Abstract
In Europe, colorectal cancer is the second most prevalent form of cancer diagnosed. Globally each year, almost one million cases of colorectal cancer are registered and almost half a million deaths are attributed to this disease. This high mortality is associated with the development of liver metastases. For oncological advances to occur, accurate in vivo models are required to study colorectal cancer metastasis development. These models, by increasing our understanding of the early stages of colorectal liver establishment, will facilitate the development of novel therapeutic interventions and allow the clinical effects of these interventions to be studied. By analysis of current in vivo models for early development of colorectal liver metastasis, this review examines available methods of the tumour cell preparation, introduction and monitoring in vivo. An insight into the technical problems which can occur will be discussed. The implications of these different techniques on the resulting metastasis picture will be analysed. Existing in vivo models are assessed regarding the accuracy of the metastatic picture they portray.
Keywords: colorectal cancer, experimental model, intravital videomicroscopy, metastasis
A European study estimated that 2.9 million cancer cases were diagnosed and over 1.7 million cancer deaths occurred in 2004. The second most prevalent form of cancer diagnosed was colorectal cancer and accounted for 13.2% of all cases. Colorectal cancer was responsible for 203,700 deaths (Boyle & Ferlay 2005). Only lung cancer was responsible for more cancer deaths. Globally each year, almost one million cases of colorectal cancer are registered and almost half a million deaths are attributed to this disease (Parkin et al. 2001). At diagnosis, as many as 25% of colorectal cancer patients have established liver metastases (Kavolius et al. 1996). The primary locus of the colorectal tumour is frequently managed by either surgery alone or in combination with neo-adjuvant and adjuvant oncological therapy (Nelson et al. 2001). Management of the metastatic spread to other organs is a therapeutic challenge. Despite advances in the oncological management of disseminated colorectal cancer (Allegra & Sargent 2005; Twelves et al. 2005), surgical resection of liver metastases remains the only therapeutic intervention that offers the possibility of long-term survival and cure (Mutsaerts et al. 2005). This therapeutic option is dependent on both patient and tumour characteristics. For many, it is not a viable option (Scheele et al. 1995).
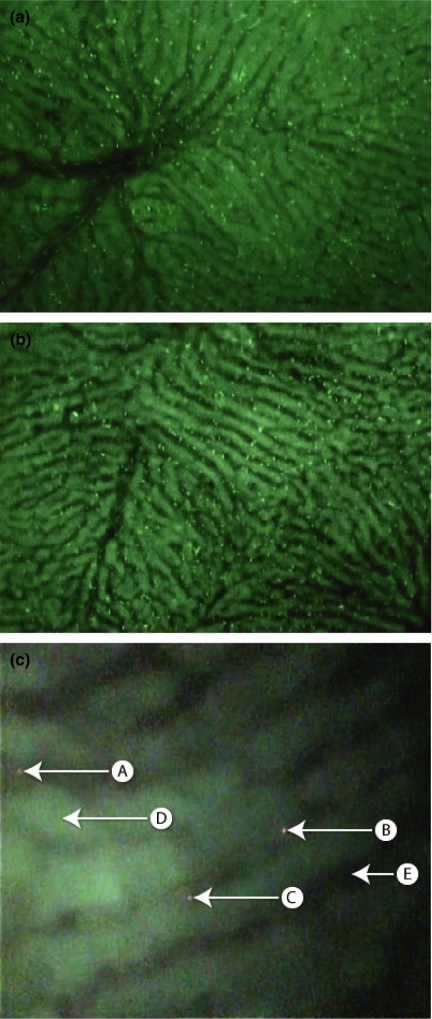
Intravital videomicroscopy (IVVM) now allows dynamic, real time imaging of biological events in an in vivo model system (Figure 1). This technique has proven particularly useful in the study of tumour blood flow and the therapeutic effects of vascular disruptive agents (Iga et al. 2006). IVVM has enabled the early development of colorectal metastasis particularly tumour cell arrest, adhesion and extravasation to be analysed in great detail (Table 1). For accurate study of novel therapeutic agents targeted at these early stages, an in vivo model must be established which closely resembles the genuine tumour development process.
Figure 1.
(a) Intravital videomicroscopy (IVVM) image shows labelled red blood cells within the hepatic circulation of rat. A hepatic venule is seen. (b) This image shows labelled red blood cells predominantly within the hepatic sinusoids. (c) The liver parenchyma has a green autofluorescence labelled (D). The sinusoid vessels are black (E). Three labelled (red) colorectal cancer cells are seen – (A, B & C). (A) is adherent to the sinusoidal walls. The other two (B & C) have migrated out of the sinusoid vessel and into the liver parenchyma.
Table 1.
Evolution of in vivo studies examining early stages of colorectal cancer liver metastasis
| Reference | Strain of animal | Cancer cell line | Molecular target (intervention) | Labelling method | Tumour inoculation | Outcomes |
|---|---|---|---|---|---|---|
| Luzzi et al. (1998) | C57BL/6 mice | B16F1 murinemelanoma | NONE (none) | Fluoresbrite carboxylated polystyrene nanospheres | Superior mesenteric vein | Adhesion and extravasation process is very efficient (>80%survive). The inefficiency of metastatic process occurs after this early stage. |
| Naumov et al. (1999) | SCID mice | CHO-K1 | NONE (none) | GFP | Mesenteric vein | GFP expressing cells enables the metastatic process to be monitored in vivo. |
| Ito et al. (2001) | Athymic nude miceof KSN strain | Rat tongue carcinoma RSC3 LM and E2 | NONE (none) | GFP | Intra portal via mesenteric vein | Metastatic cells and non-metastatic cells arrest in hepatic circulation. After 3 days all non-metastatic cells cleared from liver. |
| Ding et al. (2001) | BALB/c mice – P selectin knockout mice and wild type C57BL/6 | C26 adenocarcinoma and EL-4 lymphoma | Selectins (function blocking mAb) | PKH-26 | Mesenteric vein | Colorectal cancer cells demonstrated mechanical entrapment. Lymphoma cells metastasized although these cells were smaller than the sinusoid diameter. Cell specific adhesions using P selectin are involved. |
| Kikkawa et al. (2002) | BALB/c mice | CHO-K1 | αvβ3 (cells transfected with αvβ3) | GFP | Portal vein andtail vein | Transfected αvβ3 showed significantly higher accumulation in the liver postportal vein injection but not in the lung after tail vein injection. |
| Reinmuth et al. (2003) | BALB/c mice | Murine CT26 adenocarcinoma | αvβ3 and αvβ5 (S247 and αvβ3/αvβ5 antagonist) | None | Spleen | S247 prolonged survival in this animal model. S247 impaired both metastatic and angiogenic processes. |
| Steinbauer et al. (2003) | BALB/c mice – SCID and wild type | Murine CT26 adenocarcinoma | NONE (none) | GFP and calcein AM | Portal vein | GFP stain in longer experiments can trigger an immune reaction. |
| Sturm et al. (2003) | BALB/c mice | Murine CT26 adenocarcinoma | NONE (none) | GFP | Spleen | Created an animal model for color ectal cancer using murine cancer cells in a murine host. |
| Haier et al. (2003) | Sprague–Dawley rats | Human HT29 and rat CC531 colorectal cancer cells | NONE (none) | Calcein AM | Intra-arterially, intravenous and extrahepatic portal vein | Created animal model without GFP label, which could cause immune reaction. Showed despite method of colorectal cancer cell inoculation, cells still metastasized to liver. |
| Enns et al. (2004) | Sprague–Dawley rats | Human HT29 cells (HT29P and HT29LMM) | Integrins –α1, α3, α5, α6, β4 and α2β1. VCAM-1 and Selectins | Calcein AM | Intra cardiac | Specific integrins play a key role in colorectal cancer cell adhesion to the liver and in tumour migration. ECM of space of Disse is important in metastasis formation. |
| Enns et al. (2005) | Sprague–Dawley rats | Human HT29 colorectal cancer cells | Intergins pan αv, αvβ3 and αvβ5 | Calcein AM | Intra-arterial | αv integrins especially αvβ5 have a key role in colorectal cancer cell adhesion to the liver. |
| Schluter et al. (2006) | Sprague–Dawley or nude rats | HT29P low, KM-12C Intermediate or HT29LMM, KM-12L4 colorectal cancer cells | Calcein AM | Intra-arterial | Cell adhesion occurred in metastatic target organs only. Migration into target organs correlated with their metastatic potential. |
The purpose of this review will be to examine current in vivo models of colorectal metastasis. It will analyse the current models focusing on the different techniques used. By providing an overview and analysis of the tumour cell preparation, introduction and monitoring in vivo, this review will provide an insight into the problems that can occur. The effects that the different techniques have in altering the picture of metastasis portrayed will also be discussed.
Model of metastasis
In vivo models deployed should provide a true reflection of the physiological processes that occur in the cancer patient. As early as 1889, it was proposed that metastasis occurred in a non-random pattern. Indeed this was the basis of Stephen Paget’s ‘seed and soil’ hypothesis (Paget 1889). This hypothesis, as well as the metastasis process, has been studied intensely in the interim period. The ‘seed and soil’ hypothesis has been adapted and now consists of three separate entities (Onn & Fidler 2002; Fidler 2003). First, cancers consist of different cell subpopulations each of which has its own phenotypes. Current research (Bao et al. 2006; O’Brien et al. 2007) is closely examining these cell subpopulations to determine whether every cancer cell possesses the ability to initiate and sustain tumour growth or whether only a subset of cells, cancer stem cells, possess such potential. O’Brien et al. (2007) identified human colon cancer-initiating cells in immunodeficient mice hosts post-transplantation. All human colon cancer-initiating cells were CD133+. The majority of the tumour cells were CD133− and were unable to initiate tumour growth. Bao et al. (2006), in glioma tumours, also identified CD133+ cells. Within glioma tumours in vitro and in vivo, a higher proportion of these CD133+ cells were found to survive ionising radiation. These CD133+ cells were found to initiate DNA repair, post-radiotherapy, more effectively than CD133− cells. These results suggested that the CD133+ cells confer radioresistance on glioma tumours and could cause tumour recurrence postradiation therapy. These results suggest that a cancer hierarchy may exist. Only a proportion of tumour cells may be responsible for tumour proliferation. These stem cells may also be responsible for tumour recurrence after conventional oncological management. Significant advances in oncological management may occur through targeted cytotoxic therapies directed at these cancer stem cells. Secondly, the process of metastasis is selective for tumour cells which have the ability to embolize, invade, adhere, extravasate and establish metastasis in distant organs. Lastly, metastasis requires multiple interactions between the tumour cell and the regulatory mechanisms of the adjacent microenvironment (Liotta & Kohn 2001; Fidler 2002b).
The modified hypothesis provides a framework which any novel in vivo model should adhere. Deviation from these principles will result in physiological disruption (Figure 2) and produce an inaccurate reflection of metastasis development. Not only will a distorted image be obtained, but the model will be invalid for accurate assessment of therapeutic interventions. An in vivo model mirroring the genuine metastatic process would allow each stage of metastasis to be studied and be better understood. An accurate model would also facilitate the development of specific therapeutic interventions and enable the clinical effects of such an intervention to be analysed.
Figure 2.
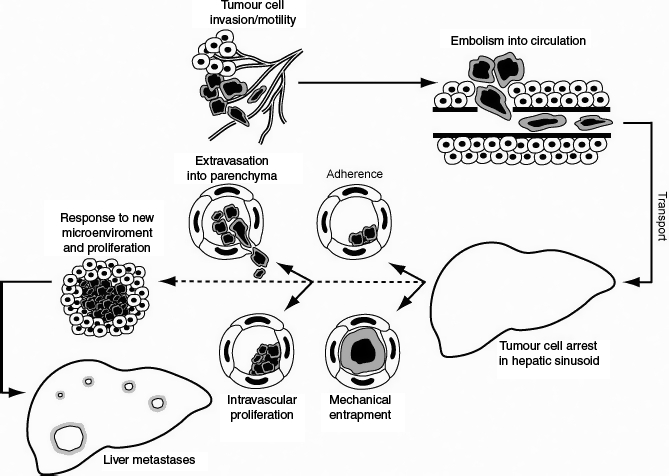
This figure graphically depicts the metastatic process, focussing on the early stages. Initially the colorectal cancer cells at the primary locus proliferate. Subsequently, these malignant cells become invasive and adopt a migratory phenotype. The invasion process if successful will allow the tumour cells to enter the circulation. In vivo, colorectal cancer cells can metastasize using a haematogenous or lymphatic method. For simplicity, only haematogenous spread is discussed. The haematogenous route of spread is utilized in many recent in vivo studies shown in Table 1. The cancer cells are then carried via the portal circulation to the liver where the sinusoids are the vessels with the smallest diameter. The figure then depicts the two different methods proposed for tumour cell arrest – mechanical entrapment and specific tumour cell adhesion. After the initial arrest, there is also evidence to support different methods of early metastatic development. Tumour cells can either undergo extravasation into the parenchyma and subsequent proliferation or undergo initial proliferation intravascularly followed by liver infiltration. If the tumour cells successfully complete these stages, they proliferate within their new environment and can develop into macroscopic liver metastasis.
Colorectal cancer cell line selection
Perhaps the most significant factor affecting the biological accuracy of the in vivo model is the selection of an appropriate colorectal cancer cell line. Some studies have utilized a synergic colorectal cancer cell line (Reinmuth et al. 2003; Steinbauer et al. 2003; Sturm et al. 2003), others have compared synergic and non-synergic lines (Haier et al. 2003),while others have deployed non-synergic human colorectal cancer cells in a Sprague–Dawley host (Enns et al. 2004, 2005; Schluter et al. 2006). Returning to the ‘seed and soil hypothesis,’ the third principle states that tumour cells interact with the microenvironment and homeostatic mechanisms (Fidler 2003). The biological accuracy of a cancer model that uses human colorectal cancer cells to develop metastasis in a rat liver has to be questioned. Although the comparison between human HT29 colorectal cells and rat CC531 colorectal cancer cells showed no differences over the 30 min observation period (Haier et al. 2003), the differences between the hepatic architecture and homeostatic mechanisms in a rat and a human are likely to produce a distorted image of metastatic development. Synergic models therefore are likely to provide a more accurate representation of metastatic development.
With cultured cell lines, there is the issue of phenotypic drift. Current colorectal cell lines have frequently undergone multiple passages. HT29 human colorectal cancer cells, for example provided by the European Collection of Cell Cultures, have undergone 135 passages. Further culturing of these cells in different laboratories and in different countries is likely to alter tumour cell characteristics. As phenotypic drift occurs, different representations of hepatic metastasis development could be obtained from colorectal cancer cells initially derived from the same cell colony.
Methods of transplantation
The ‘seed and soil hypothesis’ states that a tumour is a heterogeneous population of cells; selection occurs during the metastasis establishment and that tumour and microenvironmental interactions occur (Fidler, 2002; Fidler 2003). Many of the recent models have used colorectal cancer cells cultured in vitro. The colorectal cancer cells are labelled with calcein (Haier et al. 2003; Steinbauer et al. 2003; Enns et al. 2004, 2005) or transfected with green fluorescent protein (GFP) (Steinbauer et al. 2003; Sturm et al. 2003) prior to introduction to the animal. The tumour cells are then introduced into the circulation of the animal (Figure 2) to mimic haematogenous colorectal cancer spread in the human patient (Haier et al. 2003; Steinbauer et al. 2003; Enns et al. 2004, 2005). This model is intrinsically flawed regarding the second principle of the ‘seed and soil’ hypothesis (Fidler 2003). While the colorectal cancer introduced will be a heterogeneous population and interactions will occur between tumour cells and the microenvironment, there is no selection occurring. In the human patient, colorectal cancer cells, that reached the blood stream and disseminated, had already exhibited many aggressive or metastatic characteristics. In contrast, colorectal cells that had been passaged in vitro have demonstrated none of these characteristics. Current metastatic models could therefore portray an early metastasis development model that is far less efficient than in a human colorectal cancer patient. Genetically engineered animals, that spontaneously develop cancer and subsequent metastases, are the most biologically accurate models of metastasis. Smad3 mutant mice were shown to develop metastatic colorectal cancer (Zhu et al. 1998). Subsequent groups (Philipp-Staheli et al. 2002; Domino et al. 2007) have used these mice to examine different factors influencing colorectal cancer development and progression. However, these models are not amenable to the intravital microscope – an essential tool for real time observation of tumour development. Tumour cells must be labelled to allow detection in vivo.
Direct haematogenous introduction negates the effect of immune surveillance. Physiological immune surveillance mechanisms are artificially challenged by haematogenous bolus introduction of tumour cells to varying extents as the immune system would take time to detect and process the newly introduced cellular matter. The variability of the immune response between species and individuals within species may account for experimental differences seen in tumour natural history. Recent research using orthotopic tumour models examined human colorectal cancer in female BALB/c mice (Flatmark et al. 2004). Twelve colorectal cancer strains were implanted. The results showed considerable variation between the cell lines with regard to tumour propagation and dissemination. One of the twelve colorectal cancer cell lines produced liver metastasis and this only occurred in two of 10 animals. While the method of colorectal cancer cell introduction is different – orthotopic vs. haematogenous – immunosurveillance and clearance was occurring. This theory is further supported by other orthotopic research. A further study looking at HT29 human colorectal cancer cells produced similar results (Flatmark et al. 2004) in immunocompetent mice (Guilbaud et al. 2001). However in the immunocompromized severe combined immunodeficient (SCID) mouse, significantly higher rates of lymph node metastases and liver and lung metastases occurred. Immune reactions have also been observed in longer haematogenous experimental models. CT26 synergic murine colorectal cancer cells were introduced into a BALB/c mouse host (Steinbauer et al. 2003). Metastatic development was monitored and comparisons made between cells labelled with GFP and cells labelled with conventional techniques. No significant differences were noted in early metastasis development. However, a significant reduction in the late metastatic growth of GFP expressing CT26 colorectal cancer cells was observed. This phenomenon was only noticed in the immunocompetent mice. Further experiments in immunodeficient mice demonstrated that this cancer cell clearance was immune mediated. In the previously healthy colorectal cancer patient, colorectal cancer development and dissemination will occur despite immunosurveillance in an immunocompetent patient. A sudden introduction of 1 × 106 colorectal cancer cells (Haier et al. 2003; Enns et al. 2004, 2005) into the circulation will not accurately portray tumour cell dissemination. Instead the speed of introduction will allow at least initial escape from the immune system. This may in part explain why when human HT29 colorectal cells and rat CC531 colorectal cancer cells were introduced haematogenously into Sprague–Dawley rats (Haier et al. 2003), no significant differences were found between the adhesive properties of the two cell lines over the 30 min observation period. The introduction of a cell bolus and the short duration of the experiment will prevent immune surveillance from influencing the early stages of hepatic metastasis development. In longer experiments, significant differences are likely to be detected between the growth of these two tumour cell lines. In the rodent host, the human cancer cell line is more likely to be recognized as foreign matter and an immune response triggered. This response is likely to inhibit metastasis development.
Arguments continue whether haematogenous or orthotopic introduction of colorectal cancer cells provide a more accurate model. Orthotopic implantation (Guilbaud et al. 2001; Reinmuth et al. 2003; Sturm et al. 2003; Flatmark et al. 2004) from the principles of the ‘seed and soil hypothesis’ is likely to portray a more accurate picture of the metastasis process. A heterogeneous population of colorectal cancer cells is allowed to establish itself. Selection will occur throughout the dissemination process and tumour microenvironment interactions occur. The disadvantage is that this process of implantation makes analysis of events in early metastasis development hard to analyse with any degree of accuracy. Both theories of mechanical entrapment (Naumov et al. 1999; Chambers et al. 2001; MacDonald et al. 2002) and cell specific adhesion (Haier et al. 2003; Sturm et al. 2003; Enns et al. 2004, 2005) have been proposed for colorectal cell arrest within the hepatic microcirculation. A model of intravascular proliferation of tumour cells prior to extravasation has also been described (Al-Mehdi et al. 2000; Sturm et al. 2003). Accurate analysis of the above steps requires large numbers of tumour cells within the circulation at any specific time point. The bolus of cells, introduced in the haematogenous in vivo models, makes analysis of the early steps of metastasis development much easier. With the orthotopic model, the number of tumour cells within the circulation at any given time point will be significantly less than the haematogenous bolus. In addition in the orthotopic model, the introduction of tumour cells into circulation will be more erratic. Observations and experiments will be prolonged. This not only will increase observation error and variation, thereby reducing statistical validity but present problems with preservation of normal physiological parameters in the host.
In the haematogenous models, calcein AM is frequently used to label the colorectal cancer cells (Haier et al. 2003; Sturm et al. 2003; Enns et al. 2004, 2005). The principle focus of these experiments is to study the very early stages of metastasis development – tumour cell arrest, adhesion and extravasation. These experiments are short – minutes to hours. The calcein AM is not only an effective cellular label for this purpose but is not known to have any physiological effect on the tumour cell. The study of tumour cell arrest, adhesion and extravasation in orthotopic models is more difficult from a tumour cell labelling perspective. Orthotopic experiments are prolonged and involve colorectal cancer cell division. This cell division creates problems with calcein AM and nanosphere fluorescent probes (MacDonald et al. 2002) as will be discussed in the next section. In order to optimize tumour cell signal, either GFP or exogenous administration of polymer substrate would have to be used. GFP has been shown to stimulate an immune reaction in longer experiments (Steinbauer et al. 2003). Early metastasis development in the liver, after colorectal cancer cell dissemination from the primary site of orthotopic implantation, is likely to be affected. The alternative labelling technique involves the haematogenous administration of polymer substrate (Weissleder et al. 1999). Haematogenous polymer could influence the early stages of metastasis development. This technique (Weissleder et al. 1999) is also more appropriate for labelling metastases than individual cells and is associated with high background liver fluorescence (Hoffman 2002).
Host and surgical techniques used
To date, most in vivo models studying the early stages of colorectal liver development have used rodents (Table 1). For metastasis development to occur, multiple interactions occur between the introduced tumour cells and rodent liver. To ensure an accurate clinical picture, a cell line comparable with these hosts should be selected. This will be discussed in more detail later. At best, these early models will provide only some representation of the human metastasis process as there is no close correlation between the species. Models in higher primates are therefore likely to provide more accurate representation. However, higher order primate studies are yet to emerge.
The host and particularly techniques deployed to establish the experimental model can affect the validity of the metastasis model. Much current work relies upon the host, under anaesthetic, undergoing an abdominal incision (Haier et al. 2003; Reinmuth et al. 2003; Steinbauer et al. 2003; Sturm et al. 2003; Enns et al. 2004, 2005). The anaesthetic and the stress of surgery introduce novel variables into the metastasis model. In addition, subsequent exposition of the liver, involving mobilisation and partial exteriorisation for IVVM observation, can distort metastasis development (Haier et al. 2003; Steinbauer et al. 2003; Sturm et al. 2003; Enns et al. 2004, 2005). This mobilisation of the liver has the ability to disrupt the hepatic architecture and microenvironment of the colorectal cells. Vessels in the microcirculation could easily become compressed. It has been argued by some (Enns et al. 2005) that this compression could be in part responsible for tumour cell mechanical entrapment (Koop et al. 1995; Naumov et al. 1999). Trauma sustained during mobilisation could also trigger inflammatory responses which would add yet another variable to the metastasis model. Cannulation of the blood vessels required for colorectal cancer cell introduction has recognized complications and effects on the animal host and the introduced cell line (Kikkawa et al. 2002; Haier et al. 2003; Steinbauer et al. 2003; Enns et al. 2004, 2005). These problems and resulting distortion to the metastasis development are likely to be more significant in smaller rodents. In mice, the tissues are more friable and mobilisation of the liver can induce more significant trauma. During an experiment, alterations to the normal physiological parameters of the animal host can occur which again will be more exaggerated in a smaller host. In addition to the stresses of anaesthetic and laparotomy, further stresses from insensible loss and hypovolaemia and hypothermia can occur during the experiment. Even with close monitoring of pulse, mean arterial pressure, pulse oximetry and temperature and adherence to protocols to maintain values with in normal parameters, this is an artificial system at best. Another host factor to consider is the immune status. It has been shown in longer experiments that the immune system can play a critical role (Guilbaud et al. 2001; Steinbauer et al. 2003; Flatmark et al. 2004). SCID animal hosts used to accommodate xenogenic cell lines greatly alter the normal physiological criteria (Guilbaud et al. 2001).
The method of tumour cell introduction can have a significant effect on metastasis development. Earlier models used intra-portal routes via mesenteric veins (Luzzi et al. 1998; Naumov et al. 1999; Ding et al. 2001; Ito et al. 2001; Kikkawa et al. 2002; Steinbauer et al. 2003). This method of introduction not only caused transient disruption of a usually low pressure system but could account for the phenomenon of mechanical entrapment (Ding et al. 2001; Ito et al. 2001). Mechanical entrapment is a theory that proposes that tumour cell arrest occurs when larger tumour cells become lodged in microcirculation vessels. Many argue this is artifactual due to either compression of the hepatic architecture or circulatory disruption. Regardless of whether tumour cell arrest is due to mechanical entrapment or cell specific adhesion, these models provide useful information about cancer cell extravasation and cancer cell growth in a foreign environment (Luzzi et al. 1998; Naumov et al. 1999; Ding et al. 2001; Ito et al. 2001; Kikkawa et al. 2002; Steinbauer et al. 2003). However, clarification of the mechanism of tumour cell arrest is extremely important. If tumour cells become trapped in the microcirculation due to size restriction – mechanical entrapment, creation of a therapeutic intervention to inhibit this is futile. If, as many argue (Ding et al. 2001; Ito et al. 2001), cell specific adhesion is responsible then therapeutic interventions targeting the responsible molecules could significantly impair metastasis development. The argument, that mechanical entrapment is artifactual, is strongly supported by the findings of Haier et al. (2003). Their in vivo model introduced 1 × 106cells in 1 ml of phosphate buffered saline (PBS) solution. Venous tumour cell introduction via the jugular vein could cause cardio-pulmonary dysfunction. Intra-portal introduction was noted, due to transient dramatic pressure changes, to cause hepatic physiological disruption. The preferred method of introduction was arterial via the carotid artery. Physiological disruption was minimalized and the number of adherent cells viewed in the hepatic circulation by IVVM was greater than with other methods. Through a series of experiments (Haier et al. 2003; Enns et al. 2004, 2005; Schluter et al. 2006), this group has shown that colorectal cancer cells adhere only in metastatic target organs and this is shown to occur in vessels with diameters larger than the colorectal cancer cells. Adaptations to the original model (Haier et al. 2003) have shown that blocking specific integrins can significantly reduce colorectal cancer cell adhesion (Enns et al. 2004, 2005) and migration (Enns et al. 2004). These models clearly demonstrate specific colorectal organ targeting mediated by cell surface receptors. Most recently, comparison of colorectal cancer cell lines of varying metastatic potential has shown that migration rates correlate with metastatic potential (Schluter et al. 2006).
Reinmuth et al. (2003) created an in vivo model using a murine host and a murine colorectal cancer cell line. The tumour cells were introduced intra-splenically to induce metastases. Administration of S247, a peptide inhibitor of integrin αvβ3, was shown to prolong survival, decrease colorectal metastasis and angiogenesis. Models like these helps to elucidate the essential processes and molecules involved in metastasis establishment and development. Although identified in a distantly related species, they provide the insights that are essential for increased understanding of the metastasis process and the development of highly specific cancer targeted therapy.
Labelling
To enable the colorectal cancer cells to be viewed under the intravital microscope, the cells must be suitably labelled. Traditional labelling methods have utilized various histochemical marker genes –Escherichia coliβ-galactosidase gene (Lac Z), Drosophila alcohol dehydrogenase gene and human placenta alkaline phosphatase gene. These have been cloned into eukaryotic cells using viral vectors. The cells have subsequently been differentiated using appropriate staining protocols (Lin et al. 1990; Lin & Culp 1991). Although good for analysing cells in culture systems, these markers require cellular staining and therefore the sacrifice of the animal. These techniques are ineffective for dynamic in vivo assessment of cancer progression.
Adaptations to conventional markers have occurred. In particular modifications of the Lac Z labelling system has allowed in vivo real time detection of β-galactosidase activity (Tung et al. 2004). These adaptations utilize complex synthetic graft polymers. When cleaved by target proteases, the proteins become fluorescent in the infra-red region of the spectrum (Weissleder et al. 1999; Tung et al. 2000; Chen et al. 2002). The polymer substrate can be adapted to the specific protease. As many proteases are cellular specific, specific cancer cells can be monitored. While specifically labelling target cells in the animal, there are some limitations to this system. This system relies on an intravenous infusion (Figueiredo et al. 2006) of infra-red probes into the animal host which entails non-selective delivery to the animal organs. Due to the abundance of proteases within hepatocytes, concerns have been expressed (Hoffman 2002) about high liver background fluorescence. The study of metastasis development involves monitoring single labelled cancer cells undergoing tumour cell arrest, adhesion and extravasation (Haier et al. 2003; Enns et al. 2004, 2005). The high background fluorescence of the liver will impede accurate analysis of liver metastasis development. Experimental models investigating the early stages of colorectal metastasis development frequently use a haematogenous route of tumour cell dispersion to mimic blood borne tumour spread. The delivery of infra-red probes by intravenous infusion is likely to distort the physiological picture seen in the experimental model. Not only the volume of the infusion, but the introduction of foreign material into the blood stream simultaneously to the colorectal tumour cells, is likely to provide an inaccurate picture of the metastasis process. Recent studies (Enns et al. 2004, 2005) have shown adherence of tumour cells within the hepatic microcirculation to occur within minutes of injection. Delivery of a substrate for enzymatic cleavage would have a delay before tumour cells fluoresced due to substrate delivery to cells and cellular processing of the substrate. This makes the method unsuitable for the very early stages of metastasis development. Another method of tumour cell labelling uses the luciferase gene which involved exogenous delivery of substrate (Sweeney et al. 1999). This marker gene has been transfected into human cancer cells to monitor tumour growth and regression. Disruption to normal physiology especially during the early stages of tumour dissemination definitely occurs with this system. The luciferase enzyme requires the substrate luciferin to be delivered for light emission. In addition, due to low image resolution and signal achieved, an anaesthetic was required. Both these systems of exogenous substrate delivery are therefore better suited to the monitoring and identification of established tumour cells in the in vivo animal model. Antibodies have been used in cancer imaging but again are more suitable for imaging established tumour metastasis (Chester et al. 2004).
Fluorescent labelling of the colorectal cancers prior to the introduction into the animal host is considered favourable for monitoring the early stages of dissemination. This was achieved either by tumour cell transfection and altered protein expression (Hoffman, 2002) or introducing fluorescent compounds into the cell (MacDonald et al. 2002). These methods allow immediate visualisation of haematogenously introduced colorectal cancer cells. Green fluorescent protein cDNA extracted from Aequorea victoria has been transfected into both pro- and eukaryotic cells with stable expression of protein obtained (Chalfie et al. 1994). Optimisation of GFP expression and signal strength has subsequently been achieved (Cheng et al. 1996; Cormack et al. 1996; Zolotukhin et al. 1996). This has enabled GFP to be used extensively as a tool for cancer research. Hoffman (2002) has written an excellent review on GFP and its uses. Recently, several groups utilized GFP to study the early stages of colorectal cancer spread (Steinbauer et al. 2003; Sturm et al. 2003). This protein has many advantages compared with other methods of colorectal cancer cell labelling. In cells lines with stable expression, GFP continues to be expressed in future generations (Hoffman, 2002). This enables easy observation of tumour growth over a prolonged period of time. Comparatively, calcein AM (Uggeri et al. 2004) and other non-toxic cytoplasmic markers produce fluorescent signals that tend to be relatively short lived and susceptible to bleaching with exposure to fluorescent illumination (MacDonald et al. 2002). Fluorescent nanospheres have been deployed in cancer cell labelling. These markers tend to be photoresistant. However, the distribution of these spheres throughout the cell can be uneven and during replication the number of spheres per cell diminishes, leading to decreased signal strength (MacDonald et al. 2002). Introduction of nanospheres into cancer cells is likely to alter the characteristics of the tumour cell. GFP appeared the perfect tumour cell marker. Recently problems have been identified with this marker. GFP has now been shown to stimulate an immune reaction in longer experiments. This was noticed in murine colorectal cancer introduced into a murine host (Steinbauer et al. 2003).
As yet, the ideal colorectal cancer cell label for in vivo staining has not been discovered. Tumour cell labelling prior to introduction appears to be the most favoured and sensible approach and is utilized in recent experimental systems. Table 1 shows the experimental model development and the refinement of the model to analyse the early stages of colorectal metastasis development. Recent works have used calcein AM (Haier et al. 2003; Enns et al. 2004, 2005). Despite its disadvantages, calcein AM has not been shown to cause any immune reaction nor to have an effect on key tumour cell functions. In HT29 human colorectal cancer, calcein AM has been shown to have no effect on tumour cell viability or adhesion (Haier et al. 1999). In addition, as calcein AM is activated intra-cellularly, it will only label viable tumour cells (Haier et al. 2003; Uggeri et al. 2004). It therefore fulfils the role of an efficient tumour cell label as it causes minimal disruption of normal physiological processes.
Intravital microscope
Another factor which can influence the experimental model and metastasis development is the type of intravital microscope used. The two types of intravital microscope are upright (Haier et al. 2003; Steinbauer et al. 2003; Enns et al. 2004, 2005) and inverted (Naumov et al. 1999; MacDonald et al. 2002; Sturm et al. 2003). Both of these have effects on part of the third principle of the ‘seed and soil’ hypothesis – the tumour microenvironment. In the upright scope, the liver is mobilized, exteriorized and viewed in an animal host lying on its back. This process involves dissection of the falciform ligament, mobilising and stretching some of the vasculature. The liver is placed on a viewing platform outside and above the host. This process can distort the hepatic microenvironment. Exteriorisation of the liver involves more extensive physical handling and experimental exposure of the liver to the external environment than is associated with the inverted IVVM. All of these factors could have profound effects on the circulation, causing either vasoconstriction or vasodilatation.
The inverted microscope involves surgical exposition of the liver with minimal mobilisation. The animal is placed on its abdomen with the liver above the viewing platform. Using the inverted microscope, the liver is likely to have less physical manipulation but is likely to have more compression. This can distort the architecture of the liver, especially the microcirculation. The microcirculation, particularly the sinusoids (Naumov et al. 1999; Haier et al. 2003; Sturm et al. 2003; Enns et al. 2004, 2005), appear to play an important role in tumour cell arrest. This tumour cell arrest is vitally important. The mechanism of how this arrest occurs is still contested. Cell specific adhesions (Haier et al. 2003; Enns et al. 2004, 2005) accounting for tumour cell arrest would allow targeted interventions to be created. Mechanical entrapment (Naumov et al. 1999; Chambers et al. 2002), thought by some to be an artefact associated with compression of the hepatic microcirculation (Enns et al. 2004), would allow very little therapeutic intervention.
Conclusion
As yet, the perfect model of early colorectal cancer dissemination and metastasis development has not been established. For close analysis of early events, the haematogenous method is preferred over orthotopic as it allows studying of larger numbers of tumour cells to be examined. Calcein AM appears to be an effective mechanism of labelling with minimal distortion of the physiological picture. Syngenic cell lines are likely to provide a more accurate picture of the metastatic process while both methods of intravital microscope visualisation can contribute to the experimental variables. It is only with the establishment of a model that adheres to all of the principles of the ‘seed and soil’ hypothesis that a truly accurate picture of tumour evolution will be seen. An in vivo model closely mirroring the genuine metastatic process allows accurate study and an increased understanding of the early stages of colorectal liver metastasis establishment and development. Such a model would facilitate the development of specific therapeutic interventions and enables the biological effects of these interventions to be analysed (Eble et al. 2006).
Acknowledgments
We would like to thank Dr. Wenxuan Yang for his invaluable help and support during the writing of this review article. Tragically, he died before submission.
References
- Allegra C, Sargent DJ. Adjuvant therapy for colon cancer – the pace quickens. N. Engl. J. Med. 2005;352:2746–2748. doi: 10.1056/NEJMe058117. [DOI] [PubMed] [Google Scholar]
- Al-Mehdi AB, Tozawa K, Fisher AB, Shientag L, Lee A, Muschel RJ. Intravascular origin of metastasis from the proliferation of endothelium-attached tumor cells: a new model for metastasis. Nat. Med. 2000;6:100–102. doi: 10.1038/71429. [DOI] [PubMed] [Google Scholar]
- Bao S, Wu Q, McLendon RE, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756–760. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- Boyle P, Ferlay J. Cancer incidence and mortality in Europe, 2004. Ann. Oncol. 2005;16:481–488. doi: 10.1093/annonc/mdi098. [DOI] [PubMed] [Google Scholar]
- Chalfie M, Tu Y, Euskirchen G, et al. Green fluorescent protein as a marker for gene expression. Science. 1994;263:802–805. doi: 10.1126/science.8303295. [DOI] [PubMed] [Google Scholar]
- Chambers AF, Naumov GN, Varghese HJ, Nadkarni KV, MacDonald IC, Groom AC. Critical steps in hematogenous metastasis: an overview. Surg. Oncol. Clin. N. Am. 2001;10:563–572. [PubMed] [Google Scholar]
- Chambers AF, Groom AC, MacDonald IC. Dissemination and growth of cancer cells in metastatic sites. Nat. Rev. Cancer. 2002;2:563–572. doi: 10.1038/nrc865. [DOI] [PubMed] [Google Scholar]
- Chen J, Tung CH, Mahmood U, et al. In vivo imaging of proteolytic activity in atherosclerosis. Circulation. 2002;105:2766–2771. doi: 10.1161/01.cir.0000017860.20619.23. [DOI] [PubMed] [Google Scholar]
- Cheng L, Fu J, Tsukamoto A, et al. Use of green fluorescent protein variants to monitor gene transfer and expression in mammalian cells. Nat. Biotechnol. 1996;14:606–609. doi: 10.1038/nbt0596-606. [DOI] [PubMed] [Google Scholar]
- Chester K, Pedley B, Tolner B, et al. Engineering antibodies for clinical applications in cancer. Tumour Biol. 2004;25:91–98. doi: 10.1159/000077727. [DOI] [PubMed] [Google Scholar]
- Cormack BP, Valdivia RH, Falkow S, Cormack BP, Valdivia RH, Falkow S. FACS-optimized mutants of the green fluorescent protein (GFP) Gene. 1996;173:33–38. doi: 10.1016/0378-1119(95)00685-0. [DOI] [PubMed] [Google Scholar]
- Ding L, Sunamura M, Kodama T, et al. In vivo evaluation of the early events associated with liver metastasis of circulating cancer cells. Br. J. Cancer. 2001;85:431–438. doi: 10.1054/bjoc.2001.1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domino SE, Karnak DM, Hurd EA, Domino SE, Karnak DM, Hurd EA. Cell surface fucosylation does not affect development of colon tumors in mice with germline Smad3 mutation. Tumour Biol. 2007;28:77–83. doi: 10.1159/000099153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eble JA, Haier J, Eble JA, Haier J. Integrins in cancer treatment. Curr. Cancer Drug Targets. 2006;6:89–105. doi: 10.2174/156800906776056518. [DOI] [PubMed] [Google Scholar]
- Enns A, Gassmann P, Schluter K, et al. Integrins can directly mediate metastatic tumor cell adhesion within the liver sinusoids. J. Gastrointest. Surg. 2004;8:1049–1059. doi: 10.1016/j.gassur.2004.08.016. [DOI] [PubMed] [Google Scholar]
- Enns A, Korb T, Schluter K, et al. Avbeta5-integrins mediate early steps of metastasis formation. Eur. J. Cancer. 2005;41:1065–1072. doi: 10.1016/j.ejca.2004.12.031. [DOI] [PubMed] [Google Scholar]
- Fidler IJ. The organ microenvironment and cancer metastasis. Differentiation. 2002;70:498–505. doi: 10.1046/j.1432-0436.2002.700904.x. [DOI] [PubMed] [Google Scholar]
- Fidler IJ. The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited. Nat. Rev. Cancer. 2003;3:453–458. doi: 10.1038/nrc1098. [DOI] [PubMed] [Google Scholar]
- Figueiredo JL, Alencar H, Weissleder R, et al. Near infrared thoracoscopy of tumoral protease activity for improved detection of peripheral lung cancer. Int. J. Cancer. 2006;118:2672–2677. doi: 10.1002/ijc.21713. [DOI] [PubMed] [Google Scholar]
- Flatmark K, Maelandsmo GM, Martinsen M, Rasmussen H, Fodstad O. Twelve colorectal cancer cell lines exhibit highly variable growth and metastatic capacities in an orthotopic model in nude mice. Eur. J. Cancer. 2004;40:1593–1598. doi: 10.1016/j.ejca.2004.02.023. [DOI] [PubMed] [Google Scholar]
- Guilbaud N, Kraus-Berthier L, Meyer-Losic F, et al. Marked antitumor activity of a new potent acronycine derivative in orthotopic models of human solid tumors. Clin. Cancer Res. 2001;7:2573–2580. [PubMed] [Google Scholar]
- Haier J, Nasralla M, Nicolson GL. Different adhesion properties of highly and poorly metastatic HT-29 colon carcinoma cells with extracellular matrix components: role of integrin expression and cytoskeletal components. Br. J. Cancer. 1999;80:1867–1874. doi: 10.1038/sj.bjc.6690614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haier J, Korb T, Hotz B, Spiegel H-U, Senninger N. An intravital model to monitor steps of metastatic tumour cell adhesion within the hepatic microcirculation. J. Gastrointest. Surg. 2003;7:507–515. doi: 10.1016/S1091-255X(03)00023-4. [DOI] [PubMed] [Google Scholar]
- Hoffman R. Green fluorescent protein imaging of tumour growth, metastasis, and angiogenesis in mouse models. Lancet Oncol. 2002;3:546–556. doi: 10.1016/s1470-2045(02)00848-3. [DOI] [PubMed] [Google Scholar]
- Iga AM, Sarkar S, Sales KM, Winslet MC, Seifalian AM. Quantitating therapeutic disruption of tumour blood flow with intravital video microscopy. Cancer Res. 2006;66:11517–11519. doi: 10.1158/0008-5472.CAN-06-1743. [DOI] [PubMed] [Google Scholar]
- Ito S, Nakanishi H, Ikehara Y, et al. Real-time observation of micrometastasis formation in the living mouse liver using a green fluorescent protein gene-tagged rat tongue carcinoma cell line. Int. J. Cancer. 2001;93:212–217. doi: 10.1002/ijc.1318. [DOI] [PubMed] [Google Scholar]
- Kavolius J, Fong Y, Blumgart LH. Surgical resection of metastatic liver tumors. Surg. Oncol. Clin. N. Am. 1996;5:337–352. [PubMed] [Google Scholar]
- Kikkawa H, Kaihou M, Horaguchi N, et al. Role of integrin alpha (v)beta3 in the early phase of liver metastasis: PET and IVM analyses. Clin. Exp. Metastasis. 2002;19:717–725. doi: 10.1023/a:1021356019563. [DOI] [PubMed] [Google Scholar]
- Koop S, MacDonald IC, Luzzi K, et al. Fate of melanoma cells entering the microcirculation: over 80% survive and extravasate. Cancer Res. 1995;55:2520–2523. [PubMed] [Google Scholar]
- Lin WC, Culp LA. Selectable plasmid vectors with alternative and ultrasensitive histochemical marker genes. Biotechniques. 1991;11:344–348. 350–351. [PubMed] [Google Scholar]
- Lin WC, Pretlow TP, Pretlow TG, et al. Bacterial LacZ gene as a highly sensitive marker to detect micrometastasis formation during tumor progression. Cancer Res. 1990;50:2808–2817. [PubMed] [Google Scholar]
- Liotta LA, Kohn EC. The microenvironment of the tumour-host interface. Nature. 2001;411:375–379. doi: 10.1038/35077241. [Review] [65 Refs] [DOI] [PubMed] [Google Scholar]
- Luzzi KJ, MacDonald IC, Schmidt EE, et al. Multistep nature of metastatic inefficiency: dormancy of solitary cells after successful extravasation and limited survival of early micrometastases. Am. J. Pathol. 1998;153:865–873. doi: 10.1016/S0002-9440(10)65628-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald IC, Groom AC, Chambers AF. Cancer spread and micrometastasis development: quantitative approaches for in vivo models. Bioessays. 2002;24:885–893. doi: 10.1002/bies.10156. [DOI] [PubMed] [Google Scholar]
- Mutsaerts EL, van RS, Zoetmulder FA, Rutgers EJ, Hart AA, van CF. Prognostic factors and evaluation of surgical management of hepatic metastases from colorectal origin: a 10-year single-institute experience. J. Gastrointest. Surg. 2005;9:178–186. doi: 10.1016/j.gassur.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Naumov GN, Wilson SM, MacDonald IC, et al. Cellular expression of green fluorescent protein, coupled with high-resolution in vivo videomicroscopy, to monitor steps in tumor metastasis. J. Cell Sci. 1999;112:1835–1842. doi: 10.1242/jcs.112.12.1835. [DOI] [PubMed] [Google Scholar]
- Nelson H, Petrelli N, Carlin A, et al. Guidelines 2000 for colon and rectal cancer surgery. J. Natl Cancer Inst. 2001;93:583–596. doi: 10.1093/jnci/93.8.583. [DOI] [PubMed] [Google Scholar]
- O’Brien CA, Pollett A, Gallinger S, et al. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature. 2007;445:106–110. doi: 10.1038/nature05372. [DOI] [PubMed] [Google Scholar]
- Onn A, Fidler IJ. Metastatic potential of human neoplasms. In Vivo. 2002;16:423–429. [PubMed] [Google Scholar]
- Paget S. The distribution of secondary growths in cancer of the breast. Lancet. 1889;1:571–573. [PubMed] [Google Scholar]
- Parkin DM, Bray F, Ferlay J, Pisani P. Estimating the world cancer burden: Globocan 2000. Int. J. Cancer. 2001;94:153–156. doi: 10.1002/ijc.1440. [DOI] [PubMed] [Google Scholar]
- Philipp-Staheli J, Kim KH, Payne SR, et al. Pathway-specific tumor suppression. reduction of P27 accelerates gastrointestinal tumorigenesis in Apc mutant mice, but not in Smad3 mutant mice. Cancer Cell. 2002;1:355–368. doi: 10.1016/s1535-6108(02)00054-5. [DOI] [PubMed] [Google Scholar]
- Reinmuth N, Liu W, Ahmad SA, et al. Alphavbeta3 integrin antagonist s247 decreases colon cancer metastasis and angiogenesis and improves survival in mice. Cancer Res. 2003;63:2079–2087. [PubMed] [Google Scholar]
- Scheele J, Stang R, tendorf-Hofmann A, Paul M. Resection of colorectal liver metastases. World J. Surg. 1995;19:59–71. doi: 10.1007/BF00316981. [DOI] [PubMed] [Google Scholar]
- Schluter K, Gassmann P, Enns A, et al. Organ-specific metastatic tumor cell adhesion and extravasation of colon carcinoma cells with different metastatic potential. Am. J. Pathol. 2006;169:1064–1073. doi: 10.2353/ajpath.2006.050566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbauer M, Guba M, Cernaianu G, et al. GFP-transfected tumor cells are useful in examining early metastasis in vivo, but immune reaction precludes long-term tumor development studies in immunocompetent mice. Clin. Exp. Metastasis. 2003;20:135–141. doi: 10.1023/a:1022618909921. [DOI] [PubMed] [Google Scholar]
- Sturm JW, Keese MA, Petruch B, et al. Enhanced green fluorescent protein-transfection of murine colon carcinoma cells: key for early tumor detection and quantification. Clin. Exp. Metastasis. 2003;20:395–405. doi: 10.1023/a:1025470312074. [DOI] [PubMed] [Google Scholar]
- Sweeney TJ, Mailander V, Tucker AA, et al. Visualizing the kinetics of tumor-cell clearance in living animals. Proc. Natl Acad. Sci. USA. 1999;96:12044–12049. doi: 10.1073/pnas.96.21.12044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tung CH, Mahmood U, Bredow S, et al. In vivo imaging of proteolytic enzyme activity using a novel molecular reporter. Cancer Res. 2000;60:4953–4958. [PubMed] [Google Scholar]
- Tung CH, Zeng Q, Shah K, et al. In vivo imaging of beta-galactosidase activity using far red fluorescent switch. Cancer Res. 2004;64:1579–1583. doi: 10.1158/0008-5472.can-03-3226. [DOI] [PubMed] [Google Scholar]
- Twelves C, Wong A, Nowacki MP, et al. Capecitabine as adjuvant treatment for stage III colon cancer. N. Engl. J. Med. 2005;352:2696–2704. doi: 10.1056/NEJMoa043116. [DOI] [PubMed] [Google Scholar]
- Uggeri J, Gatti R, Belletti S, et al. Calcein-AM is a detector of intracellular oxidative activity. Histochem. Cell Biol. 2004;122:499–505. doi: 10.1007/s00418-004-0712-y. [DOI] [PubMed] [Google Scholar]
- Weissleder R, Tung CH, Mahmood U, et al. In vivo imaging of tumors with protease-activated near-infrared fluorescent probes. Nat. Biotechnol. 1999;17:375–378. doi: 10.1038/7933. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Richardson JA, Parada LF, et al. Smad3 mutant mice develop metastatic colorectal cancer. Cell. 1998;94:703–714. doi: 10.1016/s0092-8674(00)81730-4. [DOI] [PubMed] [Google Scholar]
- Zolotukhin S, Potter M, Hauswirth WW, et al. A “humanized” green fluorescent protein cDNA adapted for high-level expression in mammalian cells. J. Virol. 1996;70:4646–4654. doi: 10.1128/jvi.70.7.4646-4654.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]