P2-Il10−/− mice carry the Tlr4Lps-d allele as determined by RFLP analysis. A 325 bp PCR amplicon was digested using BstNI. The resulting two fragments (202 and 123 bp) in C3H/HeJZtm and C3.129P2-Il10−/− mice and three fragments (202, 94, and 29 bp) in C3H/HeN mice demonstrate presence of a Tlr4 point mutation in mice of the former two strains, leading to a defective TLR4. kb = DNA molecular weight marker XIV (Roche Diagnostics, Mannheim, Germany).
Discussion
Germ-free mice of different strains and stocks were exposed orally to EcN to determine the distribution of EcN in organs after inoculation and to identify a potential virulent behaviour of this probiotic. Unlike the results for mice of other genetic backgrounds, cultures from C3H/HeJZtm mice were positive for EcN with all organs investigated. Furthermore, mortality, morbidity and pathological lesions in mice of this strain were consistent with acute inflammatory reaction in the cecum, spleen, pancreas and serosal surfaces. This was corroborated by the detection of considerably high amounts of serum TNF levels in EcN-treated C3H/HeJZtm mice. In C3H/HeJZtm mice inoculated with E. coli MG1655, fewer organs were positive for E. coli and neither clinical signs nor histological lesions were observed. C3H/HeJZtm-SPF mice neither developed disease nor did they show histological evidence of inflammation, and only two organs were positive for EcN by bacterial culture. Similar but less extensive histological lesions were observed in Ztm:NMRI mice exposed to EcN for 3 months; however, these mice developed no clinical signs. In our opinion, these observations indicate that orally applied EcN has potential for inducing disease in mice of a certain genetic background under distinct environmental conditions, which to our best knowledge has not been shown before.
The mechanism of how EcN affects C3H/HeJZtm mice is unclear. One striking difference between C3H/HeJ substrains of mice and the other mice used here is the TLR4-defect of this strain. The presence of the Tlr4Lps-d allele in GF C3H/HeJZtm mice used in this study was likely because of their history and was confirmed by a molecular approach (Figure 4) using RFLP analysis of a PCR product. TLR4-defective alleles are associated with increased susceptibility to certain infections in humans (Agnese et al. 2002; Montes et al. 2006; Van der Graaf et al. 2006), and LPS-hyporesponsive C3H/HeJ mice were more susceptible than LPS-normal responsive strains to infections with gram-negative bacteria (Wang et al. 1999, 2002; Bernheiden et al. 2001; Vallance et al. 2003; Branger et al. 2004), including experimental E. coli infection of urogenital organs (Hopkins et al. 1998; Schilling et al. 2001; Elkahwaji et al. 2005), the lung (Lee et al. 2005) and systemic infection (Cross et al. 1989). Notably, bacterial counts were usually higher in C3H/HeJ substrains of mice in studies using E. coli infection models; however, higher bacterial counts were not necessarily associated with more severe clinical signs (Hopkins et al. 1998; Schilling et al. 2001; Lee et al. 2005). In this study, extra-intestinal presence of bacteria might have contributed to disease development. The highest number of positive bacterial cultures from all strains used here were from organs of C3H/HeJZtm mice; however, cultures of organs of unaffected mice of other strains were also positive for EcN. Thus, quantitative culture did not fully support the assumption, that occurrence of lesions in C3H/HeJZtm is solely because of a high extra-intestinal bacterial count. However, bacteria were not detected in most organs of EcN-treated C3.129P2-Il10−/− mice, and this went along with absence of pathological lesions and low TNF serum levels, although these mice also carry the Tlr4Lps-d allele (Figure 4). Absence of this anti-inflammatory cytokine is likely to have enhanced local defence mechanisms and bacterial clearance which in turn inhibited bacterial colonization of extra-intestinal organs in otherwise susceptible mice. Such a mechanism has also been suggested by others which showed that neutralization of IL10 enhanced resistance of C3H/HeJ mice to K. pneumoniae infection (Greenberger et al. 1995; Wang et al. 1999).
A proof for the contribution of the defective TLR4 to susceptibility of GF C3H/HeJZtm mice towards EcN induced pathology could be achieved by using GF reared C3H/HeN mice or other C3H substrains carrying the wild-type Tlr4 allele. However, other strain specific factors than the TLR4 deficiency might be also crucial for susceptibility of C3H/HeJZtm mice to EcN-induced pathology. C3H/HeJ substrains are well known to be very sensitive to intestinal inflammation when used as animal models for IBD, e.g. as interleukin 10-deficient mice (Bristol et al. 2000). Here, a defective TLR4 does not seem to be involved in disease susceptibility (Farmer et al. 2001; Mähler et al. 2002), although bacterial factors do play a role (Sellon et al. 1998; Bleich & Mähler 2005).
In previous studies, the host response to EcN (Ukena et al. 2005) in vitro or to other probiotics such as Bifidobacterium lactis (Ruiz et al. 2005) in vitro and in vivo was shown to be at least transiently proinflammatory in nature; therefore, induction of a proinflammatory response by EcN might be part of its probiotic effect. In vitro data showing a proinflammatory response to EcN treatment are consistent with our data showing higher serum TNF levels in GF mice infected with EcN compared with E. coli MG1655 inoculated animals. This was detected not only in C3H/HeJZtm mice that developed pathological lesions but also in BALB/cZtm mice; however, in BALB/cZtm mice, serum TNF levels were very low regardless of treatment.
In conclusion, presence of normal bacterial flora and particularities of the genetic background determine if EcN behaves as probiotic or pathogenic bacterium, demonstrating residual virulent capacities of this strain. Further investigations are needed to determine if the pathogenic effect of EcN in GF C3H/HeJZtm mice is linked to its probiotic properties and whether interaction between EcN and intestinal commensals, the normal primed immune response of a conventional host or both account for the different outcomes of EcN colonization of GF or SPF C3H/HeJZtm mice.
Acknowledgments
This work was supported in part by a grant from the National Institutes of Health (RR000173, JPS) and from the DFG (SFB621, HH & FG).
References
- Agnese DM, Calvano JE, Hahm SJ, et al. Human toll-like receptor 4 mutations but not CD14 polymorphisms are associated with an increased risk of gram-negative infections. J. Infect. Dis. 2002;186:1522–1525. doi: 10.1086/344893. [DOI] [PubMed] [Google Scholar]
- Bernheiden M, Heinrich JM, Minigo G, et al. LBP, CD14, TLR4 and the murine innate immune response to a peritoneal Salmonella infection. J. Endotoxin. Res. 2001;7:447–450. [PubMed] [Google Scholar]
- Blattner FR, Plunkett G, 3rd, Bloch CA, et al. The complete genome sequence of Escherichia coli K-12. Science. 1997;277:1453–1474. doi: 10.1126/science.277.5331.1453. [DOI] [PubMed] [Google Scholar]
- Bleich A, Mähler M. Environment as a critical factor for the pathogenesis and outcome of gastrointestinal disease: experimental and human inflammatory bowel disease and Helicobacter-induced gastritis. Pathobiology. 2005;72:293–307. doi: 10.1159/000091327. [DOI] [PubMed] [Google Scholar]
- Blum-Oehler G, Oswald S, Eiteljorge K, et al. Development of strain-specific PCR reactions for the detection of the probiotic Escherichia coli strain Nissle 1917 in fecal samples. Res. Microbiol. 2003;154:59–66. doi: 10.1016/s0923-2508(02)00007-4. [DOI] [PubMed] [Google Scholar]
- Branger J, Knapp S, Weijer S, et al. Role of Toll-like receptor 4 in gram-positive and gram-negative pneumonia in mice. Infect. Immun. 2004;72:788–794. doi: 10.1128/IAI.72.2.788-794.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bristol IJ, Farmer MA, Cong Y, et al. Heritable susceptibility for colitis in mice induced by IL-10 deficiency. Inflamm. Bowel Dis. 2000;6:290–302. doi: 10.1002/ibd.3780060407. [DOI] [PubMed] [Google Scholar]
- Colombel JF, Cortot A, van Kruiningen HJ. Antibiotics in Crohn’s disease. Gut. 2001;48:647. doi: 10.1136/gut.48.5.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross AS, Sadoff JC, Kelly N, Bernton E, Gemski P. Pretreatment with recombinant murine tumor necrosis factor alpha/cachectin and murine interleukin 1 alpha protects mice from lethal bacterial infection. J. Exp. Med. 1989;169:2021–2027. doi: 10.1084/jem.169.6.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkahwaji JE, Ott CJ, Janda LM, Hopkins WJ. Mouse model for acute bacterial prostatitis in genetically distinct inbred strains. Urology. 2005;66:883–887. doi: 10.1016/j.urology.2005.04.013. [DOI] [PubMed] [Google Scholar]
- Farmer MA, Sundberg JP, Bristol IJ, et al. A major quantitative trait locus on chromosome 3 controls colitis severity in IL-10-deficient mice. Proc. Natl Acad. Sci. USA. 2001;98:13820–13825. doi: 10.1073/pnas.241258698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberger MJ, Strieter RM, Kunkel SL, Danforth JM, Goodman RE, Standiford TJ. Neutralization of IL-10 increases survival in a murine model of Klebsiella pneumonia. J. Immunol. 1995;155:722–729. [PubMed] [Google Scholar]
- Grozdanov L, Raasch C, Schulze J, et al. Analysis of the genome structure of the nonpathogenic probiotic Escherichia coli strain Nissle 1917. J. Bacteriol. 2004;186:5432–5441. doi: 10.1128/JB.186.16.5432-5441.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarner F, Schaafsma GJ. Probiotics. Int. J. Food Microbiol. 1998;39:237–238. doi: 10.1016/s0168-1605(97)00136-0. [DOI] [PubMed] [Google Scholar]
- Hopkins WJ, Gendron-Fitzpatrick A, Balish E, Uehling DT. Time course and host responses to Escherichia coli urinary tract infection in genetically distinct mouse strains. Infect. Immun. 1998;66:2798–2802. doi: 10.1128/iai.66.6.2798-2802.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruis W, Fric P, Pokrotnieks J, et al. Maintaining remission of ulcerative colitis with the probiotic Escherichia coli Nissle 1917 is as effective as with standard mesalazine. Gut. 2004;53:1617–1623. doi: 10.1136/gut.2003.037747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JS, Frevert CW, Matute-Bello G, et al. TLR-4 pathway mediates the inflammatory response but not bacterial elimination in E. coli pneumonia. Am. J. Physiol. Lung Cell Mol. Physiol. 2005;289:L731–L738. doi: 10.1152/ajplung.00196.2005. [DOI] [PubMed] [Google Scholar]
- Mähler M, Most C, Schmidtke S, et al. Genetics of colitis susceptibility in IL-10-deficient mice: backcross versus F2 results contrasted by principal component analysis. Genomics. 2002;80:274–282. doi: 10.1006/geno.2002.6840. [DOI] [PubMed] [Google Scholar]
- Montes AH, Asensi V, Alvarez V, et al. The Toll-like receptor 4 (Asp299Gly) polymorphism is a risk factor for gram-negative and haematogenous osteomyelitis. Clin. Exp. Immunol. 2006;143:404–413. doi: 10.1111/j.1365-2249.2005.03002.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicklas W, Baneux P, Boot R, et al. Recommendations for the health monitoring of rodent and rabbit colonies in breeding and experimental units. Lab. Anim. 2002;36:20–42. doi: 10.1258/0023677021911740. [DOI] [PubMed] [Google Scholar]
- Nissle A. Die antagonistische Behandlung chronischer Darmstörungen mit Colibakterien. Med. Klin. 1918;2:29–30. [Google Scholar]
- Poltorak A, He X, Smirnova I, et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- Rembacken BJ, Snelling AM, Hawkey PM, Chalmers DM, Axon AT. Non-pathogenic Escherichia coli versus mesalazine for the treatment of ulcerative colitis: a randomised trial. Lancet. 1999;354:635–639. doi: 10.1016/s0140-6736(98)06343-0. [DOI] [PubMed] [Google Scholar]
- Ruiz PA, Hoffmann M, Szcesny S, Blaut M, Haller D. Innate mechanisms for Bifidobacterium lactis to activate transient pro-inflammatory host responses in intestinal epithelial cells after the colonization of germ-free rats. Immunology. 2005;115:441–450. doi: 10.1111/j.1365-2567.2005.02176.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartor RB. Therapeutic manipulation of the enteric microflora in inflammatory bowel diseases: antibiotics, probiotics, and prebiotics. Gastroenterology. 2004;126:1620–1633. doi: 10.1053/j.gastro.2004.03.024. [DOI] [PubMed] [Google Scholar]
- Schilling JD, Mulvey MA, Vincent CD, Lorenz RG, Hultgren SJ. Bacterial invasion augments epithelial cytokine responses to Escherichia coli through a lipopolysaccharide-dependent mechanism. J. Immunol. 2001;166:1148–1155. doi: 10.4049/jimmunol.166.2.1148. [DOI] [PubMed] [Google Scholar]
- Sellon RK, Tonkonogy S, Schultz M, et al. Resident enteric bacteria are necessary for development of spontaneous colitis and immune system activation in interleukin-10-deficient mice. Infect. Immun. 1998;66:5224–5231. doi: 10.1128/iai.66.11.5224-5231.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanahan F. Probiotics and inflammatory bowel disease: is there a scientific rationale? Inflamm. Bowel Dis. 2000;6:107–115. doi: 10.1097/00054725-200005000-00007. [DOI] [PubMed] [Google Scholar]
- Shanahan F. Probiotics in inflamatory bowel disease. Gut. 2001;48:609. doi: 10.1136/gut.48.5.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Gunzer F, Westendorf AM, et al. Genomic peculiarity of coding sequences and metabolic potential of probiotic Escherichia coli strain Nissle 1917 inferred from raw genome data. J. Biotechnol. 2005;117:147–161. doi: 10.1016/j.jbiotec.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Sundberg JP, Fox JG, Ward JM, Bedigian HG. Idiopathic focal hepatic necrosis in inbred mice. In: Jones TC, Popp JA, Mohr U, editors. Monographs on Pathology of Laboratory Animals: Digestive System. Berlin: Springer-Verlag; 1997. pp. 213–217. [Google Scholar]
- Ukena SN, Westendorf AM, Hansen W, et al. The host response to the probiotic Escherichia coli strain Nissle 1917: specific up-regulation of the proinflammatory chemokine MCP-1. BMC. Med. Genet. 2005;6:43. doi: 10.1186/1471-2350-6-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallance BA, Deng W, Jacobson K, Finlay BB. Host susceptibility to the attaching and effacing bacterial pathogen Citrobacter rodentium. Infect. Immun. 2003;71:3443–3453. doi: 10.1128/IAI.71.6.3443-3453.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Graaf CA, Netea MG, Morre SA, et al. Toll-like receptor 4 Asp299Gly/Thr399Ile polymorphisms are a risk factor for Candida bloodstream infection. Eur. Cytokine Netw. 2006;17:29–34. [PubMed] [Google Scholar]
- Wang M, Jeng KC, Ping LI. Exogenous cytokine modulation or neutralization of interleukin-10 enhance survival in lipopolysaccharide-hyporesponsive C3H/HeJ mice with Klebsiella infection. Immunology. 1999;98:90–97. doi: 10.1046/j.1365-2567.1999.00838.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Moser C, Louboutin JP, et al. Toll-like receptor 4 mediates innate immune responses to Haemophilus influenzae infection in mouse lung. J. Immunol. 2002;168:810–815. doi: 10.4049/jimmunol.168.2.810. [DOI] [PubMed] [Google Scholar]
- Welch RA, Burland V, Plunkett G, 3rd, et al. Extensive mosaic structure revealed by the complete genome sequence of uropathogenic Escherichia coli. Proc. Natl Acad. Sci. USA. 2002;99:17020–17024. doi: 10.1073/pnas.252529799. [DOI] [PMC free article] [PubMed] [Google Scholar]