Abstract
As methods of cancer diagnosis and treatment progress, interest in metastatic brain tumours continues to increase. There are many studies using various methods of animal model and we considered that each model reflects different pathological processes because of the unique composition of the brain. We prepared metastatic brain tumour models using three different methods. In this study, we attempted to elucidate the roles of the pia mater in brain metastasis. The metastatic foci showed an angiocentric pattern, forming collars of neoplastic cells, and were designated ‘perivascular proliferations’. Furthermore, we observed neoplastic cells that infiltrated the brain parenchyma, the border of which had become indistinct. These were labelled ‘invasive proliferations’. The internal carotid artery injection model reflects haematogenous metastasis. In this model, both perivascular and invasive proliferations were observed. The intrathecal injection model reflects leptomeningeal carcinomatosis. In this model, metastasis to the meninges was observed. In the stereotactic injection model, the tumour proliferation at the injection site and the infiltration into the brain parenchyma were observed. The pia-glial membrane serves as a scaffold when neoplastic cells spread to the perivascular space forming angiocentric pattern. The pia-glial membrane is found between the brain parenchyma and blood vessels. Blood vessels penetrate the brain through tunnels known as perivascular spaces that are covered by pia mater. Three different methods which we prepared reflect three different pathological processes. Our findings suggest that the pia mater is a critical factor in brain metastasis.
Keywords: animal model, brain metastasis, haematogenous metastasis, perivascular space, pia mater
Recently, the number of patients with metastatic brain tumours has increased. As methods of cancer diagnosis and treatment progress, interest in metastatic brain tumours continues to increase. The proliferation of neoplastic cells in the brain can sometimes be controlled by treatments that prolong survival; however, increased survival time may provide further opportunities for brain metastases (Kindt 1964; Nugent et al. 1979).
Haematogenous metastasis is a common route to brain metastasis, and animal models that mimic this process are critical in understanding the pathogenesis and treatment of brain metastases.
There are many studies using various methods of animal model (Shapiro et al. 1979; Yoshida et al. 1986; Frank et al. 1988; Schackert & Fidler 1988, 1989; Fujimaki et al. 1993; Kim et al. 2004; Mendes et al. 2005), and we considered that each model reflects different pathological processes because of the unique composition of the brain.
Blood vessels penetrate the central nervous system through tunnels covered by the pia mater, forming the perivascular space (Williams et al. 1995; Gartner & Hiatt 2001; Urabe et al. 2002; Junqueura et al. 2005). Type IV collagen is one of the major components of the basement membrane (Williams et al. 1995; Gartner & Hiatt 2001; Urabe et al. 2002). A functional barrier composed of the pia mater and the blood–brain barrier prevents the passage of certain substances from the blood (Junqueura et al. 2005).
We prepared metastatic brain tumour models using three different methods. In this study, we attempted to elucidate the roles of the pia mator in brain metastasis.
Materials and methods
Mice
C57BL/6Ncrj male mice, 8–9 weeks old, purchased from Charles River Co. (Yokohama, Japan) were housed and cared for at the animal care facility of Toho University School of Medicine in accordance with the institution’s guidelines for the care and use of laboratory animals. The experimental protocol was approved by the Animal Research Committee, Toho University School of Medicine (ARC/TUSM).
Tumour cell line & cell culture
The 3LL (Lewis lung carcinoma) tumour cell line used in this study was obtained from the Cell Resource Center for Biomedical Research, Tohoku University.
The cells were maintained in RPMI-1640 medium (Sigma-Aldrich Co., St Louis, MO, USA) supplemented with 10% fetal bovine serum (FCS; Hyclone Laboratories, Logan, UT, USA), and were incubated at 37 °C in a humidified atmosphere of 5% CO2 in air.
Preparation for injection of the tumour cells
The volume and concentration of the inoculum was 0.1 ml and 3 × 105/ml respectively.
Internal carotid artery injection
The mice were anaesthetized by an intraperitoneal injection of pentobarbital sodium and fixed on a cork board by dorsal position. In order to stabilize the head and neck, a silk suture was placed between the teeth of the upper jaw and the straightened cervix. For surgery, the neck was sterilized using 70% ethanol and a midline incision was made. The right carotid artery and the vagus nerve were located between the digastric muscle and sternomastoid muscle. The artery was exposed by blunt dissection from the common carotid artery to the point of division into the internal and external carotid arteries. Two ligatures of 5-0 silk suture material were placed in the distal and proximal regions of the common carotid artery. First, the proximal side was tied tightly, while the distal side was tied loosely. The artery was nicked using a microscissor, and a glass cannula was inserted into the lumen of the artery. An inoculum of 0.1 ml was slowly injected, and then the cannula was removed. The distal ligature was tightened and the skin was closed by a suture. All operations were carried out under a microscope (n = 5).
Intrathecal injection
Mice were anaesthetized by intraperitoneal injection of pentobarbital sodium. The mice cisterna magna was punctured with a 30-gauge needle, and injecting 0.05 ml of cell suspension.
Stereotactic injection
Mice were anaesthetized by intraperitoneal injection of pentobarbital sodium. We slightly modified an injection method described by Shapiro et al. (Shapiro et al. 1979). Briefly, 0.01 ml of cell suspension was delivered by a 30-gauge needle attached to Hamilton syringe. The needle was inserted at a depth of 3 mm dorsal to the coronal suture and 3 mm lateral to sagittal suture.
Tissue sampling
Seven days after the injection, the animals began to show neurological signs such as head tilt. In this study, the mice were killed on day 9. For histological studies, the brain was removed from the cranial cavity and preserved in 4% paraformaldehyde fixative overnight and then embedded in paraffin (n = 6, each).
Histological observation
The histological observation was performed by haematoxylin and eosin (H & E) staining.
Electron microscopy
The mice were anaesthetized by an intraperitoneal injection of pentobarbital sodium and then the aorta was canulated. The mice were perfused with physiological saline for 5 min to wash out blood cells, followed by phosphate-buffered fixative (1% glutaraldehyde and 2% paraformaldehyde in 0.1 M phosphate buffer, pH 7.3) for 15 min. After perfusion, the brain was removed from the cranial cavity and preserved in the fixative.
The brains were then processed for electron microscopy. Ultrathin sections were examined using JEM-1200EX-II (JEOL, Tokyo, Japan) (n = 5, each).
Results
Microscopic observation
Internal carotid artery injection
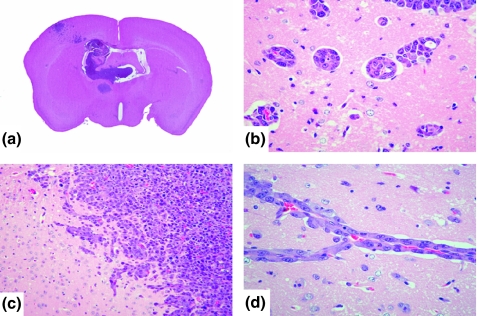
Brain metastatic foci were observed in the deep cortex of an agranular motor cortical area and somatosensory cortex, the posterior limbic area and Ammon’s horn (CA1∼CA3). They were perfused by the deep cortical branches of cortical arteries and the side branches of subcortical arteries (Akima et al. 1986; Nonaka et al. 2003a,b,c); no metastatic foci were observed in the white matter (Figure 1a).
Figure 1.
(a) Macroscopic findings (H&E staining) of brain metastasis of internal carotid artery injection. Tumour foci of brain metastasis are present in the posterior limbic area and Ammon’s horn, in addition to the agranular motor cortical area and somatosensory cortex. (b, d) The metastatic foci were localized in the perivascular lesion. (c) Invasive proliferations, neoplastic cells infiltrated the brain parenchyma, the border of which had become indistinct.
These foci were localized in the perivascular lesion (Figure 1b,d). The metastatic foci showed an angiocentric pattern, forming collars of neoplastic cells, and were designated ‘perivascular proliferations’.
In addition, we observed neoplastic cells that infiltrated the brain parenchyma, the border of which had become indistinct. These were labelled ‘invasive proliferations’ (Figure 1c). As in the case of perivascular proliferations, invasive proliferations were observed in the agranular motor cortical area, somatosensory cortex, posterior limbic area and Ammon’s horn (CA1∼CA3).
The brain metastatic foci were formed in the choroid plexus of the third and lateral ventricles. The neoplastic cells were confined to the choroid plexus, however, without disintegration of the ependymal epithelium (Figure 1a).
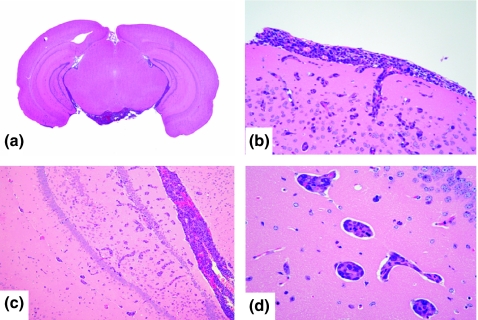
Intrathecal injection
Brain metastatic foci were observed in the base of the brain (Figure 2a). Metastasis to the meninges was formed at this site. Neoplastic cells did not infiltrate the brain parenchyma (Figure 2a,b), but angiocentric pattern to cortical arteries that invaded from the surface of the brain into the brain parenchyma were also observed (Figure 2b).
Figure 2.
(a) Macroscopic findings (H&E staining) of brain metastasis of intrathecal injection. Brain metastatic foci were observed in the base of the brain. (b) Metastasis to the meninges was observed. (c, d) Metastatic foci showed ‘perivascular proliferations’.
The brain metastatic foci were formed in the third and lateral ventricles (Figure 2a). In addition, numerous small metastatic foci were observed in the brain parenchyma away from the surface, mainly located Ammon’s horn (Figure 2c). These metastatic foci showed ‘perivascular proliferations’ without infiltration (Figure 2d).
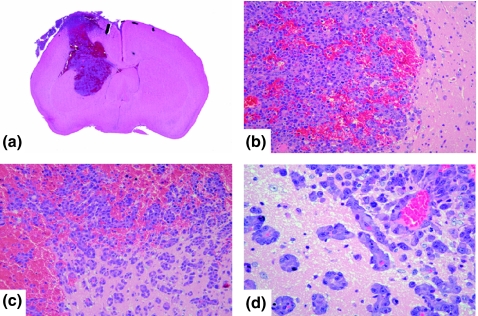
Stereotactic injection
At the injection site, the metastatic foci were formed along the direction of needle insertion (Figure 3a), and neoplastic cells infiltrated the brain parenchyma (Figure 3b,c).
Figure 3.
(a) Macroscopic findings (H&E staining) of stereotactic injection. (b, c) Invasive proliferations. (d) Perivascular proliferations of stereotactic injection.
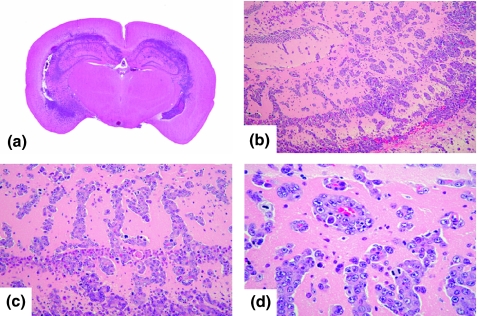
Small metastatic foci were observed slightly apart from the border between the tumour and the brain parenchyma. These sites showed perivascular proliferations (Figure 3c,d). In addition, numerous metastatic foci were observed away from the injection site without continuity (Figure 4a). These lesions were observed not only in the cortex, but also widely extended to the medulla and basal ganglia (Figure 4b–d).
Figure 4.
(a) Macroscopic findings (H&E staining) of metastasis away from the injection site. (b, c, d) These lesions were observed not only in the cortex, but also widely extended to the medulla and basal ganglia.
Electron microscopic observation
Internal carotid artery injection
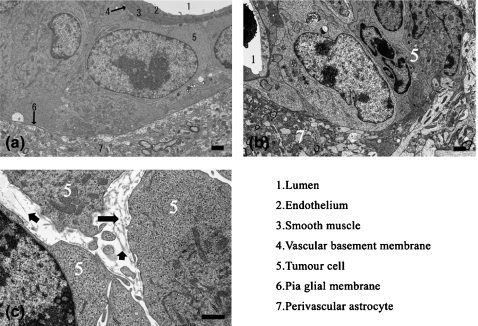
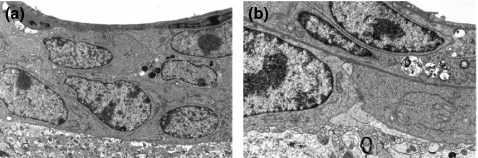
In perivascular proliferation, neoplastic cells were localized to the area between the vascular basement membrane and pia-glial membrane: the perivascular space (Akima 1972; Hutchings & Weller 1986; Pollock et al. 1997; Gartner & Hiatt 2001; Urabe et al. 2002; Junqueura et al. 2005). The pia-glial membrane was intact and no infiltration of the brain parenchyma was observed. Vascular endothelial cells and tight junctions were morphologically intact (Figure 5a).
Figure 5.
Electron microscopic observation. (a) Perivascular proliferation. Neoplastic cells were localized between the vascular basement membrane and pia-glial membrane (b) Invasive proliferation. Neoplastic cells were observed in the brain parenchyma, and the pia-glial membrane was absent. (c) Fragments of collagen fibres were observed in the gap between neoplastic cells. Arrows point to the fragment of collagen fibres.
In invasive proliferation, neoplastic cells were present in the brain parenchyma, and the pia-glial membrane was absent (Figure 5b). Fragments of collagen fibres were observed in the gap between neoplastic cells (Figure 5c), indicating that the neoplastic cells had disintegrated the pia-glial membrane.
Intrathecal injection
In perivascular proliferation, neoplastic cells were confined to the perivascular space. The pia-glial membrane, vascular endothelial cells and tight junctions were morphologically intact and no infiltration of the brain parenchyma was observed (Figure 6a).
Figure 6.
Electron microscopic observation. (a) Intrathecal injection in perivascular proliferation, neoplastic cells were confined to the perivascular space. (b) Stereotactic injection. Neoplastic cells were observed both in the perivascular space and in the brain parenchyma, across the pia-glial membrane.
Stereotactic injection
In perivascular proliferation, neoplastic cells were observed both in the perivascular space and in the brain parenchyma, across the pia-glial membrane. In this area, the pia-glial membrane was intact, and neoplastic cells were adhered to the pia-glial membrane (Figure 6b).
Discussion
The brain is frequently affected by the spread of lung cancer, and approximately 40% of metastatic brain tumours are found during the autopsy of lung cancer. In a 2-year observational study, approximately 80% of brain metastases were found to originate from small cell carcinomas (Nugent et al. 1979).
The histological findings of metastatic brain tumour in humans are usually, but not always, similar to those of the primary tumour. Infiltrations of perivascular zones that display an angiocentric pattern and form collars of neoplastic cells may be present; however, this is not considered a characteristic of brain metastasis (Burger & Scheithauer 1994). This phenomenon is not observed in metastatic foci in other organs and develops independently of the primary tumour. In the present study, these lesions were designated ‘perivascular proliferations’. We hypothesize that the unusual features of perivascular proliferations in brain metastasis are a result of the unique composition of the brain.
The pia-glial membrane is mainly composed of type IV collagen and is found between the brain parenchyma and blood vessels. Blood vessels penetrate the central nervous system through tunnels known as perivascular spaces that are covered by pia mater. The pia-glial membrane comprises the cerebrospinal fluid-brain barrier (Junqueura et al. 2005).
We assume that the perivascular space differs from the Virchow-Robin space, which has been determined microscopically to consist of the perivascular space and shrinkage artefacts of perivascular astroglial end-feet (Akima 1972; Hutchings & Weller 1986; Gartner & Hiatt 2001).
There are many studies using various methods of animal model (Shapiro et al. 1979; Yoshida et al. 1986; Frank et al. 1988; Schackert & Fidler 1988, 1989; Fujimaki et al. 1993; Kim et al. 2004; Mendes et al. 2005), and we considered that each model reflects different pathological processes.
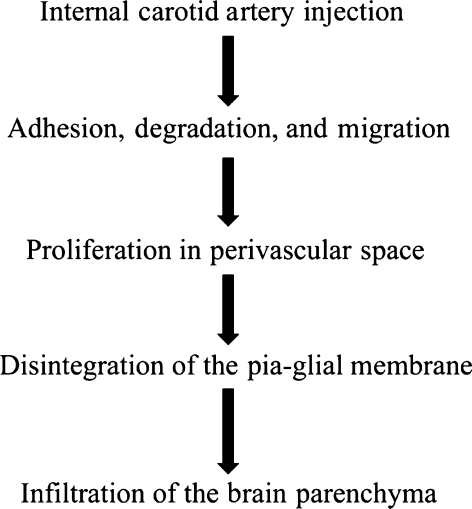
The internal carotid artery injection model reflects haematogenous metastasis (Figure 7). In this model, both perivascular and invasive proliferations were observed (Figure 1a–d). In microscopic observation of perivascular proliferations, the pia-glial membrane was intact and no infiltration of the brain parenchyma was observed. Vascular endothelial cells and tight junctions were morphologically intact (Figure 5a).
Figure 7.
Flowchart of internal carotid artery injection.
In invasive proliferation, fragments of collagen fibres were observed in the gap between neoplastic cells (Figure 5c), indicating that the neoplastic cells had disintegrated the pia-glial membrane.
In our latest report (Saito et al. 2007), we proposed a ‘double three-step theory’ for brain metastasis. This theory indicates that the perivascular proliferations evolve into invasive parenchymal proliferations.
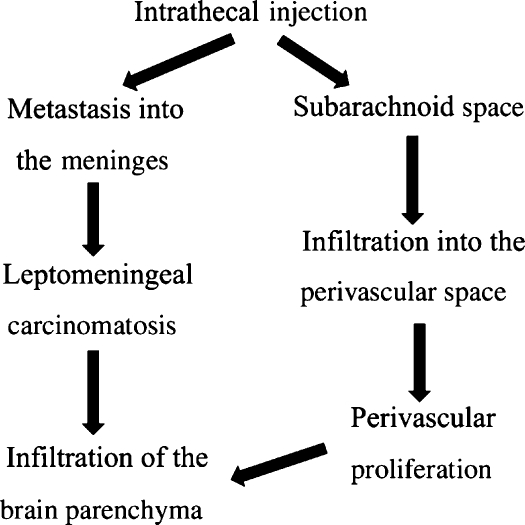
The intrathecal injection model reflects leptomeningeal carcinomatosis (Kokkoris 1983). In this model, metastasis to the meninges was observed (Figure 2a–d).
Furthermore, neoplastic cells formed perivascular proliferations and penetrated the central nervous system with blood vessels. In addition, numerous small metastatic foci were observed in the brain parenchyma away from the surface (Figure 6a).
The continuity of the subarachnoid space with the perivascular space has previously been demonstrated (Yoshida et al. 1986; Pollock et al. 1997), we considered that the neoplastic cells spread to the perivascular space along blood vessels (Figure 8).
Figure 8.
Flowchart of intrathecal injection.
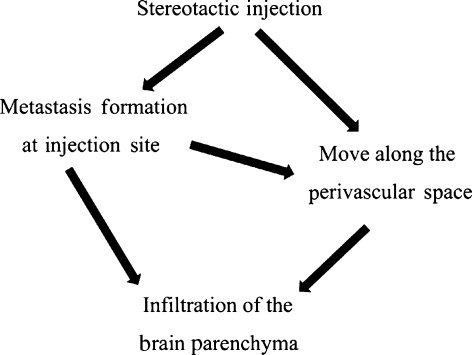
In the stereotactic injection model, the tumour proliferation at the injection site and the infiltration into the brain parenchyma were observed (Figures 3 and 4). We think that infiltrative proliferation occurs easily at the injection site because needle injection made manual destruction the brain parenchyma, the meninges, and the blood vessels. Furthermore, neoplastic cells spread to the perivascular space move along blood vessels (Figure 9), as in the intrathecal injection model, showing perivascular proliferations (Figure 4c,d).
Figure 9.
Flowchart of stereotactic injection.
The pia-glial membrane is mainly composed of type IV collagen and is found between the brain parenchyma and blood vessels. Blood vessels penetrate the brain through tunnels known as perivascular spaces that are covered by pia mater (Akima 1972; Hutchings & Weller 1986; Williams et al. 1995; Gartner & Hiatt 2001; Urabe et al. 2002). We assume that the perivascular space differs from the Virchow-Robin space, which has been determined microscopically to consist of the perivascular space and shrinkage artefacts of perivascular astroglial end-feet (Akima 1972; Hutchings & Weller 1986; Gartner & Hiatt 2001).
The pia-glial membrane serves as a scaffold when neoplastic cells spread to the perivascular space forming angiocentric pattern.
Three different methods which we prepared reflect three different pathological processes (Figures 7–9). Our findings suggest that the pia mater is a critical factor in brain metastasis.
Acknowledgments
We thank all the members of the 2nd Department of Neurosurgery and Department of Surgical Pathology, Toho University School of Medicine, for their collaboration, and Toshie Shimozeki and Akira Maeda for their skilful technical assistance.
References
- Akima M. Reaction to injuries in the brain – an electron microscopic analysis of its topographical characteristics. Acta Pathol. Jpn. 1972;22:649–680. doi: 10.1111/j.1440-1827.1972.tb00754.x. [DOI] [PubMed] [Google Scholar]
- Akima M, Nonaka H, Kagesawa M, Tanaka K. A study on the microvasculature of the cerebral cortex. Fundamental architecture and its senile change in the frontal cortex. Lab. Invest. 1986;55:482–489. [PubMed] [Google Scholar]
- Burger PC, Scheithauer BW. Atlas of tumor pathology: tumors of the central nervous system. Washington, DC: Armed Forces Institute of Pathology, Inc; 1994. 3rd series. [Google Scholar]
- Frank JA, Girton M, Dwyer AJ, Wright D, Cohen PJ, Doppman JL. Meningeal carcimatosis in the VX rabbit tumor model: detection with Gd-DTPA-enhanced MR imaging. Radiology. 1988;167:825–829. doi: 10.1148/radiology.167.3.3363148. [DOI] [PubMed] [Google Scholar]
- Fujimaki T, Fan D, Staroselsky AH, Gohji K, Bucana CD, Fidler IJ. Critical factors regulating site specific brain metastasis of murine melanomas. Int. J. Oncol. 1993;3:789–799. doi: 10.3892/ijo.3.5.789. [DOI] [PubMed] [Google Scholar]
- Gartner LP, Hiatt JL. Color atlas of histology. 4th edn. Philadelphia, PA: Lippincott, Williams & Wilkins; 2001. [Google Scholar]
- Hutchings M, Weller RO. Anatomical relationships of the pia mater to cerebral blood vessels in man. J. Neurosurg. 1986;65:316–325. doi: 10.3171/jns.1986.65.3.0316. [DOI] [PubMed] [Google Scholar]
- Junqueura LC, Carneiro J, Kelly RO. Basic histology text & atlas. New York: McGraw -Hill; 2005. [Google Scholar]
- Kim LS, Huang S, Lu W, Lev DC, Price JE. Vascular endothelial growth factor expression promotes the growth of breast cancer brain metastases in nude mice. Clin. Exp. Metastasis. 2004;21:107–118. doi: 10.1023/b:clin.0000024761.00373.55. [DOI] [PubMed] [Google Scholar]
- Kindt GW. The pattern of location of cerebral metastatic tumors. J. Neurosurg. 1964;21:54–57. doi: 10.3171/jns.1964.21.1.0054. [DOI] [PubMed] [Google Scholar]
- Kokkoris CP. Leptomeningeal carcinimatosis. How dose cancer reach the pia-arachnoid? Cancer. 1983;51:154–160. doi: 10.1002/1097-0142(19830101)51:1<154::aid-cncr2820510130>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- Mendes O, Kim HT, Stoica G. Expression of MMP2, MMP9 and MMP3 in breast cancer brain metastasis in a rat model. Clin. Exp. Metastasis. 2005;22:237–246. doi: 10.1007/s10585-005-8115-6. [DOI] [PubMed] [Google Scholar]
- Nonaka H, Akima M, Hatori T, Nagayama T, Zhang Z, Ihara F. The microvasculature of the cerebral white matter: arteries of the subcortical white matter. J. Neuropathol. Exp. Neurol. 2003a;62:154–161. doi: 10.1093/jnen/62.2.154. [DOI] [PubMed] [Google Scholar]
- Nonaka H, Akima M, Hatori T, Nagayama T, Zhang Z, Ihara F. Microvasculature of the human cerebral white matter: arteries of the deep white matter. Neuropathology. 2003b;23:111–118. doi: 10.1046/j.1440-1789.2003.00486.x. [DOI] [PubMed] [Google Scholar]
- Nonaka H, Akima M, Nagayama T, Hatori T, Zhang Z, Ihara F. Microvasculature of the human cerebral meninges. Neuropathology. 2003c;23:129–135. doi: 10.1046/j.1440-1789.2003.00487.x. [DOI] [PubMed] [Google Scholar]
- Nugent JL, Bunn PA, Jr, Matthews MJ, et al. CNS metastases in small cell bronchogenic carcinoma: increasing frequency and changing pattern with lengthening survival. Cancer. 1979;44:885–893. doi: 10.1002/1097-0142(197911)44:5<1885::aid-cncr2820440550>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Pollock H, Hutchings M, Weller RO, Zhang ET. Perivascular spaces in the basal ganglia of the human brain: their relationship to lacunes. J. Anat. 1997;191:337–346. doi: 10.1046/j.1469-7580.1997.19130337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito N, Hatori T, Murata N, et al. A double three-step theory of brain metastasis in mice: the role of the pia mater and matrix metalloproteinases. Neuropathol. Appl. Neurobiol. 2007;33:288–298. doi: 10.1111/j.1365-2990.2007.00799.x. [DOI] [PubMed] [Google Scholar]
- Schackert G, Fidler IJ. Site-specific metastasis of mouse melanomas and a fibrosarcoma in the brain or meninges of syngeneic animals. Cancer Res. 1988;6:3478–3484. [PubMed] [Google Scholar]
- Schackert HK, Fidler IJ. Development of an animal model to study the biology of recurrent colorectal cancer originating from mesenteric lymph system metastases. Int. J. Cancer. 1989;154:177–181. doi: 10.1002/ijc.2910440131. [DOI] [PubMed] [Google Scholar]
- Shapiro WR, Basler GA, Chernik NL, Posner JB. Human brain tumor transplantation into nude mice. J. Natl Cancer Inst. 1979;62:447–453. doi: 10.1093/jnci/62.3.447. [DOI] [PubMed] [Google Scholar]
- Urabe N, Naito I, Saito K, et al. Basement membrane type IV collagen molecules in the choroid plexus, pia mater and capillaries in the mouse brain. Arch. Histol. Cytol. 2002;65:133–143. doi: 10.1679/aohc.65.133. [DOI] [PubMed] [Google Scholar]
- Williams PL, Bannister LH, Berry MM. Gray’s Anatomy. 38th edn. New York: Churchill Livingstone; 1995. [Google Scholar]
- Yoshida T, Shimizu K, Ushio Y, Hayakawa T, Arita N, Mogami H. Development of experimental meningeal gliomatosis in rats. J. Neurosurg. 1986;65:503–507. doi: 10.3171/jns.1986.65.4.0503. [DOI] [PubMed] [Google Scholar]